Abstract
Polycyclic aromatic hydrocarbons (PAHs) are widespread in marine ecosystems and originate from natural sources and anthropogenic activities. PAHs enter the marine environment in two main ways, corresponding to chronic pollution or acute pollution by oil spills. The global PAH fluxes in marine environments are controlled by the microbial degradation and the biological pump, which plays a role in particle settling and in sequestration through bioaccumulation. Due to their low water solubility and hydrophobic nature, PAHs tightly adhere to sediments leading to accumulation in coastal and deep sediments. Microbial assemblages play an important role in determining the fate of PAHs in water and sediments, supporting the functioning of biogeochemical cycles and the microbial loop. This review summarises the knowledge recently acquired in terms of both chronic and acute PAH pollution. The importance of the microbial ecology in PAH-polluted marine ecosystems is highlighted as well as the importance of gaining further in-depth knowledge of the environmental services provided by microorganisms.
Keywords: marine microbes, biodegradation, biological pump, Deepwater Horizon, microbial assemblages
This review highlights the sources and fate of polycyclic aromatic hydrocarbons (PAHs) in the marine environment with particular emphasis on the microbial ecology and the biological pump controlling global PAH fluxes.
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) have attracted the interest of many scientists from different disciplines beyond biology, physics and chemistry. They are fascinating compounds due to their universality, their presence in interstellar space (Tielens 2008) and their ubiquitous distribution in earth ecosystems (Vila, Tauler and Grifoll 2015). They have even been hypothesised as crucial elements for the construction of scaffolds at the origin of life (Ruiz-Mirazo, Briones and de la Escosura 2014).
PAHs are natural compounds synthesised by organisms, produced by combustion, and derived from fossil fuels and transformation processes (Neff 2002; Hylland 2006). In the marine environment, they tend to aggregate and to sorb to particulate and organic matter because they are stable hydrophobic planar structures with low solubility and volatility. These physical-chemical properties are related to the presence of at least two benzene rings (Hites, Laflamme and Farrington 1977; Hites, Laflamme and Windsor 1980; Cerniglia 1993). As a consequence, they thus accumulate in the environment (Lu, Zhang and Fang 2011). PAHs tend to be considered persistent molecules, particularly those with a high molecular weight (HMW) (Cerniglia 1992; Wilcock et al.1996) although recent conceptual models emphasise the importance of environmental parameters in controlling their persistence (Marín-Spiotta et al.2014). Human activities have considerably increased the level of PAHs in the environment (Finlayson-Pitts and Pitts 1997), particularly in marine ecosystems (Hoffman et al.1984; Maher and Aislabie 1992), raising serious environmental issues and human health concerns. Some PAHs and their metabolites are considered highly toxic mutagens and carcinogens (Miller and Miller 1981; Dean 1985; White 1986; Mastrangelo, Fadda and Marzia 1996). Their toxicity, which depends on their nature and on environmental factors as well, is increased for PAH mixtures (for a review, see Ball and Truskewycz 2013). Sixteen PAHs have been included in the US Environmental Protection Agency's list of priority pollutants (US-EPA 1984), but this list is now under discussion as some authors propose to include metabolites such as oxy- and nitro-PAHs, which are more toxic than the parent molecules (Andersson and Achten 2015).
As natural compounds, PAHs have been circulating through biogeochemical cycles for millions of years (Henner et al.1997). Global PAH fluxes in marine environments are controlled by the microbial degradation and the biological pump, which plays a role in sequestration through bioaccumulation and particle settling (Turner 2015). The bioaccumulation of PAHs has been shown in phytoplankton (Binark et al.2000; Almeda et al.2013) and marine organisms (Meador et al.1995). Together with the particulate organic matter and the dissolved organic matter, PAHs are subject to the microbial mineralisation integrating the microbial loop. Thereby, the biological pump and the microbial loop are involved in the fate of PAHs entering the ocean. Jiao et al. (2010) proposed the concept of the ‘microbial carbon pump’ that addresses the role of microbial mineralisation of labile dissolved organic matter. The microbial activity also results in the production of recalcitrant dissolved organic matter, which was estimated to represent 155 gigatonnes of carbon with an annual production estimated between 0.008 and 0.023 gigatonnes (Benner and Herndl 2011; Jiao et al.2011). Gustafsson, Gschwend and Buesseler (1997) have shown that around 90% of PAHs reach deep-sea sediments. This observation has been recently confirmed by Adhikari, Maiti and Overton (2015) in the Gulf of Mexico where they revealed that 3.1%–6.7% of total particulate PAHs were removed daily in the euphotic zone. Microorganisms have developed biodegradation strategies to transform and utilise PAHs as carbon and energy sources. The biodegradation of PAHs by microorganisms has been extensively reviewed for both aerobic (Cerniglia 1992; Kanaly and Harayama 2000; Bamforth and Singleton 2005; Peng et al.2008; Haritash and Kaushik 2009; Seo, Keum and Li 2009; Lu, Zhang and Fang 2011) and anaerobic (Widdel and Rabus 2001; Bonin et al.2004; Meckenstock et al.2004; Foght 2008; Lu, Zhang and Fang 2011; Meckenstock and Mouttaki 2011; Meckenstock et al.2016; Rabus et al.2016) processes.
Marine environments cover a large variety of ecosystems as diverse as estuaries, coastal zones, surface and deep oceans in a broad range of latitudes, each of them having specific characteristics. In this review, we summarise the knowledge of PAH biogeochemical cycles in the different marine ecosystems, with a particular focus on the biotic and abiotic factors driving their fate.
SOURCES OF PAHs IN MARINE ECOSYSTEMS
Over the last decades, PAH contamination has been extensively studied in various marine ecosystems worldwide. Most studies aimed to identify the sources of PAH contamination based on PAH diagnostic ratios. Because PAHs are emitted as mixtures, their profiles are fingerprints reflecting their origin and weathering processes (Manoli, Kouras and Samara 2004). The application of diagnostic ratios and their limitations have been recently reviewed (Tobiszewski and Namieśnik 2012; Stogiannidis and Laane 2015). The diagnostic ratios are usually combined with chemometric approaches, multivariate analyses such as principal component analysis and more recently positive matrix factorization combined with geographic information systems (Mahmoudi et al.2013), to determine the source apportionment of PAHs (Burns et al.1997; Tobiszewski and Namieśnik 2012; Lang et al.2013; Yu et al.2015), which is pivotal information for risk assessment and management (Tobiszewski and Namieśnik 2012). The main sources of PAHs have been found to be petrogenic and pyrogenic, resulting from anthropogenic activities including direct inputs of petroleum and emissions from the combustion of oil, diesel and biomass (Baek et al.1991; Oros and Ross 2004; Lima, Farrington and Reddy 2005; Li et al.2015b; Wang et al.2015; Yu et al.2015). Natural sources from volcanic activities and forest fires have been found to be marginal (Bamforth and Singleton 2005).
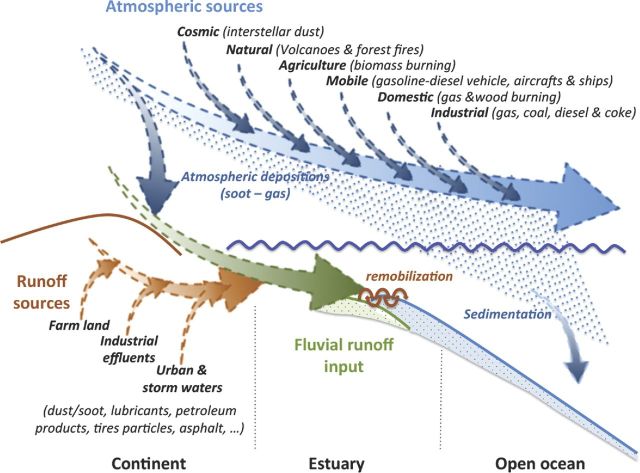
Pyrogenic PAHs, considered as chronic pollution, enter marine ecosystems mainly through fluvial run-off and atmospheric deposition (Fig. 1), whose respective contributions depend on the distance from the point sources (Liu et al.2012). In distant areas, the relative contribution of water run-off decreases, while that of atmospheric deposition increases (Gogou, Bouloubassi and Stephanou 2000; Chen et al.2006; Viñas et al.2010), the latter being almost the principal PAH source in the surface water of open oceans.
Figure 1.
Main sources of pyrogenic PAHs entering the marine environment.
The most spectacular quantities of petrogenic PAHs entering marine ecosystems are from oil spills such as the infamous examples of the Exxon Valdez in Alaska, the first Gulf War in Kuwait, the Erika in France, the Prestige in Spain and the more recent accident of the Deepwater Horizon in the Gulf of Mexico. Oil spills are considered as acute hydrocarbon pollution, with the aromatic component representing between 3% and 30% according to the type of oil (McGenity 2014). However, such accidental inputs represent only a minor share (less than 10%) of hydrocarbons (including PAHs in varying amounts according to the oil) entering the marine environment. The major sources of PAHs in oceans are from natural oil seeps representing ∼47% of all the crude oil entering the marine environment. The rest is due to transportation and other anthropogenic activities (Judd and Hovland 2007). As oil seeps are generally ancient, they are often considered as natural chronic pollution (Kvenvolden and Cooper 2003; Schroot, Klaver and Schüttenhelm 2005; Judd and Hovland 2007) although petrogenic PAHs are more bioavailable than the pyrogenic PAHs (Baumard, Budzinski and Garrigues 1998). The hydrocarbons released by oil seeps create particular habitats for benthic organisms and exert continuous selection pressure on the microbial community influencing their organization (LaMontagne et al.2004; Benedetti et al.2014).
Coastal ecosystems
Coastlines have diverse ecosystems including estuaries, salt marshes, mangroves, bays, lagoons and coral reefs. These ecosystems provide valuable ecosystemic services and play an important role in the carbon cycle (Bauer et al.2013), particularly in the mineralisation of organic matter (Burdige 2005; Blair and Aller 2012). With ∼4 billion people living within 60 km of the world's coasts (Kennish 2002), these marine coastal ecosystems are strongly impacted by human activities (Halpern et al.2008). PAHs threaten sensitive coastal ecosystems (McGenity 2014) including mangroves (Ke et al.2005; Mille et al.2006; Brito et al.2009; Bayen 2012), salt marshes (Duran and Goñi Urriza 2010; Coulon et al.2012; Chronopoulou et al.2013), coral reefs (Burns 2014; Ko, Chang and Cheng 2014; Guigue et al.2015) and even Arctic coasts with increasing traffic due to the diminution of the polar ice cap (Jörundsdóttir et al.2014).
The most urbanised coastal areas, such as estuaries, harbours and marinas, constitute hot spots of multicontaminants that may lead to the ‘coastal pollution and contamination syndrome’, whose consideration as a ‘tipping element’ (Schellnhuber 2009) in the global Earth system is discussed by Newton, Carruthers and Icely (2012). In particular, PAHs are a major concern in estuarine sediments (Christiansen et al.2009) with concentration levels often exceeding 100 ng g−1 dry weight sediment, above the lowest pollution level as defined by Baumard, Budzinski and Garrigues (1998). This value is an order of magnitude lower than the ERL (effects range-low) concentration for causing harmful effects in biota (4022 ppb per dry weight sediment, Long et al.1995). Nonetheless, many sites around the world show risks associated with PAH contamination in sediments (Burgess et al.2013) presenting PAH concentration levels above the sediment quality guideline (SQG) values proposed for total PAHs, such as the ERL and the TEL (threshold effects level) for causing occasional adverse effects (1684 ppb per dry weight sediment, Macdonald et al.1996). The different SQG approaches for sediment risk assessment have been recently reviewed by Burgess et al. (2013). However, the dilution of pollution in the vast expanse of the ocean reduces the environmental risks. For example, the PAH content during the Deepwater Horizon catastrophe decreased with distance and time in the water column (Boehm, Murray and Cook 2016) and sediments (Adhikari et al.2016). A clear decrease in PAH levels in seawater was observed after the well was capped (Wade et al.2016). A major PAH depletion (over 70%) mainly due to abiotic processes was observed in floating and stranded oils (Stout et al.2016). However, the PAH content remained high in some areas, particularly affected by the oil spill such as Louisiana coastal wetlands (Turner et al.2014). Nevertheless, most of the sediments were considered unlikely to cause toxic effects since the PAH contents were below the ERL (Wang et al.2014).
Because estuaries are ecosystems with complex hydrodynamics and the human activities carried out there are so diverse, each estuary may represent a case study with its own specificity. The estimation of PAH fluxes from the different sources is a valuable element in characterising the PAH input mechanism (Liu et al.2012) and determining the river contribution at a global scale as well (Wang et al.2007). For example, in highly urbanised areas, the urban and storm waters are the most significant PAH inputs, as shown for the Seine Estuary in France (Motelay-Massei et al.2007), Tokyo Bay in Japan (Pan et al.2014) and the estuaries of Massachusetts in the USA (Menzie et al.2002). In industrialised areas, the main sources of PAHs are mainly from industrial wastewaters as reported for the Pearl River Delta in China (Mai et al.2002; Wang et al.2007), or coal and biomass combustion deposits as determined for the estuaries of the Linhong River (Zhang, Zhang and Zhang 2013) and the Yangtze River (Guo et al.2007) in China, or petroleum combustion deposits in Malaysian estuaries (Keshavarzifard et al.2015), the San Francisco Estuary in the USA (Oros and Ross 2004) and the Mersey Estuary in the UK (Vane, Harrison and Kim 2007). The main contribution of direct petrogenic sources has been identified mainly in harbours as evidenced for the Yangshan Port in China (Li et al.2015a), the Imam Khomeini Port in Iran (Abdollahi et al.2013) and the Olbia harbour in Italy (De Luca et al.2005).
Open-Sea ecosystems
Oceans cover 70% of the Earth's surface and major gas exchanges occur at the ocean–atmosphere interface (Nizzetto et al.2008). Oceanic water bodies are thus the final sink of atmospheric pollutants that are transferred to deep waters and finally trapped into sediments (Dachs et al.2002). The pyrogenic PAH composition in the atmosphere is dependent on their sources (Tsapakis et al.2006; González-Gaya et al.2014). Atmospheric PAH fluxes are affected by physical-chemical properties and environmental variables, such as wind speed, collisions with the marine surface, seawater surface tension and hydrophobicity (González-Gaya et al.2014). PAHs are characterized by short atmospheric half-lives (Mackay, Shiu and Ma 1997) due to their affinity to soot carbon (Dachs, Eisenreich and Hoff 2000) that facilitates their deposition in seawater. Furthermore, higher primary production enhances air–water exchange and vertical PAH sinking fluxes (Dachs, Eisenreich and Hoff 2000; Dachs et al.2002). Phytoplankton influences air–water exchanges both directly by PAH sorption and indirectly. Growth rates induce the depletion of the dissolved phase concentration causing an air–water phase disequilibrium (Dachs et al.1999).
