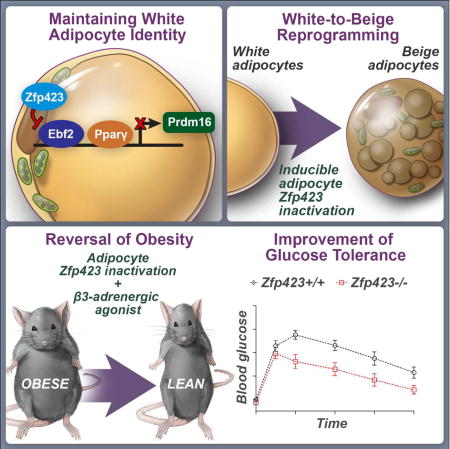
Summary
The transcriptional regulators Ebf2 and Prdm16 establish and maintain the brown/beige fat cell identity. However, the mechanisms operating in white adipocytes to suppress the thermogenic gene program and maintain an energy-storing phenotype are less understood. Here, we report that the transcriptional regulator, Zfp423, is critical for maintaining white adipocyte identity through suppression of the thermogenic gene program. Zfp423 expression is enriched in white vs. brown adipocytes and suppressed upon cold exposure. Doxycycline-inducible inactivation of Zfp423 in mature adipocytes, combined with β-adrenergic stimulation, triggers a conversion of differentiated adiponectin-expressing inguinal and gonadal adipocytes into beige-like adipocytes; this reprogramming event is sufficient to prevent and reverse diet-induced obesity and insulin resistance. Mechanistically, Zfp423 acts in adipocytes to inhibit the activity of Ebf2 and suppress Prdm16 activation. These data identify Zfp423 as a molecular brake on adipocyte thermogenesis and suggest a therapeutic strategy to unlock the thermogenic potential of white adipocytes in obesity.
TOC image
How white adipocytes suppress their thermogenic gene program has remained unclear. Here, Shao et al. identify Zfp423 as a molecular brake on adipocyte thermogenesis through inhibition of Ebf2 activity. Inducible deletion of Zfp423 in obese mice triggers a white-to-beige adipocyte reprogramming and a reversal of obesity and insulin resistance.
Introduction
Adipocytes regulate several aspects of energy homeostasis in mammalian systems. White fat cells represent the main site of energy storage, containing a single large lipid droplet and the enzymatic machinery to both synthesize and hydrolyze triglycerides. In addition, fat cells secrete numerous proteins that affect various aspects of energy homeostasis, including food intake, insulin sensitivity, and cardiovascular performance (Rosen and Spiegelman, 2014). In the setting of obesity, white adipose tissue (WAT) expands considerably to meet the increased demand for energy storage; however, this expansion can be pathological and can contribute directly to systemic metabolic dysfunction (Sun et al., 2011).
Most mammals contain a second class of adipocytes that function in adaptive thermogenesis (Cohen and Spiegelman, 2015). At least two subtypes of thermogenic adipocytes exist; brown and beige fat cells. Brown and beige fat cells are specialized to dissipate chemical energy in the form of heat, and likely evolved to protect mammals from hypothermia (Cannon and Nedergaard, 2004). This thermogenic function is mediated predominantly by the presence of UCP-1, a protein that catalyzes a proton leak across the inner mitochondrial membrane. Brown adipocytes are organized into distinct depots and originate developmentally from a Myf5+ lineage (Seale et al., 2008). Beige fat cells exist as clusters found embedded within white adipose tissue depots. Most of these cells are not derived from a Myf5-lineage (Sanchez-Gurmaches and Guertin, 2014; Sanchez-Gurmaches et al., 2016); a number of studies suggest a smooth muscle-like origin for at least a subset of these cells (Long et al., 2014; McDonald et al., 2015). There is considerable interest and excitement over the possibility of stimulating the formation and activity of beige and/or brown adipocytes in humans as a therapeutic treatment for obesity and metabolic disease (Betz and Enerback, 2015; Schrauwen et al., 2015). Adult humans have existing beige/brown adipocytes and these cells can be activated under a number of physiological or pharmacological conditions (Cypess et al., 2015; Lidell et al., 2013; Virtanen et al., 2009). However, it remains unclear as to whether there are sufficient numbers of thermogenic adipocytes present, particularly in obese individuals, to exert a long-term therapeutic effect (Cypess et al., 2015). Thus, considerable effort is now placed on elucidating the mechanisms controlling the formation of beige adipocytes from adipose precursors (adipogenesis) or other sources, with the hope of stimulating the recruitment of additional beige fat cells in obesity.
The establishment and maintenance of functional adipocytes is dependent on a number of critical events: 1) preadipose cell determination, involving the commitment of multipotent progenitors to the adipocyte lineage, 2) adipocyte differentiation, in which committed preadipose cells undergo a morphological and biochemical transition into mature adipocytes in response to appropriate cues, and 3) adipocyte maintenance, in which the cellular identity and functional properties of the terminally differentiated cells are maintained. The seminal breakthrough discovery in the field of adipogenesis was the identification of the nuclear hormone receptor, Pparγ, as a differentiation-induced “master regulator” of adipocyte differentiation (Chawla et al., 1994; Tontonoz et al., 1994). The discovery of Pparγ left open the question of how the fates of specific subtypes of adipocytes were determined and maintained. Efforts to address this question led first to the discovery of Pgc-1α, a cold-induced co-activator of Pparγ whose function is to activate the thermogenic gene program (Puigserver et al., 1998). Since then, many other transcriptional regulators of thermogenesis and Pgc-1α activity have been identified (Kong et al., 2014; Seale, 2015). Another important breakthrough came more recently with the discoveries of Prdm16, its co-regulator EHMT1, and the transcription factor, Ebf2, all three of which specify the brown/beige lineage from mesenchymal precursors (Ohno et al., 2013; Rajakumari et al., 2013; Seale et al., 2007). Ebf2 expression defines brown/beige adipocyte precursors and coordinates with Pparγ to directly regulate the expression of Prdm16 (Wang et al., 2014). Importantly, the loss of Ebf2 in mice profoundly impacts brown and beige adipocyte formation. Prdm16 and Ebf2 represent brown fat determination factors that establish and maintain the brown/beige adipocyte cellular identity; however, considerably less is known about the transcriptional components maintaining the identity and fate of white adipocytes. In particular, it remains unclear how mature white fat cells can resist activation of the thermogenic gene program, despite expressing many of same core transcriptional components. Moreover, whether this program can be sufficiently unlocked in white adipocytes of obese animals to help facilitate weight loss remains unknown.
We previously identified the C2H2 zinc-finger protein, Zfp423, as a transcriptional regulator of preadipocyte determination (Gupta et al., 2010). Zfp423, functioning in part through co-activation of Smad proteins in the bone morphogenic protein (BMP) signaling cascade, regulates preadipocyte levels of Pparγ and adipogenesis. Zfp423 expression defines committed preadipocyte mural cells that reside in the vasculature of adult adipose tissues (Gupta et al., 2012; Vishvanath et al., 2016). However, its expression persists throughout adipocyte differentiation and is present in all mature fat cells. Zfp423 regulates the formation of adipocytes; however, the function of Zfp423 in maintaining mature adipocyte function is unclear.
Here, we use a model for doxycycline-inducible deletion of Zfp423 in mature adipocytes of adult mice to reveal that Zfp423 suppresses the thermogenic gene program in fully differentiated white adipocytes. This occurs, at least in part, through direct repression of Ebf2 activity. Inducible genetic ablation of Zfp423 in white adipocytes of adult animals leads to a conversion of mature white adipocytes into functional beige adipocytes, facilitating both the prevention and reversal of diet-induced obesity and impaired glucose homeostasis. Furthermore, we find that the transcriptional complex involving Zfp423 and Ebf2 is regulated by the brown fat determination factor BMP7, providing mechanistic insight into how BMP7 can promote brown adipogenesis and thermogenesis. All together, these data implicate Zfp423 as a white adipocyte determination factor, uncover a novel mechanism by which white adipocytes suppress their thermogenic gene program, and suggest a therapeutic strategy to unlock the thermogenic potential of white fat cells as a treatment for obesity and type 2 diabetes.
Results
Adipocyte-specific inactivation of Zfp423 induced in adult mice leads to a accumulation of beige-like adipocytes in WAT depots
Our previous work revealed that Zfp423 expression identifies preadipocytes (Gupta et al., 2010; Vishvanath et al., 2016). In this context, Zfp423 regulates Pparγ expression and the potential of cells to undergo adipocyte differentiation. Zfp423 expression persists throughout adipocyte differentiation and Zfp423 mRNA levels can be readily detected in purified adipocyte fractions obtained from gonadal (gWAT), inguinal (iWAT), and interscapular brown adipose tissue (BAT) depots of adult mice (Fig. 1A). However, the abundance of Zfp423 mRNA is significantly higher in white adipocytes compared to brown adipocytes (gWAT and iWAT vs. BAT) (Fig. 1A). Differential expression of Zfp423 in mature white vs. brown adipocytes raises the possibility that this factor may play a role in controlling the functional properties of white or brown fat cells.
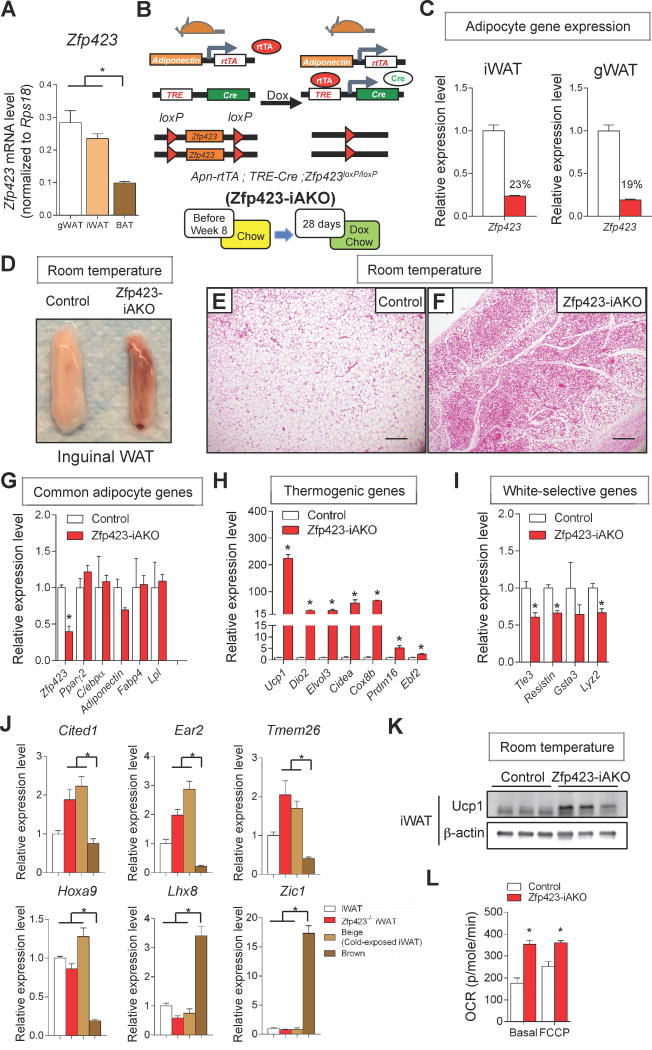
Figure 1. Widespread accumulation of beige-like adipocytes in WAT depots of inducible adipocyte-specific Zfp423 knockout mice.
