1. Introduction
Transplant glomerulopathy (TG) in an allograft biopsy is typically defined as double contours of the glomerular basement membrane (GBM) in the absence of immune complexes. It results from chronic glomerular endothelial injury and subsequent GBM remodeling and is commonly associated with antibody-mediated rejection (AMR). It may be accompanied by glomerular infiltration (glomerulitis) with cytotoxic T lymphocytes and, to a lesser extent, monocytes. We describe a case of “acute” TG, represented by mainly endothelial injury and a monocyte-predominant infiltrate resulting in unusual histology and potential for misdiagnosis.
2. Case Report
A 48-year old woman with end-stage kidney disease underwent kidney transplantation four years after initiation of dialysis. She had a history of systemic lupus erythematosis diagnosed 16 years ago. She developed class V lupus nephritis that progressed over a several years to end-stage kidney disease despite treatment with prednisone, mycophenolate and rituximab. Her extra-renal manifestations of lupus included polyarthritis, alopecia, stomatitis, photosensitivity, pleurisy and skin rash.
At the time of kidney transplant, her lupus was clinically quiescent on maintenance therapy of prednisone 5 mg daily. She received a six antigen mismatched deceased donor kidney transplant. Pre-operative class I and II PRA were 25% and 63%, respectively, with no detectable anti-donor antibodies. She received alemtuzumab induction therapy and was maintained on tacrolimus, mycophenolate mofetil (MMF) and prednisone. Valganciclovir and co-trimoxazole were administered as prophylaxis against infections. Allograft function was prompt; serum creatinine fell briskly to a nadir of 1.0 mg/dl.
Two months following transplant, she was found to be neutropenic on surveillance blood testing, prompting discontinuation of MMF, valganciclovir and co-trimoxazole. Shortly afterwards she presented with fever, malaise and sore throat. Blood and urine cultures and cytomegalovirus PCR were negative. She was treated with granulocyte colony stimulating factor and doxycyline. Her absolute neutrophil count rapidly improved from 0.209 × 103/μL to 1.665 × 103/μL (normal range; 1.8–7.7 × 103/μL) and her symptoms resolved. Mycophenolate mofetil was discontinued and she was discharged on maintenance tacrolimus and prednisone.
A week later her serum creatinine increased from 1.1 to 3.0 mg/dl. Urinalysis revealed +1 blood, no protein, no white cells. Anti-dsDNA was negative (in contrast to a titer >1:1280 three years prior to transplant). She was empirically treated with three daily doses of oral prednisone 50 mg and underwent an urgent allograft biopsy.
Light microscopy revealed 45 hypercellular glomeruli with loss of capillary luminal patency. Prominent endothelial swelling was observed along with luminal occlusion and rare infiltrating lymphocytes (Figure 1A). Except for rare foci of irregularities in the GBM, there was no significant thickening or reduplication. No glomerular deposits, karyorrhexis, GBM necrosis or crescents were identified. Mild interstitial edema was noted along with sparse mononuclear infiltrate involving approximately 40% of the renal parenchyma. Foci of tubulitis were rare with 4–5 lymphocytes per tubular cross section. Peritubular capillaritis was minimal accounting for < 10% of the sample. No tubular atrophy or interstitial fibrosis was noted and blood vessels were unremarkable. No viral inclusions were seen and immunostain for cytomegalovirus was also negative. C4d immunohistochemistry was negative in both glomeruli and peritubular capillaries.
Figure 1.
