Abstract
Neurotrophins represent some of the best candidates to enhance regeneration. In the current study, we investigated the effects of artemin, a member of the glial derived neurotrophic factor (GDNF) family, on sensory axon regeneration following a lumbar dorsal root injury and compared these effects with that observed after either NGF or GDNF expression in the rat spinal cord. Unlike previously published data, artemin failed to induce regeneration of large-diameter myelinated sensory afferents when expressed within either the spinal cord or DRG. However, artemin or NGF induced regeneration of calcitonin gene related peptide positive (CGRP+) axons only when expressed within the spinal cord. Accordingly, artemin or NGF enhanced recovery of only nociceptive behavior and showed a cFos distribution similar to the topography of regenerating axons. Artemin and GDNF signaling requires binding to different co-receptors (GFRα3 or GFRα1, respectively) prior to binding to the signaling receptor, cRet. Approximately 70% of DRG neurons express cRet, but only 35% express either co-receptor. To enhance artemin-induced regeneration, we co-expressed artemin with either GFRα3 or GDNF. Co-expression of artemin and GFRα3 only slightly enhanced regeneration of IB4+ non-peptidergic nociceptive axons, but not myelinated axons. Interestingly, this co-expression also disrupted the ability of artemin to produce topographic targeting and lead to significant increases in cFos immunoreactivity within the deep dorsal laminae. This study failed to demonstrate artemin-induced regeneration of myelinated axons, even with co-expression of GFR-α3, which only promoted mistargeted regeneration.
Keywords: Axonal guidance, Sensory system, regeneration, Artemin
Introduction
Systemic application of artemin was shown to induce topographically accurate targeting of regeneration from the majority of sensory afferents after dorsal root injury (Wang et al., 2008, Harvey et al. 2010). These axons were also observed to regenerate within the dorsal columns to precisely reinnervate the dorsal column nuclei. Artemin signal transduction is mediated by artemin first binding to the GDNF family receptor alpha-3 (GFRα3). Within the adult rat DRG it is estimated that only about 35 – 40% of DRG neuron express GFR-α3, mostly small diameter, nociceptive neurons (Bennett et al., 2000; Orozco et al., 2001; Gardell et al., 2003). The artemin/GFR-α3 complex then binds and activates cRet, a primary tyrosine kinase receptor for the GDNF-family of neurotrophins. Interestingly, over 70% of DRG neurons express cRet (Bennett et al., 2000); however, only those neurons co-expressing both GFR-α3 and cRet will respond to artemin, and this is the nociceptive population. Since GFRα3 is bound to the membrane by a glycophosphatidylinositol anchor, one could envision injury inducing release and soluble artemin/GFR-α3 complex. This complex could potential diffuse and bind to other cRet expressing neurons thus supporting regeneration of other non-GFRα3 expressing DRG neurons, thus, potentially increasing regeneration of all cRet expressing DRG neurons.
Another interesting observation of artemin-mediated regeneration and rarely observed in other regenerating systems is topographic reinnervation of previous targets. To determine if this topographic targeting was a result of the route of neurotrophin application (DRG or spinal cord expression) or the neurotrophin itself, we expressed NGF, artemin, or GDNF within the spinal cord or DRG after dorsal root injury. In this model, the lesioned sensory axons regenerate well within the peripheral nervous system (PNS), but fail to penetrate the dorsal root entry zone (DREZ), which is the transition zone between the PNS and CNS, and grow into the CNS. Different subtypes of sensory axons show prominent lamina-specific connectivity in the spinal cord: CGRP and substance P positive nociceptors restrict their terminals primarily to lamina I and outer lamina II, IB4 binding nociceptors to inner lamina II, tactile fibers to laminae II-IV, and TrkC positive proprioceptors to Clark’s column and the ventral horn. This topographic arrangement and the feasibility of immunohistochemically labeling the different classes of axons make this system suitable to study the topographic targeting of regenerated axons. In this study, we examined the extent and topographic targeting of regenerating axon subpopulations after lentiviral expression of artemin alone or in combination with GFRα3 or GDNF, and compare it with lentiviral expression of NGF within the spinal dorsal horn. We hypothesized that the pattern of regeneration elicited by individual neurotrophins is unique to that neurotrophin and can be influenced by the expressed distribution of the neurotrophin or, in the case of artemin, the neurotrophin and co-receptor complex.
Materials and Methods
Animals
Ninety adult (250–350g) female Sprague Dawley rats (Harlan, Indianapolis, IN) were used in the study. All surgical procedures and animal maintenance complied with the National Institute of Health guidelines regarding the care and use of experimental animals and were approved by the Institutional Animal Care and Use Committee of Temple University, Philadelphia, PA, USA.
Lentivirus vectors
All lentivirus used pBOB lentiviral expression vector (with CMV enhanced Chicken b-actin (CAG) promoter (Addgene, plasmid 12337; Marr et al., 2003). To generate lentivirus expressing either NGF (Romero et al., 2000), rat artemin (Origene, RN209126), rat GFR-alpha 3 (Origene, RR200905), GDNF or rat GFR-alpha1 were subcloned into the pBOB CAG vector and plasmids purified and sequenced. The coding regions of alternatively- spliced form of rat GDNF (GDNF555, NCBI reference # S75585.1) and rat GFRα1 (NCBI reference # NM_012959) were kindly supplied by Amgen. The transgene plasmid along with the lentivirus packaging plasmids (pVSVG, pMDL and pRev) were transfected into 293T human embryonic kidney cells using calcium phosphate method. Accumulated viral particles from the supernatant were purified by sucrose gradient ultracentrifugation to produce high titer in vivo quality lentivirus. Virus was resuspended in a Tris buffer containing rat serum albumin and mannitol. The virus was frozen in 10μL aliquots at −80°C. Before using the virus for in vivo experiments, the titer was determined using p24 ELISA (Zeptometrix corporation) and tested for expression by either by ELISA (NGF) or Western blot analysis (artemin and GFRα3). NGF ELISA was performed using the ELISA kit (Promega) following the manufacturers protocol. Artemin and NGF have flag tags and GFRα3 has a myc tag. The production of exogenous artemin and GFRα3 was confirmed by probing Western blots for flag (anti-M2 flag (1:1000), Sigma) and myc antibody (9E10 (1:1000), Cell Center, UPENN), respectively. GFRα1 protein expression was confirmed in vitro using Western blot analysis of lysate and supernatant of infected 293T cells (GFRα1 antibody (1:1000), R&D systems).
Surgical procedures
Dorsal root crush injury and spinal cord injection
Animals were deeply anesthetized by i.p. injections of a 2:1 mixture of ketamine (67mg/kg) and xylazine (6.7mg/kg). The dorsumof each animal was clipped and disinfected with povidone-iodine before opening the skin and fascia. A hemilaminectomy was performed under aseptic conditions at the T13-L2 vertebral segments and the dura was opened with fine scissors. After topical application of lidocaine, dorsal roots L3–L6 were identified and exposed. With the use of #5 Dumont forceps, triple-crush lesions, 10 seconds each, were inflicted at two sites separated by 3mm along the L4 and L5 afferents at 5–8 mm from the DREZ. In previous studies by our group we observed some axon sparing if crushes were done at only a single site (Romero et al., 2001). Dorsal roots L3 and L6 were tightly ligated at two regions, 1–2 mm apart with 6.0 silk sutures and then a complete cut was made through these roots to create a zone of denervation immediately rostral and caudal to L4/L5. All lesions were performed unilaterally on the right side. The spinal cord at T13-L1 was exposed to identify the L4/L5 DREZ. The anesthetized animals were held in place using a spinal clamp at levels rostral and caudal to laminectomy in order to facilitate spinal cord injections without the hindrance of respiratory movements. Eight injections of 0.3 μl lentivirus (a total of 1×107 viral particles) spaced 0.5 mm apart were given rostro-caudally along the L4/L5 DREZ using a beveled glass micropipette pulled to a diameter of between 30 and 50 μm. Injections were made at a rate of 5 nl/sec using a nano-injector (World Precision Instruments, Inc., Sarasota, FL) at a precise depth of 0.7 mm from the spinal cord dorsal surface using the coordinates on a M3301 fine micromanipulator (Narishige via World Precision Instruments). After completion of each injection, the needle remained in place for five minutes before retracting. Gelfoam soaked in sterile saline was laid on the top of the exposed spinal cord and the muscle layer was closed using chromic gut sutures followed by skin closure using surgical clips. Liquid bandage (New Skin) was applied to the right paw to help prevent autotomy which sometimes occurred. Animals showing autotomy, which included partial loss of 2 toes or inflammation of the hindpaw, were removed from the study and not included in results or animal numbers. The animals were maintained on a heated pad until complete recovery from anesthesia. Buprenorphine was administered intramuscularly (0.05 mg/kg) following surgery to help alleviate pain and discomfort.
Injections to Dorsal root ganglia
In animals under anesthesia, a 3cm skin incision was made on the dorsal midline starting from the superior iliac crest and extending in the rostral direction. The superior muscular fascia was incised and the paraspinal muscles were separated by blunt dissection exposing the midline and the lateral aspect of L4, L5 and L6 vertebra. Exposure was maintained using custom retractors. A partial laminectomy was performed on the right half of L4-L6 vertebra to remove part of the posterior articular processes and expose the L4/L5 DRGs. Replication deficient lentivirus encoding either NGF or Artemin was injected into L4/L5 DRGs using a micropipette pulled to a diameter of 0.05mm and a microinjector. The viruses were injected 1 weeks before the L4/L5 dorsal root crush. For each injection, micropipette was introduced 0.3–0.5 mm into the DRGs and a total volume of lentivirus (about 2–3 μL) containing 1×107 transducing units/μL was injected over a 10 minute injection period. The glass needle was left in place for 5 minutes after each injection.
Sciatic nerve injections
To identify regeneration of large caliber myelinated axons, a 2% solution of Alexa Fluor labeled (594) cholera toxin B (CTB) (Invitrogen) in PBS was injected into all animals 7–10 days prior to sacrifice. For these studies a small incision was made in the skin along the posterior thigh to expose the gluteus muscle. The muscle was separated to expose the sciatic nerve. The sciatic nerve was injected with 2–3 μl of 2% cholera toxin B subunit using a Hamilton syringe with a 30 gauge needle. Sciatic nerve was not crushed for these studies.
Behavioral analysis
All the animals in the study were subjected to behavioral analysis before the injury to assess the baseline and after the injury to assess the behavioral changes or behavioral improvement. We evaluated functional recovery employing four different behavioral assays at 10 day intervals; thermal nociceptive recovery, paw pressure assessment, Gridwalk test and test for allodynia using von Frey hairs. All the behavioral tests, except for thermal nociception, were performed on the same day and the thermal test was performed the following day. All the behavioral tests were performed and scored by a student blinded to the treatments.
