Abstract
Background:
Several grafting materials have been used for alveolar ridge augmentation. The literature lacks researches to compare CenoBone to other grafting materials. The aim of this study was to compare CenoBone/CenoMembrane complex to Bio-Oss/Bio-Gide complex in lateral alveolar bone augmentation in terms of radiographic, histologic, and histomorphometric parameters.
Materials and Methods:
In this randomized controlled trial, ten patients who needed lateral ridge augmentation were selected and augmentations were done using either of CenoBone/CenoMembrane or Bio-Oss/Bio-Gide complexes. In the re-entry surgery in 6 months following augmentation, core biopsies were taken and clinical, radiographic, histologic, and histomorphometric evaluations were performed.
Results:
No statistically significant difference was seen between groups except for the number of blood vessels and percentage of residual graft materials.
Conclusion:
CenoBone seems to present a comparable lateral ridge augmentation to Bio-Oss in.
Key Words: Alveolar bone grafting, alveolar ridge augmentations, Bio-Oss
INTRODUCTION
Alveolar ridge deficiency resulting from congenital disorders, trauma, pathologic conditions, infections, or periodontal diseases challenges implant therapy.[1] Besides the aforementioned factors, a more common etiology of alveolar ridge resorption named loss of functional loading is followed by tooth loss and is the leading cause of ridge deficiency and responsible for up to 40–60% of alveolar bone loss in the 1st 3 years after tooth loss.[2] Enhancement of the atrophic ridge through ridge augmentation to favor it for implant placement has become a well-recognized treatment in implantology.[1]
Guiding the regenerative tissue by means of barrier membranes was first introduced by Melcher as a guided tissue regeneration (GTR) technique.[3] Applying the principles of GTR in periodontal healing processes leads to the guided bone regeneration (GBR) concept of regeneration, which aims to promote the tissue containing osteogenic cells to fill out the defect area.[4] This goal can be achieved using barrier membranes and particulateed or block grafts.[1] Different graft and membrane materials have been designed and applied in ridge augmentation, and several experimental and clinical studies have confirmed the effectiveness of the GBR method for ridge enhancement.[5,6,7]
Barrier membranes are categorized into two main groups: Resorbable and nonresorbable membranes. A membrane of the first group experiences biological degradation, with variable sustainability which may interfere with bone regeneration.[8] Those of the latter group need to be removed by re-entry surgery and impose greater risks for postoperative complications which lessen the clinical application of these membranes.[1] Several studies have compared the bone-promoting capacity of these two membranes.[9,10,11]
Autogenous bone grafts present osteogenic, osteoinductive, and osteoconductive properties and thus have become the gold standard among graft materials for bone regenerative procedures. Despite the mentioned advantages, their clinical use is restricted by their limited availability, fast rate of resorption, and donor site morbidity.[12]
Allografts, xenografts, and alloplasts are categorized as bone substitutes and have been developed to overcome problems with autografts.
Allografts are made up of human cadaveric bone and are presented in two forms of freeze-dried bone (FDBAs) or demineralized FDBAs (DFDBAs) grafts. It has been sated that DFDBAs have osteoconductive and osteoinductive properties.[12] Exposing the bone morphogenetic proteins during the demineralization process induces osteoblastogenesis.[13] However, donor-related factors, such as age and tissue processing-related factors including sterilization method, time lag, and temperature, can adversely affect the osteoinductve potential.[14]
Xenografts which originate from animals (e.g., bovine and equine) are other biomaterials with osteoconductive properties used for periodontal augmentation. These materials undergo chemical or low-heat processing to be deproteinized and consequently lessen the potential of antigenicity.[1,15] The processing technology allows the crystalline structure to be preserved.[15] Research has shown that these materials are safe and efficient grafting materials.[12]
Alloplasts are synthetic graft materials with different compositions and properties that are always used in combination with barrier membranes. One example is the combination of hydroxyapatite and beta-tricalcium phosphate which presents both scaffolding and osteoconductive properties.[16]
CenoBone (allograft made by Hamanand Saz Baft Tissue Regeneration Corporation) is an Iranian FDBA that has not been well investigated. A preliminary study to evaluate this material in alveolar ridge augmentation has shown promising results in terms of bone regeneration and alveolar width gain.[17] The use of CenoBone in socket preservation following tooth extraction showed decreased bone loss and successful results.[18] Sarkarat et al. compared OSSEO Plus with CenoBone in ridge preservation. They showed that the Iranian sample was comparable to OSSEOPlus, and both materials were similar in their capacity to preserve the height and width of the alveolar ridge.[19]
Till date, however, the literature lacks the research comparing CenoBone to other grafting materials in alveolar ridge augmentation. The present study compares the clinical, histologic, and histomorphometric parameters of CenoBone/CenoMembrane to those of Bio-Oss/Bio-Gide in lateral alveolar bone augmentation.