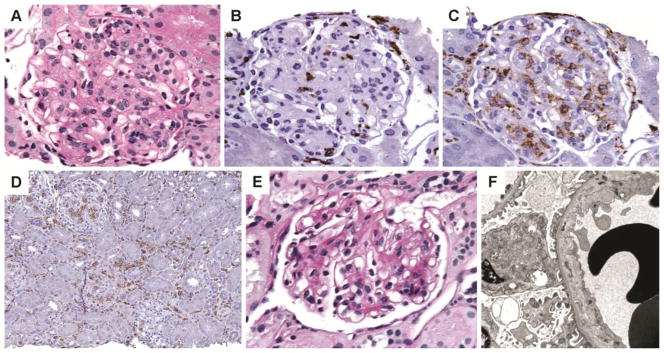
Renal allograft biopsy findings. A–D: First biopsy. A: The glomerulus shows diffuse endothelial swelling and obliterated capillary lumina (PAS). B: The glomerular intracapillary cells have a few cytotoxic CD8+ cells and C: numerous CD168+ monocytes/macrophages. D: Patchy interstitial infiltrates of monocytes/macrophages staining for CD163. E–F: Second allograft biopsy. E: Glomeruli with segmental mesangial expansion, GBM irregularities and occasional lymphocytes (PAS) F: Electron microscopy shows subendothelial widening by electron lucent material
Minimal glomerular and interstitial infiltrate prompted immunohistochemical characterization of cells occluding the glomerular capillary loops. Immunohistochemical analysis was performed using antisera against CD3 (pan T cell maker) CD4 (helper T-cell marker), CD8 (cytotoxic T-cell marker), CD163 (monocyte/macrophage marker) and CD20 (B lymphocyte marker). Although swollen endothelium accounted for several intraglomerular cells, there were abundant monocytes/macrophages in all glomeruli (g3 by Banff 2007). A few glomeruli had only 7–8 CD163+ cells, but most had 20 or more intraglomerular monocytes/macrophages (Figure 1C). The mean number of monocytes (CD163+) per glomerulus was 18.2 cells, mean number of CD3+ T cells was 4.1 cells/glomerulus and the mean glomerular monocyte-T cell ratio was 4.4. In addition, prominent interstitial CD163 infiltrate was identified that was underappreciated on light microscopy (Figure 1D). In contrast, interstitial and intraglomerular CD3+T lymphocytes were sparse and most were subtyped as CD8+ cytotoxic T cells (Figure 1B). Only occasional CD4+ helper T cells and rare CD20+ B lymphocytes were identified.
Immunofluorescence microscopy was negative except for trace mesangial IgM and fibrinogen and there was no evidence of recurrent lupus nephritis. No glomeruli were sampled for electron microscopy and hence, ultrastructural examination was performed on paraffin embedded tissue. Although electron dense deposits were not identified, features of TG such as endothelial cell swelling and loss of fenestrations could not be adequately assessed due to fixation artifact.
Laboratory tests sent at the time of biopsy revealed new complement-fixing donor specific anti-HLA antibodies [measured using the C1q single antigen bead assay(1)] to HLA loci A24 (7318–9186 mean fluorescent units) and Cw6 (5183 mean fluorescent units). Additionally, she had markedly reduced total (832/μL) and CD4 (33/μL) lymphocytes counts consistent with her alemtuzumab induction therapy.
The biopsy diagnosis was acute TG with mild acute tubulointerstitial rejection. Although C4d stain was negative, positive donor specific antibody titers were compatible with acute AMR. Per Banff 2007 criteria, the biopsy also had grade 1A acute cellular rejection and the glomerular changes were scored as g3.
The glomerular changes were rather atypical with predominantly monocyte/macrophage infiltrate and paucity of T lymphocytes. Endothelial swelling was prominent, but no GBM reduplication was evident, most compatible with acute TG occurring within 3 months after transplantation. Recurrent lupus nephritis was also considered, but lack of immune deposits, glomerular necrosis, karyorrhexis and crescents, combined with negative anti-ds DNA titers made this diagnosis unlikely. In the absence of fibrin thrombi, low platelets or schistocytes, there was no support for acute thrombotic microangiopathy; glomerular mononuclear infiltrate would also be unusual in TMA. TG like changes unrelated to membranoproliferative glomerulonephritis or cryoglobulinemia have been described in hepatitis C infection, but the patient was HCV antibody negative.
Subsequent to the biopsy, the tacrolimus dose was increased, MMF restarted and prednisone slowly tapered. She received an infusion of intravenous immunoglobulin (IVIg). Over the next few weeks her serum creatinine improved to 1.1 mg/ul and the anti-donor specific antibody titers became undetectable. A second renal allograft biopsy was performed one month later to guide treatment and revealed substantial interval improvement. Only mild segmental increase in luminal lymphocytes was observed compatible with transplant glomerulitis (g1). Segmental mesangial expansion was observed, along with rare GBM double contours (cg1) (Figure 1E). No tubulointerstitial inflammation was seen. Immunofluorescence microscopy and C4d were negative. Electron microscopy showed widening of subendothelial space, endothelial swelling and focal GBM duplication compatible with TG (Figure 1F).
Six months later she is well with a creatinine of 1.0 mg/dl, urine-albumin-creatinine ratio < 30 mg/g, neutrophils of 2–3 103/μL and undetectable anti-donor antibodies.
