Abstract
Background/Aims
Although individual contributions of TNF-α, LOX-1 and adiponectin to the regulation of endothelial function were previously studied, their interactions in the regulation of coronary endothelial function remain unclear. The aim of this study is to investigate the interactions between TNF-α, LOX-1 and adiponectin in endothelial dysfunction in atherosclerosis.
Methods
Vasodilator function was assessed in coronary arterioles isolated from wild type (WT), ApoE knockout mice (ApoE KO), ApoE KO null for TNF-α (ApoE KOTNF−/TNF−), and ApoE KO mice treated with neutralizing antibodies to either TNF-α and LOX-1, or recombinant adiponectin. Western blot analysis and immunofluorescence staining were used for mechanistic studies.
Results
Dilation to ACh was impaired in ApoE KO mice. KO of TNF-α, anti-TNF-α, anti-LOX-1 or adiponectin restored impaired vasodilation to ACh without affecting endothelium-derived hyperpolarizing factor (EDHF)-mediated vasodilation. Immunofluorescence staining demonstrated co-localization of TNF-α with vascular smooth muscle cells, and adiponectin with endothelial cells. ApoE KO mice showed increased protein expression of LOX-1, NF-κB, NADPH oxidase subunit NOX4, and nitrotyrosine (N-tyr) levels in coronary arterioles. Treatment with anti-TNF-α, anti-LOX-1 and adiponectin suppressed the protein expression of LOX-1, NOX4, NF-κB and N-tyr levels.
Conclusion
Adiponectin, anti-TNF-α and anti-LOX-1 exert vasoprotective effects in atherosclerotic ApoE KO mice.
Keywords: endothelial dysfunction, TNF-α, adiponectin, LOX-1
Introduction
Atherosclerosis is a complex, multifactorial disease with both genetic and environmental determinants. Endothelial dysfunction is one of the earliest manifestations of atherosclerosis, and the assessment of endothelial function potentially serves as a useful prognostic tool for coronary artery disease [1]. Oxidized low-density lipoprotein (ox-LDL) is recognized as a major contributor to endothelial dysfunction in atherogenesis [2]. Ox-LDL has been implicated to impair endothelium-dependent vascular tone through decreasing production/bioavailability of endothelium-derived nitric oxide (NO) and mediates several of its biological effects via LOX-1 (lectin-like ox-LDL receptor-1) [3, 4]. When ox-LDL is bound to LOX-1, it induces the generation of superoxide anions (O2·−) [5] that reduce the bioavailability of NO and activate nuclear factor-kappa B (NF-κB) [6], CD40/CD40L [7] and subsequently stimulate the expression of vasoconstrictor molecules such as endothelin-1 (ET-1), adhesion molecules, including P-selectin, vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion molecule-1 (ICAM-1), and chemokines monocyte chemoattractant protein-1 (MCP-1) [8, 9] in endothelial cells. We previously reported that oxidative stress was significantly higher in isolated coronary arterioles of ApoE KO mice, which lay the foundation for further mechanistic study of microvascular pathology in atherosclerosis [10, 11]. Despite the crucial role of LOX-1 in endothelial dysfunction, particularly within an atherosclerotic environment, the mechanism by which the expression of LOX-1 is regulated in the vasculature is poorly understood. It is plausible that multiple inflammatory cytokines and novel regulators of vascular function may converge on LOX-1 signaling, forming a complex circuit to regulate LOX-1-mediated coronary microcirculatory dysfunction in atherosclerosis. Indeed, in vitro experiments have suggested that the pro-inflammatory cytokine TNF-α increases LOX-1 expression in a concentration-dependent manner in bovine aortic endothelial cells [12]. Despite the profound pro-inflammatory effects of TNF-α, an adipose-derived hormone, adiponectin, has been shown to exhibit inhibitory effects on TNF-α expression in aortas of db/db mice [13]. The above evidence suggests a potential interaction among adiponectin/TNF-α/LOX-1 responsible for the regulation of endothelial function, but the nature of this interaction in microvessels in atherosclerosis is uncertain.
In previous studies we have established that anti-LOX-1 treatment reverses coronary arteriolar endothelial dysfunction in ApoE KO mice by preventing NADPH oxidase-mediated oxidative stress thereby restoring NO availability. We also found that anti-LOX-1 treatment improved aortic endothelial function in ApoE KO mice [11, 14]. Further, adiponectin treatment reduced LOX-1 protein expression in aortas of ApoE KO mice, and in TNF-α stimulated mouse coronary artery endothelial cells, suggesting a reciprocal relationship between adiponectin and TNF-α in the regulation of LOX-1 expression. In this study, we further delineate the role of this reciprocal regulatory mechanism in coronary microcirculatory function. We hypothesize that an interactive circuit amongst adiponectin, TNF-α and LOX-1 regulating coronary arteriolar function in atherosclerosis. To test this hypothesis, we used genetic mouse models including the ApoE KO mouse, ApoE KO mice null for TNF-α (ApoE KOTNF−/TNF−), and ApoE KO mice treated with a neutralizing antibody of TNF-α (anti-TNF-α) to neutralize TNF-α signaling, ApoE KO mice treated with exogenous recombinant adiponectin, and ApoE KO mice treated with a neutralizing antibody to LOX-1 (anti-LOX-1) to inhibit LOX-1 signaling. Using these models we aimed to determine whether 1) anti-LOX-1 and anti-TNF-α neutralizing antibody, or adiponectin supplementation improve coronary arteriolar endothelial function in ApoE KO mice; 2) adiponectin and TNF-α are co-localized with vascular cells of coronary arterioles; 3) there is reciprocal regulation in the expression of adiponectin, TNF-α and LOX-1.
Methods
Animal models
The procedures followed were in accordance with approved guidelines set by the University of Missouri Animal Care and Use Committee. Wild-type (WT, C57BL/6) control mice, ApoE KO mice and ApoE KO null for TNF-α (ApoE KOTNF−/TNF−) between 6 to 8 weeks of age were obtained from the Jackson Laboratory (Bar Harbor, ME). The ApoE and TNF-α double knockout mice (ApoE KOTNF−/TNF−) were established by breeding of ApoE KO mice (heterozygous) with TNF-α knockout (KO) mice (homozygous) and were genotyped by PCR as we described [14]. To accelerate lesion formation in atherosclerosis-prone mice on the ApoE KO background, all animals were treated with a western type diet (adjusted calorie diet; Harlan Teklad TD88137; 42% from milk fat, 0.15% cholesterol) for 12 weeks. Male mice were used in this study and animals had free access to water, and were kept at a 12 hours light/dark cycle. Body weight, abdominal circumference and glucose levels were recorded prior to euthanization and the data were reported in a previous study by our group [10].
