Abstract
Although previous studies have identified several strategies to stimulate regeneration of CNS axons, extensive regeneration and functional recovery have remained a major challenge, particularly for large diameter myelinated axons. Within the CNS, myelin is thought to inhibit axon regeneration, while modulating activity of the mTOR pathway promotes regeneration of injured axons. In this study, we examined NT-3 mediated regeneration of sensory axons through the dorsal root entry zone in a triple knockout of myelin inhibitory proteins or after activation of mTOR using a constitutively active (ca) Rheb in DRG neurons to determine the influence of environmental inhibitory or activation of intrinsic growth pathways could enhance NT-3-mediate regeneration. Loss of myelin inhibitory proteins showed modest enhancement of sensory axon regeneration. In mTOR studies, we found a dramatic age related decrease in the mTOR activation as determined by phosphorylation of the downstream marker S6 ribosomal subunit. Expression of caRheb within adult DRG neurons in vitro increased S6 phosphorylation and doubled the overall length of neurite outgrowth, which was reversed in the presence of rapamycin. In adult female rats, combined expression of caRheb in DRG neurons and NT-3 within the spinal cord increased regeneration of sensory axons almost 3 fold when compared to NT-3 alone. Proprioceptive assessment using a grid runway indicates functionally significant regeneration of large-diameter myelinated sensory afferents. Our results indicate that caRheb-induced increase in mTOR activation enhances neurotrophin-3 induced regeneration of large-diameter myelinated axons.
Keywords: Rheb, mTOR signaling pathway, neurotrophin-3 (NT-3), axonal regeneration, dorsal root ganglion (DRG)
Introduction
Of the sensory axon populations examined large diameter myelinated axons show the poorest regeneration through the dorsal root entry zone (DREZ) and into the spinal cord. Proprioceptive axons are some of the largest diameter sensory axons in the body and express the NT3 receptor trkC. Numerous studies indicate that regenerative failure is most likely due to both reduced intrinsic growth ability by adult neurons and the inhibitory environment within the spinal cord. Spinal cord application of neurotrophin-3 (NT-3) is thought to enhance the intrinsic growth state of adult DRG neurons, as well as modify the extrinsic environment (Hanna-Mitchell et al., 2008) and promote some but limited amount of sensory afferent regeneration across the dorsal root entry zone after dorsal root rhizotomies (Ramer et al., 2001; 2002). Previous studies indicated that NT-3-induced regeneration is sufficient to bypass the gliotic region at the DREZ, but regeneration fails when axons encounter degenerative domain containing myelin debris (Ramer et al., 2001). Other studies have also shown that soluble Nogo receptor can promote regeneration of sensory afferents across the DREZ (Harvey et al., 2009); however, this regeneration was limited primarily within the dorsal columns.
On the other hand, intrinsic activation of mTOR by deletion of an upstream negative regulator PTEN increased axonal regeneration of retinal ganglion neurons and cortical neurons. Likewise, inhibition of mTOR with rapamycin blocked regeneration, suggesting that modulation of mTOR activity affects intrinsic axonal growth capacity within the CNS (Park et al., 2008). mTOR is a critical regulator of protein synthesis and plays an important role in cell growth, proliferation, and survival during development (Martin and Hall, 2005; Sarbassov et al., 2005). Previous studies have suggested that mTOR expression level is correlated with increased growth capacity (Abe et al., 2010; Park et al., 2008). mTOR activity is upregulated in DRG neurons after sciatic nerve lesion (Belin et al., 2015) and downregulated in retinal and cortical neurons after lesion (Park et al., 2008, Liu et al., 2010). In cultured DRG neurons, following PTEN inhibition and neurite growth induction, rapamycin did not completely block increased neurite outgrowth, suggesting other mechanisms by which PTEN knockdown might affect regenerative growth beyond mTOR activation alone (Christie et al., 2010). Ras homolog enriched in brain (Rheb) is a direct activator of mTOR and was shown to activate the mTOR pathway in many studies (Bai et al., 2007; Inoki et al., 2003; Long et al., 2005). To date, it is not reported whether direct and specific activation of mTOR pathway can affect axonal growth capacity after central branch injury in DRG neurons.
In this study we attempted to augment NT-3 mediated regeneration of large diameter myelinated axons using two approaches. The first was to overexpress NT-3 in the spinal cord of Nogo/MAG/OMgp triple knockout mice to determine if the elimination of extrinsic, myelin-associated inhibitors would augment NT-3 mediated primary afferent regeneration. In the second part we expressed a constitutively active form of Rheb to activate mTOR to determine if further enhancement of intrinsic growth would enhance NT-3-mediated proprioceptive sensory axon regeneration through the DREZ and into the spinal cord. Here, we observed the loss of myelin-associated inhibitors to have only a modest effect on enhancing regeneration in the presence of NT-3. However, expression of ca-Rheb in DRG neurons induced greater than a 2 fold increase in NT-3 mediated regeneration.
Material and methods
Lentiviral vector construction and production
The wild-type Rheb (wtRheb) lentivirus vector was generated by PCR subcloning (the primers: 5’-AGCTCTAGAGCCATGGAGCAGAAGCTGATC-‘3;3’-AGCACCGGATATCACATCACCGAGCACGA-‘5) from pRK5-myc-wtRheb (kindly provided by Karyn Esser, University of Kentucky, Lexington, USA) into the XbaI/AgeI sites of pCSC-SP-PW vector. The constitutively active mutant RHEB (caRheb) was obtained from Zhigang He (Harvard University, Boston, USA). To generate the caRheb lentiviral vector, PCR amplified 3HA-caRheb (the primers: 5’-CCAGCTCTAGAATGTACCCATACGATGTTCCAG-‘3; 3’-AGCACCGGTATATCACATCACCGAGCACGA-‘5) and the fragment was ligated to XbaI/AgeI sites of pCSC-SP-PW vector. The pCSC-SP-PW vector contains the cytomegalovirus promoter to drive the expression of either wtRheb or caRheb. The successful recombination of these two vectors were screened by restriction enzyme analyses and verified by DNA sequencing (GENEWIZ, South Plainfield, NJ).Viral stocks were generated by calcium-phosphate transfection of 293T cells with plasmid encoding either Rheb,NT3 or GFP, Mb1, Rev and VSV-G plasmid encoding envelope glycoprotein. The supernatants were collected 72 hours after transfection and concentrated by ultracentrifugation and the viral pellet was aliquoted and stored at −70 °C until further use. The titers of Rheb, NT3 and GFP lentivirus were measured by using Aalto p24 ELISA DIY Kit (Aalto, Ireland).
AAV vector construction and production
Recombinant adeno-associated virus 2 (AAV2) carrying GFP or caRheb (kindly provided by Zhigang He, Harvard University, Boston, USA) was generated by helper virus-free system (Ayuso et al., 2010). 293T cells were grown to 70–80% confluence at which point they were transfected with two packaging plasmids using PEI (Polyethylenimine, linear, MW-25k, Warrington, PA): one carrying the AAV rep, cap genes and another helper plasmid carrying the adenovirus helper functions. Three days after transfection, the cell lysates and the supernatant were harvested and 40% PEG 8000 was added to precipitate crude virus for 2 hours. Then the viruses were purified by double-centrifugation with cesium chloride (CsCl) and the isolated virus was dialyzed in 0.1M PBS/5% sorbital overnight (Ayuso et al., 2010; Liu et al., 2014). The titers of AAV-GFP and Rheb used in this study were determined by infecting fibroblast cells. The use of fibroblast cells allows for AAV2 vector to induce rapid transgene expression. Serial dilutions of AAV2-GFP and Rheb vectors were added to a 24-well plate, which had been seeded with 0.5 × 105 fibroblast cells per well. After one day, fibroblasts cells expressing GFP or HA with staining were counted under the fluorescence microscope to determine the vector titer per ml and the genomic copy titer/mL (GC/mL). The viral titer of AAV2-GFP and AAV2-Rheb was found to be 1.6 × 1012 and 1.2 × 1012 GC/mL, respectively.
Adult sensory neuron culture, transduction and immunocytochemistry
Adult female Sprague Dawley rats (150–200g, Harlan) were killed and DRGs were dissected. Following a 60 min digestion in 0.125% collagenase at 37°C, the DRGs were mechanically dissociated by trituration and centrifuged through a 12.5%/28% Percoll gradient. The dissociated neurons were resuspended in Neurobasal A medium with B27 supplement and plated on poly-d-lysine coated glass coverslip (0.1 mg/ml), (Smith et al., 2013). Six hours after plating, DRG cultures were infected with 1 × 105 units of lentivirus per well in 12-well plate. For these studies, lentivirus was used because it induces rapid transgene expression and labels multiple DRG neuronal subpopulations (Yu et al., 2011). For mTOR inhibition experiment, rapamycin (Rap, 50 nM) was added to the culture medium after one day in vitro. For immunocytochemistry, the neurons were fixed with 4% PFA for 30 min after 72 h in culture and were blocked with 10% goat serum, 1% BSA, 0.3% Triton X-100 in PBS for 30 min and then were incubated with a chicken anti-Neurofilament-medium (NF-M) antibody (1: 1000, Neuromics, Edina, MN) and rabbit anti-phosphorylated S6 ribosomal protein (1:100, Cell Signaling) at 4°C overnight. The samples were rinsed three times 15 minutes each, incubated with Texas red-anti-rabbit IgG (1:200, Jackson ImmunoResearch, West Grove, PA) and AMCA-anti-chicken IgG (1:200, Jackson ImmunoResearch, West Grove, PA) at room temperature for 90 min. The neurons were identified by NF-M labeling and the cells that were either GFP/NF-M positive or Myc or HA/NF-M positive were imaged using a Zeiss LSM5 microscope system. For quantification of neurite outgrowth, the length of the neurite for the first 150–200 neurons encountered was determined using Image J analysis software.
