Abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD) is associated with metabolic risk factors including hypertension and dyslipidemia, and may progress to liver fibrosis. Previous studies have shown that hepatic steatosis and fibrosis are heritable but whether they have a significant shared gene effect is unknown. This study aimed to examine the shared gene effects between hepatic steatosis, fibrosis, and their associations with metabolic risk factors.
Methods
This is a cross-sectional analysis of a prospective cohort of well-characterized, community-dwelling twins (45 monozygotic, 20 dizygotic twin pairs, 130 total subjects) from Southern California. Hepatic steatosis was assessed with MRI-proton density fat fraction (MRI-PDFF) and hepatic fibrosis was assessed with magnetic resonance elastography (MRE). A standard bivariate twin AE model was used to estimate the proportion of phenotypic variance between two phenotypes accounted for by additive genetic effects (A) and individual-specific environmental effects (E). Genetic correlations (rG) estimated from this model represent the degree to which the genetic determinants of two phenotypes overlap.
Results
The mean (±SD) age and BMI were 47.1 (±21.9) years and 26.9 (±6.5) kg/m2, respectively. 20% (26/130) of the cohort had hepatic steatosis (MRI-PDFF ≥5%) and 8.2% (10/122) had hepatic fibrosis (MRE ≥3Kpa). Blood pressure (systolic and diastolic), triglycerides, glucose, homeostatic model assessment of insulin resistance (HOMA-IR), insulin, hemoglobin A1c (HbA1c), and low high-density lipoprotein (HDL) had significant shared gene effects with hepatic steatosis. Triglycerides, glucose, HOMA-IR, insulin, HbA1c, and low HDL had significant shared gene effects with hepatic fibrosis. Hepatic steatosis and fibrosis had a highly significant shared gene effect of 0.756 (95% CI: 0.716–1, p<0.0001).
Conclusions
Genes involved with steatosis pathogenesis may also be involved with fibrosis pathogenesis.
Keywords: NAFLD, steatosis, fibrosis, genetics, MRI
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) comprises of a spectrum of liver pathologies characterized by hepatic steatosis in patients with little to no history of alcohol use or secondary causes of hepatic steatosis.(1) Nonalcoholic steatohepatitis (NASH) is an advanced form of NAFLD and predisposes patients to the development of hepatic fibrosis, which is associated with increased risks of cirrhosis, mortality, and liver transplantation.(2–5) NAFLD, including its complications, is now a leading cause of liver disease in the United States and worldwide.(6–8) Due to the heavy disease burden of NAFLD and its associated morbidity and mortality, there is a great need to characterize the heritability of NAFLD to identify patients who may be at risk for the disease, improve the understanding of NAFLD pathogenesis, and identify potential targets for treatment.
Hepatic steatosis represents the initial step for the pathogenesis of NASH and hepatic fibrosis, and we have previously demonstrated in twin models that both hepatic steatosis and fibrosis are heritable traits.(9) Various genes, including PNPLA-3 and TM6SF2, are associated with the development of hepatic steatosis and fibrosis, although variations in these genes do not account for all the variance seen in hepatic steatosis and fibrosis, and additional genes remain to be identified.(10–15) But while hepatic steatosis can progress to hepatic fibrosis, it is unknown if there are direct genetic links between these two traits. Hepatic fibrosis has been shown to be the most important predictor of mortality and liver transplantation in NAFLD patients,(5, 16) and an improved understanding of the shared heritability between hepatic steatosis and fibrosis may elucidate potential targets for NAFLD prevention and treatment. If steatosis and fibrosis gene regulation significantly overlaps then it is plausible that improvement in steatosis by common shared mechanistic pathway may eventually trigger improvement in fibrosis in the context of targeting specific nodal points in the mechanistic pathway. Additionally, previous studies have shown NAFLD to be associated with metabolic risk factors including obesity, hypertension, dyslipidemia, and insulin resistance,(17–20) and there is a genetic component to this association.(21) However, further studies are needed to characterize the genetic association between hepatic steatosis, fibrosis, and individual metabolic risk factors.
Utilizing a prospective study design of community-dwelling monozygotic and dizygotic twins, we aimed to evaluate if study participants with genetic susceptibilities to hepatic steatosis also have genetic susceptibilities to hepatic fibrosis. We also aimed to evaluate the genetic susceptibilities of hepatic steatosis and fibrosis with metabolic risk factors, including blood pressure, cholesterol levels, triglycerides, insulin resistance, and diabetes. Mathematical models involved additive genetics and unique environment effects (called AE models) were constructed for the cohort to distinguish between the shared genetic versus environmental determination of individual traits. Magnetic resonance imaging – proton density fat fraction (MRI-PDFF) and magnetic resonance elastography (MRE), two novel, accurate, and non-invasive imaging biomarkers, were respectively used to assess for hepatic steatosis and fibrosis in this prospective study.
METHODS
Experimental Design
This was a cross-sectional analysis of a prospectively recruited cohort of monozygotic and dizygotic twin pairs living in Southern California. All twin pairs underwent clinical research assessments, including medical history, physical and anthropometric exams, and biochemical testing, at the University of California at San Diego (UCSD) NAFLD Research Center.(15, 21–23) Participants also underwent MRI-PDFF for hepatic steatosis and MRE for hepatic fibrosis at the UCSD MR3T Research Laboratory. Clinical and imaging visits were performed on the same day for each twin pair, and the study took place from 2012 to 2015. All participants provided written informed consent before enrolling in the study. The study protocol was approved by the UCSD Institutional Review Board.
Inclusion Criteria
Participants were included in the study if they were twins at least 18 years old who provided written informed consent. The zygosity of the majority of twin pairs as monozygotic (MZ) or dizygotic (DZ) had been previously confirmed via genetic testing before the participants enrolled in the study. A previously published questionnaire, as described by Boyd et al,(24) was used to further confirm twinship status (see Supplementary Text for details).
Exclusion Criteria
Participants were excluded from the study if they met any of the following criteria: (1) significant alcohol intake (>10 grams/day in females or >20 grams/day in males) for at least three consecutive months over the previous 12 months, or if the quantity of alcohol consumption could not be reliably ascertained; (2) clinical or biochemical evidence of liver diseases other than NAFLD, including hepatitis B, hepatitis C, alpha-1 antitrypsin deficiency, hemochromatosis, Wilson’s disease, autoimmune hepatitis, polycystic liver diseases, cholestatic liver diseases, and vascular liver diseases; (3) chronic illnesses associated with hepatic steatosis, including human immunodeficiency virus infection, type I diabetes mellitus, celiac disease, cystic fibrosis, lipodystrophy, dysbetalipoproteinemia, and glycogen storage diseases; (4) use of drugs known to cause hepatic steatosis, including amiodarone, glucocorticoids, methotrexate, L-asparaginase, and valproic acid for at least three out of the previous six months; (5) history of bariatric surgery, including roux-en-Y gastric bypass and gastroplasty; (6) presence of systemic infectious illnesses; (7) females who were pregnant or nursing at the time of the study; (8) contraindications to MRI, including metal implants, claustrophobia, and body circumference greater than that of the imaging chamber; (9) any other condition(s) which, based on the principal investigator’s opinion, may significantly affect the participant’s compliance, competence, or ability to complete the study.
