Abstract
We conducted a wide-ranging review of the literature regarding osteochondral lesions of the ankle, with the aim of presenting the current concepts, treatment options, trends and future perspectives relating to this topic.
Keywords: Ankle injuries/diagnosis, Ankle injuries/therapy, Osteochondritis/diagnosis, Osteochondritis/therapy, Talus
Resumo
Os autores fazem uma revisão ampla da literatura a respeito das lesões osteocondrais do tornozelo, com o intuito de expor os conceitos atuais sobre o tema, as opções de tratamento, as tendências e as perspectivas.
Palavras-chave: Traumatismos do tornozelo/diagnóstico, Traumatismos do tornozelo/terapia, Osteocondrite/diagnóstico, Osteocondrite/terapia, Tálus
Introduction
Lesions of chondral and osteochondral tissues of the ankle are commonly related to ankle sprain,1 which affects one in every 10,000 individuals in the United States daily.
Although there is relative agreement in the literature regarding the microtraumatic etiology of osteochondral lesions of the talus, when the focus of attention shifts to the diagnosis and treatment, this becomes a controversial and extremely dynamic subject, which justify the interest to elaborate the present study, whose main objective was to update the diagnostic and therapeutic approaches of these injuries.
Material and methods
This review and update article assessed studies related to the treatment of osteochondral lesions that affect the ankle joint. Prospective and randomized studies, case series, and systematic reviews were included.
Diagnosis
The suspected diagnosis of osteochondral lesions of the talus starts with complaints of pain related to physical activities, usually with a history of previous trauma. Joint swelling, sensation of instability, joint blockage, or extremely painful clamping may occur.
Despite the aforementioned complaints, physical examination is rather vague and is limited to diffuse tenderness of the joint during flexion and maximum extension, and touch-sensitive areas on the tibiotalar joint line.
Testing ankle stability is essential for the diagnosis of instability, which is frequently associated with or is the main cause of the osteochondral ankle injury.
Despite the great chance of false-negative diagnoses, simple ankle radiographs in AP, lateral, and oblique are the first imaging to be obtained in the diagnostic process of osteochondral lesions of the talus.2
The most common finding in simple radiology is the presence of poorly defined radiolucent area in the talar dome, in the place where the pathological process has become installed.
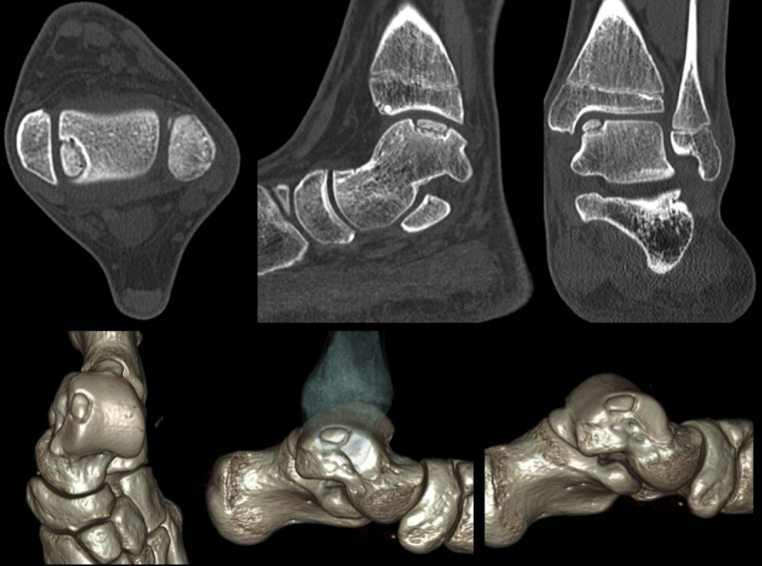
The main limitation of computed tomography (CT) is the inability to provide data on the quality of the articular cartilage; however, it is the main resource in the evaluation of bone changes associated with injury, measurement, and location, as well as in the definition of the deviations of the fragments, and therefore it has the ability to classify the lesions3 (Fig. 1).
Fig. 1.
Axial computed tomography allows for the identification, measurement, and accurate classification of osteochondral lesions of the talus. The lower images correspond to three-dimensional reconstruction.
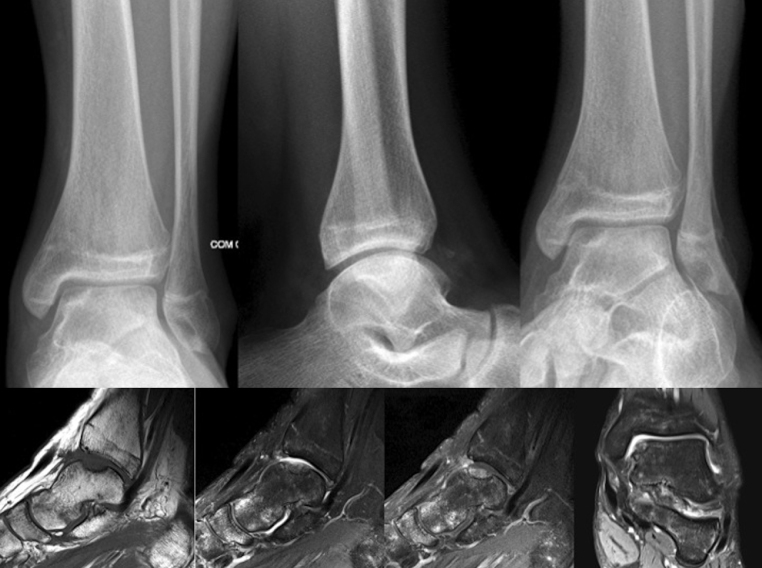
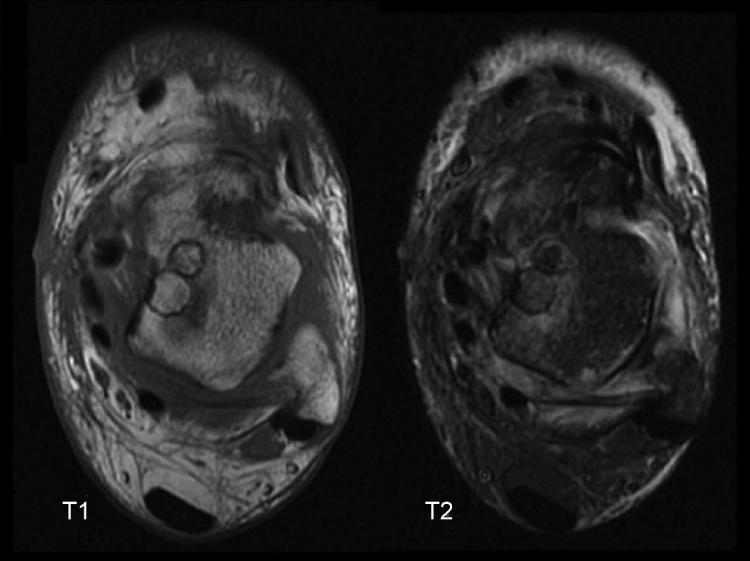
Magnetic resonance imaging (MRI) provides information, allowing for the assessment of articular cartilage and presence of subchondral inflammatory changes, as well as for the identification of the depth of the chondral lesion.4, 5 It is therefore regarded as the gold standard in the diagnosis of osteochondral lesions6, 7 (Fig. 2).