Western blot
The same treatment was performed on 293T cells, U373 cells, DRG neurons or tissues harvested from E14 embryo, P1, P5, P18 and adult rats. The cells or tissue was lysed in 0.5% Triton X-100, 3% SDS, 50 mM Tris-HCl, pH 7.4, 150mM NaCl, and 1mM EDTA containing protease inhibitors and phosphatase inhibitor. Equal quantities of total protein were loaded onto a 10% polyacrylamide gel and transferred to a nitrocellulose membrane (Amersham Pharmacia). After blocking in 5% milk for 1 hour at room temperature, the blots were incubated with anti-pS6 (1:1000, Cell Signaling), anti-β actin (1:500, Santa Cruz Biotechnology), anti-GAPDH (1:1000, Cell Signaling), anti-Rheb (1:500, R&D systems), anti-HA (1:500, Roche) or anti-Myc (1: 200, DSHB) overnight at 4°C. The corresponding conjugated second antibodies donkey anti-rabbit IRDye-800CW and goat anti-mouse IRdye-680CW (1:20,000; Invitrogen) were added the next day and incubated for 1 h at room temperature. Fluorescent blots were imaged on the Odyssey Infrared Imaging System (LI-COR Biosciences). For quantitation of bands, the blots were scanned on a two-channel infrared Odyssey scanner, the band intensities were quantified using the Odyssey software (LI-COR Biosciences) and normalized to GAPDH levels. Experiments were performed in three individual replicates.
Animals
A total of sixty adult female Sprague Dawley rats (175–200g) (Harlan) were used in this study. All surgical interventions and postoperative animal care were provided in accordance with the guide for the care and use of laboratory animals, and the guidelines for rodent survival surgery provided by the Animal Care and Use Committees of Temple University.
Congenic Nogo/OMgp/MAG triple knockout mice were generated by breeding congenice lines of Nogo/OMgp double knockout mice obtained from Dr. Binhai Zheng (University of California at San Diego) and of MAG knockout mice obtained from Dr. Jae K. Lee (University of Miami). 2–3 months old, both sexes of Nogo−/−/OMgp−/−/MAG−/− mice and age-matched C57BL/6J mice (The Jackson Laboratory) were used. Mice were genotyped at the time of weaning by tail clipping and PCR analysis using the primers in earlier study (Lee et al., 2010).
Surgical procedures
The rats were anesthetized by intraperitoneal injection of ketamine (67mg/kg) and xylazine (6.7 mg/kg). A hemilaminectomy was performed under aseptic conditions at the T13-L2 vertebral segments and the dura was opened with fine scissors. Dorsal roots L3–L6 were identified and exposed. With the use of #5 Dumont forceps, triple-crush lesions of 10 seconds were inflicted at two sites separated by 3mm along the right L4 and L5 afferents at 5–8 mm from dorsal root entry zone (DREZ). Dorsal roots L3 and L6 were tightly ligated at two regions, 1–2mm apart with 6.0 silk suture and then a complete cut was made through these roots to create a zone of denervation immediately rostral and caudal to L4/L5 (Romero et al., 2001; Tang et al., 2004). All lesions were performed unilaterally. The spinal cord at T13-L1 was exposed to identify the right L4 and L5 DREZ. Eight injections of 0.3µl lentivirus spaced 0.5 mm apart rostro-caudally along the DREZ at L4–L5 spinal segments (a total of minimum 1 × 107 viral particles), were delivered using a beveled glass micropipette pulled to a diameter of between 30 and 50 µm. Injections were made at a rate of 5 nl/sec using a nano-injector (World Precision Instruments, Inc., Sarasota, FL) at a precise depth of 0.7mm from the spinal cord dorsal surface using the coordinates on a M3301 fine micromanipulator (Narishige via World Precision Instruments). In order to label sensory axons with AAV-GFP and concurrent expression of Rheb, the AAV vectors were prepared by mixing either AAV2-Rheb with AAV2-GFP viruses at a 1:1 ratio. Two microliters of viral vector was injected into DRGs. DRG injections were performed using a nanoliter injection system (Nanoliter 2000, World Precision Instruments) mounted on a micromanipulator. Beveled tips with diameters of 20 to 40µm were used for this purpose. AAV vectors were injected at a rate of 0.10 µl /min using a Micro4 microsyringe pump controller (World Precision Instruments) and the pipette was left in place for 5 min before removal to allow viral fluid to distribute. Care was taken to avoid excess trauma to the DRG. Immediately following the injection, triple dorsal root crush 10 seconds each, repeated three times) was performed on left lumbar dorsal roots (Liu et al., 2014). The muscle was closed in layers and the skin was closed with staples. After surgery, the rats were placed on a temperature controlled heating pad to allow the animal to recover.
The survival surgery of mice was conducted as described before (Di Maio et al., 2011). Briefly, L3–S1 spinal cord segments were exposed by hemilaminectomy. A small incision was made in the dura overlying the L5 dorsal root near the L4 DRG. A fine forceps (Dumont #5) was introduced subdurally, and the L4 and L5 dorsal root was crushed for 10 seconds. For lentivirus injections, a glass pipet filled with NT3-lentivirus and attached to a micromanipulator (Nano2000 microinjector, World Precision Instruments) was lowered to the L4 and L5 DREZ and inserted into the dorsal horn. A total of five injection sites spanning rostro-caudally along the L4/L5 DREZ were injected, and each site received 120 nl of lentivirus. Infusions were made at a rate of 100 nl/min. After injection, the glass pipette was left in place for an additional 3 minutes before being slowly retracted. A piece of thin synthetic matrix membrane (Biobrane, Bertek Pharmaceuticals) was tightly applied over the exposed spinal cord to minimize scar accumulation on the dura. The musculature and skin were closed with sterile 5-0 sutures and wound clips, respectively. After surgery, mice were given subcutaneous injections of lactated Ringer’s solution to prevent dehydration, and kept on a heating pad until fully recovered from anesthesia. Buprenorphine was given as postoperative analgesia (0.05 mg/kg, subcutaneously every 12 hr for 2 days).
CTB labeling and sciatic nerve injections
To identify regeneration of large caliber myelinated axons, a 2% solution of alexa fluor labeled (594) cholera toxin B (CTB) (Invitrogen) in PBS was injected into all animals 7–10 days prior to sacrifice. For these studies a small incision was made in the skin along the posterior thigh to expose the gluteus muscle. The muscle was separated to expose the sciatic nerve. The sciatic nerve was injected with 2 – 3 ul of 2% cholera toxin B subunit using a Hamilton syringe with a 30 gauge needle.
Tissue processing and immunohistochemistry
Four weeks after dorsal root crushes, animals were sacrificed by injection of Fatal-Plus (Dearborn, MI) and perfused transcardially with PBS, followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4). For pS6 expression study of non-injured animals, DRGs were harvested from adult and postnatal 2 days Sprague Dawley rats (Harlan). The spinal cords with attached dorsal roots and DRGs were removed, post-fixed in 4% PFA at 4°C overnight and moved to 30% sucrose in 0.1 M PBS at 4°C for 2–3 days. Tissue blocks were embedded in M-1 Embedding Matrix (Kalamazoo, MI) and quick frozen on dry-ice. The spinal cords with attached DRGs were transversely sectioned at 16 µm using a cryostat and mounted directly on slides (Superfrost Plus; VWR International). For staining, the sections were permeabilized and non-specific antigenic sites blocked with 0.3% Triton X-100/10% normal goat serum in 0.1M PBS for 30 min at room temperature and then incubated with rabbit anti-phosphorylated S6 ribosomal protein (1:100, Cell Signaling), mouse anti- βIII tubulin antibody (1:1000, Promega), mouse anti-Neurofilament-200 (NF-200) antibody (RT-97, 1:200, DSHB), mouse anti-Myc antibody (1: 200, DSHB), mouse anti-HA antibody (1:200, Roche), rabbit anti-laminin antibody (1: 100, Sigma) at 4°C overnight. In order to increase GFP signals, some sections were stained with a mouse-anti-GFP antibody (1:500, Molecular Probes, Eugene, OR). In most cases, the sections were viewed directly for GFP fluorescence. The samples were rinsed three times, incubated with Texas red-anti-rabbit, Texas red-anti-mouse, FITC-anti-rabbit IgG, FITC-antimouse IgG or AMCA-anti-rabbit IgG (1:200, Jackson ImmunoResearch, West Grove, PA) at room temperature for 90 min. After staining, sections were coverslip-mounted with Fluoromount-G (Southern Biotech, San Diego, CA) and photographed under a Nikon microscope. To examine axonal regeneration through the DREZ, we counted the number of GFP or CTB labeled axons crossing the DREZ.
