Keywords: nerve regeneration, psychological stress, serotonin, 5-HT1A, 5-HT2A, brain-derived neurotrophic factor, neural regeneration
Abstract
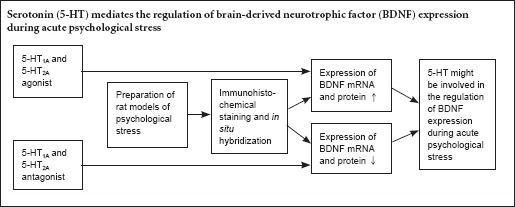
Previous studies suggest that serotonin (5-HT) might interact with brain-derived neurotrophic factor (BDNF) during the stress response. However, the relationship between 5-HT and BDNF expression under purely psychological stress is unclear. In this study, one hour before psychological stress exposure, the 5-HT1A receptor agonist 8-OH-DPAT or antagonist MDL73005, or the 5-HT2A receptor agonist DOI or antagonist ketanserin were administered to rats exposed to psychological stress. Immunohistochemistry and in situ hybridization revealed that after psychological stress, with the exception of the ventral tegmental area, BDNF protein and mRNA expression levels were higher in the 5-HT1A and the 5-HT2A receptor agonist groups compared with the solvent control no-stress or psychological stress group in the CA1 and CA3 of the hippocampus, prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, shell of the nucleus accumbens and the midbrain periaqueductal gray. There was no significant difference between the two agonist groups. In contrast, after stress exposure, BDNF protein and mRNA expression levels were lower in the 5-HT1A and 5-HT2A receptor antagonist groups than in the solvent control non-stress group, with the exception of the ventral tegmental area. Our findings suggest that 5-HT regulates BDNF expression in a rat model of acute psychological stress.
Introduction
The prevalence of psychological stress (PS) (such as bereavement, divorce and joblessness) in modern life is currently on the rise. The human health problems related to PS have become an important clinical issue for psychologists and psychiatrics (Leuner and Shors, 2013).
Previous studies have shown that chronic stress induces structural and functional changes in the brain (Romeo, 2016). For example, brain-derived neurotrophic factor (BDNF) expression in specific brain regions (i.e., mesocorticolimbic brain reward areas and the learning-related circuit) (Holly et al., 2015; Uwaya et al., 2016) is altered by social defeat stress. Some studies have demonstrated that the brain may adapt through neural plasticity to cope with stressful situations. BDNF plays a key role in neural plasticity (Rutherford et al., 1998; Morrison and Ressler, 2014). Serotonin (5-HT) plays important roles in central nervous system functions, and the dysregulation of 5-HT systems is associated with a variety of mental disorders (Murphy et al., 2013; Olivier, 2015). In addition, selective 5-HT re-uptake inhibitors clinically alleviate stress-related disorders (Amos et al., 2014). These studies indicate that 5-HT may play a key role in the body's response to stress.
A recent study shows that exogenous BDNF improves the functioning, sprouting and growth of 5-HT neurons in various rat brain regions (Alleva and Santucci, 2001). Several animal models of stress have been established for examining 5-HT and BDNF expression changes during stress (Garabadu et al., 2015; Yan et al., 2015). For example, Foltran and Diaz (2016) found that BDNF interacts with 5-HT during the stress response. Similarly, our previous study indicated that pure PS also induces BDNF alterations in rat models of acute PS (Li et al., 2016). Therefore, we hypothesized that 5-HT might regulate BDNF expression in rat models of acute PS.
Although physical stimuli-induced stresses, including electric foot shock, cold-restraint, immobilization and forced swimming, were used in almost all previous studies, only a few examined the relationship between 5-HT and BDNF expression under pure PS. To clarify the relationship between 5-HT and the regulation of BDNF expression under pure PS, we adopted the communication box paradigm (Ogawa et al., 1993; Nomura et al., 1995), which is a model of pure PS without physical stress. Moreover, we used agonists and antagonists of 5-HT1A and 5-HT2A, which are the most representative subtype receptors of the 5-HT system (Vaidya et al., 1997; Hajós-Korcsok et al., 1999), to examine the role of 5-HT in the regulation of BDNF expression in the brain after acute PS.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Central South University of China. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Second Xiangya Hospital, Central South University, China (No.2005-99). Precautions were taken to minimize suffering and the number of animals used in each experiment.
Animals
A total of 155 specific-pathogen-free 2-month-old male Sprague-Dawley rats, weighing 180–220 g, were used for all of the experiments. Rats were housed in standard polycarbonate cages (four rats per cage), under a 12-hour light/dark cycle and a temperature-controlled environment (23 ± 1°C), with free access to food and water.