MATERIALS AND METHODS
This prospective semi-clinical trial study was conducted in the Department of Periodontology and Implant, Faculty of Dentistry, Babol University of Medical Sciences, Babol, Iran. The clinical trial protocol was approved by the Ethics Committee (No: 5121) of Babol University of Medical Sciences (Babol, Iran) and registered in the Iranian Registry of Clinical Trials (No: IRCT2015031621494N1).
Patients
Samples were selected from subjects aged between 30 and 50 years seeking implant therapy and required bone augmentation who presented at the Department of Periodontology and Implant, Faculty of Dentistry, Babol University of Medical Sciences. Patients with sufficient alveolar bone height and an alveolar ridge width of 2–4 mm in the area at a distance of 3 mm from the ridge crest were included in the study. Initial alveolar ridge width and bone density were measured by cone-beam computed tomography (CBCT) radiographs (Newtom 54, 8 × 8 field of view, time duration: 4 s) and related histograms. Subjects who suffered from certain systemic conditions which affect the healing process, including uncontrolled diabetes, immune disorders, alcohol addiction, drug abuse, current smoking, pregnancy, and immune suppressor or anticoagulant drug consumption were excluded from the survey. In addition, patients with poor compliance, sufferers from active periodontal diseases which would complicate oral hygiene, and subjects unwilling to participate in the study were also excluded. The minimum required sample size was determined to be eight for conducting semi-experimental clinical trials. Each patient was thoroughly evaluated to confirm that she/he met the study's selection criteria. Finally, ten patients, six males and four females, were selected. Study samples were provided with special instructions regarding oral hygiene and pre- and post-operation considerations.
Preoperative medication
Thirty minutes before surgery, each patient was received 2 g of amoxicillin orally and rinsed his/her mouth with 0.2% chlorhexidine mouthwash.[20]
Surgical procedure
Following anesthetizing, a conventional crestal incision was made on the edentulous ridge in the posterior area of mandible (in the region of mandibular premolars), and completed with two vertical releasing incisions 3 mm beyond the edges of the bone planned to be augmented anteriorly and posteriorly (to ensure full coverage of graft material after wound closure). A full thickness flap was elevated to provide complete access to the buccal bone, and all soft tissue was thoroughly removed. Following bone scratching with back action chisel, cortical buccal bone was perforated by a small round bur, and the periosteum was released to permit tension-free suturing of the flap. A horizontal mattress suture was used for primary closure of the wound which then completed with simple interrupted sutures in the area of crestal and releasing incisions.
Grouping
Patients were randomly assigned to either of the two experimental groups. Surgery was performed using either CenoBone/CENO Membrane (FDBA allograft, Hamanand Saz Baft Tissue Regeneration Corporation, Iran) or Bio-Oss/Bio-Gide (bovine bone xenograft, Geistlich biomaterials, Wolhusen, Switzerland) in each group. From the ten subjects participating in the study, three cases required bilateral alveolar ridge augmentation and received both CenoBone and Bio-Oss on opposite sides. Seven cases were unilateral and received either CenoBone (n = 4) or Bio-Oss (n = 3) graft only.
CenoBone group
In this group, mineralized cortical cancellous powder of CenoBone, 150–2000 μm in size, which had been immersed in normal saline for 30 min was placed in the area of the bone deficiency in a manner that provided sufficient buccal and lingual bone for subsequent implant installation. Graft materials were covered with a resorbable membrane of allogeneic pericardium origin (CENO Membrane) with a thickness of 0.2–0.6 mm.
Bio-Oss group
Bio-Oss bone granules, 500–100 μm in size, which had been immersed in normal saline for 30 min (to be assure of being hydrated) were used for the augmenting procedure in this group. Sufficient volume was provided by Bio-Oss in the buccal and lingual aspect of the alveolar ridge and was covered with a Bio-Gide membrane (of pig pericardium collagen) with a thickness of 0.2–0.6 mm.
