Axitinib demonstrated clinical activity and safety in treatment-naïve Japanese patients with metastatic renal cell carcinoma. Multivariate analyses identified potential predictive factors for axitinib efficacy in first-line metastatic renal cell carcinoma.
Keywords: axitinib, Japanese, molecular targeted therapy, renal cell carcinoma, randomized clinical trial
Abstract
Objectives
To conduct Japanese subgroup analyses of a randomized, global Phase II study of axitinib with and without dose titration in first-line metastatic renal cell carcinoma and to explore predictive factors for axitinib efficacy in first-line metastatic renal cell carcinoma.
Methods
The data included 44 Japanese and 169 non-Japanese treatment-naïve patients with metastatic renal cell carcinoma. Patients received twice-daily axitinib 5 mg during a 4-week lead-in period. Patients who met the pre-defined randomization criteria were stratified by Eastern Cooperative Oncology Group performance status and randomly assigned (1:1) to axitinib or placebo titration. The primary endpoint was objective response rate; secondary endpoints included progression-free survival and safety. Predictive factors were analyzed using data from all patients.
Results
The objective response rate (95% confidence interval) was 66% (50–80%) vs. 44% (36–52%) in Japanese and non-Japanese patients, respectively. At the primary analysis, median progression-free survival could not be estimated for Japanese patients, and was 27.6 months (95% confidence interval: 16.6–33.2) in an updated analysis. Hypertension, diarrhea, hand–foot syndrome, dysphonia, hypothyroidism and proteinuria were common adverse events in Japanese patients. Due to a small number of randomized patients, effects of axitinib dose titration could not sufficiently be confirmed among Japanese patients. The multivariate analysis identified time from histopathological diagnosis to treatment and sum of the longest diameter for target lesion at baseline as independent predictive factors for progression-free survival.
Conclusions
Axitinib is effective and well tolerated as first-line metastatic renal cell carcinoma therapy in Japanese patients. Predictive factors for axitinib efficacy endpoints identified in this setting warrant further investigation.
Introduction
Axitinib, a potent and selective inhibitor of vascular endothelial growth factor receptors (VEGFR) 1, 2 and 3 (1), showed significant improvement in progression-free survival (PFS) over sorafenib in previously treated patients with metastatic renal cell carcinoma (mRCC) in the randomized Phase III AXIS trial (2). Axitinib is approved for treatment of advanced second-line renal cell carcinoma (RCC) and administered at a starting dose of 5 mg twice daily (BID), which was the maximum tolerated dose determined in a Phase I dose-escalating study in patients with solid tumors (3). Since axitinib plasma exposure is variable among individuals, dose titration is permitted to optimize drug exposure, based on patient tolerability. The population pharmacokinetic–pharmacodynamic analysis of pooled data from axitinib studies indicates a higher plasma exposure is associated with a higher response rate and longer survival in patients with mRCC (4).
To prospectively evaluate the benefit of axitinib dose titration, a randomized Phase II study was conducted in treatment-naïve patients with mRCC (5). The objective response rate (ORR) of 54% (95% confidence interval [CI]: 40–67%) in the axitinib-titration arm was significantly higher than the 34% (95% CI: 22–48%) in the placebo-titration arm (P = 0.019), supporting the clinical benefit of axitinib dose titration in a subset of patients. The study additionally showed clinical activity of axitinib in the treatment of first-line mRCC.
Efficacy and safety of axitinib in Japanese patients with previously treated mRCC has been evaluated in a limited number of clinical studies (6,7), warranting additional investigation. The aim of the current analysis was to assess the efficacy and safety of axitinib in Japanese vs. non-Japanese patients with first-line mRCC (5). Additionally, potential predictive factors for PFS in first-line mRCC were explored using data from the overall population. To the best of our knowledge, this is the first report of such analyses in patients with first-line mRCC treated with axitinib.
Patients and methods
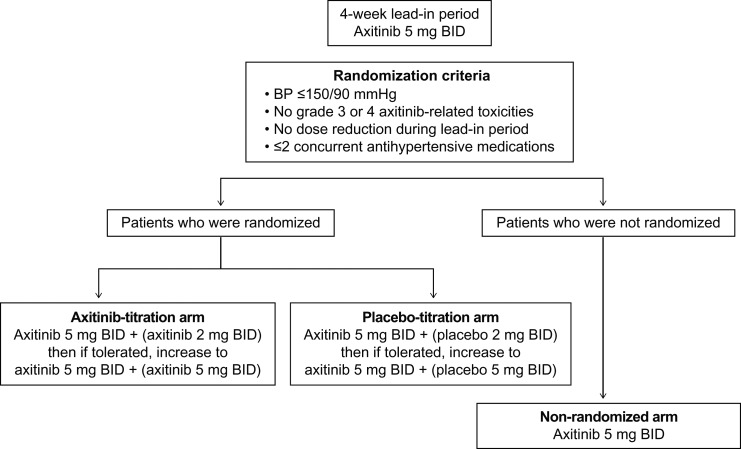
Details of this randomized, double-blind, placebo-controlled Phase II study of axitinib in patients with first-line mRCC, conducted in six countries, including Japan, have been previously reported (5). In brief, patients received axitinib 5 mg BID during a 4-week lead-in period (cycle 1); those who met the randomization criteria (Fig. 1) over 2 consecutive weeks were stratified by Eastern Cooperative Oncology Group performance status (ECOG PS) 0 vs. 1 and randomly assigned (1:1) to receive axitinib or placebo titration in 4-week cycles. Patients who did not meet the criteria continued on study (non-randomized arm). The primary endpoint was investigator-assessed ORR and secondary endpoints included PFS, overall survival (OS), safety and axitinib plasma pharmacokinetics. The study was performed with the approval of institutional review boards or independent ethics committees and in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guidelines on Good Clinical Practice and applicable local regulatory requirements. Written informed consent was provided by each patient. This study is registered with ClinicalTrials.gov (NCT00835978).
Figure 1.
Study design. Doses in parenthesis indicate blinded therapy.
As detailed previously (5), patients aged ≥18 years with histologically confirmed mRCC with a component of clear-cell histology, at least one measureable disease, no prior systemic therapy for mRCC, ECOG PS 0 or 1, adequate organ function and baseline blood pressure (BP) ≤140/90 mmHg were eligible. Key exclusion criteria included concurrent use of more than two antihypertensive medications and brain/central nervous system metastasis. Starting on cycle 2 Day 1, randomized patients received axitinib 5 mg BID (open-label) plus axitinib 2 mg BID or placebo (blinded therapy). After ≥ 2 consecutive weeks at this dosage, patients who continued to meet the randomization criteria could have their dose increased to the maximum level of axitinib 5 mg BID plus axitinib 5 mg BID or placebo. If at any time patients experienced treatment-related toxicity, study treatment was interrupted or the dose reduced (blinded therapy first) (5).