The rats were randomly divided into the following six groups: 5-HT1A receptor agonist (8-OH-DPAT) PS group (DPAT-PS group, n = 30); 5-HT1A receptor antagonist (MDL73005) PS group (MDL-PS group, n = 30); 5-HT2A receptor agonist (DOI) PS group (DOI-PS group, n = 30); 5-HT2A receptor antagonist (ketanserin) PS group (Ketan-PS group, n = 30); the solvent control no-stress group (0.9% physiological saline group, CON group); and the PS only group (PS group, n = 30). The DPAT-PS, MDL-PS, DOI-PS, Ketan-PS and PS groups were further divided into six subgroups (n = 5 each) according to the time between the stress and analysis; immediately after stress, and 0.5, 1, 2, 6 and 24 hours after stress. The CON group (n = 5) received normal feed.
Treatments
For the DPAT-PS group, 8-OH-DPAT (Sigma-Aldrich, St. Louis, MO, USA), dissolved in 0.9% physiological saline, was injected intraperitoneally at 1 mg/kg at 1 hour before each stress exposure (Tricklebank et al., 1984).
For the MDL-PS group, MDL73005 (Tocris Bioscience, Bristol, UK), dissolved in 0.9% physiological saline, was injected intraperitoneally at 2 mg/kg at 1 hour before each stress exposure (Hajós-Korcsok et al., 1999).
For the DOI-PS group, DOI (Sigma-Aldrich), dissolved in 0.9% physiological saline, was injected intraperitoneally at 3 mg/kg at 1 hour before each stress exposure (Cavus and Duman, 2003).
For the Ketan-PS group, ketanserin (Tocris Bioscience), dissolved in 0.9% physiological saline, was injected intraperitoneally at 5 mg/kg at 1 hour before each stress exposure (Niitsu et al., 1995).
For the CON group, 5 mL 0.9% physiological saline was injected into the rats.
In the PS group, the rats were only exposed to PS.
PS exposure
All rats were exposed to stress with the communication box paradigm once a day for 2 days. The communication box apparatus was modified from a protocol reported previously (Gomita et al., 1989), and was characterized by the complete removal of physical stimuli from the responder rats. PS in the responder rats was induced solely by communication between the responder rats and the sender rats. The apparatus used for this study consisted of a box with wooden walls that measured 60 cm in width, 60 cm in length, and 44 cm in height. The floor of the apparatus consisted of a grid of stainless steel rods, 5 mm in diameter and spaced 1 cm apart, center to center. The box interior was divided into nine compartments with transparent Plexiglas walls. Each compartment measured 20 cm in length and width, and 44 cm in height. Each Plexiglas wall had a single hole (6 cm from the floor, 2 cm in diameter).
The sender rats were subjected daily to 60 foot shocks (1.5–2.2 mA, 5 seconds per trial; interval: 55 seconds) while confined in the communication box for 1 hour (8:00–9:00 a.m.) for 2 consecutive days. Sender rats that responded to the foot shock stimulus were identified by behavioral reactions, such as squeals, jumps, piloerection and defecation. A thick insulated plate was placed on the floor of the “responder rat” compartments to prevent foot shock. The animals in the responder rat compartments were influenced by visual, auditory and olfactory responses of the senders, but they did not receive any direct physical stimulus. To minimize the influence of environmental factors, the sender rats underwent adaptive training in the communication box before the shock stimulus trial. Before the stress stimulus, the open field and elevated plus maze tests were performed to assess the baseline behavioral indexes of the rats in the six groups to examine the effect of the novel environment on the rats. The results indicated that there were no significant differences in the behavioral index among these groups.
Sample preparation
At each time point after stress, each rat was intraperitoneally anesthetized with pentobarbital sodium (40 mg/kg body weight). A thoracic and abdominal incision was made to expose the heart. Intubation was implemented through the left ventricle into the ascending aorta. The right atrial appendage was then cut open. Sterile saline (150 mL) was used for rapid perfusion until the effluent was clear. Then, for fixation, 250 mL of 4% paraformaldehyde was perfused rapidly at first, and then slowly for 30 minutes. The brain was then removed, and brain tissue was placed in the same fixative for 2 hours at 4°C. A 30% sucrose solution was added until the sample sank to the bottom. Continuous coronal sections were obtained using a cryostat (American HistoSTAT MicroTOME, Southbridge, MA, USA) at –20°C. The sections were 30 μm in thickness. With reference to a rat anatomical atlas (Paxinos and Watson, 1997), specimens containing the CA1 and CA3 hippocampal regions as well as the dentate gyrus, prefrontal cortex, central amygdaloid nucleus, shell of the nucleus accumbens, midbrain periaqueductal gray, ventral tegmental area and dorsomedial hypothalamic nucleus were obtained.
