Abstract
Conventional cytogenetics and interphase fluorescence in-situ hybridization (FISH) identify a high-risk multiple myeloma (HRM) population characterized by poor outcomes. We analyzed these differences among HRM versus non-HRM after upfront autologous hematopoietic cell transplantation (autoHCT). Between 2008 and 2012, 715 patients with multiple myeloma with FISH and/or cytogenetic data with upfront autoHCT were identified in the Center for International Blood and Marrow Transplant Research database. HRM was defined as del17p, t(4;14), t(14;16), hypodiploidy (< 45 chromosomes excluding -Y) or chromosome 1 p and 1q abnormalities; all others were non-HRM. Among 125 (17.5%) HRM patients, induction with bortezomib and immunomodulatory agents (imid) was higher compared to non-HRM (56% vs 43%, p <0.001) with similar pre-transplant complete response (CR) (14% vs 16%, p 0.1). At day-100 post-transplant, ≥ very good partial response was 59% in HRM and 61% in non-HRM (p=0.6). More HRM patients received post-transplant therapy with bortezomib and imids (26% vs 12%, p=0.004). Three-year post-transplant progression-free (PFS) and overall survival (OS) in HRM versus non-HRM were 37% vs 49%, p <0.001 and 72% vs 85%, p <0.001 respectively. At 3-years, PFS for HRM with and without post-transplant therapy was 46(95% confidence interval 33–59)% versus 14(4–29)% and in non-HRM with and without post-transplant therapy 55(49–62)% versus 39(32–47)%; OS for HRM with and without post-transplant therapy was 81(70–90)% versus 48(30–65)% compared to 88(84–92)% and 79(73–85)% in non-HRM with and without post-transplant therapy respectively. Among patients receiving post-transplant therapy, there was no difference in OS between HRM and non-HRM (p 0.08). In addition to HRM, higher stage, <CR pre-transplant, lack of post-transplant therapy and African-American race were associated with worse OS. In conclusion, we show HRM patients achieve similar day-100 post-transplant responses compared to non-HRM, but these responses are not sustained. Post-transplant therapy appeared to improve the poor outcomes of HRM.
Keywords: Multiple myeloma, Autologous HSCT, High risk, maintenance
Introduction
The heterogeneous clinical course of multiple myeloma is partially related to high risk prognostic molecular markers in the plasma cell clone. Using conventional metaphase cytogenetics and interphase fluorescent in-situ hybridization (FISH), 20–25% of myeloma patients are found to have high-risk myeloma (HRM) which is associated with a poor prognosis.1, 2 The International Myeloma Working Group (IMWG) 2014 consensus defines a combined high risk model incorporating International Staging System II or III and del (17p) or t(4;14). High-risk patients with these markers are expected to survive a median of 2 years despite novel agents, compared to more than 10 years for low-risk patients.3 Kapoor et al found that patients with cytogenetic abnormalities did worse after autoHCT.4 Other high risk cytogenetic/ FISH abnormalities that are associated with worse outcomes include t(14;16)5, 6 and chromosome 1 abnormalities (1q21 amplification, 1p deletion)5, 7, although conflicting data exist regarding the prognostic significance of these groups.5, 7–12
Autologous hematopoietic cell transplant (autoHCT) is available broadly, improves survival in myeloma patients, and is currently considered a standard of care for transplant-eligible patients; however data on the benefits of auto-HCT in HRM are lacking. Trends towards improved survival have been reported by University of Arkansas for Medical Sciences Myeloma Institute with the Total Therapy program, where tandem autoHCT has been preceded by induction and followed by consolidation/maintenance including bortezomib in more recent years.13 Approaches to post-transplant consolidation and maintenance specifically for HRM are evolving and range from single agent lenalidomide or bortezomib to triplet therapy combining these agent with dexamethasone.14–16 We undertook this study to examine the role of autoHCT with HRM in the era of novel agents and post-transplant therapies. We used the Center for International Blood and Marrow Transplant Research (CIBMTR) database to analyze patient and disease characteristics, response to induction therapies as well as autoHCT and post-transplant outcomes among patients undergoing autoHCT for multiple myeloma from 2008–2012.
