Abstract
Background
Neuroplasticity and neurorehabilitation have been extensively studied in animal models of stroke to guide clinical rehabilitation of stroke patients. Similar studies focused on traumatic brain injury (TBI) are lacking.
Objective
The current study was designed to examine the effects of individual and combined rehabilitative approaches, previously shown to be beneficial following stroke, in an animal model of moderate/severe TBI, the controlled cortical impact (CCI).
Methods
Rats received a unilateral CCI, followed by reach training, voluntary exercise, or unimpaired forelimb constraint, alone or in combination. Forelimb function was assessed at different time points post-CCI by tests of skilled reaching, motor coordination, and asymmetrical limb use.
Results
Following CCI, skilled reaching and motor coordination were significantly enhanced by combinations of rehabilitation strategies, not by individual approaches. The return of symmetrical limb use benefited from forelimb constraint alone. None of the rehabilitation strategies affected the size of injury, suggesting that enhanced behavioral function was not a result of neuroprotection.
Conclusions
The current study has provided evidence that individual rehabilitation strategies shown to be beneficial in animal models of stroke are not similarly sufficient to enhance behavioral outcome in a model of TBI. Motor rehabilitation strategies for TBI patients may need to be more intense and varied. Future basic science studies exploring the underlying mechanisms of combined rehabilitation approaches in TBI as well as clinical studies comparing rehabilitation approaches for stroke versus TBI would prove fruitful.
Keywords: exercise, motor rehabilitation, neuroplasticity, constraint-induced movement therapy, controlled cortical impact, recovery of function
Introduction
In the United States, an estimated 1.7 million people suffer a traumatic brain injury (TBI) yearly, leading to approximately 5 million individuals suffering long-term disabilities at any given time.1 TBI-induced deficits in cognition, memory, and mood are most commonly reported and studied; however, motor deficits are also common, especially following moderate to severe TBI, but are currently understudied.2–4 Although there are increasingly published recommendations for physical therapy,5 there is no definitive protocol for rehabilitation following TBI and only a few studies that specifically investigate the efficacy of motor rehabilitation post-TBI in patients or even in animal models.6 However, there is strong evidence from the clinical and experimental stroke literature that suggests that focused skilled motor training can reduce upper-extremity impairments and that when combined with adjuvant behavioral interventions or treatments, motor recovery may be enhanced.
Many clinical studies suggest that certain forms of motor rehabilitative practice can greatly improve motor function and drive brain remodeling following stroke.7–9 For example, a recent meta-analysis has shown that focused, intensive, highly repetitive, task-oriented, and task-specific training of the impaired limb can enhance recovery of motor ability in stroke patients.10 Furthermore, combining rehabilitative training with adjunctive treatments, such as constraint-induced movement therapy (CIMT), further enhances rehabilitation efficacy in stroke patients.11 Briefly, CIMT requires the constraint of the less-impaired limb for a large portion of the day and while an individual undergoes rehabilitative impaired limb training, reinforcing the use of the impaired limb. CIMT improves the function of the impaired limb and promotes greater movement-associated activation in the remaining cortex of the injured hemisphere.12,13 Imaging studies also suggest that CIMT treatment reduces chronic poststroke gray mater atrophy.14
Although skilled task-specific training can improve motor recovery, and this recovery may be improved with the addition of CIMT procedures in humans, it is unknown whether aerobic exercise also provides added benefit. In general, aerobic exercise or physical fitness training is seen as beneficial for many health reasons. In clinical trials, aerobic exercise has been shown to positively affect cognitive function and reduce depression following stroke and TBI.15 However, it is not clear whether aerobic exercise affects motor recovery following TBI or stroke, although studies are under way.16
In animal models of stroke and other brain injuries, there is also strong evidence that behavioral experience can drive better motor recovery and neural plasticity.17 It is thought that recovery of function is linked to appropriate neural restructuring after stroke and that motor “relearning” and task-specific practice is key to driving this neural plasticity.9,17,18 In experimental stroke models, animals trained with their impaired limb to reach and manipulate small food items (single-pellet reaching), a form of task-specific skilled forelimb motor rehabilitation, have enhanced forelimb motor recovery and neural plasticity in the remaining motor cortex.19–22 Specifically, in squirrel monkeys, rats, and mice with ischemic damage to the forelimb representation area of the primary motor cortex, extensive practice in reaching with the impaired forelimb spares cortical tissue around the lesion and prevents the loss of movement representation in the remaining motor cortex (measured with intracortical microstimulation mapping)20 and results in enlarged forelimb motor representative maps.22,23 All these studies in stroke models point to the potential therapeutic benefit of skilled motor training tasks for rehabilitation following TBI. However, to our knowledge, no study has examined the efficacy of task-specific forelimb reaching practice alone following TBI in animal models, although a recent study demonstrated that forelimb training combined with a pharmacological CIMT-like treatment did improve manual dexterity in animals, following experimental TBI.24
In noninjured rats, aerobic exercise has been shown to drive hippocampal plasticity, hippocampal neurogenesis, and upregulation of brain-derived neurotrophic factor and improve cognitive function.4,25 In noninjured animals, aerobic exercise also has been shown to increase angiogenesis and blood flow in the primary motor cortex of rats,26 but unlike reach training, it does not increase synaptogenesis, nor does it enlarge forelimb motor cortex representation.27 A recent meta-analysis of the animal literature found that following experimental stroke, aerobic exercise reduces lesion volume and inflammation and may be neuroprotective in perilesional tissue.28 Most of these beneficial effects were found in the hippocampus, but a few studies did find a reduction in inflammation in the striatum and cortex. In female rats, aerobic exercise combined with reach training after unilateral stroke, however, did not further enhance the benefits of reach training on behavioral function or plasticity.19
The effects of exercise after TBI are varied.4,29 Following TBI, several studies indicate that exercise is beneficial, but others have also reported that aerobic exercise administered too early after injury can disrupt plasticity in the hippocampus.4,30 Nevertheless, no study has explored its influence on post-TBI motor recovery. Thus, the role of aerobic exercise in recovery of motor function after TBI is unclear.