After the graft was covered with membrane, the wound was closed with a combination of mattress and interrupted 5–0 vycryl sutures to adapt the wound edges.
Postoperative medications
Patients were prescribed amoxicillin and metronidazole, three times daily for a total of 10 days. They received appropriate doses of analgesic on surgery day. Patients were instructed to rinse three times daily with chlorhexidine gluconate 0.12% for 14 days.[21] Suture removal was planned for 14 days following augmentation, and a period of 6 months was considered for the healing process. Patients were on a monthly recall interval during that time.
Taking core biopsy and implantation
During the re-entry surgery in 6 months following augmentation, the full thickness flap was elevated and core biopsies were taken from the buccal wall with a 3-mm trephine bur. The depth of trephine penetration varied based on the extent of augmentation and the primary thickness of cortical bone. To take biopsies, it was necessary to reach the cancellous bone; therefore, considering the initial alveolar ridge width which was equal to 2–4 mm, and final alveolar ridge width of at least 6 mm, the approximate depth of trephine penetration was about 4–8 mm. Biopsy sites were filled with respective graft materials in each groups and covered with membranes. Implant sites were prepared in accordance with the manufacturers' instructions.
Histologic and histomorphometric processing and evaluation
Samples were placed in 10% formalin and sent to the Department of Pathology at Babol Dental School. The specimens were kept in that solution for complete fixation for 10 days. After fixation, the samples were decalcified by being kept in 10% formic acid for 10 days and were evaluated daily during that time. Decalcified, the samples were removed from formic acid and immersed in 20% lithium bicarbonate neutralizing acidic solution. Each sample was assigned a number and divided vertically into two sections in the anteroposterior direction. The sectioned edge, which represented the middle portion of the bone, was marked with the assigned number written on it in Indian ink. The specimens were dehydrated by a series of alcohols and embedded in blocks of paraffin. Each paraffin block was cut into seven slices 5 μm thick. The slides were stained with hematoxylin and eosin staining and evaluated under BX41 Olympus light microscopy (Tokyo, Japan). The following parameters were measured in the histopathologic evaluations:
-
Degree of inflammation was determined and categorized into five grades:[22]
Grade 0: Absence of inflammatory cells
Grade 1: Presence of few and sporadic inflammatory cells
Grade 2: Presence of 5–10 foci of inflammatory cells
Grade 3: Presence of 10–50 foci of inflammatory cells
Grade 4: Presence of more than 50 foci of inflammatory cells
Presence or absence of foreign body reaction (giant cell and granulomatosis reaction)
Bone vitality (presence or absence of osteocyte containing lacuna)
Bone/biomaterial contact surface (presence or absence of connective tissue in the interface of bone and biomaterial)
-
Number of blood vessels, measured in 3 microscopic fields at ×10 and reported as follows:[23]
0: Observation of <3 blood vessels
1: Observation of 3–5 blood vessels
2: Observation of more than 5 blood vessels
Photomicrographs of the core slides were obtained using a Nikon Cooplix 4500 digital camera (Nikon Corp, Japan) at a fixed focal point and ×40. Images in JPEG format were entered in SIS LS Starter software, and the following parameters were measured:
Percentage of new bone formation: Areas of newly-formed bone were selected and the percentage of bone formation was measured by dividing the surface area of the bone by the surface area of the respective image
Trabecular thicknesses were measured as an item involved in determination of newly formed bone quality[24] and the mean value of trabecular thickness in each study group was determined and compared between study groups
Percentage of residual graft material.
All measurements were taken by an oral and maxillofacial pathologist who was blinded to the study protocol to prevent any bias in interpreting the results. To enhance accuracy, seven slides from each biopsy sample were prepared and evaluated for each of the mentioned parameters; the average value was considered for statistical analysis.
Radiographic evaluation
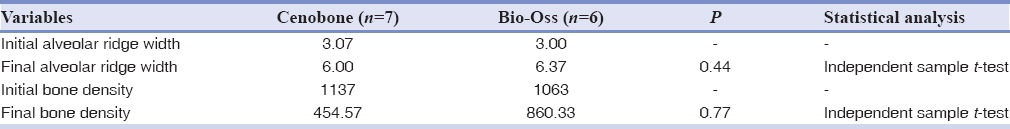
As previously described, initial alveolar ridge width and bone density were measured by CBCT radiographs (Newtom 54, 8 × 8 FOV, time duration: 4 s) and related histograms. Following 6 months period required for healing, second CBCT radiographs with similar parameters to initial ones were taken and final alveolar ridge width and bone density were determined. Related data and analyses can be seen in Table 3.