Methods
Data source
The CIBMTR is a prospectively maintained transplant database that captures transplant data from over 420 transplant centers worldwide. Data are submitted to a statistical center at the Medical College of Wisconsin in Milwaukee. Participating centers are required to report all transplants consecutively; patients are followed longitudinally and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians' review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
Patient Selection
Adults who underwent first autologous hematopoietic cell transplantation for multiple myeloma between January 1, 2008 and December 31, 2012 with high dose melphalan conditioning within 12 months of diagnosis with available molecular risk results (by FISH and/or cytogenetics) were the subjects of this retrospective observational study. We identified 715 patients limited to centers reporting at least 10% high risk patients in order to reduce center variability in evaluation of high risk status. Among them 125 patients were classified as high-risk myeloma (HRM) defined by the presence of deletion 17p13 alone (n=28), t(4;14) alone (n=28), t(14;16) alone (n=5), hypodiploid alone (n=12), chromosome 1q amplification or 1p deletion (n=25) and a combination of more than 1 of aforementioned markers (n=27). Chromosome 1 abnormalities included amplification of 1q (n=21), deletion of 1p (n=3), and both 1q amp plus 1p del (n=1). Physicians blinded to the outcome reviewed the FISH and cytogenetic reports from each reporting institution.
Outcomes and definitions
The outcomes of interest included overall survival (OS), progression-free survival (PFS) and relapse/progression of multiple myeloma after transplant. Overall survival was defined as death from any cause with censoring of surviving patients at last follow-up; PFS was defined as survival without progressive disease or relapse from complete response. Patients alive and without progression/relapse were censored at last follow-up. Relapse/progression was defined as time to first evidence of recurrence or progression of multiple myeloma and summarized by the cumulative incidence estimate with transplant-related mortality as the competing risk.
Statistical analysis
Patient, disease and transplant-related variables and outcomes of interest were evaluated. Estimates of outcomes were reported as probabilities with 95% confidence intervals (95% CI). The probability of OS and PFS was calculated with the Kaplan-Meier estimator. Multivariable analysis was performed using Cox proportional hazards regression. High-risk status was considered the main effect in the multivariable analysis. Other factors tested in the analysis are listed in Supplementary table 1. The assumption of proportional hazards was tested for each variable, and factors violating the proportionality assumption were adjusted by stratification. A stepwise model building approach was used to develop models for OS, PFS and relapse/progression. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed using SAS v 9 (Cary, NC).
Results
Baseline patient and disease characteristics as well as induction and maintenance regimens are available in table 1. Median follow up was 36 months for HRM and 44 months for non-HRM. The median age, Karnofsky and HCT comorbidity index scores were similar among cohorts. When compared to non-HRM, the HRM cohort was associated with higher stage at diagnosis (43% vs 28% p=0.003). More HRM patients received induction with bortezomib and immunomodulatory drug (imid) combinations (56% vs 43%, p <0.001) and had a similar complete response rates prior to transplant (14% vs 16%, p = 0.1).
Table 1.
Characteristics of patients
| Variable | HRM | Non-HRM | p-value |
|---|---|---|---|
| Number of patients | (17%)125 | (83%) 590 | |
| Median age at transplant, years (range) | 58 (33–72) | 58 (28–76) | 0.91 |
| Age >65 | 69 (55) | 338 (57) | 0.67 |
| Male Gender | |||
| Race | 0.55 | ||
| White | 98 (78) | 460 (78) | |
| Black | 21 (17) | 110 (19) | |
| Others1 | 5 (4) | 12 (2) | |
| Unknown | 1 (<1) | 8 (1) | |
| Karnofsky Score, < 90% | 56 (45) | 222 (38) | 0.15 |