TNF-α, LOX-1 neutralization and adiponectin treatment
TNF-α was neutralized using a 2E2 monoclonal antibody (2E2 MAb. 94021402, NCI Biological Resources Branch Frederick, MD, USA). A neutralizing antibody (R&D, AF1564), which blocks the binding of ox-LDL to LOX-1, was used to suppress LOX-1 activity. After 12 weeks of the western diet, ApoE KO mice were treated with one of, anti-TNF-α antibody (0.625 mg/ml per kilogram per day; i.p. for 3 days) [14], anti-LOX-1 antibody (16 µg/mL, 0.1 mL/day, i.p. for 7 days) [11] or recombinant murine globular adiponectin (gAcrp30, 15 µg/day, s.c. for 8 days, PeproTech) as we previously described [10, 15, 16].
Functional assessment of isolated coronary arterioles
Coronary arterioles (40 to 100 μm in diameter) from murine cardiac tissue were identified, carefully microdissected and cannulated on glass pipettes as previously described [14]. The cannulated vessels were then pressurized at 60 cm H2O in the absence of intraluminal flow in preparation for measuring vasodilatory function from the spontaneous level of pressure-induced myogenic tone. Those measurements were performed using video microscopy [14]. To determine whether TNF-α, LOX-1 or adiponectin regulate coronary vascular function in ApoE KO mice, vasodilation to the endothelium-dependent vasodilator, acetylcholine (ACh, 0.1 nmol/L to 10 µmol/L), and endothelium-independent vasodilator, sodium nitroprusside (SNP, 0.1 nmol/L to 10 µmol/L), were assessed in coronary arterioles isolated from WT, ApoE KO and ApoE KO mice treated with anti-TNFα, anti-LOX1 or adiponectin. The contribution of NO to vasodilation was determined by incubating the vessels with the NO synthase (eNOS and nNOS) inhibitor L-NAME (10 µmol/L, 20 min) [17, 18]. To determine the response of coronary arterioles to EDHF-dependent mechanisms, ACh vasodilation was performed following pretreatment (30 minutes) with both L-NAME (10 μmol/L) and the cyclooxygenase inhibitor indomethacin (Indo, 10 μmol/L). At the end of each experiment, the vessel was relaxed with 100 μmol/L SNP to obtain its maximal diameter at 60 cm H2O intraluminal pressure. All diameter changes were normalized to the vasodilation in response to 100 μmol/L SNP and expressed as a percentage of maximal dilation. For the functional experiments all drugs were administered extraluminally in the physiological salt solution superfusate.
Immunofluorescence staining
Transverse sections of murine heart were stained by immunohistochemical staining to identify and localize adiponectin and TNF-α expression with markers of endothelial cells, vascular smooth muscle cells and macrophages. Briefly, excised hearts were embedded in O.C.T. and sectioned at 5 μm. Slides were incubated with blocking solution (10% donkey serum in PBS). Primary antibodies for adiponectin (goat polyclonal, R&D, AF1119, 1:500) [19], TNF-α (goat polyclonal, R&D systems)[14], the endothelial cell marker, von Willebrand factor (rabbit polyclonal, Abcam, ab6994, 1:1000) [14], smooth muscle α-actin (rabbit polyclonal, Abcam, ab5964, 1:800) [14] or macrophage marker F4/80 (rat monoclonal, AbD Serotec, MCA497R, 1:400) were used for sequential double immunofluorescence staining. The specificity of the adiponectin antibody was confirmed in adiponectin−/ − mice as we previously described [19], and specificity of TNF-α antibody was previously validated [14]. Secondary antibodies were conjugated with the fluorophores, FITC or Texas Red. For negative controls, primary antibodies were replaced with IgG-isotype controls at the same concentration. Sections were finally mounted in an anti-fading agent (Slowfade gold with DAPI, Invitrogen). Slides were observed and analyzed using a fluorescence microscope (IX81, Olympus) with a 40× objective (0.90 N.A.) [20].
Western blot analysis of protein expression
Coronary arterioles (4–6 vessels per sample) were homogenized in lysis buffer (Cellytic™ MT Mammalian Tissue Lysis/Extraction Reagent, Sigma). Protein concentrations were assessed with a BCA™ Protein Assay Kit (Pierce) and samples were subsequently separated by SDS-PAGE and transferred to PVDF membranes. Protein expression was detected using the appropriate primary antibody: TNF-α (R&D, 1:500), adiponectin (R&D, 1:500), LOX-1 (R&D, 1:1000), NF-κB p65 (Abcam, 1:1000), NOX4 (Santa Cruz, 1:500), and Anti-Nitro tyrosine (N-Tyr) (Abcam, 1:500) and beta-actin (R&D, 1:2000). Horseradish peroxidase-conjugated secondary antibodies were used and signals were visualized by enhanced chemiluminescence (ECL, Santa-Cruz). Quantification was performed following scanning with a Fuji LAS3000 densitometer and using Multigauge software (Fujifilm). Relative amounts of protein expression were normalized to those of the corresponding WT control, which was set to a value of 1.0.
Data analysis
All diameter changes to pharmacological agonists were normalized to the passive vessel diameter at 60 cm H2O. Statistical comparisons of vasomotor responses between groups were performed with two-way analysis of variance (ANOVA) for repeated measure and intergroup differences were tested with the Bonferroni inequality using software SPSS v11.5. All data are presented as mean ± SEM except as specifically stated. Significance was accepted at P < 0.05.