The latency of paw withdrawal from a radiant heat source was used to measure the response time to noxious thermal stimuli, as described previously (Hargreaves et al., 1988; Tang et al., 2007). Paw withdrawal latencies (PWL) for each hindpaw were determined prior to (baselines) and once every 10 days following dorsal root crush and lentivirus injection. Briefly, the rats were placed in a clear plastic chamber with a glass floor and allowed to acclimate for 10 min. A halogen lamp beneath the glass surface is used to direct an intense light beam onto the plantar surface of the hind paw. Thermal intensities of 50% of the maximum power output of the lamp were tested for the entire duration of the experiment. This intensity was chosen based on preliminary studies to give baseline responses of approximately 10 seconds in normal animals. Paw withdrawal latency was detected automatically by a photocell and taken as a behavioral index of the pain threshold. A maximum exposure time of 22 sec was used as a cut off to ensure that no tissue damage occurred to the paw. Measurements in triplicate were taken, by an individual blinded to the treatment, from both the left and right hind paw and the ratio of the PWL of the right to left paw was calculated. Pre-injury baseline ratios were close to 1, as the right and left PWL are similar. Right side L4/L5 crush increased PWL close to the cut off value and raising the ratio to a value closer to 2. As regeneration progresses, this ratio trended towards the baseline value of 1. A ratio significantly lower than the baseline ratio is represented as hyperalgesia.
Mechanical Hyperalgesia
The paw pressure test was used to measure mechanical hyperalgesia, as described by Randall and Selitto (Randall and Selitto, 1957). Briefly, animals were restrained in a mitt such that the hind paws could hang free. Each paw, in turn, was placed between the apparatus surface of an Ugo-Basil Analgesymeter and a plastic point. Increasing weight was applied to the point by means of an attached metal disc. Pressure to the paw was applied at thirty two grams per second. The end point was designated as vocalization or the pulling of the hind paw from the apparatus, with a cutoff of 200 grams. Responses were recorded three times for each hind paw.
Gridwalk test
A custom made horizontal ladder with irregularly spaced rungs was used to test for proprioception. Animals were trained to walk on the horizontal ladder before they were subjected to any surgery. The percent efficiency in the right hindlimb was evaluated as a measure of proprioception. Before surgery, trained animals showed almost 100% efficiency in the right hindlimb (no errors). Immediate post-crush evaluation showed a tremendous increase in foot faults or errors on the ipsilateral (right) side of lesion, causing a drop in placement efficiency. Improvement in proprioception is expected to increase the placement efficiency in the right hind limb.
Touch sensitivity
Sensitivity to mechanical stimuli was tested using von Frey hair (VFH; North Coast Medical, Morgan Hill, CA) applied to the mid-plantar region of the hindpaws, following an up-and-down profile (Dixon, 1980; Chaplan et al., 1994). Animals were placed on a wire mesh floor and fruit cereals were provided during this behavioral testing. Measurements were taken only when the animals were eating cereals so as to rule out visual responses from seeing the presentation of monofilament (Touch Test, North Coast Medical, Gilroy, CA). Presentation of the monofilament was started from 4.93 (8 grams) and ended at 6.10 (100 grams) if there was no response. Each monofilament was applied in a smooth and steady manner over a two second period until it bends. The filament was then removed in a smooth and steady manner. This single touch constituted one single trial. A positive response was indicated by the quick lifting of the paw away from the monofilament before the monofilament was removed. If there was no response, the next higher monofilament was presented after 30 seconds to prevent adaptation. If there was a response, the next lower filament was used to determine a response. This up and down procedure for systematically applying higher or lower monofilaments based on positive or negative responses was used until we had 10 trials per paw. If there was no response at 6.10 monofilament, the same was applied again and if there was no response at 6.10, threshold was denoted as 6.45. Otherwise, threshold value is the lowest monofilament level at which 50% or more of the trials were positive and there were at least 3 trials at each threshold level. The values used for data processing were the gram force values corresponding to each filament number. Mechanical sensitivity was represented as 50% response threshold for VFH stimulus (g) to the left (normal side) and right (lesion side) hind paw of rats. As we did not observe any change in mechanical sensitivity in any of the groups, only data from the right hind paw are plotted.
Fos Stimulation
Animals were anesthetized with 1.5 g/kg urethane i.p., a dose known to block flexor-reflex responses to hindpaw stimulation ~15 min after injection (Trafton et al., 1999). For Fos induction, the right hindpaw was submerged in water heated to 52°C for 2 min and the interdigital space pinched for 15 seconds to stimulate CGRP+ and IB4+ axons respectively. The animals were placed on a heating pad for 90 min before perfusion.
Histology
Eight weeks after the injury and spinal cord injection of lentivirus, anesthetized animals were perfused transcardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.5. Lumbar spinal cord and DRGs were isolated and incubated in the same fixative for approximately 24 hours. The tissue was then cryoprotected with 30% sucrose in 0.1M phosphate buffer at 4°C for another 2 days. 30 μm sections from lumbar spinal cord were cut on a cryostat and floating sections were collected. For immunoperoxidase staining, spinal cord sections were incubated with 1 to 20,000 dilution of anti-rat CGRP (Sigma, St.Louis, MO), 1 to 1000 dilution of biotinylated anti-IB4 (Sigma) or 1 to 10,000 dilution of the cFos antibody (Calbiochem, cat# PC38). Visualization was achieved by incubating sections in biotinylated secondary antibody (except for IB4 as it is already biotinylated) followed by vectastain Elite ABC reagents (Vector laboratories, Burlingame, CA), and developed using the peroxidase substrate, 3,3-diaminobenzidine to generate brown color. For enhancing the fluorescence signal for Alexa 594 labeled CTB, the sections were incubated with rabbit anti-CTB (1to 500) (Sigma) for 2 h and further with goat anti rabbit alexa 594 (Invitrogen) for 2 h. After staining, sections were mounted on glass slides and coverslipped with either permount (immunoperoxidase stained sections) or fluoromount G (immunofluorescence sections) (Southern Biotech).
Microscopy and Image analysis
Images from immunoperoxidase stained sections were captured using bright field microscopy and fluorescent images from GFP injected animals using FITC filter (Nikon 80i). To quantify axon growth in different laminas of the spinal cord, 5–6 sections within L4/L5 spinal cord were selected randomly from each animal. Outlines of the laminas I-VI were drawn from a representative spinal cord image (Tang et al., 2007) and these outlines were used as a template and applied to appropriate locations in different spinal cord images by a technician blinded to experimental conditions. CGRP+ axon occupying area was quantified within the outline of each lamina using a macro that applied a standardized optical density threshold to each image and measured the area of staining equal to or greater than the threshold. IB4+ axon occupying area was quantified as the total area occupied by these axons in the spinal cord. The program gave the area measurements in μm2. cFos images captured at 10x was used for cFos counting. cFos activated neurons were counted manually applying the same lamina outline. cFos counts in laminae I & II were quantified as superficial dorsal horn cFos counts and laminae III-VI were counted as deep dorsal horn cFos counts. All quantification of images were performed by a trained student who was unaware of the individual treatments.
Laser scanning confocal microscopy for CTB labeled axons
Images from CTB labeled sections were acquired using confocal microscopy (Nikon C2). Z stacked images were acquired at 564 nm. Images were captured at both 10x and 20x magnifications to allow a clear representation of regenerating axons. CTB axon occupying area was quantified as the total area occupied in the ipsilateral side using thresholded intensity measurements.
Statistical analysis
Data from the image analysis of CGRP+ axon density and Fos-IR nuclei were analyzed by two-way ANOVA followed by Tukey-Kramer post hoc test to determine significant differences between groups. Data from regenerating IB4+ axons were analyzed using One-Way ANOVA and CTB labeled axons were analyzed by non-parametric test (Kruskal-Wallis test) followed by multiple comparison tests of mean ranks of the groups. Data from behavioral assessments were analyzed using one way ANOVA followed by Tukey/Dunnett’s post hoc test to determine significant differences between groups. Data represent the mean ±SEM. P values below the 5% probability level were considered significant.
Results
Expression analysis of transgenes used in the study
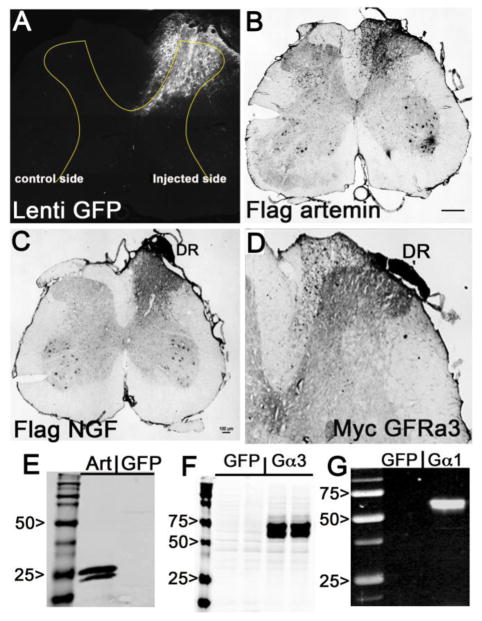
In the current study, lentivirus-encoding GFP, artemin, NGF, GDNF, GFRα3 or GFRα1were injected into the right dorsal horn or DRG following the same procedure and gene expression was analyzed by immunohistochemistry. GFP fluorescence observed throughout the right dorsal horn validated our micro-injection procedure and showed localized transgene expression in the dorsal horn (Fig. 1A). Immunohistological analysis also showed the expression of artemin, NGF and GFRα3. Expression of these exogenous proteins was confirmed by probing with flag antibody for artemin (Fig. 1B) and NGF (Fig. 1C) and myc antibody for GFRα3 (Fig. 1D). Consistent with previous observations, gene expression was restricted within the dorsal horn on the injected side of the spinal cord. In contrast to adenoviral injections, lentiviral injections showed little tissue damage or cytotoxicity. Sixty percent of the lentivirus infected cells appear to have morphology reminiscent of astrocytes. Gene expression of all the lentiviral vectors was confirmed in vitro before the in vivo experiments. Western blot analysis showed good expression of artemin (Fig. 1E, GFRα3 (Fig. 1F), and GFRα1 (Fig. 1G). As previously reported (Tang et al., 2007; Zhang et al., 2013b), the presence of secreted NGF and GDNF were confirmed by ELISA (data not shown).
Figure 1.
In vitro and in vivo analysis of lentiviral gene expression. A, Expression of GFP fluorescence in the ipsilateral lumbar spinal cord dorsal horn 10 days after injection of lenti-GFP into the L4/L5 DREZ. GFP fluorescence is localized to the ipsilateral side (right side) dorsal horn. Fluorescence also indicates the pattern and depth of injection (0.6–0.7mm deep). B,C&D shows the in vivo expression of exogenous NGF (B), artemin (C) and GFRα3 (D), 10 days after lentivirus injection into the right L4/L5 DREZ. The sections were labeled with respective epitope tags (flag for NGF and artemin, and myc for GFRα3) and processed for immunohistochemistry. The staining was observed only on the ipsilateral side of the injection and the proximal dorsal root (DR). E,F &G, Shows the presence of secreted flag tagged artemin in the supernatant of lenti-artemin infected 293Tcells (Art) and myc tagged GFRα3 (Gα3) in lysate of lenti-GFRα3 infected 293T cells and GFR α1 (Gα1) in lysates of 293T cells infected with lenti GFRα1 examined by western blot analysis. Scale bar 100μm.