| HCT-CI score, >3 | 19 (15) | 92 (16) | 0.62 |
| Disease-related | |||
| Cytogenetic abnormality (conventional or FISH) |
|||
| High Risk | |||
| t(4;14) only | 28 (22) | -- | |
| t(14;16) only | 5 (4) | -- | |
| del17p only | 28 (22) | -- | |
| Hypodiploid only | 12 (10) | -- | |
| Chromosome 1 abnormalities only | 25 (20) | -- | |
| ≥2 High risks | 27 (22) | -- | |
| ISS/Durie-Salmon Stage III | 54 (43) | 167 (28) | 0.003 |
| Beta-2 microglobulin level at diagnosis, ≥3.5 mg/l |
73 (58) | 226 (38) | <0.001 |
| Serum albumin at diagnosis, < 3.5 g/dl | 53 (42) | 175 (30) | 0.02 |
| Hemoglobin at diagnosis, g/dl | |||
| N Evaluable | 121 | 562 | |
| Median (range) | 10 (3–17) | 11 (1–18) | <0.001 |
| Serum creatinine ≥ 2 mg/dl at diagnosis | 10 (3–17) | 11 (1–18) | 0.34 |
| Treatment-related | |||
| Lines of chemotherapy | 0.81 | ||
| 1 | 102 (82) | 476 (81) | |
| 2 | 23 (18) | 114 (19) | |
| Pre-Transplant Chemotherapy | <0.001 | ||
| IMID + Bort +/− Steroids | 70 (56) | 254 (43) | |
| IMID +/− Steroids | 15 (12) | 185 (31) | |
| Bort +/− Cytoxan +/− Steroids | 27 (22) | 88 (15) | |
| Others2 | 5 (4) | 17 (3) | |
| Disease status at transplant | 0.11 | ||
| CR | 17 (14) | 92 (16) | |
| VGPR | 44 (35) | 164 (28) | |
| PR | 53 (42) | 290 (49) | |
| SD | 4 (3) | 30 (5) | |
| Rel/prog | 7 (6) | 14 (2) | |
| Time from diagnosis to transplant | 0.84 | ||
| < 6 months | 54 (43) | 249 (42) | |
| 6 – 12 months | 71 (57) | 341 (58) | |
| Year of transplant | 0.01 | ||
| 2008 | 25 (20) | 213 (36) | |
| 2009 | 18 (14) | 68 (12) | |
| 2010 | 18 (14) | 79 (13) | |
| 2011 | 36 (29) | 129 (22) | |
| 2012 | 28 (22) | 101 (17) | |
| Post-transplant therapy | 0.004 | ||
| Lenalidomide+Bortezomib+/−Steroid | 33 (26) | 72 (12) | |
| Lenalidomide+/− Steroid | 37 (30) | 218 (37) | |
| Bortezomib +/− Steroid | 3 (2) | 12 (2) | |
| Thalidomide +/− Steroid | 3 (2) | 9 (2) | |
| Others | 0 | 4 (<1) | |
| No post-transplant therapy | 36 (29) | 200 (34) | |
| Unknown | 13 (10) | 75 (13) | |
| Median follow-up of survivors (range), months |
36 (3–78) | 44 (3–83) |
Day 100 responses
At 100 day post-transplant, similar numbers of patients had achieved a response in the 2 groups. The >/= partial responses (overall response rate) for HRM and non-HRM groups were 85 and 84% respectively and >/= very good partial responses (VGPR) in the HRM and non-HRM groups were 59 and 61% respectively. Complete and stringent complete responses (CR/sCR) were 31 vs 30% and VGPR rates were 28 and 31% in the HRM and non-HRM groups respectively.
Post-transplant therapies
Based on center reporting, we were unable to discern differences between consolidation and maintenance therapies and 'post-transplant therapy' was used to encompass all therapies used in the absence of relapse and/or progression after transplant. Patients with HRM were more likely to receive planned post-transplant therapy compared to non-HRM patients (71 vs 66%, p= 0.004), with combined bortezomib and imid therapy used more frequently in HRM (26% vs 12%). While bortezomib monotherapy was used equally in both groups (2%), lenalidomide monotherapy was used more frequently in non-HRM patients (37%) compared to HRM patients (30%), p 0.004.
Disease control and survival outcomes
The median follow up of survivors in this cohort was 36 months for the HRM and 44 months for non-HRM groups. Median PFS and OS for HRM were 21 and 68 months; median PFS for non-HRM group was 36 months and OS was not reached. In the univariate analysis (Table 2) PFS at 1 year for HRM was 61% vs 82% for non-HRM, at 2 years PFS for HRM was 48% vs 64% for non-HRM, at 3 years PFS for HRM was 37% vs 49% for non-HRM and at 4 years from auto-HCT PFS was 25% for HRM vs 38% for non-HRM (p <0.001). Overall survival at 1 year from autoHCT was 89% for HRM vs 95% for non-HRM, at 2 years OS was 81% for HRM vs 91% for non-HRM, at 3 years OS was 72% for HRM vs 85% for non-HRM and at 4 years post autoHCT OS was 59% for HRM and 80% for non-HRM (p <0.001), Figure 1. Univariate analysis of PFS and OS by type of cytogenetic abnormality is shown in table 4.