(A) mRNA levels of Zfp423 in fractionated adipocytes isolated from anatomically distinct fat pads of adult C57BL/6 mice. * denotes p<0.05 from one-way ANOVA. n=6.
(B) Inducible inactivation of Zfp423 in mature adipocytes (Zfp423-iAKO mice) is achieved by breeding the AdiponectinrtTA transgenic mice to animals expressing Cre recombinase under the control of the tet-reponse element (TRE-Cre) and carrying floxed Zfp423 alleles (Zfp423loxP/loxP). Littermates carrying only AdiponectinrtTA and Zfp423loxP/loxP alleles (i.e. Cre-) were used as the control animals. Mice were kept at room temperature and fed on normal chow until 8 weeks of age before switching to doxycycline (dox)-containing chow diet for another 28 days.
(C) Relative mRNA levels of Zfp423 in fractionated adipocytes isolated from inguinal white adipose tissue (iWAT) and gonadal white adipose tissue (gWAT) of control and Zfp423-iAKO mice after dox feeding. n=4.
(D) Representative photograph of iWAT from control and Zfp423-iAKO mice after dox feeding.
(E) Representative hematoxylin and eosin (H&E) staining of iWAT section obtained from control mouse after dox feeding. Scale bar=200μM.
(F) Representative H&E staining of iWAT section obtained from Zfp423-iAKO mouse after dox feeding
(G) Relative mRNA levels of common adipocyte genes in the iWAT of control and Zfp423-iAKO mice after dox feeding. * denotes p<0.05 from Student’s t-test. n=4.
(H), Relative mRNA levels of brown/beige-selective thermogenic genes in the iWAT of control and Zfp423-iAKO mice after dox feeding. * denotes p<0.05 from Student’s t-test. n=4.
(I), Relative mRNA levels of white adipocyte-selective genes in the iWAT of control and Zfp423-iAKO mice after dox feeding. * denotes p<0.05 from Student’s t-test. n=4.
(J) Relative mRNA levels of brown adipocyte- and beige adipocyte-selective markers in the inguinal WAT depots of control mice (iWAT), Zfp423-iAKO mice (Zfp423−/− iWAT), cold-exposed control mice (Beige), and brown adipose tissue (BAT) of control mice after dox feeding. * denotes p<0.05 from one-way ANOVA. n=4–6.
(K) Western blot of Ucp1 protein levels in the iWAT of control and Zfp423-iAKO mice after dox feeding.
(L) Basal and maximal (FCCP) oxygen consumption rate (OCR) of diced iWAT isolated from control and Zfp423-iAKO mice after dox feeding. * denotes p<0.05 from Student’s t-test. n=5.
To investigate the function of Zfp423 in fully differentiated mature adipocytes, we generated a novel mouse model in which Zfp423 can be inactivated in adiponectin-expressing adipocytes in a doxycycline (Dox)-dependent manner. The model consists of one transgene that expresses the reverse tetracycline transactivator (rtTA) under the control of a 5.4 kb promoter fragment of the adiponectin locus, another transgene in which Cre recombinase is expressed from a promoter containing the Tet-response element (TRE-Cre), and two conditional (loxP-flanked) Zfp423 alleles (AdiponectinrtTA; TRE-Cre; Zfp423loxP/loxP animals, herein denoted as Zfp423-iAKO mice) (Fig. 1B). In this system, the administration of Dox-containing chow diet to adult mice triggers Cre-mediated recombination specifically in terminally differentiated adipocytes (Wang et al., 2013). Accordingly, Zfp423 mRNA levels are reduced by ~80% in the purified adipocyte fraction from both iWAT and gWAT of Zfp423-iAKO mice following the administration of a DOX-containing chow diet for 7 days (Fig. 1C). Therefore, this system allows for the assessment of Zfp423 function in both major white adipose depots.
We administered control (AdiponectinrtTA; TRE-Cre mice or AdiponectinrtTA; Zfp423loP/loP animals) and Zfp423-iAKO mice Dox-containing chow diet at room temperature for four weeks, beginning at eight weeks of age. After the four weeks period, a dramatic morphological change of white adipose tissue was observed in the Zfp423-iAKO mice. In particular, the inguinal WAT depot (iWAT) of Zfp423-iAKO mice exhibited a uniform “brown” appearance (Fig. 1D). Hematoxylin and eosin (H&E) staining of iWAT sections revealed a widespread accumulation of multilocular, brown/beige-like adipocytes in the Zfp423-deficient fat depot (Fig. 1E,F). mRNA levels of pan-adipocyte markers such as Pparγ, C/ebpa, adiponectin, Fabp4, and Lpl were not altered in Zfp423-deficient fat depots (Fig. 1G); this suggests that Zfp423 is not required in mature adipocytes to maintain a fat cell identity per se. However, mRNA levels of key components of the adipocyte thermogenic gene program were robustly enriched in Zfp423-deficient WAT (Fig. 1H), while mRNA levels of white adipocyte-selective genes were significantly downregulated (Fig. 1I). Moreover, mRNA levels of genes recently described as beige-selective were enriched in the iWAT of Zfp423-iAKO mice; classic brown adipocyte markers remained low (Fig. 1J) (Sharp et al., 2012; Shinoda et al., 2015). Most notably, levels of Ucp1 mRNA were ~200-fold higher than observed in tissues of control mice. Accordingly, protein levels of Ucp1 were also enriched in the knockout tissue lysates and observed ubiquitously across histological sections (Fig. 1K, Fig. S1A). Zfp423-deficient WAT also appears more metabolically active. Both basal and maximum rates of oxygen consumption were significantly elevated in diced iWAT tissue from the Zfp423-iAKO animals when compared to controls (Fig. 1L).
The morphology and metabolic activity of the iWAT from Zfp423-iAKO mice maintained at room temperature closely resembled beige adipocytes active in cold-exposed mice. To explore this more globally, we obtained and compared global microarray gene expression profiles of iWAT from control animals maintained at room temperature, control animals exposed to the cold for 14 days, and Zfp423-iAKO mice maintained at room temperature. 270 transcripts were differentially regulated (± 2 fold) in Zfp423-deficient iWAT when compared to iWAT from control animals maintained at room temperature (Table S1). Of these 270 transcripts, 140 (52%) were genes whose expression is regulated by cold exposure (Fig. S2A). Functional classification of these 140 genes by gene ontology analysis indicated that nearly all of these genes are related to mitochondrial function and biogenesis, a hallmark of thermogenic adipocytes (Fig.S2B).
Activation of the thermogenic gene program in WAT of the Zfp423-iAKO mice was not limited to the inguinal depot. Four weeks after the deletion of Zfp423, an induction in expression of genes of the thermogenic program were also observed in gonadal WAT, albeit to a lesser degree (Fig.S1B). However, the expression of these same genes in the interscapular BAT of Zfp423-iAKO mice was not significantly different from those observed in control animals (Fig.S1C). This may be a result of the recombination efficiency in this depot. For reasons currently unclear, the adiponectin-driven rtTA transgene is not active in all interscapular brown adipocytes (Wang et al., 2013). Accordingly, we consistently observe <50% reduction in mRNA levels of Zfp423 in this depot.
We also examined adipose depots of Zfp423-iAKO mice maintained on a DOX-containing chow diet for 16 weeks. In these animals, a significant morphological and molecular “browning” of all major WAT depots can now be observed, including iWAT, rWAT (peri-renal WAT), and gWAT (Fig. S3). These data suggest that Zfp423 deletion can unlock the thermogenic gene program in white adipocytes, but an additional signal is needed to fully activate the thermogenic function of the cells.
It is well known that anatomically distinct depots differ in their thermogenic capacity. This is due, in part, to intrinsic differences in the distinct adipocytes. However, this is likely also due to differences in sympathetic innervation (activation of β3 adrenergic signaling) or immune response, both of which can modulate “browning”. We tested whether the additional signal needed to fully trigger browning of the gWAT in Zfp423-iAKO mice was related to sympathetic outflow. In this experiment, we held animals at thermoneutrality while treating with DOX for one week to delete Zfp423 (Fig. S4). Inactivation of adipocyte Zfp423 at thermoneutrality led to an increase in expression of UCP1 and other genes of the thermogenic program; however, the effects were less robust than observed in the aforementioned experiments performed at room temperature, and morphological changes adipose depots were not observed (Fig. S4). We then treated mice at thermoneutrality with the β3-agonist, CL316243, once daily for three days total. Following treatment with the β3 receptor agonist, we observed a greater accumulation of beige adipocytes in the iWAT of Zfp423-iAKO mice when compared to tissues from treated controls. Compared to the inguinal WAT depot, the gonadal WAT depot is normally quite resistant to the effects of pharmacological β3-receptor stimulation; however, deletion of Zfp423 in gonadal adipocytes renders the depot capable of adopting a robust beige phenotype within 3 days of treatment, similar to that observed in normal iWAT depots. (Fig. S4).
Beige-like adipocytes in Zfp423-iAKO mice arise through a conversion of adiponectin-expressing Zfp423-deficient adipocytes
White adipocytes in the inguinal WAT depot of adult mice maintained on a standard chow diet at room temperature exhibit very little turnover (Wang et al., 2013). However, beige adipocytes accumulate rapidly within the white adipose depots of mice exposed to cold temperatures. Lineage tracing studies suggest that beige adipocytes can arise through a number of mechanisms. Recent studies have indicated that beige adipocytes arise, likely in large part, through de novo differentiation from beige preadipocytes during the period of cold exposure (Berry et al., 2016; Long et al., 2014; Vishvanath et al., 2016; Wang et al., 2013). Mature unilocular adipocytes can also adopt a beige adipocyte phenotype upon cold exposure. The specific unilocular adipocytes that become UCP1+ multilocular cells may represent existing dormant beige adipocytes or bona fide white adipocytes undergoing a lineage conversion (for review, see (Kajimura et al., 2015)). The latter mechanisms are difficult to distinguish from one another due to a lack of suitable molecular markers.
