Abstract
We report results in a 77-year-old male patient with visual loss from long-standing serpiginous choroidopathy treated with bone marrow derived stem cells (BMSC) within the Stem Cell Ophthalmology Treatment Study (SCOTS). SCOTS is an Institutional Review Board approved clinical trial and the largest ophthalmology stem cell study registered at the National Institutes of Health to date (ClinicalTrials.gov Identifier: NCT01920867). Eight months after treatment by a combination of retrobulbar, subtenon, intravitreal and intravenous injection of BMSC, the patient's best corrected Snellen acuity improved from 20/80– to 20/60+1 in the right eye and from 20/50– to 20/20–3 in the left eye. The Early Treatment of Diabetic Retinopathy Study (ETDRS) visual acuity continued to improve over the succeeding 8 months and the optical coherence tomography macular volume increased. The increases in visual acuity and macular volume are encouraging and suggest that the use of BMSC as provided in SCOTS may be a viable approach to treating serpiginous choroidopathy.
Keywords: serpiginous choroidopathy, serpigionous choroiditis, geographic helicoid peripapillary chroidopathy, retina, macula, stem cell therapy, uveitis
Introduction
Serpiginous choroidopathy, also known as geographic helicoid peripapillary choroidopathy, is one of the “white dot syndromes” and is a rare clinical condition responsible for less than 5% of posterior uveitis cases. It is unassociated with systemic disease, affects young to middle-aged adults and has a high relapse rate as noted by Wells and Smith (2014). As described by Nazari and Rao (2013), tuberculosis has been associated with a chorioretinitis that mimics serpiginous choroidopathy and is termed serpiginous-like choroidopathy (SLC). Other types of serpiginous choroidopathy include macular serpiginous choroidopathy and ampiginous choroidopathy.
According to Crawford and Igboeli (2013), treatment of serpiginous choroidopathy with corticosteroids is not as effective as anti-inflammatory treatment with cyclosporine, cyclophosphamide or chlorambucil in preventing recurrences.
Stem Cell Ophthalmology Treatment Study (SCOTS) uses the platelet rich bone marrow fraction which contains bone marrow derived stem cells (BMSC). The constituents of the platelet rich bone marrow may include various growth factors that can be stimulatory to the constituent BMSC such as hepatocytic growth factor and insulin-like-growth factor (Zhang et al., 2015). Paracrine factors released from mesenchymal stem cells within the BMSC fraction may be neuroprotective and stimulate neuronal regeneration (Mead et al., 2015). BMSC have been show to stimulate proliferation and differentiation of retinal progenitor cells through cytokine expression and neurotrophic factors (Xia et al., 2013). BMSC have been shown to produce nerve growth factor and glial neurotrophic factors (Garcia et al., 2004). Additional evidence has developed for the release of micro RNA (miRNA) and transfer RNA (tRNA) through exosomes in MSC from the bone marrow as established by Baglio et al. (2015). Yang et al. (2015) have noted that extracellular vesicles derived from BMSC appear to play a role in tissue repair, including microvesicles and exosomes containing diverse proteins, messenger RNA (mRNA) and miRNA. Mitochondrial transfer from BMSC has been demonstrated to protect tissues by Islam et al. (2012). Finally Ma et al. (2013) have shown that immunomodulation provided by BMSC may promote recovery of damaged tissues including neurologic tissue through interleukin (IL)-23/IL-17 in disease with an inflammatory component.
These various mechanisms all probably play a role in recovery of visual function in choroidal disease through reduction of residual inflammation and improvement in the existing cellular function. Transdifferentiation of the BMSC may also be occurring as evidenced by the increased macular thickening and improved vision, similar to neural transdifferentiation of BMSC transplanted into injured spinal cord tissue noted by Vaquero and Zurita (2009).
