INTRODUCTION
Robotic surgery has enabled expansion of minimally invasive surgery that was previously prohibitively difficult with standard laparoscopy. Robotic partial nephrectomy is a well-established technique in the management of renal cell carcinoma that is used more frequently for complex multifocal disease.1-2 We manage a large population of patients with hereditary and multifocal renal cell carcinoma conditions. Surgical treatment with partial nephrectomy remains the standard of care for most hereditary renal cancers when the largest renal mass approaches 3cm.3 As our experience with robotic partial nephrectomy has increased, we have applied this technique to increasingly complex renal tumors as well as increasing numbers of tumors.4-9 Herein we present renal functional outcomes after robotic surgery on a single kidney with more than three masses, which we have termed a “Robotic Multiplex Partial Nephrectomy” (RMxPNx). To our knowledge, this is the largest series of Robotic Multiplex partial nephrectomies to date.
METHODS
Between January 2007 and November 2013 three surgeons (ARM, GB, PP) performed resection of 3 or more masses in a single kidney (RMxPNx). Tumors are resected using enucleation and renorraphy techniques as previously described.2 Intraoperative ultrasound was used to identify endophytic tumors and resection was performed on smaller, exophytic masses before more complex tumors were removed. Data compiled included age, gender, demographics, number of previous abdominal surgeries, laterality, number of tumors, operative time, preoperative (preop) serum creatinine (SCr), and postoperative (postop) SCr daily and at 3 month postoperative follow up. Renal function was additionally assessed using eGFR (CKD-EPI-Creatinine 2009 formula), and differences were reported as percent change in eGFR from preoperative value. Renal function outcomes were further stratified based on CKD stages I, II and III. Differences in SCr, eGFR and clinical variables were compared using logistic regression, Spearman rank correlations and T-Test.
RESULTS
A total of 407 partial nephrectomies were performed at the NIH from 2007 to 2013. One hundred and twenty-one underwent robotic renal surgery and of these, 54 patients underwent RMxPNx (mean age 46, range 20 to 84). All RMxPNx cases performed at NIH have been included in this study and were performed transperitoneal. Table 1 summarizes the demographic data for these patients. Sixty six percent were male and 83% were caucasian. Mean body mass index (BMI) was 30.5 (range 22.5 to 41.6) and mean ASA at surgery was 3.0 (range 2-3). Operative characteristics are presented in Table 2. The mean number of previous abdominal surgeries patients had undergone prior to the RMxPNx was 0.63±0.8, with as many as 3 previous abdominal surgeries in the same patient. No laterality was predominant with 54% RMxPNx performed on the right vs. 46% performed on the left.
Table 1.
Patient Characteristics
| Patient Characteristics | N, (%) |
|---|---|
| Mean Age (range) | 46.1 years (20-84) |
| Gender | |
| Females (%) | 18 (33) |
| Males (%) | 36 (66) |
| Race | |
| White | 45 (83) |
| AA | 6 (11) |
| Asian | 2 (4) |
| Latino | 1 (2) |
| Mean ASA score (range) | 2.96 (2-3) |
| Mean BMI (range) | 30.5 (22.5-41.6) |
| Estimated Preoperative GFR (SD) | 85.4 (21.5) |
| Patient (n) Renal Mass Disease States | Von Hippel Lindau - 32 |
| Bilateral Multifocal - 12 | |
| Birt-Hogg-Dube - 7 | |
| Hereditary Papillary RCC - 2 | |
| Unilateral Multifocal - 1 |
AA: African American, BMl: Body Mass lndex, ASA: American Society of Anesthesiologists
Table 2.
Operative Characteristics reported as mean and SD.
| Operative Characteristics | |
|---|---|
| Previous Abdominal Surgery (mean) | 0.63 ±0.8 (Range:0.0-3.0) |
| Operative Side | |
| Right (%) | 54% |
| Left (%) | 46% |
| Mean number of masses (mode) | 8.63 (3.0) |
| EBL, ml (SD) | 1434 (1475) |
| Surgery Time, min (SD) | 385 (124) |
| Cases with Warm Ischemia (%) | 10 (18.5) |
| Mean Warm Ischemia Time, min (SD) | 23.3 (6.4 min) |
| Cases Requiring Conversion to Open (%) | 6.0 (11) |
| Conversions from Partial to Radical (%) | 1.0 (1.85) |
| Reasons for RMxPNx to Open Surgery. | |
| Endophytic Mass | 3 (5.5%) |
| Adhesions | 2 (3.7%) |
| Renal Vascular Injury | 1 (1.8%) [radical nx] |
The mean number of tumors removed was 8.63 (mode 3.0). Operative time averaged 382 minutes (6.4 hours), which included routine cystoscopy and ipsilateral ureteral catheter placement in addition to the minimally invasive renal surgery. Estimated blood loss averaged 1439mL (range 250 to 8500mL) with 79% of patients undergoing a blood transfusion intraoperatively. Renal hilar occlusion was performed for masses concerning for increased hemorrhage due to preoperative imaging and intraoperative judgment in only 10 of the 54 cases with a mean warm ischemia time of 23.3 minutes (Table 2).
