Abstract
Root canal therapy has enabled us to save numerous teeth over the years. The most desired outcome of endodontic treatment would be when diseased or nonvital pulp is replaced with healthy pulp tissue that would revitalize the teeth through regenerative endodontics. ‘A search was conducted using the Pubmed and MEDLINE databases for articles with the criteria ‘Platelet rich plasma’, ‘Platelet rich fibrin’, ‘Stem cells’, ‘Natural and artificial scaffolds’ from 1982–2015’. Tissues are organized as three-dimensional structures, and appropriate scaffolding is necessary to provide a spatially correct position of cell location and regulate differentiation, proliferation, or metabolism of the stem cells. Extracellular matrix molecules control the differentiation of stem cells, and an appropriate scaffold might selectively bind and localize cells, contain growth factors, and undergo biodegradation over time. Different scaffolds facilitate the regeneration of different tissues. To ensure a successful regenerative procedure, it is essential to have a thorough and precise knowledge about the suitable scaffold for the required tissue. This article gives a review on the different scaffolds providing an insight into the new developmental approaches on the horizon.
Key Words: Extracellular matrix, pulp, regeneration, scaffolds
INTRODUCTION
Regenerative dentistry has been popularized due to advancements in biologic therapies that apply growth and differentiation factors which hasten or induce natural biologic regeneration. Hermann in 1920 described the application of calcium hydroxide for vital pulp therapy which laid the foundation for regeneration of dental tissues. NygaardOstby in 1961 evaluated a revascularization method for re-establishing a pulp-dentin complex in permanent teeth with pulpal necrosis.[1]
Regenerative endodontics is based on the concept of tissue engineering. Regenerative endodontic procedures (REPs) have been defined as biologically-based procedures designed to replace damaged structures, including dentin and root structures, as well as cells of the pulp-dentin complex with live viable tissues, preferably of the same origin, that restore the normal physiologic functions of the pulp-dentin complex.[2] ‘An exhaustive search was conducted using the Pubmed and MEDLINE databases for articles with the criteria ‘Platelet rich plasma’, ‘Platelet rich fibrin’, ‘Stem cells’, ‘Natural and artificial scaffolds’ from 1982–2015. All articles were selected, with no inclusion or exclusion criteria’.
Pulp revascularization is defined as re-introduction of vascularity in the root canal system. Although blood vessels are indispensable constituents of dental pulp, pulp regeneration is considered incomplete without an odontoblastic layer lining the dentin surface, nociceptive as well as sympathetic and parasympathetic nerve fibers, in addition to interstitial fibroblasts and most importantly, stem/progenitor cells that serve to replenish all pulp cells in the regenerated pulp when they undergo apoptosis and turnover. Thus, a clear distinction between regeneration and revascularization can be made as follows:
Pulp revascularization = induction of angiogenesis in endodontically-treated root canal
Pulp regeneration = pulp revascularization + restoration of functional odontoblasts and/or nerve fibers.[3]
The three key ingredients for regeneration are morphogens, progenitor/stem cells, and the extracellular matrix (ECM) scaffold.[1]
STEM CELLS
Stem cells are undifferentiated embryonic or adult cells that continuously divide. They can divide and create additional stem cells and differentiate along a specified molecular pathway. Embryonic stem cells are totipotent and have the capacity to self-renew. In contrast, stem cells that reside within an adult organ or tissue have more restricted options, with ability to select a differentiation program from only a few possible pathways[4,5] [Table 1].
Table 1.