Fig. 2.
X-rays of the ankle and magnetic resonance imaging of a patient who underwent arthroscopic treatment with debridement and microfractures.
The most widespread classification for osteochondral lesions of the talus is that proposed by Berndt and Harty8 in 1959; it is based on the degree of displacement of the osteochondral fragment and has four stages: Stage I – small focal subchondral trabecular compression area; Stage II – partially loose fragment (incomplete fracture); Stage III – loose fragment (complete fracture), but not displaced; and Stage IV – loose fragment (complete fracture) and displaced from its bed.
In 2001, Scranton and McDermott9 added Stage V to the classification of Berndt & Harty, characterized by the presence of osteochondral cysts with a size corresponding to that of the original injury, just below the damaged articular surface.
Mintz et al.4 combined arthroscopic observations with MRI to design their rating for osteochondral lesions of the talus, following the same dynamics of the other classifications. Six different stages are possible: Stage 0 – normal cartilage; Stage 1 – hypersignal cartilage on MRI, but normal arthroscopic appearance; Stage 2 – fibrillation and cracks that do not reach the bone; Stage 3 – presence of cartilage flap, with exposure of the subchondral bone; Stage 4 – loose fragment, non-diverted; Stage 5 – diverted fragment.
Despite the known possibility of overestimation of injuries, there is a clear and well-defined trend in the literature to value images of osteochondral lesions of the talus obtained by MRI,10 especially those of high-resolution,11 due to their excellent correlation with arthroscopic findings, a fact which greatly helps in therapy planning and prognosis.
Prognostic factors
Size
Currently, there appears to be a consensus on the indication of arthroscopic treatment for osteochondral lesions of the talus smaller than 1.5 cm2, even for recurrent lesions.11, 12, 13 The recommended arthroscopic treatment is debridement of the injured area, with resection of free or partially-detached osteochondral fragments, followed by stimulation of the bone marrow through drilling or microfractures of the subchondral bone.14, 15
Location
The location of the lesions influences the prognosis much more due to the reparative tissue stability (retention) than to the area or the quadrant in which the lesion is positioned. It is known that lesions located in the rounded areas of the articular surface, also known as talar shoulders, offer more precarious conditions to stabilize the repair tissue – uncontained injuries – and therefore they have a less favorable prognosis.12, 16
Without good quality edges, the scar that forms is less stable, increasing the chances of mechanically unfavorable formation of fibrocartilage,17 rich in type I collagen.
Age
The patient's age at the onset of the injury is considered to be an important prognostic factor.18 However, there is controversy over this claim, as some authors failed to observe differences in results when considering only age, having denied this correlation.18, 19
Subchondral cysts and depth
The occurrence of subchondral cystic lesions indicates poor prognosis.9, 20 Poor results can be expected in 53% of patients in this group.21, 22, 23
Other possible prognostic factors
Chondral lesions vs. osteochondral lesions
Through the observation of 283 patients for an average of 52 months, Choi et al.24 observed no differences in the prognosis of injuries that involved only the chondral layers from those that surpassed the subchondral bone plate.
Bodyweight load support
While some authors believe that early loading does not interfere with the final result of small osteochondral lesions,25 Gill et al.26 histologically demonstrated that maintenance of load limitation in the postoperative period definitively influences the filling speed and the quality of the repair tissue in osteochondral lesions.
Spinal cord edema
The clinical picture and the prognosis of osteochondral lesions are inversely related to the intensity of bone marrow edema observed in the MRI.27
Joint instability and trauma28
Treatment
The treatment of osteochondral lesions of the talus should be restricted to symptomatic lesions. The occasional finding of asymptomatic osteochondral lesions, regardless of location, type, and size, should be communicated to the patient or his/her relatives; the case should be followed-up at regular intervals in search of possible joint deterioration.29
Conservative treatment
The non-surgical treatment modalities available in the literature include modification of activities of daily living, intra-articular steroid infiltration, use of orthotics, load suppression, and use of immobilizing boots and orthoses.8, 11
The results of this type of treatment do not exceed 45%30; it is neither consistent nor predictable.31
The plasma rich in growth factors is a form of PRP considered to be a biological carrier of a complex mixture of bioactive proteins essential to the normal healing process, having potential effects on the repair of osteochondral lesions and arthrosis.32, 33 These findings and therapeutic applications still require further studies to consolidate their applicability in daily practice.
Surgical treatment
Surgical treatment of osteochondral ankle injuries can be divided into five main groups of procedures17, 34:
-
1.
Reduction and fixation of osteochondral fragments
-
2.
Bone marrow stimulation
-
3.
Articular cartilage replacement
-
4.
Regenerative cell therapy
-
5.
Metal implants
Reduction and fixation of osteochondral fragments
Acute osteochondral fractures are produced mostly by injuries after ankle inversion.
Under these conditions, patient should be treated with urgency and, when feasible, the fragments should be reduced and set in their original bed. The procedure can be done arthroscopically and the fragments can be fixed with fixation darts or absorbable screws, as both of which provide excellent functional results.
Smaller or devitalized fragments are resected, and the base of the lesion is treated by stimulating the bone marrow.