Radiographic tumor assessments were performed at baseline; weeks 8, 16 and 24; and every 12 weeks thereafter, according to Response Evaluation Criteria in Solid Tumors version 1.0. Safety was monitored throughout the study and adverse events (AEs) were graded according to the Common Terminology Criteria for AEs version 3.0. BP readings were taken in clinic and at home. Serial pharmacokinetic samples were collected in a subset of patients pre-dose and 0.5, 1, 2, 4 and 6 hours post-dose on cycle 1 Day 15 (prior to dose titration: 5 mg BID) and cycle 2 Day 15 (post-dose titration: 7 mg BID). Plasma axitinib concentrations were measured using a validated high-performance liquid chromatography with tandem mass spectrometry method (3). Statistical analyses were previously described (5). Median PFS was estimated using the Kaplan–Meier method. Axitinib pharmacokinetic parameters were estimated using non-compartmental analyses. Exploratory analyses to assess predictive factors for PFS were conducted using Cox proportional hazard model, witheach variable evaluated in the univariate analysis with Wald test. The final model was constructed by a stepwise method with a 5% level of significance.
Results
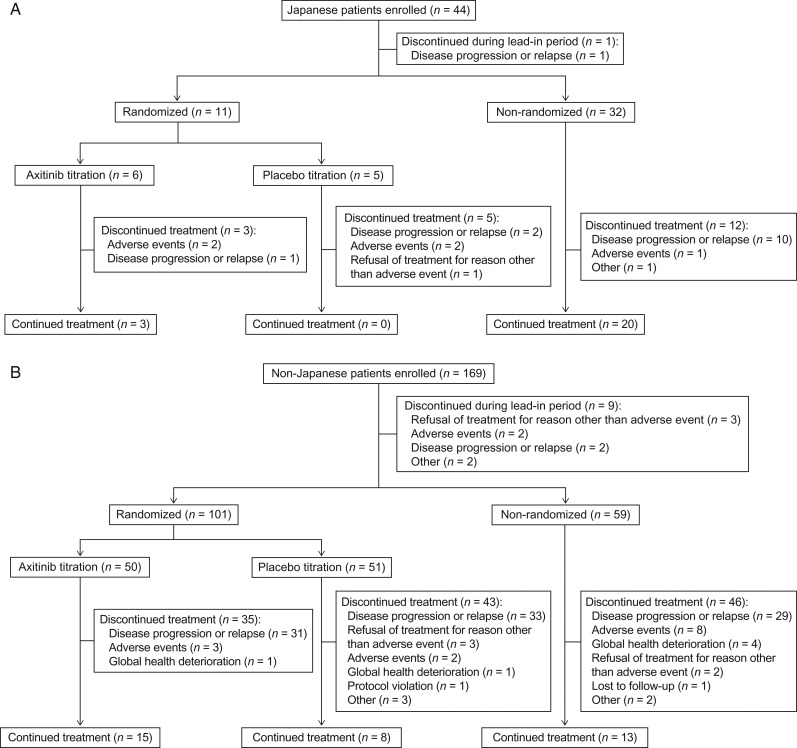
Of the 213 patients enrolled, 44 were Japanese and 169 were non-Japanese. All Japanese patients were enrolled at sites in Japan. Compared with non-Japanese patients, a higher percentage of Japanese patients had ECOG PS 0, smaller tumor size at baseline and fewer metastatic sites as well as fewer lymph node and adrenal metastases (Table 1). One Japanese and nine non-Japanese patients discontinued during the lead-in period. Following the lead-in period, 11 (25%) Japanese patients were randomly assigned to axitinib (n = 6) or placebo titration (n = 5) and the remaining 32 Japanese patients continued axitinib treatment in the non-randomized arm, whereas 50 and 51 non-Japanese patients were randomized to axitinib and placebo titration, respectively, and the remaining 59 patients to the non-randomized arm (Fig. 2). At the primary data cutoff date (12 October 2012), 48% of Japanese and 79% of non-Japanese patients discontinued treatment, mostly due to disease progression (Fig. 2). AEs were the reason for treatment discontinuation in five (11%) Japanese and 15 (9%) non-Japanese patients. Median duration of treatment was longer in Japanese vs. non-Japanese patients, respectively, in the axitinib titration (604 vs. 409 days) and the non-randomized arms (618 vs. 365 days); however, a higher percentage of Japanese than non-Japanese patients, respectively, required dose reductions (axitinib titration: 50% vs. 14%; non-randomized: 50% vs. 39%) (Table 2). The median axitinib daily dose and relative dose intensity were lower in Japanese than non-Japanese patients.
Table 1.
Patient demographics and baseline clinical characteristics in Japanese vs. non-Japanese patients
| Japanese n = 44 | Non-Japanese n = 169 | P value | |
|---|---|---|---|
| Age, median (range), years | 66 (42–81) | 61 (28–87) | 0.0231a |
| Sex, n (%) | |||
| Male | 30 (68) | 113 (67) | 1.0000b |
| Female | 14 (32) | 56 (33) | |
| Race, n (%) | |||
| White | 0 | 162 (96) | <0.0001b |
| Asian | 44 (100) | 2 (1) | |
| Black | 0 | 2 (1) | |
| Other | 0 | 3 (2) | |
| Height, mean (SD), cm | 162 (9) | 172 (10) | <0.0001a |
| Weight, mean (SD), kg | 61 (12) | 83 (18) | <0.0001a |
| ECOG PS, n (%)c | |||
| 0 | 37 (84) | 99 (59) | 0.0015b |
| ≥1 | 7 (16) | 70 (41) | |
| Prior nephrectomy, n (%) | |||
| Yes | 37 (84) | 146 (86) | 0.8077b |
| No | 7 (16) | 23 (14) | |
| No. of metastatic sites, n (%) | |||
| 1 | 17 (39) | 24 (14) | 0.0003d |
| 2 | 13 (30) | 44 (26) | |
| 3 | 6 (14) | 46 (27) | |
| ≥4 | 8 (18) | 55 (33) | |
| Metastatic sites (lung vs. lung + others), n (%) | |||
| Lung only | 9 (20) | 16 (9) | 0.0627b |
| Lung + others | 35 (80) | 153 (91) | |
| Metastatic sites (individual), n (%) | |||
| Lung | 30 (68) | 119 (70) | 0.8538b |
| Lymph node | 13 (30) | 86 (51) | 0.0169b |
| Kidney | 13 (30) | 37 (22) | 0.3194b |
| Liver | 6 (14) | 47 (28) | 0.0766b |
| Adrenal | 3 (7) | 46 (27) | 0.0042b |
| Bone | 7 (16) | 30 (18) | 1.0000b |
| Pancreas | 1 (2) | 4 (2) | 1.0000b |
| Time from histopathological diagnosis to treatment, median (range), weeks | 56 (0.1–952) | 23 (0.1–1338) | 0.9223e |
| Time from metastatic diagnosis to treatment, median (range), weeks | 7 (0.9–263) | 8 (0.7–456) | 0.4476e |
| Sum of longest diameter for target lesion, median (range), mm | 75 (10–376) | 99 (10–466) | 0.0013e |
| Presence of metastases (de novo) at initial diagnosis, n (%) | |||
| No | 25 (57) | 94 (56) | 1.0000b |
| Yes | 19 (43) | 75 (44) | |
ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation.