We sought to determine the source of the beige adipocytes accumulating in the Zfp423-iAKO mice. In principle, the accumulated beige-like adipocytes could emerge through a conversion or “reprogramming” of Zfp423-deficient adiponectin+ adipocytes. Alternatively, beige adipocytes can emerge from precursors in response to events triggered by Zfp423-deletion in mature adipocytes. We employed “pulse-chase” lineage tracing to determine if the beige adipocytes in the Zfp423-iAKO mice indeed arise through a direct conversion of existing Zfp423-deficient adiponectin+ white adipocytes. We reconstituted the Cre-dependent Rosa26RmT/mG reporter to the Zfp423-iAKO animal background (AdiponectinrtTA; TRE-Cre; Zfp423loxP/loxP; Rosa26RmTmG animals) (Fig.2A). This allows for DOX-dependent inactivation of the Zfp423 locus with simultaneous indelible membrane-bound GFP (mGFP) labeling of the targeted adipocytes. We administered DOX for seven days at room temperature to ensure complete labeling and deletion of Zfp423 (“pulse”). After this initial pulse-labeling period, Cre expression and the subsequent loss of Zfp423 expression occurs efficiently and specifically in mature adipocyte fraction of the inguinal adipose depot (Fig. 2B,C). Indirect immunofluorescence assays with anti-GFP antibodies indicated that nearly all adipocytes were expressed mGFP; however, at this time no multilocular cells were present in control or knockout mice (Fig. 2D–F). Animals were then maintained at room temperature for three weeks in the absence of DOX (“chase”). After the three-week period, large regions of multi-locular cells were present only in the Zfp423-iAKO mice (Fig.2G–I). Importantly, all of the cells we observed continued to express mGFP (Fig. 2H). This indicates that the beige adipocytes accumulating this model were derived from Zfp423-deficient adiponectin+ adipocytes. As a control experiment, we also placed a cohort of control mice (AdiponectinrtTA; TRE-Cre; Rosa26RmTmG animals) at 6 °C for 7 days after the pulse-labeling period. As expected and previously reported, both mGFP+ and mGFP- cells were observed (Fig. 2J), highlighting the ability of the model to capture the dual mechanisms used to recruit beige adipcytes upon cold exposure.
Figure 2. Beige adipocytes in Zfp423-iAKO mice arise through a conversion of adiponectin+ Zfp423-deficient adipocytes.
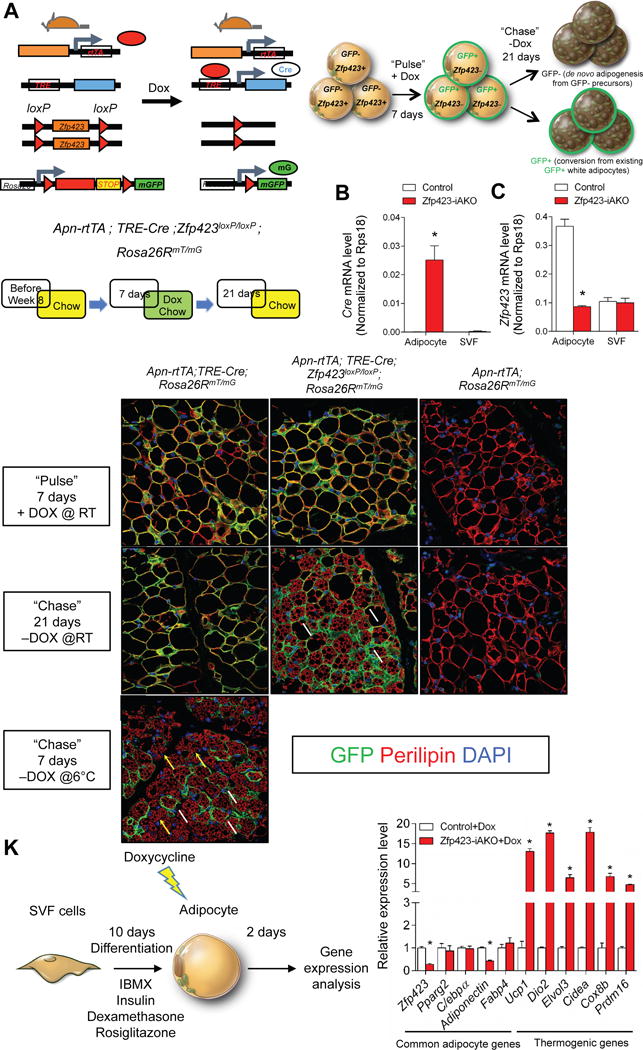
(A) The Cre-dependent Rosa26RmT/mG reporter allele was reconstituted to the Zfp423-iAKO background, allowing for indelible GFP labeling of Zfp423-deficient adipocytes. Mice were kept on normal chow until 8 weeks of age before switching to dox-containing chow for 7 days (“Pulse”). After the labeling period, the mice were switched back to a standard chow diet (devoid of Dox) for another 21 days (“Chase”). After the 21-day period, the presence of GFP- multilocular adipocytes indicates beige cells were derived from GFP- precursors. The presence of GFP+ multilocular adipocytes indicates beige cells that arise directly from mature adipocytes targeted during the pulse-labeling period.
(B) mRNA levels of Cre in purified adipocytes and stromal vascular fraction (SVF) from iWAT of control and Zfp423-iAKO mice after 7 days of dox feeding. * denotes p<0.05 from Student’s t-test. n=4.
(C) mRNA levels of Zfp423 in purified adipocytes and SVF from iWAT of control and Zfp423-iAKO mice after 7 days of dox feeding. * denotes p<0.05 from Student’s t-test. n=4.
(D) Representative immunofluorescence staining of Perilipin (red) and GFP (green) in iWAT sections obtained from control (Adpn-rtTA; TRE-Cre; Rosa26RmT/mG) mice after pulse-labeling (“Pulse”).
(E) Same as in (D) but from Zfp423-iAKO mice (Adpn-rtTA; TRE-Cre; Zfp423loxP/loxP;Rosa26RmT/mG).
(F) Same as in (D) but from additional control mice (Adpn-rtTA; Rosa26RmT/mG).
(G) Representative immunofluorescence staining of Perilipin (red) and GFP (green) in iWAT sections obtained from control (Adpn-rtTA; TRE-Cre; Rosa26RmT/mG) mice held at room temperature (RT) for 21 days after the removal of doxycycline (“Chase”).
(H) Same as in (G) but from Zfp423-iAKO mice (Adpn-rtTA; TRE-Cre; Zfp423loxP/loxP;Rosa26RmT/mG). White arrows denote GFP+ adipocytes.
(I) Same as in (G) but from control mice (Adpn-rtTA; Rosa26RmT/mG).
(J) Representative immunofluorescence staining of Perilipin (red) and GFP (green) in iWAT sections obtained from control (Adpn-rtTA; TRE-Cre; Rosa26RmT/mG) mice exposed to cold temperatures (6 °C) for 7 days after the removal of doxycycline (“Chase”). White arrows denote GFP+ adipocytes. Yellow arrows denote GFP− adipocytes.
(K) iWAT SVF isolated from control and Zfp423-iAKO mice were induced to differentiate in vitro. After differentiation, the adipocytes were treated with 5μM dox for 12 hours. Relative mRNA levels of common adipocyte genes and thermogenic genes were measured by qPCR in the differentiated adipocytes 2 days after dox treatment. * denotes p<0.05 from Student’s t-test. n=3
Finally, we asked if the white-to-beige adipocyte conversion is observed in vitro, in an adipocyte-autonomous manner. We isolated the stromal vascular fraction (SVF) of iWAT from control and Zfp423-iAKO animals and induced adipocyte differentiation in vitro for ten days. We then treated fully differentiated cultures with DOX for 12 hours in order to inactivate Zfp423 in adipocytes. Within 48 hours after the removal of DOX from differentiated cultures, we can observe a robust enrichment of mRNAs encoding key thermogenic genes in Zfp423-deficient adipocytes, including an ~15-fold induction of UCP1 and ~20-fold increase in Dio2 (Fig. 2K). These data indicate that Zfp423-deficient adipocytes can convert into UCP1+ beige cells in a cell autonomous manner. Taken all together, Zfp423 appears critical for the maintenance of white adipocyte cellular identity and the loss of Zfp423 triggers a conversion of white adipocytes into beige fat cells.
Mice lacking adipocyte Zfp423 are resistant to diet-induced obesity
A preponderance of evidence in the literature supports the notion that beige adipocytes can exert beneficial effects on glucose homeostasis and increase energy expenditure. Thus, we asked if the white-to-beige adipocyte conversion occurring in Zfp423-iAKO mice was likewise capable of protecting the animals against diet-induced obesity and impaired glucose tolerance. Starting at eight weeks of age, Zfp423-iAKO and control mice were administered a high-fat diet (HFD) (60% calories from fat) containing DOX. Body weights of the two groups began to significantly diverge after eleven weeks of HFD feeding, with Zfp423-iAKO mice gaining less weight on the HFD (Fig. 3A). Zfp423-iAKO weighed significantly less by week 16 of HFD feeding, coinciding with a reduction in WAT depot mass (Fig. 3B–D). Metabolically, the leaner Zfp423-iAKO mice were healthier; these animals were more glucose tolerant and more insulin sensitive than controls (Fig. 3E,F). Moreover, knockout animals exhibited less hepatic steatosis (Fig. 3G,H), and significant browning and UCP1 expression in the inguinal and gonadal WAT, but not BAT depots (Fig. 3I–K; Fig. S5A–C). Moreover, mRNA levels of inflammatory markers were decreased across all depots examined (Fig. S5A–C). It should be noted, however, that improvements in glucose tolerance and insulin sensitivity can be observed prior to the divergence in weight again, consistent with the ability of beige adipocytes to elicit beneficial effects on glucose homeostasis independent of its impact on body weight (Fig. S5D,E) (Seale et al., 2011).
Figure 3. Mice lacking adipocyte Zfp423 are resistant to diet-induced obesity.