Six cases (11%) were converted to open partial nephrectomy. Only 1 case was converted to open after 2010 for a conversion rate of 2.7% during the latter part of the series, and no robotic to open conversions occurred in the last 33 cases of the series. Reasons for conversion included endophytic lesions deemed unsafe to be removed robotically, adhesions and renal vascular injury. Only one case was converted from Robotic MxPNx to Radical Nephrectomy for a renal vascular injury sustained intraoperatively.
Mean preoperative SCr and eGFR were 1.02±0.26mg/dL and 85.4±21.5mL/min respectively (Table 3). Perioperatively, the mean SCr increased to 1.47±0.53mg/dL (p=0.016) and eGFR diminished to 60.8±24.3mL/min (p=0.031). At 3-month post-op follow-up the mean SCr was 1.07±0.33mg/dL, an increase of 0.05mg/dL (p=0.101) from baseline. Mean eGFR at 3-month follow-up was 82.3±23.9mL/min, a decrease from baseline of 3.1mL/min (p=0.21) (Figure 1). Perioperatively, SCr increased 23.8% (p=0.016) from baseline but reduced to a 4.9% increase in SCr at 3-month follow-up (p=0.10). The overall eGFR declined by 28.8% (p=0.03) in the perioperative period but by only 3.6% (p=0.21) at 3-month follow-up (Table 3).
Table 3.
Pre-operative and Post-operative comparisons of Creatinine and eGFR.
| Creatinine | Mean | P-Value | eGFR | Mean | P-Value |
|---|---|---|---|---|---|
| Cr Pre-Operative | 1.02±0.26 | eGFR Pre-operative | 85.4±21.5 | ||
| Cr post-op peak | 1.47±0.53 | p<0.016 | eGFR post-op nadir | 60.8±24.3 | p<0.031 |
| Cr at 3 month Follow-up | 1.07±0.33 | p=0.101 | eGFR 3 month Follow-up | 82.3±24.0 | p=0.21 |
Figure 1.
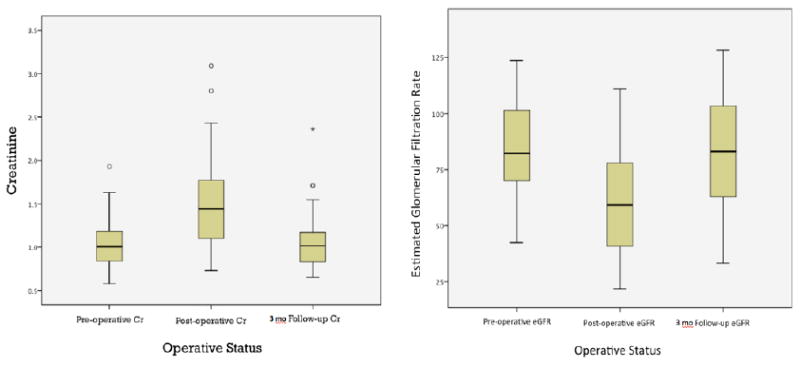
Pre-operative, Post-operative and 3-month Follow-up Trends for Serum Creatinine and eGFR.
Number of tumors removed and EBL did not correlate with change in SCr, eGFR at discharge or 3-month post-op. Longer surgery time was positively correlated with SCr (r=0.31) and negatively correlated with eGFR (r=-0.3) at 3-month follow-up.
Patients were further sub-categorized based on stages of preoperative CKD. Patients with Preoperative eGFR <60mL/min demonstrated a 37% decrease in eGFR at discharge (p= 0.02) however, at 3-month follow-up, patients with Stage III CKD were found to have only decreased eGFR 10% (p= 0.25) which did not achieve statistical significance.
