Visual symptoms are frequently reported in Parkinson’s disease. Weil et al.. relate visual changes to underlying brain regions, and consider mechanisms for visual hallucinations. They examine links between visual changes and other features of Parkinson’s disease and discuss the role of visual dysfunction as a marker of dementia.
Keywords: vision, perception, Parkinson’s disease, cognition
Visual symptoms are frequently reported in Parkinson’s disease. Weil et al.. relate visual changes to underlying brain regions, and consider mechanisms for visual hallucinations. They examine links between visual changes and other features of Parkinson’s disease and discuss the role of visual dysfunction as a marker of dementia.
Abstract
Patients with Parkinson’s disease have a number of specific visual disturbances. These include changes in colour vision and contrast sensitivity and difficulties with complex visual tasks such as mental rotation and emotion recognition. We review changes in visual function at each stage of visual processing from retinal deficits, including contrast sensitivity and colour vision deficits to higher cortical processing impairments such as object and motion processing and neglect. We consider changes in visual function in patients with common Parkinson’s disease-associated genetic mutations including GBA and LRRK2. We discuss the association between visual deficits and clinical features of Parkinson’s disease such as rapid eye movement sleep behavioural disorder and the postural instability and gait disorder phenotype. We review the link between abnormal visual function and visual hallucinations, considering current models for mechanisms of visual hallucinations. Finally, we discuss the role of visuo-perceptual testing as a biomarker of disease and predictor of dementia in Parkinson’s disease.
Introduction
The contribution of non-motor symptoms to a reduced quality of life in Parkinson’s disease is now widely recognized. In addition to complaints attributable to autonomic, gastrointestinal and cognitive dysfunction, visual symptoms are frequently reported (Davidsdottir et al., 2005). Although many groups have demonstrated changes in visual perception in Parkinson’s disease, until now, most of these have focused on changes attributed to ophthalmic pathology. Here, we review the changes in visual perception in Parkinson’s disease under four separate sections. First we examine the evidence for visual deficits from early sensory discrimination to higher visual dysfunction. Second, we consider the neurobiology underlying visual changes including location of deficits and associated genetic mutations. Third, we examine the link between visual dysfunction and other clinical manifestations such as visual hallucinations and consider current theories of visual hallucinations. Finally we consider the role for testing visual perceptual function in early detection of dementia in Parkinson’s disease.
The scope of the problem of vision in Parkinson’s disease
Patients with Parkinson’s disease frequently report problems with visual tasks, such as navigating around everyday environments and using maps (Bowen et al., 1972; McDowell and Harris, 1997b; Davidsdottir et al., 2005). In questionnaire studies, 78% of patients with Parkinson’s disease report at least one visual symptom, including difficulty reading, sometimes with double vision, and misjudging objects and distances (Archibald et al., 2011; Urwyler et al., 2014). Visual hallucinations are also common in Parkinson’s disease, with a reported prevalence as high as 74% after 20 years of disease (Fenelon et al., 2000; Hely et al., 2008). The underlying mechanisms are still not well understood, despite multiple proposed mechanisms (Barnes et al., 2003; Collerton et al., 2005; Diederich et al., 2014; Shine et al., 2014). A deeper understanding of changes in visual perception in Parkinson’s disease will greatly enhance our ability to manage visual hallucinations. Visuo-perceptual problems are well described in dementia with Lewy bodies and in Parkinson’s disease dementia, but there is growing evidence pointing to changes in visual processing earlier in the disease course. Here we examine changes in visual processing that are seen in patients with Parkinson’s disease and no other cognitive deficits. The studies cited here involve patients in the mid-stages of Parkinson’s disease at an average Hoehn and Yahr stage of 2 while on their regular Parkinson’s drugs, unless stated otherwise.
Evidence for changes in visual processing
Ophthalmic visual processing: visual acuity
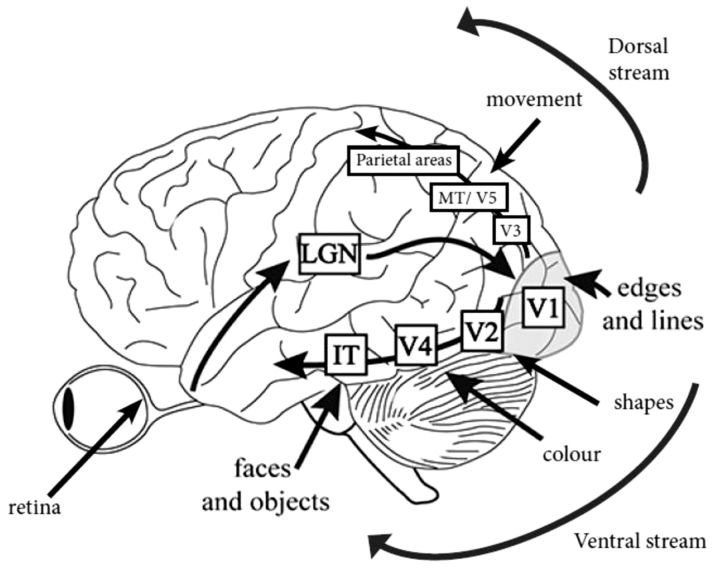
Visual acuity is the ability to resolve the details of a stimulus and is affected by ophthalmic factors, including retinal deficits, rather than cortical disease. See Fig. 1 for an overview of the functional anatomy of healthy vision. Visual acuity may be impaired in Parkinson’s disease (Jones et al., 1992; Archibald et al., 2011), and this effect is not corrected by dopamine. Higher scores on parkinsonian motor examination may predict poor visual acuity (Archibald et al., 2011). However, the differences are small (Jones et al., 1992) and are not seen in early stage untreated patients (Biousse et al., 2004) or in some studies involving mid-stage patients (Regan and Maxner, 1987), although in many studies acuity is regarded as normal if it is 6/9 or better (Bodis-Wollner et al., 1987) or is considered on a case-by-case basis, which may miss subtle group differences in acuity.
Figure 1.
Functional anatomy of healthy human vision. Information passes from the retina, via the optic nerve and optic tract to the lateral geniculate nucleus (LGN) in the thalamus. From there, signals project via the optic radiation to the primary visual cortex (V1). Cells in V1 process simple local attributes such as the orientation of lines and edges. From primary visual cortex, information is organized as two parallel hierarchical processing streams: the ventral stream identifies features of objects and passes from V1 through areas V2 and V4 to the inferior temporal cortex. The dorsal stream processes spatial relations between objects and projects through areas V2 and V3 to the superior temporal and parietal cortices. Almost all connections between regions are reciprocal, with further feedback projections from areas outside this pathway. IT = infero-temporal region; MT/V5 = motion processing region. Adapted from Manassi et al. (2013) with permission (copyright held by the Association for Research in Vision and Ophthalmology).
Contrast sensitivity
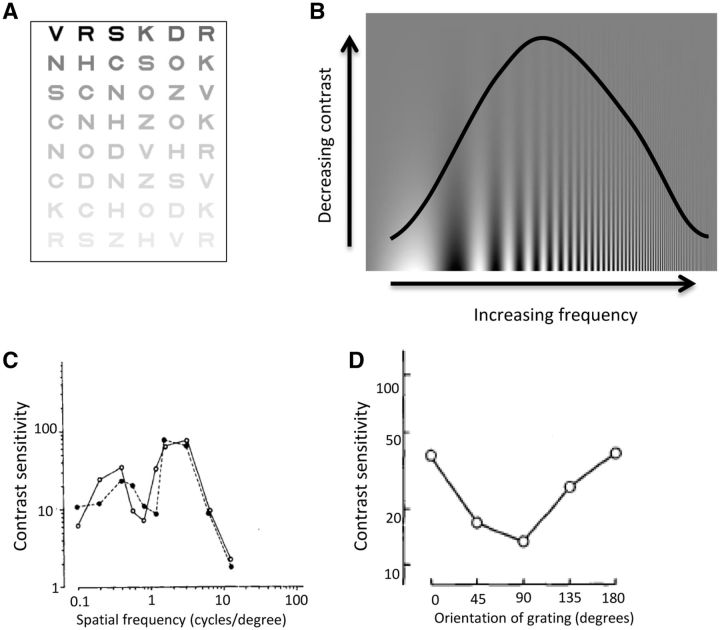
Contrast sensitivity is the ability to discriminate an object from its background and is affected by lesions in the eye (including retina) as well as in thalamic or cortical locations. In tests using vision charts with letters of the same size but reducing contrast, patients with Parkinson’s disease have normal acuity but impaired contrast detection (Regan and Neima, 1984) (Fig. 2A and B). With more quantitative measurements, patients with Parkinson’s disease show loss of contrast sensitivity across a range of spatial frequencies and in both foveal (central) and peripheral locations (Silva et al., 2005). Contrast sensitivity loss is partly reversible with l-DOPA treatment, in both early (Bulens et al., 1987) and mid-stage Parkinson’s disease (Bodis-Wollner et al., 1987) but it eventually deteriorates again as the disease advances (Diederich et al., 2002).
Figure 2.
Measuring visual function: visual contrast sensitivity. (A) Pelli-Robson Chart for measuring contrast sensitivity. Letters are of the same size but reducing contrast (Pelli et al., 1988). (B) A Campbell-Robson grating, showing increasing spatial frequency plotted against decreasing contrast. Visibility is indicated by the inverted U-shaped curve, with maximal visibility at mid-range spatial frequency, although individuals vary in their precise point of visibility (Campbell and Robson, 1968). (C) Loss of contrast sensitivity at the middle range of spatial frequencies in Parkinson’s disease. Example contrast sensitivity curve for a patient with Parkinson’s disease (each line represents one eye). Adapted from Bulens et al. (1986) with permission. (D) Effect of orientation of visual grating on contrast sensitivity in Parkinson’s disease. Contrast sensitivity for perception of gratings at orientations ranging from vertical (0 degrees), through horizontal (90 degrees) and back to vertical (180 degrees). Reduced contrast sensitivity is seen for horizontal gratings. Adapted from Regan and Maxner (1987) with permission.
Colour vision
In the healthy brain, colour is processed by photoreceptor cones in the retina and at higher levels from primary visual cortex (V1) to extra-striate visual cortex (V4). Disordered colour discrimination is recognized in Parkinson’s disease (Price et al., 1992; Buttner et al., 1995), even in the early stages of the disease, in untreated patients (Buttner et al., 1995). Abnormalities in colour vision have also been correlated with axial motor symptoms (Oh et al., 2011), with motor speed (in untreated patients) (Muller et al., 1999) and with disease duration (Price et al., 1992). Colour vision deficits progress over time in both treated and untreated patients (Diederich et al., 2002; Muller et al., 2002). Colour discrimination may be confounded by cognitive or motor deficits in some studies (Regan et al., 1998), due to the length of time needed to administer the widely used Farnsworth-Munsell 100 Hue test. This test requires patients to arrange 100 coloured discs in sequence. Colour discrimination, as measured in this way, correlates with cognitive performance and with white matter changes in posterior brain structures (Bertrand et al., 2012). However, abnormalities in colour vision can also be found using tests that are less susceptible to these confounds (Silva et al., 2005). Colour loss, when measured in this way, is distinct from normal ageing and seems to particularly affect the protan and deutan (red–green) axes (Silva et al., 2005), although other investigators have found greatest deficits in the tritan (blue–yellow) axis (Haug et al., 1995; Regan et al., 1998). It is unclear whether colour vision abnormalities in Parkinson’s disease reflect retinal or cortical disease, but given that differences can be seen along several axes (Silva et al., 2005), it is likely that changes in colour sensitivity are multifactorial.
Are visual abnormalities in Parkinson’s disease due to retinal dysfunction?