Definition of NAFLD
Participants were considered to have NAFLD if they had hepatic steatosis (MRI-PDFF ≥5%) and no secondary causes of hepatic steatosis due to factors including the use of steatogenic medications, other liver diseases, and significant alcohol intake (see Exclusion Criteria above for details).
Clinical Research Assessment
All participants underwent clinical research assessments at the UCSD NAFLD Research Center (See Supplementary Text for details).
Genotyping
DNA samples were extracted from whole blood samples collected during the clinical research visit. Genotyping was performed by Human Longevity Inc (San Diego, CA, USA).
Primary Outcome
The primary outcome was the presence of shared gene effect between hepatic steatosis and hepatic fibrosis.
Secondary Outcomes
The secondary outcome was the shared gene of hepatic steatosis and fibrosis with the following metabolic risk factors: systolic and diastolic blood pressures, total cholesterol, HDL, LDL, triglycerides, ferritin, glucose, HOMA-IR, insulin, and HbA1c.
Magnetic Resonance Imaging
MRI was performed at the UCSD MR3T Research Laboratory using the 3T research scanner (GE Signa EXCITE HDxt, GE Healthcare, Waukesha, WI, USA) with all participants in supine positions, on the same day as the clinical research visit to reduce potential confounding factors. MRI-PDFF was used to measure hepatic steatosis and MRE was used to measure hepatic fibrosis. MRI-PDFF has been previously shown to be a highly precise, accurate, and reproducible noninvasive biomarker to quantify hepatic fat content.(25, 26), correlates well with MR spectroscopy (r2=0.99, p<0.001)(22, 23), and is superior to noninvasive imaging techniques such as ultrasound and computed tomography for measuring hepatic fat content (27) even in iron-overloaded livers that may coexist with NAFLD livers. (28) MRI-PDFF has also been shown to correlate well with histology from contemporaneous liver biopsies.(29, 30) MRE has been shown to be a highly accurate, noninvasive biomarker to estimate hepatic fibrosis quantified by liver stiffness values in units of kilopascals (kPa), (31) and has been shown to be more accurate than clinical prediction rules (32) and ultrasound-based acoustic radiation force impulse imaging (33) for quantifying hepatic fibrosis. Please see Supplementary Text for a description of the MR procedures.
Justification for not using liver biopsy to assess for hepatic steatosis and fibrosis
Due to the invasive nature of liver biopsies, it would be unethical to perform liver biopsies in study participants with no clinical indications for liver biopsies.(1) Therefore, we used noninvasive imaging techniques to quantify hepatic steatosis and fibrosis. MRI-PDFF have been previously shown to be accurate for estimating hepatic steatosis and more precise than liver biopsies.(25) MRE has also been previously shown to be accurate for estimating hepatic fibrosis.(31–33)
Statistical Analysis
Patients’ demographic, anthropometric, clinical, and biochemical characteristics were summarized. Categorical variables were shown as counts and percentages and associations were tested using a Chi-square test or Fisher’s exact test. Normally distributed continuous variables were shown as mean (± standard deviation) and differences between groups were analyzed using a two independent samples t-test or Wilcoxon– Mann–Whitney test. Odds ratios were derived from generalized estimating equations (PROC GENMOD) to account for intra-pair correlations within twinships. A two-tailed p-value < 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
AE models were used to estimate the shared genetic determination (rG) and shared environmental determination (rE) between twin pairs. In the classical twin study of sets of MZ and DZ twins, four latent factors can account for the variance of any phenotype: additive genetic effects (A); non-additive genetic effects, including dominance (D); common or shared environmental effects (C); and non-shared or individual-specific environmental effects (E) (34). Because MZ twins are presumed to be genetically identical, they correlate perfectly (r = 1.0) with respect to both additive and non-additive genetic effects. DZ twins share, on average, 50% of their genes, resulting in correlations of 0.50 for additive genetic effects and 0.25 for non-additive genetic effects. The C term is defined as environmental factors that make twins similar; hence, common environmental factors correlate 1.0 across twin pairs, regardless of zygosity. The E term represents environmental factors that lead to differences between twins. Because these are individual-specific factors, they are assumed to be uncorrelated across twins. Error is assumed to be random across individuals, so measurement error forms part of the estimate of E in these analyses. These latent factors comprise what are referred to as the univariate ACE or ADE models; due to model under-identification, an ACDE model cannot be tested in the classical twin design (34).
The ACE and ADE models are easily extended to the multivariate case (34). In addition to genetic and environmental sources of variance, sources of covariance can also be examined in the bivariate model. In the present study, we used bivariate models to compute genetic correlations between two phenotypes. A phenotypic correlation measures shared variance; a genetic correlation measures shared genetic variance. More specifically, a phenotypic correlation is defined as the total covariance (genetic plus environmental) of two variables divided by the square root of the product of the total variance of variable 1 and the total variance of variable 2. After decomposing the sources of variance in the bivariate model, we computed genetic correlations. These are defined as the genetic covariance divided by the square root of the product of the genetic variance of variable 1 and the genetic variance of variable 2. The analyses were performed using OpenMx, a structural equation modeling software package for genetically informative data (http://openmx.psyc.virginia.edu). Prior to the model fitting, the measures were adjusted for controlling age, gender and ethnicity. Overall, AE models tended to provide the best fits to the data. Consequently, the genetic effects estimated in these AE models refer to broad-sense heritability, reflecting the proportion of phenotypic variance accounted for by the combined effect of all genetic influences (A+D).
Sample Size Estimation
In previous studies, the heritability of hepatic steatosis ranged from 0.37, when hepatic steatosis was assessed using ultrasound and serum ALT levels,(35) to almost 1.0, when hepatic steatosis was assessed using MRI in obese Hispanic probands and their relatives.(36) We have also previously estimated the heritability of hepatic steatosis and fibrosis to be approximately 0.5.(9) Based on these numbers, we anticipated that the heritability of hepatic steatosis and fibrosis with one another should also be approximately 0.5. It has previously been shown that, to detect an additive genetic component of 0.4 to 0.8 in an ACE model, approximately 36–74 twin pairs are needed to produce a power of 0.95 with an alpha value of 0.05.(37) Therefore, the 65 twin pairs in this study should provide adequate sample size to assess the heritability of steatosis and fibrosis in our population.