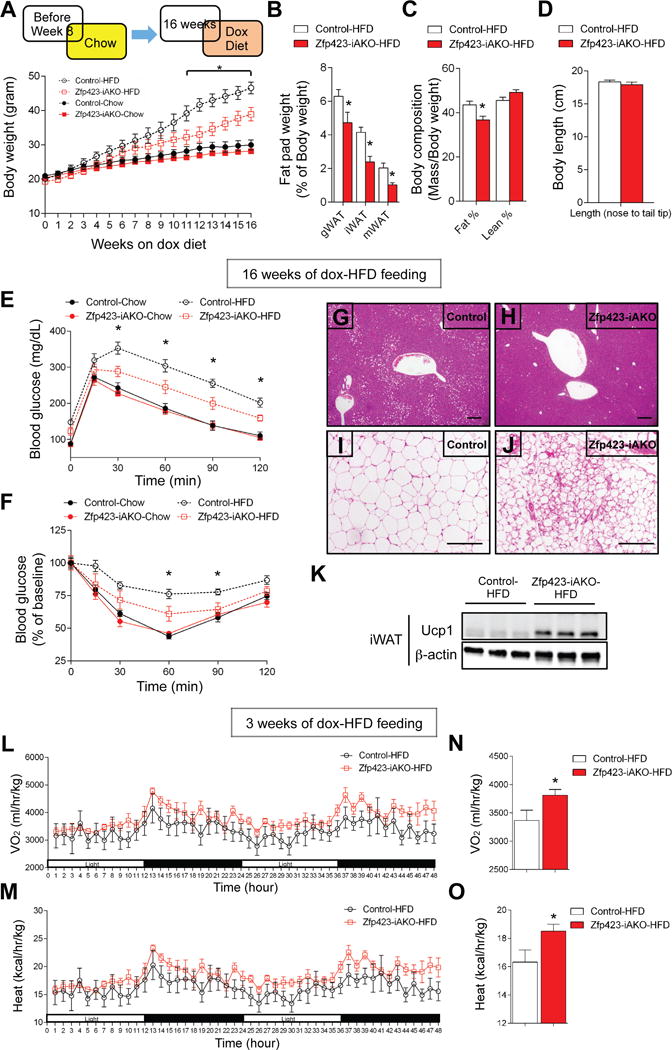
(A) Control and Zfp423-iAKO mice were fed a standard chow diet until 8 weeks of age before switching to dox-containing chow or dox-containing high fat diet (HFD). Body weights of control and Zfp423-iAKO mice were measured weekly. * denotes control-HFD vs. Zfp423-iAKO-HFD p<0.05 from two-way ANOVA. n=6–8.
(B) Fat pad weight (normalized to body weight) of control and Zfp423-iAKO mice after 16 weeks of dox-HFD feeding. * denotes p<0.05 from Student’s t-test. n=6.
(C) Total fat mass and lean mass (normalized to body weight) of control and Zfp423-iAKO mice after 16 weeks of dox-HFD feeding. * denotes p<0.05 from Student’s t-test. n=12–13.
(D) Body length of control and Zfp423-iAKO mice after 16 weeks of dox-HFD feeding. n=6.
(E) Glucose tolerance test of control and Zfp423-iAKO mice after 16 weeks of dox feeding. * denotes control-HFD vs. Zfp423-iAKO-HFD p<0.05 from two-way ANOVA. n=6–8.
(F) Insulin tolerance test of control and Zfp423-iAKO mice after 16 weeks of dox feeding. * denotes control-HFD vs. Zfp423-iAKO-HFD p<0.05 from two-way ANOVA. n=6–8.
(G) Representative H&E staining of the liver of control mice after 16 weeks of dox-HFD feeding. Scale bar=200μM.
(H) Same as in (G) but from Zfp423-iAKO mice.
(I) Representative H&E staining of the iWAT from control mice after 16 weeks of dox-HFD feeding. Scale bar=200μM.
(J) Same as in (J) but from Zfp423-iAKO mice.
(K) Western blot of Ucp1 protein levels in the iWAT of control and Zfp423-iAKO mice after 16 weeks of dox-HFD feeding.
(L) O2 consumption in control and Zfp423-iAKO mice during two complete 12 hr light and dark cycles following 3 weeks of dox-HFD feeding. n=6.
(M) Heat production in control and Zfp423-iAKO mice during two complete 12 hr light and dark cycles following 3 weeks of dox-HFD feeding. n=6.
(N) Average O2 consumption of control and Zfp423-iAKO mice during the 5-day measurement. * denotes p<0.05 from Student’s t-test. n=6.
(O) Average heat production of control and Zfp423-iAKO mice during the 5-day measurement. * denotes p<0.05 from Student’s t-test. n=6.
We also assessed the rates of energy expenditure and food intake in control and knockout HFD-fed animals at a time point before the body weights diverged. After three weeks of HFD feeding, O2 consumption, and heat production were elevated in the Zfp423-deficient mice (Fig. 3L–O). This increased energy expenditure was observed in the absence of any changes in food intake and locomotor activity (Fig. S5F,H). This suggests that increased energy expenditure induced by the loss of Zfp423 in mature adipocytes and white-to-beige cell conversion lead to resistance to diet-induced weight gain in these animals. The dependency of these phenotypes on activated beige adipocytes is supported by experiments performed at thermoneutrality. At 30 °C, deletion of Zfp423 is sufficient activate the thermogenic gene program to a certain degree (Fig. S4); however, the morphological conversion to multilocular cells and resistance to diet-induced obesity is not apparent (Fig. S6).
Zfp423 deficiency, combined with β3-adrenergic receptor activation, leads to a reversal of weight gain and improved glucose tolerance when induced in obese animals
The data above are in line with numerous mouse models demonstrating that activated beige adipocytes can be protective against weight gain and insulin resistance initiated by high-fat diet feeding. However, it has remained unclear as to whether beige adipocyte activity can be stimulated in the state of obesity (i.e. in animals already obese), leading to a reversal of the metabolic dysfunction associated with high-fat diet feeding. Pharmacological approaches/studies have correlated the browning of WAT with improved glucose homeostasis in obese animals; however, direct genetic evidence demonstrating the potential of beige adipocytes in the setting of obesity has been lacking.
We asked if the deletion of Zfp423 in mature white adipocytes, and the subsequent beige adipose accumulation, induced in obese animals can lead to the reversal of the weight again and glucose intolerance triggered by high-fat diet feeding. Following 8 weeks of HFD feeding (without DOX), control and Zfp423-iAKO male mice were switched to HFD feed containing DOX (Fig. 4A). Thus, Zfp423 deletion occurs after the mice become obese. Both groups continued to gain weight one week after switching the diet. Zfp423-iAKO gained slightly less weight during the next five weeks; however, this difference did not reach statistical significance (Fig. 4B). We also did not observe any difference in glucose tolerance at this time point (Fig. 4C). Gene expression analysis of isolated WAT depots confirmed Zfp423 inactivation and an induction of the thermogenic gene program (Fig. 4D,E). Thus, this degree of beige adipose accumulation by itself appears insufficient to increase energy expenditure to a significant degree in obese mice.
Figure 4. Reversal of weight gain and glucose intolerance by inducible inactivation of adipocyte Zfp423 in obese mice.
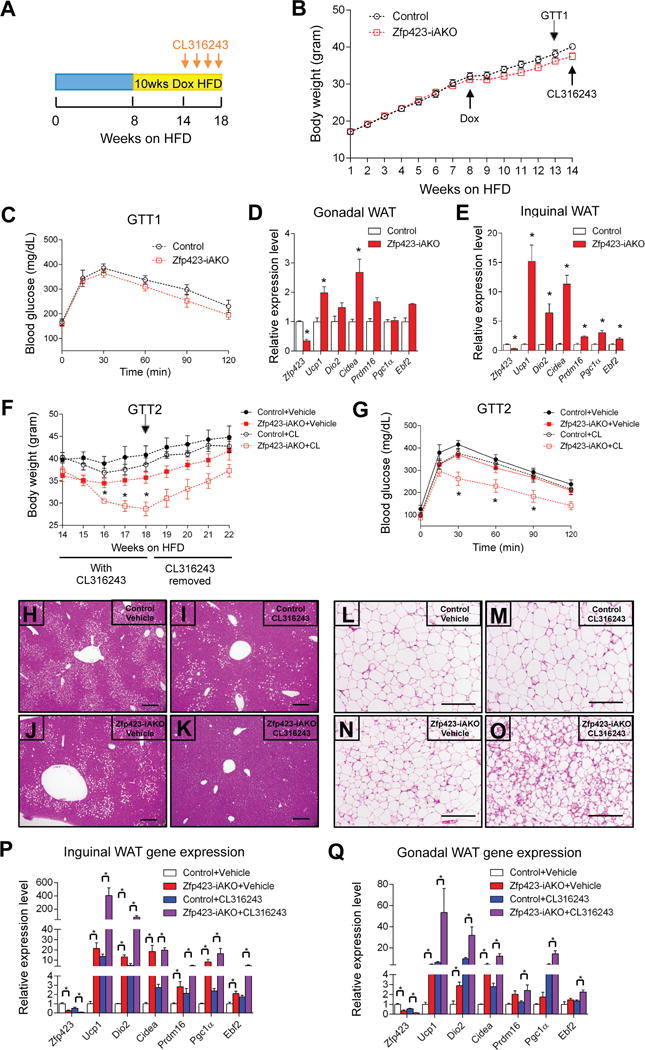
(A) Obesity was induced in 5 week-old control and Zfp423-iAKO mice by administering a high-fat diet (HFD) for 8 weeks. Then animals were switched to a dox-containing HFD (dox-HFD) for another 14 weeks. After 5 weeks of dox-HFD, the mice were treated daily with CL316243 (1mg/kg/24hr) or Vehicle (PBS) for 4 weeks via osmotic pumps.
(B) Body weights of control and Zfp423-iAKO mice prior to CL316243 administration. n=12–13.
(C) Glucose tolerance test (GTT) of control and Zfp423-iAKO mice immediately prior to CL316243 administration. n=6–7.
(D) Relative mRNA levels of indicated genes in gWAT from dox-HFD fed control and Zfp423-iAKO mice prior to CL316243 administration. * denotes p<0.05 from Student’s t-test. n=4–5.
(E) Same as in (D) but in iWAT.
(F) Body weights of control and Zfp423-iAKO mice after vehicle or CL316243 administration. * denotes control-CL316243 vs. Zfp423-iAKO-CL316243 p<0.05 from two-way ANOVA. n=5–9.
(G) Glucose tolerance test (GTT) of control and Zfp423-iAKO mice after vehicle or CL316243 administration. * denotes control-CL316243 vs. Zfp423-iAKO-CL316243 p<0.05 from two-way ANOVA. n=5.
(H) Representative H&E staining of the liver from control mice receiving vehicle treatment (Week 18 of HFD as indicated in (A)). Scale bar=200μM.
(I) Same as in (H) but from control mice receiving CL316243.
(J) Same as in (H) but from Zfp423-iAKO mice receiving vehicle.
(K) Same as in (H) but from Zfp423-iAKO mice receiving CL316243.
(L) Representative H&E staining of iWAT from control mice receiving vehicle treatment (Week 18 of HFD as indicated in (A)). Scale bar=200μM.
(M) Same as in (L) but from control mice receiving CL316243.
(N) Same as in (L) but from Zfp423-iAKO mice receiving vehicle.
(O) Same as in (L) but from Zfp423-iAKO mice receiving CL316243.
(P) Relative mRNA levels of indicated genes in iWAT from control and Zfp423-iAKO mice after vehicle or CL316243 administration. (Week 18 of HFD as indicated in (A)) * denotes p<0.05 from two-way ANOVA. n=5.