Although animal studies of rehabilitation following stroke are beginning to significantly influence clinical rehabilitation strategies, extension of these findings from stroke to TBI requires the assumption that mechanisms of brain plasticity are essentially similar after these 2 types of brain injuries. Recent studies indicate that this assumption is flawed. Research on neuroplasticity following rodent models of TBI has primarily focused on the hippocampus, and very little attention has been paid to direct examination of forelimb motor recovery.31–33 Our recent study of neuroplasticity in the motor cortex in rats demonstrates that after a unilateral controlled cortical impact (CCI) model of TBI over the forelimb sensorimotor cortex (FL-SMC), there is a decrease in dendritic density in the area surrounding the CCI and in the contralateral homotopic cortex,4,34 regions that undergo robust dendritic growth and plasticity following ischemic infarcts placed in the same anatomical location and resulting in the same lesion volume as in the CCI.35,36 This study suggested that neuroplasticity may be muted after TBI, compared with stroke models. If neuroplasticity is muted or prevented following TBI, then rehabilitation strategies modeled after stroke treatments that interact with stroke-induced neural plasticity may be less effective following a TBI model in the absence of further adjunctive treatments.
The current study utilized motor rehabilitation paradigms previously examined in animal models of stroke and investigated their efficacy in a rodent model of moderate-severe TBI (unilateral CCI of the FL-SMC). Specifically, our paradigms included the following: forelimb reaching (a motor skills training task), running in an exercise wheel (aerobic exercise), and constraining the nonimpaired forelimb (similar to CIMT). Animals were given individual and combinatorial therapies (initiated and tested at time points previously demonstrated to result in significant enhancement of some behavioral recovery following stroke), and the long-term effects on recovery of forelimb function were examined.
Methods
Animals and Experimental Design Overview
A total of 78 male hooded Long-Evans rats (250–350 g; 3 months old) were housed in the DePaul University Research Facility on a 12:12 hour light/dark cycle. The animals were weighed and placed on a restricted feeding schedule (10–15 g standard chow/daily) 48 hours prior to behavioral training, reducing their body weight by 5% to 10% of their initial weight. All animals were trained on single-pellet reaching tasks and then randomly assigned to injury groups. Following induction of a CCI over the FL-SMC or sham surgery, rats were further assigned to groups that either received a single rehabilitation task or combinations of tray reaching, forelimb constraint, and exercise (Table 1; Figure 1). Recovery of function was assessed by comparing preinjury to postinjury performance at several time points using the single-pellet reaching task, foot fault, and cylinder task (Figure 1). Animals were euthanized on day 43 postinjury via cardiac perfusion and injury size measured. All procedures were approved by DePaul’s Institutional Animal Care and Use Committee and the Department of Defense Animal Care and Use Review Office. For more details, see Methods section in supplementary material.
Table 1.
Experimental Groups.
| Group | n | Group | n |
|---|---|---|---|
| CCI + Reach Training (CCI + R) | 9 | Sham + Reach Training (Sh + R) | 6 |
| CCI + Reach + Exercise (CCI + RE) | 9 | Sham + Reach + Exercise (Sh + RE) | 6 |
| CCI + Reach + Exercise + Forelimb Constraint (CCI + REC) | 7 | Sham + Reach + Exercise + Forelimb Constraint (Sh + REC) | 7 |
| CCI + Constraint only (CCI + C) | 7 | Sham + Constraint only (Sh + C) | 6 |
| CCI + Yoked Control (CCI) | 9 | Sham + Yoked Control (Sham) | 6 |
Abbreviation: CCI, controlled cortical impact.
Figure 1.
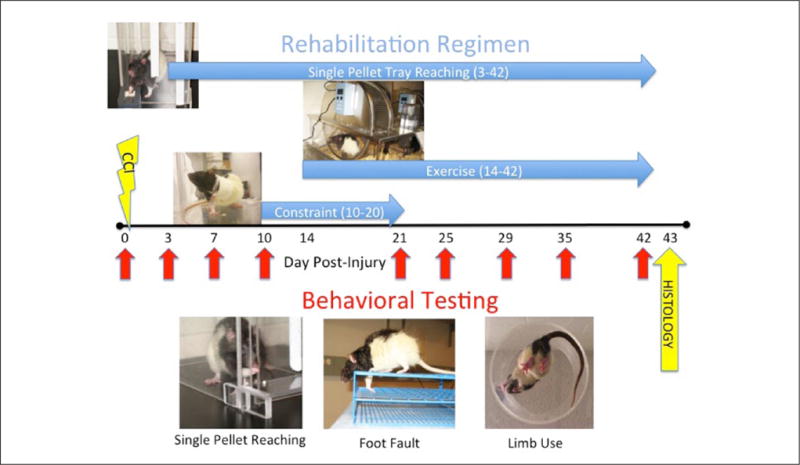
(Top) Rehabilitation consisted of single-pellet reaching, exercise, and forelimb constraint alone or in combination administered at different time points and durations following CCI. (Bottom) Behavioral testing (single-pellet reaching, foot fault, and limb use) was administered at baseline (day 0) and at different time points postinjury (red arrows). Rats were killed humanely for histology on day 43.
Rehabilitation Methods
Prior to injury, rats were randomly assigned to receive different rehabilitation regimens that included the following: tray reaching alone, less-impaired forelimb constraint alone, tray reaching + exercise, and tray reaching + exercise + constraint (see Table 1). These combinations were chosen based on their efficacy in enhancing recovery or plasticity in animal models of stroke and TBI. Reaching, forelimb constraint, and exercise were introduced at time points found to be effective in other models of recovery (see Figure 1). Control animals were assigned to appropriate yoked conditions in which rats were placed in the rehabilitation environments without actually performing motor tasks, described individually below.