Despite the good prognosis for fracture consolidation, a deterioration of the cartilage that covers the fragments can be expected in one-third of the cases.
Bone marrow stimulation
If the articular cartilage has only softened zones (chondromalacia) or fibrillation without exposure of the subchondral bone, and there is good stability of the tissue, surface debridement and “chondral sealing” with the use of radiofrequency can be performed.35
Anterograde drilling and microfractures
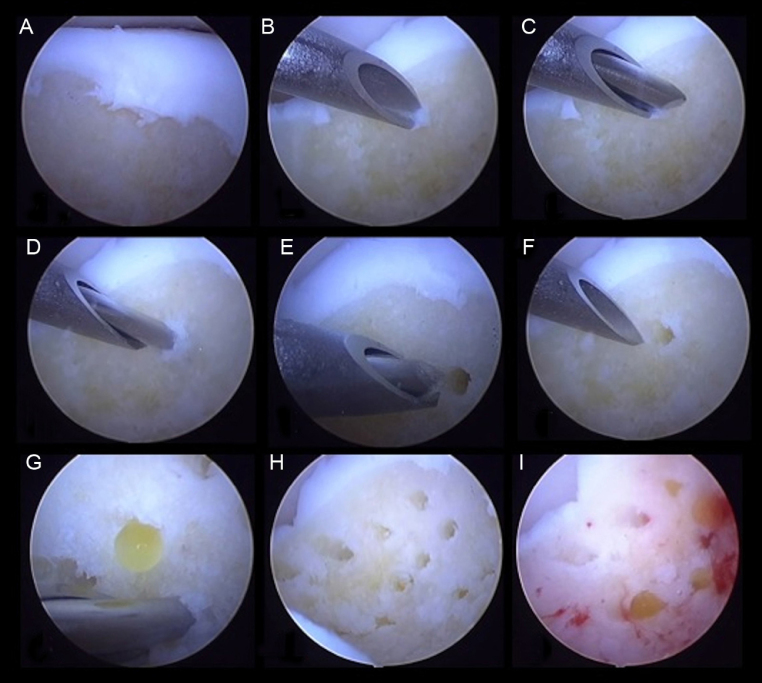
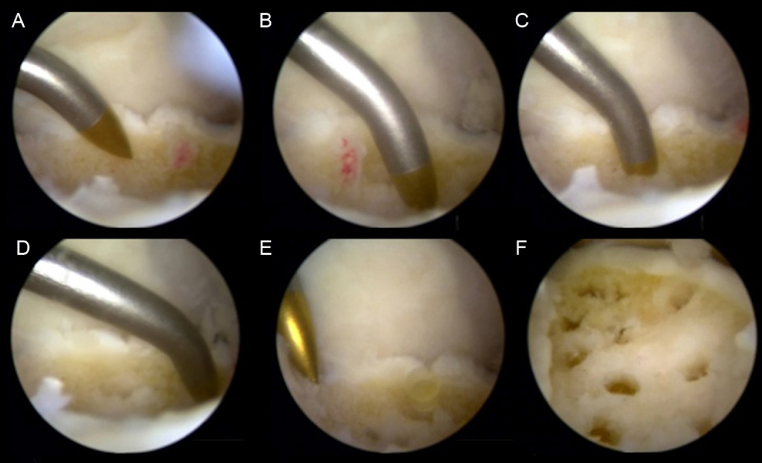
The rationale behind multidrilling or microfractures for the treatment of osteochondral lesions of the talus is based on the perforation of the subchondral bone, allowing the contact of the bone marrow with the lesion. In addition to the migration of multipotent mesenchymal cells into the bed of the wound, local neovascularization is also induced, with cellular afflux to the repair zone. Some basic rules must be followed to ensure the success of these procedures: (1) the edges of the lesion should be curetted and the softened tissue should be removed until the healthy cartilage, firmly adhered to the subchondral bone, is reached; (2) the edges should be as regular and perpendicular as possible; (3) drilling or microfractures in the subchondral bone must be made perpendicularly to the surface at 3–4-mm intervals; (4) the depth should be at least 3 mm to ensure that the subchondral bone has been crossed; and (5) the procedure should be started in the periphery of the lesion and finish in the center29 (Fig. 3). The finding of bleeding in the base of the injury from the subchondral capillary is essential. The blood that is deposited in the base of the lesion contains progenitor cells and cytokines responsible for initiating the healing process, which involves the formation of a fibrin clot and subsequent formation of fibrous scar tissue that will undergo metaplasia into fibrocartilaginous tissue (Fig. 4) with less resistance to compression and shear forces when compared with the normal articular cartilage tissue.
Fig. 3.
Drilling in the base of osteochondral lesions of the talus.
Fig. 4.
Microfractures in the base of osteochondral lesions of the talus.
A systematic review demonstrated the consistency of the results obtained with the treatment of osteochondral lesions through debridement and microfractures, with mean AOFAS score of 86.8 points, achieving 80.2% excellent and good results36; however, significant deterioration of results was observed after 4.2 years of the procedure.17
In a more recent meta-analysis, the success of combining excision of fragments, curettage of the base of the injury, and bone marrow stimulation was 85%, compared with 32% good results from isolated fragment excision and 77% good results from excision combined with curettage of the base of the injury.37
The repetition of the debridement process, curettage, and bone marrow stimulation in patients with unfavorable evolution reached 92% good results, characterized by return to prior sporting activities, including professional activity.38
The result of microfractures can be enhanced by hyaluronate intra-articular injection immediately after surgery, with improved function and pain results when compared to patients who did not receive such treatment.39
Animal models using platelet-rich plasma in conjunction with bone marrow stimulation procedures showed better repair of the joint cartilage when compared with isolated surgical procedure, although hyaline cartilage was not obtained in the final result.40 The same is true for the use of bone marrow aspirate concentrate.41
Retrograde drilling
Retrograde drilling with radiographic control is considered to be a very effective treatment option for osteochondral lesions of the intact joint cartilage.41 After placing a guidewire in the lesion with the aid of fluoroscopy and arthroscopy, a cannulated drill is passed over this guidewire and never exceeds the intact cartilage. Through the tunnel formed, it is possible to place the bone graft to fill the lesion. Currently, various models of articulated and extremely efficient guiding instruments help the surgeon to locate the lesion and reach it safely.