3. Discussion
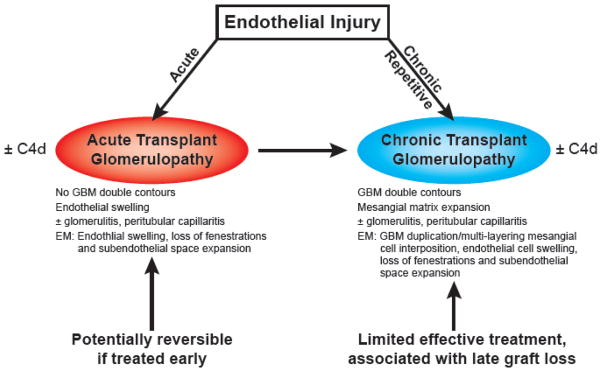
Acute transplant glomerulopathy (TG) refers to alloimmune mediated endothelial injury and glomerular inflammation that typically occurs within 6 months of transplantation (2–4) Ultrastructural evidence of acute TG has been reported as early as 1 month post transplantation (5). Acute TG is a relatively uncommon diagnosis; its light microscopic findings are relatively subtle and its frequent lack of overt clinical manifestations may not prompt an allograft biopsy. Acute TG is variably associated with peritubular capillaritis (frequently described as microcirculation inflammation). Chronic/overt TG, at the other end of the spectrum, is characterized by GBM double contours and variable mesangial matrix expansion readily recognized on PAS or silver stained sections (Figure 2). The earliest demonstrable changes of chronic TG may be manifest only at an ultrastructural level with the light microscopic findings of GBM duplication or multilamination lagging behind. Studies have shown that chronic/overt TG is usually preceded by acute TG and is associated with poor graft survival (6).
Figure 2.
Etiology of and relationship between acute and chronic transplant glomerulopathy
The earliest histologic change in acute TG is diffuse endothelial and mesangial cell swelling with lobular simplification and mesangiolysis (4). It is often associated with glomerular capillary mononuclear cell infiltration. Accumulating lymphocytes comprise of mostly cytotoxic T cells (7). Monocytes are seen but are not usually prominent (8). Immune complexes are not deposited except for occasional nonspecific IgM. The ultrastructural appearance closely parallels light microscopic findings with endothelial cytoplasmic swelling, loss of fenestrations and subendothelial electron-lucent widening (2). These early ultrastructural changes (seen in the first 1–3 months post-transplantation) are associated with subsequent development of chronic/overt TG (9).
Although earlier studies erroneously linked CMV viremia to acute TG based on temporal association between onset of viremia and allograft dysfunction, no such correlation was found subsequently (2). Chronic hepatitis C virus (HCV) infection in recipient or donor increases the prevalance of acute TG in early post transplanatation period (10, 11). Although HCV infection can also cause membanoproliferative glomerulonephritis, the absence of cryoglobulins, immune complex deposits and hypocomplementemia point toward the diagnosis of acute TG. While low level immune deposition with resorption prior to biopsy is a possibility, HCV-related thrombotic microangiopathy may underlie TG-like changes in an allograft (12, 13).
However, it is increasingly evident that acute as well as chronic/overt TG is often caused by circulating anti-HLA donor specific alloantibodies (DSA) and is frequently associated with C4d (6, 14). This causal link with alloantibodies is supported by a recent study in which the patients with electron microscopic evidence of early TG and positive DSA were treated for AMR thus preventing the development of overt TG (5). In addition to AMR, T cell mediated non-alloantibody processes may also play a role in pathogenesis of TG (15).
Acute TG is often associated with tubulointerstitial cellular rejection and results in poorer long-term prognosis possibly due to progression to overt TG and chronic AMR (6). Although distinct features of acute TG were well recognized, it was not incorporated in Banff grading of acute rejection due to lack of independent predictability of graft survival on multivariate analysis (16–18). However, newer studies have demonstrated that semi-quantitative assessment of glomerulitis does correlate with clinically important transplant outcomes (19). For instance, the ‘microinflammation score’, a quantification of glomerulitis and peritubular capillaritis in renal biopsies (the sum of g and ptc), is highly predictive of positive DSA-staining (20).