Results
Contribution of TNF-α, LOX-1 and adiponectin to coronary endothelial dysfunction in ApoE KO mice
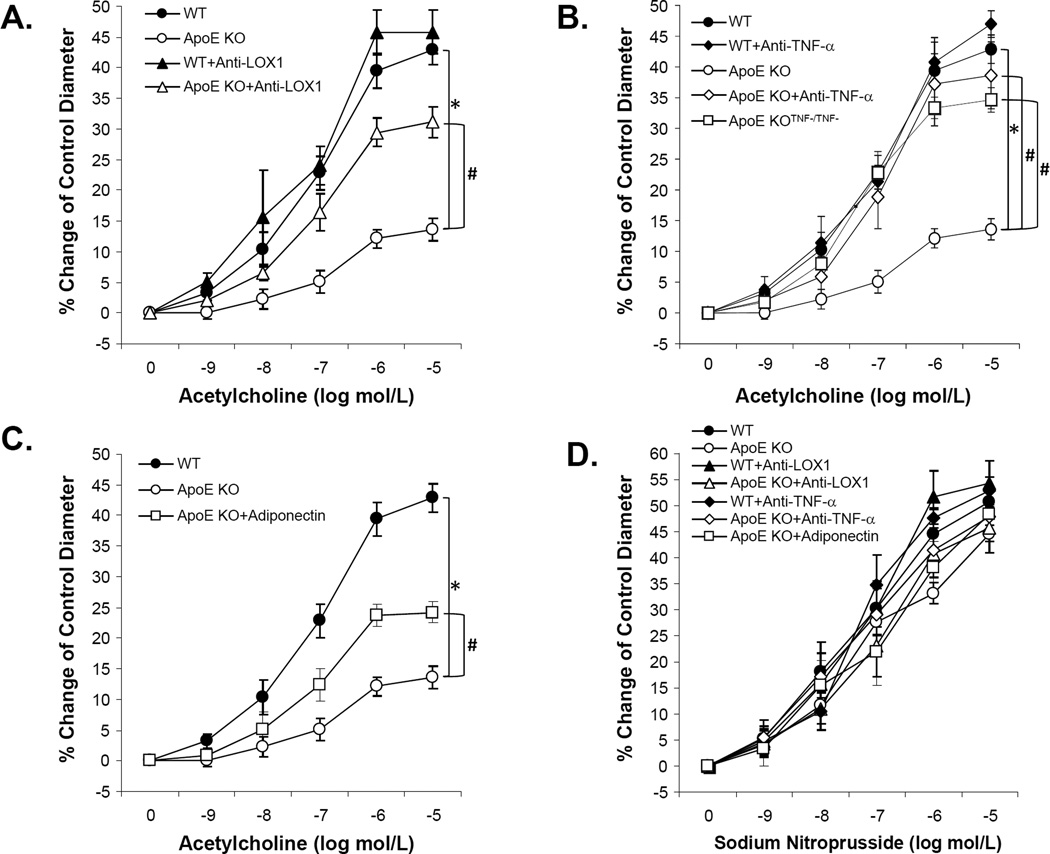
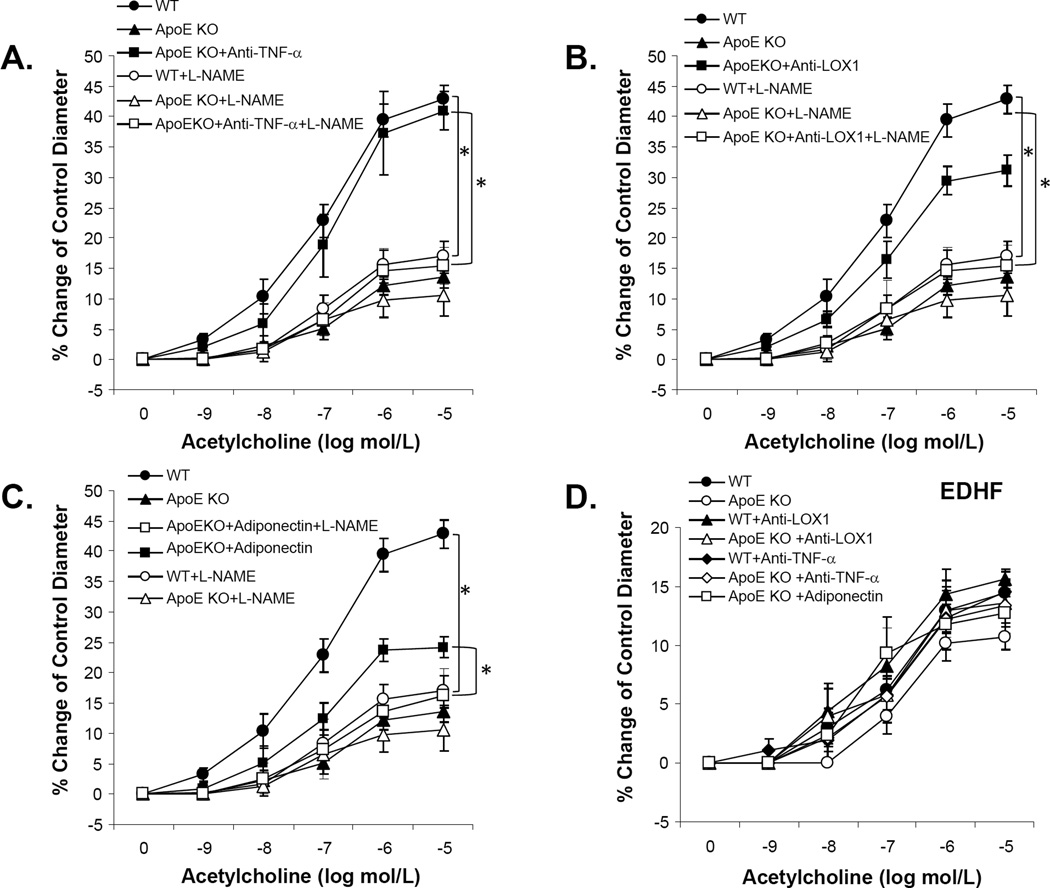
Vasodilation to the endothelium-dependent vasodilator, ACh, was significantly impaired in coronary arterioles of ApoE KO mice. Conversely, anti-LOX-1 (Figure 1A), genetic deficiency of TNF-α in ApoE KO mice (ApoE KOTNF−/TNF−), anti-TNF-α (Figure 1B), or adiponectin (Figure 1C) each partially restored ACh-induced vasodilation in ApoE KO mice. SNP-induced, endothelium-independent, vasodilation was similar among WT, ApoE KO, ApoE KO treated with anti-TNF, anti-LOX-1 or adiponectin (Figure 1D). Improvement in vasodilator function in ApoE KO mice following treatment with anti-TNF-α (Figure 2A), anti-LOX-1 (Figure 2B) or adiponectin (Figure 2C) was abolished by L-NAME. This result indicates that 1) vasodilation to ACh was NO-mediated in the coronary arterioles of both WT and ApoE KO mice; and 2) anti-TNF-α, anti-LOX-1 or adiponectin restored ACh-induced, NO-mediated, vasodilation in atherosclerotic mice. We also investigated whether EDHF interacts with anti-TNF-α, anti-LOX-1 or adiponectin in coronary arteriolar dysfunction in ApoE KO mice. Incubation with L-NAME and Indo had no apparent effect on basal arteriolar tone. Unlike NO, EDHF-induced vasodilation was not significantly different in all groups suggesting EDHF-mediated responses are preserved in atherosclerotic coronary arterioles (Figure 2D). There were no significant differences of maximal diameter and diameter after tone among all experiment groups (Table 1) and other vessels characteristics were shown in Table 1.