Articular cartilage replacement
Autologous osteochondral graft
The osteochondral autologous grafting system known as mosaicplasty involves obtaining cylindrical cartilage and bone grafts, most commonly originating from the lateral femoral condyles, and transferring them to areas of osteochondral lesion in the loading surface of the talar dome. This procedure presents encouraging results.
The indications for osteochondral grafting comprise lesions larger than 1.5 cm2, recurrent or refractory to more conservative treatment methods, and especially lesions associated with subchondral cysts.42
Mosaicoplasty has technical standards that must be followed in order to achieve better results: (1) the donor area can never be a load bearing region; (2) osteochondral cylinders must be inserted perpendicularly to the receiving surface; (3) the cartilage portion should have the shape and curvature as close to the receiving zone as possible; (4) the cylinder must be at least 15 mm in length for chondral lesions and 25 mm in the presence of subchondral cysts; (5) the cartilage plug must remain perfectly leveled with the edges of the receiving region; steps or unevenness regarding the neighboring cartilage are not acceptable29, 43 (Fig. 5).
Fig. 5.
MRI scans obtained six months after autologous osteochondral graft.
Following the indications for each procedure, the results of osteochondral autologous grafts are superior to the combination of debridement and microfractures.44
The number of grafts used, previous procedures, the need for osteotomy of the malleolus, and the presence of mild osteoarthritis of the affected joint do not influence the final outcome of this procedure.
Regarding the donor area, the most frequent residual symptoms are a feeling of instability during activities of daily living and pain, present in 11% of patients who underwent surgery.45 The occurrence of these symptoms is related to very large parapatellar incisions and exaggerated tensioning during the closure. The knee prognosis does not appear to be affected by the number of removed grafts, graft size, or age of the patient.46
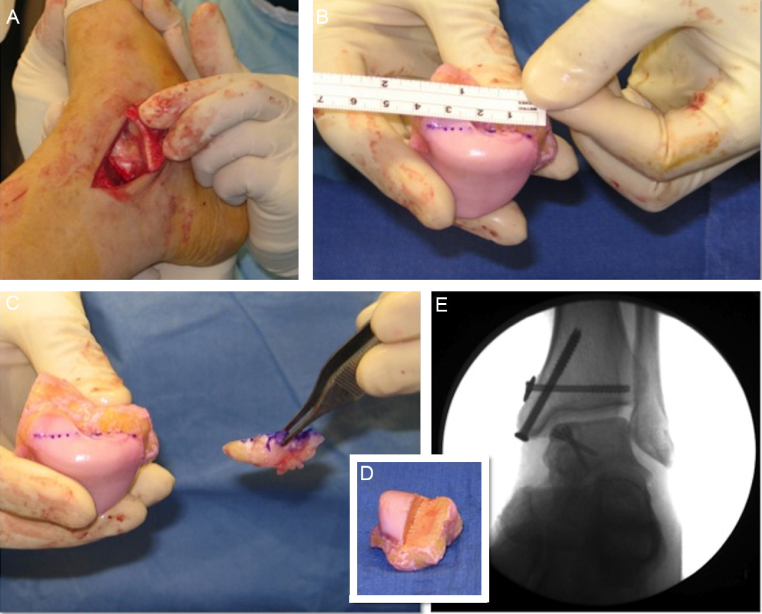
Osteochondral allograft
When osteochondral lesions exceed 3 cm2, become uncontained, affect the shoulder of the talus, or are accompanied by large subchondral cysts, osteochondral autografts present technical difficulties, with a greater chance of poor results. In such cases, fresh cadaver allograft, with viable chondrocytes and normal subchondral bone, appears as an interesting option, especially because it does not present morbidity in the donor area or areas without coverage between the grafted plugs47 (Fig. 6).
Fig. 6.
Osteochondral homograft used for the treatment of extensive medial talar shoulder injury (The authors thank Dr. Mark S. Myerson for the authorization to use these figures).
Cryopreservation leads to a significant decline in the number of viable chondrocytes. The cell survival falls to 20–30% in two weeks, the period during which the procedure should be done.
Despite the perspective of good results, the method has some main obstacles: the transmission of diseases, the possibility of adverse immune reaction, and difficulty in graft incorporation into the bed.
There is consensus among the authors to consider the osteochondral allograft as a salvage treatment for large lesions and for those where other methods have failed repeatedly. However, the high incidence of procedure failure (30%) and of secondary procedures (40%) should be taken into account.48, 49
Regenerative cell therapy
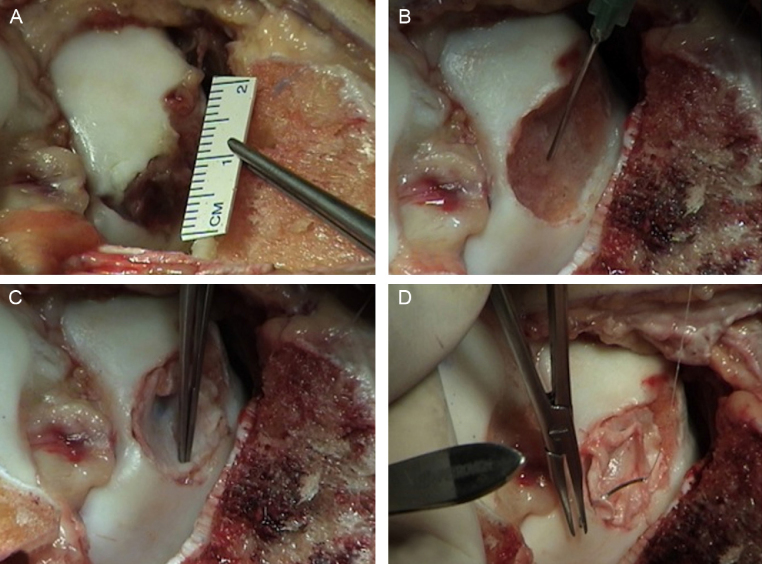
Autologous chondrocyte implantation
The procedure begins by obtaining viable chondrocytes through resection of a small fragment of the healthy cartilage tissue from the joint to be treated or from another joint from the same individual. The chondrocytes are isolated and cultured for three to six weeks in order to multiply. The second part of the procedure is the preparation of the receiving area and the implantation of the cultured cells.50 Curettage and debridement of the base and edges of the injury until they establish the limits of healthy cartilage, firmly adhered to the subchondral bone, are an integral part of this process. Possible subchondral cysts are filled with cancellous graft and periosteum blades in the appropriate dimensions which are sutured and glued with fibrin to the edges of the lesion, in order to create an airtight chamber, inside which the cultured cells are implanted (Fig. 7).