Microscopy and image analysis
Images from CTB labeled sections were acquired using confocal microscopy (Nikon C2). Z stacked images were acquired at 564 nm. Images were captured at both 10× and 20× magnifications to allow a clear representation of regenerating axons. To quantify the axon growth into the spinal cord, 5–6 sections within L4/L5 spinal cord were selected randomly from each animal. CTB axon occupying area was quantified as the total area occupied in the ipsilateral side using thresholded intensity measurements employing the image analysis software (NIS elements). Program computed the area measurements in µm2.
For quantitative analysis of axons crossing the DREZ in mouse, digital images were captured from three transverse sections taken randomly from L4 and L5 segment of each of three mice. A raw image was converted to a binary image using ImageJ with a threshold that appropriately separates GFP and background fluorescence. The CNS:PNS interface was defined by GFAP immunostaining and considered as the entry point of an axon that penetrated into the CNS. Lines were drawn at 100 µm and 200 µm beyond the entry point and the number of intersections of GFP-labeled axons was counted. The number of axons crossed the interface was normalized by the number of GFP-labeled axons in the PNS, counted at 100 µm before the interface in the peripheral root, which was then averaged by the number of evaluated sections and animals. This quantification resulted in the ‘axon number index’ to indicate the relative number and distance of axons that regenerated into the CNS in each group of mice. One-way ANOVA was used. Values of p<0.05 were considered significant.
Behavioral tests
Mechanical Hyperalgesia
The paw pressure test was used to measure mechanical hyperalgesia, as described by Randall and Selitto (Randall and Selitto, 1957). Briefly, animals were restrained in a mitt such that the hind paws could hang free. Each paw, in turn, was placed between the apparatus surface of an Ugo-Basil Analgesymeter and a plastic point. Increasing weight was applied to the point by means of an attached metal disc. Pressure to the paw was applied at thirty two grams per second. The end point was designated as vocalization or the pulling of the hindpaw from the apparatus, with a cutoff of 200 grams. Responses were recorded three times for both hindpaws.
Gridwalk test
A custom made horizontal ladder with irregularly spaced rungs was used to test for proprioception. Animals were trained to walk on the horizontal ladder with no errors before they were subjected to any surgery. The percent efficiency in the right hindlimb was evaluated as a measure of proprioception. Before the surgery, the trained animals show 100% efficiency in right hind limb (no errors). Immediate post-crush evaluation showed a tremendous increase in foot faults or errors on the ipsilateral (right) side of lesion causing a drop in efficiency. Improvement in proprioception is expected to increase the efficiency in the right hindlimb.
Results
Nogo/MAG/OMgp triple knockout mice show little enhancement of NT-3 mediated sensory afferent regeneration
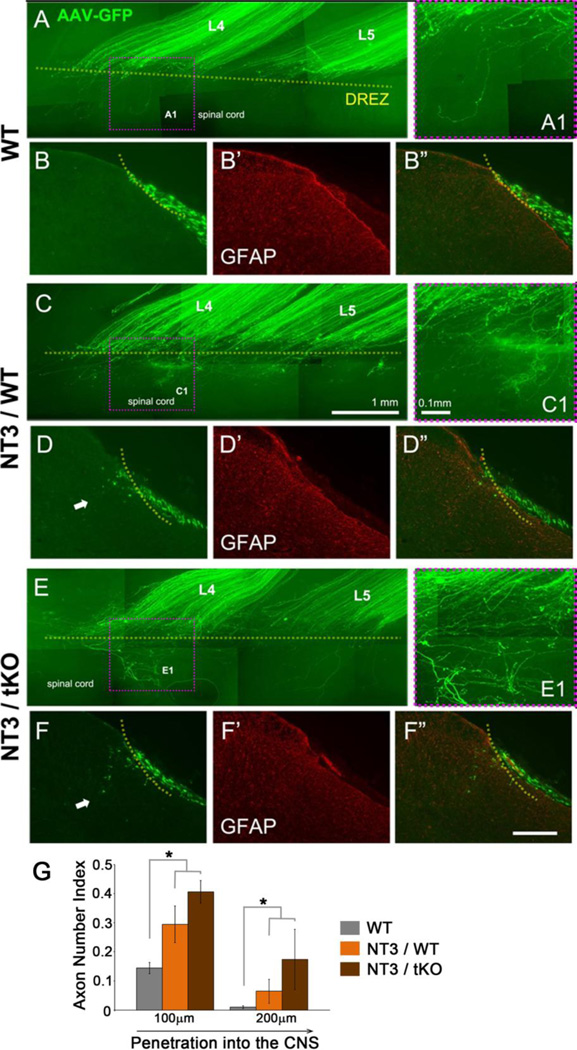
Previous studies have suggested that degenerating myelin acts as a secondary inhibitory barrier to NT-3-mediated regeneration of large diameter myelinated sensory afferents (Ramer et al., 2001). Nogo/MAG/OMgp triple knockout mice were used to examine if reduction in myelin-inhibitory proteins could enhance NT-3 mediated sensory afferent regeneration. Dorsal root injury in wildtype mice show no regeneration of sensory afferents through the DREZ and into the spinal cord as assessed in whole mount (Figure 1A, A1) or transverse sections through the spinal cord (Figure 1B). Interestingly, no enhancement of sensory afferent regeneration was observed in the untreated Nogo−/− MAG−/−OMgp−/− tKO mice (not shown; Zhai et al., in preparation). Expression of NT-3 within the spinal cord of wildtype mice induced minimal axon growth through the DREZ at 2 weeks after rhizotomy (Figure 1C, D). Expression of NT-3 within the spinal cord of Nogo−/−MAG−/−OMgp−/− tKO mice modestly enhanced regeneration, exhibiting deeper penetration of some axons across the DREZ (Figure 1E, F). Most axons however fail to regenerate through the DREZ similar to those in NT-3 treated wildtype mice. Quantitative analysis also show no significant difference between the WT and tKO mice expressing NT-3 (Figure 1G). This data indicates that myelin inhibitors play a minor role in the inhibition of NT-3 mediated regeneration through the DREZ. This data demonstrates axonal regeneration through the DREZ is only minimally influenced by the loss of myelin inhibitors, even in the presence of NT-3, indicating this extrinsic inhibitory pathway plays a minor role in preventing axon regeneration across the DREZ.
Figure 1. Sensory axons regeneration into the spinal cords of Nogo−/−MAG−/−OMgp−/− tKO mice show modest if any enhancement with expression of NT-3.
The L4 and L5 dorsal roots of Nogo−/−MAG−/−OMgp−/− tKO or C57Bl/6 control mice were crushed and analyzed in wholemounts (A, C, E) or in transverse sections (B, D, F), two weeks after rhizotomy. Regenerating axons were labeled by AAV-GFP injected into the DRG at the time of rhizotomy. The DREZ was identified by staining astrocytes with GFAP antibody (B’, D’, F’). A, B: In wildtype mice, almost all the labeled axons that regenerated along the L4 and L5 roots failed to cross the DREZ. C, D: Expression of NT-3 in the spinal cord of wildtype mice enabled some axons to penetrate the DREZ (e.g., arrow in D). E, F: Expression of NT-3 in the spinal cord of Nogo−/−MAG−/−OMgp−/− tKO mice enhanced regeneration only modestly. Although some axons regenerated slightly deeper within the CNS (e.g., arrows in F), most of the axons terminate their regeneration at the DREZ, similar to NT-3 treated wildtype mice. G: Quantifications of the axons that regenerated into the CNS, counted at different distances from the DREZ and normalized against the number of labeled axons in the PNS. Three random sections from each L4 and L5 segment were quantified (n=3 mice/group). Error bars indicate SEM. * p< 0.05, Bar in F”, 200µm.
