Abstract
Purpose
Black breast cancer survivors have high rates of obesity and low physical activity levels. Little is known about the acceptability and feasibility of interventions in this population.
Objective
A two-arm RCT was launched to assess the efficacy of a culturally targeted 12-week multimodal lifestyle intervention in overweight and obese black survivors.
Methods
Intervention components included nutrition education, exercise groups, and survivor-led motivational interviewing phone sessions. The analytic sample included women who completed the trial (intervention n = 10; control n = 12). Anthropometric measures, physical activity, and VO2max were assessed at baseline and follow-up. Change scores (intervention vs. control) were assessed with Wilcoxon rank-sum tests. A process evaluation assessed intervention acceptability.
Results
Overall adherence was 70% and overall satisfaction was high (86%). Despite the 5% weight loss target, the intervention group lost 0.8% but BMI improved. Total physical activity levels increased in the intervention vs. control arm (+3501 MET min/week vs. +965 MET min/week, respectively). VO2max improved in the intervention group (+0.10 ± 1.03 kg/L/min). Intervention participants reduced energy intake (−207.3 ± 31.5 kcals) and showed improvements in fat intake (−15.5 ± 3.8 g), fiber (+3.2 ± 1.2 g) and % energy from fat (−4.8 ± 3.1%). Survivors suggested providing diet/exercise information within a cancer context.
Conclusions
Group and individualized intervention strategies are acceptable to black survivors. Observed differences between self-report and objective outcomes may suggest reporting bias or changes in body composition. Increasing supervised intervention components and assessment of body composition will be important for future trials.
Keywords: African American, Black, Breast cancer, Survivors, Physical activity
1. Introduction
Worldwide, breast cancer survivors comprise the largest group of cancer survivors [1]. Being overweight or obese is associated with a higher risk of secondary tumors, recurrence, and mortality [2–5]. Physical activity is associated with improved outcomes in breast cancer survivors [6,7]. National guidelines for breast cancer survivors include maintaining a healthy weight, limiting consumption of high calorie foods, and engaging in physical activity [8,9]. Unfortunately, up to 71% of cancer survivors are overweight or obese, and 68–80% do not meet the above guidelines [10–12].
Black breast cancer survivors are 70% more likely to be obese than their white counterparts [13]. Though limited, data from these reports suggest that they may be more likely to gain weight after diagnosis [14] and during treatment [15], and are less likely to meet physical activity or nutrition recommendations [16–19]. In a recent review of 82 physical activity trials, Speck and colleagues (2010) [20] concluded that 76% of reviewed studies did not properly describe the sample characteristics and race was the factor most commonly overlooked.
To date, there have been only four small studies of weight loss/physical activity interventions focused on black breast cancer survivors [21–24]. While findings seem promising, only two studies incorporated physical activity and diet [21,24], only one employed randomization [24], and none integrated survivorship support. Findings from a recent large multicenter trial of survivors (N = 692; blacks = 71) suggest that a behavioral intervention may support clinically meaningful weight loss. [25,26]. Empirical data from studies with white survivors suggest that interventions are more effective when they are multifaceted, personalized, teach behavioral skills, provide social support, and increase self-efficacy [27,28]. This is also likely true for black survivors, but documentation of successful strategies for them are lacking. Guided by tenets from the Theory of Planned Behavior (TPB), [29,30] we developed and piloted an innovative intervention, Stepping STONE (Survivors Taking on Nutrition and Exercise) to begin to fill these gaps. This report expands existing knowledge about black survivors' experiences with a lifestyle intervention and describes outcomes (physical activity levels, adiposity, cardiovascular outcomes, dietary intake, and satisfaction). As black survivors have lower levels of physical activity post diagnosis compared to their white counterparts, study data will be used to inform the delivery of trials in this group.
2. Methods
The MedStar Georgetown Joint Oncology Institutional Review Board approved this study. All research staff were trained in the protection of human subjects and the study protocol. A detailed intervention-manual was developed for use in all group and individual sessions. Interventionists (i.e., nutritionists, exercise physiologists, survivor-coaches) received an overview of breast cancer and training in motivational interviewing techniques. Survivor coaches were required to be at least two years post-primary treatment.
Study recruitment lasted 14 months (December 2010 through January 2012). Eligible survivors self-identified as African American and were overweight or obese (BMI ≥ 25 kg/m2 and ≤40 kg/m2), but not morbidly obese, sedentary (exercised less than 60 min/week for the previous six months), with early stage/localized breast cancer. Additional eligibility criteria included being 6 months to 5 years post-active treatment, ability to read and speak English, and to provide informed consent. The exclusion criteria were: a history of other cancers (exception: basal or squamous cell carcinoma), a recurrence of breast cancer, current enrollment in another physical activity and/or dietary clinical trial or commercial programs like Weight Watchers ®, etc., inability to commit to the intervention schedule, telephone inaccessibility, pre-existing condition(s) that precluded adherence to an unsupervised exercise program, and failure to provide medical clearance.
Written consent was obtained for all participants. Breast cancer survivors were recruited from two local hospitals and through outreach efforts that included flyers at community events and study notices in local newspapers in the Washington, DC metropolitan area. Clinical research assistants (CRA) approached survivors from hospitals, completed the eligibility screening, and consented participants. After, the CRA conducted a phone interview and mailed additional materials to be completed by participants. Similar procedures were followed for women contacted through outreach. Participants received $25.00 gift cards at baseline and study completion.