In situ hybridization
The rats in each group were intraperitoneally anesthetized with pentobarbital sodium (40 mg/kg) and perfused transcardially with physiological saline containing 4% paraformaldehyde. Brains were removed and fixed in 4% paraformaldehyde for 1 hour at 37°C, and then immersed in 30% sucrose solution until they sank completely. Coronal sections (30 μm) were cut using an AO HistoSTAT cryomicrotome (American Optical, Southbridge, MA, USA) at –20°C. Sections containing CA1 and CA3 regions of the hippocampus as well as the prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, ventral tegmental area, shell of the nucleus accumbens and the midbrain periaqueductal gray, as confirmed by an anatomical atlas, were stored for further analysis. In situ hybridization was performed according to the manufacturer's protocol using the BDNF mRNA level detection kit (Wuhan Boster Biological Technology, Wuhan, Hubei Province, China). The sequence of the BDNF probe was 5′-GCT GAG CGT GTG TGA CAG TAT TAG TGA GTG-3′. In brief, brain sections were mounted on poly-L-lysine-coated slides. Endogenous peroxidase was inactivated, and the sections were prehybridized and then incubated with the BDNF oligonucleotide probe (20 μL, digoxin-labeled) at 37°C for 14 hours. After washing, biotinylated mouse anti-digoxin antibody (50 μL) was added. The sections were incubated at 37°C for 60 minutes. The tissues were then incubated for 20 minutes with a streptavidin-biotin-peroxidase complex. Biotinylated peroxidase (50 μL) was then added after washing, and the mixture was incubated for an additional 20 minutes. Finally, the sections were developed and mounted with a water-soluble mounting reagent, and cover slips were attached. All experimental procedures were performed under strict RNase-free conditions, and all instruments and solvents were sterilized completely. Controls were arranged on adjacent sections to ensure the specificity of the probe. Control sections were treated with RNase, followed by in situ hybridization in the presence or absence of oligonucleotide probe. The BDNF in situ hybridization signal was quantified with the aid of a computerized video imaging system (HPIAS-1000; Wuhan Champion Image Engineering Co., Ltd., Wuhan, Hubei Province, China) by determining the gray intensity in each section for each of the target brain regions (CA1 and CA3 of the hippocampus, prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, shell of the nucleus accumbens, midbrain periaqueductal gray, and ventral tegmental area).
Immunohistochemical staining
Immunohistochemical staining was performed according to a previously published protocol (Li et al., 2014) with minor modifications. In brief, the samples were incubated with primary anti-BDNF antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. The samples were then rinsed three times with 0.01 M PBS for 5 minutes and then incubated with biotinylated secondary antibody (1:100; Santa Cruz Biotechnology) for 2 hours. After washing three times with 0.01 M PBS for 5 minutes, avidin-biotin complex (1:100; Vector Laboratories, Burlingame, CA, USA) was added, and the mixture was incubated for 2 hours at room temperature. The sample was then rinsed with 0.01 M PBS and visualized with 3,3′-diaminobenzidine solution (0.03% 3,3′-diaminobenzidine, 0.01% H2O2 and 0.01 M PBS). The reaction was monitored under a microscope. When the color was fully developed, the sample was thoroughly rinsed with 0.01 M PBS to stop the reaction. The sample was then patched, dehydrated, cleared, and mounted with neutral resin. The negative control was prepared by replacing primary antibody with normal goat serum.
Data analysis
We used HPIAS-1000 image analysis software (Wuhan Champion Image Engineering Co., Ltd., Hubei Province, China) for image analysis. A light microscope (Motic, Xiamen, Fujian Province, China) was used to view the samples. A digital camera (Nikon, Tokyo, Japan) attached to the microscope was used to capture the images. Initially, established capture parameters were uniformly used for all images. Four sections from each specimen and four visual fields for each section were randomly quantified. To quantify BDNF immunoreactivity, we assessed the relative gray intensity of positive staining. All quantifications were completed by an author who was blinded to treatments. BDNF signal quantification was performed with the aid of HPIAS-1000 image analysis software by determining the gray intensity of BDNF mRNA or protein expression in each section for each of the target brain regions (CA1 and CA3 of the hippocampus, prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, shell of the nucleus accumbens, midbrain periaqueductal gray and ventral tegmental area). Samples for each group were analyzed under the same conditions for in situ hybridization and immunohistochemical staining. For each rat in each group, four brain sections containing each of the target brain regions were selected randomly, and four fields were selected randomly for analysis. Gray intensity was measured at the same anteroposterior level for each target brain region. The expression level of protein or mRNA was expressed as the gray intensity value.
Statistical analysis
Unless otherwise stated, all test results are expressed as the mean ± SD. All statistical analyses were performed using the SPSS 11.5 software package (SPSS, Chicago, IL, USA). Means among multiple groups were compared using two-way analysis of variance. Comparisons between groups were conducted using post-hoc least significant difference test. A value of P < 0.05 was considered statistically significant.