The most common dental stem cells

GROWTH FACTORS
Growth factors regulate either transplanted cells or endogenous cells in dental pulp-dentin regeneration. They are polypeptides or proteins that bind to specific receptors on the surface of target cells (e.g., bone morphogenetic protein [BMP] receptors) that affect a broad range of cellular activities including migration, proliferation, differentiation, and apoptosis of all dental pulp cells, including stem/progenitor cells.[4] Bioactive cues that recruit the proper cells are critical in pulp regeneration (transforming growth factors [TGFs] β1, β3 for odontoblast differentiation and stimulation of dentin matrix). These events of repair and regeneration can be coordinated and modulated by growth factors such as platelet-derived growth factor (PDGF), TGF, BMPs, vascular endothelial growth factor (VEGF), fibroblast growth factor, and insulin-like growth factor (IGF).[6]
SCAFFOLDS
Scaffolds are three-dimensional (3D) porous solid biomaterials designed which
Provide a spatially correct position of cell location[1]
Promote cell-biomaterial interactions, cell adhesion, and ECM deposition
Permit sufficient transport of gases, nutrients, and regulatory factors to allow cell survival, proliferation, and differentiation
Biodegrade at a controllable rate that approximates the rate of tissue regeneration
Provoke a minimal degree of inflammation or toxicity in vivo.[6]
Apart from blood cells, most of the normal cells in human tissues are anchorage-dependent residing in a solid matrix called ECM. The best scaffold for an engineered tissue should be the ECM of the target tissue in its native state.[7]
Ideal requirements of a scaffold
A high porosity and an adequate pore size are necessary to facilitate cell seeding and diffusion throughout whole structure of both cells and nutrients[8]
Should allow effective transport of nutrients, oxygen, and waste[9]
Biodegradability is essential, since scaffolds need to be absorbed by the surrounding tissues without the necessity of surgical removal[8]
The rate at which degradation occurs has to coincide with the rate of tissue formation[18]
Should be biocompatible[9]
Should have adequate physical and mechanical strength.[9]
Classification of scaffolds
Table 2.
Classification of scaffolds

Biological or natural scaffolds
See Table 3.
Table 3.
Attributes of commonly used natural scaffolds[26]
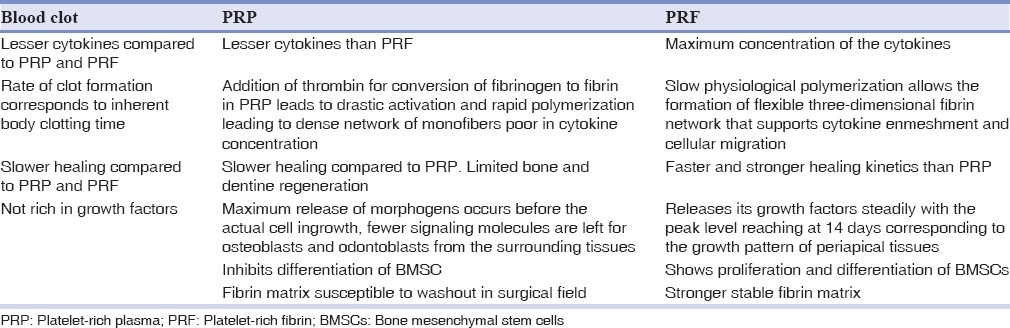
Platelet rich plasma
Platelet rich plasma (PRP), an autologous first generation platelet concentrate with a rich source of growth factors, has been proposed as a potential addendum/substitute scaffold.[18] It is easy to prepare, rich in growth factors, and forms a 3D fibrin matrix that helps entrap the growth factors. Platelet concentration in PRP exceeds 1 million/mL, which is 5 times more than that of the normal platelet count.[19] More number of platelets increases the number of growth factors secreted by them which helps in the proliferation of stem cells to induce healing and regeneration of tissues.[20] It is a concentrated suspension of different growth factors like PDGF, TGF-b, IGF, VEGF, epidermal growth factor, and epithelial cell growth factor. These are released via degranulation of alpha granules and stimulate bone and soft-tissue healing.[21] The disadvantages of this procedure include drawing blood in young patients, the need of special equipment and reagents to prepare PRP, and the increased cost of treatment.[20]
Platelet rich fibrin
Platelet rich fibrin (PRF) is second-generation platelet concentrate named as Choukroun's PRF after its inventor.[22] The procedure consists of drawing blood which is collected into test tubes without an anticoagulant and is centrifuged instantaneously. A tabletop centrifuge can be used for 10 min at 3000 rpm or for 12 min at 2700 rpm.[23]
The resultant product consists of three layers:
Acellular platelet poor plasma at peak level
PRF clot in intermediate level
Red fraction of red blood cells at the base level.