Background about SCOTS
SCOTS, the Stem Cell Ophthalmology Treatment Study, is the largest ophthalmology stem cell study registered at ClinicalTrial.gov (NCT01920867) approved by an Institutional Review Board verified by the US National Institutes of Health. Written informed consent was obtained from the patient. SCOTS is an open label, non-randomized, efficacy study. There is no placebo or sham arm.
All patients meeting eligibility criteria and enrolled in the study receive active treatment of bone marrow derived stem cells and their associated neurotrophic factors. Bone marrow aspirated from the posterior iliac crest is separated to provide BMSC within the stem cell concentrate separated through centrifugation.
Inclusion criteria for SCOTS patients:
-Have objective, documented damage to the retina or optic nerve unlikely to improve OR
-Have objective, documented damage to the retina or optic nerve that is progressive.
-AND have less than or equal to 20/40 best corrected central visual acuity in one or both eyes AND/OR an abnormal visual field in one or both eyes.
-Be at least 3 months post-surgical treatment intended to treat any ophthalmologic disease and be stable.
-If under current medical therapy (pharmacologic treatment) for a retinal or optic nerve disease be considered stable on that treatment and unlikely to have visual function improvement (for example, glaucoma with intraocular pressure stable on topical medications but visual field damage).
-Have the potential for improvement with BMSC treatment and be at minimal risk of any potential harm from the procedure.
-Be 18 years of age or older.
-Be medically stable and able to be medically cleared by their primary care physician or a licensed primary care practitioner for the procedure. Medical clearance means that in the estimation of the primary care practitioner, the patient can reasonably be expected to undergo the procedure without significant medical risk to health.
Exclusion criteria:
-Patients who are not capable of an adequate ophthalmologic examination or evaluation to document the pathology.
-Patients who are not capable or not willing to undergo follow up eye exams with the principle investigator or their ophthalmologist or optometrist as outlined in the protocol.
-Patients who are not capable of providing informed consent.
-Patients who may be at significant risk to general health or to the eyes and visual function should they undergo the procedure.
There are three arms of SCOTS with the type of treatment chosen based on the degree of visual loss, etiology of visual loss, associated risk factors for the treatment arms and the patient's medical risk status. Bilateral treatment is provided assuming both eyes meet eligibility requirements. As these are autologous stem cells, no immunosuppression is required. An FDA cleared centrifugation medical device is used to separate the bone marrow aspirate into a stem cell concentrate. This concentrate has averaged 1.2 billion total nucleated cells including mesenchymal stem cells in approximately 14–15 mL of concentrate. 3 mL of concentrate was used for retrobulbar injection, 1 mL for subtenon injection, 0.05 mL for intravitreal injection, approximately 0.1 mL for subretinal injection and approximately 0.2 mL for intra-optic nerve injection. The intravenous injection is provided from the remainder of the concentrate.
Arm 1 consists of retrobulbar and subtenon injection of stem cell concentrate followed by intravenous infusion. Patients with ophthalmic conditions which preclude safe or effective utilization of intravitreal injection of concentrate, such as the presence of silicon oil, may be offered Arm 1 if meeting inclusion criteria. Arm 2 consists of the administration of retrobulbar, subtenon and intravitreal concentrate followed by intravenous infusion. Patients meeting inclusion criteria with visual acuity between 20/40 and 20/200 in one or both eyes and/or visual field loss may be offered Arm 2. Arm 3 is reserved for retinal and optic nerve disease patients with severe visual loss meaning visual acuity of 20/200 or worse in at least one eye. Typically patients admitted to Arm 3 have much poorer vision. Arm 3 consists of the better-seeing eye receiving the same treatment as Arm 1 or more typically, Arm 2, and the eye with more extensive visual acuity loss receiving a core pars plana vitrectomy with injection of subretinal or intra-optic nerve concentrate followed by the infusion of intravenous stem cells. Monocular patients are not eligible for Arm 3. Follow up is required at 1, 3, 6 and 12 months post treatment with reporting of the eye exam results to the Principal Investigator and Study Director.