Results
5-HT1A and 5-HT2A receptor agonists upregulated BDNF immunoreactivity in various brain regions in rats subjected to PS
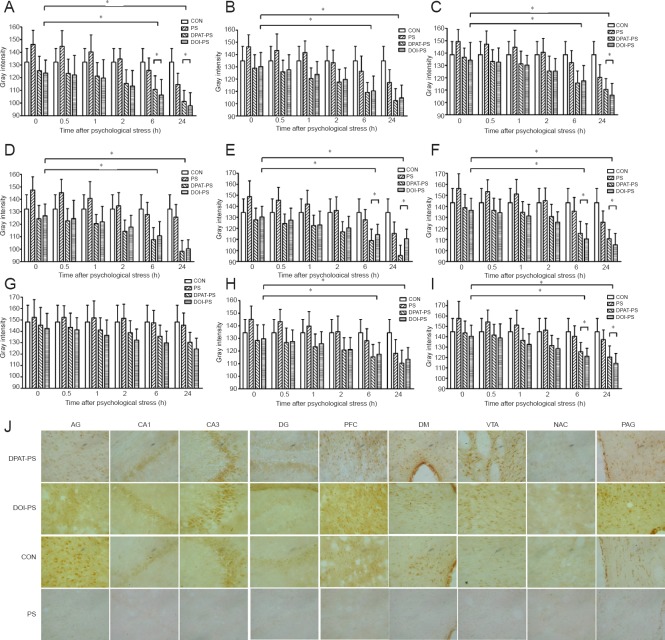
To investigate the roles of the 5-HT1A and 5-HT2A receptors in the regulation of BDNF immunoreactivity under PS, we examined BDNF immunoreactivity in the DPAT-PS and DOI-PS groups in the CA1 and CA3 regions of the hippocampus, as well as the prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, ventral tegmental area, shell of the nucleus accumbens and the midbrain periaqueductal gray in the rat model of PS (Figure 1). With the exception of the ventral tegmental area, BDNF immunoreactivity was higher in the DPAT-PS and DOI-PS groups than in the CON and PS groups immediately after PS. These results suggest that the 5-HT1A agonist DPAT and the 5-HT2A agonist DOI significantly upregulate BDNF immunoreactivity in various brain regions. Immediately after PS, BDNF immunoreactivity was lower in the PS group than in the CON group. Interestingly, at each time point after PS, BDNF immunoreactivity was higher in the DPAT-PS and DOI-PS groups compared with the CON and PS groups, and the relatively high immunoreactivity levels were maintained until the 24-hour time point. Notably, starting 2 hours after PS, BDNF immunoreactivity in the PS group recovered to the same level as that in the CON group, gradually increasing over time.
Figure 1.
Effects of stress exposure on BDNF immunoreactivity in the rat brain (immunohistochemical staining).
BDNF immunoreactivity was expressed as the gray intensity values quantified from immunohistochemical analysis of the brain regions. AG (A), CA1 (B), CA3 (C), DG (D), PFC (E), DM (F), VTA (G), NAC (H) and PAG (I) at 0, 0.5, 1, 2, 6 and 24 hours (h). Data are presented as the mean ± SD. Data were analyzed by two-way analysis of variance followed by post-hoc least significant difference test. *P < 0.05. (J) Representative images showing BDNF immunoreactivity in various brain regions at 24 hours (immunohistochemical staining, × 400). Brown indicates BDNF immunoreactivity. BDNF: Brain-derived neurotrophic factor; AG: central amygdaloid nucleus; DG: dentate gyrus; PFC: prefrontal cortex; DM: dorsomedial hypothalamic nucleus; VTA: ventral tegmental area; NAC: shell of the nucleus accumbens; PAG: midbrain periaqueductal gray; CON: control; DPAT: 5-HT1A receptor agonist (8-OH-DPAT); DOI: 5-HT2A receptor agonist; PS: psychological stress.
5-HT1A and 5-HT2A receptor agonists upregulated BDNF mRNA levels in various brain regions in rats subjected to PS
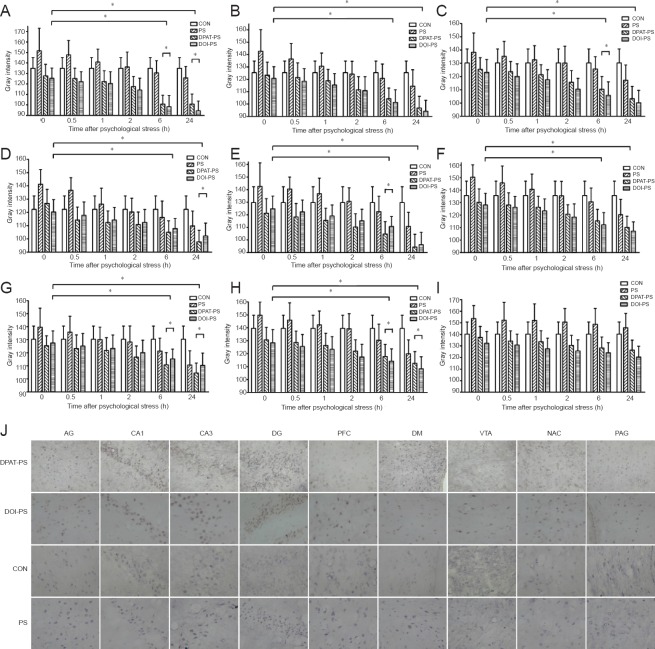
To further clarify the molecular mechanisms underlying the changes in BDNF expression induced by PS, we measured BDNF mRNA levels by in situ hybridization. Similar to the changes in BDNF immunoreactivity, with the exception of the ventral tegmental area, BDNF mRNA levels were much higher in the DPAT-PS and DOI-PS groups than in the CON and PS groups in the CA1 and CA3 regions of the hippocampus, prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, shell of the nucleus accumbens and the midbrain periaqueductal gray after PS exposure (Figure 2). This suggests that both the 5-HT1A agonist DPAT and the 5-HT2A agonist DOI significantly upregulate BDNF mRNA levels. Moreover, at each time point after PS exposure, the levels of BDNF mRNA were substantially higher in the DPAT-PS and DOI-PS groups than in the CON and PS groups until the 24-hour time point. We also noticed from the 2-hour time point after PS that BDNF mRNA levels in the PS group recovered to the same level as those in the CON group, and then gradually increased over time.