Deficits in visual acuity, contrast and colour in Parkinson’s disease are at least partly due to retinal dopamine deficiency. The healthy human retina contains dopaminergic amacrine cells (Frederick et al., 1982) and patients with Parkinson’s disease have reduced dopamine innervation around the fovea (Nguyen-Legros, 1988). Autopsy studies show decreased retinal dopamine concentrations in Parkinson’s disease (Harnois and Di, 1990). The inner retinal layer is thinner in patients with Parkinson’s disease, measured using optical coherence tomography (Hajee et al., 2009). Retinal electrical activity is decreased compared to healthy controls, measured by electroretinogram (Tagliati et al., 1996; Moschos et al., 2011) and improves with l-DOPA treatment (Peppe et al., 1995). Intriguingly, patients with Parkinson’s disease have shorter durations of negative afterimages than healthy controls (Khadjevand et al., 2010), a process thought to be mediated in health by retinal dopaminergic neuromodulation (Wink and Harris, 2000). In some patients, contrast sensitivity thresholds and visual evoked potential latencies differ between eyes (Bulens et al., 1986, 1987; Bodis-Wollner et al., 1987), raising the likelihood of prechiasmal involvement. The recent finding in the inner retinal layer of misfolded α-synuclein (Bodis-Wollner et al., 2014a) and of phosphorylated α-synuclein (Beach et al., 2014), the pathological hallmark of Parkinson’s disease, is striking evidence of retinal involvement in Parkinson’s disease. At least some aspects of visual dysfunction in Parkinson’s disease therefore involve disruption of processing at the level of the retina.
Some untreated patients also show loss of contrast sensitivity at specific intermediate spatial frequencies (Fig. 2C) (Bulens et al., 1986). This is more suggestive of a cortical rather than retinal or geniculate site of disruption as cortical neurons show stronger selectivity for different spatial frequencies than neurons in the retina or thalamus (Campbell and Robson, 1968; De Valois et al., 1982; Regan, 1982). In addition to spatial frequency selectivity, contrast sensitivity in Parkinson’s disease is often orientation-specific, with greatest deficits for horizontal gratings (Regan and Maxner, 1987; Bulens et al., 1988; Trick et al., 1994) (Fig. 2D). As receptive fields of neurons in primary visual cortex are tuned to specific orientations (Hubel et al., 1978), these findings implicate primary visual cortex as the locus for deficits in contrast sensitivity in Parkinson’s disease, rather than just the retina or the thalamus, which are not linked to orientation specificity.
Changes in eye movements
Subtle oculomotor changes can be seen in Parkinson’s disease, with voluntary saccades particularly affected. Latency and velocity are preserved but amplitude is reduced, producing hypometric movements (Ventre et al., 1992; Kimmig et al., 2002; MacAskill et al., 2002). Saccades to a remembered target are particularly impaired and show a multistep pattern (Crawford et al., 1989). These are thought to arise from deficits in oculomotor pathways in the brainstem, cerebellum, basal ganglia and frontal lobes. In addition, patients with Parkinson’s disease commonly show convergence insufficiency, that impacts on near activities and may lead to double vision on reading (Almer et al., 2012; Nowacka et al., 2014). Pronounced oculomotor abnormalities, when present, usually indicate an atypical Parkinsonian syndrome, but supranuclear vertical gaze impairment has rarely been reported in pathologically confirmed Parkinson’s disease. Early decreased saccadic velocity, preservation of saccadic latency and paresis of vertical saccades favour a diagnosis of progressive supranuclear gaze palsy (Bhidayasiri et al., 2001). Cerebellar type eye movement abnormalities, such as gaze-evoked nystagmus, abnormal vestibulo-ocular-reflex suppression, down-beat nystagmus, and excessive square wave jerks are more suggestive of multiple system atrophy (for a review see Pinkhardt and Kassubek, 2011).
Visual processing in early cortical visual areas: line orientation, pattern perception and depth perception
The earliest stages of cortical visual processing involve discrimination of local attributes. Neurons in primary visual cortex (V1) are sensitive to the orientation of lines and edges (Fig. 1) and changes in visual acuity and contrast sensitivity in patients with Parkinson’s disease, may be caused by deficits in early cortical visual processing. In tests comparing the ability to distinguish between lines of differing directions (Benton et al., 1978), several studies showed impaired performance in patients with Parkinson’s disease (Montse et al., 2001; Uc et al., 2005; Gullett et al., 2013) that correlated with duration and severity of disease. Although primary visual cortex is sensitive to changes in line orientation, judgements of matching lines may also involve higher order visuospatial processing. Indeed, in lesion studies of non-parkinsonian patients, failure in orientation judgements is most strongly associated with parietal lobe lesions (Tranel et al., 2009).
Pattern perception and figure ground discrimination involve a combination of earlier visual cortical processing and higher level interpretation. In tasks requiring completion of patterns, patients with Parkinson’s disease perform worse than healthy controls (Flowers and Robertson, 1995). Similarly, patients performing a figure–ground discrimination task (identifying figures embedded in more complex figures) made more errors than healthy controls (especially those with more advanced disease) (Flowers and Robertson, 1995). In neuroimaging studies, patients with worse performance in similar tasks also have reduced grey matter density in their superior parietal lobes (Pereira et al., 2009).
Depth perception is also affected in patients with Parkinson’s disease and is associated with worse motor dexterity and decreased colour perception (Sun et al., 2014). Untreated Parkinson’s patients with impaired depth perception have been shown, in neuroimaging studies, to have reduced grey matter volume in visual association areas (Koh et al., 2013).
Peripheral vision
Information from peripheral visual fields is processed at multiple levels from the retina to higher cortical regions. When patients are given a visual task, and presented with distracting images in their peripheral fields at the same time, the irrelevant peripheral objects interfere with the main task to a much greater extent than they do for healthy volunteers (McDowell and Harris, 1997a). Conversely, although patients with Parkinson’s disease are more distracted by targets shown in the periphery, they have greater difficulty discriminating details of peripheral images and perceive these images less strongly than healthy volunteers (Harris et al., 1992; Sampaio et al., 2011). A combination of reduced contrast sensitivity in the periphery with greater distraction from peripheral objects may provide a perceptual explanation for the common phenomenon of freezing in doorways and crowded surroundings seen in the later stages of Parkinson’s disease in many patients (McDowell and Harris, 1997a).
Object perception
In the healthy brain, object processing occurs in V2, V4 and the lateral occipital complex, especially in the right hemisphere (Grill-Spector et al., 1999; Kourtzi and Kanwisher, 2000; Large et al., 2007). Patients with early stage Parkinson’s disease are as good at recognizing common objects from silhouettes as healthy controls (Hipp et al., 2014). However, by mid-stage, they start to have difficulties in identifying overlapping objects (Pillon et al., 1989; Mosimann et al., 2004), with worse performance occurring in patients with left-sided motor symptoms (Levin et al., 1991). This is consistent with right hemisphere predominance in object recognition and suggests asymmetry in cortical as well as nigral involvement.
Mental rotation is another aspect of object recognition that may be affected in Parkinson’s disease. In a previous small study, patients with Parkinson’s disease made more errors when matching objects in 3D space (Lee et al., 1998). Greater differences were seen at larger angular discrepancies, when objects were more rotated, suggesting that the abnormality is caused by changes in the mental ability to rotate objects, rather than simply due to differences in pattern matching. Although error rates were not increased when objects were rotated in 2D space, responses were slower, suggesting some impaired performance for 2D rotation. A recent large study found impaired performance in 2D rotation in patients with early stage Parkinson’s disease, with even greater impairments in patients carrying the H1 MAPT haplotype (microtubule-associated protein tau), particularly in more difficult forms of the task (Nombela et al., 2014) (see below for further discussion on genetic associations).
Visuospatial construction
Patients with Parkinson’s disease make more errors when copying and recalling complex figures than healthy controls (Ogden et al., 1990; Uc et al., 2005). Copying intersecting pentagons is frequently used as a simple bedside measure of visuospatial function in Parkinson’s disease. It is quick to perform and is a component of the Mini-Mental State Examination (Williams-Gray et al., 2013; Lawson et al., 2014). Longitudinal studies show that it has value in predicting the development of dementia in Parkinson’s disease. In a recent large cross-sectional study, patients with Parkinson’s disease showed slightly lower scores for copying intersecting pentagons than healthy controls (Garcia-Diaz et al., 2014), with a correlation between performance in pentagon copying and reduced cortical thickness in parieto-temporal regions. However, no difference was seen by another group using a less detailed scoring system and fewer patients (Filoteo et al., 2014). This highlights the relatively low sensitivity of this test in assessing visuospatial performance. In clock drawing tests, patients with Parkinson’s disease (and no dementia) make more errors than healthy controls (Dal et al., 1989; Stella et al., 2007; Saur et al., 2012) and errors in drawing houses are also reported in Parkinson’s disease (Kulkarni et al., 2013). However, all these tests lack specificity for visuospatial function as they involve several cognitive domains in addition to visuospatial processing, including praxis, memory and executive function.
Motion perception
Parkinson’s disease impairs the perception of motion, which is strongly linked with the middle temporal area MT/V5 (Zeki et al., 1991; Tootell et al., 1995) (Fig. 1). Patients have greater difficulty in detecting a moving grating than a static one, which is a reversal of the normal pattern where motion of a grating is detected before it can be identified (Mestre et al., 1990; Haug et al., 1994). Patients with Parkinson’s disease also have higher thresholds than healthy controls for detecting the direction of motion (Trick et al., 1994), although in a speed discrimination task, patients with Parkinson’s disease performed no worse than controls (Mosimann et al., 2004). Finally, in a structure from motion task, where volunteers detect an object that is formed from the contrast of moving dots, patients with Parkinson’s disease show higher thresholds for correctly identifying objects (Uc et al., 2005).
Spatial neglect
Patients with Parkinson’s disease predominantly affecting their left side show a rightward bias when bisecting a horizontal line. (Lee et al., 2001b; Laudate et al., 2013). This reflects spatial neglect on the left, ipsilateral to the side affected by motor symptoms, and suggests involvement of right parietal cortex. Line bisection bias is not seen in patients with right-sided Parkinson’s disease. Similarly, in line cancellation tasks, patients with left-sided (but not right-sided) Parkinson’s disease show impaired line cancellation (Villardita et al., 1983). In a related phenomenon, patients with left sided Parkinson’s disease perceive objects within their left visual field as smaller than on the right (Harris et al., 2003) and experience a reduced visual representation of doorways (Lee et al., 2001a). This perceptual difference may contribute mechanistically to the phenomenon of freezing in doorways. In eye movement analyses, when searching for a target, patients with left-sided Parkinson’s disease will explore the right hemifield first, in contrast with healthy controls and patients with right-sided Parkinson’s disease, who show a leftward bias (Ebersbach et al., 1996). Similarly, patients with left-sided Parkinson’s disease show longer latencies for saccades to left-sided targets compared to the right (Ventre et al., 1992). Whether differences in spatial representation are caused by eye movement abnormalities, or eye movements reflect the differences in perception could be tested in future studies.
Face and emotion recognition
The ability to recognize faces is impaired in Parkinson’s disease (Levin et al., 1991), with performance correlated with grey matter density in the fusiform face area, the region involved in face recognition in the heathy brain (Pereira et al., 2009). There is particular difficulty in interpreting facial expressions (Sprengelmeyer et al., 2003), with greater impairment for negative emotions including disgust (Suzuki et al., 2006), sadness (Hipp et al., 2014) and fear (Jacobs et al., 1995; Dujardin et al., 2004; Clark et al., 2008; Gray and Tickle-Degnen, 2010). This greater deficit for emotional face processing in Parkinson’s disease may be explained by the presence of two distinct face processing pathways that are sensitive to different spatial frequency ranges: face identification, associated with the fusiform face area, is more activated by high spatial frequency ranges (detailed visual information). In contrast, subcortical pathways, including the amygdala, are activated by fearful faces, and are driven by coarse, low spatial frequency information (Vuilleumier et al., 2003). The finding that performance improves in patients treated with l-DOPA is consistent with the involvement of dopaminergic neurons in this pathway (Sprengelmeyer et al., 2003).
Neurobiology underlying visual changes: genetic basis for heterogeneity
Important insights into the heterogeneity of Parkinson’s disease have emerged with the discovery in recent years of genetic mutations that cause or predispose to Parkinson’s disease, some of which are associated with defined molecular pathways. Visual perceptual dysfunction associated with specific mutations may therefore implicate potential pathophysiological mechanisms. Patients carrying LRRK2 mutations have better colour discrimination (Marras et al., 2011) and fewer cognitive deficits than patients with idiopathic Parkinson’s disease (Alcalay et al., 2013) (Table 1). Parkinson’s patients who carry PARK2 mutations, which are associated with dysfunction in the mitochondrial system and have a more restricted distribution of neuropathology, perform better in cognitive tests than patients with sporadic Parkinson’s disease (Alcalay et al., 2014).
Table 1.