Expression of either NGF or artemin within the DRG failed to induce regeneration
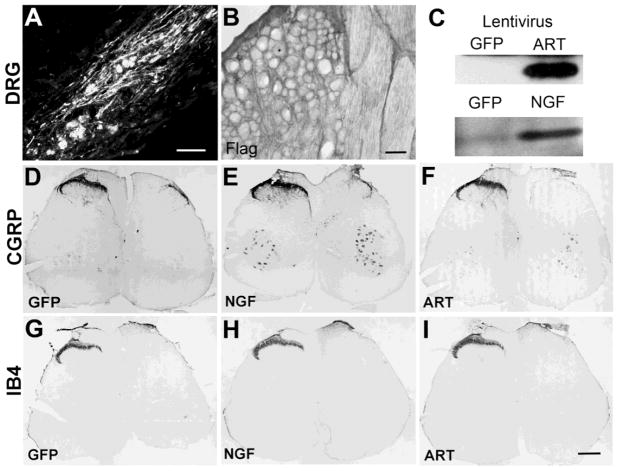
Since previous reports indicated systemic application of artemin induces topographic sensory afferent regeneration and high levels of artemin were observed within the DRG, we first injected GFP (n=8), artemin (n=8) or NGF (n=8) into DRGs to examine targeting of regenerating axons. Twenty days following injection, GFP was localized within numerous neurons and Schwann cells and axons within the DRG (Fig 2A). Lentiviral expression of either exogenous artemin or NGF in the DRG was confirmed by immunohistochemistry and western blot analysis of DRG tissue homogenates (Fig. 2B & C). Surprisingly, the expression of these neurotrophins in the DRG failed to induce regeneration of any class of sensory axons or return of any behavioral function (Fig. 2D - I). This indicates that the mechanism underlying artemin induced topographically targeted regeneration is not mediated by the availability of artemin at the neuronal soma level, at least within the lumbar region of the spinal cord. For all remaining studies all neurotrophins were injected into the dorsal horn of the spinal cord.
Figure 2.
Expression of neurotrophin in the DRG failed to promote regeneration of sensory afferents through the DREZ. Lentivirus encoding GFP, NGF or Artemin shows very good expression 20 days after direct injection into the DRG. Within the DRG numerous neurons, Schwann cells and axons could be observed labeled by GFP (A) and a generalize artemin staining surrounding neurons was identify using the Flag epitope antibody (B). Western blot analysis was also used to confirm expression of either artemin or NGF within DRGs (C). Although, we consistently observed good expression of GFP (D & G), NGF (E & H) or artemin (F & I), this expression within the DRG failed to support regeneration of either CGRP or IB4 sensory afferents into the spinal cord. Scale bars A) 100μm; B 50μm; D – I) 300μm
Differential targeting of regenerating axons by NGF or artemin
In normal spinal cord the distribution of CGRP+ fibers is restricted to the superficial dorsal horn (Fig. 3A & B). For these experiments, dorsal root lesions completely transected all the sensory axons in the L4/L5 dorsal root causing degeneration of axons innervating the spinal cord. These axons spontaneously regenerate within the peripheral nerve but terminate their growth upon contact with the spinal cord at the DREZ (Cajal, 1929; Carlstedt, 1985), as shown in the GFP control virus injected group (Fig. 3C). To determine if expression of artemin within the spinal cord could induce regeneration and if this local expression would disrupt targeting, we expressed NGF, artemin, or GDNF in the spinal cord dorsal horn using the lentiviral expression system. These neurotrophins were individually expressed along the L4/L5 DREZ in an identical fashion. Consistent with previous observations, lentiviral expression of NGF produced robust but mistargeted and ectopic regeneration (Fig. 3D & G). Excitingly, spinal cord expression of artemin induced physiologically restricted and topographically targeted regeneration of CGRP+ fibers within the superficial dorsal horn (Fig. 3E & G). GDNF expression in the dorsal horn had no effect on regeneration of CGRP+ axons (Fig. 3F & G). Statistical analysis for CGRP+ regenerated axons 8 weeks after treatment showed a significant effect (p<.0001) on lamina distribution across the groups F(4,240)=194.8, on treatment to induce regeneration, F(5,240)=22.98 and interaction between treatment and laminae distribution F(20,240)=8.634. Tukey’s multiple comparison test revealed a significant difference (*p<0.05) between sham control and GFP lesion control indicating failed regeneration. Expression of either artemin (n=12) or NGF (n=9) showed a significant increase in regeneration of CGRP+ axons into the spinal cord when compared to GFP controls (n=12) (**p<0.05) (Fig. 3G). NGF, but not artemin expressing animals, showed a significantly higher (δp<0.05) density of axons innervating lamina III, IV, V and VI when compared to sham lesion controls (Fig 3G). The distribution of CGRP+ axon regenerated by artemin treatment was not significantly different (P>0.05) from sham lesion control (normal animals) demonstrating artemin-induced regeneration of CGRP+ fibers was very similar to physiological conditions (Fig 3G). Axon density in GDNF expressed group was very similar to GFP lesion controls indicating CGRP+ axons failed to regenerate (Fig 3G).
Figure 3.
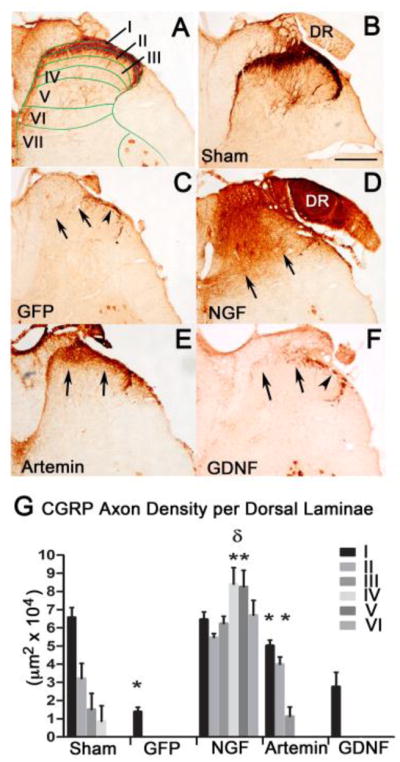
Regeneration of CGRP-positive axons with neurotrophic factor expression. In normal spinal cord (A & B), CGRP+ axons are mostly located in superficial laminas I & II as indicated by the stain patterns associated with the laminae of Rexed (A). Lenti-GFP injections following L4/L5 dorsal root crush (C) did not have any effect on regeneration of CGRP+ axons past the entry zone (arrows), but showed some staining within Lissauer’s tract (arrowhead). Lentiviral expression of NGF induced robust regeneration of CGRP + axons (D), but the regeneration was ectopic and axons grew throughout most of the dorsal horn laminae (arrows). Lentiviral expression of artemin produced topographically targeted regeneration of CGRP+ fibers (E) and the axons occupied their physiological lamina (arrows). Lentiviral expression of GDNF had no effect on regeneration of CGRP+ axons (arrows) showing axons only within Lissauer’s tract (arrowhead; F). Lamina specific quantification of the area occupied by CGRP+ axons in control GFP, NGF, artemin and GDNF expressed group (G). Dorsal root injury completely abolished CGRP fibers in all laminas in the ipsilateral dorsal horn and the axon density was significantly lower in GFP lesion control compared to no lesion controls (*p<.05). Statistical analysis by a two-way ANOVA showed a significant increase in axon occupying area in lamina I and II for NGF and artemin compared to GFP controls and GDNF (*p<.05). NGF expression resulted in a significant increase in axons occupying the deeper laminae (III, IV, V and VI) when compared to the Sham or artemin groups that targeted regenerating CGRP axons to their physiological targets (lamina1&II) (δp<.05). Values represent mean±SEM, n=12 for GFP and artemin, n=9 for NGF, =7 for GDNF. Scale bar = 300 μm.
Effect of artemin and NGF on other classes of sensory axons
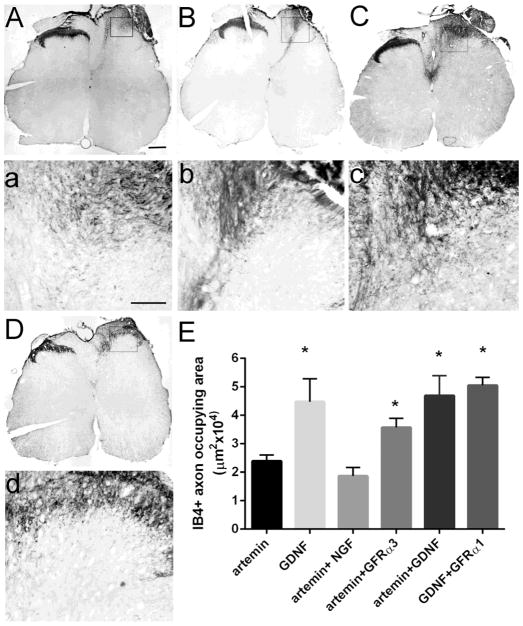
In addition to CGRP+ axons, we also evaluated the regeneration of two other classes of sensory axons, non-peptidergic nociceptive axons and the deeper myelinated sensory afferents. The non-peptidergic nociceptive axons label with the plant isolectin B4 (IB4) and terminate in inner lamina II (Fig. 4A). These axons express cRet and GFRα1 and respond to GDNF (Snider and McMahon, 1998) but are insensitive to the effects of NGF (Romero et al., 2001). As expected, NGF expression did not support the regeneration of IB4+ axons and was similar to GFP controls (Fig. 4B & C). Artemin produced a minimal and superficially restricted regeneration of IB4+ axons (Fig. 4D) that was statistically significant. In contrast, GDNF produced robust regeneration of IB4+ axons (Fig. 4E). Analysis of the total area occupied by regenerating IB4+ axons by a one-way ANOVA showed a significant effect of treatment with artemin or GDNF (F(3,39)=37.14, p<.001) to induce regeneration. Tukey’s multiple comparison analysis showed a significantly higher (*p<0.05) regeneration of IB4+ axons induced by artemin (n=12) or GDNF (n=7), when compared to NGF (n=9) or GFP lesion controls (n=12) (Fig. 4F).
Figure 4.
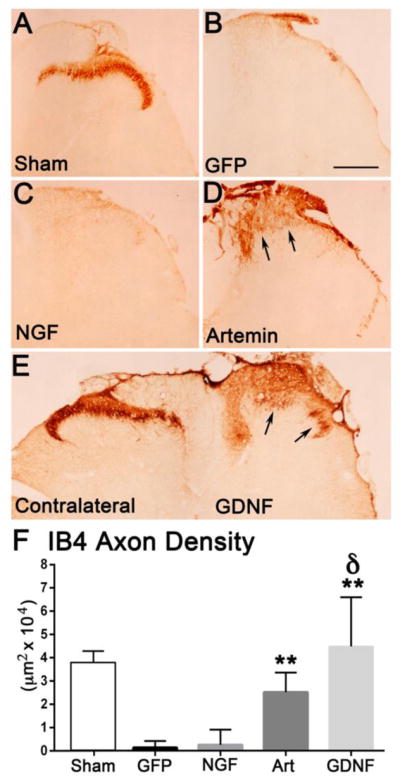
Artemin and GDNF enhanced regeneration of IB4+ axons while NGF had no effect on IB4 axons. Non-injured Sham controls show a normal distribution of IB4 axons within the inner region of laminae II (A). Dorsal root crush and injections of lenti-GFP resulted in a complete abolishment of IB4+ axons and absence of regeneration (B). NGF expression in the dorsal horn did not have any effect on IB4+ axon regeneration and looked similar to GFP lesion controls (C). Artemin produced modest regeneration of IB4+ axons just past the entry zone (arrows; D). GDNF induced robust regeneration of IB4+ axons (arrows) into the appropriate laminae (right side E). Quantification of IB4+ axon occupying area within laminae I – III (F). Compared to control and NGF, artemin and GDNF produced a statistically significant regeneration of IB4+ axons (*p<.05, Tukey’s post hoc test) and the effect of GDNF was significantly higher compared to all treatment groups after dorsal root injury (δ p<.05). Scale bar = 300 μm.