Figure 2.
Effects of stress exposure on BDNF mRNA levels in the rat brain.
After PS, BDNF mRNA levels were expressed as the gray intensity values quantified from in situ hybridization of the brain regions. AG (A), CA1 (B), CA3 (C), DG (D), PFC (E), DM (F), NAC (G), PAG (H) and VTA (I) at 0, 0.5, 1, 2, 6 and 24 hours (h). Data are presented as the mean ± SD. Data were analyzed by two-way analysis of variance followed by post-hoc least significant difference test. *P < 0.05. (J) Representative images of in situ hybridization for BDNF mRNA in various brain regions at 24 hours (× 400). Brown indicates BDNF immunoreactivity. BDNF: Brain-derived neurotrophic factor; AG: central amygdaloid nucleus; DG: dentate gyrus; PFC: prefrontal cortex; DM: dorsomedial hypothalamic nucleus; VTA: ventral tegmental area; NAC: shell of the nucleus accumbens; PAG: midbrain periaqueductal gray; DPAT: 5-HT1A receptor agonist (8-OH-DPAT); DOI: 5-HT2A receptor agonist; PS: psychological stress; CON: control.
5-HT1A and 5-HT2A receptor antagonists inhibited BDNF immunoreactivity in various brain regions in rats subjected to PS
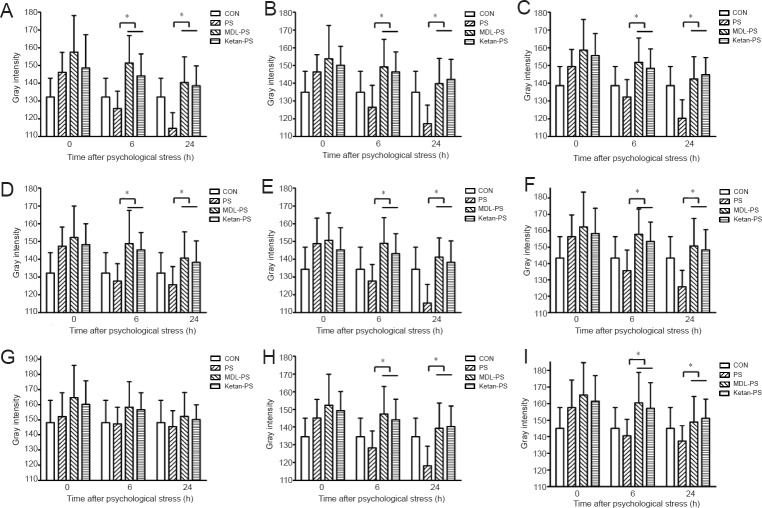
We next examined the effect of 5-HT1A and 5-HT2A receptor antagonists in the PS model. BDNF immunoreactivity was much lower in the MDL-PS and Ketan-PS groups than in the CON group, with the exception of the ventral tegmental area. This indicates that MDL-73005 and ketanserin significantly reduce BDNF protein levels in numerous brain regions (CA1 and CA3 of the hippocampus, prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, shell of the nucleus accumbens and midbrain periaqueductal gray). No significant differences were observed between these two groups in this rat model of PS. Interestingly, 6 hours after PS, immunoreactivity for BDNF was higher in the PS group than in the CON group. However, BDNF immunoreactivity remained lower in the MDL-PS and Ketan-PS groups than in the CON group (P < 0.05), which displayed a slight increase, until 24 hours after PS exposure (Figure 3).
Figure 3.
Effects of 5-HT1A and 5-HT2A receptor antagonists on BDNF immunoreactivity in various brain regions after PS treatment.
BDNF immunoreactivity was expressed as the gray intensity values quantified from immunohistochemical staining of the brain regions. AG (A), CA1 (B), CA3 (C), DG (D), PFC (E), DM (F), VTA (G), NAC (H) and PAG (I) at 0, 6 and 24 hours (h). Data are presented as the mean ± SD. Data were analyzed by two-way analysis of variance followed by post-hoc least significant difference test. *P < 0.05. BDNF: Brain-derived neurotrophic factor; AG: central amygdaloid nucleus; DG: dentate gyrus; PFC: prefrontal cortex; DM: dorsomedial hypothalamic nucleus; VTA: ventral tegmental area; NAC: shell of the nucleus accumbens; PAG: midbrain periaqueductal gray; PS: psychological stress; MDL: 5-HT1A receptor antagonist (MDL73005); Ketan: 5-HT2A receptor antagonist (ketanserin); CON: control.