The blood coagulation starts instantaneously as it comes in contact with the glass surface due to the lack of anticoagulant.[24]
Biological properties of platelet rich fibrin
PRF can be considered as an immune concentrate with specific composition and a 3D architecture. It contains multitude of growth factors such as PDGF, TGF β1, and IGF.[22]
Attributes of platelet-rich fibrin
Ideal biomaterial for pulp-dentin complex regeneration
Prevents the early encroachment of undesired cells, thereby acts as a viable barrier between desired and undesired cells
Healing and inter positional biomaterial
Accelerates wound closure and mucosal healing due to fibrin bandage and growth factor release.[25]
Biochemical analysis of platelet-rich fibrin
PRF consists of an intimate assembly of cytokines, glycan chains, structural glycoproteins enmeshed within a slowly polymerized fibrin network. These biochemical components have well known synergistic effects on healing processes. Fibrin is the natural guide of angiogenesis. Fibrin constitutes a natural support to immunity.[22]
Collagen
Collagen is the major component in extracellular matrices, and provides great tensile strength in tissues. As a scaffold, collagen allows for easy placement of cells and growth factors and allows for replacement with natural tissues after undergoing degradation.[27,28,29]
Advantages
It is biocompatible, biodegradable, has a good tensile strength, simulates natural ECM of dentin, demonstrates high alkaline phosphatase activity, allows soft tissue and hard tissue formation, forms a trap for osteoinductive factors.[29,30] Collagen may also be processed into a variety of formats, including porous sponges, gels, and sheets, and can be crosslinked with chemicals to make it stronger or to alter its degradation rate.[30]
Disadvantages
It is mechanically weak and undergoes rapid degradation, undergoes contraction (shrinkage).[31,32]
Chitosan
Chitosan is produced commercially by deacetylation of chitin, which is the structural element in the exoskeleton of crustaceans (such as crabs and shrimp) and cell walls of fungi. The properties of chitosan affect the formation of pores in the scaffolds, thereby influencing the mechanical and biological properties.[33,34]
Advantages
Chitosan is nontoxic, easily bioabsorbable, shows antibacterial activity, has gel forming ability, increases alkaline phosphatase activity, shows fibroblast and odontoblastic proliferation.[35,36] It is a porous scaffold that can be molded into any shape and its hydrophilic property enhances cell attachment and proliferation.[35,37]
Disadvantages
It has low strength and inconsistent behavior with seeded cells, difficult to accurately control the size of the hydrogel pores, chemical modifications of chitosan structure could induce toxicity.[35]
Glycosoaminoglycans
Hyaluronic acid (HA) is one of the glycosaminoglycans in ECM and plays important roles in maintaining morphologic organization by preserving extracellular spaces, and it has been reported to have excellent potential for tissue engineering. This supports osteogenesis and can provide an environment facilitating chondrogenesis when exposed to its initiating factors.[38,39]
Advantages
It helps in differentiation of dental mesenchymal cells to odontoblasts, contributes to formation of dentin matrix and dental pulp, is biocompatible, biodegradable, bioactive, non immunogenic, and nonthrombogenic, plays a beneficial role in wound healing, can be used as an injectable scaffold and also as HA sponge.[39,40,41]
Disadvantages
HA is highly water soluble, it degrades rapidly by enzymes such as hyaluronidase[42], especially when not in the form of hydrogel and lacks mechanical integrity in an aqueous environment. However, these drawbacks can be overcome by cross linking and modification of HA.[42]
Demineralized or native dentin matrix
The organic matrix of dentin is known to contain 233 total and 68 common proteins, including a variety of collagenous and non collagenous proteins. Dentin is dominated by a rich ECMand not cells.[43]
Advantages