Fig. 7.
Autologous chondrocyte implantation (sandwich technique).
Indications for this therapy include recurrent osteochondral lesions of any size and primary treatment of larger lesions than 2.5 cm2 with or without subchondral cysts in patients aged between 15 and 55 years, without degenerative arthritis or mirror-image osteochondral lesions, and without instability or changes in joint alignment.51
The complications, especially those related to periosteal hypertrophy and delamination of the membranes used in wound coverage, reach 18–33% of cases.52
In order to reduce morbidity and the technical difficulty of autologous chondrocyte implantation, various attempts have been made regarding the carrier for cultured chondral cells.
A combination of fibrin and thrombin (Chondron, Sewon Cellontech Co., Seoul, South Korea), to which the cultured chondrocytes are added for implantation in the receiving area of the talus, can be used.53
The second generation of autologous chondrocyte transplants involves the use of collagen membranes for carrying the cells, which eliminates the need for obtaining the periosteum and all the difficulties and complications inherent to this period of the operation, and the results obtained are encouraging.54
The autologous chondrocyte implantation through an arthroscopic procedure was a step forward. This achievement was only possible due to the use of a flexible collagen membrane called matrix-induced autologous chondrocyte implantation (MACI).55
Mesenchymal stem cells
Stem cell therapy is based on two mechanisms of action. At first, the cells differentiate and mimic the final cells of tissues and organs; then, there is the production of substances (cytokines and growth factors) that favorably influence the angiogenesis and the reduction of cell apoptosis, and induce the endogenous regeneration.
Giannini et al. demonstrated the efficiency of the use of stem cells obtained from concentration of aspirated bone marrow or bone marrow-derived cell transplantation (BMDCT) in clinical trials,56 albeit with a possible tendency toward deterioration of the results.57
Stem cells derived from fat have a better potential for chondrogenesis58; those obtained from the synovium apparently have good differentiation into chondrocytes, but only in animal studies.59
Intra-articular injection of mesenchymal stem cells (MSC) favorably influenced the results of treatment of patients older than 50 years with lesions larger than 109 mm2 associated with subchondral cysts, and may have some benefit in lowering the speed of evolution to degenerative disease.
Chondrogenesis induction by autologous matrix
The use a combination of collagen membranes with MSC, bone marrow concentrate (BMC), and bone marrow-derived MSC (BMSC) has been shown to be more advantageous than chondrocyte implantation.57
In the matrix-induced chondrogenesis technique, autologous chondrocytes (matrix-associated autologous chondrocyte transplantation/implantation [MACT/MACI]) are seeded into a type I and III acellular collagen matrix that is placed in the clot formed after microfracture to provide a favorable environment for chondral regeneration. Clinical results with five years of follow-up are encouraging.60
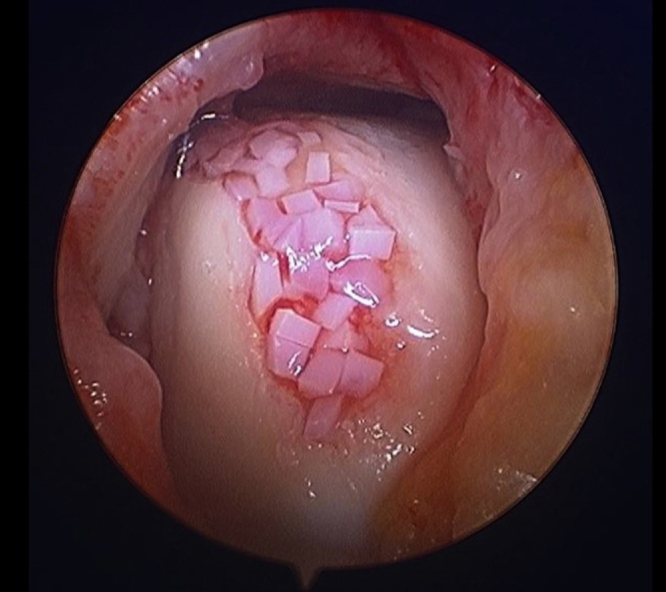
Particulated juvenile articular cartilage
Stimulation of osteochondral talar lesions repair can be made from the articular cartilage of children and adolescents cadavers, particulated into 1-mm cubes implanted in the previously prepared bed of the lesion (DeNovo Natural Tissue, Zimmer Inc., Warsaw, USA). After arthroscopical debridement and preparation of the lesion, the saline flow is interrupted and the injury is dried. A thin layer of fibrin glue is applied across the extent of chondral lesion and the particulate cartilage is inserted to cover the full osteochondral defect. A new fibrin layer is applied over the region so as to increase the stability of the graft. Studies with animal models and short-term follow-up in patients have shown good results, with the formation of hyaline cartilage in the defects (Fig. 8).
Fig. 8.
Particulated juvenile cartilage. (The authors thank Dr. Rebecca Cerrato – Mercy Hospital, Baltimore, USA – for the authorization to use these figures).
The initial clinical results are good61; in lesions smaller than 1.5 cm2 the success rate reaches 92% good results.62
Metal implants
Filling of the talar osteochondral lesion area with the use of metallic implants – surface prostheses – aims to redo the contour of the injured area and enhance the distribution of loads on the ankle joint. It is considered a valid method for the treatment of osteochondral lesions that are recurrent and refractory to other forms of treatment.63
Osteochondral lesions of the distal tibia
Osteochondral lesions of the distal third of the tibia are unusual findings, appearing in 2.6% of all osteochondral ankle injuries.64
This lower incidence may be related to the concave shape of the inferior articular surface of the tibia and to the greater resistance of the tibial cartilage to compression when compared with the talar cartilage.65, 66
The treatment of these injuries is more complex due to the difficulty of access and to the shape of the articular surface of the tibia. Curettage, excision of fragments, thermal ablation, and stimulation of bone marrow can be done through an arthroscopic approach.