Figure 1. Anti-TNF-α, anti-LOX-1 and adiponectin improved endothelium-dependent vasodilation of coronary arterioles in ApoE KO.
A. Isolated coronary arterioles from WT (n=11) and ApoE KO (n=9) mice dilated in response to acetylcholine (ACh, endothelium-dependent vasodilator) in a concentration-dependent manner. ACh-induced vasodilation was significantly attenuated in ApoE KO mice compared to WT mice. Treatment of anti-LOX-1 (ApoE KO+Anti-LOX-1, n=10) improved ACh-induced vasodilation in ApoE KO, but did not affect the vascular function of WT mice (WT+Anti-LOX-1, n=3). B. ACh-induced dilation in ApoE KO mice null for TNF (ApoE KOTNF−/TNF−, n=6) was comparable to that in WT mice and impaired ACh-induced vasodilation in ApoE KO mice was restored after treatment with neutralizing antibody to TNF-α (ApoE KO+Anti-TNF-α, n=3). Treatment of anti-TNF-α but did not affect the vascular function of WT mice (WT+Anti-TNF-α, n=3). C. Adiponectin treatment in ApoE KO mice (ApoE KO+Adiponectin, n=8) partially improved ACh-induced vasodilatation. D. The endothelium-independent vasodilator (NO donor), SNP (sodium nitroprusside)-induced vasodilation was not significantly different among all groups. Data were shown as mean ± SEM. *P<0.05 vs. WT, # P<0.05 vs. ApoE KO.
Figure 2. Improved nitric oxide (NO), but not EDHF-dependent vasodilation accounts for the vascular protective effects by anti-TNF-α, anti-LOX-1 and adiponectin.
Improvement of ACh-induced vasodilation in coronary arterioles in ApoE KO treated with anti-TNF-α antibody (A), anti-LOX-1 antibody (B) or adiponectin (C) was abolished by incubation with the nitric oxide synthase (NOS) inhibitor L-NAME. D. EDHF-induced vasodilation (ACh-induced dilation in the presence of L-NAME and indomethacin) was not significantly different among all groups. Data represent mean ± SEM. n=6–7 mice. Data were shown as mean ± SEM. *P<0.05 vs. WT, # P<0.05 vs. ApoE KO.
Table 1.
Vessel Characteristics
| Maximal Diameter |
% Tone at Baseline |
% Tone with L-NAME |
% Tone with L-NAME + Indomethacin |
|
|---|---|---|---|---|
| WT | 110.5 ± 4.4 | 29.9 ± 1.8 | 34.9 ± 1.0 * | 35.6 ± 1.4 * |
| WT + Anti-LOX1 | 111.8 ± 5.2 | 32.3 ± 1.5 | 36.6 ± 1.1 | 38.0 ± 1.5 |
| WT + Anti-TNF-α | 102.7 ± 9.7 | 27.5 ± 1.2 | 35.0 ± 1.4 * | 34.9 ± 2.7 * |
| ApoE KO | 116.7 ± 6.0 | 32.7 ± 0.7 | 35.6 ±0.3 | 37.1 ± 1.1 * |
| ApoE KO + Anti-LOX1 | 106.5 ± 4.2 | 28.3 ± 1.9 | 34.7 ± 0.5 * | 36.8 ± 1.8 * |
| ApoE KO + Anti-TNF-α | 113.0 ± 5.9 | 29.9 ± 1.7 | 33.9 ± 2.1 | 36.3 ± 1.2 * |
| ApoE KO + Adiponectin | 101.6 ± 6.0 | 31.0 ± 1.1 | 34.8 ±1.1 | 35.3 ± 1.8 |
| ApoE KOTNF−/TNF− | 98.8 ± 6.5 | 30.7 ± 2.9 | 36.2 ±1.3 | 36.2 ± 1.2 |
Data are expressed as mean ± SEM.
P<0.05 vs. % Tone at Baseline
WT, wild type mice; ApoE KO, ApoE knockout mice; ApoE KOTNF−/TNF−, ApoE KO null for TNF-α; WT + Anti-TNF-α and ApoE KO + Anti-TNF-α, WT and ApoE KO mice treated with neutralizing antibodies to TNF-α; WT + Anti-LOX1 and ApoE KO + Anti-LOX1, WT and ApoE KO mice treated with neutralizing antibodies to LOX1; ApoE KO+ Adiponectin, ApoE KO mice treated with recombinant adiponectin.