mTOR activity declines with development of cortical and DRG neurons
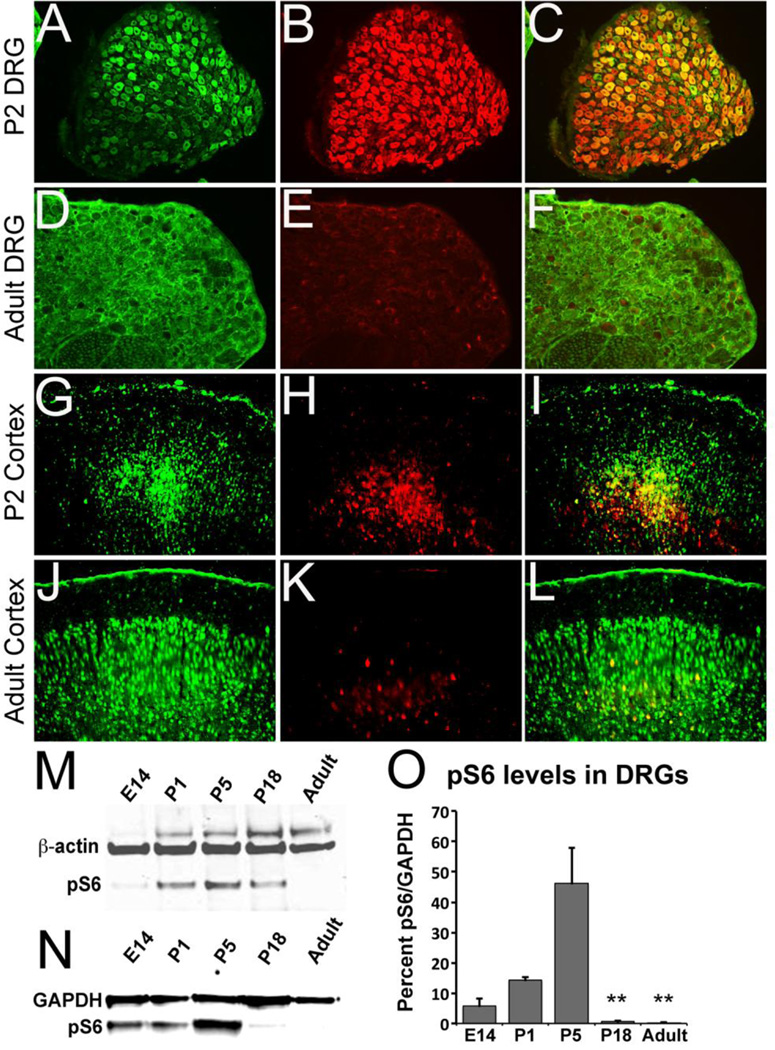
Recent studies show mTOR activity to be associated with enhanced regeneration in adult mouse CNS and PNS neurons (Abe et al., 2010; Park et al., 2008). However, the expression pattern of mTOR activity in developing and adult neurons in rat has not yet been reported. The activation of mTOR leads to phosphorylation of S6 (pS6) ribosomal protein. Antibodies directed against pS6 can therefore be used as an indicator of mTOR activity. To examine the development changes in mTOR activity, we performed immunohistochemical analysis on sections of rat DRG and cortex at two time points, postnatal day 2 (P2) and adult rat. We observed strong pS6 labeling in P2 rat DRG (Figure 2A–C) and cortical neurons (Figure 2G–I), but diminished labeling in adult DRG (Figure 2D–F) and cortical neurons (Figure 2J–L). Western blot analyses of pS6 in embryonic, postnatal and adult ages showed that pS6 levels were strongly upregulated in early postnatal rat DRGs (Figure 2M) and cortical neurons (Figure 2N) and a decrease with age. Quantitation of Westerns blots demonstrated a precipitous drop in pS6 levels in DRGs between the ages of P8 and P14. Overall statistical evaluation by one-way ANOVE demonstrates a significant development difference F(4,10)=24.58, p<0.0001.
Figure 2. Developmental activation of mTor pathway by surveillance of ribosomal subunit S6 phosphorylation.
A–F: Transverse sections of normal postnatal day 2 DRG (A–C) and adult DRG (D–F) showing NF-M (A, D) and pS6 (B, E) staining and merged images (C, F). G–L: Coronal section of cortex from postnatal day 2 (G–I) and adult rats (J–L) showing NeuN (G, J) and pS6 (H, K) staining and merged images (I, L). Western blot analysis of pS6 expression in embryonic day 14 (E14), postnatal day 1 (P1), 5 (P5), 18 (P18) and adult rat DRGs (M), and cortical tissues (N). DRG or cortical tissues were homogenized and lysates were analyzed using rabbit anti-pS6 antibody with β-actin as loading control. Quantitative analysis of pS6 bands shows a significant decrease in density between P1 and P18 or Adult and P5 and P18 or Adult (** p<0.05). Scale Bars: 500 µm (A–F) and 1000 µm (G–L).
mTOR is expressed in DRG neuronal cell bodies but not their axons
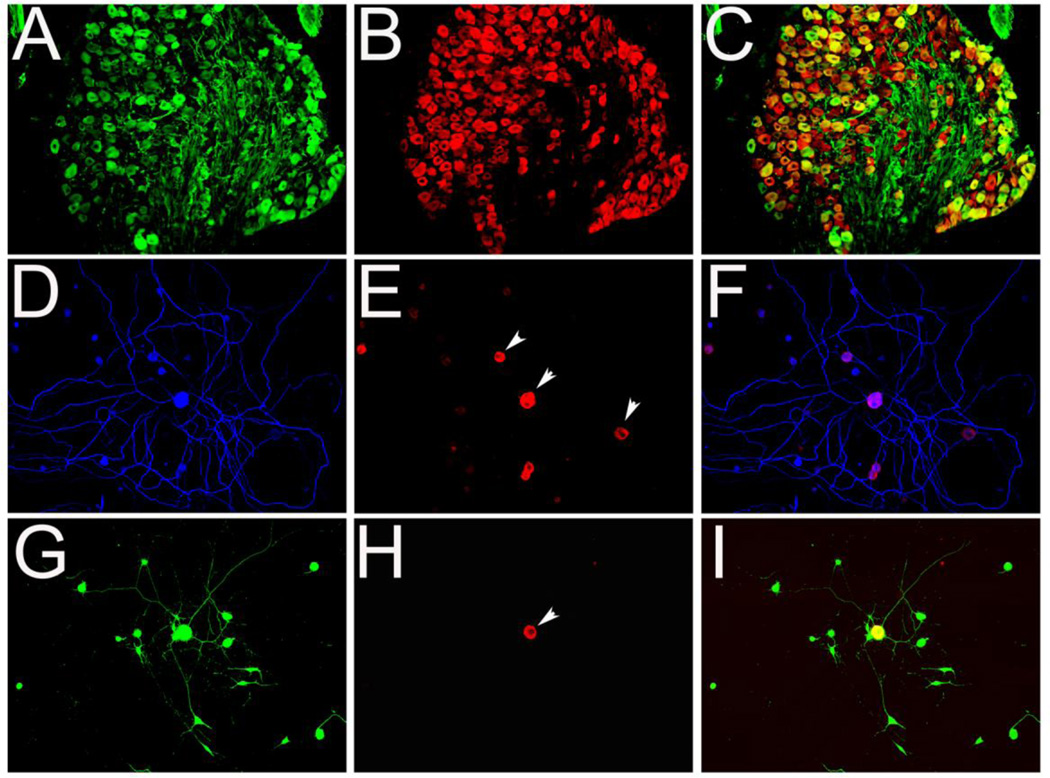
Some reports have indicated that protein synthesis in the axon is required for efficient growth cone regeneration (Nie et al., 2010), a prerequisite for effective axon regeneration (Bradke et al., 2012). Since mTOR controls protein synthesis, we examined whether its downstream effector, phosphorylated pS6 is expressed in axons and growth cones. We labeled P2 rat DRG tissue with pS6 and found pS6 was exclusively localized to the neuronal body, but not axons, or non-neuronal cells (Figure 3A–C). To differentiate between neurons and non-neuronal cells we used Neurofilament-M (NF-M) antibody, a neuronal specific marker, and lentivirus GFP, which is expressed in neurons and non-neuronal cells. Likewise, cultured DRG neurons stained for pS6 showed label restricted to neuronal cell bodies of NF-M positive neurons (Figure 3E and H, arrows) or to non-neuronal cells (Figure 3D–I, arrowheads). No labeling of pS6 was observed in axons.
Figure 3. pS6 is expressed in DRG neuronal cell body, but not observed in axons.
A–C: DRG from P2 rat immunolabeled with anti-NF-M (A) and pS6 (B) antibodies and images merged (C). Dissociated neurons and glia isolated from P2 rat DRGs were transduced with lentivirus vector to express GFP (G, I). Three days after viral transduction, cells were fixed and stained for pS6 (E, F, H, I) and NF-M to label neuronal cell bodies and axons (blue in D, F). Only neuronal bodies were labeled by pS6 (arrowheads in E and H), whereas, GFP (G) and NF-M labeled both cell bodies and neurites. Scale bar, in A–C, 500 µm; in G–I, 300 µm.
Rheb activates mTOR pathway in 293T cells and DRG
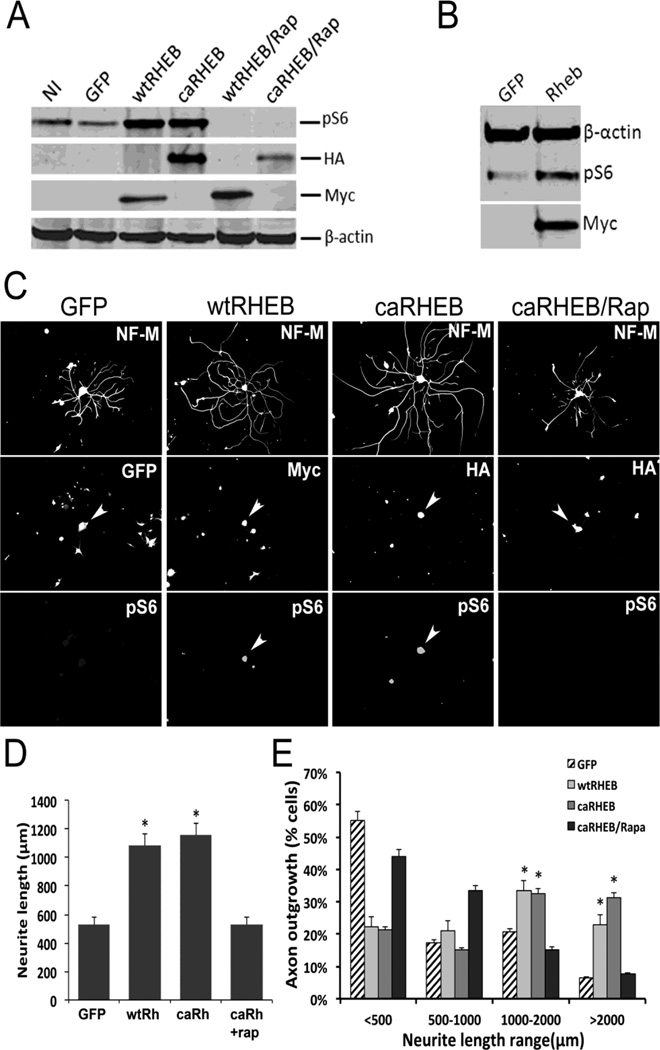
To test whether over-expression of Rheb could upregulate mTOR activity in vitro, we constructed a Myc tagged wild-type (wtRheb) and a HA tagged, constitutively active mutant Rheb (caRheb). We used lentiviral vector to overexpress Rheb constructs in 293T cells. We first tested the functions of both constructs to activate mTOR by examining pS6 in these cells. Three days following viral transduction, Western blot analyses revealed higher levels of pS6 in wtRheb and caRheb-infected cells than in GFP-infected or non-infected (NI) cells. Application of rapamycin (50 nM), an mTOR inhibitor, completely blocked S6 phosphorylation (Figure 4A), confirming that Rheb increased pS6 levels through activation of mTOR (Figure 4A). To determine whether Rheb could induce activation of mTOR in DRG neurons, we transduced cultured adult DRG neurons using wtRheb-lentivirus. We found wtRheb transduction of these neurons was capable of increasing phosphorylation of S6 ribosomal protein (Figure 4B).