RESULTS
Baseline Characteristics
130 participants (45 monozygotic twin pairs, 25 dizygotic twin pairs) who underwent clinical research assessments and imaging with MRI-PDFF and MRE were included in this study. 438 participants were initially assessed for eligibility, 152 provided informed consent, and 130 was included in the final analysis (see Supplementary Figure 1 for details). The mean (±SD) age was 47.1 (±21.9) years and the mean (±SD) BMI was 26.2 (±5.8) kg/m2. 26/130 (20%) of the cohort had hepatic steatosis (MRI-PDFF ≥5%) and 10/122 (8.2%) of the cohort had hepatic fibrosis (MRE ≥3 kPa). Compared to twins without NAFLD, twins with NAFLD were significantly older (54.9 ± 17.3 years vs. 45.2 ± 22.5 years, p = 0.04) and had higher BMI (31.5 ± 4.8 kg/m2 vs. 24.8 ± 5.1 kg/m2, p < 0.0001). As expected, twins with NAFLD also had significantly higher measurements of hepatic steatosis via MRI-PDFF (10.7 ± 5.1 vs. 2.4 ± 0.9, p < 0.0001) and hepatic fibrosis via MRE (3.0 ± 1.2 vs. 2.2 ± 0.4, p < 0.0001). Detailed demographic, biochemical, and imaging data of the cohort, stratified by the presence or absence of NAFLD, are summarized in Table 1.
Table 1.
Baseline characteristics stratified by NAFLD status in the Twin Cohort
| Overall | Twins with NAFLD (MRI-PDFF ≥ 5%) | Twins without NAFLD (MRI-PDFF < 5%) | p-value | |
|---|---|---|---|---|
| N | 130 | 26 | 104 | |
| Demographics | ||||
| Age (years) | 47.1 (21.9) | 54.9 (17.3) | 45.2 (22.5) | .0417 |
| Sex (% male) | 35 (26.9%) | 11 (42.3%) | 24 (23.1%) | .0480 |
| Race | .0510 | |||
| White | 104 (80.0%) | 18 (69.2%) | 86 (82.7%) | |
| Hispanic | 18 (13.9%) | 5 (19.2%) | 13 (12.5%) | |
| Asian | 6 (4.6%) | 1 (3.9%) | 5 (4.8%) | |
| Hawaiian/Pac Islander | 2 (1.5%) | 2 (7.7%) | 0 (0%) | |
| Physical | ||||
| Height (cm) | 165.8 (8.2) | 167.0 (10.7) | 165.4 (7.5) | .4981 |
| Weight (kg) | 72.1 (17.9) | 88.8(20.5) | 67.8(14.3) | <.0001 |
| Body mass index (kg/m2) | 26.2 (5.8) | 31.5 (4.8) | 24.8 (5.1) | <.0001 |
| Systolic blood pressure (mm Hg) | 126.0 (19.6) | 135.5 (16.7) | 123.6 (19.6) | .0052 |
| Diastolic blood pressure (mm Hg) | 78.7 (12.4) | 82.9(13.0) | 77.6(12.1) | .0494 |
| Waist circumference (cm) | 88.8 (12.9) | 100.0(10.6) | 85.9(11.9) | <.0001 |
| Hip circumference (cm) | 99.6 (11.3) | 107.5 (11.6) | 97.7(10.4) | <.0001 |
| Laboratory data | ||||
| Glucose (mg/dL) | 89.3 (18.5) | 101.6 (34.3) | 86.2 (9.5) | .0013 |
| Insulin (U/L) | 8.4(5.5) | 12.6 (7.1) | 7.4(4.5) | .0005 |
| Hemoglobin A1c | 5.8 (0.5) | 6.1(0.7) | 5.7(0.3) | .0011 |
| HOMA-IR | 1.9 (1.4) | 3.1(1.9) | 1.6(1.1) | .0001 |
| AST (U/L) | 23.6 (9.9) | 27.0 (16.6) | 22.7 (7.2) | .1676 |
| ALT (U/L) | 22.9 (18.1) | 35.0(32.8) | 19.8(10.0) | .0008 |
| Alkaline Phosphatase (U/L) | 69.1 (19.6) | 69.7(15.1) | 69.0(20.6) | .6589 |
| Total bilirubin (mg/dL) | 0.5(0.3) | 0.5(0.4) | 0.5(0.2) | .7893 |
| Direct bilirubin (mg/dL) | 0.1(0.1) | 0.1(0.1) | 0.1(0.1) | .4888 |
| Albumin (g/dL) | 4.5 (0.3) | 4.5(0.2) | 4.5(0.4) | .3867 |
| GGT (U/L) | 23.2 (19.9) | 33.1(29.6) | 20.7 (15.7) | .0031 |
| Total cholesterol (mg/dL) | 196.0 (40.8) | 200.6 (36.1) | 194.8 (42.1) | .3330 |
| HDL-cholesterol (mg/dL) | 66.4 (21.7) | 51.0(15.9) | 70.4(21.2) | <.0001 |
| LDL-cholesterol (mg/dL) | 111.0 (34.7) | 119.8 (30.7) | 108.8 (35.5) | .0489 |
| Trigylcerides (mg/dL) | 92.8 (53.3) | 150.7 (71.1) | 78.0 (35.0) | <.0001 |
| White blood cell count (x103/uL) | 5.7 (1.3) | 6.4(1.5) | 5.5(1.2) | .0055 |
| Hemoglobin (g/dL) | 13.7 (1.2) | 14.0 (1.4) | 13.6 (1.2) | .1038 |
| Hematocrit (%) | 40.6 (3.3) | 41.5 (3.8) | 40.4 (3.2) | .0832 |
| Platelet count (x103/uL) | 251.7 (51.5) | 253.0 (57.7) | 251.4 (50.2) | .8753 |
| INR | 1.1 (0.3) | 1.1(0.4) | 1.1(0.3) | .6387 |
| Ferritin (ng/mL) | 100.9 (90.7) | 134.4(144.4) | 92.2(69.2) | .1769 |
| Imaging data | ||||
| MRI-PDFF (%) | 4.034 (4.11) | 10.740 (5.08) | 2.358 (0.87) | <.0001 |
| MRE (Kpa) | 2.327 (0.74) | 3.004 (1.23) | 2.152 (0.40) | <.0001 |
| PNPLA3 phenotype (N=87) | 0.8360 | |||
| CC | 39 (44.8%) | 9 (45.0%) | 30 (47.8%) | |
| CG | 38 (43.7%) | 8 (40.0%) | 30 (44.8%) | |
| GG | 10 (11.5%) | 3 (15.0%) | 7 (10.4%) | |
Mean value are provided with standard deviation in parenthesis, unless otherwise noted as N(%). Differences between participants with and without NAFLD were evaluated with t tests or Wilcoxon Mann–Whitney for continuous variables and chi square or Fishers exact tests for categorical variables.