The petrogenic PAHs from natural oil seeps are the other source of PAHs in open seas. The petrogenic/pyrogenic dual composition of PAHs is illustrated in the Norwegian Sea, where Boitsov et al. (2013) demonstrated that the PAH composition determined in the seabed (non-pyrogenic PAHs) was different to that found in coastal areas (pyrogenic PAHs). The PAH composition in open sea surface sediments was characterized by a petrogenic/pyrogenic PAH mixture (Boitsov et al.2013; Buhl-Mortensen et al.2015), but presenting variations at some locations. Similarly, in the Arctic Ocean different PAH sources have been identified including long-range atmospheric transport (Friedman and Selin 2012) and petrogenic origin from natural oil seeps (Foster et al.2015). Conversely, in the Mediterranean Sea, the atmospheric deposition dominated by pyrogenic PAHs was found to be predominant in the open sea (Lipiatou and Saliot 1991; Tsapakis et al.2006). Additionally, Tsapakis et al. (2006) demonstrated that low molecular weight (LMW) PAHs enter the seawater via diffusive gas exchanges and HMW PAHs through dry and wet deposition. HMW PAHs are generally associated with soot particles and black carbon (BC) (Shrestha, Traina and Swanston 2010). In a north/south transect in the open Atlantic Ocean, Nizzetto et al. (2008) showed that atmospheric deposition/air–water exchanges were the principal processes by which PAHs enter the Atlantic seawater. However, the PAH concentrations were found to be higher in the North Atlantic waters than in the South Atlantic waters, showing the impact of the industrialisation.
ABIOTIC FACTORS DETERMINING THE FATE OF PAHs IN MARINE ECOSYSTEMS
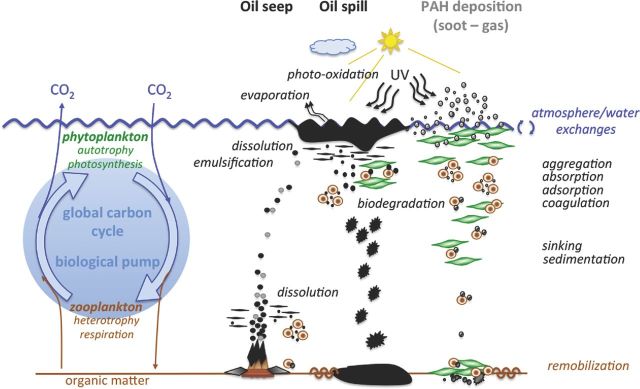
The way in which PAHs enter marine ecosystems is crucial in determining their bioavailability and fate. Petrogenic PAHs are more bioavailable than those of pyrogenic origin, because the latter are tightly bound to particles (Wade, Sweet and Klein 2008), and therefore more strongly associated to sediments (Perra et al.2009). Indeed, the degradation of fossil petrogenic PAHs was found to be higher than that of pyrogenic PAHs during transport through the water column in the Mediterranean Sea (Dachs et al.1997). Although similar general processes are involved, we make the distinction between chronic and acute PAH pollution. We thus describe specific characteristics of the abiotic fate for pyrogenic PAHs entering the marine environment by deposition on surface waters, typical of most cases of chronic pollution and for petrogenic PAHs entering the marine environment due to oil spills, which correspond to acute pollution (Fig. 2).
Figure 2.
Main processes determining the fate of oil and PAHs in the marine environment. Atmospheric PAH deposition on surface waters, corresponding to most cases of chronic pollution and petrogenic PAHs entering by oil spills, corresponding to acute pollution, undergo similar global abiotic and biotic processes. PAHs from natural oil seeps travel through the water column to the surface and finally follow the same processes as the previously described PAHs. The microbial biodegradation and the biological pump control PAH fluxes. The latter plays a crucial role in atmospheric PAH sequestration, bioaccumulation and sedimentation.
Chronic PAH pollution—fate of water-surface-deposited PAHs
The incomplete burning of biomass and fossil fuel produces BC, soot and char particles that are considered as long-term persistent in the environment because they are refractory to biological and chemical decomposition processes (Middelburg, Nieuwenhuize and Van Breugel 1999). BC is now considered a major global pollutant playing a primary role in climate change by affecting the radiation equilibrium and modifying surface albedo (for a review, see Novakov and Rosen 2013). BC is also considered as an important component of the carbon cycle (Lohmann et al.2009), being a recognised carbon sink sequestered over centuries (Bird et al.2015). The main processes involved in the formation of PAHs during BC production have been described (for a review, see Richter and Howard 2000; Lima, Farrington and Reddy 2005). PAH levels and composition in BC are dependent on the type of fuel burned and the burning conditions (Jenkins et al.1996; Schauer et al.2002; Simoneit 2002). Flores-Cervantes, Reddy and Gschwend (2009b) defined three main BC types: (i) thermally recalcitrant/highly sorptive, (ii) thermally labile/highly sorptive and (iii) thermally recalcitrant/not highly sorptive. In addition to the PAHs bound by occlusion into BC pores during soot formation, BC also binds organic pollutants, particularly PAHs through surface sorption processes (Shrestha, Traina and Swanston 2010). BC is thus an important PAH transporter both in the atmosphere and in the seawater column (Lohmann, Macfarlane and Gschwend 2005; Flores-Cervantes et al.2009a), and BC is acknowledged to play a major role in controlling the fate and bioavailability of hydrophobic organic pollutants (Shrestha, Traina and Swanston 2010). Pyrogenic PAHs emitted as soot particles and gas enter the water column by direct or rainfall depositions of soot particles (i.e. BC—HMW PAHs) and by gas exchange at the water/atmosphere interface for volatile (i.e. LMW) PAHs (Oros and Ross 2004). The annual BC production has been estimated to be between 16 and 275 Tg BC yr−1 (Flores-Cervantes et al.2009a). Jurado et al. (2008) estimated the dry deposition of total organic carbon to be 11 Tg C yr−1 and the wet deposition of particle and gaseous total organic carbon to be 47 and 187 Tg C yr−1, respectively. The global ocean BC uptake was estimated to be 2 and 10 Tg C yr−1 by dry and wet deposition, respectively (Jurado et al.2008; Shrestha, Traina and Swanston 2010).
Once in the water column, due to their low water solubility and hydrophobic nature, PAHs sorb rapidly to organic and inorganic particulate material sinking to the bottom and accumulate in sediment (Tolosa et al.2004). Deep-sea sediments and the open ocean are thus considered as the major sink for PAHs (Adhikari, Maiti and Overton 2015). Although the dynamics of PAHs in marine environments depend on diverse mechanisms including photo-oxidation, evaporation, dispersion and biodegradation (Berrojalbiz et al.2009), the vertical sinking of PAH particles is an important process in determining their fate, as summarised by Adhikari, Maiti and Overton (2015). A recent study demonstrated that 3.1%–6.7% of total particulate PAHs in the water column were lost daily via vertical sinking in the northern Gulf of Mexico (Adhikari, Maiti and Overton 2015). It has been shown that phytoplankton plays an important role in pollutant uptake (including both adsorption and absorption processes) and vertical transport (Dachs et al.2002). However, dissolved BC in marine-dissolved organic carbon constitutes an intermediate BC pool prior to sediment deposition (Masiello and Druffel 1998; Ziolkowski and Druffel 2010). BC has been estimated to contribute up to 5% of total dissolved organic carbon (DOC) (Stubbins, Niggemann and Dittmar 2012; Coppola, Walker and Druffel 2015; Coppola and Druffel 2016). The concentration of particulate PAHs in the water column is expected to decrease with depth because PAHs are desorbed and degraded during vertical transport (Lipiatou and Saliot 1991; Dachs et al.1997; Berrojalbiz et al.2009). Dachs et al. (1997) described the depth-depletion distribution of PAHs associated with suspended particulate matter, estimated from the concentration found in vertical profiles, proposing a budget of PAHs in the western Mediterranean seawater. Photo-oxidation degradation processes have been demonstrated to remove aromatic BC producing less condensed dissolved BC (Stubbins, Niggemann and Dittmar 2012; Wagner and Jaffé 2015). It is likely that the BC cycling rates throughout the surface photic zone determine the persistence of dissolved BC in the oceans (Stubbins, Niggemann and Dittmar 2012). The remineralisation of particulate organic carbon containing attached PAHs results in PAH release into the water column in colloidal and/or some soluble forms (Shrestha, Traina and Swanston 2010). Abiotic and biotic PAH degradation may also occur in a higher proportion for LMW PAHs, but degradation processes may be limited (Santín et al.2016). The transport to deep layers is determined by the biological characteristics of the ecosystem. The organic matter flux in deeper layers has been observed to be higher in eutrophic sites than in oligotrophic areas (Baines, Pace and Karl 1994).
Acute pollution—fate of PAHs from oil spills
Crude oil hydrocarbons and gas hydrocarbons released from the deep sea, as observed for the Deepwater Horizon oil spill or natural oil seeps, partially dissolve in the water column, introducing PAHs and other aromatic compounds into the seawater (Reddy et al.2012). Diercks et al. (2010) have reported the presence of PAHs at concentrations reaching 189 μg/L in the Gulf of Mexico after the Deepwater Horizon oil spill where DOC was observed at 6 mg C/L (Zhou et al.2013). The baseline values of DOC in the northern Gulf of Mexico were considerably lower (usually <1 mg C/L) before the Deepwater Horizon oil spill (Guo, Coleman and Santschi 1994; Guo, Santschi and Warnken 1995), highlighting the influence of released oil in DOC concentration. The Deepwater Horizon oil spill was characterized by the formation of a hydrocarbon plume at 1000–1300 m depth, probably as a consequence of high-pressure oil released into cold water (Socolofsky, Adams and Sherwood 2011). As described for tanker accidents, such oil spills also lead to crude oil slicks at the surface that undergo weathering processes, dispersing oil droplets through the water column, forming ‘chocolate mousse’ (water-in-oil emulsion), modifying their chemical composition and sinking to the sediment adsorbed to particles (McGenity et al.2012). The involvement of phytoplankton and marine snow in hydrocarbon sinking was demonstrated during the Deepwater Horizon oil spill (Passow et al.2012; Joye, Teske and Kostka 2014; Passow et al.2014). The main modifications include the evaporation of LMW hydrocarbons (McGenity et al.2012), the production of oxygenated PAHs (oxy-PAHs) via biological, chemical and photo-oxidation reactions (Lundstedt et al.2007) and the formation of tar balls and oil–mineral aggregates (Christiansen et al.2009) by aggregation and adsorption with minerals (Kiruri, Dellinger and Lomnicki 2013). Tar balls were found to have accumulated and be persistent in coastal sediments after the Prestige (Serrano et al.2006; Acosta-González et al.2015) and Deepwater Horizon oil spills (Beazley et al.2012; Kiruri, Dellinger and Lomnicki 2013). The production of oxy-PAHs by photo-oxidation reactions from both petrogenic and pyrogenic PAHs increases their solubility but can also result in ‘dead-end products’ resistant to biodegradation that accumulate in sediments threatening human health and the environment (Lundstedt et al.2007). Although the potential toxic effect of oxy-PAHs depends on the compounds, oxy-PAHs are often more toxic than the parent PAH molecules. The toxic effect of oxy-PAHs has been demonstrated on a large variety of aquatic and terrestrial organisms including microorganisms, algae, plants and mammalian cells (for a review, see Lundstedt et al.2007). The toxic effect of oxy-PAHs involves oxidative stress and endocrine-disruption mechanisms. They have also been found to be mutagenic and genotoxic through the formation of DNA adducts (for a review, see Bolton et al.2000; Lundstedt et al.2007).
Such oxy-PAH compounds have been found in diverse PAH-contaminated environments (Lundstedt et al.2007; Layshock, Wilson and Anderson 2010) including Deepwater Horizon oil spill impacted sediments (Aeppli et al.2012; Forsberg et al.2014). The oxy-PAH level has been estimated at up to 12% of total PAHs in marine sediments (Layshock, Wilson and Anderson 2010). The oxygenated hydrocarbon content was found to be between 50% and 65% in weathered Deepwater Horizon oil slicks and sand patties (Aeppli et al.2012). Oxy-PAHs have been found together with other products from photoreactions such as hydroxy-PAHs and nitro-PAHs in atmospheric depositions (Barrado et al.2013; Sippula et al.2014) and oil spills (Kleindienst, Paul and Joye 2015).
Fate of PAHs in sediments
Whatever the origin of contamination, PAHs finally reach the sediments, which represent the main sink for PAHs and constitute PAH reservoirs. Analyses of PAHs in sediment cores are useful chronometers for retracing the history of the pollution (Zhang, Zhang and Zhang 2013). Several studies have demonstrated the industrial PAH input with increasing levels until the 1950s and, following shifts in the use of coal/oil/gas energy sources, have highlighted a continuous decrease in PAH levels initiated in the 1960s with the first countermeasures to limit emissions (Lima, Eglinton and Reddy 2003; Louchouarn et al.2012).
Sediments, particularly coastal sediments, have been demonstrated as PAH sources for the water column (King, Readman and Zhou 2004; Sabin et al.2010). The remobilisation of PAHs, experimentally demonstrated in a sediment resuspension simulator (Feng et al.2007), has been shown to be a highly complex process in the natural environment, depending on several factors including the physical-chemical properties of PAHs and the presence of other pollutants, sediment composition, environmental conditions and hydrologic dynamics (King, Readman and Zhou 2004). It takes place when sediments are disturbed, by either mechanical (shipping, currents, storms, waves and tide) or biological processes (bioturbation), removed or relocated by dredging (for a review, see Roberts 2012). Remobilisation and resuspension processes affect benthic communities (Roberts 2012), drive particular microbial assemblages and activities by providing oxygen deeper in sediments and bioavailable PAHs amenable to biodegradation (LeBlanc et al.2006). PAH biodegradation by bacteria, fungi and algae has been shown as the main mechanism removing PAHs from marine sediments (McGenity et al.2012). PAH biodegradation has been shown even in the absence of molecular oxygen by anaerobes (for a review, see Kimes et al.2014; McGenity 2014; Meckenstock et al.2016). However, the degradation rates are low for PAHs buried in sediments, as observed after the Deepwater Horizon oil spill that resulted in PAH accumulation in deep sediments (Turner et al.2014; Chanton et al.2015; Adhikari et al.2016). Laboratory experiments have demonstrated that PAH partitioning towards the sorbed phase is controlled by sediment grain size, salinity and temperature (Frapiccini and Marini 2015).
MICROBIAL PAH DEGRADATION
Microorganisms have developed diverse strategies to activate PAH molecules, the initial steps in biodegradation pathways, even in the absence of molecular oxygen (Fig. 3). These mechanisms have been nicely reviewed recently (Kimes et al.2014; McGenity 2014). Under aerobic conditions, the first activation step involves direct incorporation of oxygen by di- and mono-oxygenases. The key enzymes are ring-hydroxylating dioxygenases (RHDs) belonging to the Rieske-type non-heme iron oxygenase family (Pieper, Martins Dos Santos and Golyshin 2004). Among the PAH-specific RHD, phylogenetic analyses discriminate between the PAH RHDs from Gram-negative bacteria and those from Gram-positive bacteria (Kweon et al.2008). The main Gram-negative representative PAH RHD genes are the nagAc gene from Ralstonia sp. U2 and the nahAc gene from Pseudomonas sp. 9816–4, while the nidA gene from Mycobacterium is representative of the PAH RHD genes from Gram-positive bacteria (Wu et al.2014). The PAH RHD incorporates two oxygen molecules into an aromatic ring forming a cis-dihydrodiol, which is converted into a catechol by a dehydrogenase. Then, a catechol dioxygenase performs the aromatic ring fission by ortho- or meta-cleavage producing aliphatic products that enter the central metabolism via the tricarboxylic acid cycle (Cerniglia 1992). Monooxygenases, such as the cytochrome P450 for eukaryotic microorganisms, are involved in detoxifying pathways rather than PAH assimilation processes (Doyle et al.2008). They are enzymes showing a wide substrate spectrum that can introduce one oxygen atom into the aromatic ring, resulting in an arene oxide intermediate subsequently transformed to either a dihydrodiol by an epoxide hydrolase or a phenol through non-enzymatic rearrangement (Cerniglia 1992; Haritash and Kaushik 2009).