5-HT1A and 5-HT2A receptor antagonists inhibited BDNF mRNA levels in various brain regions after PS
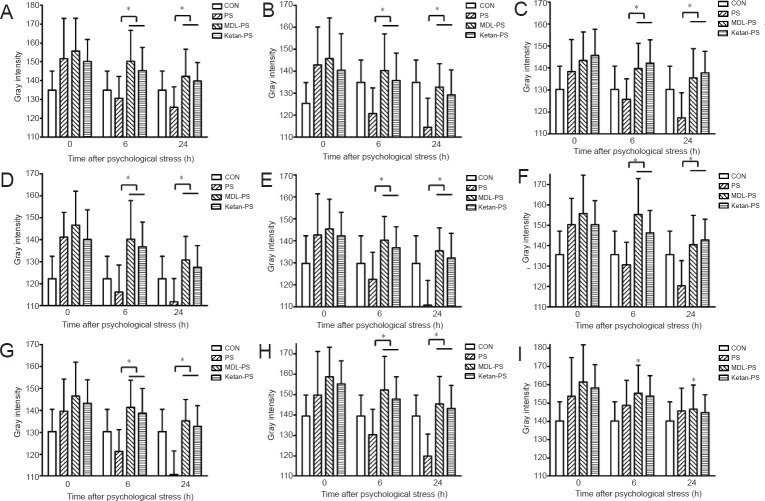
Similar with the immunohistochemical results, also with an exception of the ventral tegmental area, the levels of BDNF mRNA were lower in the MDL-PS and Ketan-PS groups compared with the CON group, indicating that 5-HT1A antagonist MDL-73005 and 5-HT2A antagonist ketanserin can significantly reduce BDNF mRNA levels in various brain regions. Besides, no obvious difference was observed in these two groups in this rat model of PS. Similar to the alteration of BDNF protein expression levels, at 6 hours after PS, BDNF mRNA levels were also greater in the PS group compared with the CON group (P < 0.05). In addition, BDNF mRNA levels were much lower in the MDL-PS and Ketan-PS groups compared with the CON group until 24 hours after PS exposure (P < 0.05; Figure 4).
Figure 4.
Effects of 5-HT1A and 5-HT2A receptor antagonists on BDNF mRNA levels in various brain regions after PS treatment.
After PS, BDNF mRNA levels were expressed as gray intensity values quantified from in situ hybridization of the brain regions. AG (A), CA1 (B), CA3 (C), DG (D), PFC (E), DM (F), NAC (G), PAG (H) and VTA (I) at 0, 6 and 24 hours (h). Data are presented as the mean ± SD. Data were analyzed by two-way analysis of variance followed by post-hoc least significant difference test. *P < 0.05. BDNF: Brain-derived neurotrophic factor; AG: central amygdaloid nucleus; DG: dentate gyrus; PFC: prefrontal cortex; DM: dorsomedial hypothalamic nucleus; VTA: ventral tegmental area; NAC: shell of the nucleus accumbens; PAG: midbrain periaqueductal gray; PS: psychological stress; MDL: 5-HT1A receptor antagonist (MDL73005); Ketan: 5-HT2A receptor antagonist (ketanserin); CON: control.
Discussion
In this study, we used a pure PS model to investigate the role of 5-HT in the regulation of BDNF expression, and we examined the dynamic relationship between 5-HT and BDNF expression in various brain regions. We found that the levels of BDNF protein and mRNA were significantly increased by the 5-HT1A receptor agonist 8-OH-DPAT and the 5-HT2A receptor agonist DOI in various regions of the rat brain. Both agonists upregulated BDNF expression in rats subjected to PS, and no significant difference was observed between these two groups. In contrast, the 5-HT1A receptor antagonist MDL73005 and the 5-HT2A receptor antagonist ketanserin significantly reduced BDNF protein and mRNA levels in the same regions of the brain.
Our findings suggest that both 5-HT1A and 5-HT2A regulate BDNF expression in various brain regions in the rat model of PS. Hence, our novel findings provide evidence that 5-HT regulates BDNF expression in the rat model of acute PS. The interaction between 5-HT and BDNF may be involved in the response to PS, and may protect the organism from stress-induced aversive processes that lead to disease. The administration of agonists significantly increased the expression levels of BDNF protein and mRNA in various brain regions, whereas antagonist treatment inhibited BDNF expression in the same regions. A previous study demonstrated that the 5-HT2A receptor regulates BDNF expression in glioma cells (Meller et al., 2002). Moreover, in another study, 5-HT was shown to be associated with learning and memory via the regulation of BDNF expression (Richter-Levin and Segal, 1993). 5-HT2A receptor agonists upregulate BDNF levels in the neocortex, but reduce BDNF expression in the hippocampal dentate gyrus, indicating that BDNF levels are differentially regulated by 5-HT2A in different brain regions (Nomura et al., 1995; Vaidya et al., 1999), which is not consistent with our present study. This lack of consistency might be due to the use of different stress paradigms, differences in the brain regions examined, or differences in the methods used for the determination of BDNF protein and mRNA expression levels. Thus, further studies using standardized materials and methods are needed to better assess the association between 5-HT and BDNF.