Tray Reaching
All animals assigned to receive reaching rehabilitation began training 3 days post-CCI and were trained once per day until postoperative day 42. For tray reach training, rats were placed in a clear Plexiglas reaching chamber (as described in the supplementary material) but using a laterally placed reaching window and the chamber wall to discourage reaching with the less-impaired forelimb (see Figure 1). Rats reached through a 1-cm-wide window with their impaired limb for 100 banana-flavored pellets (45 mg, Bioserv, Inc, Flemington, NJ) placed on a tray with a 25° incline, which was placed outside of the reaching chamber, or 20 minutes, whichever occurred first. The total time taken to eat all 100 pellets was recorded. (See supplemental results.) A yoked control group was placed in the same chamber, for the same length of time as the yoked rats undergoing rehabilitation, and received all 100 pellets on the floor of the chamber.
Forelimb Constraint
On postinjury day 10, animals in the constraint group had the forelimb ipsilateral to the CCI constrained using a limb-restricting vest (soft customized 2-holed jackets with athletic tape used to wrap around the forelimb; Lomir Biomedical, Malone, NY; Figure 1). The purpose of this was to encourage the rats to use their injured forelimb. The vests were worn continuously for 10 days and were gently removed on day 20 postinjury. A yoked control group wore a 2-holed vest, which allowed movement of both limbs.
Exercise
On day 14 postinjury, rats were given voluntary access to a running wheel (Lafayette Instruments, Lafayette, IN; Figure 1) for 6 continuous hours (3 in the light cycle, 3 in the dark cycle) every day until day 42 postinjury. The total distance run per day was measured and is presented in kilometers per hour (see supplemental Results section). A yoked control group was allowed access to a locked running wheel during the same time period.
Controlled Cortical Impact
A unilateral CCI was administered over the FL-SMC opposite the preferred reaching limb, as described previously (see Methods section in supplementary material34). Sham animals received all procedures up to but not including the craniotomy because skull removal itself produces minor acute behavioral and neurochemical asymmetries.37
Behavioral Training and Assessment
All behavioral assessment was conducted on day 0 (baseline, preinjury) and on days 3, 7, 10, 14, 21, 25, 29, 35, and 42 postinjury (see Figure 1). These tests primarily assess forelimb asymmetries directly by measuring performance of both the left and right forelimb (foot fault and limb use) or assess fine motor skills in the injured forelimb, which are usually accomplished with postural compensation by the uninjured limb38 (single-pellet reaching). On day 14, rats were wearing vests that constrained their uninjured forelimb, therefore obscuring these outcome measures that require both forelimbs. Thus, day 14 is excluded from the graphs and data analyses.
Single-Pellet Reaching
The single-pellet reaching task is a sensitive test of manual dexterity and a potent rehabilitative task of rat forelimbs that allows both quantitative and qualitative assessment of fine motor skills19,34,39,40 (see supplemental Methods section). Reaching performance was measured as the percentage of successes out of the total number of reach attempts—[(Total successes/Total reach attempts) × 100]—with the injured forelimb. Data presented are the mean ± standard error of the mean (SEM) percentage of successful reaches.
Qualitative Reaching Assessment
We also evaluated the quality of the successful reaches in the single-pellet reaching task via slow motion videoreplay of reaching performance on days 0 and days 10 and 42 postinjury. Videotapes were rated based on criteria modified from41 (see supplemental material).
Foot Fault Test
Forelimb coordination was measured by the foot fault test34,35,40,42 (see supplemental material). Data are presented as follows: [(Contralateral fault − Ipsilateral fault)/Total steps] × 100.
Cylinder Test
This test reveals asymmetries in the use of the forelimbs when animals are placed in a tall narrow cylinder34,35,40,42 (see supplemental material). The total number of ipsilateral and contralateral (to the CCI) forelimb behaviors was calculated. Data are presented as the percentage use of the ipsilateral forelimb: Percentage (Ipsilateral limb use/Total forelimb use).
Histology
Euthanasia
On day 43 postinjury, rats were given an overdose of equithesin (2.0 mL intraperitoneal) and transcardially perfused with phosphate-buffered saline + 0.01% heparin followed by 4% paraformaldehyde. Brains were then extracted, stored in 4% paraformaldehyde, and cryoprotected with 10% to 30% sucrose. Then, 7 sets of 50-μm rostral-caudal coronal sections through the cerebrum were collected and processed for Nissl stain to measure contusion size.
Contusion Size
Measurement of the estimated contusion size was obtained from 8 serial coronal Nissl-stained sections encompassing the full extent of the injury, from approximately + 1.70 A/P through −1.06 A/P bregma, as described previously34 (Methods section in supplementary material). Data are presented as the mean ± SEM of the volume of remaining cortex.
Statistical Analysis
This study was designed to use a priori planned comparisons for the primary analyses, using SPSS (SPSS Inc, Chicago, IL) general linear models procedures for analysis of variance (ANOVA), with post hoc t tests, as warranted by ANOVA, corrected with a protected Fisher’s least-significant difference (LSD) test. The primary comparisons of interest tested which treatment or treatment combination (a subset of all possible combinations) would (1) return animals to sham levels and/or (2) enhance forelimb recovery compared with spontaneous recovery (CCI + Yoked = CCI). Anatomy was analyzed by a 1-way ANOVA for group. There were no significant differences between sham groups in any of the behavioral or histological measures (see supplemental results), and thus, they were combined and labeled Sham for all results presented below.
Results
Single-Pellet Reaching Task
A comparison of percent success between all rehabilitation groups and sham animals revealed a significant effect of Group [F(5, 66) = 12.60, P < .001], Day [F(8, 528) = 30.63, P < .0001], and Day × Group interaction [F(40, 396) = 4.00, P < .001). As seen in Figure 2A, all CCI groups were significantly impaired compared to sham immediately postinjury on day 3, prior to the start of rehabilitation (P < .001), demonstrating that CCI over the FL-SMC significantly impaired forelimb function. This impairment was present until the end of the study in CCI rats that receive reaching alone, constraint alone, or no rehabilitation (P < .01). However, by day 42, CCI rats that received reaching and exercise (RE) or reaching, exercise, and constraint (REC) showed a significant improvement in reaching success, recovering to sham levels (P = .15 and .73, respectively). Additionally, the combination of these rehabilitative approaches resulted in significant increases in reaching ability when compared with CCI rats without rehabilitation (P < .02 and P < .005, respectively). Together, these data suggest that a rehabilitation regimen that combines RE or REC enhances reaching ability to the level of noninjured rats, whereas neither reach training alone nor forelimb constraint alone results in an enhancement of reaching function.