Demineralized dentin matrix (DDM) is nonimmunogenic and mechanically superior.[44] There is a release of bioactive molecules with DDM that signal associated dentinogenic events.[45] It shows direct induction of differentiating odontoblast-like cells and indirect matrix synthesis leading to odontoblast differentiation.[46] It has proved to be biocompatibile, osteoinductive, and osteoconductive.[47]
Disadvantages
Tooth demineralization is time consuming (usually 2–6 days). Drawback of demineralization is that prolonged acid exposure may negatively affect noncollagenous proteins involved in new bone formation.[48,49]
Silk
Silk-based biomaterial scaffolds have been extensively used for both soft and hard tissue engineering.[50]
Advantages
They are biocompatible and have the ability to support the attachment, proliferation, and differentiation of many different cell types. Silk fibroin (SF) is an enzymatically degradable material, which can be processed into water insoluble implants, injectable hydro gels, and porous sponges.[50] The ability of SF to support vascularization with good anticoagulant activity and platelet response is encouraging for tissue engineering research and clinical therapy in dentistry.[51] It has good mechanical strength, elasticity, biodegradability, morphologic flexibility, oxygen and water permeability, and a slow degradation rate that enables gradual replacement of fibroin with newly formed tissue.[17,52] SF is less immunogenic and inflammatory, compared with either polylactic-co-glycolic acid (PLGA) or collagens.[53]
Disadvantages
Hard tissue formation consists of osteodentin.[54] Complete degradation of silk scaffold occurs after 2 years.[55]
Artificial or synthetic scaffolds
Polymers
A number of synthetic polymers such as polylactic acid (PLA), poly-l-lactic acid (PLLA), polyglycolic acid (PGA), PLGA, and polyepsiloncaprolactone (PCL) have been used as scaffolds for pulp regeneration.[1]
Advantages
The synthetic polymers are nontoxic, biodegradable, and allow precise manipulation of the physicochemical properties such as mechanical stiffness, degradation rate, porosity, and microstructure.[3] Synthetic polymers are generally degraded by simple hydrolysis, whe natural polymers are mainly degraded enzymatically.[56]
PLLA is a very strong polymer and has found many applications where structural strength is important. Experiments were carried out by Sakai et al. and Cordeiro et al. showing PLLA scaffolds promoted dental pulp cell differentiation into endothelial cells and odontoblasts.[57,58]
PGA has been used as an artificial scaffold for cell transplantation, and degrades as the cells excrete ECM.[31]
PLA is an aliphatic polyester, more hydrophobic than PGA.[59]
PLGA was used as a scaffold to demonstrate that dentin-like tissue formed and pulp-like tissue could be regenerated after 3–4 months.[60] PLGA in a 50:50 mixture has a degradation time of about 8 weeks.[61]
PCL is a slowly degrading polymer that have been used toward tissue engineering efforts in bone, either aloneor combined with hydroxyapatite.[62]
Disadvantages
Synthetic polymers can cause a chronic or acute inflammatory host response, and localized pH decrease due to relative acidity of hydrolytically degraded byproducts.[63]
Bioceramics
This group of scaffolds refers to calcium/phosphate materials, bioactive glasses and glass ceramics.[64] Most common biomaterials in use are calcium phosphate-based (Ca-P) bioceramics.[64] Ca-P scaffolds include β-TCP or HA and have been widely tested for bone regeneration owing to their properties of resorption, biocompatibility, low immunogenicity, osteoconductivity, bone bonding, and similarity to mineralized tissues. 3D Ca-P porous granules have proved useful in dental tissue engineering by providing favorable 3D substrate conditions for human dental pulp stem cell (hDPSC) growth and odontogenic differentiation. Addition of SiO2 and ZnO dopants to pure TCP scaffolds increases its mechanical strength as well as cellular proliferation properties. Glass ceramics based on SiO2 -Na2 O-CaO-P2 O5 are bioactive and offer good crystallization conditions. Release of dissolution products such as Ca-P enhances the osteoblastic activity of the material.[65]
MODIFICATIONS