Previous studies (Barbas et al., 2003; Dremencov et al., 2003; Mattson et al., 2004; Schloss and Henn, 2004) have provided insight into the molecular biology of the 5-HT1A receptor pathway in stress. Under PS, the release of 5-HT is increased, and the 5-HT1A receptor activates adenylyl cyclase through the Gi/o and cAMP pathways. This activates protein kinase A, which in turn activates transcription factors such as CREB and NF-κB. The regulation by the 5-HT2A receptor of BDNF expression might be related to the activation of phospholipase C, which is mediated by the Gq protein. Phospholipase C hydrolyzes phosphatidylinositol, generating inositol trisphosphate and diacyl glycerol to release intracellular calcium and activate protein kinase C, which in turn activates transcription factors, including CREB and NF-κB. Thus, the activation of these transcription factors can increase BDNF protein and mRNA levels in various brain regions (Barbas et al., 2003; Dremencov et al., 2003; Mattson et al., 2004; Schloss and Henn, 2004). Therefore, we postulate that BDNF protein and mRNA levels are regulated by the 5-HT receptor and its downstream effectors.
There are several limitations of our present study. First, although we established a solvent control no-stress group (CON group) to control for the effect of stress, it did not receive PS. We did not have a sufficient number of animals to establish a solvent control PS group; however, we will include such a control in future studies. Second, we did not perform reverse transcription-polymerase chain reaction or western blot assay to determine the expression levels of BDNF mRNA and protein, respectively. We performed in situ hybridization and immunohistochemical staining to quantify the BDNF expression levels in various brain regions. We will perform reverse transcription-polymerase chain reaction and western blot assay to examine expression levels in our future study. Third, as in previous studies (Barbas et al., 2003; Dremencov et al., 2003; Mattson et al., 2004; Schloss and Henn, 2004), we will conduct a series of experiments to explore the downstream transcription factors that are involved in the interaction between 5-HT and BDNF, and which may be involved in the response to PS, protecting the organism from stress-induced aversive processes leading to disease.
Notably, BDNF protein and mRNA expression levels showed significant differences between the DPAT-PS and DOI-PS groups at 6 and 24 hours in certain brain regions. However, these differences varied among the brain regions examined, and it was difficult to determine which agonist upregulated BDNF expression levels to a greater extent. Further study is required to clarify the effects of the different agonists on BDNF expression.
In summary, both 5-HT1A and 5-HT2A receptor agonists upregulated BDNF protein and mRNA expression in various brain regions in the rat model of PS, whereas the 5-HT1A and 5-HT2A receptor antagonists downregulated expression of the neurotrophin. Our findings indicate that 5-HT might be involved in the regulation of BDNF expression at different time points after acute PS. Our novel findings provide support for an important role of 5-HT in regulating BDNF expression during acute PS, and they provide insight into the mechanisms that protect the organism from stress-induced processes that result in disease.
Footnotes
Funding: This work was supported by grants from the Natural Science Foundation of Shandong Province of China (ZR2011HM023 to GYL), the “11th Five-Year Plan”, National Supporting Program (2007BAI17B02 to GYL), the Science and Technology Project of Higher Education of Shandong Province of China (J10LF01 to GYL), a grant from Medical Science and Technology Development Project of Shandong Province of China (2011HZ011 to GYL), the China Postdoctoral Science Foundation of China (2012M 520585 to CJZ), the Fund of Tianjin Health Bureau of China (2014KR02 to CJZ), the Foundation of Hainan Li Ou Pharmaceutical Co., Ltd. and the Foundation of Xuzhou Enhua Pharmaceutical Co., Ltd. of China.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Patel B, Yajima W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Alleva E, Santucci D. Psychosocial vs. “physical” stress situations in rodents and humans: role of neurotrophins. Physiol Behav. 2001;73:313–320. doi: 10.1016/s0031-9384(01)00498-x. [DOI] [PubMed] [Google Scholar]
- Amos T, Stein DJ, Ipser JC. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2014:CD006239. doi: 10.1002/14651858.CD006239.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in aplysia: evidence of diverse serotonin receptor subtypes. Learn Mem. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavus I, Duman RS. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- Dremencov E, Gur E, Lerer B, Newman ME. Effects of chronic antidepressants and electroconvulsive shock on serotonergic neurotransmission in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:729–739. doi: 10.1016/S0278-5846(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Foltran RB, Diaz SL. BDNF isoforms: a round trip ticket between neurogenesis and serotonin? J Neurochem. 2016 doi: 10.1111/jnc.13658. doi: 10.1111/jnc.13658. [DOI] [PubMed] [Google Scholar]