Unlike pharmaceutical studies in which a single condition is treated, SCOTS focuses on the endpoint of visual loss, not the instigating cause. This allows the treatment of various retinal and optic nerve diseases, many of which are not presently the subject of clinical treatment studies, and include visual loss resulting from more than one disease.
The SCOTS procedure is patient funded and typically performed under general anesthesia. Written informed consent is obtained from each patient for publication of reports and any accompanying images. Treatment is provided in a fully licensed ambulatory surgical center in Coconut Creek, FL, USA.
Case presentation
A 77-year-old male patient experienced bilateral acute visual loss in their middle 20's to 20/100 in both eyes as a result of a choroidopathy, eventually diagnosed as serpiginous choroidopathy. There was no family history of ocular disease. The vision loss improved somewhat early in its course, but persisted throughout the patient's life.
In the patient's early 70's, following cataract surgery with intraocular lens implantations, the visual acuity remained the same at 20/100 in the right eye and improved to 20/30 in the left eye. Five years later, best corrected visual acuity was 20/80– in the right eye and 20/50– in the left eye as a result of the serpiginous choroidopathy. Visual acuities on the Early Treatment Diabetic Retinopathy Study (ETDRS) charts were 22 in the right eye and 36 in the left eye at one meter. On pre-procedure examination, old inactive scarring of the retinal pigment epithelium and choroid in both eyes and small disc drusen in both eyes were observed (Figure 1).
Figure 1.
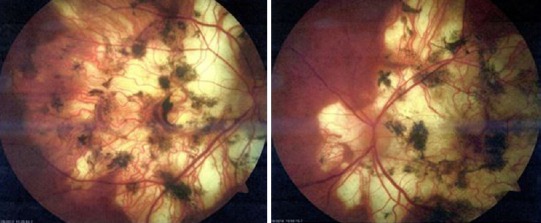
Pre-procedure retinal photographs of right eye (right image) and left eye (left image) showing extensive chorioretinal atrophy and pigmentary changes as a result of serpiginous choroidopathy.
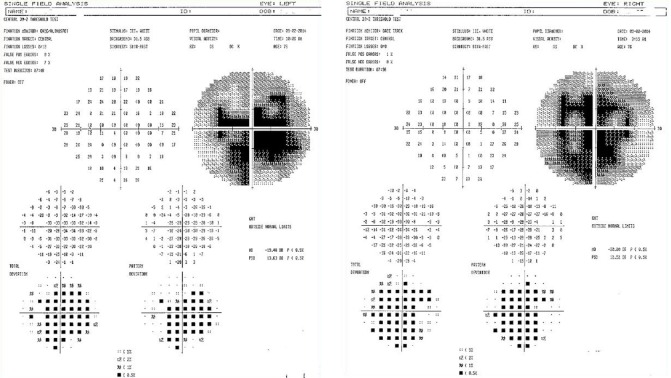
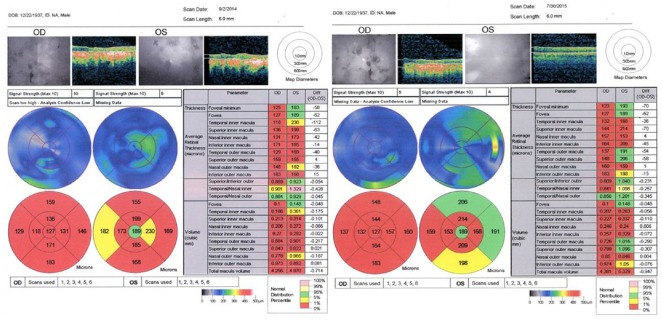
The baseline visual fields demonstrated dense central and paracentral scotomas of both eyes: Right mean deviation was –20.00 with pattern standard deviation 12.52 and left mean deviation was –19.48 with pattern standard deviation 13.63 (Figure 2). The baseline ocular coherence tomography of the maculae (Figure 3) showed a bilateral maculopathy with retinal nerve fiber layer thinning in both eyes.