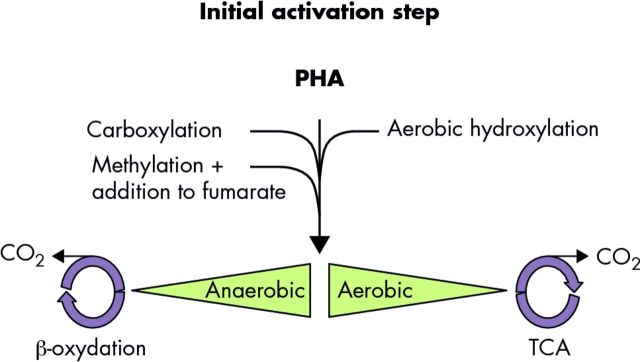
Figure 3.
Main activation processes in PAH biodegradation. The first step in the aerobic PAH catabolic pathways involves RHD. For the anaerobic PAH catabolic pathways, the activation steps presumably involve methyl-transferases for methylation, succinate synthases (glycyl radical enzymes) for addition to fumarate and carboxylases for carboxylation. The metabolic pathways have been largely reviewed (Lu, Zhang and Fang 2011; Meckenstock and Mouttaki 2011; Heider and Schühle 2013).
Under anaerobic conditions two main mechanisms have so far been described for PAH molecule activation (Fig. 3). Anaerobic activation mechanisms include direct carboxylation or methylation followed by addition to fumarate (Heider and Schühle 2013; Meckenstock et al.2016; Rabus et al.2016). The enzymes involved in the anaerobic pathways have been described in detail (for a review, see Meckenstock et al.2016). For the carboxylation route, a carboxylase belonging to the UbiD-like proteins family is proposed for the carboxylation of the aromatic ring (Rabus et al.2016). For the methylation route, it is proposed to proceed via a methyl-transferase that methylates the aromatic ring (Safinowski and Meckenstock 2006) and then a naphthyl-2-methyl-succinate synthase, a glycyl-radical-containing enzyme, catalyses the addition to fumarate (Meckenstock and Mouttaki 2011). In both cases, the degradation pathway further proceeds via β-oxidation after activation with coenzyme A (Meckenstock et al.2016).
MICROBIAL ECOLOGY OF PAH-POLLUTED MARINE ECOSYSTEMS
Although PAH microbial degradation mechanisms are well known, the ecology of microbial communities facing the presence of PAHs in marine environments requires further elucidation due to the myriad of factors that can contribute to and/or affect microbial community structure in different polluted environments (Cravo-Laureau and Duran 2014; Duran et al.2015c). The particle grain size and dissolved oxygen were revealed as the main drivers for bacterial diversity and abundance in estuarine sediments (Wang et al.2013) and deep ocean waters (Salazar et al.2015). However, specific microbial assemblages and hydrocarbonoclastic bacterial strains have been described for chronically contaminated coastal sediments (Paissé et al.2008; Ben Said et al.2010; Duran et al.2015a; Misson et al.2016) and for waters and sediments contaminated by oil spills (Bordenave et al.2004a,b; Stauffert et al.2013; Kimes et al.2014; Acosta-González et al.2015; Stauffert, Cravo-Laureau and Duran 2015a). Particular hydrocarbon degrading bacterial strains have been found in deep ocean waters such as members related to the Maribaculum, Novosphingobium, Oceanibaculum, Parvibaculum, Roseovarius and Stappia genera as well as in deep sediments including members related to the Oceanicola, Parvibaculum, Nitratireductor, Celeribacter and Bowmanella genera (for a review, see Louvado et al.2015). The genomes of the PAH-degrading bacterial isolates from deep-sea sediments carry potential new PAH RHD genes with low sequence similarity to known genes involved in the initial activation step (Louvado et al.2015). Such observation suggested that the genetic potential for PAH degradation may be greater than previously recognised. Some isolates from deep-sea sediments also harbour known genes probably acquired via horizontal gene transfer (Cao et al.2015).
The occurrence of hydrocarbon-degrading bacteria in PAH-polluted sites, their role in PAH biodegradation and their ecological significance have been largely discussed (for a review, see McGenity et al.2012; Kimes et al.2014; McGenity 2014; Louvado et al.2015). However, despite the information available, the generalisation of microbial patterns in PAH-contaminated sites is made difficult because of the wide variety of marine ecosystems, the origin of contamination and the environmental conditions (Nogales et al.2011). A recent large-scale study in the Mediterranean Sea revealed that the microbial communities in pristine sites responded in a different way to those inhabiting chronically polluted sites (Bargiela et al.2015). The latter showed a faster degradation response when an accidental oil spill occurred. Bargiela et al. (2015), by comparing metagenomic-based pollutant-degrading networks of chronically polluted sites in the Mediterranean Sea with those of the Deepwater Horizon oil spill, demonstrated that chronic pollution promotes the diversification of PAH catabolic capacities. The study also revealed a correlation between temperature and microbial diversity, with low temperature increasing bacterial richness while decreasing catabolic diversity (Bargiela et al.2015).
Oil spill microbial ecology
The microbial ecology of oil spills has been studied extensively (for a review, see McGenity et al.2012; Cravo-Laureau and Duran 2014; McGenity 2014; Acosta-González and Marqués 2016). Here we focus mainly on the most recent oil spill, the Deepwater Horizon oil spill, which largely increased the input of PAHs in the Gulf of Mexico (Reddy et al.2012), although the PAH content of the Macondo MC252 crude oil was estimated between 1.1% and 4% (Reddy et al.2012; Daling et al.2014; Yin et al.2015). It is important to note that the Gulf of Mexico is a chronically polluted environment with numerous natural oil seeps that contribute more than 400 000 barrels of oil per year (Atlas and Hazen 2011; Farrington 2013). The Deepwater Horizon disaster was thus a major oil spill in a chronically polluted environment providing an input of bioavailable hydrocarbons and PAHs to adapted microbial communities (Atlas and Hazen 2011; Atlas et al.2015). The Deepwater Horizon oil spill was characterized by the use of dispersants as a remediation strategy. The consequences and impact of dispersant application have been recently reviewed (Kleindienst, Paul and Joye 2015). The role of dispersants is to allow the dispersion of insoluble hydrocarbon compounds (Prince et al.2013). Contrasted effects of dispersants on hydrocarbon microbial degradation have been recently reported. Several studies have demonstrated the efficiency of dispersants in hydrocarbon dilution promoting their microbial degradation (Lee et al.2013; Prince et al.2013; Prince 2015), while recent studies have revealed that dispersants can exert a negative effect on microbial hydrocarbon degradation rates (Kleindienst et al.2015) and affect the macrofaunal activity (Cuny et al.2015). Thus, these observations point out the necessity to further understand the effect of dispersant on biodegradation, preferentially in realistic environmentally relevant conditions because most studies conducted so far under laboratory conditions do not reflect the in situ conditions. Studies taking into account the type and amount of dispersant as well as considering the whole complex microbial community in a holistic point of view are particularly needed for a fully conscious use of dispersants (Kleindienst, Paul and Joye 2015).
The Deepwater Horizon disaster provided a perfect ecosystem model for studies on the fate of oil in the marine environment. A large literature is now available, particularly on microbial ecology aspects, confirming the important role of microorganisms in determining the fate of spilled oil. Among this large literature, three reviews (Joye, Teske and Kostka 2014; Kimes et al.2014; King et al.2015) have been recently published addressing the microbial response to the Deepwater Horizon oil spill and the underlying ecological rules.
In this ecosystem, although environmental conditions such as nutrients or temperature could limit microbial activities, PAH biodegradation by microorganisms has been evidenced. Moreover, an ecological succession of microorganisms during the hydrocarbon degradation has been highlighted (Joye, Teske and Kostka 2014). Such ecological succession has been described as the main rule of microbial oil response (Head, Jones and Roling 2006) and observed in other oil spills such as the Prestige oil spill, mainly composed of PAHs (Acosta-González et al.2015). Similar trends have also been observed with experimental ecology approaches simulating oil spill and mimicking environmental conditions (Cravo-Laureau and Duran 2014) showing a modification in benthic microbial communities during experimental exposure (Stauffert et al.2013, 2014; Stauffert, Cravo-Laureau and Duran 2015a,b). All these observations highlight the functional redundancy in hydrocarbon degradation characterized by different compositions of microbial communities, although the efficacy in hydrocarbon degradation and removal remains similar (Head, Jones and Roling 2006; Stauffert et al.2013; Cravo-Laureau and Duran 2014).
It has been suggested that Gulf of Mexico deep-sea microbial communities were adapted to oil because they were exposed to natural oil seeps (Joye, Teske and Kostka 2014). The link between microbial degradation efficiency and pollution history has been largely discussed (Head, Jones and Roling 2006; Bordenave et al.2007) and remains under debate (Sauret et al.2012). The idea that microbial communities with pollution history respond faster than a pristine microbial community is supported by the fact that the response of the Gulf of Mexico bacterial communities to the presence of the Macondo Deepwater Horizon oil was extremely fast, characterized by the enrichment of the deep-sea oil plume (1000–1300 m depth) by indigenous oil-degrading bacteria (Hazen et al.2010) as well as by bacterial blooms and marine snow phenomena (Joye, Teske and Kostka 2014). The marine oil snow, also referred to as a ‘dirty blizzard’, was characterized by ecological succession, with the presence and dominance of members related to Cycloclasticus genus associated with the presence of PAHs (Joye, Teske and Kostka 2014). Biological snow production by the association of bacterial and/or algal mucus, with phytoplankton and zooplankton faecal debris and feeding, was studied during the Deepwater Horizon oil spill (Passow et al.2012; Vonk, Hollander and Murk 2015). The studies demonstrated that a significant proportion of the spilled oil was carried to the seafloor during snow sinking. During sedimentation, biodegradation of the most labile fraction (e.g. LMW PAHs) was demonstrated (Ziervogel, Joye and Arnosti 2016; Joye, Teske and Kostka 2014). Once at the sediment surface under oxygen-depleted conditions, anaerobic bacteria, such as sulphate-reducing Deltaproteobacteria, replaced the aerobic bacteria (Joye, Teske and Kostka 2014). In a recent review, Vonk, Hollander and Murk (2015) investigated whether marine oil snow occurred in other large oil spills by meta-analysis, and concluded that marine oil snow and related benthic contamination may be widespread phenomena in response to pollution.
Rodriguez-R et al. (2015) demonstrated that, a year after the catastrophe, the ecological succession resulted in the general recovery of diversity with the reoccurrence of specialised and sensitive microbial populations. The resistance and resilience of microbial communities are important issues in microbial ecology (Allison and Martiny 2008; Nogales et al.2011), which was essentially so far described through oil exposure in experimental microcosms oil (Bordenave et al.2007). The Deepwater Horizon disaster at the Gulf of Mexico is a perfect in situ case study to follow microbial communities behaviour. PAH biodegradation was demonstrated, for example by metatranscriptomic approaches, revealing the expression of PAH degradation genes in oiled sediments (Lamendella et al.2014), or by culture-dependent approaches using SIP (Gutierrez et al.2013). Considering the microbial community related to PAH biodegradation, microorganisms belonging to the genera Cycloclasticus, Colwellia and Alteromonas have been described as abundant in the water column and surface water (Valentine et al.2010; Gutierrez et al.2013), while in deep-sea sediments a high level of sulphate-reducing microorganisms have been observed (Orcutt et al.2010; Kimes et al.2014). Associating cultivation-based and cultivation-independent molecular analyses of bacterial communities, Kostka et al. (2011) proposed to use members of Alphaproteobacteria (Labrenzia and Rhodobacteraceae) and Gram-positive groups (Bacillus and Microbacterium) as sentinels for PAH biodegradation in Gulf beach sands. In coastal wetlands, polluted by weathered oil containing complex PAHs (Beazley et al.2012), oil degradation was enhanced by the increasing oxygenation of sediments by bioturbation or by the presence of rhizosphere marsh vegetation that also releases exudates. The microbial community was dominated by phyla containing previously described hydrocarbon-degrading bacteria (Proteobacteria, Bacteroidetes and Actinobacteria) and functional genes involved in PAH degradation were detected (Beazley et al.2012).
As evidenced by the available literature, considerable information has been obtained on bacterial communities and PAH biodegradation. Considering Archaea, their involvement in PAH degradation has been demonstrated particularly by halophilic microorganisms (McGenity 2010; Bonfá et al.2011; Andrei, Banciu and Oren 2012) as shown by Bertrand et al. (1990), who isolated the first halophilic strain able to degrade PAHs (to a lesser extent than aliphatic hydrocarbons) from a salt marsh, a marine coastal ecosystem where Archaea represent an important component (Sanni, Coulon and McGenity 2015). PAH biodegradation has also been observed under methanogenic conditions (Chang, Um and Holoman 2006). The impact of PAH pollution on archaeal communities has been indirectly addressed, by monitoring the effect of oil pollution. Only a few studies have been reported on this, with contrasting results (Röling et al.2004; Taketani et al.2010; Stauffert et al.2014; Sanni, Coulon and McGenity 2015). Modifications in the composition of archaeal communities have been described during oil spills (Newell et al.2014; Stauffert et al.2014), the nitrification community being particularly affected by hydrocarbon toxicity (Urakawa et al.2012). In contrast, no effect has been observed in the archaeal ammonia oxidizer community (Rivers et al.2013) and more recently, Yergeau et al. (2015) reported this community to be dominant in the polluted area they studied.
Although fungi have a high PAH degradation potential (Harms, Schlosser and Wick 2011), the descriptions of fungal communities and of their role in hydrocarbon degradation in marine environments remain scarce. A few reports have shown enrichment of Dothideomycetes-related members, known to be able to degrade PAHs, in oil-polluted beach sediments (Bik et al.2012) and marshes (Mahmoudi et al.2013).