Abbreviations: NAFLD, non-alcoholic fatty liver disease; HOMA-IR, homeostatic model of insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma- glutamyl transpeptidase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; INR, international normalized ratio; MRI, magnetic resonance imaging; PDFF, proton-density-fat-fraction. Significant p-values <0.05.
Shared Gene Effect
Using AE models, the shared gene effect rG between hepatic steatosis, fibrosis, and metabolic risk factors are summarized below.
Shared Gene Effects between Hepatic Steatosis and Metabolic Risk Factors
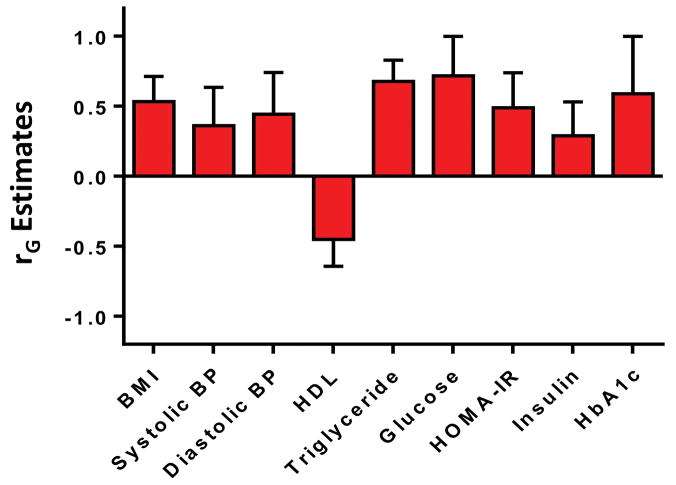
There were significant shared gene effects between hepatic steatosis, as measured by MRI-PDFF, and BMI at 0.534 (95% CI: 0.305, 0.713), p=3.19e-5; systolic blood pressure at 0.360 (95% CI: 0.052, 0.636), p=0.023; diastolic blood pressure at 0.444 (95% CI: 0.444, 0.742), p=0.0071; HDL cholesterol at −0.451 (95% CI: −0.643, −0.216), p=3.57e-4; triglycerides at 0.678 (95% CI: 0.585, 0.830), p=4.69e-8; glucose at 0.716 (95% CI: 0.716, 1), p=1.64e-4; HOMA-IR at 0.490 (95% CI: 0.212, 0.739), p=8.71e-4; insulin at 0.289 (95% CI: 0.017, 0.531), p=0.038; and HbA1c at 0.588 (95% CI: 0.588, 1), p=9.83e-4. There were no significant shared gene effects between hepatic steatosis and total cholesterol, LDL cholesterol, and ferritin (Table 2). Significant shared gene effects between hepatic steatosis and metabolic risk factors are shown in Figure 1a.
Table 2.
AE model for shared genetic determination (pleiotropy) rG between hepatic steatosis, fibrosis, and metabolic risk factors
| Hepatic Steatosis (MRI-PDFF) | Hepatic Fibrosis (MRE) | |||
|---|---|---|---|---|
|
| ||||
| Trait | rG estimate (CIs) | P-value | rG estimate (CIs) | P-value |
|
| ||||
| BMI | 0.534 (0.305, 0.713) | 3.19e-5 | 0.493 (0.493, 0.845) | 0.00649 |
|
| ||||
| Blood pressure* | ||||
| Systolic | 0.360 (0.052, 0.636) | 0.023 | 0.308 (−0.137, 0.688) | 0.160 |
| Diastolic | 0.444 (0.444, 0.742) | 0.0071 | 0.257 (0.257, 0.723) | 0.292 |
|
| ||||
| Total cholesterol | −0.021 (−0.397, −0.014) | 0.903 | −0.299 (−0.839, 0.218) | 0.243 |
|
| ||||
| LDL-cholesterol | 0.076 (−0.134, 0.090) | 0.658 | −0.127 (−0.609, −0.053) | 0.600 |
|
| ||||
| HDL-cholesterol | −0.451 (−0.643, −0.216) | 3.57e-4 | −0.614 (−0.890, −0.614) | 5.74e-4 |
|
| ||||
| Triglycerides | 0.678 (0.585, 0.830) | 4.69e-8 | 0.657 (0.657, 1) | 3.44e-4 |
|
| ||||
| Ferritin | 0.370 (−1, 1) | 0.445 | 1 (−1, 1) | 0.057 |
|
| ||||
| Glucose | 0.716 (0.716, 1) | 1.64e-4 | 0.746 (0.746, 1) | 0.0029 |
|
| ||||
| HOMA-IR | 0.490 (0.212, 0.739) | 8.71e-4 | 0.610 (0.218, 1) | 0.0025 |
|
| ||||
| Insulin | 0.289 (0.017, 0.531) | 0.038 | 0.429 (0.167, 0.735) | 0.023 |
|
| ||||
| HbA1c | 0.588 (0.588, 1) | 9.83e-4 | 0.566 (0.566, 1) | 0.015 |
|
| ||||
| Liver fibrosis (MRE) | 0.756 (0.716, 1) | 2.54e-5 | N/A | N/A |
Significant (p<0.05) coefficients are designated in bold type. rG indicates genetic co-variance that detects the shared genetic determination between two traits.
Abbreviations: MRI-PDFF, magnetic resonance imaging-proton density fat fraction; MRE, magnetic resonance elastography; BMI, body mass index; CI: confidence interval; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; HbA1c, hemoglobin A1c.
Figure 1.
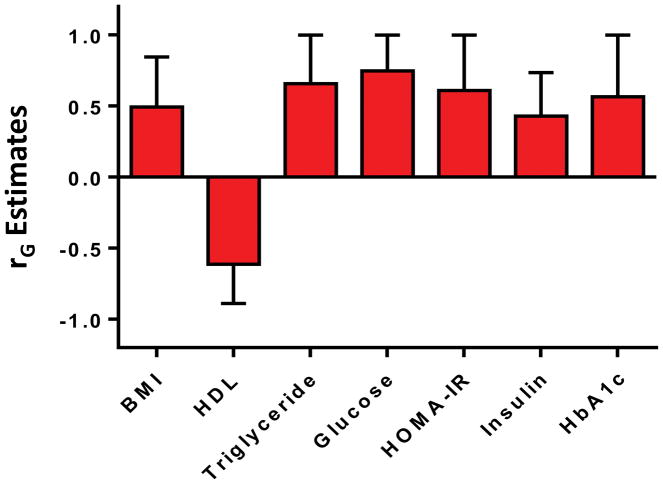
Figure 1a–1b: The significant shared gene effects rG of hepatic steatosis and hepatic fibrosis with metabolic risk factors. Hepatic steatosis (Figure 1a) had significant shared gene effects rG with BMI, systolic blood pressure, diastolic blood pressure, HDL, triglycerides, glucose, HOMA-IR, insulin, and HbA1c. Hepatic fibrosis (Figure 1b) had significant shared gene effects rG with BMI, HDL, triglycerides, glucose, HOMA-IR, insulin, and HbA1c.