Scaffolds made of ceramic can be modified to obtain desired permeability, controlled dissolution rate, and specific surface characteristics to enhance cellular activity. Change in pore size and volume affects the mechanical stiffness of the scaffold. Magnesium-based glass ceramics have improved mechanical integrity and high rate of bioactivity. Niobium doped fluorapatite glass ceramic displays excellent attachment, proliferation, and differentiation of hDPSCs on its surface.[66]
Disadvantages
Bioceramics have a time-consuming fabrication, lack of organic phase, nonhomogenous particle size and shape, large grain size, difficult porosity control, difficulty of shaping, brittleness, slow degradation rate, and high density.[66] When used alone, the bioceramics have low mechanical strength and are brittle. To overcome this disadvantage, they can be combined with polymer scaffolds.[67]
CONCLUSION
REPs have emerged as viable alternatives for the treatment of immature teeth with pulpal necrosis. The clinicians should be aware of the attributes of various scaffolds so that they can select most suitable one for successful results. Combinations of various scaffoldssuch ashydroxyapatite-polymer gels can be used to compensate for their individual shortcomings, which is a significant advantage. Through the use of computer-aided design and 3D printing technologies, scaffolds like polymers can be fabricated into precise geometries with a wide range of bioactive surfaces. Such scaffolds have the potential to provide environments conducive to the growth of specific cell types such as pulpal cells. Future in regenerative endodontics is very promising owing to the discoveries and advancements in scaffold technology.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, and financial or nonfinancial in this article.
REFERENCES
- 1.Hargreaves KM, Law AS. Regenerative endodontics. In: Hargreaves KM, Cohen S, editors. Cohen's Pathways of the Pulp. 10th ed. St. Louis, Mo.: Mosby Elsevier; 2011. pp. 602–19. [Google Scholar]
- 2.Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: A review of current status and a call for action. J Endod. 2007;33:377–90. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Mao JJ, Kim SG, Zhou J, Ye L, Cho S, Suzuki T, et al. Regenerative endodontics: Barriers and strategies for clinical translation. Dent Clin North Am. 2012;56:639–49. doi: 10.1016/j.cden.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs E, Segre JA. Stem cells: A new lease on life. Cell. 2000;100:143–55. doi: 10.1016/s0092-8674(00)81691-8. [DOI] [PubMed] [Google Scholar]
- 5.Sedgley CM, Botero TM. Dental stem cells and their sources. Dent Clin North Am. 2012;56:549–61. doi: 10.1016/j.cden.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Kim SG, Zhou J, Solomon C, Zheng Y, Suzuki T, Chen M, et al. Effects of growth factors on dental stem/progenitor cells. Dent Clin North Am. 2012;56:563–75. doi: 10.1016/j.cden.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering. J Bone Joint Surg Am. 2004;86-A:1541–58. doi: 10.2106/00004623-200407000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Patel H, Bonde M, Srinivasan G. Biodegradable polymer scaffold for tissue engineering. Trends Biomater Artif Organs. 2011;25:20–9. [Google Scholar]
- 9.Saber SE. Tissue engineering in endodontics. J Oral Sci. 2009;51:495–507. doi: 10.2334/josnusd.51.495. [DOI] [PubMed] [Google Scholar]
- 10.Marei M. Regenerative Dentistry. San Rafael, Calif: Morgan & Claypool; 2010. [Google Scholar]
- 11.Arnal-Pastor M. New Scaffolding Materials for Regeneration of Infarcted Myocardium [Doctoral Thesis].: Universitat Politecnica de Valencia, Valencia, Spain. 2013. [Google Scholar]
- 12.Sureshchandra B, Roma M. Regeneration of dental pulp: A myth or hype. Endodontology. 2013;13:139–54. [Google Scholar]