Table 3.
Comparison of radiographic variables between study groups
Statistical analysis
Histologic and histomorphometric data was entered into the statistical package for social sciences (SPSS) version 18 for Windows (SPSS, Inc., Chicago, Illinois, USA). The Kolmogorov–Smirnov test was performed to assess the mode of data distribution. For normally distributed data, the groups were compared by the two-sample t-test for independent samples. The Mann–Whitney U-test was used to compare nonparametric data. Nominal parameters were compared using Chi-square analysis. Statistical significance was considered to be P ≤ 0.05.
RESULTS
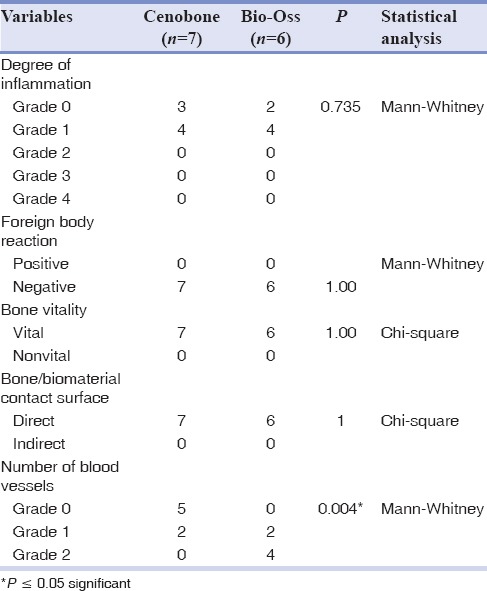
As can be seen from Table 1, all histopathologic variables except for number of blood vessels were comparable between the two experimental groups.
Table 1.
Comparison of histopathologic variables
Among the histomorphometric parameters, the percentage of residual graft material was significantly higher in the Bio-Oss group. Table 2 Also, Comparison of radiographic variables between study groups is presented in Table 3.
Table 2.
Comparison of histomorphometric variables
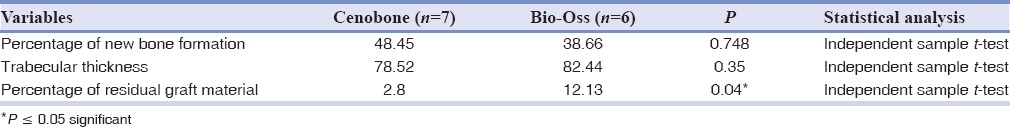
No significant differences in histomorphometric parameters were seen between the groups.
DISCUSSION
As previously described in the results section, histologic and histomorphometric analyses failed to reveal any statistically significant differences between studied parameters except for number of blood vessels and percentage of residual graft materials. CenoBone showed comparable results to those of Bio-Oss in terms of new bone formation, trabecular thickness of newly-formed bone, and inflammatory parameters.