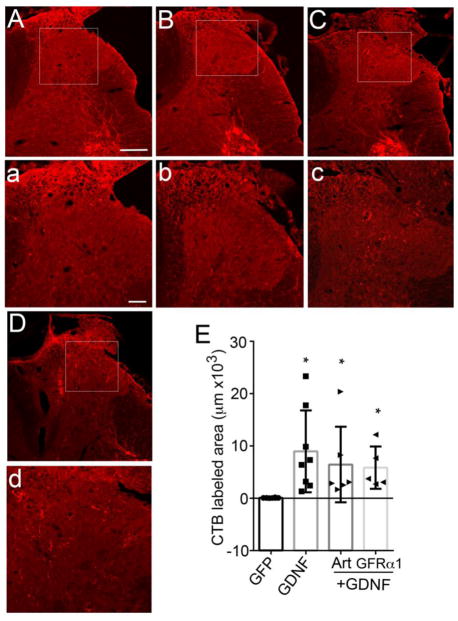
Previous studies indicate that systemic application of artemin (Wang et al., 2008) and intrathecal application of GDNF (Ramer et al.,2000) enhances regeneration of tactile and proprioceptive axons into the spinal cord. Artemin was also shown to induce regeneration within the dorsal columns to innervate the dorsal column nuclei. Hence, we next determined if artemin and GDNF expression within the spinal cord could elicit either short or long distance regeneration of large diameter myelinated axons. Regeneration of myelinated sensory afferents was examined by injecting cholera toxin B (Robertson and Grant, 1985) into the sciatic nerve. In uninjured animals, this tracer densely labels axons within laminae III – VI as well as motor neurons and their dendrites (Fig. 5A & a). Lesioning dorsal roots L3 – L6 and injection of lentivirus encoding GFP resulted in no regeneration or the presence of CTB labeled sensory axons. However, motor neurons are readily apparent, indicating good uptake of tracer and transport since the ventral roots were not injured (Fig. 5B & b). Neither NGF (Fig. 5C & c) nor artemin (Fig. 5D & d) expressed within the spinal cord induced regeneration of CTB labeled axons. In contrast, we observed robust regeneration of CTB labeled axons in GDNF expressed animals (Fig 5E & e). Hence, in contrast to the previous reports in which artemin was shown to produce multimodal topographically targeted regeneration (Wang et al., 2008; Harvey et al., 2010), our study showed topographic targeting of only CGRP+ fibers and some, but minor regeneration of IB4+ fibers and no regeneration of large, myelinated axons.
Figure 5.
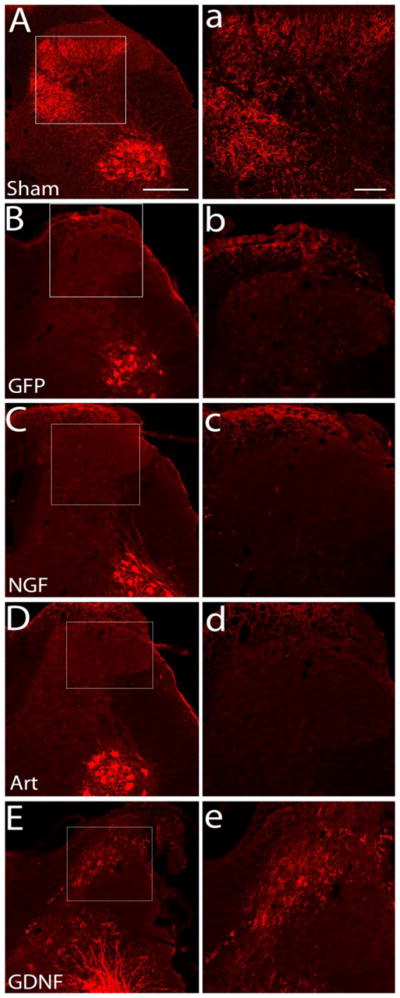
Regeneration of CTB-labeled myelinated axons after neurotrophin treatment. Sham controls show intact axons labeled by cholera toxin-B to demonstrate successful labeling and topography of the deeper myelinated afferents in a non-lesioned animal (A, a). Dorsal root crush injury completely transected all the deeper myelinated sensory afferents and there is no spontaneous regeneration of these axons in GFP control animals (B, b). Regeneration of CTB-labeled sensory afferents was absent after dorsal root injury and treatment with either NGF (C, c) or artemin (D, d). However, all animals showed very good and consistent labeling of the ventral motor neuron pools and their dendrites, indicating tracer uptake into the sciatic nerve. Many CTB-labeled sensory axons were observed to have regenerated into the spinal cord after treatment with GDNF (E, e). High magnification images (a,b,c,d, and e) are of boxed area shown in A,B,C,D, and E, respectively. n=6 for sham, GFP control and NGF, n=9 for artemin, n=7 for GDNF. Scale bar = 300μm (A,B,C,D,&E) and 100 μm (a,b,c,d, & e).
Regeneration induced by either artemin or NGF shows nearly complete return of thermal nociception but no other sensory modalities
In rats, the majority of sensory information is transferred to the spinal cord by the ipsilateral dorsal roots in which CGRP+ axons and IB4+ axons mediate cutaneous nociceptive sensation and deeper myelinated sensory afferents mediate tactile, pressure sensation and proprioception. These sensations are lost within the respective dermatomes after dorsal root lesions. Thermal nociception was tested by assessing the paw withdrawal latency (PWL) in response to a radiant heat applied to the plantar surface of the rat hind limb, while sensitivity to touch stimulus was tested using von Frey hairs, paw pressure sensation and proprioception. After dorsal root lesions all animals showed an increase in the PWL of the right hind paw to the cut off value (22 seconds), raising the ratio of right to left PWL close to 2. Compared to controls (n=12) and GDNF animals (n=7), animals treated with either lenti-NGF (n=10) or lenti-artemin (n=12) showed a decrease in PWL beginning 20 days after the treatment, recovering to near normal level about 4 weeks after injections (Fig. 6A). One way ANOVA showed a significant effect of treatment (p<0.01) on days 30, 40 and 50 with F (3,30)= 9.64, 34.89 and 29.38 respectively. Tukey’s multiple comparison test revealed that the nociceptive response significantly recovered (*p<.05) in NGF and artemin groups compared to control GFP and GDNF groups. There was no difference in nociceptive response between NGF and artemin animals at these time points, indicating that CGRP+ axon regeneration was primarily responsible for the improvement in thermal nociception and the differential distribution of CGRP fibers alone did not contribute to an alteration in pain response (Lin et al., 2014).
Figure 6.
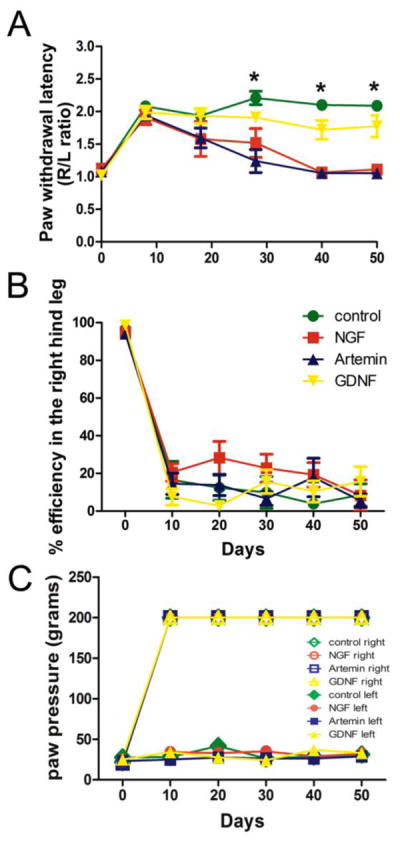
Behavioral analysis showed a return of thermal nociception, but no other behavior tested. A, Regeneration induced by either NGF or artemin resulted in nearly complete recovery of thermal nociception within 6 weeks post-rhizotomy. PWL was measured for both right and left hind paws before and once every 10 days after injury and treatment and the ratio of right to left PWL plotted. Pre-injury baseline ratios were always close to a ratio of 1. Injury to L3/L6 dorsal roots caused an immediate loss of paw withdrawal response, raising the ipsilateral PWL time with a ratio close to 2. No spontaneous recovery of PWL was found in lenti-GFP animals. Treatment with either lenti-NGF or artemin significantly improved thermal nociceptive responses from day 30 onwards. Treatment with lenti-GDNF did not have any effect on the recovery of thermal nociception (*p<.05, One way ANOVA, n=12 for GFP and artemin, n=9 for NGF and n=7 for GDNF, values represent mean±SEM). B, To evaluate recovery of proprioception a grid walkway test was used. Animals were trained to walk on a horizontal ladder without any error before surgery. Measurements represent the % efficiency for right hind limb placement by counting the number of right paw slips from the total number of steps taken to cross the ladder. The efficiency in the right hind limb is close to 100% (no errors) before injury and falls to about 20–30% after injury, remaining at that level throughout the study for all the groups (control GFP, NGF, artemin and GDNF) indicating no recovery. The left uninjured side showed no loss in paw placement onto the ladder rungs (not shown). C, Incremental increases in paw pressure was used to determine the mechanoreceptive thresholds for both right and left hind paws. The pressure causing paw withdrawal was measured before injury and every 10 days after injury and treatment. Threshold for the non-lesion side (left) and normal animals was between 30–50 g. Ipsilateral (right) paw withdrawal threshold rose to the cut off value of 200g immediately after the injury and remained at that level throughout the course of the study for both lesion (GFP) control and treatment groups (many of the symbols representing individual groups are overlaid one on top of the other), indicating no functional recovery. The left paw withdrawal threshold was not affected for any group.
The percent efficiency for placement of the right hind limb was evaluated as a measure of proprioception. None of the treatment groups showed recovery of foot fall errors on the grid walk test to evaluate proprioception. Before the surgery, trained rodents showed 100% efficiency in placement of the right hind limb (no errors). Testing performed 10 days after lesioning showed a significant increase in foot faults or errors on the ipsilateral (right) side of the lesion for all the groups with the overall placement efficiency of the right hind limb dropping to almost zero. Periodic assessment of proprioception also did not show any improvement after either artemin, NGF or GDNF treatments when compared to GFP controls (One way ANOVA, P=0.9) (Fig. 6B). Paw pressure sensation was evaluated using Randall-Selitto noxious pressure test. For sham-control rats, the threshold for the paw withdrawal was found to be 20–30 grams for hind paws. After the injury, animals did not show a withdrawal response even with the application of maximum pressure of 200g on the right hind paw while the left hind paw always showed a normal withdrawal response. Surprisingly, none of the treatments (NGF, artemin or GDNF) showed any improvement in this behavior when compared to GFP control animals (Fig. 6C). Touch sensitivity was also measured in these animals using von Frey hairs and no difference was observed in the VFH threshold values for any of the treatment groups compared to control, indicating the absence of allodynia (data not shown). These behavioral measurements are in agreement with our observations on anatomical regeneration and indicate a lack of regeneration of large diameter myelinated sensory afferents in response to either NGF or artemin.