- 13.Abou Neel EA, Chrzanowski W, Salih VM, Kim HW, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;B:915–28. doi: 10.1016/j.jdent.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Jadhav GR, Shah N, Logani A. Platelet-rich plasma supplemented revascularization of an immature tooth associated with a periapical lesion in a 40-year-old man. Case Rep Dent. 2014;2014:479584. doi: 10.1155/2014/479584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadhav GR, Shah D, Raghavendra SS. Autologus Platelet Rich Fibrin aided Revascularization of an immature, non-vital permanent tooth with apical periodontitis: A case report. J Nat Sci Biol Med. 2015;6:224–5. doi: 10.4103/0976-9668.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vemuri S, Kotha RS, Raghunath RG, Kandregula CR. Root canal revascularization via blood clotting in regenerative endodontics: Essentials and expectations. J Dr NTR Univ Health Sci. 2013;2:235–8. [Google Scholar]
- 17.Yang JW, Zhang YF, Sun ZY, Song GT, Chen Z. Dental pulp tissue engineering with bFGF-incorporated silk fibroin scaffolds. J Biomater Appl. 2015;30:221–9. doi: 10.1177/0885328215577296. [DOI] [PubMed] [Google Scholar]
- 18.Jadhav GR, Shah N, Logani A. Comparative outcome of revascularization in bilateral, non-vital, immature maxillary anterior teeth supplemented with or without platelet rich plasma: A case series. J Conserv Dent. 2013;16:568–72. doi: 10.4103/0972-0707.120932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lata P, Chhabra A, Jindal V, Kaur D, Thakur AK. In-vivo clinical evaluation of regenerative endodontics in immature necrotic permanent teeth with open apex. Dent J Adv Stud. 2015;3:26–33. [Google Scholar]
- 20.Jadhav G, Shah N, Logani A. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: A pilot clinical study. J Endod. 2012;38:1581–7. doi: 10.1016/j.joen.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Kaur P, Puneet VD. Platelet-rich plasma: A novel bioengineering concept. Trends Biomater Artif Organs. 2011;25:86–90. [Google Scholar]
- 22.Hotwani K, Sharma K. Platelet rich fibrin - A novel acumen into regenerative endodontic therapy. Restor Dent Endod. 2014;39:1–6. doi: 10.5395/rde.2014.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51–5. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Shivashankar VY, Johns DA, Vidyanath S, Sam G. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. J Conserv Dent. 2013;16:261–4. doi: 10.4103/0972-0707.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narang I, Mittal N, Mishra N. A comparative evaluation of the blood clot, platelet-rich plasma, and platelet-rich fibrin in regeneration of necrotic immature permanent teeth: A clinical study. Contemp Clin Dent. 2015;6:63–8. doi: 10.4103/0976-237X.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi N, Yamauchi S, Nagaoka H, Duggan D, Zhong S, Lee SM, et al. Tissue engineering strategies for immature teeth with apical periodontitis. J Endod. 2011;37:390–7. doi: 10.1016/j.joen.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Sumita Y, Honda MJ, Ohara T, Tsuchiya S, Sagara H, Kagami H, et al. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials. 2006;27:3238–48. doi: 10.1016/j.biomaterials.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 29.Prescott RS, Alsanea R, Fayad MI, Johnson BR, Wenckus CS, Hao J, et al. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J Endod. 2008;34:421–6. doi: 10.1016/j.joen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasir NM, Raha MG, Kadri KN, Rampado M, Azlan CA. The study of morphological structure, phase structure and molecular structure of collagen-PEO 600K blends for tissue engineering application. Am J Biochem Biotechnol. 2006;2:175–9. [Google Scholar]
- 31.Galler KM. Scaffolds for pulp regeneration and repair. In: Goldberg M, editor. The Dental Pulp: Biology, Pathology, and Regenerative Therapies. Germany: Springer; 2014. p. 252. [Google Scholar]
- 32.Vaissiere G, Chevallay B, Herbage D, Damour O. Comparative analysis of different collagen-based biomaterials as scaffolds for long-term culture of human fibroblasts. Med Biol Eng Comput. 2000;38:205–10. doi: 10.1007/BF02344778. [DOI] [PubMed] [Google Scholar]
- 33.Hatab TA, Kochaji N, Issa N, Nadra R, Saleh M, Rahmo A, et al. In vivo and immunohistochemical study of dentin and pulp tissue regeneration in the root canal. J Chem Pharm Res. 2015;7:302–10. [Google Scholar]
- 34.Wikipedia Contributors. Chitosan. Wikipedia. [Last cited on 2016 Jan 11]. Available from: https://www.en.wikipedia.org/wiki/Chitosan .