Horizontal ridge augmentation can be performed using different graft materials and membranes. Autogenous bone grafts in particulate or block forms have been shown to provide successful and predictable results.[25,26,27,28] In spite of the success of autogenous bone grafts in lateral ridge augmentation, their widespread use is hindered by inherent limitations, including morbidity related to graft harvesting and a high rate of resorption.[1] Bone substitutes, mainly those of xenogenic nature, have been introduced for the augmentation procedures and have shown successful results with low morbidity and few postoperative complications. These materials experience very slow resorption which leads to long-term stability.[1]
In a systematic review, the literature was analyzed to compare the bone substitute materials (BSMs) with autologous bone (AB) in augmentation of the edentulous jaw. The results of this study showed no evidence of the superior performance of AB over BSM.[29]
CenoBone (allograft made by Hamanand Saz Baft Tissue Regeneration Corporation) is an Iranian FDBA allograft. Few investigations have studied this allograft in ridge preservation, dehiscence defects, or lateral ridge augmentation.[17,18,19,30,31] In a study conducted to evaluate two types of DFDBA (DemoBone: American DFDBA and CenoBone: Iranian DFDBA) in the treatment of dehiscence defects around implants in dogs, no statistically significant difference was seen in bone implant contact, rate of bone formation, and implant stability quotient among the groups.[30]
In a radiographic evaluation of the effectiveness of CenoBone graft material in preventing alveolar ridge resorption following tooth extraction, it was shown that CenoBone performed appropriately in the regeneration of osseous defects and ridge preservation.[18] Comparing the ridge preservation potential of OSSEO+ (allograft, made by IMTEC Corporation) with that of CenoBone showed that both materials performed similarly in preserving the height and width of the alveolar ridge. It was mentioned that the foreign sample did not show the superior properties compared with the Iranian one.[19]
In a preliminary study to evaluate CenoBone bone strips allograft with resorbable membrane in lateral ridge augmentation, clinical, histologic, and histomorphometric analyses were done. The results of this preliminary study revealed the induction of bone formation and a significant alveolar width gain at distances of 2 and 5 mm from the alveolar crest that were obtained by CenoBone allograft.[17] This confirms the bone formation potential of CenoBone which is in line with the results.
Some studies have shown that BSMs were capable of bone formation in the range of 14–44%.[32] The percentages of bone formation by the different tested DFDBA graft materials were reported as 41.74%[33] and 58.43% (CenoBone DFDBA)[17] in previous studies. The latter is consistent with the 48.45% new bone formation measured in the current study.
Based on the results of this study, Grade 1 inflammation was the main mode in both study groups. No foreign body reaction was seen in the groups, and all bone specimens were vital.
The study groups differ significantly in terms of residual graft materials. Microscopic evaluation showed significantly more remnants of biomaterial in the Bio-Oss group (12.13%) compared with the CenoBone group (2.8%). This difference can be explained in part by the slow resorbing nature of Geistlich Bio-Oss claimed by the manufacturer, which is a desirable characteristic and ensures long-term volume preservation and complete integration of the biomaterial into living bone. These results are consistent with those of other studies which found 4.07% residual biomaterial for CenoBone (in the range of 0.56–8.19%)[17] and 15% for a combination of 50% Bio-Oss and 50% DFDBA.[31] The percentage of residual biomaterial was 8.88% for the DFDBA group compared to 25.42% for the FDBA group.[34]
The interface of bone/biomaterial in all specimens of this study revealed direct contact without any connective tissue. In contrast to this finding, a preliminary study assessing CenoBone in lateral ridge augmentation showed that 57.1% of cases had direct contact, while in 42.9% of cases, the contact was mediated with connective tissue.[17] This difference can be partly explained by the different methodologies used in augmenting procedures.
The number of blood vessels which entered the newly-formed bone was significantly higher in the Bio-Oss group. This observation can be attributed to its topographic structure which features a unique and highly efficient system of pores that support optimal bone ingrowth for healthy bone formation.
Geistlich Bio-Oss has been called the most successful bone substitute worldwide[35] and remains the best choice among different graft materials. Considering the unique properties of Bio-Oss, which ranked it as one of the best choices of bone substitutes, the comparable results obtained by the CenoBone allograft in this study seem promising.
Conducting the research on human cases was one of the advantages of this study, and it allows for extrapolation of the results. However, because of the small sample size, results should be interpreted cautiously. Further clinical trials with larger samples and longer follow-up times need to be undertaken before making conclusive statements in this regard.
CONCLUSION
This study compared two bone substitutes in alveolar ridge augmentation: CenoBone (Iranian DFDBA) and Bio-Oss (Geistlich biomaterials, Wolhusen, Switzerland Xenograft). The histopathologic and histomorphometric analyses showed comparable results for these biomaterials, except for higher blood vessels and more residual graft materials in the Bio-0ss specimens. CenoBone seems to be appropriate and successfull as a biomaterial for lateral bone augmentation.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or non-financial in this article.