Figure 2.
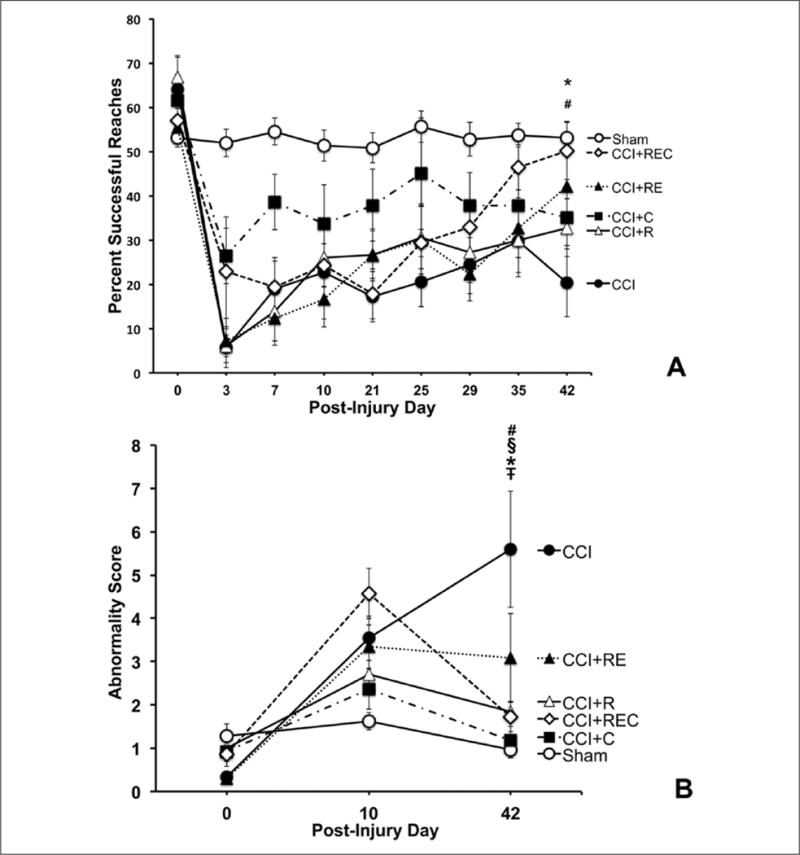
(A) Rats that received a combination of reaching and exercise (#CCI + RE) or reaching, exercise, and constraint (*CCI + REC) showed enhanced reaching behaviors compared with injured animals without rehabilitation (#P < .05, *P < .01). (B) Injured animals without rehabilitation (CCI) showed progressively more abnormal reaching behaviors over time, whereas all rehabilitation strategies reduced abnormal compensatory reaching behaviors compared with CCI: §CCI + R (P < .0001); ŦCCI + C (P < .0001); #CCI + RE (P < .01); and *CCI + REC (P < .0001).
Abbreviation: CCI, controlled cortical impact.
It should be noted that the CCI + constraint (CCI + C) group was significantly less impaired on the single-pellet reaching task compared with the CCI group on day 3 (P = .028), but they were no longer significantly different by day 42 (P = .159), likely driven by a group mean increase (possibly spontaneous recovery) in the CCI group that is not evident in the CCI + C group.
In addition to a quantitative assessment of successful reaching, we also qualitatively examined movement patterns during successful reaches. We examined the reaching movement patterns at baseline and at days 10 and 42 postinjury in a subset of animals. Reaching abnormality scores were calculated and showed an effect of Group [F(5, 37) = 5.18, P < .001], Day [F(2, 74) = 27.19, P < .001], and a Group × Day interaction [F(10, 74) = 4.39, P < .0001]; see Figure 2B. Post hoc analysis demonstrated that all groups that received a treatment intervention had decreased abnormality scores (more normalized movement patterns) on successful reaches by the end of the study compared with the group with CCI alone (P = .008−.0001). Furthermore, neither CCI + R, CCI + C, nor CCI + REC were significantly different from sham at the end of the study (P > .05). However, both CCI and CCI + RE were significantly different from sham on day 42 (P = .0001 and .02, respectively). These data suggest that although not all animals significantly improved in the quantity of their successful reaches, overall, those that received motor rehabilitation had more normalized reaching movement patterns and less abnormal compensatory strategies, compared with the untreated CCI rats.
Cylinder Test
The use of forelimbs for support during weight bearing behavior was assessed through examination of preference for ipsilateral forelimb use. In rANOVA of the combined sham group compared with CCI treatment groups, there were significant effects of Group [F(5, 66) = 28.70, P < .001], Day [F(8, 528) = 61.68, P < .001], and Day × Group [F(40, 528) = 7.32, P < .001], indicating that injury produced an asymmetry in the use of forelimbs for weight-bearing movements, with injured animals preferring to use the forelimb ipsilateral to the injury (see Figure 3). Post hoc analysis demonstrated significant differences between sham operates and all CCI groups (P < .05), suggesting that unlike what was seen in other behavioral tests, no injured rats recovered to sham levels. An examination of whether the rats that received rehabilitation showed recovery of symmetrical forelimb use compared with nonrehabilitated rats demonstrated that only rats that received constraint alone showed a significantly enhanced recovery (P < .03). Although not significant, there was a trend for rats that received all 3 rehabilitative tasks to show a return to symmetrical limb use (P = .052). This was the only behavioral measure that showed a benefit from a single rehabilitative approach.
Figure 3.