Shared Gene Effects between Hepatic Fibrosis and Metabolic Risk Factors
There were significant shared gene effects between hepatic fibrosis, as measured by MRE, and BMI at 0.493 (95% CI: 0.493, 0.845), p=0.00649; HDL cholesterol at −0.614 (95% CI: −0.890, −0.614), p=5.74e-4; triglycerides at 0.657 (95% CI: 0.657, 1), p=3.44e-4; glucose at 0.746 (95% CI: 0.746, 1), p=0.0029; HOMA-IR at 0.610 (95% CI: 0.218, 1), p=0.0025; insulin at 0.429 (95% CI: 0.167, 0.735), p=0.023; and HbA1c at 0.566 (95% CI: 0.566, 1), p=0.015. There were no significant shared gene effects between hepatic fibrosis and systolic blood pressure, diastolic blood pressure, total cholesterol, LDL cholesterol, and ferritin (Table 2). Significant shared gene effects between hepatic fibrosis and metabolic risk factors are shown in Figure 1b.
Shared Gene Effect between Hepatic Steatosis and Fibrosis
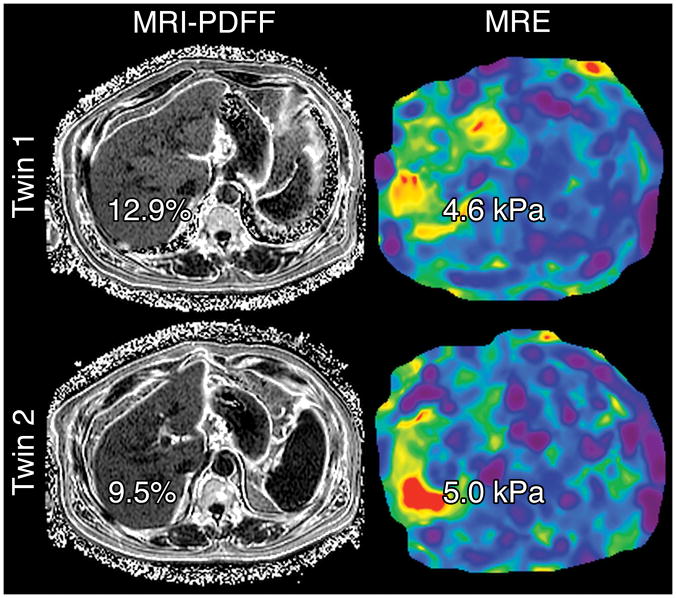
There was a significant shared gene effect between hepatic steatosis and fibrosis at 0.756 (95% CI: 0.716, 1), p=2.54e-5 (Table 2, Figure 2). Figure 3 depicts MRI-PDFF images and MRE elastograms of a representative pair of 60 year old male twins with both hepatic steatosis and fibrosis traits.
Figure 2.
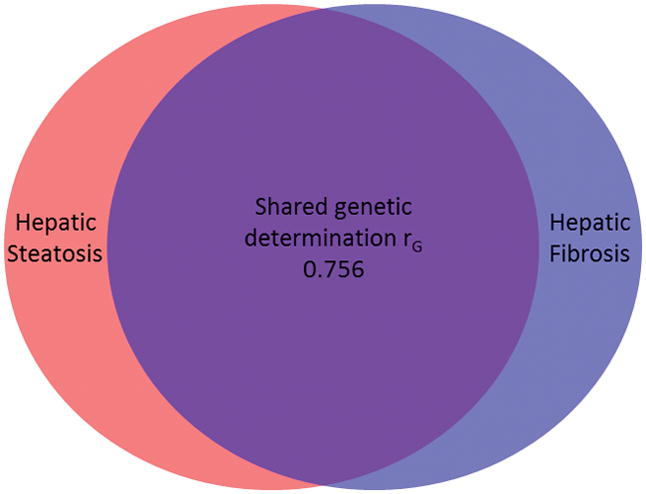
Hepatic steatosis and fibrosis has a shared genetic determination rG of 0.756.
Figure 3.
Representative MRI-PDFF and MRE of a pair of 60 year old male twins concordant for both NAFLD (MRI-PDFF ≥5%) and hepatic fibrosis (MRE >3 kPa). Hepatic steatosis and fibrosis have significant shared gene effects with one another at 0.756 (95% CI: 0.716, 1), p=2.54e-5. Hepatic steatosis also has significant shared gene effects with BMI, systolic blood pressure, diastolic blood pressure, HDL cholesterol, triglycerides, glucose, HOMA-IR, insulin, and hemoglobin A1c, and hepatic fibrosis has significant shared gene effects with BMI, HDL cholesterol, triglycerides, glucose, HOMA-IR, insulin, and hemoglobin A1c.
Shared Environmental Effect
Using AE models, the shared environmental effect rE between hepatic steatosis, fibrosis, and metabolic risk factors are summarized below.
Shared Environmental Effect between Hepatic Steatosis and Metabolic Risk Factors
There was a significant shared environmental effect between hepatic steatosis, as measured by MRI-PDFF, and ferritin at 0.307 (95% CI: 0.019, 0.544), p=0.037. There were no other significant shared environmental effects between hepatic steatosis and other metabolic risk factors (Table 3).
Table 3.
AE model for shared environmental determination (pleiotropy) rE between hepatic steatosis, fibrosis, and metabolic risk factors
| Hepatic Steatosis (MRI-PDFF) | Hepatic Fibrosis (MRE) | |||
|---|---|---|---|---|
|
| ||||
| Trait | rE estimate (CIs) | P-value | rE estimate (CIs) | P-value |
|
| ||||
| BMI | 0.087 (−0.188, 0.352) | 0.0538 | −0.067 (−0.345, 0.226) | 0.654 |
|
| ||||
| Blood pressure* | ||||
| Systolic | 0.177 (−0.098, 0.426) | 0.204 | 0.116 (−0.161, 0.378) | 0.411 |
| Diastolic | 0.181 (−0.104, 0.438) | 0.211 | 0.129 (−0.160, 0.399) | 0.381 |
|
| ||||
| Total cholesterol | 0.178 (−0.128, 0.452) | 0.252 | −0.023 (−0.322, 0.277) | 0.883 |
|
| ||||
| LDL-cholesterol | 0.213 (−0.075, 0.467) | 0.146 | −0.065 (−0.343, 0.223) | 0.660 |
|
| ||||
| HDL-cholesterol | −0.229 (−0.488, −0.069) | 0.129 | 0.030 (−0.279, 0.327) | 0.851 |
|
| ||||
| Triglycerides | 0.236 (−0.045, 0.481) | 0.097 | 0.123 (−0.190, 0.415) | 0.445 |
|
| ||||
| Ferritin | 0.307 (0.019, 0.544) | 0.037 | −0.189 (−0.415, 0.083) | 0.167 |
|
| ||||
| Glucose | −0.070 (−0.332, 0.202) | 0.617 | −0.006 (−0.271, 0.262) | 0.968 |
|
| ||||
| HOMA-IR | 0.015 (−0.271, 0.298) | 0.917 | −0.166 (−0.430, 0.125) | 0.261 |
|
| ||||
| Insulin | 0.088 (−0.211, 0.370) | 0.565 | −0.210 (−0.477, 0.096) | 0.176 |
|
| ||||
| HbA1c | 0.200 (−0.098, 0.460) | 0.185 | 0.089 (−0.195, 0.360) | 0.543 |
|
| ||||
| Liver fibrosis (MRE) | −0.052 (−0.359, 0.278) | 0.758 | N/A | N/A |
Significant (p<0.05) coefficients are designated in bold type. rE indicates environmental co-variance that suggests the shared environmental determination between two traits.