aUsing t-test.
bUsing Fisher's exact test.
cPer case report forms and the last measure taken prior to dosing. One non-Japanese patient had ECOG PS 2.
dUsing Cochran-Armitage trend exact test.
eUsing Wilcoxon test.
Figure 2.
CONSORT diagram (A) Japanese and (B) non-Japanese patients.
Table 2.
Exposure to study drug in Japanese vs. non-Japanese patients
| Japanese | Non-Japanese | |||||
|---|---|---|---|---|---|---|
| Axitinib titration | Placebo titration | Non-randomized | Axitinib titration | Placebo titration | Non-randomized | |
| n = 6 | n = 5 | n = 32 | n = 50 | n = 51 | n = 59 | |
| Days on studya | ||||||
| Median | 604 | 315 | 618 | 409 | 392 | 365 |
| Range | 106–657 | 305–591 | 64–816 | 42–1062 | 43–1075 | 33–1089 |
| Days on drugb | ||||||
| Median | 578 | 305 | 530 | 385 | 369 | 358 |
| Range | 91–636 | 229–591 | 46–787 | 38–990 | 43–1073 | 4–1084 |
| Dose reduced <5 mg BID, n (%) | 3 (50) | 1 (20) | 16 (50) | 7 (14) | 4 (8) | 23 (39) |
| Average daily dose administered, mgc | ||||||
| Median | 9.7 | 13.4 | 8.5 | 13.6 | 17.3 | 9.6 |
| Range | 8.3–13.9 | 8.0–18.6 | 3.9–10.4 | 5.2–19.5 | 7.4–19.5 | 4.1–10.0 |
| Relative dose intensity, %d | ||||||
| Median | 92 | 125 | 78 | 132 | 171 | 92 |
| Range | 80–135 | 77–186 | 12–105 | 47–195 | 65–196 | 6–100 |
aNumber of days between date of first dose and date of the last dose or data cutoff date + 1.
bTotal number of days on which axitinib was actually administered.
cTotal dose administered divided by actual number of days treated.
d(Total dose administered / 5 mg BID) × 100.
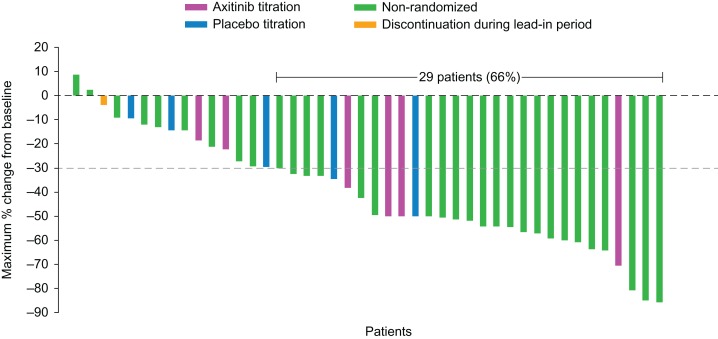
Among Japanese patients, overall ORR was 66% (95% CI: 50–80%) and axitinib titration resulted in a numerically higher ORR compared with placebo titration (67% vs. 40%, respectively). Among non-Japanese patients, overall ORR was 44% (95% CI: 36–52%), favoring axitinib titration over placebo titration (52% vs. 33%, respectively). In the non-randomized arm, ORR in Japanese and non-Japanese patients (72% vs. 53%, respectively) was similar to the corresponding axitinib-titration arm (Table 3). The mean percentage of tumor shrinkage in the axitinib-titration arm was numerically larger in Japanese than non-Japanese patients, whereas those in the placebo titration and non-randomized arms were similar (Table 4). In the overall population, the mean percentage change from baseline in tumor size was −34.7%, −26.2% and −42.7% in the axitinib titration, placebo titration and non-randomized arm, respectively. Twenty-nine Japanese patients (4 in the axitinib titration, 2 in the placebo titration and 23 in the non-randomized arm) achieved a ≥30% decrease in tumor size (Fig. 3).
Table 3.
Best response by RECIST in Japanese vs. non-Japanese patients
| Japanese | Non-Japanese | |||||||
|---|---|---|---|---|---|---|---|---|
| Totala | Axitinib titration | Placebo titration | Non-randomized | Totalb,c | Axitinib titration | Placebo titrationc | Non-randomized | |
| n = 44 | n = 6 | n = 5 | n = 32 | n = 169 | n = 50 | n = 51 | n = 59 | |
| Best response, n (%) | ||||||||
| CR | 0 | 0 | 0 | 0 | 1 (1) | 1 (2) | 0 | 0 |
| PR | 29 (66) | 4 (67) | 2 (40) | 23 (72) | 73 (43) | 25 (50) | 17 (33) | 31 (53) |
| SD | 13 (30) | 2 (33) | 3 (60) | 8 (25) | 47 (28) | 11 (22) | 21 (41) | 15 (25) |
| PD | 2 (5) | 0 | 0 | 1 (3) | 36 (21) | 13 (26) | 11 (22) | 10 (17) |
| Not assessed | 0 | 0 | 0 | 0 | 9 (5) | 0 | 1 (2) | 2 (3) |
| Indeterminate | 0 | 0 | 0 | 0 | 2 (1) | 0 | 0 | 1 (2) |
| ORR, %d | 66 | 67 | 40 | 72 | 44 | 52 | 33 | 53 |
| 95% CIe | 50–80 | 22–96 | 5–85 | 53–86 | 36–52 | 37–66 | 21–48 | 39–66 |
CR, complete response; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; CI, confidence interval.
aIncludes one patient who received treatment and withdrew during the lead-in period.
bIncludes nine patients who received treatment and withdrew during the lead-in period.
cOne patient in placebo-titration arm did not have measurable disease at baseline.
dCR plus PR.
eUsing exact method based on F distribution.