Associations between features linked with visual dysfunction and common Parkinson’s disease-associated genetic mutations
| Potential pathway | Gene | Performance compared with idiopathic Parkinson’s disease | Frequency compared with idiopathic Parkinson’s disease | ||
|---|---|---|---|---|---|
| Colour vision | Cognitive performance | Visual hallucinations | RBD | ||
| Multiple implicated | LRRK2 | Increaseda | Increasedb | Decreasedc | Decreasedd |
| Mitochondrial | PARK2 | Increasede | Increasedf | Not known | Similarg |
| Lysosomal | GBA | Possibly increasedh | Decreasedi | Increasedj | Increasedk |
| Lysosomal | SNCA | Not known | Decreasedl,m | Increasedl,m | Increasedl,n |
aMarras et al., 2011; bAlcalay et al., 2013; cSomme et al., 2015; dEhrminger et al., 2015; eKertelge et al., 2010; fAlcalay et al., 2014; gSixel-Doring et al., 2015; hSimon-Tov et al., 2015; iAlcalay et al., 2012; jNeumann et al., 2009; kGan-Or et al., 2015; lKonno et al., 2016; mPetrucci et al., 2016; nNishioka et al., 2009.
Conversely, patients with Parkinson’s disease that carry mutations in the gene encoding the lysosomal enzyme glucocerebrosidase (GBA) show deficits in visuospatial tasks (Alcalay et al., 2012), have impaired visual memory (Zokaei et al., 2014) and show a higher frequency of visual hallucinations (Neumann et al., 2009; Wang et al., 2014) than patients with idiopathic Parkinson’s disease. Intriguingly, patients with GBA mutations have decreased cerebral blood flow in the parietal lobe and precuneus (Goker-Alpan et al., 2012), regions involved in visual perception (Cavanna and Trimble, 2006) and in visual memory (Kochan et al., 2011). Patients with Parkinson’s disease that carry GBA mutations also show a higher frequency of clinical features that are often associated with visual dysfunction in Parkinson’s disease: they have a higher risk of cognitive deficits (Neumann et al., 2009; Brockmann et al., 2011; Winder-Rhodes et al., 2013) and rapid eye movement (REM) sleep disorder than patients with idiopathic Parkinson’s disease (Beavan et al., 2015; Gan-Or et al., 2015) (see below for further discussion of clinical features associated with visual dysfunction in Parkinson’s disease).
Patients with Parkinson’s disease carrying mutations in the gene for synuclein, SNCA, also show higher rates of cognitive deficits, REM sleep behaviour disorder (RBD) and hallucinations (Nishioka et al., 2009; Konno et al., 2016; Petrucci et al., 2016). Neuropathological studies of these patients show a greater α-synuclein burden in the cerebral cortex than in idiopathic Parkinson’s disease (Ikeuchi et al., 2008; Obi et al., 2008) and fluorodeoxyglucose (FDG) signal is reduced in the occipital lobes (visual processing regions) of patients carrying SNCA mutations compared with controls (Nishioka et al., 2009). Both the GBA and SNCA genes are linked with the lysosomal pathway (Mazzulli et al., 2011), suggesting the visual deficits associated with these genes may share a pathophysiological mechanism, with lysosomal dysfunction associated with cortical visual dysfunction.
Genetic polymorphisms may also play a role in predisposition to cognitive impairment in Parkinson’s disease. A common polymorphism in MAPT has been linked to dementia in Parkinson’s disease (Goris et al., 2007) and patients carrying the H1 MAPT polymorphism make more errors in difficult spatial rotation tasks, and show reduced parietal cortex activity (Nombela et al., 2014). The underlying mechanism for these findings may relate to differences in the cortical expression of 4- versus 3-repeat isoforms of tau (Williams-Gray et al., 2009). Greater understanding of these genetic differences between individuals will be crucial in future studies seeking to define the explanations for the diversity in visuospatial function found in Parkinson’s disease
Clinical relevance
Clustering of symptoms with visual dysfunction in Parkinson’s disease
There is an increasing acknowledgement of the clinical heterogeneity of idiopathic Parkinson’s disease (Selikhova et al., 2009; Halliday et al., 2011; Sieber et al., 2014) and differences in survival rates have been reported between clinical subtypes defined in longitudinal analysis (de Lau et al., 2014). Visual dysfunction frequently co-exists with cognitive impairment, visual hallucinations, postural instability with gait disorder and RBD (Davidsdottir et al., 2005; Marques et al., 2010). Two distinct neuropsychological syndromes have been proposed in Parkinson’s disease: a frontal-striatal dopamine-mediated dysexecutive syndrome that does not progress to dementia; and a second form with prominent visuospatial and sematic fluency impairments that is more frequently associated with decline to dementia. Therefore, recognizing visuospatial impairment in the context of cognitive deterioration may have importance as a prognostic marker for dementia in Parkinson’s disease. (Williams-Gray et al., 2009; Kehagia et al., 2010).
Relation with sleep abnormalities
Idiopathic RBD, characterized by loss of normal atonia during REM sleep, is considered a risk factor for Parkinson’s disease and other synucleinopathies: over 80% of idiopathic RBD patients may eventually develop a neurodegenerative disorder linked to α-synuclein accumulation in the brain (Iranzo et al., 2013; Schenck et al., 2013). Visuo-perceptual deficits have been reported in patients with RBD (who have not yet developed Parkinson’s disease), with impairments in colour vision and visuospatial construction (Boeve et al., 1998; Ferini-Strambi et al., 2004; Postuma et al., 2009; Manni et al., 2013). Those RBD patients with sensory abnormalities at baseline (including colour vision defects) have also been claimed to develop a form of Parkinson’s disease with more prominent cognitive involvement (Postuma et al., 2011). Later, at mid-stage, this same association, RBD and poor colour discrimination, is again linked with a more rapid and aggressive disease course (Fereshtehnejad et al., 2015). Although RBD is a rare prodromal sign in patients presenting with motor symptoms, up to a third of patients develop RBD in the course of their illness (Gagnon et al., 2002; Manni et al., 2010). When it occurs, it is associated with visuo-perceptual dysfunction, including errors in object recognition (Marques et al., 2010) and complex figure copying (Vendette et al., 2007). The presence of RBD in Parkinson’s disease is also predictive of progressive cognitive dysfunction (Sinforiani et al., 2008; Postuma et al., 2012; Nomura et al., 2013).
The recent discovery of a novel photoreceptor system in the retina may provide a further link between sleep and visual dysfunction in Parkinson’s disease. A subset of retinal ganglion cells, known as melanopsin photoreceptors, are believed to play a role in regulating circadian rhythms. Dysfunction of these retinal ganglion cells, possibly by α-synuclein deposition, or by a change in dopamine levels, causes unopposed melatonin production, with subsequent effects on sleep (for a review see Schmoll et al., 2011). Furthermore, the projection of these cells to brain regions involved in circadian and sleep functions as well as to visual areas such as the lateral geniculate nucleus may explain some of the daytime-dependent (not just luminance-dependent) visual symptoms of Parkinson’s disease (La Morgia et al., 2011).
Gait, postural instability and tremor
Visual perceptual deficits are frequently associated with the postural instability and gait disorder phenotype (PIGD). Visual abnormalities are more common in patients with freezing of gait (Davidsdottir et al., 2005) and correlate with the severity of gait impairment (Uc et al., 2005). Furthermore, visual contrast sensitivity predicts severity of freezing, independent of duration or severity of disease (Davidsdottir et al., 2005). In patients with freezing of gait, step length reduces on going through narrow doorways, suggesting a perceptual mechanism interfering with movement planning (Almeida and Lebold, 2010; Cowie et al., 2010).
Conversely, the tremor predominant phenotype is less associated with visual deficits. In questionnaire studies, patients without tremor report worse visual function (Seichepine et al., 2011). Patients with increased tremor perform better on tasks of line orientation, mental reconstruction and rotation tasks (Levin et al., 1991) and in longitudinal follow-up, presence of tremor is associated with a more benign course and fewer colour vision abnormalities (Fereshtehnejad et al., 2015).
The link between visual perceptual deficits and gait abnormalities may be partly explained by the critical role of vision in balance (Day and Guerraz, 2007). Patients with Parkinson’s disease show a higher dependence on visual information for motor and postural control (Bronstein et al., 1990). This may have a therapeutic role as it has been shown that visual cueing can improve walking in Parkinson’s disease (Stern et al., 1980; Azulay et al., 1999; Lewis et al., 2000). However, the association of visual deficits with the akinetic rigid phenotype and relative sparing of visual dysfunction in the tremor predominant phenotype may also reflect distinct pathophysiological patterns of disease.
Hallucinations: links with visuo-perceptual deficits
Visual hallucinations are common in Parkinson’s disease, with a prevalence of 30% (Holroyd et al., 2001). Their presence is believed to be highly specific for Lewy body pathology (Williams et al., 2008), and can be helpful in distinguishing between Parkinson’s disease and other parkinsonian syndromes such as multiple system atrophy (MSA-P) and progressive supranuclear palsy (PSP-P) (Williams and Lees, 2005). Visual hallucinations often occur in poor lighting and are associated with reduced visual acuity (Holroyd et al., 2001; Matsui et al., 2006; Archibald et al., 2011) but can also be seen in patients with normal visual acuity (Diederich et al., 1998; Biousse et al., 2004). They are also associated with impaired contrast sensitivity and colour vision (Diederich et al., 1998) and patients with visual hallucinations show greater deficits in object and face identification compared to those without hallucinations (Barnes et al., 2003; Ramirez-Ruiz et al., 2006; Gallagher et al., 2011), reflecting involvement of higher cortical visual dysfunction. Recent reports of minor visual hallucinations in patients at the earliest stages of Parkinson’s disease (Pagonabarraga et al., 2016), including in untreated patients, lends further support for changes in cortical visual processing even at the initial stages of Parkinson’s disease.
Mechanistic considerations of minor hallucinations
‘Passage hallucinations’ are illusions of objects, commonly insects and small animals, moving across the peripheries of vision (Fenelon et al., 2000). The combination of being more sensitive to irrelevant peripheral objects (McDowell and Harris, 1997a), and less able to discriminate details in the periphery could explain this phenomenon. Misinterpretations, or illusions, where innocuous objects such as piles of clothes are misidentified as animals, are also commonly reported in Parkinson’s disease. These may be generated by similar mechanisms of increased sensitivity but reduced discriminatory ability for objects in peripheral vision.
The phenomenon of blindsight might also help to explain minor illusions in Parkinson’s disease (Diederich et al., 2014). Blindsight is the syndrome of retained visual abilities in patients with bilateral occipital damage who can accurately guess locations of objects in space despite the absence of conscious visual perception. It is mediated via alternate visual pathways from the retina, involving the thalamus, colliculus and amygdala and is a form of unconscious processing of visual information. It has been suggested that these alternate pathways are damaged in Parkinson’s disease, rendering patients ‘blind to blindsight’ (Diederich et al., 2014). According to the model, patients with Parkinson’s disease have difficulty guessing locations in peripheral space, which may lead to the erroneous experience of objects in the lateral field. This conceptual framework may explain minor illusions but it cannot account for complex visual hallucinations that arise in the absence of any stimulus and at the point of fixation.
Mechanistic considerations of complex hallucinations
The complex visual hallucinations reported in Parkinson’s disease almost invariably involve animate objects, are often at fixation and are more frequently experienced in dim light. They are usually non-frightening and occasionally enjoyed, but become increasingly distressing over time (for reviews see Fenelon et al., 2000; Diederich et al., 2009; Collerton et al., 2015). Previous models to explain these hallucinations include cortical irritation caused by electrical over-activity in brain regions containing images (Levine and Finklestein, 1982). However, there is no evidence for cortical irritation in Parkinson’s disease and complex hallucinations are not strongly associated with diseases where cortical irritation is a feature, such as epilepsy.
A more attractive model considers hallucinations as ‘cortical release’ phenomena (Ffytche and Howard, 1999). In this model, that has also been used to explain the hallucinations seen in Charles Bonnet syndrome, stimulus-driven bottom-up visual processing is inhibitory in nature and when absent, this releases spontaneous activity. Visual hallucinations therefore arise from a lack of sensory input that leads to the release of stored images. However, loss of visual input alone, from eye disease or stroke, only infrequently leads to visual hallucinations and the visual deficits seen in Parkinson’s disease are not profound enough to cause absent sensory input.