Abbreviations: MRI-PDFF, magnetic resonance imaging-proton density fat fraction; MRE, magnetic resonance elastography; BMI, body mass index; CI: confidence interval; LDL, low-density lipoprotein; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; HbA1c, hemoglobin A1c.
Shared Environmental Effect between Hepatic Fibrosis and Metabolic Risk Factors
There were no significant shared environmental effects between hepatic fibrosis, as measured by MRE, and metabolic risk factors (Table 3).
Predictors of NAFLD in Overall Cohort
In our overall cohort of twins, generalized estimating equations were used to estimate the odds ratios of demographic, anthropometric, and laboratory variables for predicting NAFLD. Significant odds ratios as predictors of NAFLD included weight at 1.07 (95% CI: 1.04, 1.11), p<0.0001; BMI at 1.26 (95% CI: 1.12, 1.43), p=0.0002; BMI > 30 at 6.42 (95% CI: 2.40, 17.16), p=0.0002; systolic blood pressure at 1.03 (95% CI: 1.01, 1.04), p=0.0108; waist circumference at 1.10 (95% CI: 1.05, 1.15), p=0.0002; hip circumference at 1.09 (95% CI: 1.04, 1.14), p=0.0002; glucose at 1.03 (95% CI: 1.01, 1.05), p=0.0132; HbA1c at 4.31 (95% CI: 1.75, 10.65), p=0.0015; HOMA-IR at 1.63 (95% CI: 1.08, 2.44), p=0.0189; ALT at 1.03 (95% CI: 1.01, 1.06), p=0.0104; HDL cholesterol at 0.95 (95% CI: 0.92, 0.97), p=0.0002; triglycerides at 1.03 (95% CI: 1.01, 1.04), p<0.0001; white blood cells at 1.74 (95% CI: 1.26, 2.40), p=0.0008; and ferritin at 1.00 (95% CI: 1.00, 1.00), p=0.0479 (Table 4).
Table 4.
Odds of NAFLD and Fibrosis
| Odds of NAFLD | Odds of Fibrosis | |||
|---|---|---|---|---|
|
| ||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Demographics | ||||
| Age | 1.02 (1.00–1.05) | 0.0832 | 1.05 (0.99–1.11) | 0.1071 |
| Age ≥45 | 2.92 (0.85–10.05) | 0.0894 | 5.78 (0.69–48.21) | 0.1053 |
| Male | 2.75 (0.92–8.24) | 0.0713 | 4.65 (1.09–19.87) | 0.0379 |
| Hispanic | 1.67 (0.47–5.91) | 0.4289 | 3.26 (0.54–19.70) | 0.1971 |
| Anthropometric | ||||
| Height (cm) | 1.02 (0.95–1.09) | 0.6515 | 0.99 (0.90–1.08) | 0.7534 |
| Weight (kg) | 1.07 (1.04–1.11) | <0.0001 | 1.05 (1.02–1.08) | 0.0008 |
| Body mass index (kg/m2) | 1.26 (1.12–1.43) | 0.0002 | 1.21 (1.10–1.32) | <0.0001 |
| BMI > 30 | 6.42 (2.40–17.16) | 0.0002 | 7.72 (1.65–27.39) | 0.0079 |
| SBP (mm Hg) | 1.03 (1.01–1.04) | 0.0108 | 1.04 (1.01–1.08) | 0.0172 |
| DBP (mm Hg) | 1.02 (0.99–1.05) | 0.1533 | 1.04 (1.00–1.08) | 0.0548 |
| Waist (cm) | 1.10 (1.05–1.15) | 0.0002 | 1.08 (1.03–1.13) | 0.0018 |
| Hip (cm) | 1.09 (1.04–1.14) | 0.0002 | 1.04 (1.00–1.08) | 0.0274 |
| Laboratory data | ||||
| Glucose (mg/dL) | 1.03 (1.01–1.05) | 0.0132 | 1.03 (0.99–1.07) | 0.1075 |
| Insulin (U/L) | 1.12 (1.00–1.26) | 0.0563 | 1.10 (0.97–1.24) | 0.1253 |
| Hemoglobin A1c | 4.31 (1.75–10.65) | 0.0015 | 3.48 (1.04–11.61) | 0.0426 |
| HOMA-IR | 1.63 (1.08–2.44) | 0.0189 | 1.70 (1.04–2.79) | 0.0359 |
| AST (U/L) | 1.01 (0.98–1.04) | 0.6153 | 1.04 (1.00–1.09) | 0.0427 |
| ALT (U/L) | 1.03 (1.01–1.06) | 0.0104 | 1.03 (1.01–1.05) | 0.0046 |
| Alkaline Phosphatase (U/L) | 1.00 (0.98–1.01) | 0.5761 | 1.01 (0.99–1.02) | 0.4051 |
| Albumin (g/dL) | 1.02 (0.37–2.85) | 0.9699 | 0.62 (0.09–4.25) | 0.6250 |
| GGT (U/L) | 1.01 (0.99–1.03) | 0.3642 | 1.03 (1.01–1.06) | 0.0175 |
| Total cholesterol (mg/dL) | 1.00 (0.99–1.01) | 0.5128 | 1.00 (0.99–1.02) | 0.6757 |
| HDL-cholesterol (mg/dL) | 0.95 (0.92–0.97) | 0.0002 | 0.90 (0.85–0.95) | 0.0002 |
| LDL-cholesterol (mg/dL) | 1.01 (1.00–1.02) | 0.1528 | 1.01 (0.99–1.03) | 0.3180 |
| Trigylcerides (mg/dL) | 1.03 (1.01–1.04) | <0.0001 | 1.02 (1.01–1.03) | <0.0001 |
| WBC (x103/uL) | 1.74 (1.26–2.40) | 0.0008 | 1.27 (0.80–2.04) | 0.3122 |
| Hemoglobin (g/dL) | 1.45 (0.96–2.19) | 0.0788 | 1.01 (0.64–1.59) | 0.9581 |
| Hematocrit (%) | 1.13 (0.98–1.31) | 0.1025 | 1.01 (0.86–1.18) | 0.9357 |
| Platelets (x103/uL) | 1.00 (0.99–1.01) | 0.4749 | 1.00 (0.98–1.01) | 0.5051 |
| INR | 0.94 (0.35–2.55) | 0.9002 | 10.34 (2.03–52.69) | 0.0049 |
| Ferritin (ng/mL) | 1.00 (1.00–1.00) | 0.0479 | 1.00 (1.00–1.01) | 0.1294 |
Odds ratios derived from generalized estimating equations (PROC GENMOD) in SAS software version 9.4, to account for intra-pair correlations within twinships. A two-tailed p-value < 0.05 is considered statistically significant (in bold type). Abbreviations for table: NAFLD, non-alcoholic fatty liver disease; HOMA-IR, homeostatic model of insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma- glutamyl transpeptidase; LDL, low- density lipoprotein; HDL, high-density lipoprotein; INR, international normalized ratio. Significant p-values <0.10.