Table 4.
Tumor shrinkage in Japanese vs. non-Japanese patients
| Japanese | Non-Japanese | |||||||
|---|---|---|---|---|---|---|---|---|
| Totala | Axitinib titration | Placebo titration | Non-randomized | Totalb,c,d | Axitinib titrationd | Placebo titrationc,d | Non-randomizedd | |
| n = 44 | n = 6 | n = 5 | n = 32 | n = 169 | n = 50 | n = 51 | n = 59 | |
| Change from baseline in sum of tumor diameter for target lesion (%) | ||||||||
| Mean | −40.2 | −41.6 | −27.5 | −43.0 | −33.9 | −33.8 | −26.1 | −42.6 |
| SD | 23.2 | 19.5 | 16.3 | 23.9 | 37.3 | 41.9 | 36.9 | 31.7 |
aIncludes one patient who received treatment and withdrew during the lead-in period.
bIncludes nine patients who received treatment and withdrew during the lead-in period.
cOne patient in placebo-titration arm did not have measurable disease at baseline.
dData are missing for 12, one, three and two patients in the total, axitinib titration, placebo titration and non-randomized arm, respectively.
Figure 3.
Maximum percentage change from baseline in tumor size in Japanese patients. The dotted line indicates a 30% decrease in tumor size.
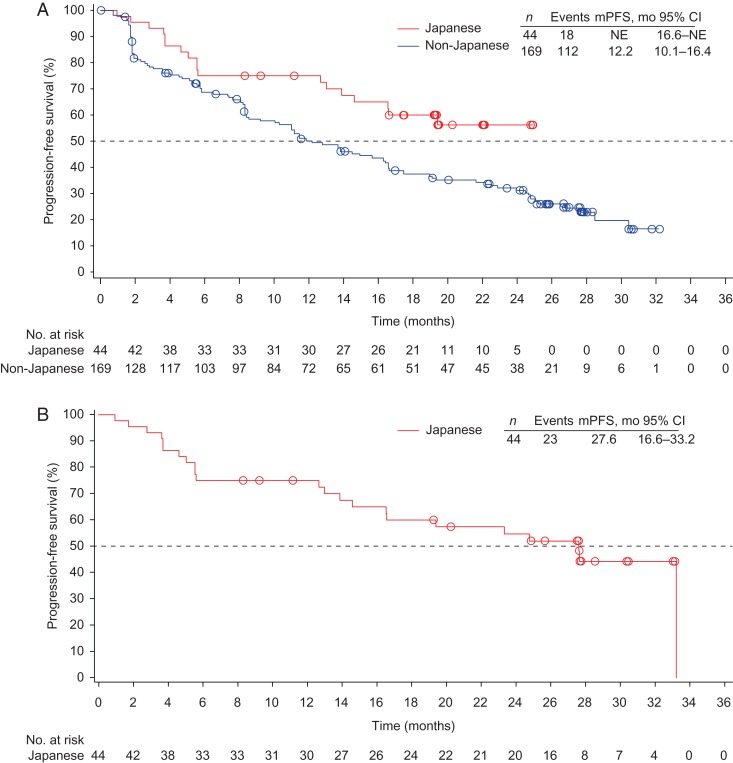
At the time of the primary endpoint analysis, median PFS could not be estimated for Japanese patients (95% CI: 16.6 months to not estimable) since only 18 events had occurred, compared with 12.2 months (95% CI: 10.1–16.4 months) among non-Japanese patients (Fig. 4A). In the updated analysis (data cutoff date, 17 May 2013) conducted in Japanese patients only, 23 patients had events and median PFS was 27.6 months (95% CI: 16.6–33.2 months; Fig. 4B). OS data were not mature in Japanese patients at the primary or follow-up analyses (12 of 44 patients died).
Figure 4.
Kaplan–Meier estimates of progression-free survival (PFS) in (A) Japanese vs. non-Japanese patients at 12 October 2012 data cutoff, and (B) Japanese patients at 17 May 2013 data cutoff. NE, not estimable.
Hypertension and diarrhea were the most common all-causality AEs in both Japanese and non-Japanese patients (Table 5). The nature of common AEs was generally similar between Japanese and non-Japanese patients, but incidence rates of some AEs, including hypertension, hand–foot syndrome, dysphonia, hypothyroidism and proteinuria, were higher in Japanese patients and more Japanese than non-Japanese patients, respectively, received antihypertensive (95% vs. 64%) and hypothyroidism (64% vs. 32%) medications. Among Japanese patients, hypothyroidism and decreased appetite were more common with axitinib titration vs. placebo titration, whereas dysphonia, proteinuria and headache were more common in the placebo-titration vs. axitinib-titration arms (Table 6).
Table 5.
Common treatment-emergent, all-causality AEs reported by all Japanese vs. non-Japanese patients
| AE, n (%)a | Japanesen = 44 | Non-Japanese n = 169 | ||
|---|---|---|---|---|
| All-grade | Grade 3/4 | All-grade | Grade 3/4 | |
| Hypertension | 40 (91)b | 29 (66)b | 98 (58) | 34 (20) |
| Diarrhea | 33 (75) | 1 (2) | 94 (56) | 16 (9) |
| Hand–foot syndrome | 32 (73)b | 6 (14) | 36 (21) | 2 (1) |
| Dysphonia | 30 (68)b | 0 | 55 (33) | 2 (1) |
| Hypothyroidism | 30 (68)b | 0 | 44 (26) | 0 |
| Proteinuria | 27 (61)b | 1 (2) | 36 (21) | 2 (1) |
| Decreased appetite | 21 (48) | 3 (7) | 55 (33) | 4 (2) |
| Fatigue | 21 (48) | 2 (5) | 83 (49) | 12 (7) |
| Stomatitis | 15 (34)b | 0 | 15 (9) | 1 (1) |
| Nasopharyngitis | 14 (32)b | 0 | 8 (5) | 0 |
| Weight decreased | 13 (30) | 2 (5) | 40 (24) | 11 (7) |
| Dysgeusia | 12 (27) | 0 | 23 (14) | 0 |
| Nausea | 11 (25) | 1 (2) | 62 (37) | 4 (2) |
| Alopecia | 9 (20) | 0 | 10 (6) | 0 |
| Constipation | 9 (20) | 0 | 39 (23) | 1 (1) |
| Epistaxis | 9 (20) | 0 | 9 (5) | 0 |
| Headache | 8 (18) | 0 | 40 (24) | 2 (1) |
| Vomiting | 4 (9) | 0 | 49 (29) | 4 (2) |
| Dyspnea | 4 (9) | 1 (2) | 36 (21) | 5 (3) |
| Pain in extremity | 3 (7) | 0 | 34 (20) | 2 (1) |
AE, adverse event.