Discussion
Urologic surgeons continue to perform more complex surgeries using robotic techniques since its introduction in 2000s. Previous studies show that laparoscopic partial nephrectomy and robotic partial nephrectomy result in less blood loss and shorter recovery with similar long-term renal function and oncologic outcomes.1,3,4,5,8 Patients with multiple complex renal masses often have bilateral disease, and the previous standard of radical nephrectomy would leave these patients on renal replacement therapy.4
Our findings demonstrate that the RMxPNx preserves renal function for multi-tumor partial nephrectomy. No statistically significant changes in either SCr or eGFR were observed over the study period that included both primary partial nephrectomies as well as reoperative RMxPNx. During the seven year study period the robotic to open conversion rate decreased by more than three-fold in the first three years (Figure 2). The mean number of tumors removed during each case also steadily increased over that same time period, likely representing improvement in surgical skill and technique as experience increased.
Figure 2.
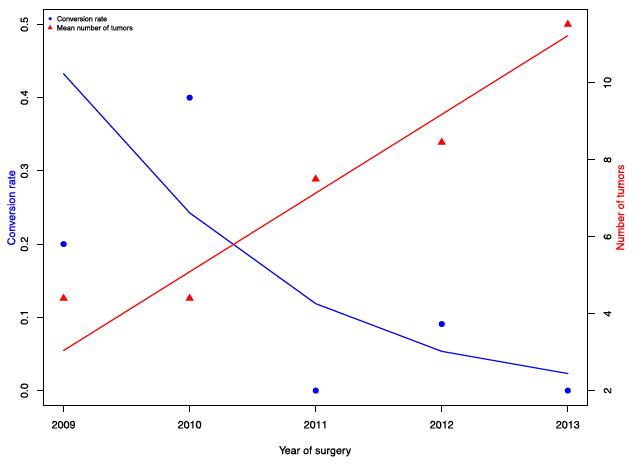
Trends in Tumors Removed and Open Conversions Over Time
Given the concern for prolonged ischemia times or the need for repeated hilar occlusion in this patient population, hilar occlusion is rarely performed except for the most endophtytic masses or those intimately associated with the vasculature seen on preoperative imaging. This may contribute to the notable preservation of renal function seen in this series of RMxPNx. Additionally, our enucleation technique also allows for maximal preservation of unaffected renal parenchyma, a primary predictor of post-operative renal function.12 As a result of the off-clamp approach to most of these tumors, the estimated blood loss in this series is markedly higher than in other published reports of robotic partial nephrectomy.
Preservation of renal function despite preoperative renal dysfunction was also observed in this cohort. Stratified by CKD stage, an acute decline in renal function was observed irrespective of preoperative eGFR. In patients with preoperative stage III CKD, a 10% decrease in eGFR was seen at follow-up but this did not achieve statistical significance. This is likely due to the current sample size and will delineate itself with further statistical analysis as more RMxPNx cases accrue.
Given excellent renal function preservation demonstrated by standard measures, volume-adjusted functional outcomes and differential renal function by radio-nucleotide imaging were deemed unnecessary. More important will be longer follow-up to determine long-term renal function outcomes in this patient population that is at increased risk for needing subsequent renal surgeries.
Although this study is the largest of its kind to date, limitations exist regarding the sample size and retrospective nature of the study. We utilize the 3cm approach described by Duffey et al as the cutoff for surgical intervention.11 The vast majority of the patients in our cohort had at least one mass that was approaching 3cm at the time of surgery. The remaining masses removed from the kidneys were typically less than 1-1.5cm each. Pathologic margin status is also not reported given the technique of enucleation performed on these masses, however to this date no cases of metastatic disease has been recorded if patients undergo partial nephrectomy before the renal mass grows larger than 3cm. This study also does not compare robotic assistance to other approaches for partial nephrectomy; rather, it presents a feasible technique for a minimally invasive approach to management of multiple renal tumors.
Conclusion
Robotic partial nephrectomy for 3 or more tumors in a single kidney is safe and feasible in selected patients. More importantly, these data demonstrate exceptional renal function preservation despite the highly challenging nature of this type of surgery.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Supported by Grants No. ZIA1BC011028-05, ZIA BC011038-05, ZIA BC011043-05, ZID BC011089-05, and ZIE BC 011023-05 from the National Institutes of Health.