When cysts are present, filling with bone graft can be done through the transmalleolar approach, with arthroscopic assistance.
The consensus among authors is that osteochondral lesions of the tibia have a worse prognosis than osteochondral lesions of the talus when considering the same physical characteristics of the lesions.
Assessment of the repaired cartilage
T2-weighted MRI mapping is becoming the most useful and popular resource for the assessment of the repaired articular cartilage, as an important alternative to arthroscopy, which is an invasive procedure not free from complications. As an added advantage, MRI assessments include the repaired region as a whole, while arthroscopy has a more restricted and superficial field of vision than a local biopsy would provide.67
The integration of the Mocart morphological scale parameters and biochemical mapping through T2-weighted MRI is essential for a complete and accurate noninvasive assessment of the repaired cartilage, improving the interpretation of the clinical scales. Mapping is suitable for a qualitative assessment of cartilage, being able to distinguish hyaline cartilage from fibrocartilage and to correlate with clinical outcomes.67
The flowchart (Fig. 9) presents the most common occurrences, as well as solutions that are supported in the literature.
Fig. 9.
Diagnostic and treatment flowchart for osteochondral lesions of the talus, based on the literature.
Some of the solutions presented are not available in Brazil, which does not preclude knowledge of them and research options for patients who present the problems listed in this article.
Final remarks
Systematic reviews of the literature, due to the heterogeneity of the available studies, do not allow for the definition of absolute standards of conduct.41 However, there is important information about the efficiency of the treatment methods with their respective success rates. With this information, orthopedists are able to support their choices, despite the lack of mathematical confirmation, until the much-expected prospective, comparative, well-controlled studies are conducted.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Study conducted at Hospital Israelita Albert Einstein, São Paulo, SP, Brazil, and Hospital for Special Surgery, New York, United States.
References
- 1.Baltzer A.W., Arnold J.P. Bone-cartilage transplantation from the ipsilateral knee for chondral lesions of the talus. Arthroscopy. 2005;21(2):159–166. doi: 10.1016/j.arthro.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Dheer S., Khan M., Zoga A.C., Morrison W.B. Limitations of radiographs in evaluating non-displaced osteochondral lesions of the talus. Skeletal Radiol. 2012;41(4):415–421. doi: 10.1007/s00256-011-1242-z. [DOI] [PubMed] [Google Scholar]
- 3.Ferkel R.D., Flannigan B.D., Elkins B.S. Magnetic resonance imaging of the foot and ankle: correlation of normal anatomy with pathologic conditions. Foot Ankle. 1991;11(5):289–305. doi: 10.1177/107110079101100506. [DOI] [PubMed] [Google Scholar]
- 4.Mintz D.N., Tashjian G.S., Connell D.A., Deland J.T., O’Malley M., Potter H.G. Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy. 2003;19(4):353–359. doi: 10.1053/jars.2003.50041. [DOI] [PubMed] [Google Scholar]
- 5.Lüsse S., Claassen H., Gehrke T., Hassenpflug J., Schünke M., Heller M. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn Reson Imaging. 2000;18(4):423–430. doi: 10.1016/s0730-725x(99)00144-7. [DOI] [PubMed] [Google Scholar]
- 6.Cuttica D.J., Smith W.B., Hyer C.F., Philbin T.M., Berlet G.C. Osteochondral lesions of the talus: predictors of clinical outcome. Foot Ankle Int. 2011;32(11):1045–1051. doi: 10.3113/FAI.2011.1045. [DOI] [PubMed] [Google Scholar]
- 7.O’Loughlin P.F., Heyworth B.E., Kennedy J.G. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38(2):392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 8.Berndt A.L., Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41-A:988–1020. [PubMed] [Google Scholar]
- 9.Scranton P.E., Jr., McDermott J.E. Treatment of type V osteochondral lesions of the talus with ipsilateral knee osteochondral autografts. Foot Ankle Int. 2001;22(5):380–384. doi: 10.1177/107110070102200504. [DOI] [PubMed] [Google Scholar]
- 10.Bae S., Lee H.K., Lee K., Lim S., Rim N.J., Kim J.S. Comparison of arthroscopic and magnetic resonance imaging findings in osteochondral lesions of the talus. Foot Ankle Int. 2012;33(12):1058–1062. doi: 10.3113/FAI.2012.1058. [DOI] [PubMed] [Google Scholar]
- 11.Griffith J.F., Lau D.T., Yeung D.K., Wong M.W. High-resolution MR imaging of talar osteochondral lesions with new classification. Skeletal Radiol. 2012;41(4):387–399. doi: 10.1007/s00256-011-1246-8. [DOI] [PubMed] [Google Scholar]
- 12.Chuckpaiwong B., Berkson E.M., Theodore G.H. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24(1):106–112. doi: 10.1016/j.arthro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Choi W.J., Park K.K., Kim B.S., Lee J.W. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974–1980. doi: 10.1177/0363546509335765. [DOI] [PubMed] [Google Scholar]
- 14.Christensen J.C., Driscoll H.L., Tencer A.F., William J. Stickel Gold Award. Contact characteristics of the ankle joint. Part 2. The effects of talar dome cartilage defects. J Am Pediatr Med Assoc. 1994;84(11):537–547. doi: 10.7547/87507315-84-11-537. [DOI] [PubMed] [Google Scholar]
- 15.Giannini S., Ceccarelli F., Girolami M., Coppola G., Ferrari A. Biological osteosynthesis in osteochondral lesions of the talus. Ital J Orthop Traumatol. 1989;15(4):425–432. [PubMed] [Google Scholar]
- 16.Furukawa T., Eyre D.R., Koide S., Glimcher M.J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62(1):79–89. [PubMed] [Google Scholar]