Regenerated nociceptive axons form functional synaptic connections in spinal cord
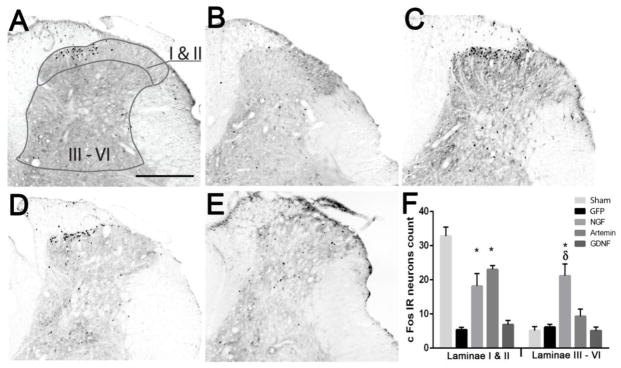
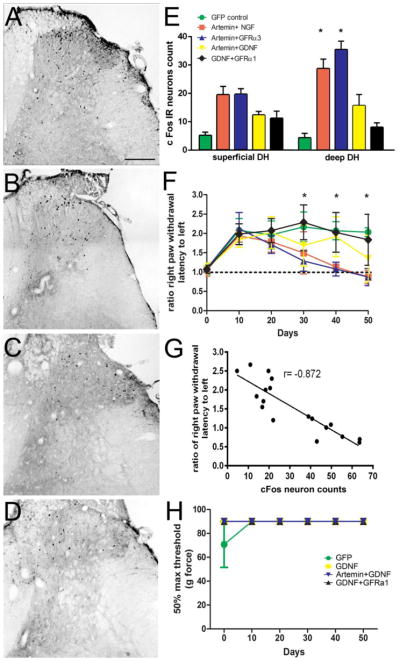
The recovery of nociceptive function in either NGF or artemin expressing animals indicates the establishment of functional synapses by regenerating axons. In this study, we provide further evidence for the formation of functional synaptic circuitry by demonstrating cFos immunoreactivity (IR) in second order neurons in response to thermal stimulation and toe pinch. cFos is an immediate early gene that gets activated in projection neurons undergoing high levels of neuronal activity. In normal uninjured animals, the majority of cFos expression was localized to the superficial dorsal horn (lamina I&II) (Fig. 7A & F); whereas, after lesioning dorsal roots L3–L6, cFos is virtually absent in GFP lesion controls (Fig. 7B&E). Statistical analysis using a two-way ANOVA showed a significant effect (p<0.05) of treatment, F (4,66)=22.73, of lamina distribution, F(1,66)=35.66 and of interaction between treatment and lamina distribution, F(4,66)=21.21. Tukey’s multiple comparison test revealed a significantly higher (*p<0.05) cFos IR in the superficial dorsal horn of NGF (Fig 7C & F n=6) and artemin (Fig 7D & F, n=8) expressed animals when compared to the GFP lesion control (n=8) and GDNF (Fig 7E & F, n=7) group. This indicates that regenerating axons form functional connections onto second order neurons. Although cFos stimulation paradigm was expected to stimulate both CGRP+ and IB4+ axons, the results probably show only synapses innervated by regenerated CGRP+ axons as we did not observe significant cFos activation in GDNF animals despite the robust IB4+ axon regeneration. On the other hand, low cFos immunoreactivity in GDNF animals is in agreement with the lack of return of thermal nociception in these animals (Fig. 6A). We observed no significant difference in cFos IR in the superficial dorsal horn between NGF and artemin group. Although NGF and artemin expression restored some functional synaptic connectivity, the magnitude of cFos expression in the superficial dorsal horn was still significantly lower (p<0.05) than no lesion controls (n=5) indicating only a fraction of the regenerating axons formed synapses. In the case of GDNF, cFos immunoreactivity was very low and they were distributed randomly without a clear pattern (Fig. 7E). In the deep dorsal horn, there was no significant difference in cFos IR for the artemin treatment group compared to lesion control, no lesion control or the GDNF treatment group (p>0.05). The lack of difference in the cFos distribution pattern between artemin treatment and sham controls indicate that regeneration induced by artemin also promotes topographic patterning of synapse formation. In contrast, NGF expressing animals showed a significantly higher cFos expression within laminae III – VI when compared to sham controls, GFP/lesion controls and the artemin treated group (Tukeys multiple comparison test, δp<0.05). The significant difference between NGF and sham controls (Tukeys multiple comparison test, p<0.05) indicates ectopic formation of synapses within the deep dorsal horn. These data indicate axonal regeneration in response to NGF or artemin results in the formation of functional synapses, but only artemin induces topographic reformation of synaptic connections
Figure 7.
Topography of cFos expression follows the pattern of CGRP axon regeneration. cFos immune reactivity (IR) was examined within two general regions, laminae I and II (superficial dorsal horn) or laminae III – VI (deep dorsal horn). A, In normal non-lesioned animals, Fos-IR-positive neurons are predominantly localized to the superficial dorsal horn (laminas I and II), with very few labeled cells in the deeper laminae. B, Following dorsal root crush injury a dramatic loss of Fos-IR cells is found on the ipsilateral side indicating no spontaneous regeneration in Lenti-GFP treated animals. C, NGF treatment resulted in high numbers of cFos positive cells localized throughout the ipsilateral superficial as well as deep dorsal horn. D, Artemin treated animals show cFos positive cells localized to ipsilateral superficial dorsal horn with very few cFos expressing cells in the deep dorsal horn. E, GDNF treated animals show very low cFos IR and the cFos IR neurons were randomly distributed in the spinal cord. F, Graph showing the distribution of cFos positive cells in the superficial and deep dorsal horn regions as shown in panel A. As seen in the images, the majority of cFos positive cells were present in the superficial laminae in normal and artemin treated animals, while NGF expressed animals showed cFos-IR cells in superficial as well as deep dorsal horn laminae in almost equal proportion. Values represent mean ±SEM, n=5 for no lesion control, n=6 for NGF, n=8 for artemin and GFP control, n=7 for GDNF *, δ p<0.05, Tukey’s post hoc test). Scale bar = 300 μm.
Co-expression of either NGF or GFRα3 with artemin disrupts the topographic targeting of artemin
We next wanted to determine if the mechanism of artemin-induced laminar specific regeneration was sensitive to NGF-induced sprouting or highly restricted localization of its co-receptor GFRα3. Co-expression of NGF with artemin produced similar ectopic regeneration as NGF alone indicating that NGF-induced sprouting can overcome artemin-induced targeting (Fig 8A & a). Similarly, we also examined whether the ability of artemin to topographically target regenerating CGRP+ fibers can be attributed to the restricted distribution/availability of GFRα3 co-receptor by co-expressing artemin with GFRα3 (Fig. 8B & b). We also expressed GDNF along with artemin to determine whether robust CGRP+ axon regeneration we observed with GDNF would affect artemin’s ability to topographically target regenerating axons (Fig. 8C & c). Similarly, we also expressed GFRα1, the cognate receptor for GDNF, along with GDNF in the spinal dorsal horn (Fig. 8D & d) to evaluate whether the effects on targeting is influenced by expression of GDNF family of receptors or depends on the phenotype of regenerating axons (CGRP fibers in NGF or artemin vs. IB4 fibers in GDNF). For this experiment, lentiviral expression of GFRα3 and GFRα1 within the spinal cord would create an ectopic expression pattern among resident astrocytes and neurons. Artemin and GFRα3 co-expression in the adult spinal cord disrupted the topographic targeting of artemin and produced ectopic regeneration of CGRP+ axons (Fig 8B & b). The expression of GFRα3 alone in the dorsal horn failed to induce any regeneration (n=5, data not shown). Artemin + GDNF co-expression did not disrupt the targeting of CGRP fibers by artemin (Fig 8C & c). Similarly, the co-expression of GFRα1 along with GDNF had no effect on CGRP+ fibers probably because GDNF was unable to entice these axons into the spinal cord (Fig. 8D & d). Interestingly, axons within all GDNF treatment groups showed a corkscrew patterning indicative of the coiling observed when axons become trapped within a GDNF gradient and stop growing (Eggers et al., 2013).
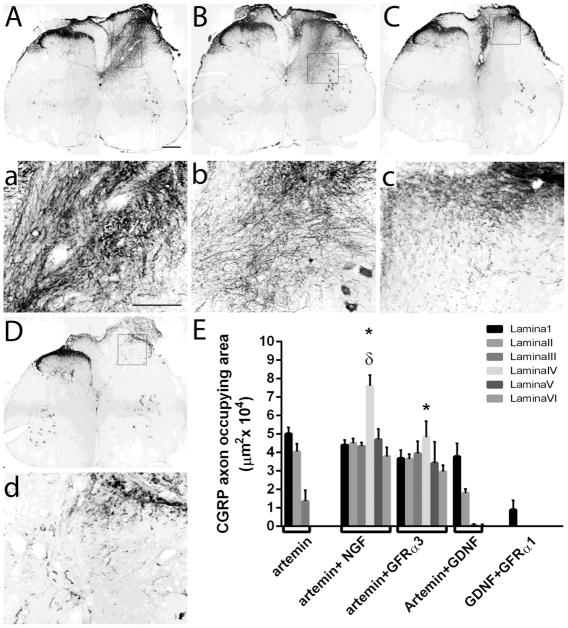
Figure 8.
Regeneration of CGRP+ axons with combined treatment of neurotrophic factors or GDNF-family co-receptors. CGRP+ axons exhibit robust ectopic regeneration and mistargeting occupying most of the dorsal horn laminae in animals co-expressing artemin with either NGF (A, a) or GFRα3 (B, b). Magnified images of the dorsal horn for each treatment group in the upper panel (a & b). C, Co-expression of GDNF with artemin did not alter the ability of artemin to topographically target regenerating CGRP+ axons. D, Co-expression of GDNF with GFRα1 induce very little, if any, regeneration of CGRP+ axons. Magnified images of C & D in upper panel (c and d, respectively). Scale bar = 300 μm (A, B, C, & D) or 100 μm (a, b, c, & d). E, Quantification of the area occupied by CGRP positive axons in the dorsal horn. For the quantification, lamina specific regions of the dorsal horn were measured as described in figure 2. Compared to artemin (from figure 2) there was a significant increase in axon occupying area in laminas III-VI in both artemin+NGF and artemin+GFRα3 groups (*p<.05, Tukey’s posthoc test). Also lamina IV showed a significant increase in CGRP+ axon occupying area in artemin+NGF group (n=6) compared to artemin+GFRα3 (n=6) (δ p<0.05, Tukey’s posthoc test). Artemin+GDNF (n=7) and GDNF+GFRα1 (n=5) had no effect on topography of CGRP+ axons.