REFERENCES
- 1.Rios HF, Vignoletti F, Giannobile WV, Sanz M. Ridge augmentation procedures. In: Lang NP, Lindhe J, editors. Clinical periodontology and implant dentistry. 6th ed. Oxford: Blackwell Munksgaard; 2015. pp. 1092–114. [Google Scholar]
- 2.Carlsson GE, Bergman B, Hedegård B. Changes in contour of the maxillary alveolar process under immediate dentures. A longitudinal clinical and x-ray cephalometric study covering 5 years. Acta Odontol Scand. 1967;25:45–75. doi: 10.3109/00016356709072522. [DOI] [PubMed] [Google Scholar]
- 3.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–60. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 4.Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81:672–6. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto MA, Filho HN, Francischone AE, Consolaro A. Microscopic analysis of reconstructed maxillary alveolar ridges using autogenous bone grafts from the chin and iliac crest. Int J Oral Maxillofac Implants. 2002;17:507–16. [PubMed] [Google Scholar]
- 6.Glowacki J, Altobelli D, Mulliken JB. Fate of mineralized and demineralized osseous implants in cranial defects. Calcif Tissue Int. 1981;33:71–6. doi: 10.1007/BF02409414. [DOI] [PubMed] [Google Scholar]
- 7.Alonso N, Machado de Almeida O, Jorgetti V, Amarante MT. Cranial versus iliac onlay bone grafts in the facial skeleton: A macroscopic and histomorphometric study. J Craniofac Surg. 1995;6:113–8. doi: 10.1097/00001665-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Warrer K, Karring T, Nyman S, Gogolewski S. Guided tissue regeneration using biodegradable membranes of polylactic acid or polyurethane. J Clin Periodontol. 1992;19(9 Pt 1):633–40. doi: 10.1111/j.1600-051x.1992.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 9.Hürzeler MB, Quiñones CR, Schüpbach P. Guided bone regeneration around dental implants in the atrophic alveolar ridge using a bioresorbable barrier. An experimental study in the monkey. Clin Oral Implants Res. 1997;8:323–31. doi: 10.1034/j.1600-0501.1997.080411.x. Erratum in: Clin Oral Implants Res 1997;8:535-6. [DOI] [PubMed] [Google Scholar]
- 10.Hürzeler MB, Kohal RJ, Naghshbandi J, Mota LF, Conradt J, Hutmacher D, et al. Evaluation of a new bioresorbable barrier to facilitate guided bone regeneration around exposed implant threads. An experimental study in the monkey. Int J Oral Maxillofac Surg. 1998;27:315–20. doi: 10.1016/s0901-5027(05)80623-x. [DOI] [PubMed] [Google Scholar]
- 11.Rothamel D, Schwarz F, Sculean A, Herten M, Scherbaum W, Becker J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin Oral Implants Res. 2004;15:443–9. doi: 10.1111/j.1600-0501.2004.01039.x. [DOI] [PubMed] [Google Scholar]
- 12.Yukna RA. Synthetic bone grafts in periodontics. Periodontol 2000. 1993;1:92–9. [PubMed] [Google Scholar]
- 13.Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50:1392–406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- 14.Moore TM, Artal R, Arenas M, Gendler E. Influence of postmortem time and temperature on osteoinductive activity of demineralized microperforated ethylene oxide-sterilized syngeneic bone implant in the rat. Clin Orthop Relat Res. 1990;259:239–44. [PubMed] [Google Scholar]
- 15.Gross JS. Bone grafting materials for dental applications: A practical guide. Compend Contin Educ Dent. 1997;18:1013. [PubMed] [Google Scholar]
- 16.Rogers KD, Daniels P. An X-ray diffraction study of the effects of heat treatment on bone mineral microstructure. Biomaterials. 2002;23:2577–85. doi: 10.1016/s0142-9612(01)00395-7. [DOI] [PubMed] [Google Scholar]
- 17.Amooian B, Seyyed Majidi M, Haji Ahmadi M, Kiakojuri A. Clinical, histologic and histomorphometric evaluation of bone strip allograft with resorbable membrane in horizontal alveolar ridge augmentation: A preliminary study. Beheshti Univ Dent J. 2014;32:17–26. [Google Scholar]
- 18.Azimi H, Jalayer T, Babaee H. Radiographic evaluation of the inhibitory effect of ceno-bone on alveolar bone resorption after tooth extraction. J Res Dent Sci. 2012;9:156–60. [Google Scholar]