aReported by ≥20% of either Japanese or non-Japanese patients.
b≥20% higher in Japanese than non-Japanese patients.
Table 6.
Common treatment-emergent, all-causality AEs reported by Japanese patients in each treatment arm
| AE, n (%)a | Axitinib titration n = 6 | Placebo titration n = 5 | Non-randomized n = 32 | |||
|---|---|---|---|---|---|---|
| All-grade | Grade 3/4 | All-grade | Grade 3/4 | All-grade | Grade 3/4 | |
| Hypertension | 5 (83) | 3 (50) | 5 (100) | 2 (40) | 29 (91) | 23 (72) |
| Diarrhea | 5 (83) | 0 | 5 (100) | 0 | 23 (72) | 1 (3) |
| Hypothyroidism | 5 (83)b | 0 | 3 (60) | 0 | 22 (69) | 0 |
| Hand–foot syndrome | 5 (83) | 1 (17) | 5 (100) | 0 | 22 (69) | 5 (16) |
| Dysphonia | 4 (67) | 0 | 5 (100)c | 0 | 21 (66) | 0 |
| Proteinuria | 3 (50) | 1 (17) | 4 (80)c | 0 | 20 (63) | 0 |
| Fatigue | 3 (50) | 0 | 3 (60) | 0 | 15 (47) | 2 (6) |
| Decreased appetite | 5 (83)b | 0 | 3 (60) | 0 | 13 (41) | 3 (9) |
| Nasopharyngitis | 1 (17) | 0 | 1 (20) | 0 | 12 (38) | 0 |
| Stomatitis | 2 (33) | 0 | 2 (40) | 0 | 11 (34) | 0 |
| Alopecia | 0 | 0 | 0 | 0 | 9 (28) | 0 |
| Epistaxis | 1 (17) | 0 | 0 | 0 | 8 (25) | 0 |
| Hyperuricemia | 0 | 0 | 0 | 0 | 8 (25) | 1 (3) |
| Weight decreased | 3 (50) | 1 (17) | 2 (40) | 0 | 8 (25) | 1 (3) |
| Dysgeusia | 3 (50) | 0 | 2 (40) | 0 | 7 (22) | 0 |
| Headache | 0 | 0 | 1 (20)c | 0 | 7 (22) | 0 |
| Nausea | 2 (33) | 0 | 2 (40) | 0 | 7 (22) | 1 (3) |
aReported by ≥20% of patients in the non-randomized arm.
b≥20% higher in the axitinib-titration than the placebo-titration arm.
c≥20% higher in the placebo-titration than the axitinib-titration arm.
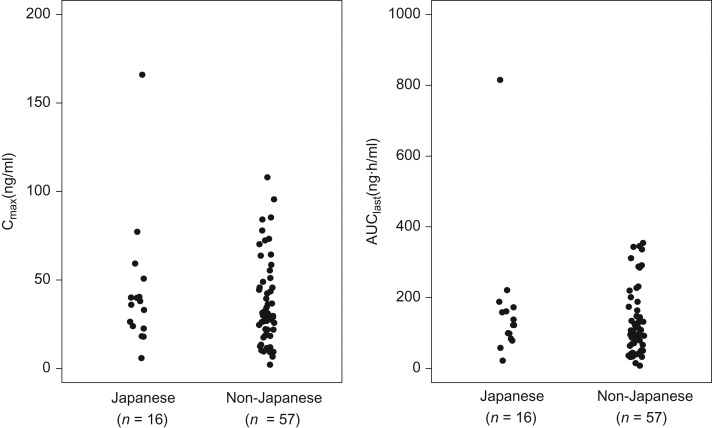
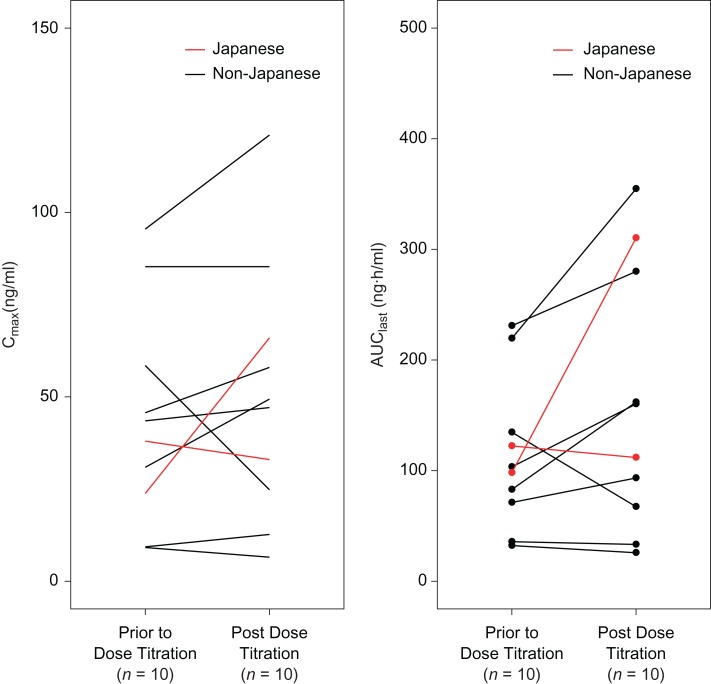
The range for the maximum observed plasma concentration (Cmax) and area under the plasma concentration–time curve from time zero to last time of measurable concentration (AUClast) of axitinib on cycle 1 Day 15 (i.e. at steady-state prior to dose titration) in Japanese patients generally overlapped with those in non-Japanese patients (Fig. 5). Dose titration from 5 mg BID to 7 mg BID generally resulted in increased axitinib plasma exposure (Fig. 6). The median Cmax and AUClast, respectively, in 10 patients, including two Japanese, increased from 40.8 ng/ml and 101 ng·h/ml before the dose titration to 48.3 ng/ml and 136 ng·h/ml following the dose titration. Based on the limited patient data, axitinib pharmacokinetic parameters for Japanese patients were within the range of those for non-Japanese patients.