References
- 1.Rogers CG, Singh A, Blatt AM, Linehan WM, Pinto PA. Robotic Partial Nephrectomy for Complex Renal Tumors: Surgical Technique. Eur Urol. 2008;53:514–523. doi: 10.1016/j.eururo.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boris R, Proano M, Linehan WM, Pinto PA, Bratslavsky G. Initial Experience With Robot Assisted Partial Nephrectomy for Multiple Renal Masses. J Urol. 2009;182:1280–1286. doi: 10.1016/j.juro.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herring JC, Enquist EG, Chernoff A, Linehan WM, Choyke PL, Walther MM. Parenchymal Sparing Surgery in Patients with Hereditary Renal Cell Carcinoma: 10-Year Experience. J Urol. 2001;165:777–781. [PubMed] [Google Scholar]
- 4.Drachenberg DE, Mena OJ, Choyke PL, Linehan WM, Walther MM. Parenchymal Sparing Surgery for Central Renal Tumors in Patients with Hereditary Renal Cancers. J Urol. 2004;172:49–53. doi: 10.1097/01.ju.0000130930.70356.28. [DOI] [PubMed] [Google Scholar]
- 5.Liangkuan B, Zhang C, Li K, Fan X, Xu K, Han J, Huang H, Liu H, Dong W, Yang X, Huang J, Lin T. Robotic Partial Nephrectomy for Renal Tumors Larger than 4cm: A Systematic Review and Meta-analysis. Plus One. 2013;8:1–6. doi: 10.1371/journal.pone.0075050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, Pinto PA. Robotic Partial Nephrectomy for Renal Hilar Tumors: A Multi-Institutional Analysis. J Urol. 2008;180:2353–2356. doi: 10.1016/j.juro.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong Y, Du C, Josephson DY, Wilson TG, Nelson R. Four-arm Robotic Partial Nephrectomy for Complex Renal Cell Carcinoma. World J Urol. 2010;28:111–115. doi: 10.1007/s00345-009-0427-8. [DOI] [PubMed] [Google Scholar]
- 8.Borghesi M, Schiavina R, Gan M, Novara G, Mottrie A, Ficarra V. Expanding Utilization of Robotic Partial Nephrectomy for Clinical T1b and complex T1a Renal Masses. World J Urol. 2013;31:499–504. doi: 10.1007/s00345-013-1095-2. [DOI] [PubMed] [Google Scholar]
- 9.Petros F, Sukumar S, Haber G, Dulabon L, Bhayani S, Stifelman M, Kaouk J, Rogers C. Multi-Institutional Analysis of Robot-Assisted Partial Nephrectomy for Renal Tumors >4cm Versus <4cm in 445 Consecutive Patients. J EndoUrol. 2012;26:642–646. doi: 10.1089/end.2011.0340. [DOI] [PubMed] [Google Scholar]
- 10.Ghani KR, Sukumar S, Sammon JD, Rogers CG, Trinh Q, Menon M. Practice Patterns and Outcomes of Open and Minimally Invasive Partial Nephrectomy Since the Introduction of Robotic Partial Nephrectomy: Results from the Nationwide Inpatient Sample. J Urol. 2013;191:1–7. doi: 10.1016/j.juro.2013.10.099. [DOI] [PubMed] [Google Scholar]
- 11.Duffey BG, Choyke PL, Glenn G, Grubb RL, Venzon D, Linehan WM, Walther MM. The Relationship Between Renal Tumor Size and Metastases in Patients With von Hippel-Lindau Disease. J Urol. 2004;172:63–5. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 12.Lane BR, Babineau DC, Poggio ED, Weight CJ, Larson BT, Gill IS, Novick AC. Factors Predicting Renal Functional Outcome After Partial Nephrectomy. J Urol. 2008;180:2363–2369. doi: 10.1016/j.juro.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 13.Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, Gill IS, Blute ML, Campbell SC. Renal Function After Partial Nephrectomy: Effect of Warm Ischemia Relative to Quantity and Quality of Preserved Kidney. J Urol. 2011;79:356–360. doi: 10.1016/j.urology.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Johnson A, Sudarshan S, Liu J, et al. Feasibility and outcomes of repeat partial nephrectomy. J Urol. 2008;180:89–93. doi: 10.1016/j.juro.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadahunsi AT, Sanford T, Linehan WM, et al. Feasibility and outcomes of partial nephrectomy for resection of at least 20 tumors in a single renal unit. J Urol. 185:49–53. doi: 10.1016/j.juro.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