3. Study design and intervention arms
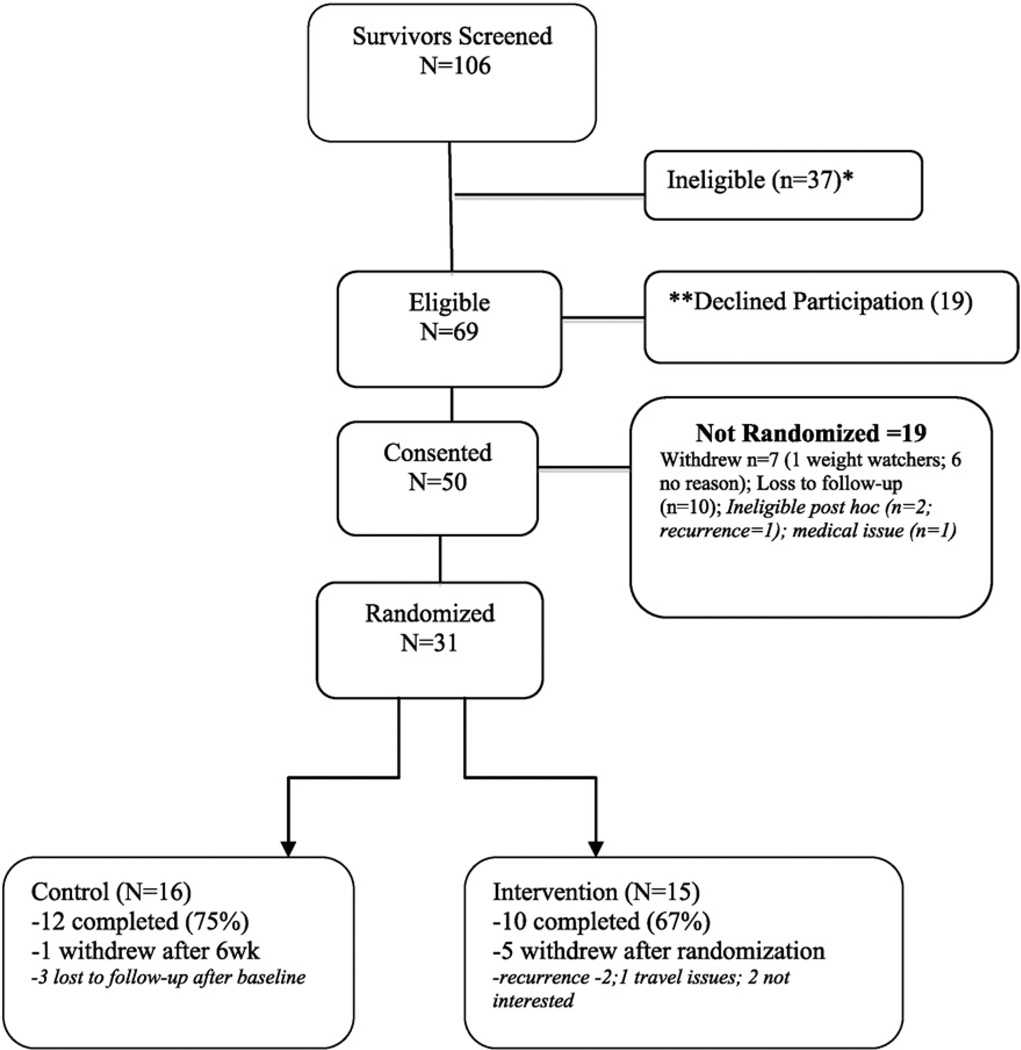
The design was a two-arm randomized controlled trial (RCT) with 31 women randomized to usual care (n = 16) or intervention (n = 15) arms (see Consort diagram in Fig. 1). Participants in the control arm received general health information for cancer survivors, i.e., the NCI booklet “Facing Forward Life After Cancer Treatment,” [31] and were offered the intervention at the end of the study. Stepping Stone was targeted toward black survivors using several culturally adaptation strategies [26]. For example when developing and refining activities and tenets, constituent-involving strategies were used to plan and implement the intervention. Socio-cultural strategies were used enhance delivery with the use of themes and content relevant to many survivors such as faith and spirituality, barriers and traditional/cultural foods and body image perceptions. Finally, program materials (e.g., survivor toolkit) were adapted to include risk-related information relevant to black survivors versus the general population.
Fig. 1.
Consort diagram.
The Stepping STONE intervention was based on the Theory of Planned Behavior (TPB) and Social Cognitive Theory (SCT). The TPB posits that the intention to perform a behavior is the most proximal determinant of behavior. Table 1 summarizes key intervention features relevant to the primary constructs. Attitudes, subjective norms, and perceived control are influenced by beliefs and independently impact behavioral intentions [30]. Thus, individualized sessions were tailored to baseline intentions, attitudes, and subjective norms. For example, if a woman reported high ratings of negative subjective norms and/or lack of support within her social network to making dietary changes, the interventionist would help her develop a strategy of actionable steps within her personal implementation plan. Example of actionable steps would include helpful language and/or discussion points to share with family members and identification of meal options with the nutritionist. The nutritionist would assist her in identifying healthier meal options that her family may also be willing to try.
Table 1.
Exemplary of Stepping STONE intervention sessions by module.
| Module | Week | Exemplar of issues addressed | Strategies | |
|---|---|---|---|---|
| 1 | Taking control | 1–3 | TPB components of — perceived control, as related to making lifestyle changes, SCT components of self-efficacy, goal setting |
Review the role of energy balance in relation to weight control and physical activity/diet recommendations Learn to make decisions about food choices |
| 2 | New attitudes | 4–6 | TPB-behavioral attitudes regarding physical activity/diet, SCT expectancies and role modeling of behaviors |
Discussion of challenges to physical activity/healthy diet, how to enjoy physical activity/healthy diet and improve attitudes (e.g. use positive self-talk) |
| 3 | Healthy choices | 7–9 | TPB — subjective and cultural norms, SCT aspects of role modeling behaviors |
Management of beliefs, norms, and perceptions about physical activity and diet, problem solving techniques, and action plans |
| 4 | Keeping the faith | 10–12 | Future maintenance of physical activity/diet behaviors, coaching using MI techniques to address ambivalence |
Setting specific short-term goals, create a support system to maintain behaviors |
Survivor Toolkit: Personalized implementation plan (goals, targets), physical activity tracker, session notebook, recipes, and resources.