(Q) Same as in (P) but from gWAT.
As described above, thermogenic adipocytes rely on β-adrenergic signaling for their activation in vivo. It is well known that obesity is associated with augmented sympathetic activity (Tentolouris et al., 2006). Therefore, we reasoned that the beige cells induced by Zfp423-deficiency in obese mice would require a stimulus to fully activate their thermogenic function in this setting. To test this, we utilized subcutaneous osmotic pumps to deliver the β3-adrenergic receptor agonist, CL316243, daily at a dose of 1mg/kg/day for four continuous weeks. The agonist was given to obese control and Zfp423 iAKO mice after 14 weeks of HFD feeding (Fig. 4A). Control animals were largely resistant to the effects of the β3-receptor agonist. However, Zfp423-iAKO mice given the β3-agonist lost a significant amount of body weight and exhibited markedly improved glucose tolerance after four weeks of treatment (Fig. 4F,G). This was accompanied by reduced hepatic steatosis (Fig. 4H–K) and significant accumulation of beige adipocytes in the inguinal WAT (Fig. 4L–Q). The effects on body weight appear reversible as weight rebounded nearly back to base line after the osmotic pumps released all of their content (Fig. 4F). These data suggest that white adipocytes can be reprogrammed into beige-like adipocytes in obese animals, and when activated, can reverse weight gain and metabolic dysfunction triggered by HFD feeding.
Levels of Zfp423 in adipocytes are suppressed by cold exposure/β-adrenergic signaling
The robust white to beige-like adipocyte conversion in Zfp423-iAKO mice indicates that white adipocytes require Zfp423 in order to suppress their thermogenic gene program and maintain a white adipocyte phenotype. This raises the question as to whether the suppression of Zfp423 expression is part of the natural mechanisms by which thermogenic adipocytes accumulate and/or become active under physiological conditions. To address this, we examined proteins levels of Zfp423 in WAT and BAT depots of C57BL/6 animals held at room temperature or exposed to 6 °C for 1 week. Levels of Zfp423 drop considerably in all depots examined (Fig. 5A). Notably, mRNA levels of Zfp423 are suppressed specifically in the adipocyte fraction of adipose tissue in response to cold (Fig. 5B). This suppression can occur quickly, within 24 hours of transferring animals from 30 °C to 6 °C, or in response to β3-adrenergic receptor agonism. Thus, the suppression of adipocyte Zfp423 expression may allow the transcriptional program regulating adaptive thermogenesis to quickly activate upon cold exposure. Indeed, the transcriptional response to cold exposure occurs much more rapidly and robustly following the inactivation of Zfp423 in adipocytes of Zfp423-iAKO mice (Fig. 5C).
Figure 5. Physiological regulation of brown and white adipocyte Zfp423 expression.
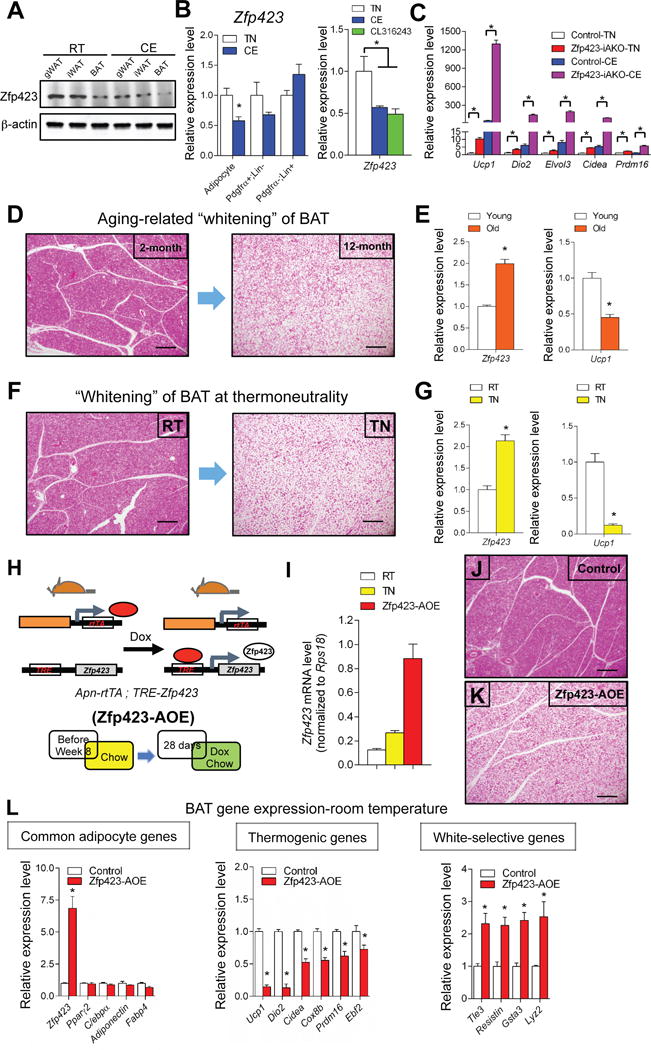
(A) Western blot of endogenous Zfp423 proteins in adipose depots isolated from C57BL/6 mice held at room temperature or exposed to cold (6°C) for 3 days.
(B) Relative mRNA levels of Zfp423 in fractionated iWAT adipocytes, Pdgfrα+; Lin-(CD31- and CD45-) cells and Pdgfrα-; Lin+ (CD31+ and CD45+) cells isolated from C57BL/6 mice held at room temperature or exposed to cold (6°C) for 3 days. Relative mRNA levels of Zfp423 in iWAT isolated from C57BL/6 mice held at thermoneutrality, exposed to cold (6°C) for 1 day or injected with CL 316243 (1mg/kg/day) for 3 days. * denotes p<0.05 from Student’s t-test or one-way ANOVA. n=4–6.
(C) Relative mRNA levels of Zfp423 and thermogenic genes in iWAT of control and Zfp423-iAKO mice held at thermoneutrality (TN) or exposed to cold (CE) (6°C) for 1 day. * denotes p<0.05 from two-way ANOVA. n=5.
(D) Representative H&E staining of interscapular BAT from 2 months-old and 12 months-old C57BL/6 mice. Scale bar=200μM.
(E) Relative mRNA levels of Zfp423 and Ucp1 in interscapular BAT from 2 months-old and 12 months-old C57BL/6 mice. * denotes p<0.05 from Student’s t-test. n=6.
(F) Representative H&E staining of interscapular BAT from C57BL/6 mice held at room temperature (RT) or thermoneutrality (TN). Scale bar=200μM.
(G) Relative mRNA levels of Zfp423 and Ucp1 in interscapular BAT from C57BL/6 mice held at room temperature (RT) or thermoneutrality (TN). * denotes p<0.05 from Student’s t-test. n=6.
(H) Animals conferring adipocyte-specific Zfp423 overexpression (Zfp423-AOE mice) were derived by breeding AdiponectinrtTA transgenic mice with transgenic mice expressing full-length murine Zfp423 under the control of a promoter containing the tet-response element (TRE-Zfp423). Littermates carrying only the AdiponectinrtTA allele were used as control animals. Mice were kept at room temperature and fed on standard chow until 8 weeks of age before switching to dox-containing chow diet for another 28 days.
(I) mRNA levels of Zfp423 in the BAT of C57BL/6 mice held at room temperature (RT) or thermoneutrality (TN), and BAT of Zfp423-AOE mice held at room temperature. n=4–6.
(J) Representative H&E staining of the BAT from control mice after dox feeding. Scale bar=200μM.
(K) Same as in (J) but from Zfp423-AOE mice.
(L) Relative mRNA levels of common adipocyte genes, thermogenic genes, and white adipocyte-selective genes in the BAT of control and Zfp423-AOE mice after dox feeding. * denotes p<0.05 from Student’s t-test. n=4.
Elevated Zfp423 expression in brown adipocytes drives a “whitening” of BAT
We also assayed the expression of Zfp423 in settings where brown adipose tissues adopt a white adipocyte-like phenotype. In aged animals, BAT depots acquire a white adipose-like phenotype characterized by the increasing presence of unilocular adipocytes and reduction in UCP1 expression (Fig. 5D,E). A similar “whitening” of BAT depots occurs in animals transitioned from room temperature to 30 °C (Fig. 5F,G). We observed that in both settings the expression of Zfp423 increases, inversely correlating with UCP1 expression. These data suggest that the activation of Zfp423 expression in brown adipocytes can switch cells to more “white-like” phenotype. We tested this hypothesis directly by generating a transgenic model in which Zfp423 expression can be induced in adiponectin+ adipocytes in the presence of DOX. This bi-transgenic model consists of the aforementioned adiponectin promoter-driven rtTA allele (AdiponectinrtTA) and a transgenic allele in which full-length Zfp423 is expressed from a promoter containing the TRE (AdiponectinrtTA; TRE-Zfp423, herein “Zfp423-AOE”) (Fig. 5H). As described above and previously reported, activity of the AdiponectinrtTA transgene is relatively weak in adult brown adipocytes (Wang et al., 2013). As a result, we achieve a modest, but physiological, overexpression of Zfp423 in the BAT of transgenic adult mice at room temperature. Importantly, levels of Zfp423 are induced in BAT to a level that is only a few-fold higher than what is observed in BAT of animals housed at thermoneutrality (Fig.5I). Strikingly, an ~7-fold overexpression of Zfp423 is sufficient to drive a conversion of BAT to a more unilocular white adipocyte-like phenotype at room temperature (Fig. 5J,K). Expression of pan-adipocyte selective genes is not affected; however, the thermogenic gene program is largely suppressed (~70–80% reduction in UCP1 mRNA) while expression of white adipocyte-selective genes is induced (Fig.5L). In cold-exposed Zfp423-AOE mice, UCP1 expression was not impacted by the Zfp423 transgene; however, transgene activity was noticeably even weaker under these conditions (Fig.S7). Overexpression of Zfp423 in iWAT is also modest (~4-fold). However, this level is sufficient to partially inhibit “browning” of the inguinal WAT depot in animals exposed to cold (Fig.S7). All together, these data indicate that Zfp423 acts a dominant suppressor of the thermogenic gene program in mature adipocytes. Importantly, the data suggest that a doubling of Zfp423 expression in brown adipocytes, combined with a gradual age-related reduction in sympathetic tone, may contribute to the natural age-related alterations in BAT phenotype.