Collerton and colleagues (2005) have proposed a Perception and Attention Deficit model based on proto-objects that are part of normal scene perception. These are object templates from higher visual processing regions projected to lower visual regions during normal scene perception. In health, visual input activates several competing proto-objects but attentional binding allows only one object to be perceived. During hallucinations, impaired sensory input causes activation of an incorrect proto-object. This, combined with impaired attentional binding, causes it to be incorrectly projected onto the intact visual scene. Although the combination of early perceptual deficits with higher attentional networks is likely to be important in generating visual hallucinations, this model has limitations. It cannot adequately explain the appearance of incongruent objects during hallucinations. It also implies that properly perceived objects should displace hallucinatory objects, causing the hallucination to disappear, which does not occur [see commentary by Morrison and David (2005) on Collerton et al., 2005)].
The Activation-Input-Modulation model proposed by Hobson, is another hypothesis (Hobson et al., 2000; Diederich et al., 2005). The first dimension, activation, relates to level of alertness; the second is input, or sensory information. The third dimension, modulation, is the integration of the first two dimensions over time and invokes aminergic and cholinergic transmission. According to this model, movement along each dimension increases the likelihood for visual hallucinations. In this way, aberrant visual processing increases the propensity for visual hallucinations, as would changes in arousal levels. The modulation dimension is affected by pharmacological influences. One weakness of the model is that it fails to explain how visual hallucinations that are initially benign can become increasingly distressing with loss of insight.
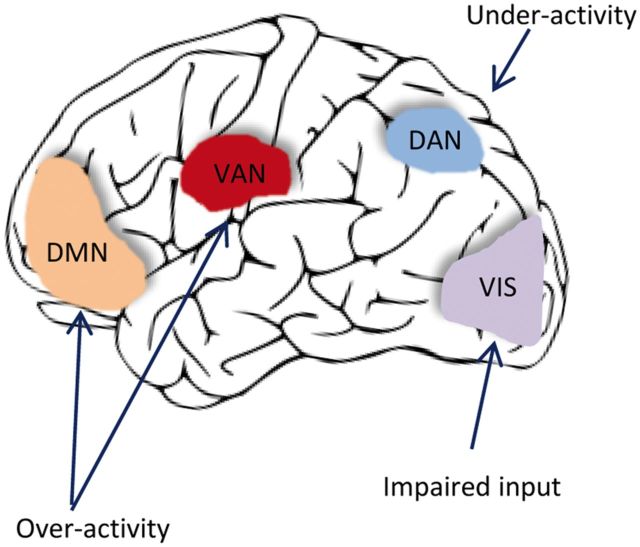
Visual hallucinations in Parkinson’s disease have also been claimed to be due to breakdown in the connectivity in the neural networks subserving attention and conscious perception (Shine et al., 2014) (Fig. 3). These comprise the Dorsal Attention Network (DAN), the Ventral Attention Network (VAN) and the Default Mode Network (DMN). The DAN is activated during tasks requiring exogenous attention. The DMN is associated with internally focused tasks, e.g. mind wandering, whereas the VAN is used for salience monitoring and mediates the switch between the DAN and the DMN. The attentional networks hypothesis predicts that visual hallucinations in Parkinson’s disease are caused by overactivity of the DMN and VAN reinforcing false images that are then unchecked due to failure to engage the DAN. This leads to over reliance on the DMN and VAN that are poorly suited to interpret ambiguous sensory images.
Figure 3.
Theories for visual hallucinations in Parkinson’s disease. The attention networks hypothesis for visual hallucinations in Parkinson’s disease (Shine et al., 2014). The DAN (involving dorsolateral prefrontal cortex and posterior parietal cortex) is engaged in voluntary orienting; the VAN (involving the lateral and inferior prefrontal cortex and amygdala) engages attention to salient stimuli and mediates activation of other networks; the DMN (involving the medial temporal and medial prefrontal cortex) is engaged in task independent introspective tasks); VIS = visual cortex, processing of visual information. In this model, visual hallucinations are caused by over-reliance on the DMN and the VAN in processing ambiguous percepts, with relative inability to recruit the DAN. Adapted from Shine et al. (2015) with permission.
Change in visual perceptual function seems at least necessary, if not sufficient, for complex hallucinations. Visual dysfunction is more common in Parkinson’s disease patients experiencing hallucinations (Gallagher et al., 2011) and in neuroimaging studies, glucose consumption is reduced in occipito-temporal regions in Parkinson’s patients with visual hallucinations compared to those without (Boecker et al., 2007). Executive dysfunction may also have a role in mediating visual hallucinations: Parkinson’s patients with hallucinations perform worse on tests of executive function compared to those without visual hallucinations (Grossi et al., 2005; Barnes and Boubert, 2008; Shin et al., 2012) and higher cortical Lewy body density is seen in the middle frontal gyrus in patients with visual hallucinations (Harding et al., 2002; Papapetropoulos et al., 2006). Structural neuroimaging shows grey matter atrophy in the medial frontal lobe in Parkinson’s disease patients with visual hallucinations compared to those without (Ramirez-Ruiz et al., 2007; Goldman et al., 2014) as well as in visual processing regions. Hallucinations are difficult to capture using functional neuroimaging due to their transient nature, but a recent study has shown increased activation in frontal regions and decreased activation in visual cortex (Goetz et al., 2014), although no correction for multiple comparisons was reported, making the data difficult to interpret. The prevailing models could provide a framework for the conjunction of visual perceptual deficits with frontal executive dysfunction seen in patients with Parkinson’s disease who experience complex hallucinations.
Drug modulation of visual symptoms
Parkinson’s drugs can improve ophthalmic visual processing. l-DOPA enhances colour vision and contrast sensitivity in Parkinson’s disease (Bulens et al., 1987; Buttner et al., 1994) and abnormal visual evoked potentials in patients with Parkinson’s disease improve with l-DOPA (Bodis-Wollner and Yahr, 1978), supporting a dopaminergic basis for these deficits in the lower visual pathways. On the other hand, the effects of l-DOPA on higher visual function are less well documented. Emotional face recognition improves with l-DOPA (Sprengelmeyer et al., 2003), but effects of l-DOPA or other Parkinson’s drugs, on other aspects of cortical visual processing are not known.
The effects of Parkinsonian drugs on visual hallucinations associated with Parkinson’s disease are also not fully established. Early reports correlated the incidence of visual hallucinations with dose and duration of treatment with l-DOPA (Sweet et al., 1976; Weiner et al., 1980). However, recent reports are more equivocal. Some groups found no difference in l-DOPA dose or duration between patients with Parkinson’s disease with and without hallucinations (Diederich et al., 1998). Off period visual hallucinations are a recognized, if rare, phenomenon in l-DOPA-treated patients with motor fluctuations (Steiger et al., 1991). Others found that while patients with hallucinations were on a slightly higher daily dose of l-DOPA, their levodopa equivalent dose was no different (Fenelon et al., 2000). A recent study reported that a higher proportion of patients with visual hallucinations were taking l-DOPA (Gallagher et al., 2011) and no association was found with other parkinsonian medications. However, double-blind controlled trials with dopamine agonists consistently show a higher rate of hallucinations than on placebo or l-DOPA (Poewe, 2003; Baker et al., 2009; Bonuccelli et al., 2009; Kulisevsky and Pagonabarraga, 2010; Zhou et al., 2014). The conventional approach to treatment of visual hallucinations reflects this by slowly withdrawing dopamine agonists (Connolly and Lang, 2014). Anticholinergic drugs are a well-recognized cause of visual hallucinations and delirium in elderly patients with Parkinson’s disease (Goetz et al., 1982). Amantadine is also hallucinogenic and both drugs should be withdrawn when hallucinations occur (Postma and Van, 1975).
Impact on driving
There is a relation between contrast sensitivity and driving performance in Parkinson’s disease and between visual perception and driving ability, particularly in tests measuring visual attention, motion perception and visuospatial construction (Uc et al., 2006, 2009; Devos et al., 2013; for a review see Crizzle et al., 2012). The postural instability and gait disorder phenotype is predictive of poorer on-road driving performance, beyond motor severity scores (Devos et al., 2013), consistent with the association of visuo-perceptual deficits with this phenotype. Although visuo-perceptual tests are useful predictors of driving ability, there is insufficient evidence for them to be used alone to determine driver safety. General advice is to consider visuo-perceptual ability and if there is any doubt, to refer the patient for a formal driving assessment via the national driving authority (Crizzle et al., 2012).
The role of visuo-perceptual changes as a marker for disease
Visuo-perceptual measures as diagnostic markers of Parkinson’s disease
Changes in visual performance may be a sensitive marker of Parkinson’s disease. In one study, deficits in colour and contrast sensitivity were shown to have better discriminatory power for early diagnosis of Parkinson’s disease than any other non-motor symptom, including hyposmia and sleep disturbance, in patients within 3 years of diagnosis (Diederich et al., 2010). One theory of disease progression in Parkinson’s disease has suggested that the cerebral cortex is not damaged until the later stages of the disease (Braak et al., 2004). Although the exact pathological correlates of colour and contrast sensitivity changes are unclear, changes in visual perception at the earliest stages of the disease raise concerns about this notion. It is likely that visual measures will be a useful marker of early disease only in specific subgroups of patients with Parkinson’s disease (such as those with prominent early cognitive involvement). Visuo-perceptual deficits (unlike oculomotor changes) are rarely reported in atypical forms of parkinsonism such as progressive supranuclear palsy, multiple system atrophy or corticobasal syndrome. Visual processing abnormalities may prove helpful in differentiating these other diseases from idiopathic Parkinson’s disease.
Visuo-perceptual measures as predictors of dementia in Parkinson’s disease
Visual function may be important in predicting dementia in Parkinson’s disease. In a prospective study, abnormal colour vision at baseline tripled the odds of developing dementia (Anang et al., 2014). In newly diagnosed patients with Parkinson’s disease, impaired pentagon copying at diagnosis was a strong predictor for development of dementia at 5- (Williams-Gray et al., 2009) and 10-year follow-up (Williams-Gray et al., 2013) and pentagon copying deficits show power to predict dementia even at 2-year follow-up (Kaul and Elble, 2014). Similarly, patients with new multi-domain dementia at 3-year follow-up showed impaired performance in an overlapping figures test at baseline (Shoji et al., 2014). These findings are supported by functional neuroimaging findings: in a longitudinal FDG-PET study of Parkinson’s disease, patients who developed dementia, showed occipito-parietal hypometabolism at baseline (Bohnen et al., 2011). However, pentagon copying involves multiple cognitive domains including executive function, praxis and motor planning areas, in addition to visuo-perceptual processes, making it difficult to make confident claims regarding the role for visual perception in dementia prediction.
Visual symptoms may also predict outcome. Visual hallucinations are strong predictors for nursing home placement (Goetz and Stebbins, 1993) and have been suggested as a harbinger for the onset of dementia in Parkinson’s disease (Santangelo et al., 2007). In nursing home residents with Parkinson’s disease, the presence of visual problems is a predictor of mortality (with age, cognitive impairment and pressure ulcers also predictive) (Fernandez and Lapane, 2002). Identifying the presence of visuo-perceptual deficits may therefore have a critical role in stratifying patients in the clinic for early treatment with cholinesterase inhibitors and for defining enriched populations for disease-modifying interventions in clinical trials aimed at preventing dementia in Parkinson’s disease.