Figure 5.
Pharmacokinetics of axitinib on cycle 1 Day 15 in a subset of Japanese and non-Japanese patients following 5 mg twice daily administration.
Figure 6.
Pharmacokinetics of axitinib prior to dose titration (5 mg twice daily) and post dose titration (7 mg twice daily).
Univariate analysis of the data in the overall population identified a significant (P ≤ 0.05) association between PFS and Asian ethnicity, ECOG PS, numbers and site (lung plus others vs. lung only) of metastasis, time from histopathological diagnosis to treatment, sum of the longest diameter for target lesion (tumor size), presence of de novo metastasis at initial diagnosis and baseline hemoglobin (Table 7). In the multivariate analysis, time from histopathological diagnosis to treatment <1 year or sum of the longest diameter for target lesion greater than median (89 mm) at baseline remained significantly associated with shorter PFS (Table 7). In an additional univariate analysis to evaluate the effect of individual metastatic sites, results showed presence of baseline lymph node, liver and bone metastases to be potential predictors for PFS (Table 8). However, baseline lactate dehydrogenase and hemoglobin levels were found not to be substantially different between patients with or without lymph node, liver or bone metastases (Table S1).
Table 7.
Univariate and multivariate analyses of predictive factors for progression-free survival (PFS) in the overall population
| n | mPFS, mo (95% CI) | Hazard ratio (95% CI)a | P valueb | |
|---|---|---|---|---|
| Univariate analyses | ||||
| Age, years | ||||
| <65 | 136 | 11.1 (8.3–16.6) | 1 | |
| ≥65 | 77 | 17.5 (13.9–26.7) | 0.73 (0.50–1.06) | 0.0941 |
| Sex | ||||
| Male | 143 | 15.7 (11.1–22.5) | 1 | |
| Female | 70 | 13.9 (9.2–17.4) | 1.14 (0.79–1.64) | 0.4736 |
| Race | ||||
| White | 162 | 12.8 (10.1–16.5) | 1 | |
| Asian | 46 | NE (14.6–NE) | 0.58 (0.36–0.94) | 0.0279 |
| Black | 2 | 6.5 (1.8–11.2) | 2.67 (0.65–10.89) | 0.1709 |
| Other | 3 | 23.9 (2.8–25.1) | 1.15 (0.37–3.64) | 0.8088 |
| Body weight, kg | ||||
| ≤65 | 56 | 14.9 (8.3–19.3) | 1 | |
| >65 to ≤76 | 51 | 9.2 (5.7–24.8) | 0.93 (0.57–1.52) | 0.7736 |
| >76 to ≤89 | 53 | 15.7 (11.1–23.9) | 0.88 (0.55–1.42) | 0.5957 |
| >89 | 53 | 16.6 (10.3–25.1) | 0.82 (0.51–1.34) | 0.4380 |
| ECOG PS | ||||
| 0 | 136 | 16.6 (13.7–24.8) | 1 | |
| ≥ 1 | 77 | 8.1 (4.7–16.6) | 1.58 (1.11–2.26) | 0.0115 |
| Prior nephrectomy | ||||
| Yes | 183 | 14.6 (11.2–19.0) | 1 | |
| No | 30 | 12.7 (5.6–22.2) | 1.31 (0.79–2.15) | 0.2942 |
| No. of metastatic sites | ||||
| 1 | 41 | 24.8 (13.7–NE) | 1 | |
| 2 | 57 | 22.2 (10.3–27.6) | 1.19 (0.67–2.11) | 0.5624 |
| 3 | 52 | 13.8 (7.4–16.6) | 1.97 (1.13–3.42) | 0.0166 |
| ≥4 | 63 | 11.1 (5.6–16.6) | 2.12 (1.24–3.63) | 0.0064 |
| Metastatic sites (lung vs. lung + others) | ||||
| Lung only | 25 | 25.1 (13.9–NE) | 1 | |
| Lung + others | 188 | 13.8 (11.1–16.6) | 1.91 (1.03–3.54) | 0.0411 |
| Time from histopathological diagnosis to treatment, years | ||||
| ≥1 | 88 | 22.5 (16.3–26.7) | 1 | |
| <1 | 125 | 10.3 (8.1–14.5) | 1.81 (1.26–2.59) | 0.0013 |
| Time from metastatic diagnosis to treatment, years | ||||
| ≥1 | 20 | 16.6 (8.3–NE) | 1 | |
| <1 | 193 | 14.5 (11.1–17.5) | 1.15 (0.62–2.14) | 0.6563 |
| Sum of longest diameter for target lesion | ||||
| ≤Medianc | 107 | 21.6 (13.9–25.1) | 1 | |
| >Medianc | 105 | 11.1 (7.6–14.5) | 1.74 (1.23–2.47) | 0.0018 |
| Presence of metastases (de novo) at initial diagnosis | ||||
| No | 119 | 16.6 (11.5–25.1) | 1 | |
| Yes | 94 | 12.7 (8.3–16.6) | 1.44 (1.02–2.04) | 0.0376 |
| Baseline lactate dehydrogenased | ||||
| ≤1.5 × ULN | 198 | 15.7 (12.2–19.1) | 1 | |
| >1.5 × ULN | 11 | 2.8 (1.7–NE) | 2.12 (0.99–4.56) | 0.0536 |
| Baseline hemoglobin | ||||
| ≥LLN | 119 | 16.6 (13.8–24.5) | 1 | |
| <LLN | 94 | 11.5 (8.1–14.9) | 1.43 (1.01–2.03) | 0.0414 |
| Multivariate analysese | ||||
| Time from histopathological diagnosis to treatment, years | ||||
| <1 vs. ≥1 | 1.66 (1.15–2.40) | 0.0068 | ||
| Sum of longest diameter for target legion | ||||
| >Medianc vs. ≤Medianc | 1.63 (1.14–2.32) | 0.0069 | ||
LLN, lower limit of normal; mPFS, median progression-free survival; NE, not estimable; ULN, upper limit of normal.
a1 equals reference.
bUsing Wald test.
cMedian equals 89 mm in the overall population.
dBaseline lactate dehydrogenase levels were missing for four patients.
eFinal model constructed by a stepwise method with a 0.05 significance level.