The distribution of regenerating CGRP+ axons was statistically analyzed using a 2-way ANOVA with treatment and lamina as factors in artemin alone, artemin + NGF, artemin + GFRα3, artemin + GDNF and GDNF + GFRα1 expressing groups (Fig. 8E). Analysis by 2- way ANOVA showed a significant effect of treatment (p<0.01) Ftreatment(4,231)=91.55, significant effect on lamina distribution (p<0.05) Flamina(5,231)=9.882 and a significant effect of interaction between distribution and treatment (p<0.01) Finteraction(20,231)=7.862. Tukey’s multiple comparison test showed no statistically significant difference in the distribution of axons regenerating into lamina I and II (p>0.05). For all the other laminae (lamina III, IV, V and VI) CGRP axon occupying area was found to be significantly higher in artemin+NGF and artemin+GFRα3 groups (*p<0.01) when compared to artemin alone, artemin+GDNF or GDNF+GFRα1 groups. Within lamina IV, CGRP+ axon occupying area was significantly higher for artemin+NGF (δp<0.01) compared to artemin+GFRα3 group (Fig. 8E). Altogether, this data demonstrates that co-expressing artemin with NGF or GFRα3 disrupts the capacity of artemin to promote topographic targeting and results in mistargeting of CGRP fibers. The data from artemin+GFRα3 regeneration strongly indicate the distribution of GFRα3 to substrate cells greatly affects the distribution of these axons.
Effect of the above co-expression patterns on other classes of sensory axons
In addition to CGRP+ axons, we also investigated the effect of co-expressing artemin with NGF, GFRα3 or GDNF and GDNF with GFRα1 on+axons (Fig. 9) and deeper myelinated sensory afferents (Fig. 10). In Figure 3 we show that artemin and GDNF, but not NGF, induces regeneration of IB4+ axons. When GFRα3 (Fig. 9B & b) or GDNF (Fig. 9C & c) was co-expressed with artemin, we observed a significantly higher regeneration of IB4+ axons into the spinal cord, mostly restricted to superficial laminae. In general, GFRα3 co-expression with artemin induced regeneration of IB4 positive axons only to the superficial dorsal horn, except in 2 animals in which some of these axons regenerated into the deeper dorsal horn (Fig. 9B); however, this patterning was not statistically significant. Analysis of the IB4+ axon density using a one-way ANOVA showed an effect of treatment Ftreatment(5,54)=9.49, p<0.01. Tukey’s multiple comparison analysis revealed a significantly greater (p<0.01) regeneration of IB4+ fibers in artemin+GFRα3 group (n=6), artemin + GDNF (n=7) and GDNF+GFRα1 group (n=5) compared to artemin group (n=10) and artemin+NGF (n=6) group. These data indicate a possible role for GFRα3 co-receptor in artemin-mediated regeneration of IB4+ fibers and no additive effect of artemin with GDNF in enhancing IB4+ fibers regeneration. There was no significant difference in IB4+ axon density between GDNF and GDNF+GFRα1, indicating that sprouting and ectopic distribution could be a property of the sensory axon phenotype (Fig. 9E).
Figure 9.
Co-expression of artemin with NGF, GFRα3 or GDNF augmented the regeneration of IB4+ axons. NGF (A, a), GFRα3 (B, b) or GDNF (C, c) when co-expressed with artemin produced robust regeneration of IB4+ axons while, co-expression of GFRα1 with GDNF (D, d) did not increase IB4+ axon regeneration compared to GDNF alone. Magnified images of the boxed region from respective ipsilateral dorsal horn (a, b, c, & d). Scale bar = 300 μm A, B, C, & D. Scale bar = 100 μm for a, b, c, & d. E, Quantification of IB4+ axon occupying area in artemin+NGF, artemin+GFRα3, artemin+GDNF and GDNF+GFRα1 animals compared to artemin and GDNF from figure 3. Co-expression of GFRα3 or GDNF along with artemin produced a statistically significant increase in regeneration of IB4+ axons compared to artemin alone and artemin+NGF (one way ANOVA, Tukey’s post hoc test, *p<.05).
Figure 10.
Cholera-toxin β-subunit-labeled myelinated sensory afferents regenerate only with treatments co-expressing GDNF. Artemin+NGF (A & a, n=6) and artemin+GFRα3 group (B & b, n=6) did not show any regeneration of CTB labeled axons. GDNF co-expressed groups, artemin+GDNF (C & c) and GDNF+GFRα1 (D & d) co-expressed groups showed regeneration of CTB labeled axons. Magnified images of ipsilateral dorsal horn from boxed area (a, b, c, & d). Scale bar = 300μm (A, B, C, & D). Scale bar = 100μm (a, b, c, & d). E, Statistical analysis by nonparametric test (Kruskal-Wallis test) showed significantly higher regeneration of CTB labeled axons for GDNF alone and GDNF co-expressed animals compared to GFP control (*p<.05). There was no statistically significant difference among GDNF (n=8), artemin+GDNF (n=6) and GDNF+ GFRα1 (n=5) groups.
Cholera toxin labeled myelinated sensory afferents did not show any regeneration with the co-expression of artemin+NGF (Fig. 10A & a) or artemin+GFRα3 (Fig. 10B & b). This is in agreement with our previous data with NGF and artemin (Fig. 5C & D). Because GDNF was able to induce regeneration of these axons (Fig. 5E), we evaluated the effect of combining GDNF with artemin (Fig. 10C & c) or GFRα1 (Fig. 10D & d). Statistical analysis of total area occupied by CTB labeled axons by non-parametric test (Kruskal-Wallis’ ANOVA) showed a significant effect of treatment (p< 0.05, Fig. 10E). Multiple comparison analysis showed a statistically significant increase in regeneration of CTB axons in GDNF expressed animals compared to GFP controls (p< 0.05). However, there was no significant difference between GDNF alone group and artemin+GDNF or GDNF+GFRα1, again indicating that the sensory phenotype of regenerating fibers might be a determining factor in affecting distribution and topography.
Co-expressing NGF or GFRα3 with artemin produces aberrant synaptic connectivity and hyperalgesia
We examined cFos IR in 2nd order projection neurons in animals co-expressing artemin with either NGF, GFRα3 or GDNF and GDNF with GFRα1. For artemin+NGF (Fig. 11A) and artemin+GFRα3 (Fig. 10B), the topography of cFos IR followed the distribution of regenerated CGRP+ axons with a number of projection neurons showing activated cFos within the deeper dorsal horn. In the artemin+GDNF group, there was lower cFos IR and it was distributed almost equally in superficial and in the deep dorsal horn (Fig. 11C). Similarly, the GDNF+GFRα1 group showed very minimal and randomly distributed cFos IR (Fig. 11D). Statistical analysis of cFos-IR by 2-way ANOVA among artemin (from Fig. 6) (n=9), artemin+NGF (n=6) artemin+GFRα3 (n=6), artemin+GDNF (n=7) and GDNF+GFRα1(n=5) groups using topography (superficial vs. deep dorsal horn) and treatment as two factors did not show significance for the topography (p=0.34), but found a statistically significant effect of treatment, Ftreatment(5,80)=18.6, p<0.01 and an effect of interaction, Finteraction(5,80)=7.664, p<0.01. The animals co-expressing artemin with NGF or GFRα3 showed significantly higher cFos IR in the deep dorsal horn compared to artemin (p<0.05) confirming that ectopically regenerated fibers formed functional synaptic contacts. Tukey’s multiple comparison showed a significantly lower cFos IR in the superficial as well as deep dorsal horn for the GDNF alone group (from Fig. 7E) compared to artemin+NGF and artemin+GFRα3 (p<0.05). cFos IR in GDNF+GFRα1 and artemin+GDNF expressing animals was not significantly different from GDNF (Fig. 11E). Further, we confirmed the functional significance of regeneration by employing various behavioral measurements specific to distinct classes of axons. In agreement with the data on anatomical regeneration of nociceptive axons, these animals showed a return of only thermal nociception (Fig. 11F), *p<0.05). Moreover, these animals exhibited slight thermal hyperalgesia concordant with the data on aberrant synaptic connectivity while artemin+GDNF and GDNF+GFRα1 did not show any recovery of thermal nociception. Beginning at day 40, animals co-expressing artemin with NGF or GFRα3 showed a hyperalgesic trend with the ratio of the right PWL to left PWL falling below 1. By day 50, comparison of artemin+NGF or artemin+ GFRα3 to baseline control values from day zero (before crush) were statistically significant (δp<0.05), as determined by a one way ANOVA, indicating mild hyperalgesia (Dunnett’s multiple comparison test). Hence, these data indicate that the ectopic regeneration of CGRP+ fibers, in the presence of IB4+ axon regeneration, established aberrant synaptic connectivity and may consequently lead to abnormal pain behavior in these animals. Correlational analysis of thermal hyperalgesic index (ratio of right PWL to left PWL) with the count of cFos IR neurons in the spinal cord (Fig. 11G) indicated that nociceptive recovery is correlated well with the cFos IR (r= −0.872). Evaluation of touch sensitivity using von Frey hairs in these groups (only data from 4 groups shown as they overlap tightly) showed no statistical difference from VFH threshold for GFP control indicating the absence of allodynia (Fig. 11H). This shows that even though there was thermal hyperalgesia in artemin+NGF and artemin+GFRα3, there was no allodynia. The lack of robust IB4 regeneration in these groups might be contributing to the absence of allodynia (Zhang et al., 2013a). On the other hand, despite robust IB4 regeneration, absence of ectopic CGRP regeneration in GDNF, artemin+GDNF and GDNF+GFRα1 groups might be the reason for lack of recovery of thermal nociception.
Figure 11.
Behavioral analysis of animals co-expressing artemin along with NGF or GFRα3 or GDNF with artemin and GFRα1. Identification of Fos IR cells in the right dorsal horn following thermal stimulation of the ipsilateral hindlimb in artemin+NGF (A), artemin+GFRα3 (B), artemin+GDNF (C) and GDNF+GFRα1 (D) groups. Scale bar = 300 μm. E, Quantification of cFos measurements and comparison with artemin and GDNF from figure 6. Average number of cFos IR cells in the superficial dorsal horn is similar for artemin, artemin+NGF and artemin+GFRα3. Deep dorsal horn shows a significant increase in cFos IR cells for artemin+NGF and artemin+GFRα3 groups compared to artemin, GDNF and GDNF co-expressed groups (*p<.05, Tukeys post hoc test, n=6 for artemin+NGF, and artemin+GFRα3, n=7 for artemin+GDNF and n=5 for GDNF+GFRα1). F, Animals co-expressing artemin with NGF or GFRα3 shows a recovery of thermal nociception from day 30 onwards (*p<.05, Tukey’s post hoc test) and the ratio of right PWL to left PWL was significantly below the pre-injury baseline ratio of 1 by day 50 indicating hyperalgesia (δp<.05, Dunnett’s post hoc test). Animals co-expressing GDNF did not show a recovery of thermal nociceptive behavior. Color and character legend similar to E. G, Correlation analysis of the total count of cFos IR neurons from all treatment groups with the ratio of right PWL to left PWL on day 50 (last behavioral assessment) of behavioral measurement indicates a strong correlation between the two (r= −0.872). Negative correlation coefficient indicates a lower latency of withdrawal with higher c-Fos counts. H, Evaluation of touch sensitivity and allodynia using von Frey hairs. None of the treatment groups showed a difference in vfh threshold compared to control indicating the absence of development of any allodynia. Data from only 4 groups shown as all of them tightly overlap.