C4d staining in peritubular capillaries and glomeruli (by immunohistochemistry) is a marker of AMR. It has been shown that predominance of macrophages in acute TG, especially when associated with prominent glomerulitis (g2/g3 by Banff), is frequently associated with C4d (8, 21, 22). The absence of C4d in our patient may represent C4d-negative AMR (8, 23) or may represent a false negative immunohistochemical C4d stain. Gene expression microarrays have demonstrated that despite the absence of C4d, overexpression of endothelial-associated genes in the presence of DSA is associated with increased graft loss, providing the initial evidence of C4d negative AMR (23). C4d negative AMR clearly increases the risk of progression to chronic TG, but is less aggressive in comparison to C4d positive AMR (24, 25).
Prominent infiltration of monocytes in glomeruli correlates with worse prognosis (26), independent of C4d status (27). Although not routinely immunophenotyped, interstitial macrophage infiltrate in acute rejection may also be a poor prognostic sign. While the quantitative threshold for ”monocyte rich” transplant glomerulitis is not defined, one study showed an association between 3.01 ± 2.35 (versus 0.95 ± 1.00) monocytes per glomerulus with C4d positive transplant glomerulitis. In the same study, no significant differences were found in the T cell infiltrate per glomerulus in C4d positive versus C4d negative transplant glomerulitis (1.4 ± 0.9 versus 1.1 ±1.7)(8).
Glomerular lesions observed in allograft biopsies from patients with a history of lupus nephritis include proliferative glomerulonephritis with scant immune deposits (28). The histological features in such cases, while distinct from typical TG or acute transplant glomerulitis, can mimic acute TG. However, in the absence of serological support for active lupus and lack of glomerular necrosis, crescents and tubuloreticular inclusions in our patient, we considered recurrent lupus or lupus-related glomerular lesions unlikely. The progression of histological changes to typical TG in the repeat biopsy also supports our impression.
Alemtuzumab, a CD52-specific monoclonal antibody, binds to surface CD52 receptor on T cells, B ells and to lesser extent on monocytes and natural killer cells. Alemtuzumab induction is comparable to anti-thymocyte globulin in in terms of acute rejection rate in immunologically high-risk kidney transplant patients (29). Humoral rejection has been reported early following transplantation with alemtuzumab and one large trial have showed a non-statistically significant trend toward increased C4d staining on acute rejection biopsies in alemtuzumab versus thymoglobulin induction in sensitized patients in a post-hoc analysis (29, 30). Thus, there is a distinct possibility that alemtuzumab induction may have predisposed our patient to develop early AMR.
Alemtuzumab results in transient but marked peripheral and secondary lymphoid T cell depletion (31) due to complement mediated cell lysis. B cells and monocytes are also suppressed to a lesser degree. Despite this profound T cell depletion, early (2–4 weeks post transplantation) rejection episodes occur in alemtuzumab-induced patients in the absence of other adjuvant and chronic immunosuppressive therapy. Transcriptional analysis (using RT-PCR) of these allograft biopsies had significantly elevated chemokines of macrophage and monocyte lineage relative to T lymphocytes (31). Immunophenotypic analysis of allograft biopsies has demonstrated that percentage of interstitial monocyte infiltrate is significantly higher with alemtuzumab induction than with other protocols or no induction (31, 32). In fact, monocyte infiltration precedes clinical manifestations of acute rejection as evidenced by serial protocol biopsies (31). In alemtuzumab-treated patients with acute cellular rejection there is relative paucity of T lymphocytes with those present exhibiting a memory phenotype (31, 32). We propose that monocyte rich acute TG in our patients is due to profound T cell depletion and relative abundance of monocytes and macrophages associated with alemtuzumab therapy.
In our patient, the episode of rejection and development of new DSA was preceded by a period of protracted neutropenia for which her anti-metabolite (MMF) therapy was withheld. There are several reports linking neutropenia and prolonged time off anti-metabolite therapy to adverse outcomes including allograft rejection, graft loss and death in transplant recipients (33, 34). However, the use of granulocyte colony stimulating factor in these patients appears to safely expedite recovery without increasing the risk of adverse events.
The treatment of acute transplant glomerulitis, at least when accompanied by pathological features of AMR, C4d staining or DSA is designed to reduce circulating anti-donor antibody with IVIG, plasmapharesis and rituximab.
4. Conclusion
Although C4d was negative in our biopsy, the monocyte infiltrate, transplant glomerulitis and detectable alloantibody indicate an acute AMR developing in a patient who received alemtuzumab induction. The monocyte/macrophage influx in the glomerulus and interstitium in our patient was likely exacerbated by alemtuzumab induction, and this together with severe endothelial swelling resulted in an unusual histological picture.