Microbial ecology in chronically PAH-contaminated sites
We consider here chronic pollution corresponding to pyrogenic PAHs, which are considered less bioavailable than petrogenic PAHs (see above sections). They enter the marine environment by deposition on surface waters. Atmospheric PAH depositions have toxic effects on the oceanic phytoplankton communities thus perturbing the first level of marine food webs (Hjorth et al.2007; Echeveste et al.2010) and indirectly influencing the other trophic levels, the bacterial and zooplankton communities, through cascading effects (Hjorth, Forbes and Dahllöf 2008). Atmospheric PAHs entering the seawater accumulate in the sea surface microlayer (SML), the ocean/atmosphere interface, with an enrichment factor of up to 500 times in comparison to the concentrations found in the water column (Wurl and Obbard 2004). The role of the biological pump in the sequestration of atmospheric organic pollutants has been described (Galbán-Malagón et al.2012). This sequestration can be explained by uptake (adsorption and absorption) of organic compounds by phytoplankton, and by fluxes of settling particles rich in organic matter, driving and enhancing air–water diffusive fluxes. The SML is home to a characteristic bacterial community known as Bacterioneuston that has the potential to cope with the presence of contaminants (Sauret et al.2015). On the French Mediterranean coast, the PAH-degrading bacteria composition was found to be dependent on the PAH source, with the presence of bacterial species different to the well-known PAH degraders and pyrogenic PAHs that were shown to be the most abundant. The PAH degraders in the SML were significantly correlated with the dissolved total PAH concentrations that formed a gradient from the shore to near-shore waters (Sauret et al.2015).
As described above for the Deepwater Horizon oil spill, chronic PAHs were also detected in marine snow (Li et al.2014), coagulated to small particles and aggregated to organic matter (De La Rocha 2007). As it sinks, marine snow transports PAHs rapidly to the seabed sediments (Berrojalbiz et al.2011; Nizzetto et al.2012). In deep-sea sediments in the Arctic Ocean, the PAH concentration decreases with sediment depth and movement from the south to the north (Dong et al.2015). Using both culture-dependent and independent methods, Cycloclasticus, Pseudomonas, Pseudoalteromonas, Marinomonas, Halomonas and Dietzia have been revealed in the sediment and suggested as major players in PAH degradation under low temperatures (Dong et al.2015). Populations containing genes encoding the α-subunit of PAH dioxygenase have been described as fairly stable and relatively abundant within the indigenous microbial community in chronically polluted Subantarctic marine sediments (Marcos, Lozada and Dionisi 2009). All these findings reveal that PAHs and PAH-degrading bacteria are widespread in the deep-sea sediments of polar oceans.
Interactions and environmental parameters affecting microbial communities and PAH biodegradation
Microorganisms constitute community assemblages in natural environments, living in association with other (micro) organisms, ensuring the microbial loop through top-down regulation and ecosystem services by metabolic networks. These interactions, characterized by the coexistence of different species involved in positive and/or negative interactions, are an integral part of the functioning of ecosystems and govern microbial processes involved in the hydrocarbons biodegradation (Head, Jones and Roling 2006; McGenity et al.2012). Moreover, it is known that in natural or anthropogenic environments some pollutants can only be eliminated through the action of several microorganisms performing additional reactions (Müller 1992; Singleton 1994). Indeed, it is known that natural microbial consortia or networks allow microorganisms to perform complex tasks, including the degradation of recalcitrant molecules such as PAHs (for a review, see McGenity et al.2012).
In situ characterisation of PAH microbial assemblages
In situ studies comparing contaminated and uncontaminated marine sediments have revealed specific microbial assemblages related to the presence of PAHs (Bordenave et al.2008; Todorova, Mironova and Karamfilov 2014; Wu et al.2014). The degradation capacities of these microbial assemblages were further attested by the detection of aromatic ring hydroxylating dioxygenase genes (rhd), genes involved in the first step of aerobic PAH biodegradation (Bordenave et al.2008; Todorova, Mironova and Karamfilov 2014; Wu et al.2014). These genes have been used as target genes for the characterisation of PAH-degrading microorganisms (Ben Said et al.2008; Guermouche M'rassi et al.2015) as well as for the estimation of the PAH-degradation potential of microbial communities in the environment (Chadhain et al.2006; Bacosa and Inoue 2015; Meynet et al.2015). Interestingly, Xia et al. (2015) demonstrated that the diversity of rhd genes was correlated with bioavailable PAH contents. The diversity of rhd genes was higher in deposited sediments than in suspended sediments and overlaying water (Xia et al.2015). The role of rhd genes in environmental studies has been further demonstrated by targeting their transcripts, which were shown to be induced just after the addition of fresh bioavailable PAHs contained in heavy crude oil (Paissé et al.2012).
In the absence of functional evidence, the contribution of PAHs to structuring microbial communities could be difficult to evaluate because of the presence of multicontaminants. To overcome these difficulties, the high-throughput sequencing technology associated with appropriate statistical analyses, such as co-occurrence network analyses, offers the possibility of identifying pollutant–degrader interactions (Yergeau et al.2012; Llado et al.2015). Applying such an approach, a recent study revealed Actinobacteria ‘specialists’ associated with PAHs and heavy metals (Hg, Cd, Cu, Pb and Zn) in harbour marine sediments (Duran et al.2015a). Such an observation highlights the complexity of the interactions prevailing in the microbial assemblages. The microbial collective metabolic diversity copes with the multiple factors that control PAH biodegradation including oxygen and nutrient availability, pH, salinity and the presence of surfactants, co-substrates and multicontaminants (Lu, Zhang and Fang 2011) and facilitates PAH biodegradation (Gallego et al.2014).
Experimental ecology for understanding PAH microbial assemblages
Because of the sediment complexity, the environmental observations alone are insufficient to fully understand the mechanisms controlling the microbial assemblages in the presence of PAHs. Laboratory studies, including single-cell physiology as well as appropriate microcosm and mesocosm experiments, are methods that facilitate understanding complex microbial assemblages (Cravo-Laureau and Duran 2014; Röling and Van Bodegom 2014). We relate here the effect of fluctuations in oxygen availability on PAH microbial assemblages and microbial interactions with macroorganisms.
Oxygen and redox oscillations are major fluctuations in estuarine and tidal sediments (Cravo-Laureau and Duran 2014). The effect of such fluctuations on the dynamics of microbial communities and PAH degradation capacities has been demonstrated in several microcosm (Duran et al.2015b; Militon et al.2015) and bioreactor studies (Cravo-Laureau et al.2011; Vitte et al.2011, 2013). Different PAH-degrading microbial assemblages have been obtained according to the oxygenation regimes (Vitte et al.2011) with optimal degradation capacities observed either under permanent oxic conditions (Militon et al.2015) or under anoxic/oxic oscillations (Vitte et al.2011). However, degradation under oxic/anoxic oscillations was less effective in removing the toxicity, suggesting incomplete PAH mineralisation with the accumulation of toxic metabolites, probably because the microbial assemblage lacked key microorganisms for a complete PAH-degradation metabolic network (Vitte et al.2013). These observations illustrate the need for better understanding the biogeochemical functioning of polluted environments under fluctuating redox conditions as highlighted by Borch et al. (2010).
The influence of PAHs on the interactions between microbial communities and meiofauna has been revealed by microcosms for studies into bioremediation strategies (Louati et al.2013a,b; Ben Said et al.2015). Meio- and macrofauna influence the microbial assemblages, directly as the main predators and indirectly by modifying the environmental conditions. Louati et al. (2013b) demonstrated that top-down control by meiofauna was more effective in shaping microbial assemblages than the selective pressure exerted by PAHs. Microbial interactions, including interactions between microbes during hydrocarbon degradation as well as interactions with meio- and macrofauna and plants in oil-polluted marine sediments, have been recently reviewed (McGenity et al.2012), highlighting the need for a better understanding of microbial interactions, to achieve a more rational approach to the bioremediation of hydrocarbons.
CONCLUDING REMARKS
Although the issues related to oil pollution, particularly the concerns regarding to PAHs, are increasingly taken into consideration with the introduction of specific countermeasures, PAHs are still threatening environmental health. The marine environment is the major sink and receptacle of PAHs, which enter through different forms whose bioavailability varies according to their origin and their weathering processes. Microorganisms are the main actors in controlling and determining their fate in all marine ecosystems. The biological pump plays a pivotal role in the surface water and water-column trapping of PAHs, originating from both oil spills and atmospheric deposition, and then transporting them to the bottom. The utilisation of this spectacular phenomenon, by promoting it with fertilisers, for cleaning the open oceans and for carbon sequestration is currently under debate (De La Rocha 2007; Nogales et al.2011). The wide diversity of microorganisms carrying genes involved in both aerobic and anaerobic PAH degradation together, with the presence of specialist degraders, are found in all the ecosystems examined, ensuring the most important depletion route. The degradation activities depend on a complex set of environmental conditions driving the microbial assemblages that microbial ecology ambitions to understand, a challenging objective from both an academic point of view and an applied perspective. Systems biology tools involving meta-omics approaches are promising tools to address the challenge.
The exploitation of oil reservoirs in more and more extreme conditions, such as deep sea and cold areas, in the context of climate change complicates the challenge. The study of oil reservoirs in more extreme conditions is required because these colder environments present different environmental parameters, including hydrostatic pressure, low temperature and water acidification, that may shape microbial processes. Further knowledge of the ecology of the adapted bacterial populations described in deep-sea sediments and of the PAH degraders found in cold Arctic Ocean sediments, both responsible for low PAH-degradation rates, is required to complete the global model of the fate of PAHs in the marine environment.
Additionally, it is of paramount importance to gain information on how the modification of the resulting environmental parameters may affect the microbial assemblages, the biological pump and the global carbon cycle in a climate change scenario.
Acknowledgments
We would like to thank all partners of the DECAPAGE and DHYVA projects and MELODY group for their useful discussions. We thank Sally Ferguson (Alba Traduction) for carefully checking the English language. Reviewers are gratefully acknowledged for their helpful comments.
FUNDING
We thank the support of the for their support through the () and () projects.
Conflict of interest. None declared.
REFERENCES
- Abdollahi S, Raoufi Z, Faghiri I, et al. Contamination levels and spatial distributions of heavy metals and PAHs in surface sediment of Imam Khomeini Port, Persian Gulf, Iran. Mar Pollut Bull. 2013;71:336–45. doi: 10.1016/j.marpolbul.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Acosta-González A, Marqués S. Bacterial diversity in oil-polluted marine coastal sediments. Curr Opin Biotech. 2016;38:24–32. doi: 10.1016/j.copbio.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Acosta-González A, Martirani-von Abercron SM, Rosselló-Móra R, et al. The effect of oil spills on the bacterial diversity and catabolic function in coastal sediments: a case study on the Prestige oil spill. Environ Sci Pollut R. 2015;22:15200–14. doi: 10.1007/s11356-015-4458-y. [DOI] [PubMed] [Google Scholar]
- Adhikari PL, Maiti K, Overton EB. Vertical fluxes of polycyclic aromatic hydrocarbons in the northern Gulf of Mexico. Mar Chem. 2015;168:60–8. [Google Scholar]
- Adhikari PL, Maiti K, Overton EB, et al. Distributions and accumulation rates of polycyclic aromatic hydrocarbons in the northern Gulf of Mexico sediments. Environ Pollut. 2016;212:413–23. doi: 10.1016/j.envpol.2016.01.064. [DOI] [PubMed] [Google Scholar]
- Aeppli C, Carmichael CA, Nelson RK, et al. Oil weathering after the Deepwater Horizon disaster led to the formation of oxygenated residues. Environ Sci Technol. 2012;46:8799–807. doi: 10.1021/es3015138. [DOI] [PubMed] [Google Scholar]
- Allison SD, Martiny JBH. Resistance, resilience, and redundancy in microbial communities. P Natl Acad Sci USA. 2008;105:11512–9. doi: 10.1073/pnas.0801925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeda R, Wambaugh Z, Chai C, et al. Effects of crude oil exposure on bioaccumulation of polycyclic aromatic hydrocarbons and survival of adult and larval stages of gelatinous zooplankton. PLoS One. 2013;8:15. doi: 10.1371/journal.pone.0074476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JT, Achten C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycycl Aromat Comp. 2015;35:330–54. doi: 10.1080/10406638.2014.991042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei AS, Banciu HL, Oren A. Living with salt: metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol Lett. 2012;330:1–9. doi: 10.1111/j.1574-6968.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- Atlas RM, Hazen TC. Oil biodegradation and bioremediation: a tale of the two worst spills in U.S. history. Environ Sci Technol. 2011;45:6709–15. doi: 10.1021/es2013227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas RM, Stoeckel DM, Faith SA, et al. Oil Biodegradation and oil-degrading microbial populations in marsh sediments impacted by oil from the Deepwater Horizon well blowout. Environ Sci Technol. 2015;49:8356–66. doi: 10.1021/acs.est.5b00413. [DOI] [PubMed] [Google Scholar]