Zfp423 suppresses the thermogenic gene program in white adipocytes through repression of Ebf2 transcriptional activity
Zfp423 is a multi zinc-finger (ZF) protein that contains 30 C2H2 zinc-finger motifs, organized into five distinct domains (Fig. 6A) (Tsai and Reed, 1998). We previously mapped the ZF domains critical for the ability of Zfp423 to enhance BMP-induced adipogenesis and Pparγ expression in cultured cells (Gupta et al., 2010). This function was found to be dependent on its ability to interact with Smad proteins (ZF 14–20). We sought to elucidate the mechanism by which Zfp423 suppresses the thermogenic gene program by using a similar domain-mapping approach. We utilized retroviral vectors to express either full-length Zfp423 (ZF1–30), a variant incapable of Smad-interaction (ΔSBD), or a previously described variant lacking the C-terminal zinc fingers (ZF1–25) (Fig. 6A) (Hata et al., 2000). Primary brown stromal vascular cultures transduced with these viruses all differentiated into adipocytes to a similar degree (Fig. 6B,C). However, cells expressing full-length Zfp423 adopted a white adipocyte-like gene expression profile; levels of Ucp1 and other thermogenic genes were significantly lower than control cultures (Fig. 6D). This effect was not dependent on Smad-protein interactions as expression of a mutant Zfp423 lacking the Smad-binding domain (ΔSBD; ZF 14–20) was equally effective in suppressing thermogenic gene expression. However, expression of a variant of Zfp423 lacking the C-terminal zinc fingers (ZF26–30) was entirely ineffective at suppressing the thermogenic genes (Fig. 6D), despite being expressed at similar levels as full-length Zfp423 or the Zfp423 ΔSBD mutant (Fig. 6A). This indicates that the ability of Zfp423 to suppress brown adipocyte phenotype in cells is dependent on the C-terminal zinc finger domain. Moreover, this suggests that the mechanism by which Zfp423 functions in precursors to promote adipogenesis is distinct from the mechanism it employs to inhibit the thermogenic gene program.
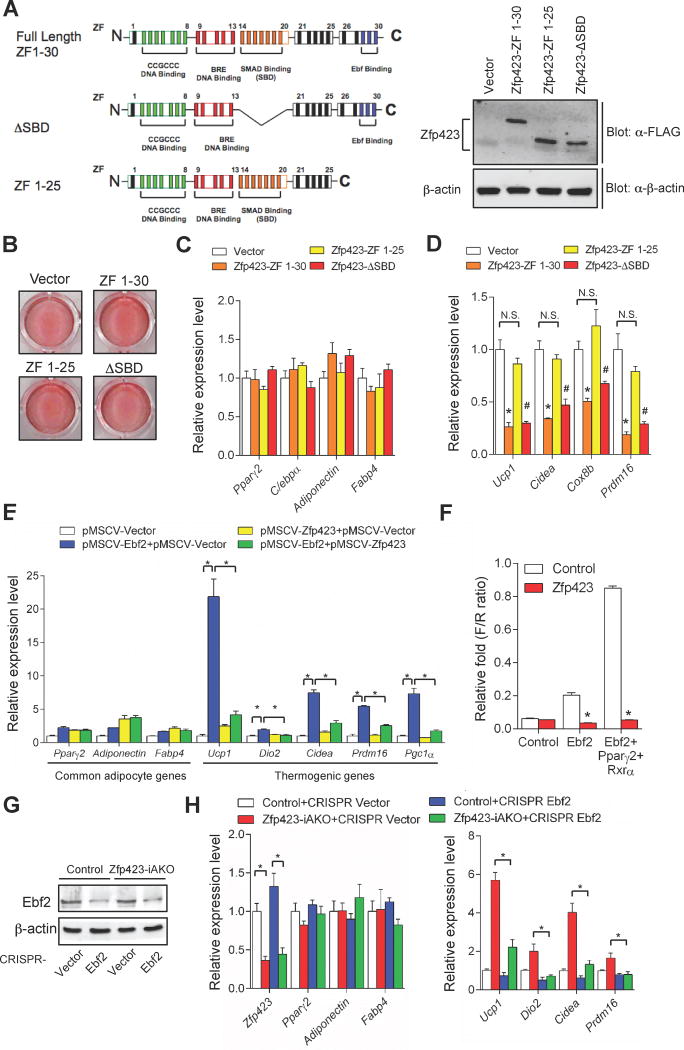
Figure 6. Zfp423 suppresses the thermogenic gene program in white adipocytes through repression of Ebf2 transcriptional activity.
(A) Schematic illustration of the zinc finger (ZF) domains of full-length Zfp423 (ZF 1–30), Zfp423 smad-binding mutant (ΔSBD), and Zfp423 C-terminal mutant (ZF 1–25). Western blots of FLAG-tagged Zfp423 protein levels in in vitro differentiated primary brown adipocytes expressing full-length Zfp423 and Zfp423 mutants.
(B) Oil red O staining of in vitro differentiated brown adipocytes expressing full-length Zfp423 and Zfp423 mutants.
(C) Relative mRNA levels of common adipocyte genes in in vitro differentiated brown adipocytes expressing full-length Zfp423 and Zfp423 mutants. n=3.
(D) Relative mRNA levels of thermogenic genes in in vitro differentiated brown adipocytes expressing full-length Zfp423 and Zfp423 mutants. * denotes Vector vs. ZF 1–30 p<0.05 from one-way ANOVA; # denotes Vector vs. ZF 1-ΔSBD p<0.05 from one-way ANOVA; N.S= not significant. n=3.
(E) Relative mRNA levels of common adipocyte genes and thermogenic genes in in vitro differentiated iWAT adipocytes virally expressing Zfp423, Ebf2, or Zfp423 and Ebf2. * denotes p<0.05 from one-way ANOVA. n=3.
(F) Firefly luciferase activity (normalized to Renilla activity) in cells transfected with the Prdm16 enhancer-driven firefly luciferase reporter construct and Ebf2 or Ebf2/Pparγ/Rxrα vectors, with or without Zfp423 co-expression. * denotes p<0.05 from Student’s t-test. n=3.
(G) iWAT SVF cells were isolated from control and Zfp423-iAKO mice and transduced with the indicated CRISPR lentivirus before in vitro differentiation. Ebf2 protein levels in the differentiated adipocyte cultures were detected by western blotting.
(H) Relative mRNA levels of common adipocyte genes and thermogenic genes in in vitro differentiated iWAT adipocytes with indicated genotype and CRISPR lentivirus transduction. * denotes p<0.05 from two-way ANOVA. n=3.
Zfp423 was originally cloned as an interacting partner of the Ebf family of transcription factors in the olfactory epithelium; ZF 28–30 of Zfp423 mediates this direct interaction (Tsai and Reed, 1997). We confirmed that this interaction could occur in differentiated white adipocytes (Fig. 7A). As described above, the Ebf2 family member is required for the development of brown and beige adipocytes. The ability of Zfp423 to suppress the thermogenic gene program in white adipocytes is dependent on the same zinc fingers that mediate its potential to interact with Ebf proteins; therefore, we postulated that Zfp423 functions in white adipocytes to control Ebf2 activity.
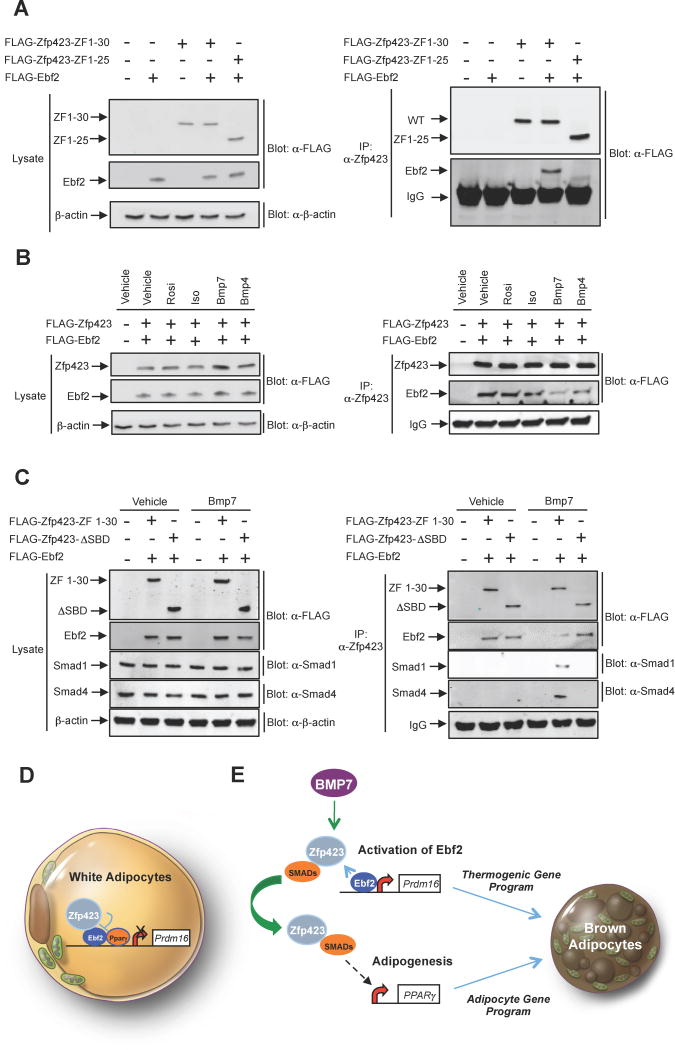
Figure 7. BMP7 signaling disrupts the Zfp423-Ebf2 protein complex.
(A) iWAT SVF cultures from C57BL/6 mice were co-transduced with retroviruses expressing either FLAG-tagged full-length (ZF 1–30) Zfp423 or mutant Zfp423 (ZF 1–25), along with full-length FLAG-Ebf2. After adipocyte differentiation, protein-protein interactions were analyzed by Ebf2 immunoblotting (anti-FLAG antibody) of Zfp423 immunoprecipitates (anti-Zfp423 antibody immunoprecipitation).
(B) iWAT SVF cells from C57BL/6 mice were transduced with retrovirus expressing FLAG-tagged Zfp423 and Ebf2 before in vitro differentiation. After differentiation, the adipocytes were treated with vehicle or browning agents (10 μM rosiglitazone, 10 μM isoproterenol, or 10 nM BMPs) for 6 hr. Protein-protein interaction was analyzed by immunoblotting of Zfp423 immunoprecipitates.
(C) iWAT SVF cells from C57BL/6 were transduced with retrovirus expressing FLAG-tagged full-length Zfp423 (ZF 1–30) or Zfp423 smad-binding mutant (ΔSBD) together with retrovirus expressing Ebf2 before in vitro differentiation. After differentiation, the differentiated adipocytes were treated with vehicle or 10 nM BMP7 for 6 hr. Protein-protein interaction was analyzed by immunoblotting of Zfp423 immunoprecipitates.
(D) Proposed model for the inhibition of the thermogenic gene program in white adipocytes by Zfp423. Zfp423 binds to Ebf2 and serves as a transcriptional co-repressor at key Ebf2-target genes, such as Prdm16. This leads to a suppression of the overall thermogenic gene program in white adipocytes.