Figure 4. Rheb enhances neurite outgrowth through activation of mTOR signaling pathway.
A: 293T Cells were transduced with lentivirus expressing GFP, Myc tagged wtRheb construct, or HA tagged caRheb. Non-infected (NI) cells were used as negative control. Twenty-four hours after viral transduction, rapamycin (Rap, 50 nM), an mTOR inhibitor, was added into the medium. Two days after viral transduction, cells were harvested for Western blot analysis. B: The DRG neurons isolated from P2 rat were transduced with lentivirus expressing GFP or Rheb. Western blot analysis of cell lysates of GFP or Rheb transduced DRG neurons were examined for expression of pS6 level and actin for a loading control. Myc antibody was used to identify wtRheb expression. C: Adult DRG neurons transduced with lentiviruses expressing GFP, wtRheb or caRheb were cultured on poly-D-lysine-coated plates. After 72 hours, cells were fixed for immunofluorescence staining: NF-M antibody was used to label neurons, myc antibody was used to label wtRheb, HA antibody was used to label caRheb, as pS6 to show mTor activation (arrowheads). D, The length of at least 150 randomly selected neurons was analyzed. All neurite outgrowth assays were repeated three times. There was significantly greater outgrowth of the longest neurite (D, p<0.05*) as well as total neurite outgrowth (E, p<0.05*) from dissociated DRGs expressing either wildtype or constitutively active Rheb (Rh) when compared to either GFP control or caRheb +rapamicin (rap) groups. Error bars indicate SEM. Scale bars, 300 µm.
Rheb enhances neurite outgrowth in adult DRG neurons through activation of mTOR signaling pathway
Deletion of PTEN was shown to enhance axonal regeneration of retinal ganglion neuron and cortical neurons (Liu et al., 2010; Park et al., 2008). In these knockout animals, regeneration could be inhibited with treatment of rapamycin indicating upregulation of mTOR activity to be an important mediator of regeneration (Park et al., 2008). To determine whether the direct activation of mTOR by Rheb also enhances axonal growth capacity in vitro, we cultured DRG neurons from adult rats and measured axonal outgrowth 3 days following Rheb or GFP lentiviral transduction (Figure 4C). We used lentivirus-expressing GFP to transduce DRG neurons to determine transduction efficiency, which was higher than 50% of the total neurons (data not shown). Only neurons transfected with wtRheb or caRheb were positive for pS6 (Figure 4C) and showed a 2-fold increase in neurite outgrowth when compared to control (GFP-transduced) neurons (Figure 4D). The addition of rapamycin (Rap) completely blocked both pS6 expression and any increase in neurite outgrowth in Rheb-transduced neurons (Figure 4C–D). Analysis showed a significantly higher proportion of neurons grew axons to a length between 1000 to 2000µm when they were transduced with wtRheb or caRheb compared with neurons transduced with GFP lentivirus or with caRheb-transduced neurons treated with rapamycin. Fifty seven percent of wtRheb-transduced and 64% of caRheb-transduced neurons produced neurites that were longer than 1000µm compared with 27% of GFP-transduced and 23% of rapamycin treated caRheb neurons (Figure 4E). These results suggest that Rheb overexpression can increase axonal growth capacity in DRG neurons in vitro through activation of mTOR in the absence of neurotrophins.
caRheb expression by adeno-associated virus vector in vitro and in vivo
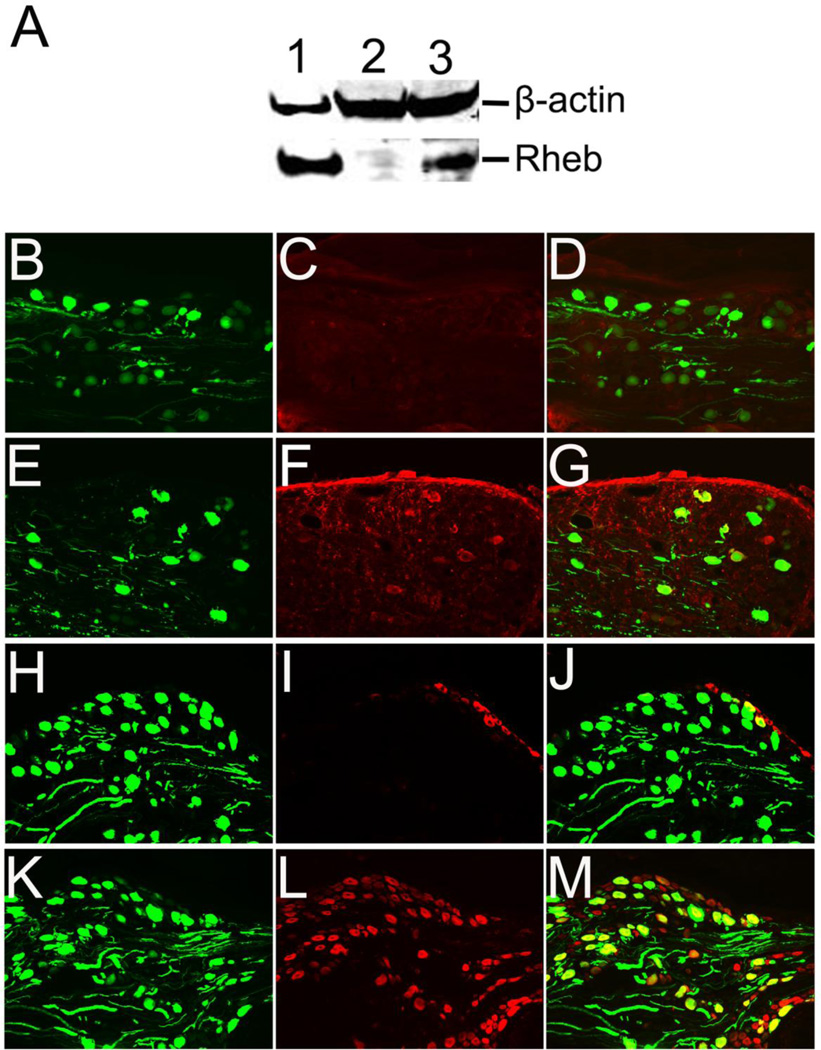
To examine the potential effects of Rheb expression in mediating or enhancing sensory axon regeneration in vivo, we generated caRheb-AAV vector. To confirm the expression of caRheb, we transfected U373 astrocytoma cell line with AAV-Rheb in vitro and analyzed Rheb expression by Western blot. The expression of Rheb in this cell line also induced phosphorylation of ribosomal subunit S6 (not shown). We further confirmed AAV-Rheb expression after direct injection into DRGs in vivo, immediately after dorsal root crushes. AAV-Rheb was co-injected with an equal concentration of AAV-GFP to identify axons regenerating through the dorsal root entry zone. Four weeks after AAV-Rheb/AAV-GFP injections, Rheb expression, as determined by HA staining, was observed in many GFP-positive neurons within the adult rat DRG (Figure 5E, F and G) when compared to AAVGFP injected controls (Figure 5B, C and D). We also examined mTOR activity in Rheb transduced DRGs by labeling with pS6 (Figure 5H – M). Immunohistochemical analysis showed that pS6 expression was upregulated in Rheb-transduced DRGs (Figure 5L) compared with GFP-transduced DRGs (Figure 5I).
Figure 5. Rheb expression by adeno-associated virus vector in vitro and in vivo.
A, Western blot analysis of cell lysates of lentivirus-Rheb transduced U373 cells (lane 1), or AAV-GFP transduced U373 cells (lane 2), and AAV-Rheb transduced U373 cells (lane 3). Four weeks after AAV-GFP (B, C, D, H, I, J) or AAV-caRheb (E, F, G, K, L, M) injection, GFP was detected in many DRG neurons and attached axons (B, E, H, K). Immunostaining of HA tagged Rheb (C & F) showed expression of caRheb only in DRG injected with AAV-caRheb (F) and no expression of caRheb in AAV-GFP control sections (C). Likewise, pS6 expression was observed only in DRGs transduced by AAV-Rheb DRGs (L), compared to AAV-GFP controls (I). Scale Bars: in B–M, 500 µm.