Optical coherence tomography for measuring disease presence and progression
Optical coherence tomography enables high resolution structural imaging of the retina, with measurement of all retinal layers, in vivo. Recent technical advances have led to increasing interest in the role of the retina in monitoring disease activity in Parkinson’s disease (Bodis-Wollner et al., 2014b; Lee et al., 2014a). The nerve fibre layer is thinned in Parkinson’s disease (Inzelberg et al., 2004; Altintas et al., 2008) and at the macula, thinning is found in the inner retinal layer (Hajee et al., 2009; Shrier et al., 2012). Interocular differences are seen in retinal thickness in Parkinson’s disease (Shrier et al., 2012) with more thinning contralateral to the side of motor symptoms (La Morgia et al., 2013), consistent with an asymmetric process involving the eye on the same side as the affected substantia nigra. Recently, retinal morphology, measured using optical coherence tomography, combined with visual electrophysiology was shown to have high diagnostic yield in Parkinson’s disease (Miri et al., 2016). A correlation between retinal thickness, disease severity (Altintas et al., 2008; Miri et al., 2015) and duration (Miri et al., 2015; Sari et al., 2015) has been reported in some but not all studies (La Morgia et al., 2013). In Parkinson’s disease dementia, there is a correlation between retinal thickness and Mini-Mental State Examination scores (Moreno-Ramos et al., 2013), although this is not found in patients without dementia (Garcia-Martin et al., 2012). Whether this reflects insensitivity of the Mini-Mental State Examination, or an absence of relationship between dementia and retinal thickness is not yet known. Intriguingly, retinal thinning is associated with presence of minor hallucinations (Lee et al., 2014b), although this was not found for patients with complex visual hallucinations, consistent with the involvement of higher cognitive domains in these hallucinations. Optical coherence tomography therefore shows promise as a potential biomarker for presence of disease in Parkinson’s disease, but more studies will be needed to assess the role of the retina in predicting dementia in Parkinson’s disease.
Neuroimaging signatures of visual dysfunction as a marker of disease in Parkinson’s disease
Structural and functional neuroimaging studies are consistent with early involvement of cortical visual processing regions in Parkinson’s disease. Cortical thinning in the occipital cortex, measured using MRI, correlates with disease duration (Jubault et al., 2011), with maximal cortical thinning seen in the lateral occipital complex and parietal regions (Pereira et al., 2012). Metabolic deficits have been reported using PET in occipital cortex (Eberling et al., 1994; Bohnen et al., 1999) and occipital and parietal hypoperfusion in Parkinson’s disease can be detected by measuring regional cerebral blood flow (Abe et al., 2003), that worsens with disease progression (Nagamachi et al., 2008). Arterial spin labelled perfusion MRI, a non-invasive measure of perfusion, similarly shows regions of perfusion deficit in early stages of Parkinson’s disease affecting frontal, parietal and also occipital brain regions (Fernandez-Seara et al., 2012).
Recently, network approaches have been applied to structural and functional MRI data in Parkinson’s disease. These reveal changes in patterns of connectivity between brain regions and strongly implicate early involvement of visual processing regions in Parkinson’s disease. Reduced connectivity is seen in temporo-occipital regions, using resting state functional MRI in patients within 3 years of diagnosis, with connectivity losses strongly correlated with reduced visuospatial performance (Luo et al., 2015). In patients at mid-stage Parkinson’s disease, resting state functional MRI network analysis shows connectivity changes especially affecting visual regions (Gottlich et al., 2013). In Parkinson’s disease patients with mild cognitive impairment, the efficiency of connections in parietal, as well as frontal regions is reduced (Pereira et al., 2015), consistent with involvement of visuo-perceptual processing brain regions in the early stages of dementia in Parkinson’s disease.
Conclusion and future directions
Alterations in visual function from the retina to higher cortical brain regions have been found in Parkinson’s disease, with some aspects of visuo-perceptual processing worsening with disease progression (Diederich et al., 2002; Gullett et al., 2013) and there are emerging data showing impaired pentagon copying as one of the earliest cognitive deficits in Parkinson’s disease. There are, however, no studies of the power of other markers of visuo-perpetual function to predict dementia in Parkinson’s disease. Further studies examining visuo-perceptual function in Parkinson’s disease are required to correlate performance with other disease features such as laterality of motor symptoms and with clinical phenotypes such as postural instability or tremor predominance. Visuo-perceptual performance also needs to be correlated with common Parkinson’s disease-associated genetic mutations to gain insights into pathophysiological pathways underlying deficits; and with neuroimaging to link visuo-perceptual impairment with anatomical regions. These future combined approaches should enhance understanding of the mechanisms underlying progression of Parkinson’s disease to involve cognition.
Funding
R.S.W. is supported by a UCL Excellence Fellowship, a grant from the Academy of Medical Sciences and received funding from the department of health’s NIHR Biomedical Research Centre funding scheme (UCL/UCLH). J.D.W. is supported by a Wellcome Senior Clinical Fellowship: grant no. 091673/Z/10/Z. S.J.C. is supported by grants from ESRC/NIHR (ES/L001810/1), EPSRC (EP/M006093/1) and an Alzheimer’s Research UK Senior Research Fellowship. H.R.M. has been supported by Parkinson’s UK (grants 8047, J-0804) and the Medical Research Council (G0700943)
Glossary
Abbreviations
- DAN
dorsal attention network
- DMN
default mode network
- RBD
REM sleep behaviour disorder
- REM
rapid eye movement
- V1
primary visual cortex
- VAN
ventral attention network
References
- Abe Y, Kachi T, Kato T, Arahata Y, Yamada T, Washimi Y, et al. Occipital hypoperfusion in Parkinson's disease without dementia: correlation to impaired cortical visual processing. J Neurol Neurosurg Psychiatry 2003; 74: 419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalay RN, Caccappolo E, Mejia-Santana H, Tang M, Rosado L, Orbe RM, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology 2012; 78: 1434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Orbe RM, et al. Cognitive and motor function in long-duration PARKIN-associated Parkinson disease. JAMA Neurol 2014; 71: 62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalay RN, Mirelman A, Saunders-Pullman R, Tang MX, Mejia SH, Raymond D, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord 2013; 28: 1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida QJ, Lebold CA. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry 2010; 81: 513–18. [DOI] [PubMed] [Google Scholar]
- Almer Z, Klein KS, Marsh L, Gerstenhaber M, Repka MX. Ocular motor and sensory function in Parkinson's disease. Ophthalmology 2012; 119: 178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintas O, Iseri P, Ozkan B, Caglar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson's disease. Doc Ophthalmol 2008; 116: 137–46. [DOI] [PubMed] [Google Scholar]
- Anang JB, Gagnon JF, Bertrand JA, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014; 83: 1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Visual symptoms in Parkinson's disease and Parkinson's disease dementia. Mov Disord 2011; 26: 2387–95. [DOI] [PubMed] [Google Scholar]
- Azulay JP, Mesure S, Amblard B, Blin O, Sangla I, Pouget J. Visual control of locomotion in Parkinson's disease. Brain 1999; 122 (Pt 1): 111–20. [DOI] [PubMed] [Google Scholar]
- Baker WL, Silver D, White CM, Kluger J, Aberle J, Patel AA, et al. Dopamine agonists in the treatment of early Parkinson's disease: a meta-analysis. Parkinsonism Relat Disord 2009; 15: 287–94. [DOI] [PubMed] [Google Scholar]
- Barnes J, Boubert L. Executive functions are impaired in patients with Parkinson's disease with visual hallucinations. J Neurol Neurosurg Psychiatry 2008; 79: 190–92. [DOI] [PubMed] [Google Scholar]
- Barnes J, Boubert L, Harris J, Lee A, David AS. Reality monitoring and visual hallucinations in Parkinson's disease. Neuropsychologia 2003; 41: 565–74. [DOI] [PubMed] [Google Scholar]
- Beach TG, Carew J, Serrano G, Adler CH, Shill HA, Sue LI, et al. Phosphorylated alpha-synuclein-immunoreactive retinal neuronal elements in Parkinson's disease subjects. Neurosci Lett 2014; 571: 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol 2015; 72: 201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Varney NR, Hamsher KD. Visuospatial judgment: a clinical test. Arch Neurol 1978; 35: 364–7. [DOI] [PubMed] [Google Scholar]
- Bertrand JA, Bedetti C, Postuma RB, Monchi O, Genier MD, Jubault T, et al. Color discrimination deficits in Parkinson's disease are related to cognitive impairment and white-matter alterations. Mov Disord 2012; 27: 1781–8. [DOI] [PubMed] [Google Scholar]
- Bhidayasiri R, Riley DE, Somers JT, Lerner AJ, Buttner-Ennever JA, Leigh RJ. Pathophysiology of slow vertical saccades in progressive supranuclear palsy. Neurology 2001; 57: 2070–7. [DOI] [PubMed] [Google Scholar]
- Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C, Newman NJ. Ophthalmologic features of Parkinson's disease. Neurology 2004; 62: 177–80. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Kozlowski PB, Glazman S, Miri S. alpha-synuclein in the inner retina in parkinson disease. Ann Neurol 2014a; 75: 964–6. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Marx MS, Mitra S, Bobak P, Mylin L, Yahr M. Visual dysfunction in Parkinson's disease. Loss in spatiotemporal contrast sensitivity. Brain 1987; 110 (Pt 6): 1675–98. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Miri S, Glazman S. Venturing into the no-man's land of the retina in Parkinson's disease. Mov Disord 2014b; 29: 15–22. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I, Yahr MD. Measurements of visual evoked potentials in Parkinson's disease. Brain 1978; 101: 661–71. [DOI] [PubMed] [Google Scholar]
- Boecker H, Ceballos-Baumann AO, Volk D, Conrad B, Forstl H, Haussermann P. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol 2007; 64: 984–8. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ, Kokmen E, Smith GE, Ivnik RJ, et al. REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease. Neurology 1998; 51: 363–70. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Koeppe RA, Minoshima S, Giordani B, Albin RL, Frey KA, et al. Cerebral glucose metabolic features of Parkinson disease and incident dementia: longitudinal study. J Nucl Med 2011; 52: 848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Minoshima S, Giordani B, Frey KA, Kuhl DE. Motor correlates of occipital glucose hypometabolism in Parkinson's disease without dementia. Neurology 1999; 52: 541–6. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Del DP, Rascol O. Role of dopamine receptor agonists in the treatment of early Parkinson's disease. Parkinsonism Relat Disord 2009; 15 (Suppl 4): S44–53. [DOI] [PubMed] [Google Scholar]
- Bowen FP, Hoehn MM, Yahr MD. Parkinsonism: alterations in spatial orientation as determined by a route-walking test. Neuropsychologia 1972; 10: 355–61. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del TK. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res 2004; 318: 121–34. [DOI] [PubMed] [Google Scholar]
- Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, Gasser T, et al. GBA-associated PD presents with nonmotor characteristics. Neurology 2011; 77: 276–80. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Hood JD, Gresty MA, Panagi C. Visual control of balance in cerebellar and parkinsonian syndromes. Brain 1990; 113 (Pt 3): 767–79. [DOI] [PubMed] [Google Scholar]
- Bulens C, Meerwaldt JD, Van der Wildt GJ. Effect of stimulus orientation on contrast sensitivity in Parkinson's disease. Neurology 1988; 38: 76–81. [DOI] [PubMed] [Google Scholar]
- Bulens C, Meerwaldt JD, Van der Wildt GJ, Keemink CJ. Contrast sensitivity in Parkinson's disease. Neurology 1986; 36: 1121–5. [DOI] [PubMed] [Google Scholar]