- 19.Sarkarat F, Sadri D, Bohlooli B, Lozani S. Ridge preservation with OSSEO+compared to cenobone for implant site development: A clinical and histologic study in humans. J Res Dent Sci. 2012;7:1–7. [Google Scholar]
- 20.Cortellini P, Tonetti MS. Regenerative periodontal therapy. In: Lindhe J, Lang NP, Karring T, editors. Clinical Periodontology and Implant Dentistry. 5th ed. Oxford: Blackwell Munksgaard; 2008. p. 937. [Google Scholar]
- 21.Cortellini P, Tonetti MS. Regenerative periodontal therapy. In: Lindhe J, Lang NP, Karring T, editors. Clinical Periodontology and Implant Dentistry. 5th ed. Oxford: Blackwell Munksgaard; 2008. p. 926. [Google Scholar]
- 22.Paknejad M, Rokn A, Rouzmeh N, Heidari M, Titidej A, Kharazifard MJ, et al. Histologic evaluation of bone healing capacity following application of inorganic bovine bone and a new allograft material in rabbit calvaria. J Dent (Tehran) 2015;12:31–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Hasheminia SM, Feizi G, Razavi SM, Feizianfard M, Gutknecht N, Mir M. A comparative study of three treatment methods of direct pulp capping in canine teeth of cats: A histologic evaluation. Lasers Med Sci. 2010;25:9–15. doi: 10.1007/s10103-008-0584-9. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim N, Parsa A, Hassan B, van der Stelt P, Wismeijer D. Diagnostic imaging of trabecular bone microstructure for oral implants: A literature review. Dentomaxillofac Radiol. 2013;42:20120075. doi: 10.1259/dmfr.20120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorellini JP, Nevins ML. Localized ridge augmentation/preservation. A systematic review. Ann Periodontol. 2003;8:321–7. doi: 10.1902/annals.2003.8.1.321. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz-Arad D, Levin L. Intraoral autogenous block onlay bone grafting for extensive reconstruction of atrophic maxillary alveolar ridges. J Periodontol. 2005;76:636–41. doi: 10.1902/jop.2005.76.4.636. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz-Arad D, Levin L, Sigal L. Surgical success of intraoral autogenous block onlay bone grafting for alveolar ridge augmentation. Implant Dent. 2005;14:131–8. doi: 10.1097/01.id.0000165031.33190.0d. [DOI] [PubMed] [Google Scholar]
- 28.Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: Systematic assessment of available options (barrier membranes, bone grafts, split osteotomy) J Clin Periodontol. 2008;35(8 Suppl):173–202. doi: 10.1111/j.1600-051X.2008.01269.x. [DOI] [PubMed] [Google Scholar]
- 29.Al-Nawas B, Schiegnitz E. Augmentation procedures using bone substitute materials or autogenous bone - A systematic review and meta-analysis. Eur J Oral Implantol. 2014;7(Suppl 2):S219–34. [PubMed] [Google Scholar]
- 30.Abed AM, Pestekan RH, Yaghini J, Razavi SM, Tavakoli M, Amjadi M. A comparision of two types of decalcified freeze-dried bone allograft in treatment of dehiscence defects around implants in dogs. Dent Res J (Isfahan) 2011;8:132–7. [PMC free article] [PubMed] [Google Scholar]
- 31.Noumbissi SS, Lozada JL, Boyne PJ, Rohrer MD, Clem D, Kim JS, et al. Clinical, histologic, and histomorphometric evaluation of mineralized solvent-dehydrated bone allograf (Puros) in human maxillary sinus grafts. J Oral Implantol. 2005;31:171–9. doi: 10.1563/1548-1336(2005)31[171:CHAHEO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Tadjoedin ES, de Lange GL, Holzmann PJ, Kulper L, Burger EH. Histological observations on biopsies harvested following sinus floor elevation using a bioactive glass material of narrow size range. Clin Oral Implants Res. 2000;11:334–44. doi: 10.1034/j.1600-0501.2000.011004334.x. [DOI] [PubMed] [Google Scholar]
- 33.Cammack GV, 2nd, Nevins M, Clem DS, 3rd, Hatch JP, Mellonig JT. Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent. 2005;25:231–7. [PubMed] [Google Scholar]
- 34.Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol. 2012;83:329–36. doi: 10.1902/jop.2011.110270. [DOI] [PubMed] [Google Scholar]
- 35.US Dental Bone Graft Substitutes and Other Biomaterials Market. IData Research Inc. 2012. [Last accessed on 2016 Feb 15]. Available at: https://www.idataresearch.com/.../us-market-for-dental-bone-graft-substit .