- 35.Croisier F, Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur Polym J. 2013;49:780–92. [Google Scholar]
- 36.Matsunaga T, Yanagiguchi K, Yamada S, Ohara N, Ikeda T, Hayashi Y. Chitosan monomer promotes tissue regeneration on dental pulp wounds. J Biomed Mater Res A. 2006;76:711–20. doi: 10.1002/jbm.a.30588. [DOI] [PubMed] [Google Scholar]
- 37.Muzzarelli RA. Chitosan composites with inorganic, morphogenetic proteins and stem cells for bone regeneration. Carbohydr Polym. 2011;83:1433–45. [Google Scholar]
- 38.Tziafas D, Amar S, Staubli A, Meyer JM, Ruch JV. Effects of glycosaminoglycans on in vitro mouse dental cells. Arch Oral Biol. 1988;33:735–40. doi: 10.1016/0003-9969(88)90007-6. [DOI] [PubMed] [Google Scholar]
- 39.Inuyama Y, Kitamura C, Nishihara T, Morotomi T, Nagayoshi M, Tabata Y, et al. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J Biomed Mater Res B Appl Biomater. 2010;92:120–8. doi: 10.1002/jbm.b.31497. [DOI] [PubMed] [Google Scholar]
- 40.Tan L, Wang J, Yin S, Zhu W, Zhou W, Cao Y, et al. Regeneration of dentin-pulp-like tissue using an injectable tissue engineering technique. RSC Adv. 2015;5:59723–37. [Google Scholar]
- 41.Yuan Z, Nie H, Wang S, Lee CH, Li A, Fu SY, et al. Biomaterial selection for tooth regeneration. Tissue Eng Part B Rev. 2011;17:373–88. doi: 10.1089/ten.teb.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bronzino J. Tissue Engineering and Artificial Organs. Boca Raton: CRC/Taylor and Francis; 2006. [Google Scholar]
- 43.Park ES, Cho HS, Kwon TG, Jang SN, Lee SH, An CH, et al. Proteomics analysis of human dentin reveals distinct protein expression profiles. J Proteome Res. 2009;8:1338–46. doi: 10.1021/pr801065s. [DOI] [PubMed] [Google Scholar]
- 44.Guo W, He Y, Zhang X, Lu W, Wang C, Yu H, et al. The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials. 2009;30:6708–23. doi: 10.1016/j.biomaterials.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, Xu G, Gao Z, Liu Z, Xu J, Wang J, et al. Demineralized dentin matrix induces odontoblastic differentiation of dental pulp stem cells. Cells Tissues Organs. 2016;201:65–76. doi: 10.1159/000440952. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27. doi: 10.1177/154411130401500103. [DOI] [PubMed] [Google Scholar]
- 47.Murata M, Sato D, Hino J, Akazawa T, Tazaki J, Ito K, et al. Acid-insoluble human dentin as carrier material for recombinant human BMP-2. J Biomed Mater Res A. 2012;100:571–7. doi: 10.1002/jbm.a.33236. [DOI] [PubMed] [Google Scholar]
- 48.Movin S, Borring-Møller G. Regeneration of infrabony periodontal defects in humans after implantation of allogenic demineralized dentin. J Clin Periodontol. 1982;9:141–7. doi: 10.1111/j.1600-051x.1982.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 49.Pietrzak WS, Ali SN, Chitturi D, Jacob M, Woodell-May JE. BMP depletion occurs during prolonged acid demineralization of bone: Characterization and implications for graft preparation. Cell Tissue Bank. 2011;12:81–8. doi: 10.1007/s10561-009-9168-6. [DOI] [PubMed] [Google Scholar]