Expression of Rheb in DRG neurons alone failed to induce regeneration of sensory axons through the DREZ
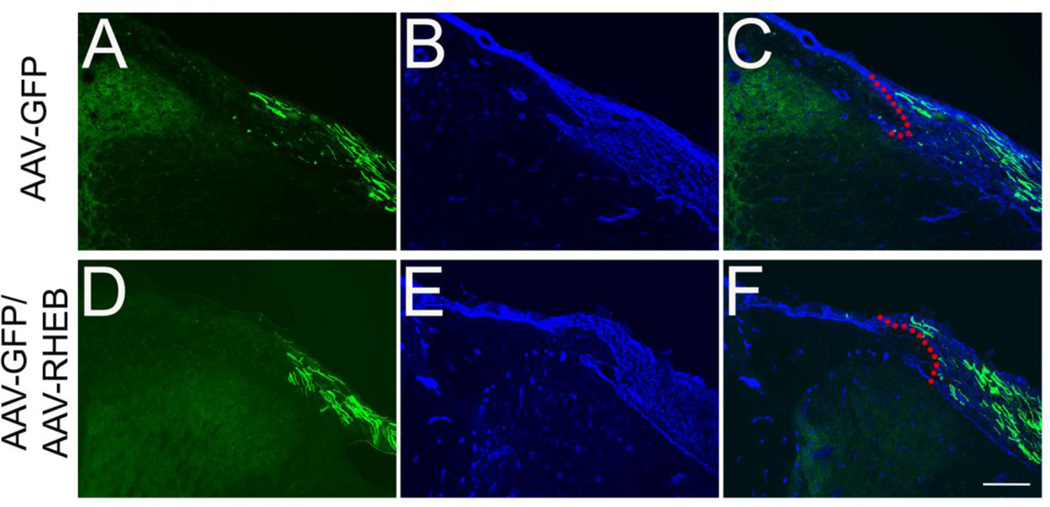
Having demonstrated that Rheb expression enhances neurite outgrowth in vitro, we asked whether the transduction of Rheb would also promote axonal regeneration in vivo. We delivered AAV-caRheb/AAV-GFP or AAV-GFP alone to adult rat sensory neurons within DRGs and examined axonal regeneration after dorsal root injury. In the control group (Figure 6A–C), axons regenerated within the PNS but stop at the peripheral zone of the DREZ indicated by laminin (LN) labeling. Likewise, DRG neurons in the AAV-Rheb/AAV-GFP group (Figure 6D–F) also exhibited a similar pattern of axons growth up to the DREZ but no axons were observed growing into the spinal cord (N=5, both groups).
Figure 6. Rheb alone does not promote the growth of regenerating sensory axons into the spinal cord.
Cross sections of crushed dorsal root with attached spinal cord showed that numerous GFP-labeled axons were in the dorsal root 4 weeks after DRGs transduced with AAV-GFP or with AAV Rheb plus AAV-GFP (A and D). The DREZ boundary is indicated with laminin (LN) labeling (B and E) and merged images showed that many of GFP-expressing axons grew and stopped at the DREZ (red dashed lines outline the borders of DREZ). Scale Bars: 200 µm.
Co-expression of Rheb within DRG neurons and NT-3 within the spinal cord results in regeneration of sensory axons
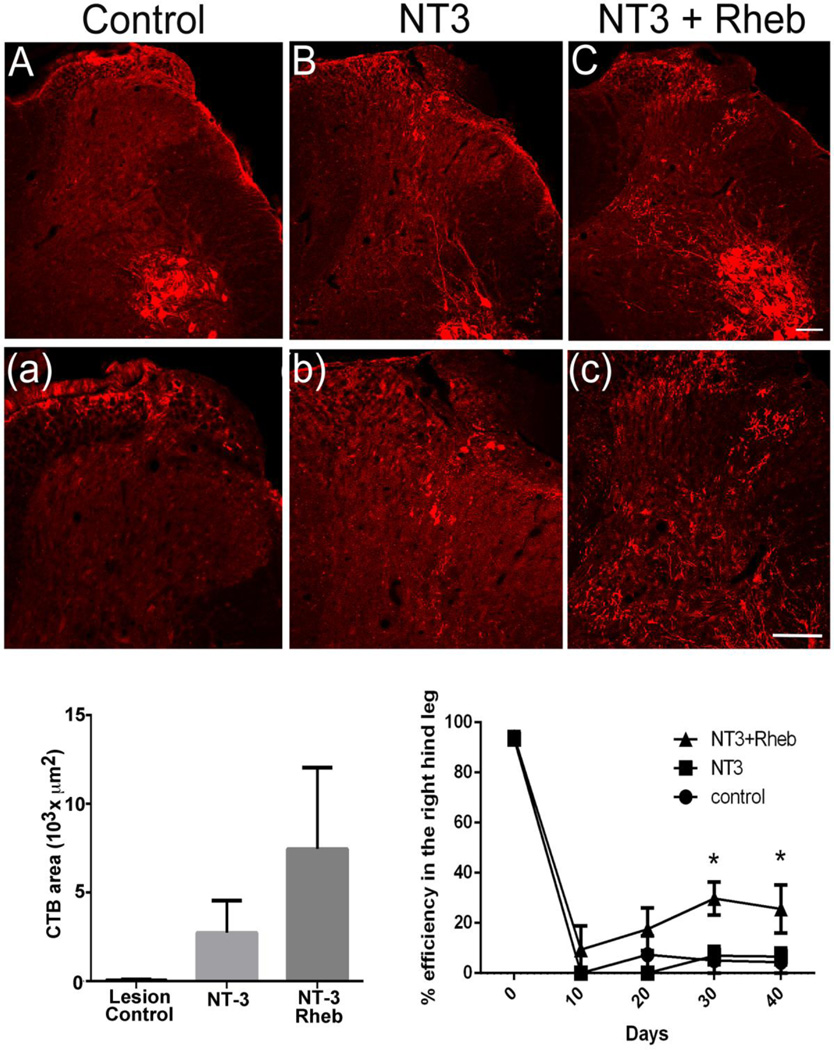
In order to avoid possible dilution of the virus by mixing AAV-GFP vector to label axons, we used Cholera toxin B tracer to label regenerated dorsal root axons. For this study, NT-3 was expressed within the spinal cord because previous experiments show NT-3 promotes some but limited regeneration of proprioceptive axons through the DREZ (Ramer 2001, 2002). We hypothesized that caRheb expression in DRG neurons would increase NT-3-mediated regeneration into the spinal cord. We expressed caRheb within the DRG using AAV2 and delivered NT-3 to the spinal cord using lentivirus (See Materials and Methods). After a dorsal root crush and treatment, regenerating axons appeared morphologically distinct from normal large diameter axons, which enter the dorsal horn more medially. Again, injections of only AAV-Rheb into the DRG showed no regeneration past the DREZ (Figure 7A), whereas, injections of only Lenti-NT-3 into the spinal cord showed some but very little regeneration past the DREZ (Figure 7B). With combined treatment of both Rheb and NT-3, many more axons regenerated past the DREZ and some axons were able to penetrate to a depth of about 800 µm corresponding to lamina VIII under combined treatment (Figure 7C, (c)). Labeled axons also appeared patchy often following a tortuous course when compared to normal axons (Kelamangalath et al., 2015). Analysis of the total area occupied by regenerating axons using one-way ANOVA revealed a significant effect (p<0.05) of treatment (F (2,17) =12.15). Multiple comparison analysis (Bonferroni’s test) showed a significantly increased axon density (p<0.05) in NT-3+Rheb treated group compared to NT-3 treatment or caRheb/lesion control (Figure 8D). No significant regenerative effect was observed between NT-3 and the lesion control group (Figure 8D). This indicates that expression of caRheb in sensory neurons can enhance NT-3 mediated regeneration of myelinated sensory axons after dorsal rhizotomy.
Figure 7. Rheb expression in the DRG enhances functional regenerative in response to NT3 expression in the spinal cord.
A&(a), AAV-GFP injection in the right L4/L5 DRG following L4/L5 dorsal root crush (right side) did not have any effect on regeneration of CTB labeled deeper myelinated sensory afferents (n=6). B&(b), Lentiviral expression of NT3 in the right L4 and L5 dorsal horn induced a modest regeneration of CTB labeled axons, but the regeneration was very minimal and superficial. C&(c), Lentiviral expression of NT3 in the right dorsal horn along with the adeno associated viral expression of Rheb in the right L4/L5 DRGs produced a robust regeneration of CTB labeled axons compared to control and NT3 group. Moreover, regenerated axons were found to occupy deeper laminae similar to the physiological pattern. D, NT3+Rheb group (n=5) showed a significantly high (p<0.05, One way ANOVA) regeneration of myelinated sensory afferents in laminae 1 – V as indicated by the presence of CTB labeled axons compared to control (n=6) and NT3 groups (n=4). E. NT3+Rheb group (n=5) showed a modest but statistically significant (p<0.05) reduction of right foot slip errors when the animals walked on a horizontal ladder compared to NT3 (n=4) and the lesion control group (n=6) (two-way ANOVA). Tukey’s multiple comparison analysis showed a significantly high improvement (p<0.05) in NT3+Rheb group compared to lesion control and NT3 group on day 30, but statistically significant improvement was only seen between lesion control and NT3+Rheb group on day 40.