Table 2.
Univariate analysis of outcomes
| HRM, N=125 Prob (95% CI) |
Non-HRM, N=590 Prob (95% CI) |
p-value | |
|---|---|---|---|
| Relapse | <0.001 | ||
| 1-year | 38 (29–46)% | 18 (15–21)% | |
| 2-year | 51 (42–60)% | 35 (31–39)% | |
| 3-year | 62 (52–70)% | 49 (44–53)% | |
| 4-year | 73 (61–82)% | 61 (56–66)% | |
| Progression free survival | <0.001 | ||
| 1-year | 61 (53–70)% | 82 (79–85)% | |
| 2-year | 48 (39–57)% | 64 (60–68)% | |
| 3-year | 37 (28–47)% | 49 (45–54)% | |
| 4-year | 25 (16–35)% | 38 (33–43)% | |
| Overall survival | <0.001 | ||
| 1-year | 89 (82–94)% | 95 (94–97)% | |
| 2-year | 81 (73–87)% | 91 (89–93)% | |
| 3-year | 72 (63–80)% | 85 (81–88)% | |
| 4-year | 59 (47–71)% | 80 (76–84)% |
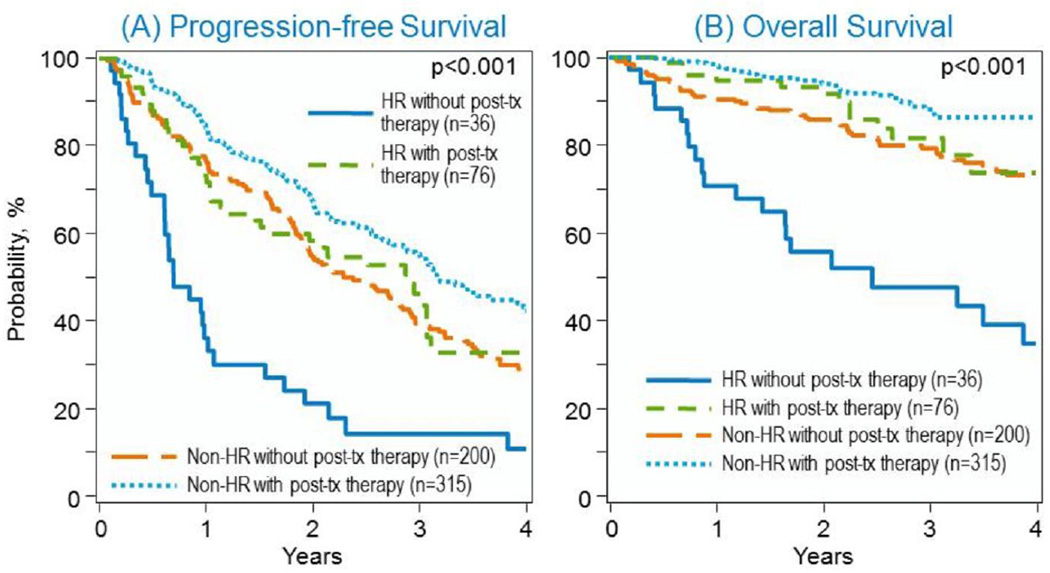
Figure 1.
A. Progression-free and (B) overall survival in HRM and non-HRM after autoHCT
Table 4.
Outcomes at 3 years post-transplant. Values are expressed as probabilities with 95% confidence intervals.
| nonHRM | t(4;14) | del 17p | 1q/1p abn | ≥2 HR | P-value | |
|---|---|---|---|---|---|---|
| PFS | 49 (45–54)% | 28 (11–50)% | 43 (24–62)% | 50 (29–72)% | 27 (10–47)% | 0.14 |
| OS | 85 (81–88)% | 60 (39–80)% | 78 (57–93)% | 91 (77–99)% | 67 (48–84)% | 0.15 |
In the multivariable analysis (Table 3), HRM was independently associated with higher relapse rate (hazard ratio 1.82, 95% confidence interval 1.4–2.4, p < 0.0001) and overall mortality (HR 2.24, 95% CI 1.5–3.3, p < 0.0001), A deeper response status prior to transplant and planned post-transplant therapy (HR1.94, 95% CI 1.4–2.8, p = 0.001) independently reduced relapse, and improved PFS and OS. Higher stage at diagnosis and black race also adversely affected survival in this cohort.