- 50.Jindal SK. Silk scaffolds for dental tissue engineering. In: Kundu S, editor. Silk Biomaterials for Tissue Engineering and Regenerative Medicine. Amsterdam: Woodhead Publishing; 2014. pp. 403–28. [Google Scholar]
- 51.Blitterswijk C, Thomsen P. Tissue Engineering. London: Academic Press; 2008. [Google Scholar]
- 52.Park JY, Yang C, Jung IH, Lim HC, Lee JS, Jung UW, et al. Regeneration of rabbit calvarial defects using cells-implanted nano-hydroxyapatite coated silk scaffolds. Biomater Res. 2015;19:7. doi: 10.1186/s40824-015-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–55. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 54.Xu WP, Zhang W, Asrican R, Kim HJ, Kaplan DL, Yelick PC. Accurately shaped tooth bud cell-derived mineralized tissue formation on silk scaffolds. Tissue Eng Part A. 2008;14:549–57. doi: 10.1089/tea.2007.0227. [DOI] [PubMed] [Google Scholar]
- 55.Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci. 2009;10:1514–24. doi: 10.3390/ijms10041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16. doi: 10.22203/ecm.v005a01. [DOI] [PubMed] [Google Scholar]
- 57.Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, Shi S, et al. SHED differentiate into functional odontoblasts and endothelium. J Dent Res. 2010;89:791–6. doi: 10.1177/0022034510368647. [DOI] [PubMed] [Google Scholar]
- 58.Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–9. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly (lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15:3640–59. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–15. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singhal AR, Agrawal CM, Athanasiou KA. Salient degradation features of a 50:50 PLA/PGA scaffold for tissue engineering. Tissue Eng. 1996;2:197–207. doi: 10.1089/ten.1996.2.197. [DOI] [PubMed] [Google Scholar]
- 62.Horst OV, Chavez MG, Jheon AH, Desai T, Klein OD. Stem cell and biomaterials research in dental tissue engineering and regeneration. Dent Clin North Am. 2012;56:495–520. doi: 10.1016/j.cden.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chan G, Mooney DJ. New materials for tissue engineering: Towards greater control over the biological response. Trends Biotechnol. 2008;26:382–92. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Sharma S, Srivastava D, Grover S, Sharma V. Biomaterials in tooth tissue engineering: A review. J Clin Diagn Res. 2014;8:309–15. doi: 10.7860/JCDR/2014/7609.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burdick J, Mauck R. Biomaterials for Tissue Engineering Applications. Vienna: Springer; 2011. [Google Scholar]
- 66.Garg T, Bilandi A, Kapoor B, Kumar S, Joshi R. Scaffold: Tissue engineering and regenerative medicine. Int Res J Pharm. 2011;2:37–42. [Google Scholar]
- 67.Khanna-Jain R, Mannerström B, Vuorinen A, Sándor GK, Suuronen R, Miettinen S. Osteogenic differentiation of human dental pulp stem cells on â-tricalcium phosphate/poly (l-lactic acid/caprolactone) three-dimensional scaffolds. J Tissue Eng. 2012;3:1–11. doi: 10.1177/2041731412467998. [DOI] [PMC free article] [PubMed] [Google Scholar]