- 17.Choi W.J., Choi G.W., Kim J.S., Lee J.W. Prognostic significance of the containment and location of osteochondral lesions of the talus: independent adverse outcomes associated with uncontained lesions of the talar shoulder. Am J Sports Med. 2013;41(1):126–133. doi: 10.1177/0363546512453302. [DOI] [PubMed] [Google Scholar]
- 18.Becher C., Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26(8):583–589. doi: 10.1177/107110070502600801. [DOI] [PubMed] [Google Scholar]
- 19.Choi W.J., Kim B.S., Lee J.W. Osteochondral lesion of the talus: could age be an indication for arthroscopic treatment? Am J Sports Med. 2012;40(2):419–424. doi: 10.1177/0363546511423739. [DOI] [PubMed] [Google Scholar]
- 20.Ferkel R.D., Zanotti R.M., Komenda G.A., Sgaglione N.A., Cheng M.S., Applegate G.R. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750–1762. doi: 10.1177/0363546508316773. [DOI] [PubMed] [Google Scholar]
- 21.Robinson D.E., Winson I.G., Harries W.J., Kelly A.J. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br. 2003;85(7):989–993. doi: 10.1302/0301-620x.85b7.13959. [DOI] [PubMed] [Google Scholar]
- 22.Han S.H., Lee J.W., Lee D.Y., Kang E.S. Radiographic changes and clinical results of osteochondral defects of the talus with and without subchondral cysts. Foot Ankle Int. 2006;27(12):1109–1114. doi: 10.1177/107110070602701218. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura I., Kanazawa K., Takeyama A., Angthong C., Ida T., Hagio T. Arthroscopic bone marrow stimulation techniques for osteochondral lesions of the talus: prognostic factors for small lesions. Am J Sports Med. 2013;41(3):528–534. doi: 10.1177/0363546512472979. [DOI] [PubMed] [Google Scholar]
- 24.Choi G.W., Choi W.J., Youn H.K., Park Y.J., Lee J.W. Osteochondral lesions of the talus: are there any differences between osteochondral and chondral types? Am J Sports Med. 2013;41(3):504–510. doi: 10.1177/0363546512472976. [DOI] [PubMed] [Google Scholar]
- 25.Lee D.H., Lee K.B., Jung S.T., Seon J.K., Kim M.S., Sung I.H. Comparison of early versus delayed weightbearing outcomes after microfracture for small to midsized osteochondral lesions of the talus. Am J Sports Med. 2012;40(9):2023–2028. doi: 10.1177/0363546512455316. [DOI] [PubMed] [Google Scholar]
- 26.Gill T.J., McCulloch P.C., Glasson S.S., Blanchet T., Morris E.A. Chondral defect repair after the microfracture procedure: a nonhuman primate model. Am J Sports Med. 2005;33(5):680–685. doi: 10.1177/0363546504271744. [DOI] [PubMed] [Google Scholar]
- 27.Cuttica D.J., Shockley J.A., Hyer C.F., Berlet G.C. Correlation of MRI edema and clinical outcomes following microfracture of osteochondral lesions of the talus. Foot Ankle Spec. 2011;4(5):274–279. doi: 10.1177/1938640011411082. [DOI] [PubMed] [Google Scholar]
- 28.Goldstone R.A., Pisani A.J. Osteochondritis dissecans of the talus. N Y State J Med. 1965;65(19):2487–2494. [PubMed] [Google Scholar]
- 29.Easley M.E., Latt L.D., Santangelo J.R., Merian-Genast M., Nunley J.A., 2nd. Osteochondral lesions of the talus. J Am Acad Orthop Surg. 2010;18(10):616–630. doi: 10.5435/00124635-201010000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Mei-Dan O., Maoz G., Swartzon M., Onel E., Kish B., Nyska M. Treatment of osteochondritis dissecans of the ankle with hyaluronic acid injections: a prospective study. Foot Ankle Int. 2008;29(12):1171–1178. doi: 10.3113/FAI.2008.1171. [DOI] [PubMed] [Google Scholar]
- 31.Sánchez M., Anitua E., Azofra J., Aguirre J.J., Andia I. Intra-articular injection of an autologous preparation rich in growth factors for the treatment of knee OA: a retrospective cohort study. Clin Exp Rheumatol. 2008;26(5):910–913. [PubMed] [Google Scholar]
- 32.Deol P.P., Cuttica D.J., Smith W.B., Berlet G.C. Osteochondral lesions of the talus: size, age, and predictors of outcomes. Foot Ankle Clin. 2013;18(1):13–34. doi: 10.1016/j.fcl.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 33.David T.S., Shields C.L. Radiofrequency and articular cartilage. Tech Knee Surg. 2004;3(3):193–197. [Google Scholar]
- 34.Verhagen R.A., Struijs P.A., Bossuyt P.M., van Dijk C.N. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233–242. doi: 10.1016/s1083-7515(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 35.Zengerink M., Struijs P.A., Tol J.L., van Dijk C.N. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238–246. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savva N., Jabur M., Davies M., Saxby T. Osteochondral lesions of the talus: results of repeat arthroscopic debridement. Foot Ankle Int. 2007;28(6):669–673. doi: 10.3113/FAI.2007.0669. [DOI] [PubMed] [Google Scholar]
- 37.Doral M.N., Bilge O., Batmaz G., Donmez G., Turhan E., Demirel M. Treatment of osteochondral lesions of the talus with microfracture technique and postoperative hyaluronan injection. Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1398–1403. doi: 10.1007/s00167-011-1856-7. [DOI] [PubMed] [Google Scholar]
- 38.Milano G., Sanna Passino E., Deriu L., Careddu G., Manunta L., Manunta A. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(7):971–980. doi: 10.1016/j.joca.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Fortier L.A., Potter H.G., Rickey E.J., Schnabel L.V., Foo L.F., Chong L.R. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927–1937. doi: 10.2106/JBJS.I.01284. [DOI] [PubMed] [Google Scholar]
- 40.Anders S., Lechler P., Rackl W., Grifka J., Schaumburger J. Fluoroscopy-guided retrograde core drilling and cancellous bone grafting in osteochondral defects of the talus. Int Orthop. 2012;36(8):1635–1640. doi: 10.1007/s00264-012-1530-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emre T.Y., Ege T., Cift H.T., Demircioğlu D.T., Seyhan B., Uzun M. Open mosaicplasty in osteochondral lesions of the talus: a prospective study. J Foot Ankle Surg. 2012;51(5):556–560. doi: 10.1053/j.jfas.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Latt L.D., Glisson R.R., Montijo H.E., Usuelli F.G., Easley M.E. Effect of graft height mismatch on contact pressures with osteochondral grafting of the talus. Am J Sports Med. 2011;39(12):2662–2669. doi: 10.1177/0363546511422987. [DOI] [PubMed] [Google Scholar]