Predictors of Fibrosis in Overall Cohort
Using generalized estimating equations, significant odds ratios as predictors of fibrosis in our overall cohort included male gender at 4.65 (95% CI: 1.09, 19.87), p=0.0379; weight at 1.05 (95% CI: 1.02, 1.08), p=0.0008; BMI at 1.21 (95% CI: 1.10, 1.32), p<0.0001; BMI > 30 at 7.72 (95% CI: 1.65, 27.39), p=0.0079; SBP at 1.04 (95% CI: 1.01, 1.08), p=0.0172; DBP at 1.04 (95% CI: 1.00, 1.08), p=0.0548; waist circumference at 1.08 (95% CI: 1.03, 1.13), p=0.0018; hip circumference at 1.04 (1.00, 1.08), p=0.0274; HbA1c at 3.48 (95% CI: 1.04, 11.61), p=0.0426; HOMA-IR at 1.70 (95% CI: 1.04, 2.79), p=0.0359; AST at 1.04 (95% CI: 1.00, 1.09), p=0.0427; ALT at 1.03 (95% CI: 1.01, 1.05), p=0.0046; GGT at 1.03 (95% CI: 1.01, 1.06), p=0.0175; HDL cholesterol at 0.90 (95% CI: 0.85, 0.95), p=0.0002; triglycerides at 1.02 (95% CI: 1.01, 1.03), p<0.0001; and INR at 10.34 (95% CI: 2.03, 52.69), p=0.0049 (Table 4).
DISCUSSION
Main findings
In this study, we utilized a well-characterized, prospective, community-dwelling twin cohort study design to demonstrate that hepatic steatosis and hepatic fibrosis have statistically and clinically significant shared gene effect. This builds on our previous findings that both hepatic steatosis and fibrosis are each individually heritable traits.(9) We also demonstrated significant shared genetic effects between hepatic steatosis and fibrosis and a wide number of metabolic risk factors, including HDL, triglycerides, insulin resistance, and HbA1c. These results suggest a genetic basis underlying the pathogenesis of both hepatic steatosis and fibrosis, and also with metabolic risk factors. This is a paradigm changing finding, as most expert believe that hepatic steatosis is inconsequential and only hepatic fibrosis is associated with worse outcomes, including mortality and liver transplantation.(5) The shared gene effects between hepatic steatosis and hepatic fibrosis suggests that development of hepatic steatosis may itself portend a worse outcome. However, the time horizon for hepatic steatosis to reach these adverse outcomes may be long, and studies with 10 to 20 years of follow-up may be needed to assess these outcomes. It also has implications in developing targeted therapies for the treatment of NASH. It provides a biologic plausibility that reduction of hepatic steatosis over a sustained period of time may also influence the expression of genes associated with fibrosis progression/regression and may be viable target for the treatment of NASH.
In context of published literature
Previous studies have shown that both hepatic steatosis (9, 35, 36) and fibrosis are heritable traits.(9) We build on the results of these previous studies to show additional heritabilities between hepatic steatosis and fibrosis. Additionally, NAFLD has been shown to be associated with metabolic risk factors, (17–20) although it is unknown from these studies the relative contributions of genetic versus environmental factors to these associations. While we have previously demonstrated genetic covariance between NAFLD and metabolic risk factors in a prospective twin study design, gamma-glutamyl transferase was used as a marker of hepatic steatosis, and liver fat content was not measured directly.(21) Additionally, no previous studies have demonstrated genetic covariance between hepatic fibrosis and metabolic risk factors. This is the first study to demonstrate genetic covariance between metabolic risk factors and both hepatic steatosis and fibrosis in a community-dwelling cohort of twins, with accurate quantification of steatosis and fibrosis throughout the liver achieved through the use of non-invasive MRI-based imaging techniques.
There are currently few effective medical therapies to manage NAFLD and its complications. Vitamin E and thiazolidinediones have been shown to improve hepatic steatosis in NAFLD patients.(38–40) However, few treatments have been shown to be effective in reversing NAFLD-associated hepatic fibrosis. The genes PNPLA3 (10, 41) and TM6SF2,(42, 43) have been shown to modify the risks of hepatic steatosis and fibrosis, and other genetic pathways associated with steatosis, fibrosis, and metabolic traits remain to be elucidated. Future identification of these genetic pathways may lead to individualized, targeted therapies that may prevent and/or reverse hepatic steatosis and fibrosis.
Strengths and Limitations
The strength of this study lies in its use of a twin study design that allows for the evaluation of the heritability of steatosis, fibrosis, and metabolic risk factors. The cohort consisted of well-characterized, community-dwelling twins in which twins with conditions such as excessive alcohol use, use of steatogenic medications, viral hepatitis, and secondary causes of steatosis were systemically excluded. The use of MRI-PDFF allowed for detailed mapping and steatosis quantification throughout the entire liver, and the use of MRE allowed for an accurate, non-invasive way to quantify hepatic fibrosis.