Table 3.
Multivariate analysis of outcomes
| Outcome | Number | Hazard ratio (95% CI) | p-value |
|---|---|---|---|
| Relapse | |||
| HRM vs non-HRM | 125 | 1.83 (1.41–2.36) | <0.0001 |
| Pre-transplant CR | 109 | 1 | <0.0001 |
| VGPR | 208 | 1.2 (0.8–1.7) | 0.3032 |
| PR | 343 | 1.7 (1.2–2.3) | 0.0014 |
| SD | 34 | 2.0 (1.1–3.3) | 0.0084 |
| Progression | 21 | 3.2 (1.8–5.7) | <0.0001 |
| Planned post-transplant therapy | 391 | 1 | 0.0002 |
| None | 235 | 1.5 (1.2–1.9) | 0.0002 |
| Missing | 88 | 0.9 (0.6–1.3) | 0.6177 |
| Progression-free survival | |||
| HRM vs non-HRM | 125 | 1.8 (1.4–2.4) | <0.0001 |
| Pre-transplant CR | 109 | 1 | <0.0001 |
| VGPR | 208 | 1.2 (0.8–1.7) | 0.3984 |
| PR | 343 | 1.6 (1.1–2.2) | 0.0026 |
| SD | 34 | 1.8 (1.1–3.1) | 0.0156 |
| Progression | 21 | 3.0 (1.7–5.3) | 0.0001 |
| Planned post-transplant therapy | 391 | 1 | <0.0001 |
| None | 235 | 1.6 (1.2–2.0) | <0.0001 |
| Missing | 88 | 0.9 (0.6–1.4) | 0.6926 |
| Overall survival | |||
| HRM vs non-HRM | 125 | 2.24 (1.53–3.27) | <0.0001 |
| Black vs White | 131 | 1.7 (1.1–2.5) | 0.0061 |
| ISS/DSS III Yes vs No | 221 | 1.8 (1.2–2.5) | 0.0034 |
| Pre-transplant CR | 109 | 1 | 0.0042 |
| VGPR | 208 | 2.0 (1.0–3.8) | 0.0244 |
| PR | 343 | 2.2 (1.2–4.0) | 0.0054 |
| SD | 34 | 2.2 (0.8–5.4) | 0.0996 |
| Progression | 21 | 5.0 (2.1–11.3) | 0.0001 |
| Planned post-transplant therapy | 391 | 1 | 0.0010 |
| None | 236 | 1.9 (1.3–2.8) | 0.0002 |
| Missing | 88 | 1.3 (0.7–2.4) | 0.2892 |
Progressive disease was the predominant cause of death in both groups (87% vs 83%). Infection (3% vs 4%), organ failure (5% vs 3%) and secondary malignancy (0 vs 2%) rates were similar in HRM and non-HRM respectively.
Impact of post-transplant therapies on survival
Comparing survival of HRM versus non-HRM based on whether they received post-transplant therapy or not, the 3 year post-transplant PFS for HRM receiving post-transplant therapy was 46(33–59)% compared to 14(4–29)% for HRM not receiving it (p-value <0.001). For non-HRM with post-transplant therapy, 3 year PFS was 55(49–62)% compared to 39(32–47)% for non-HRM without it (p-value 0.002). The 3-year OS for HRM with post-transplant therapy was 81(70–90)% versus 48(30–65)% without (p-value <0.001), compared to non-HRM with post-transplant therapy 88(84–92)% versus 79(73–85)% without it (p-value 0.02). Overall survival for HRM versus non-HRM without post-transplant therapy (Figure 1A) was significantly worse (p-value <0.0001) but not statistically significant for HRM versus non-HRM on post-transplant therapy (p-value 0.08) (Figure 1B). Details of type of post-transplant therapy and outcomes are shown in Supplementary table.