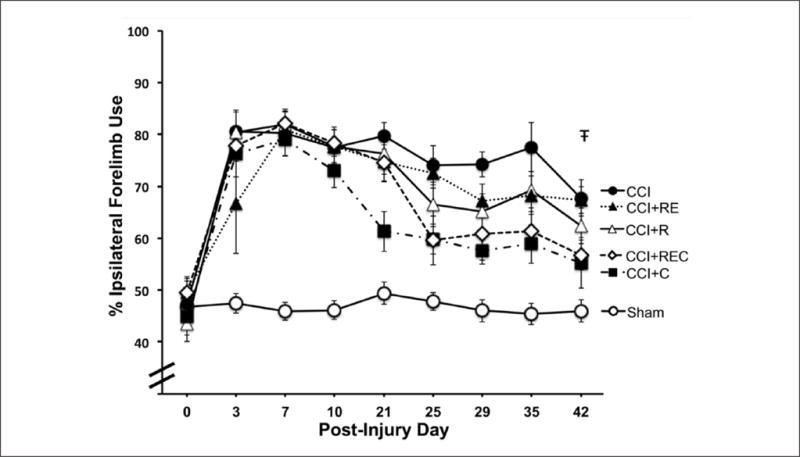
Reliance on the forelimb ipsilateral to injury was significantly increased by CCI. A recovery to symmetrical forelimb use was seen in rats that received forelimb constraint but not in any of the other rehabilitation groups (ŦCCI + C vs CCI, P < .05).
Abbreviations: CCI, controlled cortical impact; R, reaching; E, exercise; C, constraint.
Foot Fault Test
There were significant differences between the combined sham group and the CCI groups—Group: F(5, 66) = 52.46, P < .0001; Days: F(8, 528) = 82.37, P < .0001; Days × Groups: F(40, 528) = 9.46, P < .0001. Overall, CCI groups had significantly more foot faults in the injured forelimb compared with the sham group (all P < .001; Figure 4).
Figure 4.
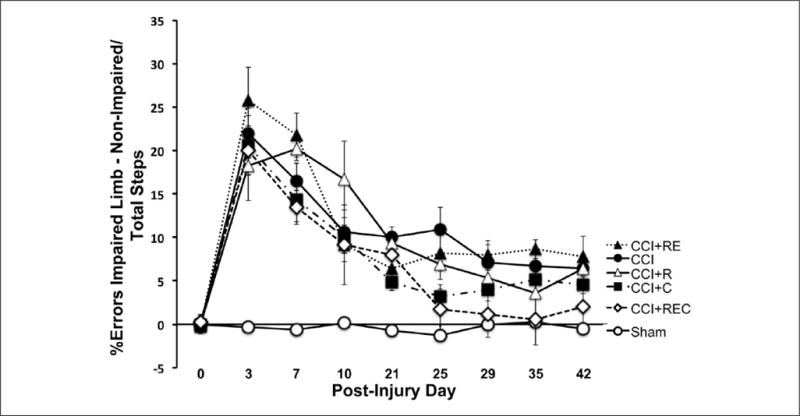
Percentage contralateral forelimb faults were significantly reduced in rats that received all 3 rehabilitation regimens (*CCI + REC vs CCI, P < .05).
Abbreviations: CCI, controlled cortical impact; R, reaching; E, exercise; C, constraint.
We used a post hoc analysis of the final testing day (LSD corrected) to determine if our treatments reduced foot faults compared with the CCI group or with the combined sham group. Only animals that received all 3 rehabilitation tasks combined (CCI + REC) were significantly different from those in the CCI group (P < .049) by day 42. Furthermore, this was also the only group that recovered to Sham levels (day 42: P = .18). All other groups were significantly different from sham by day 42 (P ≤ .008).
Contusion Size
To assess the consistency in the size of the contusion delivered between groups, the remaining cortical area was measured, and from this, the remaining cortical volume was determined (Figure 5). All animals that received a CCI showed a remaining cortical volume that was substantially smaller than that of the sham animals (P < .05) but not significantly different from one another, indicating that the size of CCI was consistent between groups. This is important to note because previous studies have demonstrated that too intense rehabilitation too soon could result in an exaggeration of the injury.43 This was not the case in this study. However, the CCI + REC group showed a remaining cortical volume that was slightly larger than that of the CCI group (approaching statistical significance P = .0512), though initial behavioral impairment levels were similar.
Figure 5.
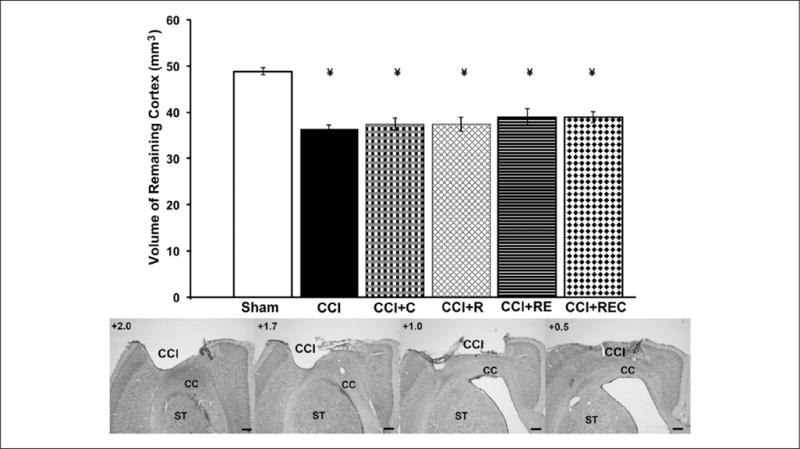
(Top) Volume of remaining cortex is significantly reduced in all injury groups compared with sham (¥P < .05). There were no significant differences in contusion size between treatment groups. (Bottom) Coronal sections representing injury size and location from 0.5 to 2.0 mm anterior to the bregma.
Abbreviations: CCI, contusion; R, reaching; E, exercise; C, constraint; CC, corpus callosum; ST, striatum (scale bar = 250 μm).