Discussion
Reestablishment of synaptic connections in the appropriate target locations is of paramount significance in axonal regeneration. In many parts of the central nervous system (CNS), laminar specificity determines functional specificity (Sanes and Yamagata, 1999). Hence, it is important to instruct regenerating axons to grow to their original synaptic locations. Gene therapy offers a reliable method to express the genes of interest in a spatially restricted pattern (Tang et al., 2004; 2007). Neurotrophins induce chemoattractive guidance of regenerating axons specifically by activating receptors at the axon tip (Campenot, 1982; Kimpinski et al., 1997; Zhou and Snider, 2006). Similar mechanisms operate in axonal guidance during development. Regulating surface expression of axon guidance receptors could provide a potent mechanism to influence guidance (O’Donnell et al., 2009) and achieve the exquisite organization of the CNS. Typically, neurotrophins are applied to the target location in order to attract regenerating axons, but NGF-induced ectopic sprouting and regeneration of CGRP+ axons throughout the region of expression (Romero et al., 2000; Tang et al., 2004; Tang et al., 2007). Such ectopic growth and sprouting is thought to occur when the neurotrophin overwhelms the endogenous guidance cues and elicits growth towards and throughout the region of neurotrophin expression (Blesch et al., 2002).
Disruption of topographic organization by nociceptive axons has been associated with the development of hyperalgesia in several models (Lewin et al., 1994; Romero et al., 2000; Tang et al., 2004). Under such conditions, the application of anti-NGF to injured spinal cord reduces sprouting of CGRP+ axons, neuropathic pain and autonomic dysreflexia (Christensen and Hulsebosch, 1997; Krenz and Weaver, 2000; Brown et al., 2004). Interestingly, after dorsal rhizotomy, NGF-induced regeneration and mistargeting of only CGRP+ axons in the absence of other sensory modalities showed normal responses to thermal nociception, without the development of hyperalgesia (Lin et al., 2014). This phenomenon most likely is either due to the lack of sufficient synaptic connectivity to drive hyperexcitability or the requirement of other sensory modalities for co-activation of pain pathways (Lin et al., 2014). On the other hand, artemin-induced regeneration of both peptidergic and non-peptidergic nociceptive axons into their normal laminar distribution also showed normal thermal nociceptive responses. Interestingly, co-application of either NGF or GFR-α3 with artemin induced regeneration of CGRP-IR axons into the deeper dorsal horn. This led to the development of mild thermal hyperalgesia, strongly indicating that regeneration of both the peptidergic and non-peptidergic axons are required for mistargeted axons to induce hyperalgesic responses. Although very few studies have examined selective ablation of either population, ablation of only IB4+ DRG neurons using IB4-saporin (kills neurons by inhibiting protein synthesis) resulted in reduced thermal and mechanical nociception (Vulchanova et al., 2001; Tarpley et al., 2004), demonstrating the importance of both pathways in the establishment of neuropathic pain.
Very few studies have examined the relationship between specificity of synaptic connections at targets after axonal regeneration and behavioral improvement. Several studies attribute recovery to supraspinal reorganization of circuits or contralateral sprouting establishing new functional circuits (Fouad et al., 2001; Kim et al., 2006). Although a few studies have examined the synaptic function after dorsal root regeneration by electrophysiology measurements, laminar specificity of post-synaptic neurons remained undetermined in those experiments (Ramer et al., 2001; Wang et al., 2008). cFos activation in the dorsal horn has been indicated as a neural correlate of nociception (Harris, 1998; Coggeshall, 2005) and cFos IR in the lumbar dorsal horn is topographically correlated to the primary afferent projection pattern from the hind paw (Hunt et al., 1987). Thermal stimulation increased cFos activity in all groups showing regeneration and functional return of thermal nociception. Interestingly, these groups, whether or not they developed hyperalgesia, showed a similar level of cFos activation within the superficial region of the dorsal horn. Within the deeper dorsal horn (laminae III, IV, V, & VI), NGF expression either alone or with artemin led to increased cFos labeled neurons, with co-expression of NGF with artemin showing a slightly (138%) higher level, but not reaching statistical significance (p=0.059). Increased cFos expression (166%) was also evident with co-expression of artemin and GFR-α3 when compared to NGF treatment alone (p<0.05). Both NGF and artemin have been shown to promote hyperalgesia (Malin and Davis, 2008), increase the expression of TRPV1 (Jankowski et al., 2010), TRPM8 (Lippoldt et al., 2013), and increase cutaneous innervation of skin (Elitt et al., 2006). Interesting, although injections of either NGF or artemin lead to transient increases in thermal hyperagesia lasting a few hours, co-injections of both lead to further increases and prolongation of hyperalgesia up to 6 days (Malin et al., 2006). Many of these artemin-induced responses appear mediated through GFR-α3. Co-expression of both artemin with GFR-α3 could increase nociceptive signaling receptors, including nicotinic acetylcholine receptor (Albers et al., 2014), to potentiate hyperagesia and cfos expression within the spinal cord. Unlike artemin, GDNF has been implicated in producing an analgesic effect against injury-induced pain when injected or expressed within the spinal cord (Boucher et al., 2000; Pezet et al., 2006; Salio et al., 2014). The exact mechanism for this analgesic effect is unknown, but could be mediated by down regulation of several sodium channel subunits, which results in reduced spontaneous activity (Boucher et al., 2000).
Although multiple mechanisms for NGF induced CGRP-IR axon sprouting or mistargeted regeneration have been hypothesized (Tang et al., 2004; Hannila and Kawaja, 2005; Hancock et al., 2011), our present data indicate this phenomenon to be related to the well documented role of NGF in axon collateral branch formation (Diamond et al., 1985; Doubleday and Robinson, 1992; Harper et al., 1999). Theoretically, if NGF induced axonal trapping within the region of NGF expression, then synapses associated with trapped axons should be randomly distributed in this region. Even though the distribution and density of CGRP-IR axons is relatively uniform within the dorsal horn, the expression of cFos is concentrated within the superficial dorsal lamina, similar to the cFos distribution established by topographically targeted regeneration mediated by artemin. These data indicate a topographic targeting of synapses to their normal laminar locations. Since it is well established that NGF-promotes sprouting from uninjured CGRP-IR axons (Diamond et al., 1992) or the formation of collateral branches during regeneration (Devor and Govrin-Lippmann., 1979; Doucette and Diamond, 1987), the extension of axons into the deeper dorsal horn may simply represent additional sprouting of these axons within the region of NGF expression. Indeed, NGF-induces filopodial and collateral branch formation by activation of PI3 kinase pathway along axon shafts (Gallo and Letourneau, 1998; Ketschek and Gallo, 2010). Likewise similar results are observed with co-expression of both artemin and NGF, in which the density of cFos labeling in the superficial horn is similar to treatment by artemin alone, but the density of CGRP-IR fibers within the deeper dorsal laminae is quite extensive.
Unlike NGF, which binds directly to its signaling receptor, artemin needs to bind to a non-signaling co-receptor (GFR-α3) prior to binding to its signaling receptor cRet (Baloh et al., 1998) The GFR-α3 co-receptor is expressed primarily by neurons within the peripheral nervous system and very little, if any, hybridization signal has been identified in spinal cord (Widenfalk et al., 1998). However, by immunohistochemistry we identified GFR-α3 staining within laminae I and II, which disappeared after dorsal root rhizotomy (not shown), indicating GFR-α3 to be confined to the axons of primary afferents. In adult rat DRGs, it is estimated that about 20 – 40% of DRG neuron express GFR-α3, mostly small diameter peptidergic nociceptive neurons (Bennett et al., 2000) (Orozco et al., 2001; Gardell et al., 2003). To induce regeneration, artemin must diffuse into the DREZ and interact with the GFR-α3 and cRet to form the signaling complex. Under this condition, artemin binds to GFR-α3 on axons prior to binding onto cRet in the cis confirmation, inducing dimerization and signal transduction (Santoro et al., 2004). The GDNF family of ligands (GFLs) can also bind to GFR-α on non-cRet expressing cells and interact with cRet on axons in a trans configuration (Ledda et al., 2007). Signaling through the trans complex is thought to be different to that of cis binding, being slightly delayed with a longer duration (Paratcha et al., 2001). Trans activation may also potentiate cis signal transduction to further enhance axon growth and targeting (Crone and Lee, 2002; Ledda et al., 2002; Paratcha and Ledda, 2008). Although, trans signaling has been implicated in guidance, targeting and axonal growth in the presence of GDNF or neurturin (Worley et al., 2000; Ledda et al., 2002), it has not been described for artemin mediated signaling. Here we show, in the presence of artemin, ectopic expression of GFR-α3 within the spinal cord can alter the growth of axons, thus possibly functioning through a similar trans mechanism. However, since cfos patterning shows near normal activity in the superficial dorsal horn, GFR-α3 could be acting similar to NGF and induce ectopic sprouting of regenerating CGRP+ axons into deeper regions via trans activation. Since IB4 axons are known to show poorer regeneration after injury, even in the presence of GDNF, this could reduce sprouting of these axons (Leclere et al., 2007). Under such conditions, all neurotrophins and co-receptors combinations induce regeneration and connectivity to appropriate superficial laminae; however, secondary sprouting by persistent neurotrophin expression could lead to phenotype specific sprouting of CGRP+ but not IB4+ axons contributing to ectopic growth and cfos expression.
In conclusion, we demonstrate the application of artemin induces topographic regeneration of only the nociceptive afferents, with no observable regeneration of other sensory axons as reported previously (Wang et al., 2008; Harvey et al., 2010). We also show that co-expression of GFR-α3 can disrupt targeting, most likely by enhancing growth along transduced glia by interacting with neuronal cRet in a trans configuration. Similarly, artemin could also enhance the formation of synapses, resulting in a significantly greater density of cFos labeling in the deeper dorsal horn by acting as a ligand to induce GFR-α3 crosslinking.
Highlights.
Axons fail to regenerate into the spinal cord after dorsal root rhizotomy.
Sensory afferent regeneration is induced by expression of neurotrophins in the spinal cord.
Overexpression of Artemin only induced regeneration of nociceptive axons after dorsal rhizotomy.
Co-expression of artemin and its co-receptor GFR-α3 disrupted topography of regenerating axons.
Mistargeting of regenerating axons might be due to secondary sprouting induced by neurotrophin.