- Garabadu D, Reddy BC, Krishnamurthy S. Citalopram protects against cold-restraint stress-induced activation of brain-derived neurotrophic factor and expression of nuclear factor kappa-light-chain-enhancer of activated B cells in rats. J Mol Neurosci. 2015;55:355–366. doi: 10.1007/s12031-014-0334-3. [DOI] [PubMed] [Google Scholar]
- Gomita Y, Yamori M, Furuno K, Araki Y. Influences of psychological stress produced by intraspecies emotional communication on nicorandil plasma levels in rats. Pharmacology. 1989;38:388–396. doi: 10.1159/000138562. [DOI] [PubMed] [Google Scholar]
- Hajós-Korcsok É, McQuade R, Sharp T. Influence of 5-HT 1A receptors on central noradrenergic activity: microdialysis studies using (+/-)-MDL 73005EF and its enantiomers. Neuropharmacology. 1999;38:299–306. doi: 10.1016/s0028-3908(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Holly EN, DeBold JF, Miczek KA. Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology (Berl) 2015;232:4469–4479. doi: 10.1007/s00213-015-4082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Li CQ, Luo YW, Bi FF, Cui TT, Song L, Cao WY, Zhang JY, Li F, Xu JM, Hao W, Xing XW, Zhou FH, Zhou XF, Dai RP. Development of anxiety-like behavior via hippocampal IGF-2 signaling in the offspring of parental morphine exposure: effect of enriched environment. Neuropsychopharmacology. 2014;39:2777–2787. doi: 10.1038/npp.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang Y, Yan M, Ma H, Gao Y, Li Z, Li C, Tian H, Zhuo C. Time-dependent co-relation of BDNF and CREB mRNAs in adult rat brains following acute psychological stress in the communication box paradigm. Neurosci Lett. 2016;624:34–41. doi: 10.1016/j.neulet.2016.04.039. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Meller R, Babity JM, Grahame-Smith DG. 5-HT 2A receptor activation leads to increased BDNF mRNA expression in C6 glioma cells. Neuromolecular Med. 2002;1:197–205. doi: 10.1385/NMM:1:3:197. [DOI] [PubMed] [Google Scholar]
- Morrison FG, Ressler KJ. From the neurobiology of extinction to improved clinical treatments. Depress Anxiety. 2014;31:279–290. doi: 10.1002/da.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Moya PR, Fox MA, Rubenstein LM, Wendland JR, Timpano KR. Anxiety and affective disorder comorbidity related to serotonin and other neurotransmitter systems: obsessive-compulsive disorder as an example of overlapping clinical and genetic heterogeneity. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120435. doi: 10.1098/rstb.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu Y, Hatnada S, Hamaguchi K, Mikuni M, Okado N. Regulation of synapse density by 5-HT 2A receptor agonist and antagonist in the spinal cord of chicken embryo. Neurosci Lett. 1995;195:159–162. doi: 10.1016/0304-3940(95)11805-7. [DOI] [PubMed] [Google Scholar]
- Nomura K, Maeda N, Kuratani K, Yamaguchi I. Sulpiride specifically attenuates psychological stress-induced gastric lesions in rodents. Jpn J Pharmacol. 1995;68:33–39. doi: 10.1254/jjp.68.33. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Hara C, Takaki S. Anxiolytic activity of SC-48274 compared with those of buspirone and diazepam in experimental anxiety models. Jpn J Pharmacol. 1993;61:115–121. doi: 10.1254/jjp.61.115. [DOI] [PubMed] [Google Scholar]
- Olivier B. Serotonin: a never-ending story. Eur J Pharmacol. 2015;753:2–18. doi: 10.1016/j.ejphar.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR. The Rat Brain in Stereotaxic Coordinates. San Jose: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Segal M. Age-related cognitive deficits in rats are associated with a combined loss of cholinergic and serotonergic functions. Ann N Y Acad Sci. 1993;695:254–257. doi: 10.1111/j.1749-6632.1993.tb23063.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD. The impact of stress on the structure of the adolescent brain: Implications for adolescent mental health. brainres 2016. 2016:021. doi: 10.1016/j.brainres.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Schloss P, Henn FA. New insights into the mechanisms of antidepressant therapy. Pharmacol Ther. 2004;102:47–60. doi: 10.1016/j.pharmthera.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT 1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino)tetralin in the rat. Eur J Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- Uwaya A, Lee H, Park J, Lee H, Muto J, Nakajima S, Ohta S, Mikami T. Acute immobilization stress following contextual fear conditioning reduces fear memory: timing is essential. Behav Brain Funct. 2016;12:8. doi: 10.1186/s12993-016-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT 2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT 2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci. 1997;17:2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WJ, Tan YC, Xu JC, Tang XP, Zhang C, Zhang PB, Ren ZQ. Protective effects of silibinin and its possible mechanism of action in mice exposed to chronic unpredictable mild stress. Biomol Ther (Seoul) 2015;23:245–250. doi: 10.4062/biomolther.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]