Interventionist Toolkit: Implementation manual, baseline parameters (self-efficacy, nutrition, diet information, etc.), contact forms, food and fat models, study protocol.
Note: TBP — theory of planned behaviors; SCT — social cognitive theory.
Key aspects of SCT that were integrated into intervention strategies were increasing self-efficacy, role modeling, and addressing environmental influences on behavior (e.g., barriers to exercise) [29]. The interventionists incorporated MI techniques to elicit participant's internal motivation to pursue behavioral change and to assist them in making changes [28]. Stepping STONE was designed to facilitate a 5% gradual and sustained weight control through modification of physical activity and diet to promote approximately 1 lb of weight loss per week. Intervention content was based on the ACS Guide for Diet and Physical Activity for Cancer Survivors [32], which included physical activity recommendations (moderate intensity exercise of 30+ min/day, ≥5 days/week) and dietary recommendations (>5 fruits and vegetables/ day; <35% kcal from total fat).
The intervention lasted for 12 weeks. During this time, participants met once every two weeks for 90-min group sessions (30min of supervised group physical activity and 60-min education sessions) that were co-led by an exercise physiologist and a nutritionist. On weeks when participants did not meet as a group they have individual telephone coaching sessions led by a trained survivor coach. The nutrition education component included sessions about fiber, calories, cooking demonstrations to help women meet their dietary goals, and integration of MI techniques. A certified survivor coach delivered six individualized phone MI sessions (15 min every other week) to encourage women to express their concerns and motivations around physical activity, make individualized physical activity changes, overcome barriers, and to examine discrepancies between current behaviors and core values or personal goals [28]. The survivor coach also addressed survivorship issues (e.g., concerns about recurrence) and/or made resource referrals as necessary. Interventionists were trained by study PIs and were given detailed manuals that included session objectives, tasks, scripts, activities, and resources. Participants were given pedometers, notebooks, individualized step goals that gradually increased toward meeting and maintaining a goal of 10,000 steps/day for 12 weeks, tools to monitor and track their daily food intake, and binders to store resources and session materials.
3.1. Data collection and measurements
A trained CRA administered a standardized survey and an exercise physiologist took anthropometric measures and facilitated the maximal oxygen treadmill (VO2max) test. VO2max testing is a well-established and validated measure to determine cardiovascular fitness, especially in breast cancer survivors [33]. The following criteria was used to determine VO2max during the graded exercise test: [1] volitional fatigue, [2] maximal respiratory exchange ratio (RER) >1.1, or [3] maximal HR within 10 b/min of the age-predicted maximum (220 — age).
Intervention outcomes included physical activity, anthropometric measures, cardiovascular fitness, and intervention satisfaction (intervention arm only). Intermediate outcomes were self-efficacy regarding performing physical activity/diet behaviors and intentions toward physical activity/dietary behaviors.
Physical activity was measured using the International Physical Activity Questionnaire Short Form (IPAQ-SF) [34], which assessed the amount of physical activity performed during the previous seven days in three different intensity levels activities: 1) vigorous-intensity (e.g., aerobics); 2) moderate-intensity (e.g., cycling); and 3) walking. Energy requirements of each type of activity are defined by metabolic equivalents (METs) to yield a score in MET-minutes. Each MET-minute is computed by multiplying the MET score of an activity (8.0, 4.0, and 3.3 for vigorous-intensity, moderate intensity, and walking, respectively) by the total minutes performed per week. MET-minutes per week are then given a categorical score to determine overall activity level (i.e., moderate activity level equals at least 600 MET-minutes per week). Individuals who do not meet criteria for vigorous/moderate activity levels are considered to have ‘low’ physical activity [34].
3.1.1. Anthropometric measures
Weight, height, BMI (kg/m2), and waist and hip circumference were measured [35].
3.1.2. Dietary intake
Intervention participants were instructed to record their daily food/beverage intake. No dietary data were collected in the control group. Dietary intake, including daily energy intake, percentage of energy from fat, and grams of fat and fiber, was assessed using 4-day food intake records that were reviewed for accuracy and completeness prior to analysis using the NDS-R nutritional analysis software (NDS-R, University of Minnesota, Minneapolis, MN).
3.1.3. Cardiovascular fitness
Resting heart rate and blood pressure were determined using a standard Dinamap blood pressure monitor. Cardiovascular fitness was determined by a graded exercise test using the multistage treadmill Bruce Protocol. In this protocol, changing the treadmill speed increases the workload and percent grade every 3 min until voluntary fatigue is reported.
3.1.4. Intervention satisfaction
Satisfaction with the nutritionist, exercise physiologist, and survivor coach was assessed with Likert-formatted items respectively that captured participants' ratings of their performance, interpersonal, and technical skills [36]. Higher scores represented higher levels of satisfaction.
3.1.5. Process and intermediate outcomes
Using seven Likert-type 5-point items, participants were asked to rate their intentions to continue pursuing nutrition and physical activity habits and goals as well as engaging with related services. Perceived control in achieving weight loss goals was evaluated with an item in both intervention and control arms. Additionally, women in the intervention arm were asked to rate their perceived control in maintaining their weight loss and their dietary and exercise habits. An item regarding their information sharing behavior was also included. Finally, participants completed an overall evaluation of the Stepping Stone study.
In-depth perspectives of survivors' experiences were collected via a focus group with intervention participants. The focus group guide included semi-structured questions that elicited participant's experiences during each phase and component of the project and lasted 1.5 h. Participants received a $10.00 gift card. The focus group was audiotaped and transcribed. Two members of the research team identified main themes (e.g., positive and negative aspects of the intervention). Each researcher independently coded the transcripts using a directed content analysis approach [37]. Disagreements were solved by consensus. Each main theme was summarized and exemplified using quotes from the participants.