TNF-α, LOX-1 and adiponectin expression in coronary arterioles of ApoE KO mice
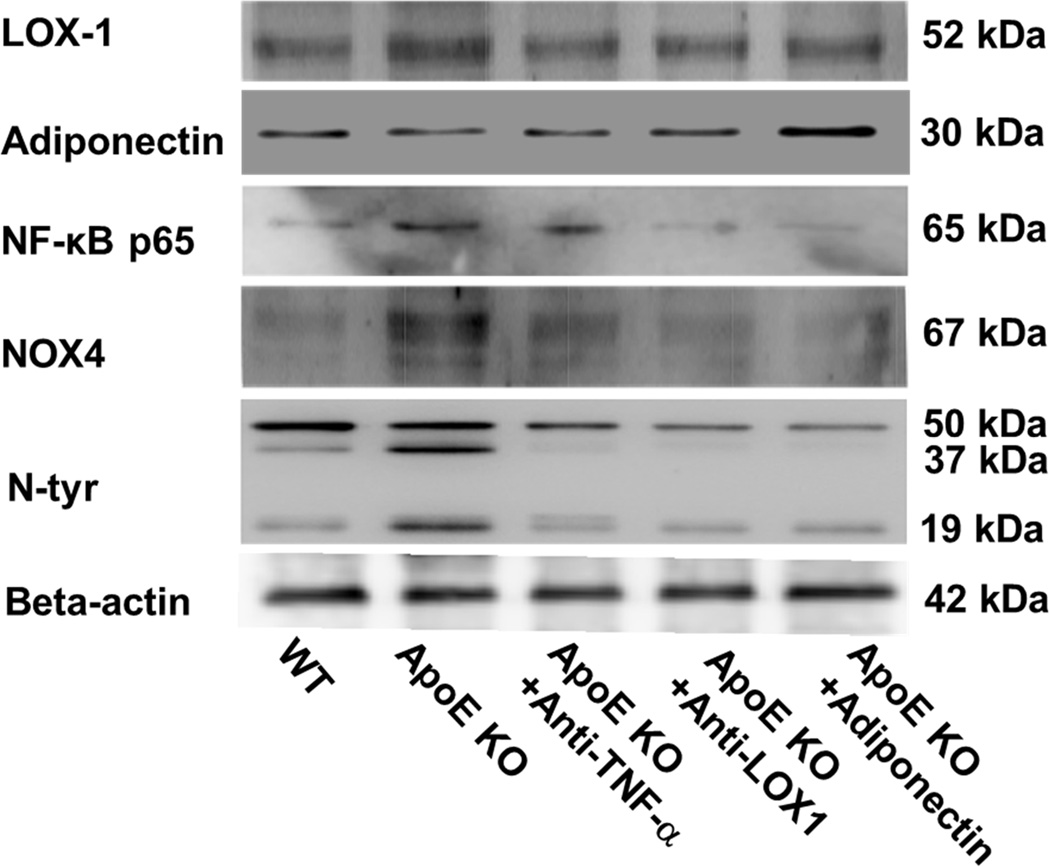
Protein expression of LOX-1 and adiponectin (Figure 3) in isolated coronary arterioles was determined for WT, ApoE KO and ApoE KO mice treated with anti-TNF-α, anti-LOX-1 or adiponectin. Anti-TNF-α, anti-LOX-1 or adiponectin treatment remarkably decreased LOX-1 expression, while adiponectin expression was identical in WT and ApoE KO mice. Although anti-TNF-α, or anti-LOX-1 did not affect adiponectin expression, adiponectin treatment increased adiponectin expression in coronary arterioles of ApoE KO mice.
Figure 3. Interactive regulation TNF-α, LOX-1 and adiponectin in coronary arterioles.
LOX-1 protein expression was higher in coronary arterioles of ApoE KO mice. Anti-TNF-α, anti-LOX-1 and adiponectin reduced LOX-1 protein expression. Treatment of adiponectin, but not anti-TNF-α or anti-LOX-1, increased adiponectin levels in the coronary arterioles of ApoE KO mice. NF-κB and NOX4 levels were higher in ApoE KO, and were suppressed by anti-TNF-α, anti-LOX-1 and adiponectin treatment. N-tyr signals showed three bands at the size of 50 kDa, 37 kDa, and 19 kDa respectively. Both 37 kDa and 19 kDa signals were higher in ApoE KO mice compared with WT mice. Anti-LOX1, anti-TNF-α and adiponectin reduced levels of 37 kDa and 19 kDa signals. In contrast, the 50 kDa signal was similar between WT and ApoE mice. Data shown were representative blots of 4 separate experiments.
TNF-α, LOX-1 and adiponectin regulate protein expression of NF-κB, NOX4 and N-Tyr
The protein expression of NF-κB p65, NOX4, and N-Tyr (37 kDa and 19 kDa) were significantly increased in coronary arterioles in ApoE KO mice. Anti-TNF-α, anti-LOX-1, and adiponectin treatment dramatically attenuated NF-κB, NOX4, and N-Tyr (37 kDa and 19 kDa) levels in the ApoE KO mice (Figure 3).
Colocalization of TNF-α and adiponectin with vascular cells in atherosclerosis
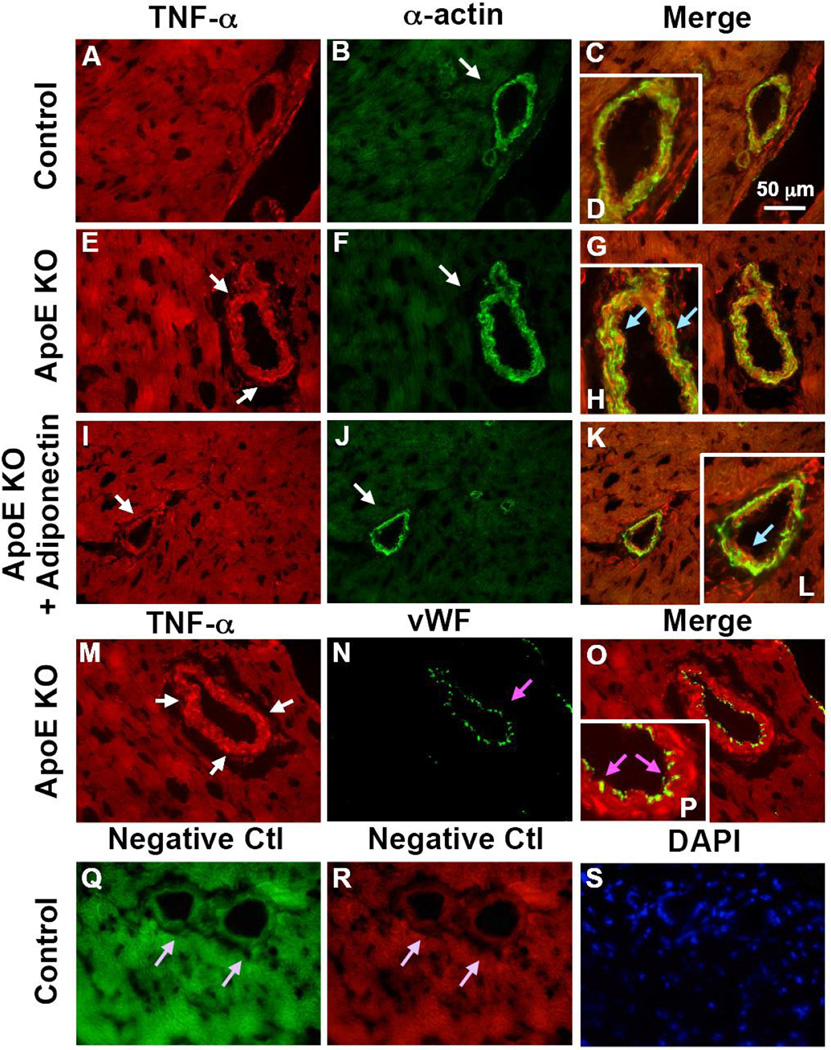
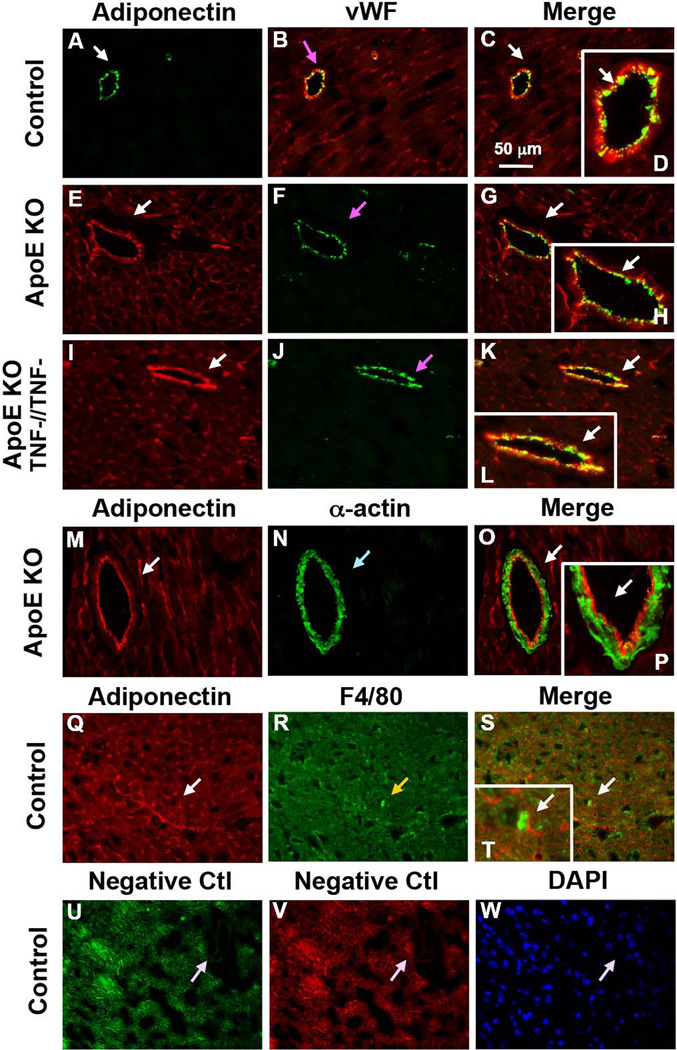
Immunostaining results showed that TNF-α (red, Figure 4 A–L) was co-localized with vascular smooth muscle cells and adiponectin (red, Figure 5 A–L) was co-localized with endothelial cells in WT, ApoE KO and ApoE KOTNF−/TNF− mice. TNF-α was not co-localized with endothelial cells (Figure 4 M–P), while adiponectin was not expressed in vascular smooth muscle cells (Figure 5 M–P) of coronary microvessels or in macrophages (Figure 5 Q–T). For negative controls, primary antibodies were replaced with IgG-isotype controls (Figure 4 Q–S and Figure 5 U–W)
Figure 4. TNF-α is colocalized with vascular smooth muscle cells in coronary arterioles.