Impact of chromosomal abnormalities on survival
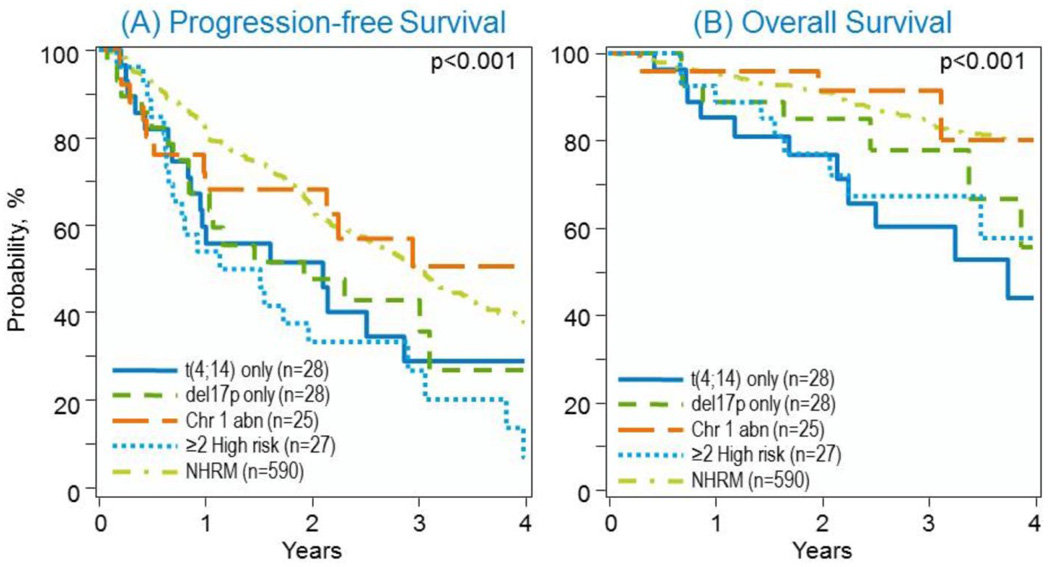
Comparing survival based on specific chromosomal abnormalities, 3 year PFS for non-HRM was 49(45–54)%; 28 (11–50)% for t(4;14); 43(24–62)% for del 17p; 50(29–72)% for chromosome 1 abnormalities and 27(10–47)% for >1 high-risk marker (p<0.001). Three year OS for non-HRM was 85(81–88)%, 60(39–80)% for t(4;14); 78(57–93)% for del 17p; 91(77–97)% for chromosome 1 abnormalities and 67(48–84)% for >1 high-risk marker (p<0.001) (Table 4 and Figure 2).
Figure 2.
(A) Progression-free and (B) overall survival in non-HRM, t(4;14), del 17p, chromosome 1 abnormalities, ≥ 2 high risk abnormalities after auto-HCT
Discussion
We describe outcomes after upfront auto-HCT in a recent cohort of multiple myeloma patients with cytogenetic and FISH data available from the CIBMTR. We make a number of clinically important observations: 1) Despite having similar day 100 post-transplant responses, HRM patients have worse outcomes as early as 1 year post-auto HCT, 2) Certain molecular abnormalities are associated with worse outcomes than others in this cohort but the presence of more than 1 high-risk marker identifies a subgroup of patients that have the worst prognosis, 3) The 3 year PFS was similar in the non –HRM and chromosome one abnormality group, 4) Post-transplant therapies are associated with lower relapse rates, longer PFS and OS in both HRM and non-HRM groups, 5) The relative improvement in PFS and OS seen in HRM receiving post-transplant therapy is greater than the improvement seen in the non-HRM groups, however OS is clearly distinguishable visually among all 4 categories (figure 1B) and 6) Black race is associated with worse OS than Whites without an association with worse relapse/progression or PFS in multivariable analysis.