3.2. Statistical analysis
In addition to descriptive analysis, continuous baseline characteristics were compared between the intervention and control groups using Wilcoxon rank-sum test. Categorical baseline characteristics were compared using chi-square test or Fisher's exact test. Change scores were computed for anthropometric, physical activity, and cardiovascular fitness variables by subtracting the baseline scores from the score at 12 weeks. These change scores were compared between the intervention and control groups using Wilcoxon rank-sum test. Results were declared significant at the 0.05 level and all analyses were carried out using SPSS version 22 for Windows (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.).
4. Results
Randomization was successful, as no differences were noted in baseline factors by study arm. Women were 54.7 years old on average (SD = 9.8) and 51.6% had education above high school. Most survivors were diagnosed with breast cancer stage I (25.8%) or stage II (32.3%). On average, survivors were 1.7 years (SD = 0.88) from having completed their primary treatment. Most women were obese (80.6%) and had at least one comorbid condition (75%). Few women withdrew from the study post-randomization most (70%) women completed the study (75% of control; 67% intervention); outcomes were assessed for women (n = 22) that completed the study. No differences in baseline factors were noted between those who completed or did not complete the study (p > .05).
4.1. Anthropometric, physical activity, and dietary outcomes
Despite the 5% weight loss target, the intervention group only lost 0.8% of their total bodyweight. As shown in Table 2, there was a tendency for lower bodyweight, body mass index, and lower waist/hip ratio in the intervention group (Δ-mean = −1.7, −0.3, −0.03, respectively). Change was greatest for vigorous activity in the intervention group compared to lower levels of activity. Total physical activity levels increased in the intervention group 3.6-fold when compared to the control group (3501.1 MET min/week vs. 965.3 MET min/week, respectively). Cardiovascular fitness, as measured by VO2max, improved from baseline to study completion in the intervention group by 0.10 kg/L/min but appeared to decline in the control group by −0.83 kg/L/min. Compared to baseline, at 12-weeks the intervention group also showed trends toward a reduced total energy intake (−207.3±31.5 kcals; p = .06), total fat (−15.5 ± 3.8 g; p = .08), percent of energy from fat (−4.8 ± 3.1%; p = .10), and an increase in fiber intake (+3.2 ± 1.2 g, p = .20) were also noted (p values > .05).
Table 2.
Means, standard deviations, medians and IQR's at baseline and 12 weeks by study group.
| Control*(N = 12) | Intervention* (N = 10) | |||||
|---|---|---|---|---|---|---|
| Baseline Mean ± SD Median (IQR) |
Final (12 weeks) Mean ± SD Median (IQR) |
Δ-Mean Δ-Median |
Baseline Mean ± SD Median (IQR) |
Final (12 weeks) Mean ± SD Median (IQR) |
Δ-Mean Δ-Median |
|
| Anthropometrics | ||||||
| Body weight (lbs) | 215.6 ± 46.5 | 216.0 ± 47.3 | 0.4 | 216.4 ± 43.1 | 214.7 ± 44.1 | −1.7 |
| 211.8(187.5,233.7) | 214.0(190.0,225.7) | 2.2 | 210.5(176.0,257.5) | 203.2(173.3,257.5) | −7.3 | |
| Body mass index (kg/m2) | 37.4 ± 8.6 | 37.5 ± 8.7 | 0.1 | 35.2 ± 4.8 | 34.9 ± 5.0 | −0.3 |
| 37.0(29.7,41.3) | 36.8(29.7,41.0) | −0.2 | 35.6(30.7,39.1) | 35.3(30.4,38.6) | −0.3 | |
| Waist (cm) | 105.7 ± 13.0 | 104.9 ± 11.5 | −0.8 | 101.5 ± 14.0 | 102.10 ± 12.85 | 0.6 |
| 103.0(96.0,112.0) | 104.0(98.0,110.0) | 1.0 | 99.5(89.0,109.3) | 100.5(89.3,114.3) | 1.0 | |
| Hip (cm) | 124.5 ± 15.3 | 121.1 ± 12.8 | −3.4 | 114.40 ± 13.17 | 119.30 ± 16.90 | 4.9 |
| 124.0(111.0,130.0) | 123.0(109.0,128.0) | −1.0 | 107.5(104.8,124.3) | 118.0(103.0,132.5) | 10.5 | |
| Waist/hip ratio (cm) | 0.85 ± 0.048 | 0.87 ± 0.049 | 0.02 | 0.89 ± 0.058 | 0.86 ± 0.093 | −0.03 |
| 0.84(0.82,0.90) | 0.86(0.83,0.91) | 0.02 | 0.88(0.85,0.92) | 0.86(0.78,0.9) | −0.22 | |
| Physical activity (mins/week) | ||||||
| Vigorous | 37.5 ± 71.4 | 93.3 ± 122.6 | 55.8 | 37.5 ± 84.50 | 121.9 ± 294.0 | 84.4 |
| 0.0(0.0,67.5) | 50.0(0.0,142.5) | 50.0 | 0.0(0.0,30.0) | 0.0(0101.3) | 0.0 | |
| Moderate | 53.6 ± 60.9 | 89.1 ± 139.2 | 35.5 | 62.5 ± 129.8 | 120.0 ± 109.9 | 57.5 |
| 55.0(0.0,112.5) | 20.0(0.0,120.0) | −35.0 | 0.0(0.0,70.0) | 135.0(0.0,232.5) | 135.0 | |
| Walking | 100.0 ± 87.6 | 219.5 ± 374.2 | 119.5 | 140.7 ± 229.0 | 165.0 ± 99.5 | 24.3 |
| 75.0(7.5,210.0) | 120.0(33.8,210.0) | 45.0 | 45.0(10.0,528.8) | 157.5(93.8,255.0) | 112.5 | |
| Total | 205.0 ± 196.9 | 357.9 ± 458.7 | 152.9 | 291.7 ± 387.0 | 366.7 ± 395.8 | 75.0 |
| 160.0(35.0,375.0) | 215.0(138.8,345.0) | 55.0 | 50.0(0.0,652.5) | 210.0(127.5,480.0) | 160.0 | |