(E) Proposed model for the interaction between BMP signaling and Ebf2 signaling in brown adipocyte differentiation. BMP7 triggers a Smad1/4 interaction with Zfp423 that leads to activation of Pparγ expression and adipogenesis. This sequesters Zfp423 from Ebf2, thereby allowing Ebf2 to drive the thermogenic gene program of brown adipose tissue.
Inguinal WAT stromal vascular cultures transduced with Ebf2-expressing retrovirus differentiate into brown-like adipocytes (Fig. 6E) (Rajakumari et al., 2013). However, co-expression of Zfp423 in Ebf2-expressing cells strongly inhibits the induction of Prdm16 and the thermogenic gene program, while having no discernible effect on overall adipocyte differentiation (Fig. 6E). This suggests that Zfp423 inhibits the transcriptional activity of Ebf2. We tested this directly by employing classic luciferase-based gene reporter assays. Ebf2 alone, or in conjunction with Pparγ/RXR heterodimers, bind and activate an Ebf2-dependent enhancer element located at the Prdm16 locus (Fig. 6F) (Rajakumari et al., 2013). Co-transfection of Zfp423 with Ebf2 or Ebf2/Pparγ/RXR completely abolishes the ability of Ebf2 to activate the Prdm16 enhancer element (Fig. 6F). This suggests a model in which Zfp423 serves as a transcriptional co-repressor of Ebf2 activity in white adipocytes; inactivation of Zfp423 then results in increased Ebf2-mediated induction of Prdm16 and the beige adipocyte gene program. In support of this model, CRISPR-Cas9 mediated inactivation of Ebf2 in Zfp423-deficient adipocyte cultures largely prevents the induction of the beige cell phenotype (Fig. 6G,H). These data indicate that the white to beige fat cell conversion induced by Zfp423 inactivation is dependent on Ebf2.
Activation of bone morphogenic protein signaling in adipocytes disrupts the interaction between Zfp423 and Ebf2
As described above, Zfp423 expression is regulated in a depot- and temperature-dependent manner. Brown adipocytes express relatively lower levels of Zfp423 than white adipocytes; however, protein levels remain detectable and are not entirely lost upon cold exposure. Thus, we reasoned that mechanisms beyond the control of gene expression might exist to alter Zfp423 function in adipocytes. The robust conversion of the white to beige adipocytes in the absence of Zfp423, and the dependence of this phenotype on Ebf2, prompted us to ask whether natural or pharmacological signals that promote beige/brown adipocyte development modulate the interaction between these two proteins. We treated differentiated adipocyte cultures with ligands that promote activation of the thermogenic gene program. Pparγ agonism (Rosiglitazone) or adrenergic receptor activation (isoproterenol) did not effect the interaction between virally expressed Zfp423 and Ebf2, at least under the conditions utilized here. However, treatment with BMP7, and to a lesser extent BMP4, reduced the degree of interaction between Ebf2 and Zfp423 (Fig. 7B). The strong effect elicited by BMP7 is noteworthy as there is considerable evidence pointing to BMP7 signaling as a key determinant of brown adipocyte development, triggering brown adipogenesis through a number of mechanisms (Tseng et al., 2008). Canonical BMP signaling leads to the activation of Smad1/4-mediated transcription. As described earlier, BMP signaling in the adipose lineage activates Pparγ expression and adipogenesis; Zfp423 serves as Smad co-activator and amplifies the pro-adipogenic effects of BMPs (Gupta et al., 2010). Accordingly, BMP7 treatment triggers an interaction between Zfp423 and Smad1 and Smad4 (Fig. 7C). Interestingly, the interaction between Ebf2 and the variant of Zfp423 lacking the Smad-binding domain (Zfp423-ΔSBD) is not inhibited by BMP treatment (Fig. 7C). This indicates that BMP7 disrupts the Zfp423-Ebf2 complex through induction of a Zfp423-Smad complex, and provides evidence of a cross-talk between BMP7 signaling and Ebf2 signaling pathways in the formation of thermogenic adipocytes. These data suggest that Zfp423 activity can be controlled at the transcriptional and post-translational level by physiological stimuli of brown adipocyte development and thermogenic gene activation.
Discussion
Tremendous progress has been made in identifying transcriptional components functioning within the brown or beige adipocyte lineage that either positively or negatively impact the differentiation of their respective precursors. As described above, Prdm16 and Ebf2 are among a growing group of factors that drive the activation of the thermogenic gene program. Inhibitors of brown and beige cell precursor differentiation have also been described, and include Rb/p107, MTFRA, and TLE3 (De Sousa et al., 2014; Hansen et al., 2004; McDonald et al., 2015; Villanueva et al., 2013). TLE3, similar to Zfp423, promotes adipocyte differentiation though activation of Pparγ while also suppressing Prdm16 activity and the thermogenic gene program (Villanueva et al., 2013; Villanueva et al., 2011). The precise step in the adipose lineage affected by TLE3 and these other proteins is not entirely clear; however, many of these factors appear to function at the level of the brown or beige precursor to effect lineage commitment and/or differentiation. Here, we reveal a mechanism that acts at the level of a fully mature white adipocyte to suppress the thermogenic gene program characteristic of brown and beige fat cells.
We previously identified Zfp423 as a molecular marker and functional regulator of committed preadipocytes. Zfp423 regulates preadipocyte levels of Pparγ, and is required for proper white and brown adipocyte differentiation in vitro and in vivo. The expression of Zfp423 persists throughout adipocyte differentiation; therefore, in designing these experiments we initially hypothesized that Zfp423 would also be required to maintain Pparγ expression, and thus the general adipocyte gene program, in mature adipocytes. On the contrary, our data here reveals that Zfp423 is dispensable for maintaining the adipocyte identity per se. Other known regulators of Pparγ expressed in mature fat cells, such as C/ebp family members, may collectively compensate for the loss of Zfp423 to maintain adipocyte Pparγ gene expression. Instead, Zfp423 functions in mature white adipocytes to suppress the thermogenic gene program associated with beige/brown adipocytes. The dual roles for Zfp423 in the adipose lineage are seemingly disparate. However, taken together, the data on Zfp423 implicates this factor as a white adipocyte determination factor; Zfp423 regulates the initial formation of a white adipocyte and later plays a role in maintaining the energy-storing, or “white” phenotype of white adipose tissue.
Lineage tracing studies using the same inducible system utilized here have definitively established that cold-induced beiging occurs via de novo differentiation from precursors (adiponectin− cells), and to a lesser degree by UCP1 activation in existing adiponectin+ adipocytes. Recently, Kajimura and colleagues have synthesized the available data and proposed a model in which at least two general mechanisms lead to the natural formation of beige adipocytes during cold exposure: 1) de novo differentiation from precursors (adiponectin− cells), and 2) activation of dormant “unilocular” beige adipocytes (adiponectin+ cells) that were present from a prior cold-exposure (Kajimura et al., 2015). Our lineage tracing data indicates that the beige-like adipocytes that accumulate in Zfp423-iAKO mice originate from adiponectin+ adipocytes through a lineage conversion. These adiponectin+ adipocytes may represent “dormant” beige adipocytes being activated by Zfp423 deletion or bona fide white adipocytes that are being reprogrammed. The browning we observe in our animals is widespread and can be triggered in all major WAT depots, even those relatively resistant to cold-induced browning. Thus, a “reprogramming” of bona fide white adipocytes is likely occurring in this model. The accepted model of physiological white adipose browning highlights the importance of de novo beige adipogenesis; however, our data highlight the potential for white adipocytes in obesity to activate a thermogenic program when the “brake” is removed.
Importantly, levels of Zfp423 appear to be regulated physiologically in settings where the thermogenic potential of adipocytes is altered. Zfp423 expression in vivo is suppressed in mature adipocytes upon cold exposure or pharmacological activation of β3 adrenergic receptors. This regulation happens quickly (1 day of cold) and we observe this at the protein and mRNA level. This suppression of Zfp423 occurs in BAT, likely contributing to the activation of the thermogenic program in this depot. Downregulation of Zfp423 also happens in inguinal adipocytes, likely in pre-existing dormant beige cells that activate UCP1, or even in newly formed beige adipocytes originating in response to cold exposure. Interestingly, this also happens in gWAT, a depot relatively resistant to browning. As highlighted here, cells of this depot require additional stimuli to fully activate their thermogenic capacity, even in the absence of Zfp423. Moreover, we find that Zfp423 expression in BAT increases in settings where “whitening” of BAT occurs, and that expression of Zfp423 in brown adipocytes is sufficient to suppress UCP1 and other genes of the thermogenic program. All together, these data reveal that the suppression of Zfp423 is part of the normal physiology of cold-induced browning. However, the loss of function data here shows that Zfp423 is a major part of the normal physiology of how adipocytes stay or become “white”.
In the model we propose here, Zfp423 functions to suppress the thermogenic gene program in adipocytes by antagonizing the actions of Ebf2, a brown fat determination factor (Fig. 7D). Our cellular experiments indicate that Zfp423 inhibits the ability of Ebf2 to convert white stromal cells into beige adipocytes. This interaction seems important, as the ability of Zfp423 to suppress the thermogenic gene program is dependent on its Ebf-interaction domain. Moreover, depletion of Ebf2 in Zfp423-deficient adipocyte cultures largely blocks the induction of the thermogenic gene program. Inhibition of Ebf2 likely explains how Zfp423 suppresses the thermogenic gene program in BAT and iWAT; however, gonadal WAT naturally expresses much lower levels of Ebf2. The highly related Ebf1 is present in gonadal WAT and is also capable of driving brown/beige adipocyte gene expression (Rajakumari et al., 2013). Thus, Zfp423 may function in this depot through inhibition of Ebf1 rather than Ebf2. It is certainly also possible that Zfp423 functions through additional mechanisms that remain unidentified.
The suppression of UCP1 by transgenic Zfp423 expression in classical brown adipocytes is somewhat surprising given that BAT development is significantly impaired in Zfp423-deficient embryos, and that Zfp423 is expressed to some degree in brown and beige cell precursors (Gupta et al., 2010; Gupta et al., 2012). However, the mechanisms by which Zfp423 regulates adipocyte differentiation per se and thermogenic gene regulation are distinct. Moreover, the interaction between Zfp423 and Ebf2 appears to be inhibited by at least some signals that promote brown/beige adipogenesis. These data offer a model for how Zfp423 can regulate Pparγ expression and adipogenesis in the brown adipose lineage, but not inhibit Ebf2 function and the thermogenic gene program (Fig. 7E). BMP7, in particular is a potent driver of brown adipocyte differentiation, regulating both Pparγ expression and activating Prdm16. A number of mechanisms have been put forth to explain the actions of BMP7 in brown adipocytes (Townsend et al., 2013; Zhang et al., 2010). Our data provide an additional possibility that involves a Zfp423-mediated cross talk between BMP/Smad signaling and Ebf2 signaling.