- Bacosa HP, Inoue C. Polycyclic aromatic hydrocarbons (PAHs) biodegradation potential and diversity of microbial consortia enriched from tsunami sediments in Miyagi, Japan. J Hazard Mater. 2015;283:689–97. doi: 10.1016/j.jhazmat.2014.09.068. [DOI] [PubMed] [Google Scholar]
- Baek SO, Field RA, Goldstone ME, et al. A review of atmospheric polycyclic aromatic hydrocarbons: sources, fate and behavior. Water Air Soil Poll. 1991;60:279–300. [Google Scholar]
- Baines SB, Pace ML, Karl DM. Why does the relationship between sinking flux and planktonic primary production differ between lakes and oceans? Limnol Oceanogr. 1994;39:213–26. [Google Scholar]
- Ball A, Truskewycz A. Polyaromatic hydrocarbon exposure: an ecological impact ambiguity. Environ Sci Pollut R. 2013;20:4311–26. doi: 10.1007/s11356-013-1620-2. [DOI] [PubMed] [Google Scholar]
- Bamforth SM, Singleton I. Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Technol Biot. 2005;80:723–36. [Google Scholar]
- Bargiela R, Mapelli F, Rojo D, et al. Bacterial population and biodegradation potential in chronically crude oil-contaminated marine sediments are strongly linked to temperature. Sci Rep. 2015;5:11651. doi: 10.1038/srep11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrado AI, García S, Castrillejo Y, et al. Exploratory data analysis of PAH, nitro-PAH and hydroxy-PAH concentrations in atmospheric PM10-bound aerosol particles. Correlations with physical and chemical factors. Atmos Environ. 2013;67:385–93. [Google Scholar]
- Bauer JE, Cai WJ, Raymond PA, et al. The changing carbon cycle of the coastal ocean. Nature. 2013;504:61–70. doi: 10.1038/nature12857. [DOI] [PubMed] [Google Scholar]
- Baumard P, Budzinski H, Garrigues P. PAHs in Arcachon Bay, France: origin and biomonitoring with caged organisms. Mar Pollut Bull. 1998;36:577–86. [Google Scholar]
- Bayen S. Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: a review. Environ Int. 2012;48:84–101. doi: 10.1016/j.envint.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Beazley MJ, Martinez RJ, Rajan S, et al. Microbial community analysis of a coastal salt marsh affected by the Deepwater Horizon oil spill. PLoS One. 2012;7:e41305. doi: 10.1371/journal.pone.0041305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Said O, Goñi-Urriza M, Bour ME, et al. Bacterial community structure of sediments of the bizerte lagoon (Tunisia), a southern mediterranean coastal anthropized lagoon. Microb Ecol. 2010;59:445–56. doi: 10.1007/s00248-009-9585-x. [DOI] [PubMed] [Google Scholar]
- Ben Said O, Goñi-Urriza MS, El Bour M, et al. Characterization of aerobic polycyclic aromatic hydrocarbon-degrading bacteria from Bizerte lagoon sediments, Tunisia. J Appl Microbiol. 2008;104:987–97. doi: 10.1111/j.1365-2672.2007.03621.x. [DOI] [PubMed] [Google Scholar]
- Ben Said O, Louati H, Soltani A, et al. Changes of benthic bacteria and meiofauna assemblages during bio-treatments of anthracene-contaminated sediments from Bizerta lagoon (Tunisia) Environ Sci Pollut R. 2015;22:15319–31. doi: 10.1007/s11356-015-4105-7. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Gorbi S, Fattorini D, et al. Environmental hazards from natural hydrocarbons seepage: integrated classification of risk from sediment chemistry, bioavailability and biomarkers responses in sentinel species. Environ Pollut. 2014;185:116–26. doi: 10.1016/j.envpol.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Benner R, Herndl G. Bacterially derived dissolved organic matter in the microbial carbon pump. In: Jiao N, Azam F, Sanders S, editors. Microbial Carbon Pump in the Ocean. Washington, DC: Science/AAAS; 2011. pp. 46–8. [Google Scholar]
- Berrojalbiz N, Dachs J, Del Vento S, et al. Persistent organic pollutants in mediterranean seawater and processes affecting their accumulation in plankton. Environ Sci Technol. 2011;45:4315–22. doi: 10.1021/es103742w. [DOI] [PubMed] [Google Scholar]
- Berrojalbiz N, Lacorte S, Calbet A, et al. Accumulation and cycling of polycyclic aromatic hydrocarbons in zooplankton. Environ Sci Technol. 2009;43:2295–301. doi: 10.1021/es8018226. [DOI] [PubMed] [Google Scholar]
- Bertrand JC, Almallah M, Acquaviva M, et al. Biodegradation of hydrocarbons by an extremely halophilic archaebacterium. Lett Appl Microbiol. 1990;11:260–3. [Google Scholar]
- Bik HM, Halanych KM, Sharma J, et al. Dramatic shifts in benthic microbial eukaryote communities following the deepwater horizon oil spill. PLoS One. 2012;7:e38550. doi: 10.1371/journal.pone.0038550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binark N, Güven K, Gezgin T, et al. Oil pollution of marine algae. B Environ Contam Tox. 2000;64:866–72. doi: 10.1007/s0012800083. [DOI] [PubMed] [Google Scholar]
- Bird MI, Wynn JG, Saiz G, et al. The pyrogenic carbon cycle. 2015;43:273–98. [Google Scholar]
- Blair NE, Aller RC. The fate of terrestrial organic carbon in the marine environment. In: Carlson CA, Giovannoni SJ, editors. Annual Review of Marine Science. Vol. 4. Palo Alto: Annual Reviews; 2012. pp. 401–23. [DOI] [PubMed] [Google Scholar]
- Boehm PD, Murray KJ, Cook LL. Distribution and attenuation of polycyclic aromatic hydrocarbons in gulf of Mexico seawater from the Deepwater Horizon oil accident. Environ Sci Technol. 2016;50:584–92. doi: 10.1021/acs.est.5b03616. [DOI] [PubMed] [Google Scholar]
- Boitsov S, Petrova V, Jensen HKB, et al. Sources of polycyclic aromatic hydrocarbons in marine sediments from southern and northern areas of the Norwegian continental shelf. Mar Environ Res. 2013;87–88:73–84. doi: 10.1016/j.marenvres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Trush MA, Penning TM, et al. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–60. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- Bonfá MRL, Grossman MJ, Mellado E, et al. Biodegradation of aromatic hydrocarbons by Haloarchaea and their use for the reduction of the chemical oxygen demand of hypersaline petroleum produced water. Chemosphere. 2011;84:1671–6. doi: 10.1016/j.chemosphere.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Bonin P, Cravo-Laureau C, Michotey V, et al. The anaerobic hydrocarbon biodegrading bacteria: an overview. Ophelia. 2004;58:243–54. [Google Scholar]
- Borch T, Kretzschmar R, Skappler A, et al. Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol. 2010;44:15–23. doi: 10.1021/es9026248. [DOI] [PubMed] [Google Scholar]
- Bordenave S, Fourçans A, Blanchard S, et al. Structure and functional analyses of bacterial communities changes in microbial mats following petroleum exposure. Ophelia. 2004a;58:195–203. [Google Scholar]
- Bordenave S, Goñi-Urriza M, Vilette C, et al. Diversity of ring-hydroxylating dioxygenases in pristine and oil contaminated microbial mats at genomic and transcriptomic levels. Environ Microbiol. 2008;10:3201–11. doi: 10.1111/j.1462-2920.2008.01707.x. [DOI] [PubMed] [Google Scholar]
- Bordenave S, Goñi-Urriza MS, Caumette P, et al. Effects of heavy fuel oil on the bacterial community structure of a pristine microbial mat. Appl Environ Microb. 2007;73:6089–97. doi: 10.1128/AEM.01352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenave S, Jézéquel R, Fourçans A, et al. Degradation of the “Erikardquo; oil. Aquat Living Resour. 2004b;17:261–7. [Google Scholar]
- Brito EMS, Duran R, Guyoneaud R, et al. A case study of in situ oil contamination in a mangrove swamp (Rio De Janeiro, Brazil) Mar Pollut Bull. 2009;58:418–23. doi: 10.1016/j.marpolbul.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Buhl-Mortensen L, Buhl-Mortensen P, Dolan MFJ, et al. The MAREANO programme – A full coverage mapping of the Norwegian off-shore benthic environment and fauna. Mar Biol Res. 2015;11:4–17. [Google Scholar]
- Burdige DJ. Burial of terrestrial organic matter in marine sediments: a re-assessment. Global Biogeochem Cy. 2005;19:Gb4011. [Google Scholar]
- Burgess RM, Berry WJ, Mount DR, et al. Mechanistic sediment quality guidelines based on contaminant bioavailability: equilibrium partitioning sediment benchmarks. Environ Toxicol Chem. 2013;32:102–14. doi: 10.1002/etc.2025. [DOI] [PubMed] [Google Scholar]
- Burns KA. PAHs in the Great Barrier Reef Lagoon reach potentially toxic levels from coal port activities. Estuar Coast Shelf S. 2014;144:39–45. [Google Scholar]
- Burns WA, Mankiewicz PJ, Bench AE, et al. A principal-component and least-squares method for allocating polycyclic aromatic hydrocarbons in sediment to multiple sources. Environ Toxicol Chem. 1997;16:1119–31. [Google Scholar]
- Cao JW, Lai QL, Yuan J, et al. Genomic and metabolic analysis of fluoranthene degradation pathway in Celeribacter indicus P73(T) Sci Rep. 2015;5:12. doi: 10.1038/srep07741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerniglia C. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–68. [Google Scholar]
- Cerniglia C. Biodegradation of polycyclic aromatic hydrocarbons. In: Rosenberg E, editor. Microorganisms to Combat Pollution. Netherlands: Springer; 1993. pp. 227–44. [Google Scholar]
- Chadhain SMN, Norman RS, Pesce KV, et al. Microbial dioxygenase gene population shifts during polycyclic aromatic hydrocarbon biodegradation. Appl Environ Microb. 2006;72:4078–87. doi: 10.1128/AEM.02969-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Um Y, Holoman TRP. Polycyclic aromatic hydrocarbon (PAH) degradation coupled to methanogenesis. Biotechnol Lett. 2006;28:425–30. doi: 10.1007/s10529-005-6073-3. [DOI] [PubMed] [Google Scholar]
- Chanton J, Zhao T, Rosenheim BE, et al. Using natural abundance radiocarbon to trace the flux of petrocarbon to the seafloor following the Deepwater Horizon oil spill. Environ Sci Technol. 2015;49:847–54. doi: 10.1021/es5046524. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Luo XJ, Mai BX, et al. Distribution and mass inventories of polycyclic aromatic hydrocarbons and organochlorine pesticides in sediments of the pearl river estuary and the northern South China Sea. Environ Sci Technol. 2006;40:709–14. doi: 10.1021/es052060g. [DOI] [PubMed] [Google Scholar]
- Christiansen C, Leipe T, Witt G, et al. Selected elements, PCBs, and PAHs in sediments of the North Sea—Baltic Sea transition zone: sources and transport as derived from the distribution pattern. Geogr Tidsskr. 2009;109:81–94. [Google Scholar]
- Chronopoulou P-M, Fahy A, Coulon F, et al. Impact of a simulated oil spill on benthic phototrophs and nitrogen-fixing bacteria in mudflat mesocosms. Environ Microbiol. 2013;15:242–52. doi: 10.1111/j.1462-2920.2012.02864.x. [DOI] [PubMed] [Google Scholar]
- Coppola AI, Druffel ERM. Cycling of black carbon in the ocean. Geophys Res Lett. 2016;43:4477–82. [Google Scholar]
- Coppola AI, Walker BD, Druffel ERM. Solid phase extraction method for the study of black carbon cycling in dissolved organic carbon using radiocarbon. Mar Chem. 2015;177:697–705. [Google Scholar]
- Coulon F, Chronopoulou P-M, Fahy A, et al. Central role of dynamic tidal biofilms dominated by aerobic hydrocarbonoclastic bacteria and diatoms in the biodegradation of hydrocarbons in coastal mudflats. Appl Environ Microb. 2012;78:3638–48. doi: 10.1128/AEM.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo-Laureau C, Duran R. Marine coastal sediments microbial hydrocarbon degradation processes: contribution of experimental ecology in the omics'era. Front Microbiol. 2014;5:39. doi: 10.3389/fmicb.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo-Laureau C, Hernandez-Raquet G, Vitte I, et al. Role of environmental fluctuations and microbial diversity in degradation of hydrocarbons in contaminated sludge. Res Microbiol. 2011;162:888–95. doi: 10.1016/j.resmic.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Cuny P, Gilbert F, Militon C, et al. Use of dispersant in mudflat oil-contaminated sediment: behavior and effects of dispersed oil on micro- and macrobenthos. Environ Sci Pollut R. 2015;22:15370–6. doi: 10.1007/s11356-015-4800-4. [DOI] [PubMed] [Google Scholar]
- Dachs J, Bayona JM, Raoux C, et al. Spatial, vertical distribution and budget of polycyclic aromatic hydrocarbons in the western Mediterranean seawater. Environ Sci Technol. 1997;31:682–8. [Google Scholar]
- Dachs J, Eisenreich SJ, Baker JE, et al. Coupling of phytoplankton uptake and air-water exchange of persistent organic pollutants. Environ Sci Technol. 1999;33:3653–60. [Google Scholar]
- Dachs J, Eisenreich SJ, Hoff RM. Influence of eutrophication on air-water exchange, vertical fluxes, and phytoplankton concentrations of persistent organic pollutants. Environ Sci Technol. 2000;34:1095–102. [Google Scholar]
- Dachs J, Lohmann R, Ockenden WA, et al. Oceanic biogeochemical controls on global dynamics of persistent organic pollutants. Environ Sci Technol. 2002;36:4229–37. doi: 10.1021/es025724k. [DOI] [PubMed] [Google Scholar]
- Daling PS, Leirvik F, Almås IK, et al. Surface weathering and dispersibility of MC252 crude oil. Mar Pollut Bull. 2014;87:300–10. doi: 10.1016/j.marpolbul.2014.07.005. [DOI] [PubMed] [Google Scholar]
- De La Rocha CL. 6.04 - the biological pump. In: Turekian HDHK, editor. Treatise on Geochemistry. Oxford: Pergamon; 2007. pp. 1–29. [Google Scholar]
- De Luca G, Furesi A, Micera G, et al. Nature, distribution and origin of polycyclic aromatic hydrocarbons (PAHs) in the sediments of Olbia harbor (Northern Sardinia, Italy) Mar Pollut Bull. 2005;50:1223–32. doi: 10.1016/j.marpolbul.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Dean BJ. Recent findings on the genetic toxicology of benzene, toluene, xylenes and phenols. Mutat Res. 1985;154:153–81. doi: 10.1016/0165-1110(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Diercks AR, Highsmith RC, Asper VL, et al. Characterization of subsurface polycyclic aromatic hydrocarbons at the Deepwater Horizon site. Geophys Res Lett. 2010;37:L20602. [Google Scholar]
- Dong C, Bai X, Sheng H, et al. Distribution of PAHs and the PAH-degrading bacteria in the deep-sea sediments of the high-latitude Arctic Ocean. Biogeosciences. 2015;12:2163–77. [Google Scholar]
- Doyle E, Muckian L, Hickey AM, et al. Microbial PAH degradation. Adv Appl Microbiol. 2008;65:27–66. doi: 10.1016/S0065-2164(08)00602-3. [DOI] [PubMed] [Google Scholar]
- Duran R, Bielen A, Paradžik T, et al. Exploring Actinobacteria assemblages in coastal marine sediments under contrasted human influences in the West Istria Sea, Croatia. Environ Sci Pollut R. 2015a;22:15215–29. doi: 10.1007/s11356-015-4240-1. [DOI] [PubMed] [Google Scholar]
- Duran R, Bonin P, Jezequel R, et al. Effect of physical sediments reworking on hydrocarbon degradation and bacterial community structure in marine coastal sediments. Environ Sci Pollut R. 2015b;22:15248–59. doi: 10.1007/s11356-015-4373-2. [DOI] [PubMed] [Google Scholar]