Table 8.
Univariate analysis of baseline individual metastatic sites for PFS in the overall population
| n | mPFS, mo (95% CI) | Hazard ratio (95% CI)a | P valueb | |
|---|---|---|---|---|
| Lung metastases | ||||
| No | 64 | 16.4 (11.1–25.1) | 1 | |
| Yes | 149 | 13.9 (11.0–17.4) | 1.21 (0.83–1.78) | 0.3220 |
| Lymph node metastases | ||||
| No | 114 | 19.4 (13.7–26.7) | 1 | |
| Yes | 99 | 11.0 (8.1–16.4) | 1.79 (1.26–2.53) | 0.0010 |
| Kidney metastases | ||||
| No | 163 | 14.6 (11.0–17.5) | 1 | |
| Yes | 50 | 14.5 (11.9–22.2) | 1.01 (0.67–1.52) | 0.9743 |
| Liver metastases | ||||
| No | 160 | 16.6 (14.6–24.5) | 1 | |
| Yes | 53 | 5.5 (3.0–11.1) | 2.32 (1.60–3.36) | <0.0001 |
| Adrenal metastases | ||||
| No | 164 | 16.3 (11.5–19.0) | 1 | |
| Yes | 49 | 12.8 (7.6–22.9) | 1.14 (0.77–1.69) | 0.5248 |
| Bone metastases | ||||
| No | 176 | 16.6 (13.7–22.9) | 1 | |
| Yes | 37 | 5.6 (1.9–12.8) | 2.26 (1.50–3.41) | 0.0001 |
| Pancreatic metastases | ||||
| No | 208 | 14.5 (11.1–16.6) | 1 | |
| Yes | 5 | NE (11.5–NE) | 0.35 (0.09–1.44) | 0.1461 |
a1 equals reference.
bUsing Wald test.
Discussion
Subgroup analyses of the data from the randomized Phase II study showed that axitinib has anti-tumor activity in first-line mRCC in Japanese patients. The ORR in the axitinib-titration arm was higher than that in the placebo-titration arm among Japanese patients, consistent with the results of the overall population (5). Furthermore, axitinib treatment resulted in higher ORR and longer median PFS (>2 years) in Japanese patients compared with non-Japanese patients, which might have been due, at least in part, to a higher percentage of Japanese patients with more favorable baseline clinical characteristics (ECOG PS 0, one site of metastasis, smaller baseline tumor size and fewer lymph node or liver metastases). Axitinib plasma pharmacokinetics do not appear to contribute substantially to the differences in clinical outcomes since Cmax and AUClastwere generally comparable between Japanese and non-Japanese patients in this study, which is in agreement with previous findings from Phase I studies of axitinib (8–10). The comparable pharmacokinetics between Japanese and non-Japanese patients observed for axitinib in this study are similar to the results reported for other VEGFR-tyrosine kinase inhibitors (TKIs), sunitinib, sorafenib and pazopanib (11–13). In the absence of any major racial/ethnic differences in axitinib pharmacokinetics, other patient factors and/or clinical practice would have accounted for the better clinical outcome in Japanese than non-Japanese patients. In this study, fewer Japanese than non-Japanese patients, respectively, were assigned to titration arms (25% vs. 60%) because more Japanese than non-Japanese patients, respectively, failed to meet randomization criteria (diastolic BP > 90 mmHg [16% vs. 8%] or treatment-related grade 3/4 AEs [28% vs. 8%]) during the lead-in period. Additionally, despite meeting randomization criteria, more Japanese than non-Japanese patients (30% vs. 19%, respectively) were not randomized, at investigator's discretion, for reasons such as presence of multiple AEs.
While cross-study comparisons need to be interpreted with caution, median PFS of 27.6 months in axitinib-treated Japanese patients was almost twice as long as the 12.2 months reported in treatment-naïve Japanese patients treated with sunitinib (14), and longer than those treated with other anti-angiogenic inhibitors in first- or second-line mRCC (15–20). The results of the current study, therefore, generate a hypothesis for potential therapeutic use of axitinib in Japanese patients with first-line mRCC. It should be noted that in a randomized Phase III study of axitinib in treatment-naïve patients with mRCC (one-quarter of whom were Asian), the difference in the primary endpoint of PFS between axitinib and sorafenib did not reach statistical significance in the overall population (median PFS: 10.1 vs. 6.5 months; hazard ratio 0.77 [95% CI: 0.56–1.05]; one-sided P = 0.038), but median PFS was significantly longer with axitinib than sorafenib in patients with ECOG PS 0 (13.7 vs. 6.6 months; hazard ratio 0.64 [95% CI: 0.42–0.99]; one-sided P = 0.022) (21).
Axitinib was reasonably well tolerated, as evidenced by treatment discontinuation due to AEs in 11% and 9% of Japanese and non-Japanese patients, respectively. Common AEs with axitinib, known class effects of antiangiogenic TKIs (18–20), were generally similar between Japanese and non-Japanese patients and were manageable. However, the incidence of several AEs (hypertension, hand–foot syndrome, dysphonia, hypothyroidism and proteinuria) was higher in Japanese patients. Differences in the frequency of some AEs between Asian and non-Asian patients have been described for other antiangiogenic TKIs (15,22–26), which might partly be explained by differences in genetic background (27,28). Although the number of Japanese patients in each randomized arm was too small to draw any meaningful conclusion, no major differences in the safety profile were noted between the axitinib- and placebo-titration arms. The common AEs in Japanese patients in this first-line mRCC study were comparable to those reported in the second-line setting (6,7).