The recent discoveries of active brown and beige adipocytes in adult humans have renewed the interest in elucidating the mechanisms controlling the formation of these cells and deriving novel strategies to stimulate their activity as a treatment for metabolic disease. It has remained unclear as to whether the numbers of beige or brown adipocytes present in obese individuals are sufficient to increase energy expenditure in a therapeutic manner. The data presented here indicate that the loss of Zfp423, combined with pharmacological stimulation, is sufficient to unlock the thermogenic potential of existing white adipocytes in the setting of obesity. Thus, direct programming of white adipocytes into beige-like cells may be an effective strategy to increase the supply of beige adipocytes in obese individuals. Our observation that natural brown adipocyte differentiation factors, such as BMPs, disrupt the anti-thermogenic Zfp423-Ebf2 complex suggests a plausible therapeutic strategy to trigger this reprogramming event. Further insight into the regulation of adipocyte Zfp423 expression as well as its regulation and function in other adult tissues may lead to novel strategies to combat obesity and metabolic disease.
Experimental procedures
Mice
The AdiponectinrtTA transgenic line was a kind gift of P. Scherer (UTSW) and previously described (Wang et al., 2013). TRE-Cre transgenic mice and the Rosa26-loxP-stop-loxP-Lacz reporter line were obtained from Jackson Laboratories. Zfp423loxP/loxP mice were a generous gift from Dr. S. Warming (Genentech, Inc.) (Warming et al., 2006). TRE-Zfp423 transgenic mice were derived by the UTSW transgenic core facility as described in the Supplemental Text. Mice were maintained at room temperature or at 6°C when indicated with a 12 -hour light/dark cycle and free access to food and water. All animal experiments were performed according to procedures approved by the UTSW Institutional Animal Care and Use Committee. Metabolic cage studies were conducted using a Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments) at the USTW Metabolic Phenotyping Core. Further details of CLAMS analysis can be found in the Supplemental Experimental Procedures.
Histological analysis
Tissues were dissected and fixed in 4% paraformaldehyde overnight. Paraffin processing, embedding, sectioning and standard hematoxylin and eosin (H&E) staining were performed at the Molecular Pathology Core Facility at UTSW. Detailed protocols for β-gal staining and indirect immunofluorescence can be found in the Supplemental Experimental Procedures.
Isolation of adipose stromal vascular fraction (SVF) and in vitro differentiation
Stromal vascular cells isolated by collagenase digestion of minced WAT or BAT (see Supplemental Experimental Procedures) were plated onto collagen-coated dishes and cultured in 10% CO2 at 37°C. WAT SVF cells were expanded in growth media containing DMEM/F12 (Invitrogen) and 10% FBS. BAT SVF cells were maintained in growth media containing high glucose DMEM (Invitrogen) supplemented with 20% FBS and 20 mM HEPES PH 7.4. For white adipocyte differentiation, confluent cultures were stimulated with adipogenic cocktail (growth media supplemented with 5 μg ml−1 insulin, 1 μM dexamethasone, 0.5 mM isobutylmethyxanthine, and 1 μM rosiglitazone) for 48 hours. After 48 hours, the cells were then maintained in growth media supplemented with 5 μg ml-1 insulin until harvest. For brown adipocyte differentiation, confluent cultures were stimulated with adipogenic cocktail (high glucose DMEM media supplemented with 10% FBS, 20 mM HEPES PH 7.4, 10 nM tri-iodothyronine, 0.1 mg ml−1 insulin, 1 μM dexamethasone, 0.5 mM isobutylmethyxanthine, and 0.125 mM indomethacin) for 48 hours. After 48 hours, the cells were then maintained in maintenance media (high glucose DMEM media supplemented with 10% FBS, 20 mM HEPES PH 7.4, 10 nM tri-iodothyronine, and 0.1 mg ml−1 insulin) until harvest.
Gene Expression Analysis
Total RNA from tissue or cultured cells was extracted and purified using the TRIzol reagent (Invitrogen) and the RNeasy Mini Kit (Qiagen). cDNA was synthesized with M-MLV reverse transcriptase (Invitrogen) and random hexamer primers (Invitrogen). Relative expression of mRNAs was determined by quantitative PCR using SYBR Green PCR system (Applied Biosystems) and values were normalized to Rps18 levels using the ΔΔ-Ct method. All primer sequences are provided in Supplemental Table 2 (Table S2). Global gene expression profiling by Affymetrix microarray analysis was conducted at the UTSW Genomics and Microarray Core. Total RNA samples from inguinal WAT were hybridized to GeneChip Mouse Transcriptome Assay 1.0 (Affymetrix) according to the manufacturer’s protocols. Normalization and statistical analysis was performed using Transcriptome Analysis Console 3.0 (Affymetrix). Gene ontology analysis was performed using NIH DAVID. All microarray data have been deposited to Gene Expression Omnibus (accession # GSE74899).
Plasmids and retrovirus production
Retroviruses were constructed using the pMSCV viral backbone (Clontech). pMSCV Zfp423 full-length, pMSCV Zfp423 ΔSBD, and pMSCV Ebf2 plasmids were described previously (Gupta et al., 2010; Rajakumari et al., 2013). The Ebf-binding mutant of Zfp423 lacking zinc fingers 26–30 was generated by PCR and cloned into pMSCV vector. Retroviral production in phoenix packaging cells was achieved as previously described (Gupta et al., 2010).
Supplementary Material
Highlights.
Deletion of adipocyte Zfp423 triggers a white-to-beige fat cell conversion.
Converted beige fat cells, when activated, reverse diet-induced obesity.
Zfp423 inhibits the adipocyte thermogenic gene program through repression of Ebf2.
The Zfp423-Ebf2 protein complex is inhibited by BMP7-Smad signaling.
Acknowledgments
The authors are grateful to members of the UTSW Touchstone Diabetes Center for useful discussions, and P. Scherer and O. Gupta for critical reading of the manuscript. The authors thank the UTSW Animal Resource Center, Metabolic Phenotyping Core, Transgenic Core Facility, Pathology Core, Live Cell Imaging Core, and Microarray Core for excellent guidance and assistance with experiments performed here. This study was supported by NIDDK R03 DK099428, R01 DK104789, the Searle Scholars Program, and the American Heart Association 15BGIA22460021 to R.K.G, the American Heart Association postdoctoral fellowship 16POST26420136 to M.S., NIDDK K01 DK107788 to Q.A.W., NIDDK R00 DK094973 to W.L.H., and NIDDK 5R01DK10300802 to P.S. The graphical abstract accompanying this manuscript was designed by Visually Medical (www.visuallymedical.com).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicting interests statement. The authors declare that they have no competing financial interests.
Author Contributions
M.S., J.I., C.K., Q.W., C.H., L.V., K.M., and S.S., conducted the experiments, K.S. generated critical reagents, M.S., J.I., C.K., W.H., P.S., and R.G. designed the experiments, and M.S. and R.G. wrote the manuscript. All authors contributed to data analysis.
References
- Berry DC, Jiang Y, Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun. 2016;7:10184. doi: 10.1038/ncomms10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz MJ, Enerback S. Human Brown Adipose Tissue: What We Have Learned So Far. Diabetes. 2015;64:2352–2360. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- Cohen P, Spiegelman BM. Brown and Beige Fat: Molecular Parts of a Thermogenic Machine. Diabetes. 2015;64:2346–2351. doi: 10.2337/db15-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sousa M, Porras DP, Perry CG, Seale P, Scime A. p107 is a crucial regulator for determining the adipocyte lineage fate choices of stem cells. Stem Cells. 2014;32:1323–1336. doi: 10.1002/stem.1637. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA, Petrovic N, Enerback S, Nedergaard J, Cinti S, et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci U S A. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A, Massague J. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell. 2000;100:229–240. doi: 10.1016/s0092-8674(00)81561-5. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Spiegelman BM, Seale P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015;22:546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Banks A, Liu T, Kazak L, Rao RR, Cohen P, Wang X, Yu S, Lo JC, Tseng YH, et al. IRF4 is a key thermogenic transcriptional partner of PGC-1alpha. Cell. 2014;158:69–83. doi: 10.1016/j.cell.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell. 2015;160:105–118. doi: 10.1016/j.cell.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504:163–167. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Guertin DA. Emerging Complexities in Adipocyte Origins and Identity. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen P, van Marken Lichtenbelt WD, Spiegelman BM. The future of brown adipose tissues in the treatment of type 2 diabetes. Diabetologia. 2015;58:1704–1707. doi: 10.1007/s00125-015-3611-y. [DOI] [PubMed] [Google Scholar]
- Seale P. Transcriptional Regulatory Circuits Controlling Brown Fat Development and Activation. Diabetes. 2015;64:2369–2375. doi: 10.2337/db15-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentolouris N, Liatis S, Katsilambros N. Sympathetic system activity in obesity and metabolic syndrome. Ann N Y Acad Sci. 2006;1083:129–152. doi: 10.1196/annals.1367.010. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, Tseng YH. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid Redox Signal. 2013;19:243–257. doi: 10.1089/ars.2012.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RY, Reed RR. Cloning and functional characterization of Roaz, a zinc finger protein that interacts with O/E-1 to regulate gene expression: implications for olfactory neuronal development. J Neurosci. 1997;17:4159–4169. doi: 10.1523/JNEUROSCI.17-11-04159.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RY, Reed RR. Identification of DNA recognition sequences and protein interaction domains of the multiple-Zn-finger protein Roaz. Mol Cell Biol. 1998;18:6447–6456. doi: 10.1128/mcb.18.11.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva CJ, Vergnes L, Wang J, Drew BG, Hong C, Tu Y, Hu Y, Peng X, Xu F, Saez E, et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARgamma specifies lipid storage versus thermogenic gene programs. Cell Metab. 2013;17:423–435. doi: 10.1016/j.cmet.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva CJ, Waki H, Godio C, Nielsen R, Chou WL, Vargas L, Wroblewski K, Schmedt C, Chao LC, Boyadjian R, et al. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab. 2011;13:413–427. doi: 10.1016/j.cmet.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, Gupta RK. Pdgfrbeta(+) Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab. 2016;23:350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kissig M, Rajakumari S, Huang L, Lim HW, Won KJ, Seale P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci U S A. 2014;111:14466–14471. doi: 10.1073/pnas.1412685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S, Rachel RA, Jenkins NA, Copeland NG. Zfp423 is required for normal cerebellar development. Mol Cell Biol. 2006;26:6913–6922. doi: 10.1128/MCB.02255-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schulz TJ, Espinoza DO, Huang TL, Emanuelli B, Kristiansen K, Tseng YH. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.