- Duran R, Cuny P, Bonin P, et al. Microbial ecology of hydrocarbon-polluted coastal sediments. Environ Sci Pollut R. 2015c;22:15195–9. doi: 10.1007/s11356-015-5373-y. [DOI] [PubMed] [Google Scholar]
- Duran R, Goñi Urriza MS. Impact of pollution on microbial mats. Microbes and communities utilizing hydrocarbons, oils and lipids. In: Timmis KN, editor. Handbook of Hydrocarbon and Lipid Microbiology. Berlin, Heidelberg: Springer; 2010. pp. 2339–48. [Google Scholar]
- Echeveste P, Dachs J, Berrojalbiz N, et al. Decrease in the abundance and viability of oceanic phytoplankton due to trace levels of complex mixtures of organic pollutants. Chemosphere. 2010;81:161–8. doi: 10.1016/j.chemosphere.2010.06.072. [DOI] [PubMed] [Google Scholar]
- Farrington JW. Oil pollution in the marine environment i: inputs, big spills, small spills, and dribbles. Environment. 2013;55:3–13. [Google Scholar]
- Feng J, Yang Z, Niu J, et al. Remobilization of polycyclic aromatic hydrocarbons during the resuspension of Yangtze River sediments using a particle entrainment simulator. Environ Pollut. 2007;149:193–200. doi: 10.1016/j.envpol.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Finlayson-Pitts BJ, Pitts JN. Tropospheric air pollution: ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science. 1997;276:1045–51. doi: 10.1126/science.276.5315.1045. [DOI] [PubMed] [Google Scholar]
- Flores-Cervantes DX, Plata DL, MacFarlane JK, et al. Black carbon in marine particulate organic carbon: inputs and cycling of highly recalcitrant organic carbon in the Gulf of Maine. Mar Chem. 2009a;113:172–81. [Google Scholar]
- Flores-Cervantes DX, Reddy CM, Gschwend PM. Inferring black carbon concentrations in particulate organic matter by observing pyrene fluorescence losses. Environ Sci Technol. 2009b;43:4864–70. doi: 10.1021/es900043c. [DOI] [PubMed] [Google Scholar]
- Foght J. Anaerobic biodegradation of aromatic hydrocarbons: pathways and prospects. J Mol Microb Biotech. 2008;15:93–120. doi: 10.1159/000121324. [DOI] [PubMed] [Google Scholar]
- Forsberg ND, O'Connell SG, Allan SE, et al. Passive sampling coupled to ultraviolet irradiation: a useful analytical approach for studying oxygenated polycyclic aromatic hydrocarbon formation in bioavailable mixtures. Environ Toxicol Chem. 2014;33:177–81. doi: 10.1002/etc.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KL, Stern GA, Carrie J, et al. Spatial, temporal, and source variations of hydrocarbons in marine sediments from Baffin Bay, Eastern Canadian Arctic. Sci Total Environ. 2015;506–507:430–43. doi: 10.1016/j.scitotenv.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Frapiccini E, Marini M. Polycyclic aromatic hydrocarbon degradation and sorption parameters in coastal and open-sea sediment. Water Air Soil Poll. 2015;226:246. [Google Scholar]
- Friedman CL, Selin NE. Long-range atmospheric transport of polycyclic aromatic hydrocarbons: a global 3-D model analysis including evaluation of arctic sources. Environ Sci Technol. 2012;46:9501–10. doi: 10.1021/es301904d. [DOI] [PubMed] [Google Scholar]
- Galbán-Malagón C, Berrojalbiz N, Ojeda MJ, et al. The oceanic biological pump modulates the atmospheric transport of persistent organic pollutants to the Arctic. Nat Commun. 2012;3:862. doi: 10.1038/ncomms1858. [DOI] [PubMed] [Google Scholar]
- Gallego S, Vila J, Tauler M, et al. Community structure and PAH ring-hydroxylating dioxygenase genes of a marine pyrene-degrading microbial consortium. Biodegradation. 2014;25:543–56. doi: 10.1007/s10532-013-9680-z. [DOI] [PubMed] [Google Scholar]
- Gogou A, Bouloubassi I, Stephanou EG. Marine organic geochemistry of the Eastern Mediterranean: 1. Aliphatic and polyaromatic hydrocarbons in Cretan Sea surficial sediments. Mar Chem. 2000;68:265–82. [Google Scholar]
- González-Gaya B, Zúñiga-Rival J, Ojeda MJ, et al. Field measurements of the atmospheric dry deposition fluxes and velocities of polycyclic aromatic hydrocarbons to the global oceans. Environ Sci Technol. 2014;48:5583–92. doi: 10.1021/es500846p. [DOI] [PubMed] [Google Scholar]
- Guermouche M'rassi A, Bensalah F, Gury J, et al. Isolation and characterization of different bacterial strains for bioremediation of n-alkanes and polycyclic aromatic hydrocarbons. Environ Sci Pollut R. 2015;22:15332–46. doi: 10.1007/s11356-015-4343-8. [DOI] [PubMed] [Google Scholar]
- Guigue C, Bigot L, Turquet J, et al. Hydrocarbons in a coral reef ecosystem subjected to anthropogenic pressures (La Reúnion Island, Indian Ocean) Environ Chem. 2015;12:350–65. doi: 10.1016/j.scitotenv.2011.01.058. [DOI] [PubMed] [Google Scholar]
- Guo L, Coleman CH, Jr, Santschi PH. The distribution of colloidal and dissolved organic carbon in the Gulf of Mexico. Mar Chem. 1994;45:105–19. [Google Scholar]
- Guo L, Santschi PH, Warnken KW. Dynamics of dissolved organic carbon (DOC) in oceanic environments. Limnol Oceanogr. 1995;40:1392–403. [Google Scholar]
- Guo Z, Lin T, Zhang G, et al. The sedimentary fluxes of polycyclic aromatic hydrocarbons in the Yangtze River Estuary coastal sea for the past century. Sci Total Environ. 2007;386:33–41. doi: 10.1016/j.scitotenv.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Gustafsson Ö, Gschwend PM, Buesseler KO. Using 234Th disequilibria to estimate the vertical removal rates of polycyclic aromatic hydrocarbons from the surface ocean. Mar Chem. 1997;57:11–23. [Google Scholar]
- Gutierrez T, Singleton DR, Berry D, et al. Hydrocarbon-degrading bacteria enriched by the Deepwater Horizon oil spill identified by cultivation and DNA-SIP. ISME J. 2013;7:2091–104. doi: 10.1038/ismej.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern BS, Walbridge S, Selkoe KA, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–52. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater. 2009;169:1–15. doi: 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- Harms H, Schlosser D, Wick LY. Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat Rev Microbiol. 2011;9:177–92. doi: 10.1038/nrmicro2519. [DOI] [PubMed] [Google Scholar]
- Hazen TC, Dubinsky EA, DeSantis TZ, et al. Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science. 2010;330:204–8. doi: 10.1126/science.1195979. [DOI] [PubMed] [Google Scholar]
- Head IM, Jones DM, Roling WFM. Marine microorganisms make a meal of oil. Nat Rev Microbiol. 2006;4:173–82. doi: 10.1038/nrmicro1348. [DOI] [PubMed] [Google Scholar]
- Heider J, Schühle K. Anaerobic biodegradation of hydrocarbons including methane. In: Rosenberg E, DeLong EF, Lory S, et al., editors. The Prokaryotes: Prokaryotic Physiology and Biochemistry. Berlin Heidelberg: Springer; 2013. pp. 605–34. [Google Scholar]
- Henner P, Schiavon M, Morel J-L, et al. Polycyclic aromatic hydrocarbon (PAH) occurrence and remediation methods. Analusis. 1997;25:M56–9. [Google Scholar]
- Hites RA, Laflamme RE, Farrington JW. Sedimentary polycyclic aromatic hydrocarbons: the historical record. Science. 1977;198:829–31. doi: 10.1126/science.198.4319.829. [DOI] [PubMed] [Google Scholar]
- Hites RA, Laflamme RE, Windsor JG. Polycyclic aromatic hydrocarbons in marine/aquatic sediments: their ubiquity. In: Petrakis L, Weiss FT, editors. Petroleum in the Marine Environment. Vol. 185. Washington, DC: American Chemical Society; 1980. pp. 289–311. [Google Scholar]
- Hjorth M, Forbes VE, Dahllöf I. Plankton stress responses from PAH exposure and nutrient enrichment. Mar Ecol-Prog Ser. 2008;363:121–30. [Google Scholar]
- Hjorth M, Vester J, Henriksen P, et al. Functional and structural responses of marine plankton food web to pyrene contamination. Mar Ecol-Prog Ser. 2007;338:21–31. [Google Scholar]
- Hoffman EJ, Mills GL, Latimer JS, et al. Urban runoff as a source of polycyclic aromatic hydrocarbons to coastal waters. Environ Sci Technol. 1984;18:580–7. doi: 10.1021/es00126a003. [DOI] [PubMed] [Google Scholar]
- Hylland K. Polycyclic aromatic hydrocarbon (PAH) ecotoxicology in marine ecosystems. J Toxicol Env Heal A. 2006;69:109–23. doi: 10.1080/15287390500259327. [DOI] [PubMed] [Google Scholar]
- Jenkins BM, Jones AD, Turn SQ, et al. Particle concentrations, gas-particle partitioning, and species intercorrelations for polycyclic aromatic hydrocarbons (PAH) emitted during biomass burning. Atmos Environ. 1996;30:3825–35. [Google Scholar]
- Jiao N, Herndl GJ, Hansell DA, et al. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat Rev Microbiol. 2010;8:593–9. doi: 10.1038/nrmicro2386. [DOI] [PubMed] [Google Scholar]
- Jiao N, Herndl GJ, Hansell DA, et al. The microbial carbon pump and the oceanic recalcitrant dissolved organic matter pool. Nat Rev Microbiol. 2011;9:555. doi: 10.1038/nrmicro2386. [DOI] [PubMed] [Google Scholar]
- Jörundsdóttir HÓ, Jensen S, Hylland K, et al. Pristine arctic: background mapping of PAHs, PAH metabolites and inorganic trace elements in the North-Atlantic Arctic and sub-Arctic coastal environment. Sci Total Environ. 2014;493:719–28. doi: 10.1016/j.scitotenv.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Joye SB, Teske AP, Kostka JE. Microbial dynamics following the macondo oil well blowout across gulf of Mexico environments. Bioscience. 2014;64:766–77. [Google Scholar]
- Judd A, Hovland M. Seabed Fluid Flow: The Impact on Geology, Biology and the Marine Environment. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Jurado E, Dachs J, Duarte CM, et al. Atmospheric deposition of organic and black carbon to the global oceans. Atmos Environ. 2008;42:7931–9. [Google Scholar]
- Kanaly RA, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182:2059–67. doi: 10.1128/jb.182.8.2059-2067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke L, Yu KSH, Wong YS, et al. Spatial and vertical distribution of polycyclic aromatic hydrocarbons in mangrove sediments. Sci Total Environ. 2005;340:177–87. doi: 10.1016/j.scitotenv.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Kennish MJ. Environmental threats and environmental future of estuaries. Environ Conserv. 2002;29:78–107. [Google Scholar]
- Keshavarzifard M, Zakaria MP, Hwai TS, et al. Distributions and source apportionment of sediment-associated polycyclic aromatic hydrocarbons (PAHs) and hopanes in rivers and estuaries of Peninsular Malaysia. Environ Sci Pollut R. 2015;22:9424–37. doi: 10.1007/s11356-015-4093-7. [DOI] [PubMed] [Google Scholar]
- Kimes NE, Callaghan AV, Suflita JM, et al. Microbial transformation of the Deepwater Horizon oil spill – past, present, and future perspectives. Front Microbiol. 2014;5:603. doi: 10.3389/fmicb.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Readman JW, Zhou JL. Dynamic behaviour of polycyclic aromatic hydrocarbons in Brighton marina, UK. Mar Pollut Bull. 2004;48:229–39. doi: 10.1016/S0025-326X(03)00393-X. [DOI] [PubMed] [Google Scholar]
- King GM, Kostka JE, Hazen TC, et al. Microbial responses to the Deepwater Horizon oil spill: from coastal wetlands to the deep sea. Ann Rev Mar Sci. 2015;7:377–401. doi: 10.1146/annurev-marine-010814-015543. [DOI] [PubMed] [Google Scholar]
- Kiruri LW, Dellinger B, Lomnicki S. Tar balls from deep water horizon oil spill: environmentally persistent free radicals (EPFR) formation during crude weathering. Environ Sci Technol. 2013;47:4220–6. doi: 10.1021/es305157w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst S, Paul JH, Joye SB. Using dispersants after oil spills: impacts on the composition and activity of microbial communities. Nat Rev Microbiol. 2015;13:388–96. doi: 10.1038/nrmicro3452. [DOI] [PubMed] [Google Scholar]
- Kleindienst S, Seidel M, Ziervogel K, et al. Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. P Natl Acad Sci USA. 2015;112:14900–5. doi: 10.1073/pnas.1507380112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko FC, Chang CW, Cheng JO. Comparative study of polycyclic aromatic hydrocarbons in coral tissues and the ambient sediments from Kenting National Park, Taiwan. Environ Pollut. 2014;185:35–43. doi: 10.1016/j.envpol.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Kostka JE, Prakash O, Overholt WA, et al. Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the deepwater horizon oil spill. Appl Environ Microb. 2011;77:7962–74. doi: 10.1128/AEM.05402-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvenvolden KA, Cooper CK. Natural seepage of crude oil into the marine environment. Geo-Mar Lett. 2003;23:140–6. [Google Scholar]
- Kweon O, Kim SJ, Baek S, et al. A new classification system for bacterial Rieske non-heme iron aromatic ring-hydroxylating oxygenases. BMC Biochem. 2008;9:11. doi: 10.1186/1471-2091-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamendella R, Strutt S, Borglin S, et al. Assessment of the deepwater horizon oil spill impact on gulf coast microbial communities. Front Microbiol. 2014;5:130. doi: 10.3389/fmicb.2014.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne MG, Leifer I, Bergmann S, et al. Bacterial diversity in marine hydrocarbon seep sediments. Environ Microbiol. 2004;6:799–808. doi: 10.1111/j.1462-2920.2004.00613.x. [DOI] [PubMed] [Google Scholar]
- Lang YH, Yang X, Wang H, et al. Diagnostic ratios and positive matrix factorization to identify potential sources of PAHS in sediments of the Rizhao Offshore, China. Polycycl Aromat Comp. 2013;33:161–72. [Google Scholar]
- Layshock JA, Wilson G, Anderson KA. Ketone and quinone-substituted polycyclic aromatic hydrocarbons in mussel tissue, sediment, urban dust, and diesel particulate matrices. Environ Toxicol Chem. 2010;29:2450–60. doi: 10.1002/etc.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc LA, Gulnick JD, Brownawell BJ, et al. The influence of sediment resuspension on the degradation of phenanthrene in flow-through microcosms. Mar Environ Res. 2006;61:202–23. doi: 10.1016/j.marenvres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lee K, Nedwed T, Prince RC, et al. Lab tests on the biodegradation of chemically dispersed oil should consider the rapid dilution that occurs at sea. Mar Pollut Bull. 2013;73:314–8. doi: 10.1016/j.marpolbul.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Li H, Lu L, Huang W, et al. In-situ partitioning and bioconcentration of polycyclic aromatic hydrocarbons among water, suspended particulate matter, and fish in the Dongjiang and Pearl Rivers and the Pearl River Estuary, China. Mar Pollut Bull. 2014;83:306–16. doi: 10.1016/j.marpolbul.2014.04.036. [DOI] [PubMed] [Google Scholar]
- Li JY, Cui Y, Su L, et al. Polycyclic aromatic hydrocarbons in the largest deepwater port of East China Sea: impact of port construction and operation. Environ Sci Pollut R. 2015a;22:12355–65. doi: 10.1007/s11356-015-4402-1. [DOI] [PubMed] [Google Scholar]