- Bulens C, Meerwaldt JD, Van der Wildt GJ, Van Deursen JB. Effect of levodopa treatment on contrast sensitivity in Parkinson's disease. Ann Neurol 1987; 22: 365–9. [DOI] [PubMed] [Google Scholar]
- Buttner T, Kuhn W, Muller T, Patzold T, Heidbrink K, Przuntek H. Distorted color discrimination in ‘de novo' Parkinsonian patients. Neurology 1995; 45: 386–7. [DOI] [PubMed] [Google Scholar]
- Buttner T, Kuhn W, Patzold T, Przuntek H. L-Dopa improves colour vision in Parkinson's disease. J Neural Transm Park Dis Dement Sect 1994; 7: 13–19. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol 1968; 197: 551–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006; 129: 564–83. [DOI] [PubMed] [Google Scholar]
- Clark US, Neargarder S, Cronin-Golomb A. Specific impairments in the recognition of emotional facial expressions in Parkinson's disease. Neuropsychologia 2008; 46: 2300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collerton D, Mosimann UP, Perry E. The neuroscience of visual hallucinations. Oxford: Wiley Blackwell; 2015. [Google Scholar]
- Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci 2005; 28: 737–57. [DOI] [PubMed] [Google Scholar]
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014; 311: 1670–83. [DOI] [PubMed] [Google Scholar]
- Cowie D, Limousin P, Peters A, Day BL. Insights into the neural control of locomotion from walking through doorways in Parkinson's disease. Neuropsychologia 2010; 48: 2750–7. [DOI] [PubMed] [Google Scholar]
- Crawford TJ, Henderson L, Kennard C. Abnormalities of nonvisually-guided eye movements in Parkinson's disease. Brain 1989; 112 (Pt 6): 1573–86. [DOI] [PubMed] [Google Scholar]
- Crizzle AM, Classen S, Uc EY. Parkinson disease and driving: an evidence-based review. Neurology 2012; 79: 2067–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal PG, Stern Y, Sano M, Mayeux R. Clock-drawing in neurological disorders. Behav Neurol 1989; 2: 39–48. [DOI] [PubMed] [Google Scholar]
- Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res 2005; 45: 1285–96. [DOI] [PubMed] [Google Scholar]
- Day BL, Guerraz M. Feedforward versus feedback modulation of human vestibular evoked balance responses by visual self-motion information. J Physiol 2007; 582: 153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LM, Verbaan D, van Rooden SM, Marinus J, van Hilten JJ. Relation of clinical subtypes in Parkinson's disease with survival. Mov Disord 2014; 29: 150–1. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Albrecht DG, Thorell LG. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res 1982; 22: 545–59. [DOI] [PubMed] [Google Scholar]
- Devos H, Vandenberghe W, Tant M, Akinwuntan AE, De WW, Nieuwboer A, Uc EY. Driving and off-road impairments underlying failure on road testing in Parkinson's disease. Mov Disord 2013; 28: 1949–56. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Fenelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol 2009; 5: 331–42. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG, Raman R, Pappert EJ, Leurgans S, Piery V. Poor visual discrimination and visual hallucinations in Parkinson's disease. Clin Neuropharmacol 1998; 21: 289–95. [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson's disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord 2005; 20: 130–40. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Pieri V, Hipp G, Rufra O, Blyth S, Vaillant M. Discriminative power of different nonmotor signs in early Parkinson's disease: a case-control study. Mov Disord 2010; 25: 882–7. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Raman R, Leurgans S, Goetz CG. Progressive worsening of spatial and chromatic processing deficits in Parkinson disease. Arch Neurol 2002; 59: 1249–52. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Stebbins G, Schiltz C, Goetz CG. Are patients with Parkinson's disease blind to blindsight? Brain 2014; 137: 1838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin K, Blairy S, Defebvre L, Duhem S, Noel Y, Hess U, et al. Deficits in decoding emotional facial expressions in Parkinson's disease. Neuropsychologia 2004; 42: 239–50. [DOI] [PubMed] [Google Scholar]
- Eberling JL, Richardson BC, Reed BR, Wolfe N, Jagust WJ. Cortical glucose metabolism in Parkinson's disease without dementia. Neurobiol Aging 1994; 15: 329–35. [DOI] [PubMed] [Google Scholar]
- Ebersbach G, Trottenberg T, Hattig H, Schelosky L, Schrag A, Poewe W. Directional bias of initial visual exploration: a symptom of neglect in Parkinson's disease. Brain 1996; 119 (Pt 1): 79–87. [DOI] [PubMed] [Google Scholar]
- Ehrminger M, Leu-Semenescu S, Cormier F, Corvol JC, Vidailhet M, Debellemaniere E, et al. Sleep aspects on video-polysomnography in LRRK2 mutation carriers. Mov Disord 2015; 30: 1839–43. [DOI] [PubMed] [Google Scholar]
- Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 2000; 123 (Pt 4): 733–45. [DOI] [PubMed] [Google Scholar]
- Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New Clinical subtypes of parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 2015; 72: 863–73. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Di Gioia MR, Castronovo V, Oldani A, Zucconi M, Cappa SF. Neuropsychological assessment in idiopathic REM sleep behavior disorder (RBD): does the idiopathic form of RBD really exist? Neurology 2004; 62: 41–5. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Lapane KL. Predictors of mortality among nursing home residents with a diagnosis of Parkinson's disease. Med Sci Monit 2002; 8: CR241–6. [PubMed] [Google Scholar]
- Fernandez-Seara MA, Mengual E, Vidorreta M, Aznarez-Sanado M, Loayza FR, Villagra F, et al. Cortical hypoperfusion in Parkinson's disease assessed using arterial spin labeled perfusion MRI. Neuroimage 2012; 59: 2743–50. [DOI] [PubMed] [Google Scholar]
- Ffytche DH, Howard RJ. The perceptual consequences of visual loss: ‘positive' pathologies of vision. Brain 1999; 122 (Pt 7): 1247–60. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Reed JD, Litvan I, Harrington DL. Volumetric correlates of cognitive functioning in nondemented patients with Parkinson's disease. Mov Disord 2014; 29: 360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers KA, Robertson C. Perceptual abnormalities in Parkinson's disease: top-down or bottom-up processes? Perception 1995; 24: 1201–21. [DOI] [PubMed] [Google Scholar]
- Frederick JM, Rayborn ME, Laties AM, Lam DM, Hollyfield JG. Dopaminergic neurons in the human retina. J Comp Neurol 1982; 210: 65–79. [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompre S, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson's disease. Neurology 2002; 59: 585–9. [DOI] [PubMed] [Google Scholar]
- Gallagher DA, Parkkinen L, O'Sullivan SS, Spratt A, Shah A, Davey CC, et al. Testing an aetiological model of visual hallucinations in Parkinson's disease. Brain 2011; 134: 3299–309. [DOI] [PubMed] [Google Scholar]
- Gan-Or Z, Mirelman A, Postuma RB, Arnulf I, Bar-Shira A, Dauvilliers Y, et al. GBA mutations are associated with Rapid Eye Movement Sleep Behavior Disorder. Ann Clin Transl Neurol 2015; 2: 941–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Diaz AI, Segura B, Baggio HC, Marti MJ, Valldeoriola F, Compta Y, et al. Structural MRI correlates of the MMSE and pentagon copying test in Parkinson's disease. Parkinsonism Relat Disord 2014; 20: 1405–10. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin E, Satue M, Fuertes I, Otin S, Alarcia R, Herrero R, et al. Ability and reproducibility of Fourier-domain optical coherence tomography to detect retinal nerve fiber layer atrophy in Parkinson's disease. Ophthalmology 2012; 119: 2161–7. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson's disease. Neurology 1993; 43: 2227–9. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tanner CM, Klawans HL. Pharmacology of hallucinations induced by long-term drug therapy. Am J Psychiatry 1982; 139: 494–7. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Vaughan CL, Goldman JG, Stebbins GT. I finally see what you see: Parkinson's disease visual hallucinations captured with functional neuroimaging. Mov Disord 2014; 29: 115–17. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O, Masdeu JC, Kohn PD, Ianni A, Lopez G, Groden C, et al. The neurobiology of glucocerebrosidase-associated parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain 2012; 135: 2440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Stebbins GT, Dinh V, Bernard B, Merkitch D, de Toledo-Morrell L, et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson's disease with hallucinations. Brain 2014; 137: 849–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris A, Williams-Gray CH, Clark GR, Foltynie T, Lewis SJ, Brown J, et al. Tau and alpha-synuclein in susceptibility to, and dementia in, Parkinson's disease. Ann Neurol 2007; 62: 145–53. [DOI] [PubMed] [Google Scholar]
- Gottlich M, Munte TF, Heldmann M, Kasten M, Hagenah J, Kramer UM. Altered resting state brain networks in Parkinson's disease. PLoS One 2013; 8: e77336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray HM, Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in Parkinson's disease. Neuropsychology 2010; 24: 176–91. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron 1999; 24: 187–203. [DOI] [PubMed] [Google Scholar]
- Grossi D, Trojano L, Pellecchia MT, Amboni M, Fragassi NA, Barone P. Frontal dysfunction contributes to the genesis of hallucinations in non-demented Parkinsonian patients. Int J Geriatr Psychiatry 2005; 20: 668–73. [DOI] [PubMed] [Google Scholar]
- Gullett JM, Price CC, Nguyen P, Okun MS, Bauer RM, Bowers D. Reliability of three Benton Judgment of Line Orientation short forms in idiopathic Parkinson's disease. Clin Neuropsychol 2013; 27: 1167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol 2009; 127: 737–41. [DOI] [PubMed] [Google Scholar]
- Halliday GM, Holton JL, Revesz T, Dickson DW. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 2011; 122: 187–204. [DOI] [PubMed] [Google Scholar]
- Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain 2002; 125: 391–403. [DOI] [PubMed] [Google Scholar]
- Harnois C, Di PT. Decreased dopamine in the retinas of patients with Parkinson's disease. Invest Ophthalmol Vis Sci 1990; 31: 2473–5. [PubMed] [Google Scholar]
- Harris JP, Atkinson EA, Lee AC, Nithi K, Fowler MS. Hemispace differences in the visual perception of size in left hemi Parkinson's disease. Neuropsychologia 2003; 41: 795–807. [DOI] [PubMed] [Google Scholar]
- Harris JP, Calvert JE, Phillipson OT. Processing of spatial contrast in peripheral vision in Parkinson's disease. Brain 1992; 115 (Pt 5): 1447–57. [DOI] [PubMed] [Google Scholar]
- Haug BA, Kolle RU, Trenkwalder C, Oertel WH, Paulus W. Predominant affection of the blue cone pathway in Parkinson's disease. Brain 1995; 118 (Pt 3): 771–8. [DOI] [PubMed] [Google Scholar]
- Haug BA, Trenkwalder C, Arden GB, Oertel WH, Paulus W. Visual thresholds to low-contrast pattern displacement, color contrast, and luminance contrast stimuli in Parkinson's disease. Mov Disord 1994; 9: 563–70. [DOI] [PubMed] [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord 2008; 23: 837–44. [DOI] [PubMed] [Google Scholar]
- Hipp G, Diederich NJ, Pieria V, Vaillant M. Primary vision and facial emotion recognition in early Parkinson's disease. J Neurol Sci 2014; 338: 178–82. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci 2000; 23: 793–42. [DOI] [PubMed] [Google Scholar]
- Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson's disease. J Neurol Neurosurg Psychiatry 2001; 70: 734–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, Stryker MP. Anatomical demonstration of orientation columns in macaque monkey. J Comp Neurol 1978; 177: 361–80. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T, Kakita A, Shiga A, Kasuga K, Kaneko H, Tan CF, et al. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol 2008; 65: 514–19. [DOI] [PubMed] [Google Scholar]
- Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res 2004; 44: 2793–7. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013; 12: 443–53. [DOI] [PubMed] [Google Scholar]
- Jacobs DH, Shuren J, Bowers D, Heilman KM. Emotional facial imagery, perception, and expression in Parkinson's disease. Neurology 1995; 45: 1696–702. [DOI] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Timmings PL. Impairment of high-contrast visual acuity in Parkinson's disease. Mov Disord 1992; 7: 232–8. [DOI] [PubMed] [Google Scholar]
- Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC, et al. Patterns of cortical thickness and surface area in early Parkinson's disease. Neuroimage 2011; 55: 462–7. [DOI] [PubMed] [Google Scholar]
- Kaul S, Elble RJ. Impaired pentagon drawing is an early predictor of cognitive decline in Parkinson's disease. Mov Disord 2014; 29: 427–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010; 9: 1200–13. [DOI] [PubMed] [Google Scholar]
- Kertelge L, Bruggemann N, Schmidt A, Tadic V, Wisse C, Dankert S, et al. Impaired sense of smell and color discrimination in monogenic and idiopathic Parkinson's disease. Mov Disord 2010; 25: 2665–9. [DOI] [PubMed] [Google Scholar]
- Khadjevand F, Shahzadi S, Abbassian A. Reduction of negative afterimage duration in Parkinson's disease patients: a possible role for dopaminergic deficiency in the retinal Interplexiform cell layer. Vision Res 2010; 50: 279–83. [DOI] [PubMed] [Google Scholar]
- Kimmig H, Haussmann K, Mergner T, Lucking CH. What is pathological with gaze shift fragmentation in Parkinson's disease? J Neurol 2002; 249: 683–92. [DOI] [PubMed] [Google Scholar]
- Kochan NA, Valenzuela M, Slavin MJ, McCraw S, Sachdev PS, Breakspear M. Impact of load-related neural processes on feature binding in visuospatial working memory. PLoS One 2011; 6, e23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SB, Suh SI, Kim SH, Kim JH. Stereopsis and extrastriate cortical atrophy in Parkinson's disease: a voxel-based morphometric study. Neuroreport 2013; 24: 229–32. [DOI] [PubMed] [Google Scholar]
- Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK. Autosomal dominant Parkinson's disease caused by SNCA duplications. Parkinsonism Relat Disord 2016; 22 (Suppl 1): S1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Cortical regions involved in perceiving object shape. J Neurosci 2000; 20: 3310–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulisevsky J, Pagonabarraga J. Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson's disease: meta-analysis of randomized controlled trials. Drug Saf 2010; 33: 147–61. [DOI] [PubMed] [Google Scholar]
- Kulkarni O, Lafaver K, Tarsy D. The “floating door sign” in Parkinson's disease. Parkinsonism Relat Disord 2013; 19: 825–6. [DOI] [PubMed] [Google Scholar]
- La Morgia C, Barboni P, Rizzo G, Carbonelli M, Savini G, Scaglione C, et al. Loss of temporal retinal nerve fibers in Parkinson disease: a mitochondrial pattern? Eur J Neurol 2013; 20: 198–201. [DOI] [PubMed] [Google Scholar]
- La Morgia C, Ross-Cisneros FN, Hannibal J, Montagna P, Sadun AA, Carelli V. Melanopsin-expressing retinal ganglion cells: implications for human diseases. Vision Res 2011; 51: 296–302. [DOI] [PubMed] [Google Scholar]
- Large ME, Aldcroft A, Vilis T. Task-related laterality effects in the lateral occipital complex. Brain Res 2007; 1128: 130–8. [DOI] [PubMed] [Google Scholar]
- Laudate TM, Neargarder S, Cronin-Golomb A. Line bisection in Parkinson's disease: investigation of contributions of visual field, retinal vision, and scanning patterns to visuospatial function. Behav Neurosci 2013; 127: 151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RA, Yarnall AJ, Duncan GW, Khoo TK, Breen DP, Barker RA, et al. Quality of life and mild cognitive impairment in early Parkinson's disease: does subtype matter? J Parkinsons Dis 2014; 4: 331–6. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Fowler MS. 2001a. Disruption of estimation of body-scaled aperture width in Hemiparkinson's disease. Neuropsychologia 39: 1097–104. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Fowler MS. 2001b. Evidence from a line bisection task for visuospatial neglect in left hemiparkinson's disease. Vision Res 41: 2677–86. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Calvert JE. Impairments of mental rotation in Parkinson's disease. Neuropsychologia 1998; 36: 109–14. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ahn J, Kim TW, Jeon BS. Optical coherence tomography in Parkinson's disease: is the retina a biomarker? J Parkinsons Dis 2014a; 4: 197–204. [DOI] [PubMed] [Google Scholar]
- Lee JY, Kim JM, Ahn J, Kim HJ, Jeon BS, Kim TW. Retinal nerve fiber layer thickness and visual hallucinations in Parkinson's Disease. Mov Disord 2014b; 29: 61–7. [DOI] [PubMed] [Google Scholar]
- Levin BE, Llabre MM, Reisman S, Weiner WJ, Sanchez-Ramos J, Singer C, et al. Visuospatial impairment in Parkinson's disease. Neurology 1991; 41: 365–9. [DOI] [PubMed] [Google Scholar]
- Levine DN, Finklestein S. Delayed psychosis after right temporoparietal stroke or trauma: relation to epilepsy. Neurology 1982; 32: 267–73. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD, Walt SE. Stride length regulation in Parkinson's disease: the use of extrinsic, visual cues. Brain 2000; 123 (Pt 10): 2077–90. [DOI] [PubMed] [Google Scholar]
- Luo CY, Guo XY, Song W, Chen Q, Cao B, Yang J, et al. Functional connectome assessed using graph theory in drug-naive Parkinson's disease. J Neurol 2015; 262: 1557–67. [DOI] [PubMed] [Google Scholar]
- MacAskill MR, Anderson TJ, Jones RD. Adaptive modification of saccade amplitude in Parkinson's disease. Brain 2002; 125: 1570–82. [DOI] [PubMed] [Google Scholar]
- Manassi M, Sayim B, Herzog MH. When crowding of crowding leads to uncrowding. J Vis 2013; 13: 10. [DOI] [PubMed] [Google Scholar]
- Manni R, Sinforiani E, Pacchetti C, Zucchella C, Cremascoli R, Terzaghi M. Cognitive dysfunction and REM sleep behavior disorder: key findings in the literature and preliminary longitudinal findings. Int J Psychophysiol 2013; 89: 213–17. [DOI] [PubMed] [Google Scholar]
- Manni R, Terzaghi M, Repetto A, Zangaglia R, Pacchetti C. Complex paroxysmal nocturnal behaviors in Parkinson's disease. Mov Disord 2010; 25: 985–90. [DOI] [PubMed] [Google Scholar]
- Marques A, Dujardin K, Boucart M, Pins D, Delliaux M, Defebvre L, et al. REM sleep behaviour disorder and visuoperceptive dysfunction: a disorder of the ventral visual stream? J Neurol 2010; 257: 383–91. [DOI] [PubMed] [Google Scholar]
- Marras C, Schule B, Munhoz RP, Rogaeva E, Langston JW, Kasten M, et al. Phenotype in parkinsonian and nonparkinsonian LRRK2 G2019S mutation carriers. Neurology 2011; 77: 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Udaka F, Tamura A, Oda M, Kubori T, Nishinaka K, et al. Impaired visual acuity as a risk factor for visual hallucinations in Parkinson's disease. J Geriatr Psychiatry Neurol 2006; 19: 36–40. [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 2011; 146: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell SA, Harris J. Irrelevant peripheral visual stimuli impair manual reaction times in Parkinson's disease. Vision Res 1997a; 37: 3549–58. [DOI] [PubMed] [Google Scholar]
- McDowell SA, Harris JP. Visual problems in Parkinson's disease: a questionnaire survey. Behav Neurol 1997b; 10: 77–81. [DOI] [PubMed] [Google Scholar]
- Mestre D, Blin O, Serratrice G, Pailhous J. Spatiotemporal contrast sensitivity differs in normal aging and Parkinson's disease. Neurology 1990; 40: 1710–14. [DOI] [PubMed] [Google Scholar]
- Miri S, Glazman S, Mylin L, Bodis-Wollner I. A combination of retinal morphology and visual electrophysiology testing increases diagnostic yield in Parkinson's disease. Parkinsonism Relat Disord 2016; 22 (Suppl 1), S134–7. [DOI] [PubMed] [Google Scholar]
- Miri S, Shrier EM, Glazman S, Ding Y, Selesnick I, Kozlowski PB, et al. The avascular zone and neuronal remodeling of the fovea in Parkinson disease. Ann Clin Transl Neurol 2015; 2: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montse A, Pere V, Carme J, Francesc V, Eduardo T. Visuospatial deficits in Parkinson's disease assessed by judgment of line orientation test: error analyses and practice effects. J Clin Exp Neuropsychol 2001; 23: 592–8. [DOI] [PubMed] [Google Scholar]
- Moreno-Ramos T, Benito-Leon J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson's disease, dementia with Lewy bodies, and Alzheimer's disease. J Alzheimers Dis 2013; 34: 659–64. [DOI] [PubMed] [Google Scholar]
- Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol 2011; 21: 24–9. [DOI] [PubMed] [Google Scholar]
- Mosimann UP, Mather G, Wesnes KA, O'Brien JT, Burn DJ, McKeith IG. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology 2004; 63: 2091–6. [DOI] [PubMed] [Google Scholar]
- Morrison J, David A S. Now you see it, now you don't: More data at the cognitive level needed before the PAD model can be accepted. Behav Brain Sci 2005; 28: 770–1. [Google Scholar]
- Muller T, Kuhn W, Buttner T, Przuntek H. Colour vision abnormalities and movement time in Parkinson's disease. Eur J Neurol 1999; 6: 711–15. [DOI] [PubMed] [Google Scholar]
- Muller T, Woitalla D, Peters S, Kohla K, Przuntek H. Progress of visual dysfunction in Parkinson's disease. Acta Neurol Scand 2002; 105: 256–60. [DOI] [PubMed] [Google Scholar]
- Nagamachi S, Wakamatsu H, Kiyohara S, Fujita S, Futami S, Tamura S, et al. Usefulness of rCBF analysis in diagnosing Parkinson's disease: supplemental role with MIBG myocardial scintigraphy. Ann Nucl Med 2008; 22: 557–64. [DOI] [PubMed] [Google Scholar]
- Neumann J, Bras J, Deas E, O'Sullivan SS, Parkkinen L, Lachmann RH, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 2009; 132: 1783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Legros J. Functional neuroarchitecture of the retina: hypothesis on the dysfunction of retinal dopaminergic circuitry in Parkinson's disease. Surg Radiol Anat 1988; 10: 137–44. [DOI] [PubMed] [Google Scholar]
- Nishioka K, Ross OA, Ishii K, Kachergus JM, Ishiwata K, Kitagawa M, et al. Expanding the clinical phenotype of SNCA duplication carriers. Mov Disord 2009; 24: 1811–19. [DOI] [PubMed] [Google Scholar]
- Nombela C, Rowe JB, Winder-Rhodes SE, Hampshire A, Owen AM, Breen DP, et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain 2014; 137: 2743–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Inoue Y, Kagimura T, Nakashima K. Clinical significance of REM sleep behavior disorder in Parkinson's disease. Sleep Med 2013; 14: 131–5. [DOI] [PubMed] [Google Scholar]
- Nowacka B, Lubinski W, Honczarenko K, Potemkowski A, Safranow K. Ophthalmological features of Parkinson disease. Med Sci Monit 2014; 20: 2243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obi T, Nishioka K, Ross OA, Terada T, Yamazaki K, Sugiura A, et al. Clinicopathologic study of a SNCA gene duplication patient with Parkinson disease and dementia. Neurology 2008; 70: 238–41. [DOI] [PubMed] [Google Scholar]
- Ogden JA, Growdon JH, Corkin S. Deficits on visuospatiaol tests involving forward planning in high-functioning Parkinsonians. Neuropsychiatry Neuropsychol Behav Neurol 1990; 3: 125–39. [Google Scholar]
- Oh YS, Kim JS, Chung SW, Song IU, Kim YD, Kim YI, et al. Color vision in Parkinson's disease and essential tremor. Eur J Neurol 2011; 18: 577–83. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga J, Martinez-Horta S, Fernandez de BR, Perez J, Ribosa-Nogue R, Marin J, et al. Minor hallucinations occur in drug-naive Parkinson's disease patients, even from the premotor phase. Mov Disord 2016; 31: 45–52. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos S, McCorquodale DS, Gonzalez J, Jean-Gilles L, Mash DC. Cortical and amygdalar Lewy body burden in Parkinson's disease patients with visual hallucinations. Parkinsonism Relat Disord 2006; 12: 253–6. [DOI] [PubMed] [Google Scholar]
- Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci 1988; 2: 187–99. [Google Scholar]
- Peppe A, Stanzione P, Pierelli F, De AD, Pierantozzi M, Bernardi G. Visual alterations in de novo Parkinson's disease: pattern electroretinogram latencies are more delayed and more reversible by levodopa than are visual evoked potentials. Neurology 1995; 45: 1144–8. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Aarsland D, Ginestet CE, Lebedev AV, Wahlund LO, Simmons A, et al. Aberrant cerebral network topology and mild cognitive impairment in early Parkinson's disease. Hum Brain Mapp 2015; 36: 2980–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Ibarretxe-Bilbao N, Marti MJ, Compta Y, Junque C, Bargallo N, et al. Assessment of cortical degeneration in patients with Parkinson's disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp 2012; 33: 2521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Junque C, Marti MJ, Ramirez-Ruiz B, Bargallo N, Tolosa E. Neuroanatomical substrate of visuospatial and visuoperceptual impairment in Parkinson's disease. Mov Disord 2009; 24: 1193–9. [DOI] [PubMed] [Google Scholar]