Based on the favorable efficacy results observed in Japanese patients, exploratory analyses were performed to investigate baseline patient characteristics that may be predictive for PFS in the overall population. Several factors (e.g. prior therapy, ECOG PS, hemoglobin level) have been identified as risk factors for PFS and/or OS in patients with second-line mRCC treated with axitinib (4,29), but there has been no report of similar analyses in first-line mRCC. In univariate analyses using data from 213 treatment-naïve patients in this study, several patient baseline characteristics were found to be significantly (P < 0.05) associated with median PFS. In the multivariate analysis, time from histopathological diagnosis to treatment and baseline tumor size remained as independent predictive factors. Incidentally, another multivariate analysis of pooled data in patients with mRCC (74% of whom were treatment-naïve) treated with sunitinib identified time from diagnosis to treatment as one of the independent predictors for PFS (30). In an additional univariate analysis for individual metastatic sites in the current study, significantly shorter PFS was observed in patients with lymph node, liver or bone metastasis. However, interestingly, no significant difference was found in baseline levels of lactate dehydrogenase or hemoglobin, which are other prognostic factors used in the Memorial Sloan-Kettering Cancer Center (MSKCC) risk scoring system, between patients with and without lymph node, liver or bone metastasis. In this study, calcium concentrations were not measured; hence, the limitation of the analyses was that MSKCC or Heng risk factors (31) could not be included. The factors identified for PFS mostly coincide with baseline characteristics that were significantly different between Japanese and non-Japanese patients, and might explain the better clinical outcomes in Japanese patients. Therefore, the current analyses would help identify patients who would likely or unlikely benefit from axitinib treatment, not only for Japanese patients, but also non-Japanese patients.
In conclusion, axitinib was effective and well tolerated in Japanese patients with previously untreated mRCC. The time from histopathological diagnosis to treatment and baseline tumor size were found to be associated with axitinib efficacy in first-line treatment of mRCC. The predictive factors for PFS identified in the current analyses warrant further investigation.
Supplementary data
Supplementary data are available at http://www.jjco.oxfordjournals.org.
Acknowledgements
This study was sponsored by Pfizer. Pfizer was involved in study design, data collection and interpretation of data. We thank Hisanaga Ohashi of Pfizer for data collection and Hiroko Godai of Pfizer for data analysis. Medical writing support was provided by Mariko Nagashima, PhD, of Engage Scientific Solutions (Southport, CT, USA) and was funded by Pfizer.
Conflicts of interest statement
The Author Responsibility-COI forms were submitted with the manuscript.
Appendix
The following authors have contributed equally to the current study, in addition to the authors listed in the author field.
Yosuke Fujii13, Akiyuki Suzuki13, Yoichi Kamei13, Yoshiko Umeyama13
13Pfizer Japan Inc, Tokyo, Japan
References
- 1.Hu-Lowe DD, Zou HY, Grazzini ML, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 2008;14:7272–83. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 2011;378:1931–9. [DOI] [PubMed] [Google Scholar]
- 3.Rugo HS, Herbst RS, Liu G, et al. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 2005;23:5474–83. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Garrett M, Poland B, et al. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. J Clin Pharmacol 2013;53:491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rini BI, Melichar B, Ueda T, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncol 2013;14:1233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomita Y, Uemura H, Fujimoto H, et al. Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell Carcinoma. Eur J Cancer 2011;47:2592–602. [DOI] [PubMed] [Google Scholar]
- 7.Ueda T, Uemura H, Tomita Y, et al. Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized Phase 3 AXIS trial. Jpn J Clin Oncol 2013;43:616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pithavala YK, Tortorici M, Toh M, et al. Effect of rifampin on the pharmacokinetics of Axitinib (AG-013736) in Japanese and Caucasian healthy volunteers. Cancer Chemother Pharmacol 2010;65:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukohara T, Nakajima H, Mukai H, et al. Effect of axitinib (AG-013736) on fatigue, thyroid-stimulating hormone, and biomarkers: a phase I study in Japanese patients. Cancer Sci 2010;101:963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara Y, Kiyota N, Chayahara N, et al. Management of axitinib (AG-013736)-induced fatigue and thyroid dysfunction, and predictive biomarkers of axitinib exposure: results from phase I studies in Japanese patients. Invest New Drugs 2012;30:1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirao K, Nishida T, Doi T, et al. Phase I/II study of sunitinib malate in Japanese patients with gastrointestinal stromal tumor after failure of prior treatment with imatinib mesylate. Invest New Drugs 2010;28:866–75. [DOI] [PubMed] [Google Scholar]
- 12.Minami H, Kawada K, Ebi H, et al. Phase I and pharmacokinetic study of sorafenib, an oral multikinase inhibitor, in Japanese patients with advanced refractory solid tumors. Cancer Sci 2008;99:1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inada-Inoue M, Ando Y, Kawada K, et al. Phase 1 study of pazopanib alone or combined with lapatinib in Japanese patients with solid tumors. Cancer Chemother Pharmacol 2014;73:673–83. [DOI] [PubMed] [Google Scholar]
- 14.Tomita Y, Shinohara N, Yuasa T, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2010;40:1166–72. [DOI] [PubMed] [Google Scholar]
- 15.Akaza H, Tsukamoto T, Murai M, Nakajima K, Naito S. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol 2007;37:755–62. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 2007;370:2103–11. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26:5422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34. [DOI] [PubMed] [Google Scholar]
- 19.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28:1061–8. [DOI] [PubMed] [Google Scholar]
- 20.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 21.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol 2013;14:1287–94. [DOI] [PubMed] [Google Scholar]
- 22.Uemura H, Shinohara N, Yuasa T, et al. A phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma: insights into the treatment, efficacy and safety. Jpn J Clin Oncol 2010;40:194–202. [DOI] [PubMed] [Google Scholar]
- 23.Hong MH, Kim HS, Kim C, et al. Treatment outcomes of sunitinib treatment in advanced renal cell carcinoma patients: a single cancer center experience in Korea. Cancer Res Treat 2009;41:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang H, Dong B, Lu JJ, et al. Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer 2009;9:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang E, Lee HJ, Sul CK, Lim JS. Efficacy and safety of sunitinib on metastatic renal cell carcinoma: a single-institution experience. Korean J Urol 2010;51:450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo C, Kim JE, Lee JL, et al. The efficacy and safety of sunitinib in korean patients with advanced renal cell carcinoma: high incidence of toxicity leads to frequent dose reduction. Jpn J Clin Oncol 2010;40:980–5. [DOI] [PubMed] [Google Scholar]
- 27.Tsuchiya N, Narita S, Inoue T, et al. Risk factors for sorafenib-induced high-grade skin rash in Japanese patients with advanced renal cell carcinoma. Anticancer Drugs 2013;24:310–4. [DOI] [PubMed] [Google Scholar]
- 28.Kim HR, Park HS, Kwon WS, et al. Pharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patients. Cancer Chemother Pharmacol 2013;72:825–35. [DOI] [PubMed] [Google Scholar]
- 29.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552–62. [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Escudier B, Bukowski R, et al. Prognostic factors for survival in 1059 patients treated with sunitinib for metastatic renal cell carcinoma. Br J Cancer 2013;108:2470–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.