Discussion
Rehabilitation is one of the only effective therapeutic options for individuals with TBI, yet very little data are available from both animal and clinical studies supporting appropriate approaches that lead to optimal therapeutic benefit. Additionally, the underlying mechanisms of rehabilitation following TBI have not been fully explored. The current study utilized 3 rehabilitation approaches in a rodent model of TBI—reaching, exercise, and forelimb constraint—individually shown to enhance recovery or plasticity in animal models of stroke and focal lesions.19,20,36,44 These approaches were applied individually and in specific combinations to explore their capacity to enhance recovery of forelimb function following CCI. Together, our data indicate that enhancement of behavioral outcome on sensitive motor skills tests is optimal when rehabilitation approaches are combined. Specifically, our data indicate that the ability to accurately reach for and manipulate small objects using the impaired paw was significantly enhanced only when a combinatorial therapeutic approach was provided that included reaching and exercise, with or without forelimb constraint. Similarly, forelimb coordination during locomotion was enhanced only when reaching, exercise, and constraint were combined. The recovery of function in these tasks was not just enhanced, but in both tasks, an approach that combined multiple rehabilitation tasks was necessary to enhance function to sham levels. Analysis of reaching movement strategies suggest that although not all animals significantly improved in the quantity of their successful reaches, overall, many rats that received any form of rehabilitation did reach with more normalized reaching patterns and not with compensatory, abnormal strategies. That is, the functional improvements observed appear to reflect a degree of true recovery, as opposed to enhanced compensatory strategies.
These data support previous studies demonstrating that experimental CCI centered over the FL-SMC results in long-lasting impairments in the contralateral forelimb.34,45,46 Additionally, it supports previous studies demonstrating that exercise alone following unilateral fluid percussion or CCI centered over the hippocampus enhances recovery, albeit in a cognitive rather than motor task.29,47,48 The present study is novel in its combination of different behavioral treatment approaches following TBI. Although others have demonstrated the benefits of combinatorial therapeutic approaches for TBI using exercise or enriched environments combined with pharmacotherapy, such as amphetamines or antidepressants,49,50 none have attempted multiple rehabilitative combinations following TBI, in contrast to studies in animal models of stroke.19,51 We provide evidence that, unlike what is seen following stroke-like damage to the sensorimotor cortex,19,20,36,44 neither rehabilitative reach training of the impaired forelimb alone nor constraint of the less-impaired forelimb alone were effective in enhancing recovery of function on tests of skilled reaching and motor coordination. Enhanced function in these tasks resulted only from a combination that included RE or REC. Interestingly, combining motor skill training and exercise in a focal ischemic model of the FL-SMC did not show the additive effect seen in this study,19 but combining constraint and exercise in a hemorrhagic stroke model did.51 These differences could be a result of the timing of the therapies, the types of therapies, and the types and severity of insults to the brain. Following focal ischemia, although the reaching training started early postischemia (as in the current study), the exercise was initiated earlier than in the present study. Perhaps the early initiation of the exercise following stroke damage prevented an additive effect. Early exercise post-TBI has been shown to be detrimental.30,52 However, when reach training was combined with forelimb constraint following hemorrhagic stroke, it proved to be beneficial. As found in the current study, the addition of constraint to RE therapies proved to be the most beneficial in tests of skilled forelimb use and motor coordination. Future studies are needed to investigate whether constraint in addition to just exercise or just reaching enhances functional recovery following TBI.
The only behavioral task to benefit from a single therapeutic approach was the recovery of symmetrical forelimb use for exploratory and weight-bearing behaviors (cylinder test). Rats that received constraint alone recovered more symmetrical limb use than any of the other treatment groups, although there was a nonsignificant recovery in the CCI + REC group. This further supports previous findings that forelimb constraint can be beneficial in a variety of unilateral injury models and in humans.12,13,51,53–55 The benefit of forelimb constraint alone and in the combination approaches may be a result of the increased demand of utilizing the impaired forelimb (food handling and grooming) and robust home-cage practice with impaired limb body weight support, as is tested in this task. The effects of constraint may also arise from its reduction of learned disuse of the injured forelimb, which can negatively affect behavioral recovery.56 Combining the robust impaired forelimb practice provided by constraint with the other rehabilitative tasks that focus on different physical modalities, such as motor skill learning (reaching) and enhanced muscular movement and cardiovascular conditioning (exercise), may be what is necessary in an injury model such as CCI, one which produces a much more global effect on the brain than does stroke. The need for more intense and combined rehabilitation approaches may also be a result of the limitations in plasticity seen following CCI. We have demonstrated that experience-dependent, injury-induced plastic changes in dendritic arborization and synapse number previously seen in a similarly placed and sized focal infarct were not present following CCI.34 If rehabilitation’s effectiveness relies, in part, on the brain’s potential to undergo experience-dependent plastic changes,20 then it is quite possible that more varied and intense rehabilitation may be required in a brain possessing diminished potential for plasticity.
Combining rehabilitation strategies did not promote recovery of function via the neuroprotective effects on brain injury size, although there was a nonsignificant tendency for increased cortical volume in the CCI + REC group. Overall, there were no significant differences in contusion size between injury groups. Therefore, rehabilitation did not decrease neuronal death, but alternatively, it also was not detrimental. Previous studies have shown that starting rehabilitation strategies too early can result in decreased behavioral recovery and detrimental effects on the brain.29,57 Carefully choosing time points for the delivery of the individual rehabilitation strategies previously shown to be beneficial avoided such negative consequences.
Mechanistically, each of these rehabilitation tasks has been shown to affect the brain in different ways. Reaching and constraint both can enhance neural plasticity in the form of increased spines, dendritic arbors, sprouting, and cortical map reorganization55,58,59 as well as increased myelination.60 Aerobic exercise increases angiogenesis, neurogenesis, and growth factor upregulation.29,61 Thus, it is possible, but not examined in this study, that the combined rehabilitation regimen provided an opportunity for increased vasculature, neurogenesis, and plastic functional neural connections that resulted in more successful reaching, enhanced motor coordination, and symmetrical limb use. Future studies are needed to explore these potential underlying mechanisms to elucidate which may have played a role in the behavioral findings presented in the current study.