Figure 2.
Pre-procedure (September 2, 2014) Humphrey visual fields, with the right eye (right image) and left eye (left image) showing visual field loss.
Figure 3.
Pre-procedure (September 2, 2014) and post-procedure (July 30, 2015) macular thickness measurements showing improvement in each eye approximately 8 months following the Stem Cell Ophthalmology Treatment Study procedure.
OD: Right eye; OS: left eye.
The patient was assigned to SCOTS Arm 2 in each eye, and BMSC were administered through retrobulbar, subtenon and intravitreal approaches on December 12, 2014 without complication.
At 1 month after surgery, the patient reported a 2 week history of dramatic visual acuity improvement sufficient to regain his driver's license. The visual acuities improved in each eye by Snellen and ETDRS (near and far) testing. At 1 month after surgery, best corrected Snellen visual acuity improved in the right eye from 20/80– to 20/60+1 and in the left eye from 20/50– to 20/20–3. ETDRS showed steady improvement over this time with gains of 46 letters for the right eye and 33 letters for the left eye at 1 meter finally achieving a score of 68 in the right eye and 69 in the left eye. Table 1 shows the visual acuities at baseline, 1 and 8 months after SCOTS treatment.
Table 1.
Early Treatment Diabetic Retinopathy Study (ETDRS) and Snellen pre and post Stem Cell Ophthalmology Treatment Study (SCOTS) treatment showing visual acuity improvement over time
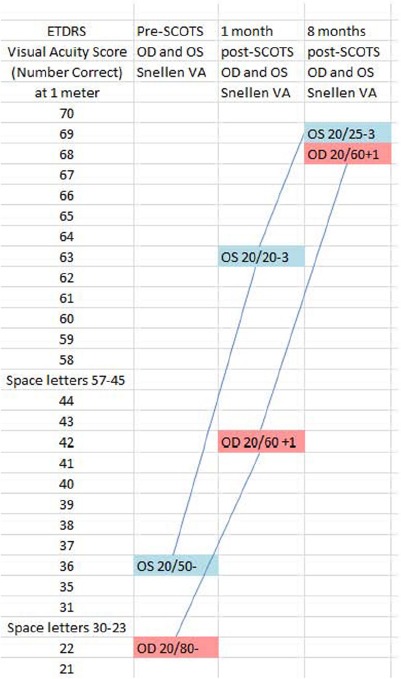
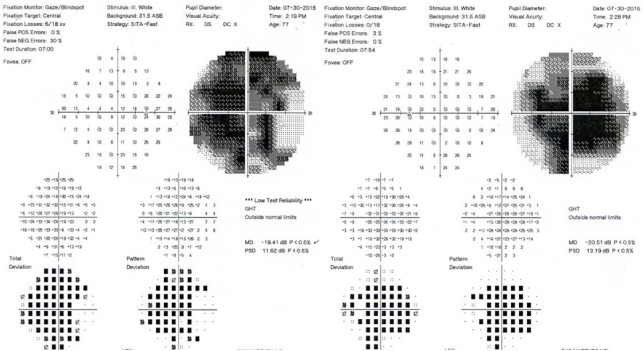
In July 2015, at 8 months after SCOTS, there was mild improvement centrally in the Humphrey visual fields: The right eye appeared slightly better with mean deviation –19.41 and pattern standard deviation 11.62; the left eye showed mean deviation of –20.51 with pattern standard deviation 13.19 (Figure 4). Ocular coherence tomography showed a thickening of the retinal nerve fiber layer in the macula of each eye at 8 months after SCOTS as compared with the baseline measurements. Figure 3 Baseline macular volumes and macular volumes 8 months after therapy demonstrate the ocular coherence tomography fast macular scans. During the post-SCOTS procedure, the patient did not receive any potential medications to treat serpiginous choroidopathy.
Figure 4.