| Physical activity (MET-mins/week) | ||||||
| Vigorous | 300.0 ± 570.9 | 746.7 ± 980.6 | 446.7 | 300.0 ± 675.8 | 975.0 ± 2351.9 | 675.0 |
| 0.0(0.0,67.5) | 50.0(0.0,142.5) | 50.0 | 0.0(0.0,30.0) | 0.0(0.0,487.5) | 0.0 | |
| Moderate | 214.5 ± 243.5 | 356.4 ± 557.0 | 141.8 | 250.0 ± 519.2 | 480.0 ± 439.7 | 230.0 |
| 30.0(0.0,90.0) | 20.0(0.0,120.0) | 10.0 | 0.0(0.0,250.0) | 180.0(0.0,240.0) | 180.0 | |
| Walking | 313.5 ± 283.9 | 323.8 ± 292.8 | 10.3 | 464.4 ± 755.7 | 544.5 ± 328.3 | 80.1 |
| 75.0(22.5,210.0) | 120.0(33.7,210.0) | 45.0 | 45.0(10.0,506.3) | 157.5(93.7,303.7) | 112.5 | |
| Total | 688.5 ± 794.2 | 1653.8 ± 1301.2 | 965.3 | 1158.1 ± 1436.3 | 1793.8 ± 2599.52 | 635.7 |
| 160.0(35.0,375.0) | 215.0(138.8,345.0) | 55.0 | 145.0(0.0,877.5) | 277.5(161.3,746.3) | 132.5 | |
| Cardiovascular fitness | ||||||
| VO2 max (kg/L/min) | 15.6 ± 3.9 | 14.7 ± 6.7 | −0.83 | 15.83 ± 4.57 | 15.93 ± 5.70 | 0.10 |
| 15.6(12.0,16.8) | 15.6(9.6,17.6) | 0.0 | 14.5(12.7,18.4) | 15.9(13.4,18.5) | 1.4 | |
| Intermediate outcomes | ||||||
| Dietary intake | ||||||
| Energy intake kcals | 1463.6 ± 360.5 | – | – | 1621.7 ± 349.6 | 1421.9 ± 329.9 | −207.3 |
| 1406 (1173, 1603) | 1738.5 (1227.0, 1881.0) | 1405(1137, 1761) | −239.5 | |||
| Fat, grams | 67.4 ± 23.1 | – | – | 68.0 ± 21.8 | 52.8 ± 21.6 | −15.5 |
| 53.2 (50.3, 86.5) | 71.7 (46.2, 78.4) | 49.3 (46.9, 71.1) | −12.5 | |||
| Fat, % kcals | 40.0 ± 5.6 | – | – | 36.8 ± 6.5 | 31.9 ± 10.2 | −4.8 |
| 38.2 (37.7, 43.0) | 37.3 (32.8, 42.7) | 31 (23.4,41.2) | −5.2 | |||
| Fiber, grams | 13.1 ± 2.8 | – | – | 13.4 ± 5.4 | 19.2 ± 12.2 | 3.2 |
| 11.9 (11.8, 14.2) | 12.9 (8.6, 16.4) | 18.9 (9.3, 25.1) | 1.5 | |||
| Perceived control◆ | – | 2.3 ± 1.2 | – | 4.0 ± 1.0 | ||
| 2.5 (1.0, 3.0) | 4 (3.0, 5.0) | |||||
| Overall satisfaction | – | 9.4 ± 0.9 | – | 9.3 ± 0.8 | ||
| – | 10 (8.0, 10.0) | 9 (9.0, 10.0) | ||||
| Satisfaction w/contact | – | 9.0 ± 1.4 | – | 9.8 ± 0.4 | ||
| – | 9.5 (8.0, 10.0) | 10 (10.0, 10.0) | ||||
| Convenience | – | 8.6 ± 1.1 | – | 8.5 ± 2.1 | ||
| 8.5 (8.0, 9.5) | 9 (9.0, 10.0) | |||||
IQR = Interquartile range.
p > 0.05 for all comparisons of baseline between treatment arms.
Perceived control regarding weight loss.
4.2. Process and intermediate outcomes
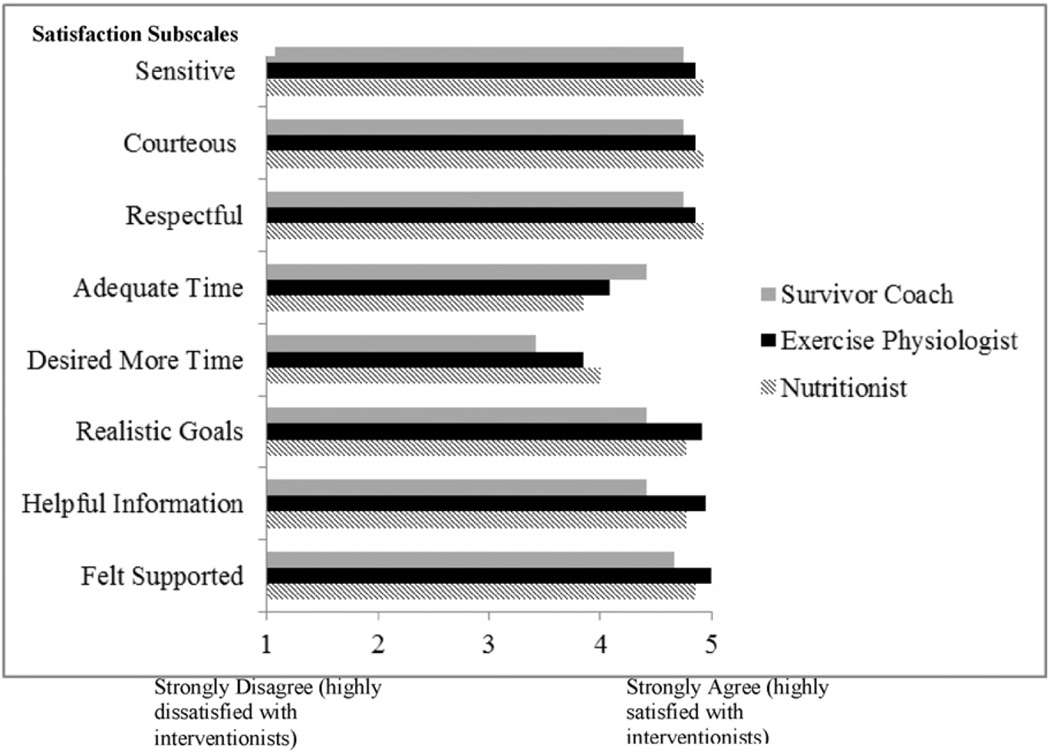
Women were highly satisfied with all aspects of the intervention including the responsiveness in being contacted, the assessments, the information packets, and the overall program. Women who participated in the intervention group positively evaluated interventionists' performance and skills (Fig. 2). Perceived control in achieving and maintaining dietary and physical activity goals was also high (see Table 2). Participants identified a range of 1 to 10 aspects in which Stepping Stones was different from other weight programs they had previously participated in (M = 5.23; SD = 2.83). Distinctive components endorsed by participants included having a nutritionist (61.5%), an exercise physiologist (69.2%), and a survivor coach (61.5%), and the targeted design for African American women (53.8%).Most participants strongly agreed or agreed that they intended to follow physical activity recommendations (100%), self-monitor their physical activity and dietary goals (92.3%), and weigh themselves weekly (77%). However, a lower percentage intended to pursue working with a coach (38.5%), joining another weight loss program (38.5%), joining a fitness club (23.1%), or hiring a trainer (15.4%). A higher percentage of women in the intervention arm agreed or strongly agreed that they could achieve their weight loss (69.3% vs. 37.5%). Moreover, most women in the intervention group (61.6%) reported trying to encourage family or friends to follow recommendations learned in the study.
Fig. 2.
Mean satisfaction ratings scores of study interventionists.
4.3. Suggestions to improve the intervention trial
Survivors praised the multifaceted nature of the program and the opportunity to engage in a healthy lifestyle change program in which they were held accountable. They also valued the usefulness of the materials and the prospect of applying their learning more broadly. For some women, participating in this project meant “moving to the next step” to continue taking care of their health by improving their nutrition and physical activity habits to better manage their future cancer risk. Participants suggested including more opportunities to enhance interaction with other group members and the study staff and tailoring the program to a greater extent to their condition as breast cancer survivors and to their particular context and life situations (e.g. employment status, and children). Participants' suggestions and exemplary quotes are presented in Table 3.