There are also several limitations in this study that will need further exploration. First, not all possible combinations of treatment were included in the study; instead, we focused on treatments previously shown to improve forelimb function following stroke. Second, we chose to use noncraniotomy sham controls to avoid acute mechanical and ischemic damage to underlying tissue because our focus was on the chronic effects of behavioral manipulations following moderate to severe TBI. The proper type of sham for CCI is subject to debate,62 but it seems unlikely that the effects of craniotomies in the sham operates would have substantially altered the main inferences and conclusions. Third, our aerobic exercise treatment was limited to a total of 6 hours (3 light: 3 dark). Although rats are more active in the dark cycle, Belke63 demonstrated that when rats were restricted in the amount of time they had access to a running wheel, the light:dark period had no effect on the amount of time spent exercising. Future studies are needed to explore the effects of longer running periods, alone and in combination with other behavioral treatments, to investigate its potential beneficial role in forelimb recovery after TBI.
Conclusions and Implications
The current study has provided evidence that individual rehabilitation strategies shown to be beneficial in animal models of stroke are not sufficient to provide behavioral enhancement in an animal model of TBI. Enhancing behavioral recovery following an animal model of TBI relies on an approach that combines varied rehabilitation tasks, relying on different skill sets. Although behavioral impairments seen in stroke and TBI patients may appear similar, this study suggests that the approach to neurorehabilitation may need to be more intense and varied after TBI. Clinical studies comparing rehabilitation approaches for functional deficits based on deficit etiology are warranted.
Supplementary Material
Acknowledgments
The authors would like to thank Nicole Donlan for assistance with histology and Elena Ramos, Michael Collela, Stacey Seidl, and Roxanne DeLaTorre for assistance with behavior and surgeries.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study has been supported by DePaul University Research Council (DAK) and the Department of Defense #W81XWH-08-1-0624 (DAK, DLA, TAJ).
Footnotes
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ICMJE Uniform Disclosure Form for Potential Conflicts of Interest: Dr Kozlowski reports a grant from US Department of Defense during the conduct of the study.
Supplementary Material
Supplementary material for this article is available on the Neurorehabilitation & Neural Repair website at http://nnr.sage-pub.com/content/by/supplemental-data.
References
- 1.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention; 2010. [Google Scholar]
- 2.Krauss JK, Jankovic J. Movement disorders after TBI. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury Medicine: Principles and Practice. New York, NY: Demos; 2007. pp. 469–489. [Google Scholar]
- 3.Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J Rehabil Res Dev. 2007;44:975–982. doi: 10.1682/jrrd.2006.12.0158. [DOI] [PubMed] [Google Scholar]
- 4.Kozlowski DA, Leasure JL, Schallert T. The control of movement following traumatic brain injury. Compr Physiol. 2013;3:121–139. doi: 10.1002/cphy.c110005. [DOI] [PubMed] [Google Scholar]
- 5.Weightman MM, Bolgla R, McCulloch KL, Peterson MD. Physical therapy recommendations for service members with mild traumatic brain injury. J Head Trauma Rehabil. 2010;25:206–218. doi: 10.1097/HTR.0b013e3181dc82d3. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald BD, Rigg JL. Neurorehabilitation in traumatic brain injury: does it make a difference? Mt Sinai J Med. 2009;76:182–189. doi: 10.1002/msj.20103. [DOI] [PubMed] [Google Scholar]
- 7.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobkin BH. Neurobiology of rehabilitation. Ann N Y Acad Sci. 2004;1038:148–170. doi: 10.1196/annals.1315.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson BB. Brain plasticity and stroke rehabilitation: the Willis lecture. Stroke. 2000;31:223–230. doi: 10.1161/01.str.31.1.223. [DOI] [PubMed] [Google Scholar]
- 10.Veerbeek JM, van Wegen E, van Peppen R, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS One. 2014;9:e87987. doi: 10.1371/journal.pone.0087987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntyre A, Viana R, Janzen S, Mehta S, Pereira S, Teasell R. Systematic review and meta-analysis of constraint-induced movement therapy in the hemiparetic upper extremity more than six months post stroke. Top Stroke Rehabil. 2012;19:499–513. doi: 10.1310/tsr1906-499. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 13.Fritz SL, Butts RJ, Wolf SL. Constraint-induced movement therapy: from history to plasticity. Expert Rev Neurother. 2012;12:191–198. doi: 10.1586/ern.11.201. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise EK, Hoffman JM, Powell JM, Bombardier CH, Bell KR. Benefits of exercise maintenance after traumatic brain injury. Arch Phys Med Rehabil. 2012;93:1319–1323. doi: 10.1016/j.apmr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Brazzelli M, Saunders DH, Greig CA, Mead GE. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316. doi: 10.1002/14651858.CD003316.pub4. [DOI] [PubMed] [Google Scholar]
- 17.Jones TA, Adkins DL. Behavioral influences on neuronal events after stroke, section I. In: Cramer SC, Nudo RJ, editors. Brain Repair After Stroke. Cambridge, UK: Cambridge University Press; 2010. chap 3. [Google Scholar]
- 18.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 19.Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008;22:250–261. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 21.Castro-Alamancos MA, Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience. 1995;68:793–805. doi: 10.1016/0306-4522(95)00178-l. [DOI] [PubMed] [Google Scholar]
- 22.Tennant KA, Kerr AL, Adkins DL, et al. Age-dependent reorganization of peri-infarct “premotor” cortex with task-specific rehabilitative training in mice. Neurorehabil Neural Repair. 2015;29:193–202. doi: 10.1177/1545968314541329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Lam TI, Bingham D, Chang TJ, et al. Beneficial effects of minocycline and botulinum toxin-induced constraint physical therapy following experimental traumatic brain injury. Neurorehabil Neural Repair. 2013;27:889–899. doi: 10.1177/1545968313491003. [DOI] [PubMed] [Google Scholar]