- Li P, Xue R, Wang Y, et al. Influence of anthropogenic activities on PAHs in sediments in a significant gulf of low-latitude developing regions, the Beibu Gulf, South China Sea: distribution, sources, inventory and probability risk. Mar Pollut Bull. 2015b;90:218–26. doi: 10.1016/j.marpolbul.2014.10.048. [DOI] [PubMed] [Google Scholar]
- Lima ALC, Eglinton TI, Reddy CM. High-resolution record of pyrogenic polycyclic aromatic hydrocarbon deposition during the 20th century. Environ Sci Technol. 2003;37:53–61. doi: 10.1021/es025895p. [DOI] [PubMed] [Google Scholar]
- Lima ALC, Farrington JW, Reddy CM. Combustion-derived polycyclic aromatic hydrocarbons in the environment - A review. Environ Forensics. 2005;6:109–31. [Google Scholar]
- Lipiatou E, Saliot A. Fluxes and transport of anthropogenic and natural polycyclic aromatic hydrocarbons in the western Mediterranean Sea. Mar Chem. 1991;32:51–71. [Google Scholar]
- Liu L-Y, Wang J-Z, Wei G-L, et al. Polycyclic aromatic hydrocarbons (PAHs) in continental shelf sediment of China: implications for anthropogenic influences on coastal marine environment. Environ Pollut. 2012;167:155–62. doi: 10.1016/j.envpol.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Llado S, Covino S, Solanas AM, et al. Pyrosequencing reveals the effect of mobilizing agents and lignocellulosic substrate amendment on microbial community composition in a real industrial PAH-polluted soil. J Hazard Mater. 2015;283:35–43. doi: 10.1016/j.jhazmat.2014.08.065. [DOI] [PubMed] [Google Scholar]
- Lohmann R, Bollinger K, Cantwell M, et al. Fluxes of soot black carbon to South Atlantic sediments. Global Biogeochem Cy. 2009;23:141–8. [Google Scholar]
- Lohmann R, Macfarlane JK, Gschwend PM. Importance of black carbon to sorption of native PAHs, PCBs, and PCDDs in Boston and New York Harbor sediments. Environ Sci Technol. 2005;39:141–8. doi: 10.1021/es049424+. [DOI] [PubMed] [Google Scholar]
- Long ER, Macdonald DD, Smith SL, et al. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage. 1995;19:81–97. [Google Scholar]
- Louati H, Ben Said O, Got P, et al. Microbial community responses to bioremediation treatments for the mitigation of low-dose anthracene in marine coastal sediments of Bizerte lagoon (Tunisia) Environ Sci Pollut R. 2013a;20:300–10. doi: 10.1007/s11356-012-0860-x. [DOI] [PubMed] [Google Scholar]
- Louati H, Ben Said O, Soltani A, et al. The roles of biological interactions and pollutant contamination in shaping microbial benthic community structure. Chemosphere. 2013b;93:2535–46. doi: 10.1016/j.chemosphere.2013.09.069. [DOI] [PubMed] [Google Scholar]
- Louchouarn P, Kuo LJ, Brandenberger JM, et al. Pyrogenic inputs of anthropogenic Pb and Hg to sediments of the Hood Canal, Washington, in the 20th century: source evidence from stable Pb isotopes and PAH signatures. Environ Sci Technol. 2012;46:5772–81. doi: 10.1021/es300269t. [DOI] [PubMed] [Google Scholar]
- Louvado A, Gomes NCM, Simões MMQ, et al. Polycyclic aromatic hydrocarbons in deep sea sediments: microbe-pollutant interactions in a remote environment. Sci Total Environ. 2015;526:312–28. doi: 10.1016/j.scitotenv.2015.04.048. [DOI] [PubMed] [Google Scholar]
- Lu X-Y, Zhang T, Fang H-P. Bacteria-mediated PAH degradation in soil and sediment. Appl Microbiol Biot. 2011;89:1357–71. doi: 10.1007/s00253-010-3072-7. [DOI] [PubMed] [Google Scholar]
- Lundstedt S, White PA, Lemieux CL, et al. Sources, fate, and toxic hazards of oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) at PAH-contaminated sites. Ambio. 2007;36:475–85. doi: 10.1579/0044-7447(2007)36[475:sfatho]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Macdonald DD, Carr RS, Calder FD, et al. Development and evaluation of sediment quality guidelines for Florida coastal waters. Ecotoxicology. 1996;5:253–78. doi: 10.1007/BF00118995. [DOI] [PubMed] [Google Scholar]
- Mackay D, Shiu WY, Ma K-C. Illustrated Handbook of Physical-Chemical Properties of Environmental Fate for Organic Chemicals. New York: CRC Press; 1997. [Google Scholar]
- Maher WA, Aislabie J. Polycyclic aromatic hydrocarbons in nearshore marine sediments of Australia. Sci Total Environ. 1992;112:143–64. doi: 10.1016/0048-9697(92)90184-t. [DOI] [PubMed] [Google Scholar]
- Mahmoudi N, Porter TM, Zimmerman AR, et al. Rapid degradation of Deepwater Horizon spilled oil by indigenous microbial communities in Louisiana saltmarsh sediments. Environ Sci Technol. 2013;47:13303–12. doi: 10.1021/es4036072. [DOI] [PubMed] [Google Scholar]
- Mai BX, Fu JM, Sheng GY, et al. Chlorinated and polycyclic aromatic hydrocarbons in riverine and estuarine sediments from Pearl River Delta, China. Environ Pollut. 2002;117:457–74. doi: 10.1016/s0269-7491(01)00193-2. [DOI] [PubMed] [Google Scholar]
- Manoli E, Kouras A, Samara C. Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere. 2004;56:867–78. doi: 10.1016/j.chemosphere.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Marcos MS, Lozada M, Dionisi HM. Aromatic hydrocarbon degradation genes from chronically polluted Subantarctic marine sediments. Lett Appl Microbiol. 2009;49:602–8. doi: 10.1111/j.1472-765X.2009.02711.x. [DOI] [PubMed] [Google Scholar]
- Marín-Spiotta E, Gruley KE, Crawford J, et al. Paradigm shifts in soil organic matter research affect interpretations of aquatic carbon cycling: transcending disciplinary and ecosystem boundaries. Biogeochemistry. 2014;117:279–97. [Google Scholar]
- Masiello CA, Druffel ERM. Black carbon in deep-sea sediments. Science. 1998;280:1911–3. doi: 10.1126/science.280.5371.1911. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G, Fadda E, Marzia V. Polycyclic aromatic hydrocarbons and cancer in man. Environ Health Perspect. 1996;104:1166–70. doi: 10.1289/ehp.961041166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGenity TJ. Halophilic hydrocarbon degraders. In: Timmis KN, editor. Handbook of Hydrocarbon and Lipid Microbiology. Berlin: Springer; 2010. pp. 1939–51. [Google Scholar]
- McGenity TJ. Hydrocarbon biodegradation in intertidal wetland sediments. Curr Opin Biotech. 2014;27:46–54. doi: 10.1016/j.copbio.2013.10.010. [DOI] [PubMed] [Google Scholar]
- McGenity TJ, Folwell BD, McKew BA, et al. Marine crude-oil biodegradation: a central role for interspecies interactions. Aquat Biosyst. 2012;8:10. doi: 10.1186/2046-9063-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador J, Stein J, Reichert W, et al. Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. In: Ware GW, editor. Reviews of Environmental Contamination and Toxicology. New York: Springer; 1995. pp. 79–165. [DOI] [PubMed] [Google Scholar]
- Meckenstock RU, Boll M, Mouttaki H, et al. Anaerobic degradation of benzene and polycyclic aromatic hydrocarbons. J Mol Microb Biotech. 2016;26:92–118. doi: 10.1159/000441358. [DOI] [PubMed] [Google Scholar]
- Meckenstock RU, Morasch B, Griebler C, et al. Stable isotope fractionation analysis as a tool to monitor biodegradation in contaminated acquifers. J Contam Hydrol. 2004;75:215–55. doi: 10.1016/j.jconhyd.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Meckenstock RU, Mouttaki H. Anaerobic degradation of non-substituted aromatic hydrocarbons. Curr Opin Biotech. 2011;22:406–14. doi: 10.1016/j.copbio.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Menzie C, Hoeppner S, Cura J, et al. Urban and suburban storm water runoff as a source of polycyclic aromatic hydrocarbons (PAHs) to Massachusetts estuarine and coastal environments. Estuaries. 2002;25:165–76. [Google Scholar]
- Meynet P, Head IM, Werner D, et al. Re-evaluation of dioxygenase gene phylogeny for the development and validation of a quantitative assay for environmental aromatic hydrocarbon degraders. FEMS Microbiol Ecol. 2015;91 doi: 10.1093/femsec/fiv049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelburg JJ, Nieuwenhuize J, Van Breugel P. Black carbon in marine sediments. Mar Chem. 1999;65:245–52. [Google Scholar]
- Militon C, Jézéquel R, Gilbert F, et al. Dynamics of bacterial assemblages and removal of polycyclic aromatic hydrocarbons in oil-contaminated coastal marine sediments subjected to contrasted oxygen regimes. Environ Sci Pollut R. 2015;22:15260–72. doi: 10.1007/s11356-015-4510-y. [DOI] [PubMed] [Google Scholar]
- Mille G, Guiliano M, Asia L, et al. Sources of hydrocarbons in sediments of the Bay of Fort de France (Martinique) Chemosphere. 2006;64:1062–73. doi: 10.1016/j.chemosphere.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Miller EC, Miller JA. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981;47:2327–45. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Misson B, Garnier C, Lauga B, et al. Chemical multi-contamination drives benthic prokaryotic diversity in the anthropized Toulon Bay. Sci Total Environ. 2016;556:319–29. doi: 10.1016/j.scitotenv.2016.02.038. [DOI] [PubMed] [Google Scholar]
- Motelay-Massei A, Ollivon D, Garban B, et al. Fluxes of polycyclic aromatic hydrocarbons in the Seine estuary, France: mass balance and role of atmospheric deposition. Hydrobiologia. 2007;588:145–57. [Google Scholar]
- Müller R. Bacterial degradation of xenobiotics. In: Fry JC, Gadd GM, Herbert RA, et al., editors. Microbial Control of Pollution. Vol. 48. Avon, UK: Cambridge University Press; 1992. pp. 35–57. [Google Scholar]
- Neff JM. Polycyclic aromatic hydrocarbons in the ocean. In: Neff JM, editor. Bioaccumulation in Marine Organisms. Oxford: Elsevier; 2002. pp. 241–318. [Google Scholar]
- Newell SE, Eveillard D, McCarthy MJ, et al. A shift in the archaeal nitrifier community in response to natural and anthropogenic disturbances in the northern Gulf of Mexico. Environ Microbiol Rep. 2014;6:106–12. doi: 10.1111/1758-2229.12114. [DOI] [PubMed] [Google Scholar]
- Newton A, Carruthers TJB, Icely J. The coastal syndromes and hotspots on the coast. Estuar Coast Shelf S. 2012;96:39–47. [Google Scholar]
- Nizzetto L, Gioia R, Li J, et al. Biological pump control of the fate and distribution of hydrophobic organic pollutants in water and plankton. Environ Sci Technol. 2012;46:3204–11. doi: 10.1021/es204176q. [DOI] [PubMed] [Google Scholar]
- Nizzetto L, Lohmann R, Gioia R, et al. PAHs in air and seawater along a North-South Atlantic transect: trends, processes and possible sources. Environ Sci Technol. 2008;42:1580–5. doi: 10.1021/es0717414. [DOI] [PubMed] [Google Scholar]
- Nogales B, Lanfranconi MP, Piña-Villalonga JM, et al. Anthropogenic perturbations in marine microbial communities. FEMS Microbiol Rev. 2011;35:275–98. doi: 10.1111/j.1574-6976.2010.00248.x. [DOI] [PubMed] [Google Scholar]
- Novakov T, Rosen H. The black carbon story: early history and new perspectives. Ambio. 2013;42:840–51. doi: 10.1007/s13280-013-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orcutt BN, Joye SB, Kleindienst S, et al. Impact of natural oil and higher hydrocarbons on microbial diversity, distribution, and activity in Gulf of Mexico cold-seep sediments. Deep-Sea Res Pt II. 2010;57:2008–21. [Google Scholar]
- Oros DR, Ross JRM. Polycyclic aromatic hydrocarbons in San Francisco Estuary sediments. Mar Chem. 2004;86:169–84. [Google Scholar]
- Paissé S, Coulon F, Goñi-Urriza M, et al. Structure of bacterial communities along a hydrocarbon contamination gradient in a coastal sediment. FEMS Microbiol Ecol. 2008;66:295–305. doi: 10.1111/j.1574-6941.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- Paissé S, Goñi-Urriza M, Stadler T, et al. Ring-hydroxylating dioxygenase (RHD) expression in a microbial community during the early response to oil pollution. FEMS Microbiol Ecol. 2012;80:77–86. doi: 10.1111/j.1574-6941.2011.01270.x. [DOI] [PubMed] [Google Scholar]
- Pan S, Kadokami K, Li X, et al. Target and screening analysis of 940 micro-pollutants in sediments in Tokyo Bay, Japan. Chemosphere. 2014;99:109–16. doi: 10.1016/j.chemosphere.2013.10.038. [DOI] [PubMed] [Google Scholar]
- Passow U, De La Rocha CL, Fairfield C, et al. Aggregation as a function of PCO2 and mineral particles. Limnol Oceanogr. 2014;59:532–47. [Google Scholar]
- Passow U, Ziervogel K, Asper V, et al. Marine snow formation in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ Res Lett. 2012;7:035301. [Google Scholar]
- Peng R-H, Xiong A-S, Xue Y, et al. Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev. 2008;32:927–55. doi: 10.1111/j.1574-6976.2008.00127.x. [DOI] [PubMed] [Google Scholar]
- Perra G, Renzi M, Guerranti C, et al. Polycyclic aromatic hydrocarbons pollution in sediments: distribution and sources in a lagoon system (Orbetello, Central Italy) Transitional Waters Bull. 2009;3:45–58. [Google Scholar]
- Pieper DH, Martins Dos Santos VAP, Golyshin PN. Genomic and mechanistic insights into the biodegradation of organic pollutants. Curr Opin Biotech. 2004;15:215–24. doi: 10.1016/j.copbio.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Prince RC. Oil spill dispersants: boon or bane? Environ Sci Technol 2015496376–84. [DOI] [PubMed] [Google Scholar]
- Prince RC, McFarlin KM, Butler JD, et al. The primary biodegradation of dispersed crude oil in the sea. Chemosphere. 2013;90:521–6. doi: 10.1016/j.chemosphere.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Rabus R, Boll M, Heider J, et al. Anaerobic microbial degradation of hydrocarbons: from enzymatic reactions to the environment. J Mol Microb Biotech. 2016;26:5–28. doi: 10.1159/000443997. [DOI] [PubMed] [Google Scholar]
- Reddy CM, Arey JS, Seewald JS, et al. Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill. P Natl Acad Sci USA. 2012;109:20229–34. doi: 10.1073/pnas.1101242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H, Howard JB. Formation of polycyclic aromatic hydrocarbons and their growth to soot-a review of chemical reaction pathways. Prog Energ Combust. 2000;26:565–608. [Google Scholar]
- Rivers AR, Sharma S, Tringe SG, et al. Transcriptional response of bathypelagic marine bacterioplankton to the Deepwater Horizon oil spill. ISME J. 2013;7:2315–29. doi: 10.1038/ismej.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DA. Causes and ecological effects of resuspended contaminated sediments (RCS) in marine environments. Environ Int. 2012;40:230–43. doi: 10.1016/j.envint.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Rodriguez-R LM, Overholt WA, Hagan C, et al. Microbial community successional patterns in beach sands impacted by the Deepwater Horizon oil spill. ISME J. 2015;9:1928–40. doi: 10.1038/ismej.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röling WFM, Couto De Brito IR, Swannell RPJ, et al. Response of archaeal communities in beach sediments to spilled oil and bioremediation. Appl Environ Microb. 2004;70:2614–20. doi: 10.1128/AEM.70.5.2614-2620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röling WFM, Van Bodegom PM. Toward quantitative understanding on microbial community structure and functioning: a modeling-centered approach using degradation of marine oil spills as example. Front Microbiol. 2014;5:125. doi: 10.3389/fmicb.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Mirazo K, Briones C, de la Escosura A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem Rev. 2014;114:285–366. doi: 10.1021/cr2004844. [DOI] [PubMed] [Google Scholar]