Post-procedure (July 30, 2015) Humphrey visual fields, with the right eye (right image) showing mild central improvement.
Conclusions
This patient with serpiginous choroidopathy included in this study initially experienced bilateral acute visual loss (20/100 in the right eye and 20/100 in the left eye). In the patient's mid 70's, the visual acuity was best corrected 20/80– in the right eye and 20/50– in the left eye. The patient chose to participate in SCOTS and underwent retrobulbar, subtenon and intravitreal injection of autologous BMSC in each eye. At 1 month after treatment, the visual acuity improved to 20/60+1 in the right eye and 20/20–3 in the left eye. At 8 months after surgery, the visual acuity was 20/60+1 in the right eye and 20/25–3 in the left eye. Ocular coherence tomography examination showed improvements in both eyes. The patient reported expanded independence regaining the ability to read and drive an automobile.
This patient with long term serpiginous choroidopathy experienced significant improvement in his visual acuity and ocular coherence tomography outcomes following treatment with autologous BMSC as proscribed in the protocols developed for the SCOTS. Autologous BMSC may prove a valuable addition to the treatment of serpiginous choroidopathy and other retinal conditions demonstrating an immunologic or choroidal component.
Acknowledgments
We would like to thank Aaron Mack, M.D. of the Columbus Eye Surgery Center, Columbus, OH, USA, who helped establish the diagnosis and follow the patient.
Footnotes
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CM, Igboeli O. A review of the inflammatory chorioretinopathies: the white dot syndromes. ISRN Inflamm 2013. 2013 doi: 10.1155/2013/783190. 783190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García R, Aguiar J, Alberti E, de la Cuétara K, Pavón N. Bone marrow stromal cells produce nerve growth factor and glial cell line-derived neurotrophic factors. Biochem Biophys Res Commun. 2004;316:753–754. doi: 10.1016/j.bbrc.2004.02.111. [DOI] [PubMed] [Google Scholar]
- Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Zhong D, Chen H, Zheng Y, Sun Y, Luo J, Li H, Li G, Yin Y. The immunomodulatory effect of bone marrow stromal cells (BMSCs) on interleukin (IL)-23/IL-17-mediated ischemic stroke in mice. J Neuroimmunol. 2013;257:28–35. doi: 10.1016/j.jneuroim.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. Stem cell treatment of degenerative eye disease. Stem Cell Res. 2015;14:243–257. doi: 10.1016/j.scr.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari KH, Rao NA. Serpiginous choroiditis and infectious multifocal choroiditis. Surv Ophthalmol. 2013;58:203–232. doi: 10.1016/j.survophthal.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero J, Zurita M. Bone marrow stromal cells for spinal cord repair: A challenge for contemporary neurobiology. Histol Histopathol. 2009;24:107–116. doi: 10.14670/HH-24.107. [DOI] [PubMed] [Google Scholar]
- Wells JM, Smith JR. Riding the wave: challenges in the management of serpiginous choroiditis. Clin Experiment Ophthalmol. 2014;42:601–602. doi: 10.1111/ceo.12411. [DOI] [PubMed] [Google Scholar]
- Xia J, Luo M, Ni N, Chen J, Hu Y, Deng Y, Ji J, Zhou J, Fan X, Gu P. Bone marrow mesenchymal stem cells stimulate proliferation and neuronal differentiation of retinal progenitor cells. PLoS One. 2013;8:e76157. doi: 10.1371/journal.pone.0076157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y, Deng SJ, Liu YJ. Extracellular vesicles derived from bone marrow mesenchymal stem cells protect against experimental colitis via attenuating colon inflammation, oxidative stress and apoptosis. PLoS One. 2015;10:e0140551. doi: 10.1371/journal.pone.0140551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GW, Gu TX, Guan XY, Sun XJ, Qi X, Li XY, Wang XB, Lv F, Yu L, Jiang DQ, Tang R. HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Prolif. 2015;48:661–670. doi: 10.1111/cpr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]