- 25.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 27.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 28.Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res. 2014;87:8–15. doi: 10.1016/j.neures.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Griesbach GS. Exercise after traumatic brain injury: is it a double-edged sword? PM R. 2011;3(6, suppl 1):S64–S72. doi: 10.1016/j.pmrj.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Griesbach GS, Gomez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 31.Hicks RR, Martin VB, Zhang L, Seroogy KB. Mild experimental brain injury differentially alters the expression of neurotrophic and neurotrophin receptor mRNAs in the hippocampus. Exp Neurol. 1999;160:469–478. doi: 10.1006/exnr.1999.7216. [DOI] [PubMed] [Google Scholar]
- 32.Li HH, Lee SM, Cai Y, Sutton RL, Hovda DA. Differential gene expression in hippocampus following experimental brain trauma reveals distinct features of moderate and severe injuries. J Neurotrauma. 2004;21:1141–1153. doi: 10.1089/neu.2004.21.1141. [DOI] [PubMed] [Google Scholar]
- 33.Thompson SN, Gibson TR, Thompson BM, Deng Y, Hall ED. Relationship of calpain-mediated proteolysis to the expression of axonal and synaptic plasticity markers following traumatic brain injury in mice. Exp Neurol. 2006;201:253–265. doi: 10.1016/j.expneurol.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Jones TA, Liput DJ, Maresh EL, et al. Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J Neurotrauma. 2012;29:1455–1468. doi: 10.1089/neu.2011.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–486. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–2152. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams FS, Schwarting RK, Huston JP. Behavioral and neurochemical asymmetries following unilateral trephination of the rat skull: is this control operation always appropriate? Physiol Behav. 1994;55:947–952. doi: 10.1016/0031-9384(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 38.Miklyaeva EI, Castaneda E, Whishaw IQ. Skilled reaching deficits in unilateral dopamine-depleted rats: impairments in movement and posture and compensatory adjustments. J Neurosci. 1994;14(11, pt 2):7148–7158. doi: 10.1523/JNEUROSCI.14-11-07148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein A, Sacrey LA, Whishaw IQ, Dunnett SB. The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neurosci Biobehav Rev. 2012;36:1030–1042. doi: 10.1016/j.neubiorev.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212:14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alaverdashvili M, Leblond H, Rossignol S, Whishaw IQ. Cineradiographic (video X-ray) analysis of skilled reaching in a single pellet reaching task provides insight into relative contribution of body, head, oral, and forelimb movement in rats. Behav Brain Res. 2008;192:232–247. doi: 10.1016/j.bbr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez TD, Schallert T. Seizures and recovery from experimental brain damage. Exp Neurol. 1988;102:318–324. doi: 10.1016/0014-4886(88)90226-9. [DOI] [PubMed] [Google Scholar]
- 43.Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–4786. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur J Neurosci. 2005;22:2069–2080. doi: 10.1111/j.1460-9568.2005.04370.x. [DOI] [PubMed] [Google Scholar]
- 45.Soblosky JS, Matthews MA, Davidson JF, Tabor SL, Carey ME. Traumatic brain injury of the forelimb and hindlimb sensorimotor areas in the rat: physiological, histological and behavioral correlates. Behav Brain Res. 1996;79:79–92. doi: 10.1016/0166-4328(95)00264-2. [DOI] [PubMed] [Google Scholar]
- 46.Nishibe M, Barbay S, Guggenmos D, Nudo RJ. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma. 2010;27:2221–2232. doi: 10.1089/neu.2010.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 48.Hicks RR, Boggs A, Leider D, et al. Effects of exercise following lateral fluid percussion brain injury in rats. Restor Neurol Neurosci. 1998;12:41–47. [PubMed] [Google Scholar]
- 49.Griesbach GS, Hovda DA, Gomez-Pinilla F, Sutton RL. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kline AE, Olsen AS, Sozda CN, Hoffman AN, Cheng JP. Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT(1A) receptor agonist buspirone after experimental traumatic brain injury. J Neurotrauma. 2012;29:1960–1969. doi: 10.1089/neu.2012.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–1026. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- 52.Crane AT, Fink KD, Smith JS. The effects of acute voluntary wheel running on recovery of function following medial frontal cortical contusions in rats. Restor Neurol Neurosci. 2012;30:325–333. doi: 10.3233/RNN-2012-120232. [DOI] [PubMed] [Google Scholar]
- 53.Shaw SE, Morris DM, Uswatte G, McKay S, Meythaler JM, Taub E. Constraint-induced movement therapy for recovery of upper-limb function following traumatic brain injury. J Rehabil Res Dev. 2005;42:769–778. doi: 10.1682/jrrd.2005.06.0094. [DOI] [PubMed] [Google Scholar]
- 54.Joo HW, Hyun JK, Kim TU, Chae SH, Lee YI, Lee SJ. Influence of constraint-induced movement therapy upon evoked potentials in rats with cerebral infarction. Eur J Neurosci. 2012;36:3691–3697. doi: 10.1111/ejn.12014. [DOI] [PubMed] [Google Scholar]
- 55.Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 2008;28:9386–9403. doi: 10.1523/JNEUROSCI.1697-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol. 2008;210:172–181. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dromerick AW, Lang CE, Birkenmeier RL, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology. 2009;73:195–201. doi: 10.1212/WNL.0b013e3181ab2b27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishibe M, Urban ET, III, Barbay S, Nudo RJ. Rehabilitative training promotes rapid motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model [published online July 22, 2014] Neurorehabil Neural Repair. doi: 10.1177/1545968314543499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones TA, Allred RP, Adkins DL, Hsu JE, O’Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke. 2009;40(3, suppl):S136–S138. doi: 10.1161/STROKEAHA.108.533653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sampaio-Baptista C, Khrapitchev AA, Foxley S, et al. Motor skill learning induces changes in white matter microstructure and myelination. J Neurosci. 2013;33:19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotting K, Roder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev. 2013;37(9, pt B):2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Cole JT, Yarnell A, Kean WS, et al. Craniotomy: true sham for traumatic brain injury, or a sham of a sham? J Neurotrauma. 2011;28:359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belke TW. Postreinforcement pause duration varies within a session and with a variable response requirement but not as a function of prior revolutions. Psychol Rec. 2011;61:213–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