Acknowledgments
This work was funded by a grant from the National Institute of Neurological Disorders and Stroke R01 NS060784 and the Shriners Hospital for Pediatric Research grants SHC 84050 and SHC 85200.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Albers KM, Zhang XL, Diges CM, Schwartz ES, Yang CI, Davis BM, Gold MS. Artemin growth factor increases nicotinic cholinergic receptor subunit expression and activity in nociceptive sensory neurons. Mol Pain. 2014;10:31. doi: 10.1186/1744-8069-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Jr, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- Bennett DLH, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. Journal of Neuroscience. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DLH, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, Phillips HS, McMahon SB, Shelton DL. The Glial Cell Line-Derived Neurotrophic Factor Family Receptor Components Are Differentially Regulated within Sensory Neurons after Nerve Injury. The Journal of Neuroscience. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Lu P, Tuszynski MH. Neurotrophic factors, gene therapy, and neural stem cells for spinal cord repair. Brain research bulletin. 2002;57:833–838. doi: 10.1016/s0361-9230(01)00774-2. [DOI] [PubMed] [Google Scholar]
- Brown A, Ricci MJ, Weaver LC. NGF message and protein distribution in the injured rat spinal cord. Experimental neurology. 2004;188:115–127. doi: 10.1016/j.expneurol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Cajal Ramon Y. Bull N Y Acad Med. 1929;5 [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures: II. Local control of neurite survival by nerve growth factor. Developmental Biology. 1982;93:13–21. doi: 10.1016/0012-1606(82)90233-0. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. Regenerating Axons Form Nerve-Terminals at Astrocytes. Brain Res. 1985;347:188–191. doi: 10.1016/0006-8993(85)90911-4. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. The Journal of pharmacology and experimental therapeutics. 1994;269:1117–1123. [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Experimental neurology. 1997;147:463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos, nociception and the dorsal horn. Progress in neurobiology. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Coulpier M, Anders J, Ibanez CF. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. The Journal of biological chemistry. 2002;277:1991–1999. doi: 10.1074/jbc.M107992200. [DOI] [PubMed] [Google Scholar]
- Crone SA, Lee KF. The bound leading the bound: Target-derived receptors act as guidance cues. Neuron. 2002;36:333–335. doi: 10.1016/s0896-6273(02)01009-7. [DOI] [PubMed] [Google Scholar]
- Devor M, Govrin-Lippmann R. Selective regeneration of sensory fibers following nerve crush injury. Experimental neurology. 1979;65:243–254. doi: 10.1016/0014-4886(79)90094-3. [DOI] [PubMed] [Google Scholar]
- Diamond J, Coughlin M, Macintyre L. Evidence that endogenous beta-nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult-rats. Proceedings of the National Academy of Sciences of the United States of America. 1985;84:6596–6600. doi: 10.1073/pnas.84.18.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J, Holmes M, Coughlin M. Endogenous Ngf and Nerve Impulses Regulate the Collateral Sprouting of Sensory Axons in the Skin of the Adult-Rat. Journal of Neuroscience. 1992;12:1454–1466. doi: 10.1523/JNEUROSCI.12-04-01454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Doubleday B, Robinson PP. The Role of Nerve Growth-Factor in Collateral Reinnervation by Cutaneous C-Fibers in the Rat. Brain Res. 1992;593:179–184. doi: 10.1016/0006-8993(92)91306-y. [DOI] [PubMed] [Google Scholar]
- Doucette R, Diamond J. Normal and precocious sprouting of heat nociceptors in the skin of adult rats. The Journal of comparative neurology. 1987;261:592–603. doi: 10.1002/cne.902610410. [DOI] [PubMed] [Google Scholar]
- Eggers R, de Winter F, Hoyng SA, Roet KC, Ehlert EM, Malessy MJ, Verhaagen J, Tannemaat MR. Lentiviral vector-mediated gradients of GDNF in the injured peripheral nerve: effects on nerve coil formation, Schwann cell maturation and myelination. PLoS One. 2013;8:e71076. doi: 10.1371/journal.pone.0071076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernsberger U. The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell and tissue research. 2008;333:353–371. doi: 10.1007/s00441-008-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Current biology : CB. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Localized Sources of Neurotrophins Initiate Axon Collateral Sprouting. The Journal of Neuroscience. 1998;18:5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardell LR, et al. Multiple actions of systemic artemin in experimental neuropathy. Nature medicine. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- Hancock ML, Nowakowski DW, Role LW, Talmage DA, Flanagan JG. Type III neuregulin 1 regulates pathfinding of sensory axons in the developing spinal cord and periphery. Development. 2011;138:4887–4898. doi: 10.1242/dev.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannila SS, Kawaja MD. Nerve growth factor-mediated collateral sprouting of central sensory axons into deafferentated regions of the dorsal horn is enhanced in the absence of the p75 neurotrophin receptor. The Journal of comparative neurology. 2005;486:331–343. doi: 10.1002/cne.20537. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A New and Sensitive Method for Measuring Thermal Nociception in Cutaneous Hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Harper SJ, Buchman VL, Owen D. Denervation of the skin following section of the inferior alveolar nerve leads to increased NGF accumulation without change in NGF mRNA expression. Experimental neurology. 1999;155:327–330. doi: 10.1006/exnr.1998.7000. [DOI] [PubMed] [Google Scholar]
- Harris JA. Using c-fos as a neural marker of pain. Brain research bulletin. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- Harvey P, Gong B, Rossomando AJ, Frank E. Topographically specific regeneration of sensory axons in the spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11585–11590. doi: 10.1073/pnas.1003287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Anderson CE, Koerber HR. Enhanced artemin/GFRα3 levels regulate mechanically insensitive, heat-sensitive C-fiber recruitment after axotomy and regeneration. J Neurosci. 2010;30:16272–16283. doi: 10.1523/JNEUROSCI.2195-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek A, Gallo G. Nerve Growth Factor Induces Axonal Filopodia through Localized Microdomains of Phosphoinositide 3-Kinase Activity That Drive the Formation of Cytoskeletal Precursors to Filopodia. The Journal of Neuroscience. 2010;30:12185–12197. doi: 10.1523/JNEUROSCI.1740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Experimental neurology. 2006;198:401–415. doi: 10.1016/j.expneurol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kimpinski K, Campenot RB, Mearow K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. J Neurobiol. 1997;33:395–410. doi: 10.1002/(sici)1097-4695(199710)33:4<395::aid-neu5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J Neurochem. 2000;74:730–739. doi: 10.1046/j.1471-4159.2000.740730.x. [DOI] [PubMed] [Google Scholar]
- Leclere PG, Norman E, Groutsi F, Coffin R, Mayer U, Pizzey J, Tonge D. Impaired axonal regeneration by isolectin B4-binding dorsal root ganglion neurons in vitro. J Neurosci. 2007;27:1190–1199. doi: 10.1523/JNEUROSCI.5089-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Ibanez CF. Target-derived GFR alpha 1 as an attractive guidance signal for developing sensory and sympathetic axons via activation of Cdk5. Neuron. 2002;36:387–401. doi: 10.1016/s0896-6273(02)01002-4. [DOI] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. GDNF and GFR alpha 1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nature neuroscience. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Rueff A, Mendell LM. Peripheral and Central Mechanisms of Ngf-Induced Hyperalgesia. European journal of neuroscience. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Lin C-L, Heron P, Hamann SR, Smith GM. Functional distinction between NGF-mediated plasticity and regeneration of nociceptive axons within the spinal cord. Neuroscience. 2014:272. doi: 10.1016/j.neuroscience.2014.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippoldt EK, Elmes RR, McCoy DD, Knowlton WM, McKemy DD. Artemin, a glial cell line-derived neurotrophic factor family member, induces TRPM8-dependent cold pain. J Neurosci. 2013;33:12543–12552. doi: 10.1523/JNEUROSCI.5765-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM. Postnatal roles of GDNF family members in nociceptors plasticity. Sheng Li Xue Bao. 2008;60:571–578. [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell M, Chance RK, Bashaw GJ. Axon Growth and Guidance: Receptor Regulation and Signal Transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco OE, LW, Sah DW, Pepinsky RB, MS GFRalpha3 is expressed predominantly in nociceptive sensory neurons. European journal of neuroscience. 2001;13:2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F. GDNF and GFR alpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF. Released GFR alpha 1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29:171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- Pezet S, Krzyzanowska A, Wong LF, Grist J, Mazarakis ND, Georgievska B, McMahon SB. Reversal of neurochemical changes and pain-related behavior in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol Ther. 2006;13:1101–1109. doi: 10.1016/j.ymthe.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Ramer MS. Endogenous neurotrophins and plasticity following spinal deafferentation. Experimental neurology. 2012;235:70–77. doi: 10.1016/j.expneurol.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. Journal of Neuroscience. 2001;21:2651–2660. doi: 10.1523/JNEUROSCI.21-08-02651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axone into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Archives internationales de pharmacodynamie et de therapie. 1957;111:409–419. [PubMed] [Google Scholar]
- Robertson B, Grant G. A Comparison between Wheat-Germ Agglutinin Horseradish and Choleragenoid-Horseradish Peroxidase as Anterogradely Transported Markers in Central Branches of Primary Sensory Neurons in the Rat with Some Observations in the Cat. Neuroscience. 1985;14:895–905. doi: 10.1016/0306-4522(85)90152-6. [DOI] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20:4435–4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Ferrini F, Muthuraju S, Merighi A. Presynaptic modulation of spinal nociceptive transmission by glial cell line-derived neurotrophic factor (GDNF) J Neurosci. 2014;34:13819–13833. doi: 10.1523/JNEUROSCI.0808-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Formation of lamina-specific synaptic connections. Current Opinion in Neurobiology. 1999;9:79–87. doi: 10.1016/s0959-4388(99)80010-5. [DOI] [PubMed] [Google Scholar]
- Santoro MI, Melillo RM, Carlomagno F, Vecchio G, Fusco A. Minireview: RET: Normal and Abnormal Functions. Endocrinology. 2004;145:5448–5451. doi: 10.1210/en.2004-0922. [DOI] [PubMed] [Google Scholar]
- Smith GM, Falone AE, Frank E. Sensory axon regeneration: rebuilding functional connections in the spinal cord. Trends Neurosci. 2012;35:156–163. doi: 10.1016/j.tins.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27:6068–6078. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley JW, Kohler MG, Martin WJ. The behavioral and neuroanatomical effects of IB4-saporin treatment in rat models of nociceptive and neuropathic pain. Brain Res. 2004;1029:65–76. doi: 10.1016/j.brainres.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: How critical is the regulation of substance P signaling? Journal of Neuroscience. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, Cariani P, Frank E, Sah DW, Porreca F. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nature neuroscience. 2008;11:488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenfalk J, Tomac A, Lindqvist E, Hoffer B, Olson L. GFR alpha-3, a protein related to GFR alpha-1, is expressed in developing peripheral neurons and ensheathing cells. European journal of neuroscience. 1998;10:1508–1517. doi: 10.1046/j.1460-9568.1998.00192.x. [DOI] [PubMed] [Google Scholar]
- Worley DS, Pisano JM, Choi ED, Walus L, Hession CA, Cate RL, Sanicola M, Birren SJ. Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development. 2000;127:4383–4393. doi: 10.1242/dev.127.20.4383. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cavanaugh DJ, Nemenov MI, Basbaum AI. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. J Physiol. 2013a;591:1097–1110. doi: 10.1113/jphysiol.2012.242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Jin Y, Ziemba KS, Fletcher AM, Ghosh B, Truit E, Yurek DM, Smith GM. Long distance directional growth of dopaminergic axons along pathways of netrin-1 and GDNF. Experimental neurology. 2013b;250:156–164. doi: 10.1016/j.expneurol.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Schmidt RE, Yan Q, Snider WD. Ngf and Nt-3 Have Differing Effects on the Growth of Dorsal-Root Axons in Developing Mammalian Spinal-Cord. Journal of Neuroscience. 1994;14:5187–5201. doi: 10.1523/JNEUROSCI.14-09-05187.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F-Q, Snider WD. Intracellular control of developmental and regenerative axon growth. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361:1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]