Highlights.
Acute transplant glomerulitis typically occurs early post-transplant.
We describe a case of acute transplant glomerulitis with monocyte-rich infiltrate.
We hypothesize that alemtuzumab is responsible for the unusual histological findings.
We discuss the association between acute transplant glomerulitis, chronic glomerulitis and antibody mediated rejection.
Acknowledgments
Sources of support: None
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91(3):342–347. doi: 10.1097/TP.0b013e318203fd26. [DOI] [PubMed] [Google Scholar]
- 2.Axelsen RA, Seymour AE, Mathew TH, Canny A, Pascoe V. Glomerular transplant rejection: a distinctive pattern of early graft damage. Clin Nephrol. 1985;23(1):1–11. [PubMed] [Google Scholar]
- 3.Husain S, Sis B. Advances in the Understanding of Transplant Glomerulopathy. Am J Kidney Dis. 2013 doi: 10.1053/j.ajkd.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 4.Maryniak RK, First MR, Weiss MA. Transplant glomerulopathy: evolution of morphologically distinct changes. Kidney Int. 1985;27(5):799–806. doi: 10.1038/ki.1985.83. [DOI] [PubMed] [Google Scholar]
- 5.Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant. 2011;11(10):2123–2131. doi: 10.1111/j.1600-6143.2011.03647.x. [DOI] [PubMed] [Google Scholar]
- 6.Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopathy. Am J Transplant. 2008;8(3):492–496. doi: 10.1111/j.1600-6143.2007.02104.x. [DOI] [PubMed] [Google Scholar]
- 7.Batal I, Azzi J, El-Haddad N, Riella LV, Lunz JG, Zeevi A, et al. Immunohistochemical markers of tissue injury in biopsies with transplant glomerulitis. Hum Pathol. 2012;43(1):69–80. doi: 10.1016/j.humpath.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Magil AB. Infiltrating cell types in transplant glomerulitis: relationship to peritubular capillary C4d deposition. Am J Kidney Dis. 2005;45(6):1084–1089. doi: 10.1053/j.ajkd.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Wavamunno MD, O’Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, et al. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant. 2007;7(12):2757–2768. doi: 10.1111/j.1600-6143.2007.01995.x. [DOI] [PubMed] [Google Scholar]
- 10.Cosio FG, Sedmak DD, Henry ML, Al Haddad C, Falkenhain ME, Elkhammas EA, et al. The high prevalence of severe early posttransplant renal allograft pathology in hepatitis C positive recipients. Transplantation. 1996;62(8):1054–1059. doi: 10.1097/00007890-199610270-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gallay BJ, Alpers CE, Davis CL, Schultz MF, Johnson RJ. Glomerulonephritis in renal allografts associated with hepatitis C infection: a possible relationship with transplant glomerulopathy in two cases. Am J Kidney Dis. 1995;26(4):662–667. doi: 10.1016/0272-6386(95)90606-1. [DOI] [PubMed] [Google Scholar]
- 12.Haas M. Transplant glomerulopathy: it’s not always about chronic rejection. Kidney Int. 2011;80(8):801–803. doi: 10.1038/ki.2011.192. [DOI] [PubMed] [Google Scholar]
- 13.Baid-Agrawal S, Farris AB, Pascual M, Mauiyyedi S, Farrell ML, Tolkoff-Rubin N, et al. Overlapping pathways to transplant glomerulopathy: chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int. 2011;80(8):879–885. doi: 10.1038/ki.2011.194. [DOI] [PubMed] [Google Scholar]
- 14.Sis B, Campbell PM, Mueller T, Hunter C, Cockfield SM, Cruz J, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant. 2007;7(7):1743–1752. doi: 10.1111/j.1600-6143.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 15.Akalin E, Dinavahi R, Dikman S, de Boccardo G, Friedlander R, Schroppel B, et al. Transplant glomerulopathy may occur in the absence of donor-specific antibody and C4d staining. Clin J Am Soc Nephrol. 2007;2(6):1261–1267. doi: 10.2215/CJN.02420607. [DOI] [PubMed] [Google Scholar]
- 16.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ‘09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10(3):464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 17.Olsen S, Spencer E, Cockfield S, Marcussen N, Solez K. Endocapillary glomerulitis in the renal allograft. Transplantation. 1995;59(10):1421–1425. doi: 10.1097/00007890-199505270-00011. [DOI] [PubMed] [Google Scholar]