- Sabin LD, Maruya KA, Lao W, et al. Exchange of polycyclic aromatic hydrocarbons among the atmosphere, water, and sediment in coastal embayments of southern California, USA. Environ Toxicol Chem. 2010;29:265–74. doi: 10.1002/etc.54. [DOI] [PubMed] [Google Scholar]
- Safinowski M, Meckenstock RU. Methylation is the initial reaction in anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Environ Microbiol. 2006;8:347–52. doi: 10.1111/j.1462-2920.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- Salazar G, Cornejo-Castillo FM, Borrull E, et al. Particle-association lifestyle is a phylogenetically conserved trait in bathypelagic prokaryotes. Mol Ecol. 2015;24:5692–706. doi: 10.1111/mec.13419. [DOI] [PubMed] [Google Scholar]
- Sanni GO, Coulon F, McGenity TJ. Dynamics and distribution of bacterial and archaeal communities in oil-contaminated temperate coastal mudflat mesocosms. Environ Sci Pollut R. 2015;22:15230–47. doi: 10.1007/s11356-015-4313-1. [DOI] [PubMed] [Google Scholar]
- Santín C, Doerr SH, Kane ES, et al. Towards a global assessment of pyrogenic carbon from vegetation fires. Global Change Biol. 2016;22:76–91. doi: 10.1111/gcb.12985. [DOI] [PubMed] [Google Scholar]
- Sauret C, Christaki U, Moutsaki P, et al. Influence of pollution history on the response of coastal bacterial and nanoeukaryote communities to crude oil and biostimulation assays. Mar Environ Res. 2012;79:70–8. doi: 10.1016/j.marenvres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Sauret C, Tedetti M, Guigue C, et al. Influence of PAHs among other coastal environmental variables on total and PAH-degrading bacterial communities. Environ Sci Pollut R. 2015;23:4242–56. doi: 10.1007/s11356-015-4768-0. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, et al. Measurement of emissions from air pollution sources. 5. C1 - C32 organic compounds from gasoline-powered motor vehicles. Environ Sci Technol. 2002;36:1169–80. doi: 10.1021/es0108077. [DOI] [PubMed] [Google Scholar]
- Schellnhuber HJ. Tipping elements in the Earth System. P Natl Acad Sci USA. 2009;106:20561–3. doi: 10.1073/pnas.0911106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroot BM, Klaver GT, Schüttenhelm RTE. Surface and subsurface expressions of gas seepage to the seabed - Examples from the Southern North Sea. Mar Petrol Geol. 2005;22:499–515. [Google Scholar]
- Seo J-S, Keum Y-S, Li QX. Bacterial degradation of aromatic compounds. Int J Environ Res Public Health. 2009;6:278–309. doi: 10.3390/ijerph6010278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano A, Sánchez F, Preciado I, et al. Spatial and temporal changes in benthic communities of the Galician continental shelf after the Prestige oil spill. Mar Pollut Bull. 2006;53:315–31. doi: 10.1016/j.marpolbul.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Shrestha G, Traina SJ, Swanston CW. Black carbon's properties and role in the environment: a comprehensive review. Sustainability. 2010;2:294–320. [Google Scholar]
- Simoneit BRT. Biomass burning - A review of organic tracers for smoke from incomplete combustion. Appl Geochem. 2002;17:129–62. [Google Scholar]
- Singleton I. Microbial metabolism of xenobiotics - fundamental and applied research. J Chem Technol Biot. 1994;59:9–23. [Google Scholar]
- Sippula O, Stengel B, Sklorz M, et al. Particle emissions from a marine engine: chemical composition and aromatic emission profiles under various operating conditions. Environ Sci Technol. 2014;48:11721–9. doi: 10.1021/es502484z. [DOI] [PubMed] [Google Scholar]
- Socolofsky SA, Adams EE, Sherwood CR. Formation dynamics of subsurface hydrocarbon intrusions following the Deepwater Horizon blowout. Geophys Res Lett. 2011;38:L09602. [Google Scholar]
- Stauffert M, Cravo-Laureau C, Duran R. Dynamic of sulphate-reducing microorganisms in petroleum-contaminated marine sediments inhabited by the polychaete Hediste diversicolor. Environ Sci Pollut R. 2015a;22:15273–84. doi: 10.1007/s11356-014-3624-y. [DOI] [PubMed] [Google Scholar]
- Stauffert M, Cravo-Laureau C, Duran R. Structure of hydrocarbonoclastic nitrate-reducing bacterial communities in bioturbated coastal marine sediments. FEMS Microbiol Ecol. 2015b;89:580–93. doi: 10.1111/1574-6941.12359. [DOI] [PubMed] [Google Scholar]
- Stauffert M, Cravo-Laureau C, Jézéquel R, et al. Impact of oil on bacterial community structure in bioturbated sediments. PLoS One. 2013;8:e65347. doi: 10.1371/journal.pone.0065347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffert M, Duran R, Gassie C, et al. Response of archaeal communities to oil spill in bioturbated mudflat sediments. Microb Ecol. 2014;67:108–19. doi: 10.1007/s00248-013-0288-y. [DOI] [PubMed] [Google Scholar]
- Stogiannidis E, Laane R. Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. In: Whitacre DM, editor. Reviews of Environmental Contamination and Toxicology. Vol. 234. Switzerland: Springer International Publishing; 2015. pp. 49–133. [DOI] [PubMed] [Google Scholar]
- Stout SA, Payne JR, Emsbo-Mattingly SD, et al. Weathering of field-collected floating and stranded Macondo oils during and shortly after the Deepwater Horizon oil spill. Mar Pollut Bull. 2016;105:7–22. doi: 10.1016/j.marpolbul.2016.02.044. [DOI] [PubMed] [Google Scholar]
- Stubbins A, Niggemann J, Dittmar T. Photo-lability of deep ocean dissolved black carbon. Biogeosciences. 2012;9:1661–70. [Google Scholar]
- Taketani RG, Franco NO, Rosado AS, et al. Microbial community response to a simulated hydrocarbon spill in mangrove sediments. J Microbiol. 2010;48:7–15. doi: 10.1007/s12275-009-0147-1. [DOI] [PubMed] [Google Scholar]
- Tielens AG. Interstellar polycyclic aromatic hydrocarbon molecules. Annu Rev Astron Astr. 2008;46:289–337. [Google Scholar]
- Tobiszewski M, Namieśnik J. PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut. 2012;162:110–9. doi: 10.1016/j.envpol.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Todorova NH, Mironova RS, Karamfilov VK. Comparative molecular analysis of bacterial communities inhabiting pristine and polluted with polycyclic aromatic hydrocarbons Black Sea coastal sediments. Mar Pollut Bull. 2014;83:231–40. doi: 10.1016/j.marpolbul.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Tolosa I, de Mora S, Sheikholeslami MR, et al. Aliphatic and aromatic hydrocarbons in coastal Caspian Sea sediments. Mar Pollut Bull. 2004;48:44–60. doi: 10.1016/S0025-326X(03)00255-8. [DOI] [PubMed] [Google Scholar]
- Tsapakis M, Apostolaki M, Eisenreich S, et al. Atmospheric deposition and marine sedimentation fluxes of polycyclic aromatic hydrocarbons in the eastern Mediterranean basin. Environ Sci Technol. 2006;40:4922–7. doi: 10.1021/es060487x. [DOI] [PubMed] [Google Scholar]
- Turner JT. Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump. Prog Oceanogr. 2015;130:205–48. [Google Scholar]
- Turner RE, Overton EB, Meyer BM, et al. Distribution and recovery trajectory of Macondo (Mississippi Canyon 252) oil in Louisiana coastal wetlands. Mar Pollut Bull. 2014;87:57–67. doi: 10.1016/j.marpolbul.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Urakawa H, Garcia JC, Barreto PD, et al. A sensitive crude oil bioassay indicates that oil spills potentially induce a change of major nitrifying prokaryotes from the Archaea to the Bacteria. Environ Pollut. 2012;164:42–45. doi: 10.1016/j.envpol.2012.01.009. [DOI] [PubMed] [Google Scholar]
- US-EPA. List of the Sixteen PAHs with Highest Carcinogenic Effect. London: IEA Coal Research; 1984. [Google Scholar]
- Valentine DL, Kessler JD, Redmond MC, et al. Propane respiration jump-starts microbial response to a deep oil spill. Science. 2010;330:208–11. doi: 10.1126/science.1196830. [DOI] [PubMed] [Google Scholar]
- Vane CH, Harrison I, Kim AW. Polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in sediments from the Mersey Estuary, U.K. Sci Total Environ. 2007;374:112–26. doi: 10.1016/j.scitotenv.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Vila J, Tauler M, Grifoll M. Bacterial PAH degradation in marine and terrestrial habitats. Curr Opin Biotech. 2015;33:95–102. doi: 10.1016/j.copbio.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Viñas L, Angeles Franco M, Antonio Soriano J, et al. Sources and distribution of polycyclic aromatic hydrocarbons in sediments from the Spanish northern continental shelf. Assessment of spatial and temporal trends. Environ Pollut. 2010;158:1551–60. doi: 10.1016/j.envpol.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Vitte I, Duran R, Hernandez-Raquet G, et al. Dynamics of metabolically active bacterial communities involved in PAH and toxicity elimination from oil-contaminated sludge during anoxic/oxic oscillations. Appl Microbiol Biot. 2013;97:4199–211. doi: 10.1007/s00253-012-4219-5. [DOI] [PubMed] [Google Scholar]
- Vitte I, Duran R, Jézéquel R, et al. Effect of oxic/anoxic switches on bacterial communities and PAH biodegradation in an oil-contaminated sludge. Environ Sci Pollut R. 2011;18:1022–32. doi: 10.1007/s11356-010-0435-7. [DOI] [PubMed] [Google Scholar]
- Vonk SM, Hollander DJ, Murk ATJ. Was the extreme and wide-spread marine oil-snow sedimentation and flocculent accumulation (MOSSFA) event during the Deepwater Horizon blow-out unique? Mar Pollut Bull. 2015;100:5–12. doi: 10.1016/j.marpolbul.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Wade TL, Sericano JL, Sweet ST, et al. Spatial and temporal distribution of water column total polycyclic aromatic hydrocarbons (PAH) and total petroleum hydrocarbons (TPH) from the Deepwater Horizon (Macondo) incident. Mar Pollut Bull. 2016;103:286–93. doi: 10.1016/j.marpolbul.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Wade TL, Sweet ST, Klein AG. Assessment of sediment contamination in Casco Bay, Maine, USA. Environ Pollut. 2008;152:505–21. doi: 10.1016/j.envpol.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Wagner S, Jaffé R. Effect of photodegradation on molecular size distribution and quality of dissolved black carbon. Org Geochem. 2015;86:1–4. [Google Scholar]
- Wang J-Z, Guan Y-F, Ni H-G, et al. Polycyclic aromatic hydrocarbons in riverine runoff of the Pearl River Delta (China): concentrations, fluxes, and fate. Environ Sci Technol. 2007;41:5614–9. doi: 10.1021/es070964r. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu L, Zheng B, et al. Analysis of the bacterial community in the two typical intertidal sediments of Bohai Bay, China by pyrosequencing. Mar Pollut Bull. 2013;72:181–7. doi: 10.1016/j.marpolbul.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Wang M, Wang C, Hu X, et al. Distributions and sources of petroleum, aliphatic hydrocarbons and polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Bohai Bay and its adjacent river, China. Mar Pollut Bull. 2015;90:88–94. doi: 10.1016/j.marpolbul.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu Z, Xu K, et al. Concentrations and sources of polycyclic aromatic hydrocarbons in surface coastal sediments of the northern Gulf of Mexico. Geochem T. 2014;15:1–12. doi: 10.1186/1467-4866-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL. An overview of immunotoxicology and carcinogenic polycyclic aromatic hydrocarbons. Environ Carcin R. 1986;4:163–202. [Google Scholar]
- Widdel F, Rabus R. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Curr Opin Biotech. 2001;12:259–76. doi: 10.1016/s0958-1669(00)00209-3. [DOI] [PubMed] [Google Scholar]
- Wilcock RJ, Corban GA, Northcott GL, et al. Persistence of polycyclic aromatic compounds of different molecular size and water solubility in surficial sediment of an intertidal sandflat. Environ Toxicol Chem. 1996;15:670–6. [Google Scholar]
- Wu P, Wang YS, Sun FL, et al. Bacterial polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenases in the sediments from the Pearl River estuary, China. Appl Microbiol Biot. 2014;98:875–84. doi: 10.1007/s00253-013-4854-5. [DOI] [PubMed] [Google Scholar]
- Wurl O, Obbard JP. A review of pollutants in the sea-surface microlayer (SML): a unique habitat for marine organisms. Mar Pollut Bull. 2004;48:1016–30. doi: 10.1016/j.marpolbul.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Xia X, Xia N, Lai Y, et al. Response of PAH-degrading genes to PAH bioavailability in the overlying water, suspended sediment, and deposited sediment of the Yangtze River. Chemosphere. 2015;128:236–44. doi: 10.1016/j.chemosphere.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Yergeau E, Lawrence JR, Sanschagrin S, et al. Next-generation sequencing of microbial communities in the Athabasca River and its tributaries in relation to oil sands mining activities. Appl Environ Microb. 2012;78:7626–37. doi: 10.1128/AEM.02036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yergeau E, Maynard C, Sanschagrin S, et al. Microbial community composition, functions, and activities in the gulf of mexico 1 year after the deepwater horizon accident. Appl Environ Microb. 2015;81:5855–66. doi: 10.1128/AEM.01470-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, John GF, Hayworth JS, et al. Long-term monitoring data to describe the fate of polycyclic aromatic hydrocarbons in Deepwater Horizon oil submerged off Alabama's beaches. Sci Total Environ. 2015;508:46–56. doi: 10.1016/j.scitotenv.2014.10.105. [DOI] [PubMed] [Google Scholar]
- Yu W, Liu R, Wang J, et al. Source apportionment of PAHs in surface sediments using positive matrix factorization combined with GIS for the estuarine area of the Yangtze River, China. Chemosphere. 2015;134:263–71. doi: 10.1016/j.chemosphere.2015.04.049. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang F, Zhang T-C. Sedimentary records of PAHs in a sediment core from tidal flat of Haizhou Bay, China. Sci Total Environ. 2013;450–451:280–8. doi: 10.1016/j.scitotenv.2013.02.029. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Guo L, Shiller AM, et al. Characterization of oil components from the Deepwater Horizon oil spill in the Gulf of Mexico using fluorescence EEM and PARAFAC techniques. Mar Chem. 2013;148:10–21. [Google Scholar]
- Ziervogel K, Joye SB, Arnosti C. Microbial enzymatic activity and secondary production in sediments affected by the sedimentation pulse following the Deepwater Horizon oil spill. Deep-Sea Res Pt II. 2016;129:241–8. [Google Scholar]
- Ziolkowski LA, Druffel ERM. Aged black carbon identified in marine dissolved organic carbon. Geophys Res Lett. 2010;37:L16601. [Google Scholar]