Table 3.
Participants' perspectives: opportunities for improvement.
| Intervention component | Lessons learned | Exemplar quotes |
|---|---|---|
| Overall | Provide participants opportunities to share more about themselves/experiences via groups and through data collection. Increase the length of the study (24 weeks and follow-up booster sessions) and schedule weekly group sessions. |
“The questionnaire did not ask a lot of relative questions…you did not know I have two children…you did not know me…Some type of questionnaire needs to ask those questions “tell your story in a paragraph” “The program gave you a baseline of information to cherry pick what was applicable to you….that's what was wonderful about the program…I go back to it [speaking of the tool-kit] very often.” |
| Bi-weekly groups Co-led: exercise physiologist & nutritionist. Group-based physical activity (e.g., walking) Education: physical activity & Diet Didactic, demonstration |
Enhance team-building in groups Encourage group interactions Group connections were valued Women valued opportunities to “tell their story” |
“Having a one-on-one (session) to find out about my story what my situation [is]. If you do that with each individual person, it will help to know more about the dynamics of the participants, the breast cancer patients, and how we're all connected and how we're, in some cases, not connected.” “It would have been nice if you guys encouraged partnerships with us going forward….accountability partners.” “Our particular group was exceptional…Great attendance. We were there on a common basis, interacted very effectively with everyone as we developed a common basis.” |
| Individual interventionist — participant interactions |
One-on-one sessions were valued. One size doesn't fit all. Data collection tools should serve as resources to support participants rather than be too cumbersome (e.g. food diaries) or stressful (treadmill test equipment). |
“One thing I did like about (the nutritionist) is that after we had the initial meeting and she kind of gave us the basics, then she did meet with each one of us, and then she went over. And then, the next time, she brought individual information for each of us.” “I found it to be very cumbersome (to complete the food diary), even though they put it in a little folder and said you can carry it…I don't like carrying a whole bunch of stuff in my purse or paper.” |
| Biweekly individual coaching Phone-based (delivered by trained survivor) Tailored to baseline intentions & psychosocial factors Integrated MI |
Clarifying the role that the survivor coach is not providing mental health. Meeting the survivor coach in person before starting the calls to build rapport. Ensure that there is flexibility in MI calls. |
“It was good to talk to someone who had already gone through it… So, it was good to talk to someone. I'd say it was helpful.” “I feel a sense of rigidity that she (the survivor coach) was working within some confinements or some parameters.” “The psychology piece — mental health, I think, needs to be stepped up a little bit… You have a survivor coach and you have a psychologist who specialized with people who have gone through these experiences.” |
5. Discussion
Despite the benefits of maintaining a healthy weight after breast cancer, almost half of breast cancer survivors are either overweight or obese and there have been surprisingly few RCTs developed for Black survivors [24]. In this RCT feasibility pilot, 80.6% of survivors were obese and 75% had at least one comorbid condition. Nevertheless, we found that survivors were motivated to make lifestyle changes as evidenced by high uptake, and study completion rates. Our innovative multimodal approach that included a trained peer was acceptable to women and garnered high ratings of satisfaction with the overall intervention and the group and individual formats. The observed improvements in physical activity and dietary behaviors suggest that the strategies in the intervention may be useful to impact these behaviors and reduce overweight and/or obesity in black survivors. We observed a three-fold increase in physical activity levels, as well as improvements in dietary behaviors, suggesting feasibility for future studies. Qualitative results revealed that while women were aware of the importance of making lifestyle changes, they had not received opportunities to engage in a formal structured program to do so. Because few studies have reported on black survivors' experiences with a lifestyle intervention, these in-depth data will be useful to improve recruitment and adherence in future RCTs.