The survival of multiple myeloma patients has significantly improved over the past two decades, however, this improvement has not been uniform, with some patients achieving long-term remissions and living for more than 10 years while others succumb to their disease and die within 3 years. This heterogeneity in outcomes is partially explained by the presence of high risk molecular markers.17
The definition of HRM has evolved over time as studies incorporating more detailed disease, patient and tumor specific markers are completed and the natural history of the disease has been examined.18 In the current era, while there is certainty regarding the poor prognostic significance of del (17p); the other cytogenetic abnormities are not equivocally poor across studies, and treatment with bortezomib appears to abrogate the poor prognosis associated with t(4;14). The International Myeloma Workshop 2011 consensus suggested that cytogenetic chromosome 13q deletion, t(4;14); del (17p); t(14;16) by FISH or cytogenetics should be considered high risk.19 The IMWG 2014 consensus defined a combined high risk model incorporating International Staging System II or III and del (17p) or t(4;14). Using this model, high-risk patients are projected to survive under 2 years despite novel agents while low-risk patients survive for more than 10 years.3, 5 Conflicting data exist regarding prognostic significance of t(14;16) whereby the Mayo Clinic6 and the Medical Research Council5 groups show that it is associated with poorer outcome, whereas the IFM studies8 did not show this. Similarly, conflicting data exist for chromosome 1 abnormalities (1q21 amplification, 1p deletion and others). Some reports have shown amplification 1q21 to be an independent prognostic factor5, 7 whereas our data as well as others have not.1, 11 According to the IMWG, although its role as a poor prognostic factor is controversial, the lack of 1q21amplification may be useful in identifying patients with good prognosis.3 Deletion of 1p has also been shown to be an independent prognostic factor associated with shorter survival.9, 10, 12 In 2015, Palumbo, et al. published a ‘revised ISS’ incorporating serum LDH, cytogenetic abnormalities [del 17p, t(4;14) and t(14;16)] and ISS.20 In our study, stage was analyzed as a separate variable but because LDH at diagnosis was not reported uniformly, we could not analyze it. The depth of pre-transplant response is also associated with outcomes.21 In our study, we found similar results with stage, depth of pre-transplant response and presence of HRM associated with worse outcomes.
A previous CIBMTR study which assessed auto-HCT outcomes based on race in an older cohort (1995–2005) did not show any difference in outcomes among Blacks compared to Whites.22 The current study which assesses a more recent cohort does identify that despite no significant differences in relapse rates or PFS, black patients had worse overall survival than white patients. This finding was unexpected and should be investigated further.
Our study highlights the benefit of high dose therapy and the importance of post-transplant consolidation and/or maintenance therapies for HRM patients and suggests that worse outcomes due to HRM are mitigated by this strategy, with no difference in OS between HRM and non-HRM groups. Historically, the impact of induction and maintenance bortezomib on t(4;14) may overcome its poor prognosis; however the impact on del (17p) has been mixed.16, 23, 24 Lenalidomide maintenance has also been shown to be beneficial in HRM.14
This is the largest CIBMTR analysis addressing the impact of chromosomal abnormalities. However, limitations include the possibility of false negative FISH results, heterogeneous FISH methodology and variable plasma cell enrichment. Thus, it is possible that our non-HRM cohort includes patients with HRM and the differences in outcomes that we show may be an under-estimation. We minimized this bias by excluding centers that reported a low number of high risk cases. Independent physician review of FISH and cytogenetic data when available were also conducted to ensure that center reporting was confirmed.
In conclusion, our results reveal worse outcomes for HRM after auto-HCT despite similar early responses and that patients with more than one high risk chromosomal abnormality fare particularly poorly, followed closely by t(4;14). In this cohort, patients with chromosome 1 abnormalities had similar outcomes to patients without high risk cytogenetic abnormalities, suggesting that single chromosome 1 abnormalities may not be considered high risk unless accompanied by other high risk cytogenetic abnormalities. Strategies to maintain early post-transplant responses such as consolidation and maintenance treatments should be considered in all HRM patients although the benefit of any particular therapy could not be gleaned from the current analysis due to the heterogeneity of post-autoHCT treatment strategies employed.
Supplementary Material
Acknowledgments
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; Oxford Immunotec; Perkin Elmer, Inc.; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Telomere Diagnostics, Inc.; TerumoBCT; Therakos, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part as a poster at the 57th Annual Meeting of the American Society of Hematology, 2015.