- 18.Messias NC, Eustace JA, Zachary AA, Tucker PC, Charney D, Racusen LC. Cohort study of the prognostic significance of acute transplant glomerulitis in acutely rejecting renal allografts. Transplantation. 2001;72(4):655–660. doi: 10.1097/00007890-200108270-00016. [DOI] [PubMed] [Google Scholar]
- 19.Batal I, Lunz JG, Aggarwal N, Zeevi A, Sasatomi E, Basu A, et al. A critical appraisal of methods to grade transplant glomerulitis in renal allograft biopsies. Am J Transplant. 2010;10(11):2442–2452. doi: 10.1111/j.1600-6143.2010.03261.x. [DOI] [PubMed] [Google Scholar]
- 20.Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, et al. A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012;12(5):1168–1179. doi: 10.1111/j.1600-6143.2011.03931.x. [DOI] [PubMed] [Google Scholar]
- 21.Papadimitriou JC, Drachenberg CB, Munivenkatappa R, Ramos E, Nogueira J, Sailey C, et al. Glomerular inflammation in renal allografts biopsies after the first year: cell types and relationship with antibody-mediated rejection and graft outcome. Transplantation. 2010;90(12):1478–1485. doi: 10.1097/TP.0b013e3181ff87f5. [DOI] [PubMed] [Google Scholar]
- 22.Fahim T, Böhmig GA, Exner M, Huttary N, Kerschner H, Kandutsch S, et al. The cellular lesion of humoral rejection: predominant recruitment of monocytes to peritubular and glomerular capillaries. Am J Transplant. 2007;7(2):385–393. doi: 10.1111/j.1600-6143.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 23.Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Curr Opin Organ Transplant. 2010;15(1):42–48. doi: 10.1097/MOT.0b013e3283352a50. [DOI] [PubMed] [Google Scholar]
- 24.Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9(11):2561–2570. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 25.Kieran N, Wang X, Perkins J, Davis C, Kendrick E, Bakthavatsalam R, et al. Combination of peritubular c4d and transplant glomerulopathy predicts late renal allograft failure. J Am Soc Nephrol. 2009;20(10):2260–2268. doi: 10.1681/ASN.2009020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magil AB, Tinckam K. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int. 2003;63(5):1888–1893. doi: 10.1046/j.1523-1755.2003.00921.x. [DOI] [PubMed] [Google Scholar]
- 27.Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005;68(4):1866–1874. doi: 10.1111/j.1523-1755.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 28.Meehan SM, Chang A, Khurana A, Baliga R, Kadambi PV, Javaid B. Pauci-immune and immune glomerular lesions in kidney transplants for systemic lupus erythematosus. Clin J Am Soc Nephrol. 2008;3(5):1469–1478. doi: 10.2215/CJN.00790208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364(20):1909–1919. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 30.Hill P, Gagliardini E, Ruggenenti P, Remuzzi G. Severe early acute humoral rejection resulting in allograft loss in a renal transplant recipient with Campath-1H induction therapy. Nephrol Dial Transplant. 2005;20(8):1741–1744. doi: 10.1093/ndt/gfh867. [DOI] [PubMed] [Google Scholar]
- 31.Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76(1):120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 32.Gallon L, Gagliardini E, Benigni A, Kaufman D, Waheed A, Noris M, et al. Immunophenotypic analysis of cellular infiltrate of renal allograft biopsies in patients with acute rejection after induction with alemtuzumab (Campath-1H) Clin J Am Soc Nephrol. 2006;1(3):539–545. doi: 10.2215/CJN.01741105. [DOI] [PubMed] [Google Scholar]
- 33.Zafrani L, Truffaut L, Kreis H, Etienne D, Rafat C, Lechaton S, et al. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: a retrospective study. Am J Transplant. 2009;9(8):1816–1825. doi: 10.1111/j.1600-6143.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- 34.Hurst FP, Belur P, Nee R, Agodoa LY, Patel P, Abbott KC, et al. Poor outcomes associated with neutropenia after kidney transplantation: analysis of United States Renal Data System. Transplantation. 2011;92(1):36–40. doi: 10.1097/TP.0b013e31821c1e70. [DOI] [PubMed] [Google Scholar]