In concert with other interventions, we found evidence for dietary improvements [21,23] and a trend toward a lower BMI, body weight, and waist/hip ratio [21,22,24]. Cardiovascular fitness levels improved in the intervention group, but appeared to decline in the control group. Given the small sample, a larger sample is needed to confirm this finding. To our knowledge, this is the only intervention study that reports cardiovascular fitness in black breast cancer patients.
The improvement in cardiovascular fitness supports findings from studies in white breast cancer survivors [38–40]. Changes in cardiovascular fitness are typically expected after at least 6 months of regular physical activity. Thus, the observed trends in our 12-week pilot suggest that a longer intervention might glean significant results that may impact outcomes in black survivors. An intervention with a longer duration and observational time frame that includes multiple objective measures may also be useful to observe meaningful changes in the overall weight loss. Studies of longer interventions and observational times have been associated with greater percent loss of body weight. (Rock, et al., 2015). It will also be important to observe changes in body composition as this may also confound changes in weight alone.
Taken together, this study has implications for future interventions and research with black survivors. One implication of our findings is that more interventions are needed for black survivors who are overweight and obese. Most samples have had few Black survivors and only a handful of interventions have tailored approaches to social constructs (e.g., spirituality) shown to be relevant to many black survivors.
While breast cancer survivors in general are at increased risk of death from cardiovascular disease, black survivors have higher mortality than whites [41]. Not increasing physical activity in black survivors after a diagnosis may lead to declines in cardiovascular fitness. While our data are only preliminary, an analysis of more than 26,000 women in the U.S. women found that black survivors had a 1.4-fold greater risk of heart failure than white survivors [42]. Thus, trends with regard to physical activity in this group have potential to reduce disparities in black women diagnosed with breast cancer [43]. In addition to patient report, it will be important to collect objective physical activity data using tools such as actigraph monitors.
Stepping STONE was acceptable and may provide a platform for engaging black survivors in cancer survivorship research. While this study is a feasibility study and hypothesis generating, it has several strengths, including a focus on an underrepresented minority group, a RCT design, and the use of culturally-sensitive intervention strategies [44,45]. Study findings support previous promising results observed by the few studies conducted with black survivors [21–24] and provides a unique contribution due to: [1] delivery of intervention strategies not previously tested in overweight black survivors (MI to address motivators and barriers identified at baseline); [2] inclusion of objective validated measures of fitness (VO2max); [3] use of a trained survivor coach; and [4] use of a mixed methods approach to evaluate participants' satisfaction with the intervention. Employment of these integrated approaches has not been previously reported in black survivors.
Despite several strengths, some limitations should be considered. First, the small sample limits statistical power and our ability to conduct multivariate analysis and to detect significant differences. Next, group sessions were offered every other week, which may have hindered our ability to detect the impact of sequential weeks of exercise. Future studies will benefit from sequential groups as suggested by our focus group participants. Also, we found that study participants felt that completion of food diaries was cumbersome. Women may benefit from the use of computerized tracking of food intake. Because we did not collect dietary data beyond baseline in the control group we do not know what dietary changes (if any) were made in this group. Future studies would benefit from assessment of dietary behaviors in control and/or comparison groups.
Although all women met the sedentary eligibility screening criteria, baseline IPAQ showed higher physical activity levels, suggesting that some participants may have been more active at baseline. However, there is evidence that physical activity levels tend to be overestimated with the IPAQ [46,47] and participant's VO2max levels indicate that all participants were sedentary [10,44]. Future intervention trials may benefit from the use of emergent technology such as the Fitbit® to facilitate tracking of physical activity and food intake. Women in our study were from an urban area and were largely educated so results may not be generalizable to women from more rural areas or lower education. Compared to whites, black survivors have higher risks of dying from breast cancer, breast cancer recurrence, non-breast cancer death, comorbid conditions, and cardiovascular risk factors. Reducing obesity through increased physical activity and an improved diet has potential to impact many of these disparate outcomes. Approaches are needed to improve physical activity, diet, and sustain the health benefits of behavior change and many Black survivors appear ready to make that change, as one participant stated: “This is the next step in making sure you're living a more healthy life.”
Acknowledgments
This study was funded by the National Cancer Institute R21CA149996. It was also supported in part by the Biostatistics and Bioinformatics Shared Resource (Makambi) and the Tissue Culture Shared Resource at Lombardi Comprehensive Cancer Center under NCI Grant # P30CA51008 and the NCI grant # P30 CA051008. Researchers are indebted to the survivors who took time to participate in the study and appreciate the interventional role of Dr. Elizabeth Dennis Parker and Ms. Wanda Lucas.