References
- 1.Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 2.Chang H, Qi XY, Samiee S, Yi QL, Chen C, Trudel S, et al. Genetic risk identifies multiple myeloma patients who do not benefit from autologous stem cell transplantation. Bone marrow transplantation. 2005;36(9):793–796. doi: 10.1038/sj.bmt.1705131. [DOI] [PubMed] [Google Scholar]
- 3.Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S, et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28(2):269–277. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(36):4529–4535. doi: 10.1200/JCO.2013.49.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd KD, Ross FM, Chiecchio L, Dagrada GP, Konn ZJ, Tapper WJ, et al. A novel prognostic model in myeloma based on co-segregating adverse FISH lesions and the ISS: analysis of patients treated in the MRC Myeloma IX trial. Leukemia. 2012;26(2):349–355. doi: 10.1038/leu.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca R, Blood E, Rue M, Harrington D, Oken MM, Kyle RA, et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood. 2003;101(11):4569–4575. doi: 10.1182/blood-2002-10-3017. [DOI] [PubMed] [Google Scholar]
- 7.Hanamura I, Stewart JP, Huang Y, Zhan F, Santra M, Sawyer JR, et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood. 2006;108(5):1724–1732. doi: 10.1182/blood-2006-03-009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avet-Loiseau H, Malard F, Campion L, Magrangeas F, Sebban C, Lioure B, et al. Translocation t(14;16) and multiple myeloma: is it really an independent prognostic factor? Blood. 2011;117(6):2009–2011. doi: 10.1182/blood-2010-07-295105. [DOI] [PubMed] [Google Scholar]
- 9.Boyd KD, Ross FM, Walker BA, Wardell CP, Tapper WJ, Chiecchio L, et al. Mapping of chromosome 1p deletions in myeloma identifies FAM46C at 1p12 and CDKN2C at 1p32.3 as being genes in regions associated with adverse survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(24):7776–7784. doi: 10.1158/1078-0432.CCR-11-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chng WJ, Gertz MA, Chung TH, Van Wier S, Keats JJ, Baker A, et al. Correlation between array-comparative genomic hybridization-defined genomic gains and losses and survival: identification of 1p31–32 deletion as a prognostic factor in myeloma. Leukemia. 2010;24(4):833–842. doi: 10.1038/leu.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseca R, Van Wier SA, Chng WJ, Ketterling R, Lacy MQ, Dispenzieri A, et al. Prognostic value of chromosome 1q21 gain by fluorescent in situ hybridization and increase CKS1B expression in myeloma. Leukemia. 2006;20(11):2034–2040. doi: 10.1038/sj.leu.2404403. [DOI] [PubMed] [Google Scholar]
- 12.Hebraud B, Leleu X, Lauwers-Cances V, Roussel M, Caillot D, Marit G, et al. Deletion of the 1p32 region is a major independent prognostic factor in young patients with myeloma: the IFM experience on 1195 patients. Leukemia. 2014;28(3):675–679. doi: 10.1038/leu.2013.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaughnessy JD, Zhou Y, Haessler J, van Rhee F, Anaissie E, Nair B, et al. TP53 deletion is not an adverse feature in multiple myeloma treated with total therapy 3. British journal of haematology. 2009;147(3):347–351. doi: 10.1111/j.1365-2141.2009.07864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2012;366(19):1782–1791. doi: 10.1056/NEJMoa1114138. [DOI] [PubMed] [Google Scholar]
- 15.Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28(3):690–693. doi: 10.1038/leu.2013.335. [DOI] [PubMed] [Google Scholar]
- 16.Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(24):2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–2221. doi: 10.1038/leu.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Usmani SZ, Rodriguez-Otero P, Bhutani M, Mateos MV, Miguel JS. Defining and treating high-risk multiple myeloma. Leukemia. 2015;29(11):2119–2125. doi: 10.1038/leu.2015.209. [DOI] [PubMed] [Google Scholar]
- 19.Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117(18):4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(26):2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harousseau JL, Avet-Loiseau H, Attal M, Charbonnel C, Garban F, Hulin C, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 Trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(34):5720–5726. doi: 10.1200/JCO.2008.21.1060. [DOI] [PubMed] [Google Scholar]
- 22.Hari PN, Majhail NS, Zhang MJ, Hassebroek A, Siddiqui F, Ballen K, et al. Race and outcomes of autologous hematopoietic cell transplantation for multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(3):395–402. doi: 10.1016/j.bbmt.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avet-Loiseau H, Leleu X, Roussel M, Moreau P, Guerin-Charbonnel C, Caillot D, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(30):4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- 24.Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.