TNF-α is colocalized with vascular smooth muscle cells in coronary arterioles as shown by dual fluorescence labeling for TNF-α and markers for endothelial cells [von Willebrand factor (vWF)] and vascular smooth muscle cells (α-actin) on frozen heart sections. A, B, C and D, dual labeling of TNF-α (red) and α-actin (green) in control mice. E, F, G and H, dual labeling of TNF-α (red) and α-actin (green) in ApoE KO mice. I, J, K and L, dual labeling of TNF-α (red) and α-actin (green) in ApoE KO + Adiponectin mice. The arrows in E and I show staining of TNF-α (red) while the arrows in B, F and J show staining of vascular smooth muscle cells (green). The blue arrows in H and L indicate colocalization of TNF-α and vascular smooth muscle cells (yellow). M, N, O and P, dual labeling of TNF-α (red) and vWF (green) in ApoE KO mice. Enlargements of panels C, G, K and O are shown in panel D, H, L and P, respectively. The pink arrows in N and P show the specific vWF staining with absence of TNF-α staining. Q and R, negative control: the arrows show an absence of staining with primary antibodies being replaced by IgG-isotype controls. S shows nuclear staining with DAPI (blue) in control mice. Magnification ×40. Data shown are representative of 4 separate experiments.
Figure 5. Adiponectin is colocalized with endothelial cells in coronary arterioles.
Dual fluorescence labeling for adiponectin and markers for endothelial cells (vWF) and vascular smooth muscle cells (α-actin) on frozen heart sections. A, B, C and D, dual labeling of adiponectin (red) and vWF (green) in control mice. E, F, G and H, dual labeling of adiponectin (red) and vWF (green) in ApoE KO mice. I, J, K and L, dual labeling of adiponectin (red) and vWF (green) in ApoE and TNF-α double knockout mice (ApoE KOTNF−/TNF−). The white arrows in C, G and K indicate colocalization of adiponectin and endothelial cells (yellow). M, N, O and P, dual labeling of adiponectin (red) and α-actin (green) in ApoE KO mice. The arrow in O shows specific α-actin staining with absence of adiponectin staining. Q, R, S and T, dual labeling of adiponectin (red) and F4/80, marker of macrophages. The arrow in S indicates specific F4/80 staining with absence of adiponectin staining. U and V, negative control: the arrows show an absence of staining with primary antibodies being replaced by IgG-isotype controls. W shows nuclear staining with DAPI (blue) in control mice. Magnification×40. Data shown are representative of 4 separate experiments.
Discussion
The major findings of this study include: 1) in addition to anti-LOX-1 treatment, adiponectin supplementation and anti-TNF-α treatment improved coronary endothelial function of ApoE KO mice through NO, but not through an EDHF-mediated mechanism; 2) both adiponectin and anti-TNF-α reduced LOX-1 expression; 3) adiponectin was co-localized with vascular endothelial cells, but TNF-α was co-localized with vascular smooth muscle cells; 4) adiponectin, anti-LOX-1 and anti-TNF-α treatment reduced oxidative stress possibly through NOX4, a mechanism not previously reported in coronary microvessels in ApoE KO mice. The results of this study provide the first evidence of interactive regulation among adiponectin, TNF-α and LOX-1. Furthermore, the results reveal the complexity of mechanisms underlying coronary microvascular dysfunction in atherosclerosis.