Rheb/NT-3 treatment shows partial recovery of proprioceptive behavioral response
We evaluated recovery of behavioral functions subserved by deeper myelinated sensory axons by assessing paw pressure sensation and proprioception. Paw pressure threshold remained at cut-off levels in all the lesioned groups and showed no improvement towards normal baseline levels (data not shown). To assess proprioceptive response animals were trained to walk on a grid runway. Before dorsal rhizotomies these animals made very few footfall errors (Figure 7D). Four to six weeks after injury and treatment, only the animals treated with Rheb/NT-3 showed a modest but statistically significant (p<0.05) improvement in foot placement. Reduction of right foot slips was observed in the Rheb/NT-3 treatment group (F-treatment (2,37)=9.315) when compared to NT-3 and the lesion control groups. We also observed a statistically significant reduction in footfall error overtime in the Rheb/NT-3 treatment group (F-days (4,37)=144.3) (Figure 7E). Tukey’s multiple comparison analysis showed Rheb/NT-3 to significantly improve foot placement (p<0.05) when compared to either NT-3 or lesion control groups at day 30, but only to lesion control at day 40 (Figure 7E). This data indicates that increased mTOR activation of large diameter DRG neurons increased their regeneration potential in the presence of NT-3.
Discussion
Myelin inhibitory proteins are thought to play an important role in inhibiting axon regrowth after spinal cord injury; however knockout of all three known myelin inhibitory proteins (Nogo, MAG and OMgp) affect on axonal regeneration remains controversial (Lee et al., 2010; Cafferty et al., 2010). Several previous studies have indicated that after dorsal root injury soluble Nogo receptor administration enhanced regeneration of sensory afferents into the spinal cord (Harvey et al., 2009; Peng et al., 2010). However, we observed no change in regeneration in the triple knockout mice, suggesting these three myelin inhibitory proteins had little effect on mediating sensory afferent regeneration (Zhai et al., in preparation). Even with the expression of NT-3 within the spinal cord, we observed very little regeneration of large diameter proprioceptive axons. Unfortunately, they failed to penetrate deeply into the dorsal horn, and the vast majority terminated at a similar location as observed in wildtype mice.
Several studies have indicated mTOR signaling to play an important role in promoting regeneration of injured axons within the central nervous system (Liu et al., 2010; Park et al., 2008). Conditional deletion of PTEN increased retinal ganglion neuron (RGN) survival and axon regeneration after optic nerve injury. This effect was largely abolished by the addition of the mTOR inhibitor, rapamycin (Park et al., 2008). Furthermore, deletion of PTEN increased sprouting of uninjured CST axons and enhanced regeneration of injured CST axons past the lesion site (Liu et al., 2010). Similar increases in axon regeneration were observed in PNS neurons, in which upregulation of mTOR activity by genetic ablation of TSC2, a negative regulator of mTOR activity, was able to enhance axonal outgrowth in culture and promoted axonal regeneration after peripheral nerve injury (Abe et al., 2010). These studies suggest increased mTOR activation can enhance axon regeneration of either central or peripheral axons. During development mTOR activity in retinal ganglion neurons (RGCs) and corticospinal neurons is high as determined by phosphorylation of S6 ribosomal protein. After development, mTOR activity in these neurons decline to low levels shortly after birth. Moreover, axon injury to these neurons caused further down regulation of mTOR activity (Liu et al., 2010; Park et al., 2008). Similar to what was observed in these studies, we found that mTOR activity in the rat was high during cortical and DRG neuron development, but decreased during maturation. This developmental decline in mTOR activity may partially explain the poor regenerative ability of adult central neurons. Injury to the sciatic nerve, however, induces increase in pS6 in DRG neurons within the first week after injury (Belin et al., 2015). This is most likely due to the high regenerative ability of these neurons. Unlike, lesions to the peripheral branch, we did not observe increased pS6 four weeks after dorsal root injuries. This is not surprising since it is well established that after dorsal root lesions axons regenerate much slower (Wujek and Lasek, 1983) and these lesions do not induce a conditional response (Oblinger and Lasek, 1984) or induce an increase in GAP-43 expression (Schreyer and Skene, 1993) as typically observed with sciatic nerve injuries. In addition, we observed Rheb expression and phosphorylation of S6 to be confined to the neuronal body and not within the axon. Previous studies suggested that local protein synthesis in the axon is required for axonal guidance and regeneration (Campbell and Holt, 2001; Hanz et al., 2003), but in our studies we failed to find pS6 labeling within the developing or regenerating DRG axon. It is possible that mTOR activation could play a larger role in neuronal growth at the cell body compared with local protein synthesis in the axon or our current staining technique was not able to detect the signal in axons. Our results confirmed active mTOR within the neuronal soma and dendrites and may account for the increase in cell body size in relation to disorders that abnormally increase mTOR activation during development (Lee et al., 2012; Lim and Crino, 2013; Mirzaa and Poduri, 2014).
In the present study, we assessed the effect of activated mTOR by an upstream factor, Rheb, on neurite outgrowth in DRG neurons and axon regeneration of lesioned dorsal root axons into the spinal cord. Rheb is a direct activator of mTOR and the only known downstream target of Rheb is mTOR (Inoki et al., 2003; Long et al., 2005). Rheb has been shown to exclusively activate the mTOR pathway without affecting other signaling pathways (Inoki et al., 2003; Li et al., 2008; Long et al., 2005). Our study in 293T cells and cultured DRG neurons confirmed that Rheb overexpression is a potent activator of mTOR signaling pathway and this effect could be specifically abolished with the mTOR inhibitor, rapamycin. To test whether Rheb overexpression in adult DRG neurons could increase neurite outgrowth in vitro, we used lentivirus to express Rheb in DRG neurons and glial cells. Interestingly, transduction with either wtRheb or caRheb lentivirus significantly enhanced neurite outgrowth and phosphorylation of S6. Previous studies have shown that deletion of either PTEN or TSC1, which both indirectly influence mTOR activation, enhance peripheral nerve regeneration (Abe et al., 2010; Christie et al., 2010). We next determined if expression of Rheb could influence regeneration of sensory axons into the spinal cord after lesioning the central DRG branch. We found that expression of caRheb within DRG neurons was insufficient to achieve successful axonal regeneration across the dorsal root entry zone and into the spinal cord. The molecular mechanism that stops axons at the DREZ is complicated and this regenerative failure maybe attributed to both growth-inhibitory molecules at the DREZ and lack of intrinsic growth capability by adult DRG neurons (Ramer et al., 2000; Ramer et al., 2001; Steinmetz et al., 2005; Smith et al., 2012; Tang et al., 2004). Although, dorsal rhizotomy does not cause direct injury to the CNS, the formation of an astrocytic barrier (McPhail et al., 2005), the upregulation of inhibitory extracellular matrix molecules and tissue inhibitors (Pindzola et al., 1993; Zhang et al., 2001; Beggah et al., 2005; Smith et al., 2012) also contribute to regenerative failure, as with direct spinal cord lesions. Our study suggests that activating the mTOR signaling pathway might be necessary to induce regeneration through the DREZ but not sufficient, requiring activation of other signaling pathways to boost axon extension.
Previous studies indicate that neurotrophic factor can enhance regeneration of sensory afferents into the spinal cord after dorsal rhizotomy (Ramer et al., 2000; Romero et al., 2001; Tang et al., 2004; Kal). However, the majority of axons regenerating are small diameter nociceptive axons and very few large diameter myelinated axons regenerate (Romero et al., 2001; Ramer et al., 2001; Ramer et al., 2002). Proprioceptive axons from muscle spindles are responsive to NT-3 and during development Bax/NT3 double knockout mice show deficits in proprioceptive growth into the spinal cord (Oakley et al., 1997; Wright et al., 1997; Patel et al., 2003; Genc et al., 2004). Expression of NT-3 in motor neurons rescues this phenotype (Wright et al., 1997). After injury to adult dorsal roots, several studies show some but limited regeneration of dorsal root A-fiber afferents using NT-3 (Ramer et al., 2001; Ramer et al., 2002; Zhang et al., 1998). On the basis of these studies, we examined whether co-expression of NT-3 in the spinal cord with Rheb in DRG neurons could improve regeneration of myelinated afferents. The combination of Rheb and NT-3 led to significantly better regeneration than either treatment alone. Moreover, many regenerating CTB labeled axons show diffuse and tortuous regeneration extending into the motor neuron pools. To induce proprioceptive axon growth into the ventral horn during development, NT-3/TrkC signaling modulates the post-translational processing of SAD-A kinase (synapses of amphids defective kinase-A). Activation of Trk-C by NT-3 prevents SAD-A degradation and supports its activation (Lilley et al., 2013). Mice with double knockouts of both SAD-A/B show abnormal termination of proprioceptive axons within the dorsal horn of the spinal cord (Lilley et al., 2013). In addition, the expression of SAD-A is modulated by mTOR activation (Wildonger et al., 2008; Choi et al., 2008; Nie et al., 2013). Increased proprioceptive axon regeneration and growth into the ventral horn could be due to increased expression and activation of SAD-A by combining Rheb and NT-3 expression; however, this possibility will require further investigation.
In conclusion, we show little to no regeneration of sensory afferent regeneration into the spinal cord in Nogo, OMgp, and MAG triple knockouts or after expression of caRheb within DRG neurons. Interestingly, NT-3 supplementation to the spinal cord of triple knockout mice showed no apparent increase in regeneration, however, when spinal cord expression of NT-3 was combined with DRG neuronal expression of caRheb an almost 3 fold enhancement of sensory afferent regeneration was observed. This resulted in a reduction in foot placement errors on a grid walkway. This is the first study to combine treatments to increase mTor activity with neurotrophin supplementation to enhance axonal regeneration.