Footnotes
Ethical declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Conflict of interest
Vanessa B. Sheppard, Ph.D., Jennifer Hicks, MS, Kepher Makambi, Ph.D., Alejandra Hurtado-de-Mendoza, Ph.D., Wendy Demark-Wahnefried, Ph.D., R.D., and Lucile Adams-Campbell, Ph.D. declare that they have no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin PJ, Boyd NF. Body size and breast cancer prognosis: a critical review of the evidence. Breast Cancer Res. Treat. 1990;16:205–214. doi: 10.1007/BF01806329. [DOI] [PubMed] [Google Scholar]
- 3.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, et al. Bodymass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol. Biomark. Prev. 2009;18:1403–1409. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res. Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J. Natl. Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 6.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med. Oncol. 2011;28:753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 8.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J. Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 9.Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Dizon D, et al. Survivorship: nutrition and weight management, version 2.2014. Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2014;12:1396–1406. doi: 10.6004/jnccn.2014.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin. Breast Cancer. 2008;8:70–79. doi: 10.3816/CBC.2008.n.005. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard CM, Courneya KS, Stein K. American Cancer Society's SCS-II, Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J. Clin. Oncol. 2008;26:2198–2204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 12.Harrison S, Hayes SC, Newman B. Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psychooncology. 2009;18:387–394. doi: 10.1002/pon.1504. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- 14.Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, et al. Factors associated with weight gain in women after diagnosis of breast cancer. Women's Healthy Eating and Living Study Group. J. Am. Diet. Assoc. 1999;99:1212–1221. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 15.Sheppard VB, Chiranjeeu D, Oppong B, Hirpa F, Dennis EP, Willey S, et al. Weight Changes in Black and White Breast Cancer Patients Undergoing Chemotherapy. 2013 [Google Scholar]
- 16.Smith AW, Alfano CM, Reeve BB, Irwin ML, Bernstein L, Baumgartner K, et al. Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer Epidemiol. Biomark. Prev. 2009;18:656–663. doi: 10.1158/1055-9965.EPI-08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paxton RJ, Phillips KL, Jones LA, Chang S, Taylor WC, Courneya KS, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118:4024–4031. doi: 10.1002/cncr.27389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason C, Alfano CM, Smith AW, Wang CY, Neuhouser ML, Duggan C, et al. Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol. Biomark. Prev. 2013;22:1153–1161. doi: 10.1158/1055-9965.EPI-13-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014 doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J. Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 21.Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Prev. Chronic Dis. 2009;6:A22. [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson DB, Porter JS, Parker G, Kilpatrick J. Anthropometric changes using a walking intervention in African American breast cancer survivors: a pilot study. Prev. Chronic Dis. 2005;2:A16. [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith KA, Royak-Schaler R, Nesbitt K, Zhan M, Kozlovsky A, Hurley K, et al. A culturally specific dietary plan to manage weight gain among African American breast cancer survivors: a feasibility study. Nutr. Health. 2012;21:97–105. doi: 10.1177/0260106012459938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djuric Z, Mirasolo J, Kimbrough L, Brown DR, Heilbrun LK, Canar L, et al. A pilot trial of spirituality counseling for weight loss maintenance in African American breast cancer survivors. J. Natl. Med. Assoc. 2009;101:552–564. doi: 10.1016/s0027-9684(15)30940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, et al. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J. Clin. Oncol. 2015;33:3169–3176. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong A, Tussing-Humphreys LM, Odoms-Young AM, Stolley MR, Fitzgibbon ML. Systematic review of behavioural interventions with culturally adapted strategies to improve diet and weight outcomes in African American women. Obes. Rev. 2014;15(Suppl. 4):62–92. doi: 10.1111/obr.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardcastle S, Blake N, Hagger MS. The effectiveness of a motivational interviewing primary-care based intervention on physical activity and predictors of change in a disadvantaged community. J. Behav. Med. 2012;35:318–333. doi: 10.1007/s10865-012-9417-1. [DOI] [PubMed] [Google Scholar]
- 28.Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York: Guilford Press; 2008. New York. [Google Scholar]
- 29.Bandura A. Health promotion by social cognitive means. Health Educ. Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 30.Ajzen I. The theory of planned behavior. Organizational behavior and human decision process. Organ. Behav. Hum. Decis. Process. 1991;50:179–211. [Google Scholar]
- 31.National Cancer Institute. Facing forward life after cancer treatment. [Last updated in May 2014]; [WWW Document] URL http://www.cancer.gov/publications/patient-education/facing-forward.
- 32.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J. Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 33.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J. Am. Heart. Assoc. 2014;3:e000432. doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8 doi: 10.1186/1479-5868-8-115. (115-5868-8-115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croft JB, Keenan NL, Sheridan DP, Wheeler FC, Speers MA. Waist-to-hip ratio in a biracial population: measurement, implications, and cautions for using guidelines to define high risk for cardiovascular disease. J. Am. Diet. Assoc. 1995;95:60–64. doi: 10.1016/S0002-8223(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 36.Sheppard VB, Wallington SF, Willey SC, Hampton RM, Lucas W, Jennings Y, et al. A peer-led decision support intervention improves decision outcomes in black women with breast cancer. J. Cancer Educ. 2013;28:262–269. doi: 10.1007/s13187-013-0459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual. Health Res. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 38.Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: a randomized controlled trial. Cancer Causes Control. 2013;24:181–191. doi: 10.1007/s10552-012-0104-x. [DOI] [PubMed] [Google Scholar]
- 39.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res. Treat. 2008;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 40.Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J. Clin. Oncol. 2005;23:2378–2388. doi: 10.1200/JCO.2005.04.106. [DOI] [PubMed] [Google Scholar]
- 41.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valina-Toth A. African American race is a correlate of heart failure in breast cancer survivors: a study of 26,347 women identified with breast cancer from 1973–2007. 2013 Mar 9; [Google Scholar]
- 43.Scott JM, Lakoski S, Mackey JR, Douglas PS, Haykowsky MJ, Jones LW. The potential role of aerobic exercise to modulate cardiotoxicity of molecularly targeted cancer therapeutics. Oncologist. 2013;18:221–231. doi: 10.1634/theoncologist.2012-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prentice AM, Jebb SA. Beyond body mass index. Obes. Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 45.Khalil SF, Mokhtar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors. 2014;14:10895–10928. doi: 10.3390/s140610895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med. Sci. Sports Exerc. 2014;46:99–106. doi: 10.1249/MSS.0b013e3182a0595f. [DOI] [PubMed] [Google Scholar]
- 47.Oyeyemi AL, Umar M, Oguche F, Aliyu SU, Oyeyemi AY. Accelerometer-determined physical activity and its comparison with the International Physical Activity Questionnaire in a sample of Nigerian adults. PLoS One. 2014;9:e87233. doi: 10.1371/journal.pone.0087233. [DOI] [PMC free article] [PubMed] [Google Scholar]