- Petrucci S, Ginevrino M, Valente EM. Phenotypic spectrum of alpha-synuclein mutations: new insights from patients and cellular models. Parkinsonism Relat Disord 2016; 22 (Suppl 1), S16–20. [DOI] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Bonnet AM, Esteguy M, Guimaraes J, Vigouret JM, et al. Cognitive slowing in Parkinson's disease fails to respond to levodopa treatment: the 15-objects test. Neurology 1989; 39: 762–8. [DOI] [PubMed] [Google Scholar]
- Pinkhardt EH, Kassubek J. Ocular motor abnormalities in Parkinsonian syndromes. Parkinsonism Relat Disord 2011; 17: 223–30. [DOI] [PubMed] [Google Scholar]
- Poewe W. Psychosis in Parkinson's disease. Mov Disord 2003; 18 (Suppl 6), S80–7. [DOI] [PubMed] [Google Scholar]
- Postma JU, Van TW. Visual hallucinations and delirium during treatment with amantadine (Symmetrel). J Am Geriatr Soc 1975; 23: 212–15. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Bertrand JA, Montplaisir J, Desjardins C, Vendette M, Rios RS, et al. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson's disease: a prospective study. Mov Disord 2012; 27: 720–6. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 2011; 69: 811–18. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M, Montplaisir JY. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson's disease. Brain 2009; 132: 3298–307. [DOI] [PubMed] [Google Scholar]
- Price MJ, Feldman RG, Adelberg D, Kayne H. Abnormalities in color vision and contrast sensitivity in Parkinson's disease. Neurology 1992; 42: 887–90. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E. Neuropsychological deficits in Parkinson's disease patients with visual hallucinations. Mov Disord 2006; 21: 1483–7. [DOI] [PubMed] [Google Scholar]
- Ramirez-Ruiz B, Marti MJ, Tolosa E, Gimenez M, Bargallo N, Valldeoriola F, et al. Cerebral atrophy in Parkinson's disease patients with visual hallucinations. Eur J Neurol 2007; 14: 750–6. [DOI] [PubMed] [Google Scholar]
- Regan BC, Freudenthaler N, Kolle R, Mollon JD, Paulus W. Colour discrimination thresholds in Parkinson's disease: results obtained with a rapid computer-controlled colour vision test. Vision Res 1998; 38: 3427–31. [DOI] [PubMed] [Google Scholar]
- Regan D. Visual information channeling in normal and disordered vision. Psychol Rev 1982; 89: 407–44. [PubMed] [Google Scholar]
- Regan D, Maxner C. Orientation-selective visual loss in patients with Parkinson's disease. Brain 1987; 110 (Pt 2): 415–32. [DOI] [PubMed] [Google Scholar]
- Regan D, Neima D. Low-contrast letter charts in early diabetic retinopathy, ocular hypertension, glaucoma, and Parkinson's disease. Br J Ophthalmol 1984; 68: 885–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio J, Bobrowicz-Campos E, Andre R, Almeida I, Faria P, Januario C, et al. Specific impairment of visual spatial covert attention mechanisms in Parkinson's disease. Neuropsychologia 2011; 49: 34–42. [DOI] [PubMed] [Google Scholar]
- Santangelo G, Trojano L, Vitale C, Ianniciello M, Amboni M, Grossi D, et al. A neuropsychological longitudinal study in Parkinson's patients with and without hallucinations. Mov Disord 2007; 22: 2418–25. [DOI] [PubMed] [Google Scholar]
- Sari ES, Koc R, Yazici A, Sahin G, Ermis SS. Ganglion cell-inner plexiform layer thickness in patients with Parkinson disease and association with disease severity and duration. J Neuroophthalmol 2015; 35: 117–21. [DOI] [PubMed] [Google Scholar]
- Saur R, Maier C, Milian M, Riedel E, Berg D, Liepelt-Scarfone I, et al. Clock test deficits related to the global cognitive state in Alzheimer's and Parkinson's disease. Dement Geriatr Cogn Disord 2012; 33: 59–72. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med 2013; 14: 744–8. [DOI] [PubMed] [Google Scholar]
- Schmoll C, Lascaratos G, Dhillon B, Skene D, Riha RL. The role of retinal regulation of sleep in health and disease. Sleep Med Rev 2011; 15: 107–13. [DOI] [PubMed] [Google Scholar]
- Seichepine DR, Neargarder S, Miller IN, Riedel TM, Gilmore GC, Cronin-Golomb A. Relation of Parkinson's disease subtypes to visual activities of daily living. J Int Neuropsychol Soc 2011; 17: 841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson's disease. Brain 2009; 132: 2947–57. [DOI] [PubMed] [Google Scholar]
- Shin S, Lee JE, Hong JY, Sunwoo MK, Sohn YH, Lee PH. Neuroanatomical substrates of visual hallucinations in patients with non-demented Parkinson's disease. J Neurol Neurosurg Psychiatry 2012; 83: 1155–61. [DOI] [PubMed] [Google Scholar]
- Shine JM, Keogh R, O'Callaghan C, Muller AJ, Lewis SJ, Pearson J. Imagine that: elevated sensory strength of mental imagery in individuals with Parkinson's disease and visual hallucinations. Proc Biol Sci 2015; 282: 20142047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, O'Callaghan C, Halliday GM, Lewis SJ. Tricks of the mind: visual hallucinations as disorders of attention. Prog Neurobiol 2014; 116: 58–65. [DOI] [PubMed] [Google Scholar]
- Shoji Y, Nishio Y, Baba T, Uchiyama M, Yokoi K, Ishioka T, et al. Neural substrates of cognitive subtypes in Parkinson's disease: a 3-year longitudinal study. PLoS One 2014; 9, e110547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrier EM, Adam CR, Spund B, Glazman S, Bodis-Wollner I. Interocular asymmetry of foveal thickness in Parkinson disease. J Ophthalmol 2012; 2012: 728457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber BA, Landis S, Koroshetz W, Bateman R, Siderowf A, Galpern WR, et al. Prioritized research recommendations from the National Institute of Neurological Disorders and Stroke Parkinson's Disease 2014 conference. Ann Neurol 2014; 76: 469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MF, Faria P, Regateiro FS, Forjaz V, Januario C, Freire A, et al. Independent patterns of damage within magno-, parvo- and koniocellular pathways in Parkinson's disease. Brain 2005; 128: 2260–71. [DOI] [PubMed] [Google Scholar]
- Simon-Tov S, Dinur T, Giladi N, Bar-Shira A, Zelis M, Zimran A, et al. Color Discrimination in Patients with Gaucher Disease and Parkinson Disease. J Parkinsons Dis 2015; 5: 525–31. [DOI] [PubMed] [Google Scholar]
- Sinforiani E, Pacchetti C, Zangaglia R, Pasotti C, Manni R, Nappi G. REM behavior disorder, hallucinations and cognitive impairment in Parkinson's disease: a two-year follow up. Mov Disord 2008; 23: 1441–5. [DOI] [PubMed] [Google Scholar]
- Sixel-Doring F, Lohmann K, Klein C, Trenkwalder C, Mollenhauer B. REM sleep-associated motor behaviors in Parkinson's disease patients with heterozygous Parkin mutations. Mov Disord 2015; 30: 597–8. [DOI] [PubMed] [Google Scholar]
- Somme JH, Molano Salazar A, Gonzalez A, Tijero B, Berganzo K, Lezcano E, et al. Cognitive and behavioual symptoms in Parkinson's disease patients with the G2019S and R1441G mutations of the LRRK2 gene. Parkinsonism Relat Disord 2015; 21: 494–9. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Young AW, Mahn K, Schroeder U, Woitalla D, Buttner T, et al. Facial expression recognition in people with medicated and unmedicated Parkinson's disease. Neuropsychologia 2003; 41: 1047–57. [DOI] [PubMed] [Google Scholar]
- Steiger MJ, Quinn NP, Toone B, Marsden CD. Off-period screaming accompanying motor fluctuations in Parkinson's disease. Mov Disord 1991; 6: 89–90. [DOI] [PubMed] [Google Scholar]
- Stella F, Gobbi LT, Gobbi S, Oliani MM, Tanaka K, Pieruccini-Faria F. Early impairment of cognitive functions in Parkinson's disease. Arq Neuropsiquiatr 2007; 65: 406–10. [DOI] [PubMed] [Google Scholar]
- Stern GM, Lander CM, Lees AJ. Akinetic freezing and trick movements in Parkinson's disease. J Neural Transm Suppl 1980; 16: 137–41. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhang H, Gu Z, Cao M, Li D, Chan P. Stereopsis impairment is associated with decreased color perception and worse motor performance in Parkinson's disease. Eur J Med Res 2014; 19: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Hoshino T, Shigemasu K, Kawamura M. Disgust-specific impairment of facial expression recognition in Parkinson's disease. Brain 2006; 129: 707–17. [DOI] [PubMed] [Google Scholar]
- Sweet RD, McDowell FH, Feigenson JS, Loranger AW, Goodell H. Mental symptoms in Parkinson's disease during chronic treatment with levodopa. Neurology 1976; 26: 305–10. [DOI] [PubMed] [Google Scholar]
- Tagliati M, Bodis-Wollner I, Yahr MD. The pattern electroretinogram in Parkinson's disease reveals lack of retinal spatial tuning. Electroencephalogr Clin Neurophysiol 1996; 100: 1–11. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, et al. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 1995; 15: 3215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Vianna E, Manzel K, Damasio H, Grabowski T. Neuroanatomical correlates of the Benton facial recognition test and judgment of line orientation test. J Clin Exp Neuropsychol 2009; 31: 219–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick GL, Kaskie B, Steinman SB. Visual impairment in Parkinson's disease: deficits in orientation and motion discrimination. Optom Vis Sci 1994; 71: 242–5. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology 2005; 65: 1907–13. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson JD. Impaired visual search in drivers with Parkinson's disease. Ann Neurol 2006; 60: 407–13. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Johnson AM, Dastrup E, Anderson SW, Dawson JD. Road safety in drivers with Parkinson disease. Neurology 2009; 73: 2112–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwyler P, Nef T, Killen A, Collerton D, Thomas A, Burn D, et al. Visual complaints and visual hallucinations in Parkinson's disease. Parkinsonism Relat Disord 2014; 20: 318–22. [DOI] [PubMed] [Google Scholar]
- Vendette M, Gagnon JF, Decary A, Massicotte-Marquez J, Postuma RB, Doyon J, et al. REM sleep behavior disorder predicts cognitive impairment in Parkinson disease without dementia. Neurology 2007; 69: 1843–9. [DOI] [PubMed] [Google Scholar]
- Ventre J, Zee DS, Papageorgiou H, Reich S. Abnormalities of predictive saccades in hemi-Parkinson's disease. Brain 1992; 115 (Pt 4): 1147–65. [DOI] [PubMed] [Google Scholar]
- Villardita C, Smirni P, Zappala G. Visual neglect in Parkinson's disease. Arch Neurol 1983; 40: 737–9. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci 2003; 6: 624–31. [DOI] [PubMed] [Google Scholar]
- Wang C, Cai Y, Gu Z, Ma J, Zheng Z, Tang BS, et al. Clinical profiles of Parkinson's disease associated with common leucine-rich repeat kinase 2 and glucocerebrosidase genetic variants in Chinese individuals. Neurobiol Aging 2014; 35: 725–6. [DOI] [PubMed] [Google Scholar]
- Weiner WJ, Koller WC, Perlik S, Nausieda PA, Klawans HL. Drug holiday and management of Parkinson disease. Neurology 1980; 30: 1257–61. [DOI] [PubMed] [Google Scholar]
- Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson's disease: a retrospective autopsy study. Lancet Neurol 2005; 4: 605–10. [DOI] [PubMed] [Google Scholar]
- Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson's disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry 2008; 79: 652–5. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 2009; 132: 2958–69. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, et al. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry 2013; 84: 1258–64. [DOI] [PubMed] [Google Scholar]
- Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, et al. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community-based incident cohort. Brain 2013; 136: 392–9. [DOI] [PubMed] [Google Scholar]
- Wink B, Harris J. A model of the Parkinsonian visual system: support for the dark adaptation hypothesis. Vision Res 2000; 40: 1937–46. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J Neurosci 1991; 11: 641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CQ, Zhang JW, Wang M, Peng GG. Meta-analysis of the efficacy and safety of long-acting non-ergot dopamine agonists in Parkinson's disease. J Clin Neurosci 2014; 21: 1094–101. [DOI] [PubMed] [Google Scholar]
- Zokaei N, McNeill A, Proukakis C, Beavan M, Jarman P, Korlipara P, et al. Visual short-term memory deficits associated with GBA mutation and Parkinson's disease. Brain 2014; 137: 2303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]