Vascular effects of TNF-α, LOX-1 and adiponectin
LOX-1, the main receptor for ox-LDL in endothelial cells, plays an important role in atherosclerosis [21, 22]. However, the precise mechanisms and effects of LOX-1 on vascular dysfunction remain unclear. Mehta et al. [23] reported that compared with WT mice, basal relaxation of aortic rings from LOX-1 KO mice in response to ACh was enhanced (compared to WT) and anti-LOX-1 antibody pretreatment protected the WT mice aortic rings from the adverse effects of ox-LDL. Our previous studies showed that LOX-1 expression was higher in coronary arterioles from ApoE KO mice [11], and serum LOX-1 levels were significantly enhanced in ApoE KO mice [10]. These results support our current finding that anti-LOX-1 treatment in ApoE KO mice is vasoprotective by partially restoring impaired ACh-induced coronary vasodilation in ApoE KO mice (Figure 1). Thus, the results implicate the role of LOX-1 in the progression of endothelial dysfunction in both macro- and micro- vasculature.
TNF-α, a proinflammatory cytokine, has been reported to be associated with plaque vulnerability [24, 25]. Herein, we found that TNF-α is colocalized with vascular smooth muscle cells of coronary arteries (Figure 4) in ApoE KO mice. Furthermore, coronary endothelial function was partially restored in ApoE KO null for TNF-α, or ApoE KO mice treated with anti-TNF-α (Figure 1B and Figure 2A) further supporting the interactive regulation at both expression and functional levels.
By treating the mice with recombinant globular adiponectin, we found that repetitive adiponectin administration partially rescues coronary microvascular dysfunction in ApoE KO mice (Figure 1C and Figure 2C). This vasoprotection by adiponectin may be, in part, be attributed to the direct stimulation of endothelial NO production. However, the current study does not fully support that this enhanced vasodilatory function is primarily due to an increase in adiponectin expression in the endothelium. This vasoprotective effect could be obtained from any secondary mechanisms evoked by adiponectin administration.
In contrast, endothelium-independent vasodilatory effects of anti-LOX-1, anti-TNF-α or adiponectin do not appear to contribute to vascular dysfunction in ApoE KO mice since sodium nitroprusside (SNP)-induced vasodilation was similar in control mice, ApoE KO mice and ApoE KO mice treated with adiponectin, LOX-1 or anti-TNF-α (Figure 1D). Our results (Figure 2, A–C) also showed that anti-TNF-α, anti-LOX-1 or adiponectin restored ACh-induced NO-mediated vasodilation in the atherosclerotic mice.
Unlike NO-mediated vasodilation, EDHF-induced vasodilation was not significantly different in all groups (Figure 2D), suggesting EDHF-induced vasodilation is preserved in coronary arterioles in our model of atherosclerosis. It suggests that the rescue of endothelium-dependent vasodilation by anti-TNF-α, anti-LOX-1 or adiponectin in ApoE KO mice was mainly through NO-dependent mechanisms but not that of EDHF. Thus, our results indicate that in our model, LOX-1, adiponectin and TNF-α exert vascular effects primarily by affecting vascular NO bioavailability, but not EDHF-induced vasodilation in coronary arterioles in atherosclerosis. In contrast, EDHF-mediated vasodilation was previously shown to be significantly compromised in coronary microvessels of db/db mice (leptin receptor deficient mice as models of type 2 diabetes). However, the relative proportion of EDHF-mediated vasodilation in db/db mice was substantially higher compared to normal wild type mice (50% vs, 81%) suggesting that EDHF in these diabetic mice is a key factor in compensating for severely diminished NO-dependent vasodilation [26]. Thus, mechanisms of endothelial dysfunction may vary greatly between different disease processes/models.
Reciprocal regulation among TNF-α, LOX-1, and adiponectin
Reciprocal regulation among TNF-α, LOX-1 and adiponectin has been implicated in vitro, but has not been extensively investigated in vivo. Up-regulation of LOX-1 by TNF-α has been documented in bovine aortic endothelial cells [12], human and murine macrophages [27]. TNF-α inhibited the expression and secretion of adiponectin from adipocytes [28, 29]. In contrast, adiponectin suppresses LPS-induced secretion of TNF-α from macrophages [30]. Furthermore, adiponectin knockout mice showed higher levels of TNF-α mRNA in adipose tissue and higher plasma TNF-α levels [31]. In the current study, we first determined the cellular localization of TNF-α and adiponectin in coronary vessels of ApoE KO mice by immunostaining. The results showed that TNF-α (Figure 4) was predominantly co-localized with vascular smooth muscle cells while adiponectin (Figure 5) was co-localized within the endothelial cells. Although, in our model, we were not able to obtain a strong immunofluorescent signal for LOX-1 expression to confirm its cellular localization, Li et al. previously reported that LOX-1 was co-localized with macrophages and proliferating smooth muscle cells [32]. Furthermore, Western-blotting of isolated coronary arterioles suggested that anti-TNF-α, anti-LOX-1, and adiponectin treatment reduced LOX-1 expression in coronary arterioles of ApoE KO. Adiponectin expression was not markedly different in coronary arterioles of ApoE KO vs. WT. Thus, these data indicate that LOX-1, adiponectin, and TNF-α act interactively to participate in the complex regulation of their vascular expression in coronary arterioles.