- 43.Gobbi A., Francisco R.A., Lubowitz J.H., Allegra F., Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22(10):1085–1092. doi: 10.1016/j.arthro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Woelfle J.V., Reichel H., Nelitz M. Indications and limitations of osteochondral autologous transplantation in osteochondritis dissecans of the talus. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1925–1930. doi: 10.1007/s00167-013-2483-2. [DOI] [PubMed] [Google Scholar]
- 45.Fraser E., Harris M.C., Pradp M.P., Kennedy J.G. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1272–1279. doi: 10.1007/s00167-015-3606-8. [DOI] [PubMed] [Google Scholar]
- 46.Giannini S., Buda R., Grigolo B., Vannini F., De Franceschi L., Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2005;13(7):601–607. doi: 10.1016/j.joca.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Woelfle J.V., Reichel H., Javaheripour-Otto K., Nelitz M. Clinical outcome and magnetic resonance imaging after osteochondral autologous transplantation in osteochondritis dissecans of the talus. Foot Ankle Int. 2013;34(2):173–179. doi: 10.1177/1071100712467433. [DOI] [PubMed] [Google Scholar]
- 48.Paul J., Sagstetter A., Kriner M., Imhoff A.B., Spang J., Hinterwimmer S. Donor-site morbidity after osteochondral autologous transplantation for lesions of the talus. J Bone Joint Surg Am. 2009;91(7):1683–1688. doi: 10.2106/JBJS.H.00429. [DOI] [PubMed] [Google Scholar]
- 49.Haene R., Qamirani E., Story R.A., Pinsker E., Daniels T.R. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105–1110. doi: 10.2106/JBJS.J.02010. [DOI] [PubMed] [Google Scholar]
- 50.Bugbee W.D., Khanna G., Cavallo M., McCauley J.C., Görtz S., Brage M.E. Bipolar fresh osteochondral allografting of the tibiotalar joint. J Bone Joint Surg Am. 2013;95(5):426–432. doi: 10.2106/JBJS.L.00165. [DOI] [PubMed] [Google Scholar]
- 51.Johnson B., Lever C., Roberts S., Richardson J., McCarthy H., Harrison P. Cell cultured chondrocyte implantation and scaffold techniques for osteochondral talar lesions. Foot Ankle Clin. 2013;18(1):135–150. doi: 10.1016/j.fcl.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Niemeyer P., Salzmann G., Schmal H., Mayr H., Südkamp N.P. Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1696–1703. doi: 10.1007/s00167-011-1729-0. [DOI] [PubMed] [Google Scholar]
- 53.Lee K.T., Kim J.S., Young K.W., Lee Y.K., Park Y.U., Kim Y.H. The use of fibrin matrix-mixed gel-type autologous chondrocyte implantation in the treatment for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1251–1260. doi: 10.1007/s00167-012-2096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anders S., Goetz J., Schubert T., Grifka J., Schaumburger J. Treatment of deep articular talus lesions by matrix associated autologous chondrocyte implantation – results at five years. Int Orthop (SICOT) 2012;36:2279–2285. doi: 10.1007/s00264-012-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aurich M., Bedi H.S., Smith P.J., Rolauffs B., Mückley T., Clayton J. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39(2):311–319. doi: 10.1177/0363546510381575. [DOI] [PubMed] [Google Scholar]
- 56.Giannini S., Buda R., Cavallo M., Ruffilli A., Cenacchi A., Cavallo C. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41(11):1196–1203. doi: 10.1016/j.injury.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 57.Giannini S., Buda R., Battaglia M., Cavallo M., Ruffilli A., Ramponi L. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med. 2013;41(3):511–518. doi: 10.1177/0363546512467622. [DOI] [PubMed] [Google Scholar]
- 58.Kim Y.S., Park E.H., Kim Y.C., Koh Y.G. Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med. 2013;41(5):1090–1099. doi: 10.1177/0363546513479018. [DOI] [PubMed] [Google Scholar]
- 59.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 60.Valderrabano V., Miska M., Leumann A., Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41(3):519–527. doi: 10.1177/0363546513476671. [DOI] [PubMed] [Google Scholar]
- 61.Adams S.B., Yao J.Q., Schon L.C. Particulated juvenile articular cartilage allograft transplantation for osteochondral lesions of the talus. Tech Foot Ankle Surg. 2011;10(2):92–98. [Google Scholar]
- 62.Coetzee J.C., Giza E., Schon L.C., Berlet G.C., Neufeld S., Stone R.M. Treatment of osteochondral lesions of the talus with particulated juvenile cartilage. Foot Ankle Int. 2013;34(9):1205–1211. doi: 10.1177/1071100713485739. [DOI] [PubMed] [Google Scholar]
- 63.van Bergen C.J., Reilingh M.L., van Dijk C.N. Tertiary osteochondral defect of the talus treated by a novel contoured metal implant. Knee Surg Sports Traumatol Arthrosc. 2011;19(6):999–1003. doi: 10.1007/s00167-011-1465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mologne T.S., Ferkel R.D. Arthroscopic treatment of osteochondral lesions of the distal tibia. Foot Ankle Int. 2007;28(8):865–872. doi: 10.3113/FAI.2007.0865. [DOI] [PubMed] [Google Scholar]
- 65.Cuttica D.J., Smith W.B., Hyer C.F., Philbin T.M., Berlet G.C. Arthroscopic treatment of osteochondral lesions of the tibial plafond. Foot Ankle Int. 2012;33(8):662–668. doi: 10.3113/FAI.2012.0662. [DOI] [PubMed] [Google Scholar]
- 66.Battaglia M., Vannini F., Buda R., Cavallo M., Ruffilli A., Monti C. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1376–1384. doi: 10.1007/s00167-011-1509-x. [DOI] [PubMed] [Google Scholar]
- 67.Zwingmann J., Südkamp N.P., Schmal H., Niemeyer P. Surgical treatment of osteochondritis dissecans of the talus: a systematic review. Arch Orthop Trauma Surg. 2012;132(9):1241–1250. doi: 10.1007/s00402-012-1544-1. [DOI] [PubMed] [Google Scholar]