However, this study is limited by the lack of biopsy, which remains the gold standard for diagnosing liver steatosis and fibrosis. While biopsies are limited by their interobserver variability and sampling bias, they allow for the diagnosis of lobular inflammation, hepatocyte ballooning, and NASH that cannot be diagnosed non-invasively. However, because it is unethical to perform liver biopsies in normal control patients with no suspicion of NAFLD, a study involving liver biopsies can only be performed if at least one twin has suspected NAFLD. Our use of noninvasive biomarkers instead of liver biopsy to assess hepatic steatosis and fibrosis allowed us to utilize a community-dwelling cohort of patients, rather than pre-selected patients with increased risk of NAFLD. Although MRI-PDFF has been shown to have high inter-reader reproducibility in non-twin studies,(44) and the interobserver variability of MR readings in our study was minimized with only one analyst performing all the image analysis, the general interobserver variability of MR readings in similar twin study designs remain unknown. Additionally, MRI-PDFF has been shown to be highly accurate for mapping hepatic steatosis throughout the entire liver without the sampling variability associated with liver biopsies, and MRE has also been shown to be highly accurate for the diagnosis of hepatic fibrosis, so we believe our noninvasive diagnostic tests can reliably measure steatosis and fibrosis.(31–33)
Implication for future study
In this study, we demonstrate in a prospective, community-dwelling cohort of twins that patients with genetic susceptibility to hepatic steatosis also have genetic susceptibility to hepatic fibrosis. We also demonstrate that both hepatic steatosis and fibrosis have shared genetic effects with metabolic risk factors. Additional studies with larger sample sizes will be needed to identify individual genes or pathways that may be implicated in hepatic steatogenesis and/or fibrogenesis. The identification of these genes may allow for further individualized, targeted therapy that may prevent and even reverse hepatic steatosis and fibrosis.
Supplementary Material
Derivation of cohort
Acknowledgments
Funding Support: The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award. Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303.
Role of Funding Agencies: Funding agencies did not have any role in the design and conduct of the study, collection, management, analysis or interpretation of the data; preparation, review, or approval of the manuscript. There is no conflict of interest.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- MRI-PDFF
magnetic resonance imaging – proton density fat fraction
- MRE
magnetic resonance elastography
- UCSD
University of California at San Diego
- MZ
monozygotic
- DZ
dizygotic
- kPa
kilopascals
- rG
shared genetic determination
- rE
shared environmental determination
Footnotes
Conflict of interests: The authors report no conflict of interests.
Author contributions: Jeffrey Cui – interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission
Chi-Hua Chen – study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission
Min-Tzu Lo - analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission
Nicholas Schork – study concept and design, critical revision of the manuscript, approved final submission
Ricki Bettencourt – analysis and interpretation of data, critical revision of the manuscript, approved final submission
Monica P Gonzalez – data collection, analysis and interpretation of data, critical revision of the manuscript, approved final submission
Archana Bhatt – data collection, critical revision of the manuscript, approved final submission
Jonathan Hooker – data collection, drafting of the manuscript, critical revision of the manuscript, approved final submission
Katherine Shaffer – drafting of the manuscript, critical revision of the manuscript, approved final submission
Karen E Nelson - critical revision of the manuscript, approved final submission Michelle T Long - critical revision of the manuscript, approved final submission David A Brenner - critical revision of the manuscript, approved final submission
Claude B Sirlin – Study concept and design, analysis and interpretation of data, drafting of the manuscript, MRI analysis, critical revision of the manuscript, obtained funding, study supervision, approved final submission.
Rohit Loomba – Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
References
- 1.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–397. e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 7.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 8.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 9.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, Nguyen P, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015;149:1784–1793. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, Cui J, et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic Fatty liver disease. Gastroenterology. 2010;139:1567–1576. 1576.e1561–1566. doi: 10.1053/j.gastro.2010.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speliotes EK, Yerges-Armstrong LM, Wu J, Hernaez R, Kim LJ, Palmer CD, Gudnason V, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet. 2011;7:e1001324. doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, Vogt TF, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anstee QM, Day CP. The Genetics of Nonalcoholic Fatty Liver Disease: Spotlight on PNPLA3 and TM6SF2. Semin Liver Dis. 2015;35:270–290. doi: 10.1055/s-0035-1562947. [DOI] [PubMed] [Google Scholar]
- 15.Zarrinpar A, Gupta S, Maurya MR, Subramaniam S, Loomba R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut. 2015 doi: 10.1136/gutjnl-2015-309456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomba R, Chalasani N. The Hierarchical Model of NAFLD: Prognostic Significance of Histologic Features in NASH. Gastroenterology. 2015;149:278–281. doi: 10.1053/j.gastro.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: A spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40:475–483. doi: 10.1002/hep.20323. [DOI] [PubMed] [Google Scholar]
- 18.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113–119. doi: 10.1111/j.1478-3231.2008.01718.x. [DOI] [PubMed] [Google Scholar]
- 20.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 21.Loomba R, Rao F, Zhang L, Khandrika S, Ziegler MG, Brenner DA, O'Connor DT. Genetic covariance between gamma-glutamyl transpeptidase and fatty liver risk factors: role of beta2-adrenergic receptor genetic variation in twins. Gastroenterology. 2010;139:836–845. 845.e831. doi: 10.1053/j.gastro.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, Cohen BL, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–932. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyd NF, Dite GS, Stone J, Gunasekara A, English DR, McCredie MR, Giles GG, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347:886–894. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 25.Noureddin M, Lam J, Peterson MR, Middleton M, Hamilton G, Le TA, Bettencourt R, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–1940. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeder SB. Emerging quantitative magnetic resonance imaging biomarkers of hepatic steatosis. Hepatology. 2013;58:1877–1880. doi: 10.1002/hep.26543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34 doi: 10.1002/jmri.22580. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bydder M, Shiehmorteza M, Yokoo T, Sugay S, Middleton MS, Girard O, Schroeder ME, et al. Assessment of liver fat quantification in the presence of iron. Magn Reson Imaging. 2010;28:767–776. doi: 10.1016/j.mri.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–29. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–431. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, Valasek M, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui J, Ang B, Haufe W, Hernandez C, Verna EC, Sirlin CB, Loomba R. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–1280. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453–461. doi: 10.1002/hep.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. [Google Scholar]
- 35.Brouwers MC, Cantor RM, Kono N, Yoon JL, van der Kallen CJ, Bilderbeek-Beckers MA, van Greevenbroek MM, et al. Heritability and genetic loci of fatty liver in familial combined hyperlipidemia. J Lipid Res. 2006;47:2799–2807. doi: 10.1194/jlr.M600312-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visscher PM. Power of the classical twin design revisited. Twin Res. 2004;7:505–512. doi: 10.1375/1369052042335250. [DOI] [PubMed] [Google Scholar]
- 38.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 39.Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 40.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, Allison ME, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, Motta BM, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 44.Hooker JCPC, Liao S, Le TA, Chen J, Wolfson T, Middleton MS, Loomba R, Sirlin C. Inter-reader reproducibility of MRI hepatic proton density fat fraction (PDFF) estimation in adults with biopsy-proven NASH. Abstract presentation at ESGAR; Paris. June 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Derivation of cohort