Highlights.
Neurotrophin-3 induces minimal regeneration of proprioceptive afferents following dorsal root rhizotomy.
NT-3 expression in Nogo, OMgp, MAG triple knockout mice did not enhance regeneration.
Activation of the mTor pathway by overexpressing Rheb does not enhance sensory axon regeneration.
Combined expression of NT-3 and Rheb increased proprioceptive axon regeneration.
Combined expression of NT-3 and Rheb lead to reduced footfall errors on grid walkway.
Acknowledgments
We thank Drs. Binhai Zheng and Jae K. Lee for providing us with congenic knockout mice lacking Nogo and OMgp (Dr. Zheng) or MAG (Dr. Lee). We would also like to thank Dr. Zhigang He for the original caRheb construct. This work was funded by a grant from the National Institute of Neurological Disorders and Stroke R01 NS060784 (GMS), R01 NS079631 (YJS), and the Shriners Hospital for Pediatric Research grants SHC 84050 and SHC 85200.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. The Journal of biological chemistry. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso E, Mingozzi F, Bosch F. Production, purification and characterization of adeno-associated vectors. Current gene therapy. 2010;10:423–436. doi: 10.2174/156652310793797685. [DOI] [PubMed] [Google Scholar]
- Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- Beggah AT, Dours-Zimmermann MT, Barras FM, Brosius A, Zimmermann DR, Zurn AD. Lesion-induced differential expression and cell association of Neurocan, Brevican, Versican V1 and V2 in the mouse dorsal root entry zone. Neuroscience. 2005;133:749–762. doi: 10.1016/j.neuroscience.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Belin S, Nawabi H, Wang C, Tang S, Latremoliere A, Warren P, Schorle H, Uncu C, Woolf CJ, He Z, Steen JA. Injury-induced decline of intrinsic regenerative ability revealed by quantitative proteomics. Neuron. 2015;86:1000–1014. doi: 10.1016/j.neuron.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nature reviews. Neuroscience. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X. Tuberous sclerosis complex proteins control axon formation. Genes & development. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J. Neuroscience. 2010;30:9306–9315. doi: 10.1523/JNEUROSCI.6271-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, Bishop D, Son YJ. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J. Neurosci. 2011;31:4569–4582. doi: 10.1523/JNEUROSCI.4638-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc B, Ozdinler PH, Mendoza AE, Erzurumlu RS. A chemoattractant role for NT-3 in proprioceptive axon guidance. PLoS biology. 2004;2:e403. doi: 10.1371/journal.pbio.0020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmicimportins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Harvey PA, Lee DH, Qian F, Weinreb PH, Frank E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci. 2009;29:6285–6295. doi: 10.1523/JNEUROSCI.5885-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. RhebGTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes & development. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Ziemba KS, Smith GM. Axon growth across a lesion site along a preformed guidance pathway in the brain. Experimental neurology. 2008;210:521–530. doi: 10.1016/j.expneurol.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelamangalath L, Tang X, Bezik K, Sterling N, Son YJ, Smith GM. Neurotrophin selectivity in organizing topographic regeneration of nociceptive afferents. Exp Neurol. 2015;271:262–278. doi: 10.1016/j.expneurol.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A, Funari V, Russ C, Gabriel SB, Mathern GW, Gleeson JG. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nature genetics. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–70. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Werner H, Puschel AW. Rheb and mTOR regulate neuronal polarity through Rap1B. The Journal of biological chemistry. 2008;283:33784–33792. doi: 10.1074/jbc.M802431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley BN, Pan YA, Sanes JR. SAD kinases sculpt axonal arbors of sensory neurons through long- and short-term responses to neurotrophin signals. Neuron. 2013;79:39–53. doi: 10.1016/j.neuron.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KC, Crino PB. Focal malformations of cortical development: new vistas for molecular pathogenesis. Neuroscience. 2013;252:262–276. doi: 10.1016/j.neuroscience.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nature neuroscience. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Keefe K, Tang X, Lin S, Smith GM. Use of self-complementary adeno-associated virus serotype 2 as a tracer for labeling axons: implications for axon regeneration. PloS one. 2014;9:e87447. doi: 10.1371/journal.pone.0087447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Current biology: CB. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Martin DE, Hall MN. The expanding TOR signaling network. Current opinion in cell biology. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- McPhail LT, Plunet WT, Das P, Ramer MS. The astrocytic barrier to axonal regeneration at the dorsal root entry zone is induced by rhizotomy. The European journal of neuroscience. 2005;21:267–270. doi: 10.1111/j.1460-9568.2004.03837.x. [DOI] [PubMed] [Google Scholar]
- Mirzaa GM, Poduri A. Megalencephaly and hemimegalencephaly: breakthroughs in molecular etiology. American journal of medical genetics. Part C, Seminars in medical genetics. 2014;166C:156–172. doi: 10.1002/ajmg.c.31401. [DOI] [PubMed] [Google Scholar]
- Nie D, Di Nardo A, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, Sahin M. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nature neuroscience. 2010;13:163–172. doi: 10.1038/nn.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J, Liu X, Lilley BN, Zhang H, Pan YA, Kimball SR, Zhang J, Zhang W, Wang L, Jefferson LS, Sanes JR, Han X, Shi Y. SAD-A kinase controls islet beta-cell size and function as a mediator of mTORC1 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13857–13862. doi: 10.1073/pnas.1307698110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RA, Lefcort FB, Clary DO, Reichardt LF, Prevette D, Oppenheim RW, Frank E. Neurotrophin-3 promotes the differentiation of muscle spindle afferents in the absence of peripheral targets. J. Neuroscience. 1997;17:4262–4274. doi: 10.1523/JNEUROSCI.17-11-04262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblinger MM, Lasek RJ. A conditioning lesion of the peripheral axons of dorsal root ganglion cells accelerates regeneration of only their peripheral axons. J Neurosci. 1984;4:1736–1744. doi: 10.1523/JNEUROSCI.04-07-01736.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, Snider WD. Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron. 2003;38:403–416. doi: 10.1016/s0896-6273(03)00261-7. [DOI] [PubMed] [Google Scholar]
- Peng X, Zhou Z, Hu J, Fink DJ, Mata M. Soluble Nogo receptor down-regulates expression of neuronal Nogo-A to enhance axonal regeneration. J Biol Chem. 2010;285:2783–2795. doi: 10.1074/jbc.M109.046425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- Ramer LM, Borisoff JF, Ramer MS. Rho-kinase inhibition enhances axonal plasticity and attenuates cold hyperalgesia after dorsal rhizotomy. J. Neuroscience. 2004;24:10796–10805. doi: 10.1523/JNEUROSCI.3337-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS, Bishop T, Dockery P, Mobarak MS, O'Leary D, Fraher JP, Priestley JV, McMahon SB. Neurotrophin-3-mediated regeneration and recovery of proprioception following dorsal rhizotomy. Molecular and cellular neurosciences. 2002;19:239–249. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J. Neurosci. 2001;21:2651–2660. doi: 10.1523/JNEUROSCI.21-08-02651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Archives internationales de pharmacodynamie et de therapie. 1957;111:409–419. [PubMed] [Google Scholar]
- Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J. Neuroscience. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Current opinion in cell biology. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J Neurobiol. 1993;24:959–970. doi: 10.1002/neu.480240709. [DOI] [PubMed] [Google Scholar]
- Smith GM, Falone AE, Frank E. Sensory axon regeneration: rebuilding functional connections in the spinal cord. Trends in neurosciences. 2012;35:156–163. doi: 10.1016/j.tins.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Liu Y, Hong JW. Quantitative assessment of neurite outgrowth over growth promoting or inhibitory substrates. Methods in molecular biology. 2013;1078:153–161. doi: 10.1007/978-1-62703-640-5_14. [DOI] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25:8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neuroscience. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildonger J, Jan LY, Jan YN. The Tsc1-Tsc2 complex influences neuronal polarity by modulating TORC1 activity and SAD levels. Genes & development. 2008;22:2447–2453. doi: 10.1101/gad.1724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Zhou L, Kucera J, Snider WD. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron. 1997;19:503–517. doi: 10.1016/s0896-6273(00)80367-0. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Lasek RJ. Correlation of axonal regeneration and slow component B in two branches of a single axon. J Neurosci. 1983;3:243–251. doi: 10.1523/JNEUROSCI.03-02-00243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Fischer G, Jia G, Reiser J, Park F, Hogan QH. Lentiviral gene transfer into the dorsal root ganglion of adult rats. Mol Pain. 2011;7:63. doi: 10.1186/1744-8069-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dijkhuizen PA, Anderson PN, Lieberman AR, Verhaagen J. NT-3 delivered by an adenoviral vector induces injured dorsal root axons to regenerate into the spinal cord of adult rats. J. Neuroscience Res. 1998;54:554–562. doi: 10.1002/(SICI)1097-4547(19981115)54:4<554::AID-JNR12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tohyama K, Winterbottom JK, Haque NS, Schachner M, Lieberman AR, Anderson PN. Correlation between putative inhibitory molecules at the dorsal root entry zone and failure of dorsal root axonal regeneration. Molecular and cellular neurosciences. 2001;17:444–459. doi: 10.1006/mcne.2000.0952. [DOI] [PubMed] [Google Scholar]