Regulation of arteriole function by TNF-α, LOX-1 and adiponectin involve NF-κB and NOX4
NADPH oxidase, a class of multicomponent enzymes, has been considered as one of the main sources of ROS in vascular cells especially in endothelial cells [33, 34]. The NADPH oxidase subunits (NOX1, NOX2, NOX4, and NOX5) are expressed in virtually all cardiovascular cells, and regulate diverse functions including differentiation, proliferation, apoptosis, senescence, inflammatory responses and oxygen sensing [35]. In regard to isoform specificity, particular interest has focused on NOX4 due to its high level of expression in cardiovascular tissues and unique enzymatic properties in ROS formation [36]. Both TNF-α and LOX-1 lie downstream of NADPH oxidase-derived ROS [5, 37] while adiponectin has been shown to suppress hyperglycaemia-induced ROS production in endothelial cells [38]. LOX-1 is known to mediate the activation of NF-κB through an increased production of intracellular ROS [5, 39]. Furthermore, the binding of ox-LDL to LOX-1 reduces the intracellular concentration of NO through increased production of superoxide in endothelial cell [5, 39]. We previously [11] demonstrated that LOX-1 leads to superoxide production and suggested NADPH oxidase to be the major source of superoxide. Here, we found that NOX4 protein expression was increased in coronary arterioles in ApoE KO mice, which was suppressed by anti-TNF-α, anti-LOX-1 and adiponectin administration (Figure 3). This suggests that NOX4 might contribute to the ROS formation in ApoE mice. We also previously demonstrated that TNF-α contributed to endothelial dysfunction in type 2 diabetes by inducing activation of NADPH oxidase and production of ROS in both aortas and coronary microcirculation [40]. These observations were confirmed by the findings in our present study. Most importantly, NF-κB and N-Tyr protein expression (Figure 3) were increased in coronary arterioles of ApoE KO mice, which were inhibited by anti-TNF-α, anti-LOX-1 and adiponectin (Figure 3). These results suggest that the impaired endothelium-dependent vasodilation in ApoE KO might be due to increased vascular oxidative stress induced by NOX4/NF-κB signaling. This is partially supported by previous studies showing that impaired coronary vasodilation to ACh in ApoE KO mice was partially restored by NAD(P)H oxidase inhibitors, apocynin or DPI [11]. In order to support the direct involvement of oxidative stress in endothelial dysfunction of ApoE KO mice and the anti-oxidative effects by anti-TNF-α, anti-LOX-1 and adiponectin in vasoprotection, further studies are needed to examine the effects of superoxide scavengers or other specific NAD(P)H oxidase inhibitors on coronary endothelial function of ApoE KO mice and ApoE KO mice treated with anti-TNF-α, anti-LOX-1 and adiponectin.
In conclusion, adiponectin, TNF-α, and LOX-1 exert complex regulatory effects on the coronary microvascular endothelial function in atherosclerotic ApoE KO mice. Administration of exogenous adiponectin, adiponectin agonist or inhibition of TNF-α and LOX-1 may serve as effective therapeutic strategies to ameliorate endothelial dysfunction in atherosclerosis.
Acknowledgments
Sources of Funding
This study was supported by grants from Pfizer Atorvastatin Research Award (2004–37, to C.Z.), American Heart Association SDG (110350047A, to C.Z.) and NIH grants (RO1-HL077566 and RO1-HL085119, to C.Z. and M.A.H). H.Z. was supported by American Heart Association Predoctoral Fellowship (10PRE4300043) and is currently supported by American Heart Association Postdoctoral Fellowship (15POST25620017).
Footnotes
Disclosures
None.
Author contributions
X.C., H.Z, C.Z. and Y.P. contributed to conception and design of the experiments, collection, analysis and interpretation of data, and drafting the article and revising; M.A.H contributed to writing and revision of the article. X.C., H.Z, and Y.P. approved the final version for publication.
Reference
- 1.Erl W, Weber PC, Weber C. Monocytic cell adhesion to endothelial cells stimulated by oxidized low density lipoprotein is mediated by distinct endothelial ligands. Atherosclerosis. 1998;136:297–303. doi: 10.1016/s0021-9150(97)00223-2. [DOI] [PubMed] [Google Scholar]
- 2.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185:219–226. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 4.Mehta JL, Li DY. Identification and autoregulation of receptor for OX-LDL in cultured human coronary artery endothelial cells. Biochem Biophys Res Commun. 1998;248:511–514. doi: 10.1006/bbrc.1998.9004. [DOI] [PubMed] [Google Scholar]
- 5.Cominacini L, Rigoni A, Pasini AF, Garbin U, Davoli A, Campagnola M, et al. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J Biol Chem. 2001;276:13750–13755. doi: 10.1074/jbc.M010612200. [DOI] [PubMed] [Google Scholar]
- 6.Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review) Int J Mol Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Liu L, Chen H, Sawamura T, Mehta JL. LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:816–821. doi: 10.1161/01.ATV.0000066685.13434.FA. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Chen H, Romeo F, Sawamura T, Saldeen T, Mehta JL. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J Pharmacol Exp Ther. 2002;302:601–605. doi: 10.1124/jpet.102.034959. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Zhang H, McAfee S, Zhang C. The reciprocal relationship between adiponectin and LOX-1 in the regulation of endothelial dysfunction in ApoE knockout mice. American journal of physiology Heart and circulatory physiology. 2010;299:H605–H612. doi: 10.1152/ajpheart.01096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 12.Kume N, Murase T, Moriwaki H, Aoyama T, Sawamura T, Masaki T, et al. Inducible expression of lectin-like oxidized LDL receptor-1 in vascular endothelial cells. Circ Res. 1998;83:322–327. doi: 10.1161/01.res.83.3.322. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Zhang H, Chen J, Dellsperger KC, Hill MA, Zhang C. Adiponectin abates diabetes-induced endothelial dysfunction by suppressing oxidative stress, adhesion molecules, and inflammation in type 2 diabetic mice. American journal of physiology Heart and circulatory physiology. 2012;303:H106–H115. doi: 10.1152/ajpheart.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, et al. Tumor necrosis factor-alpha induces endothelial dysfunction in LepRdb) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 15.Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Park Y, Zhang C. Coronary and aortic endothelial function affected by feedback between adiponectin and tumor necrosis factor alpha in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2010;30:2156–2163. doi: 10.1161/ATVBAHA.110.214700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension. 2004;44:956–962. doi: 10.1161/01.HYP.0000147559.10261.a7. [DOI] [PubMed] [Google Scholar]
- 18.Richter A, Loschmann PA, Loscher W. Antidystonic efficacy of nitric oxide synthase inhibitors in a rodent model of primary paroxysmal dystonia. Br J Pharmacol. 2000;131:921–926. doi: 10.1038/sj.bjp.0703609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Park Y, Dellsperger KC, Zhang C. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. American journal of physiology Heart and circulatory physiology. 2011;301:H306–H314. doi: 10.1152/ajpheart.01306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X, Zhang H, Belmadani S, Wu J, Xu X, Elford H, et al. Role of TNF-{alpha}-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H2242–H2249. doi: 10.1152/ajpheart.00587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu J, Mitra S, Wang X, Khaidakov M, Mehta JL. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxidants & redox signaling. 2011;15:2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 22.Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 2011;25:419–429. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- 23.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 24.Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Tumor necrosis factor gene expression in human vascular intimal smooth muscle cells detected by in situ hybridization. Am J Pathol. 1990;137:503–509. [PMC free article] [PubMed] [Google Scholar]
- 25.Tipping PG, Hancock WW. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993;142:1721–1728. [PMC free article] [PubMed] [Google Scholar]
- 26.Park Y, Capobianco S, Gao X, Falck JR, Dellsperger KC, Zhang C. Role of EDHF in type 2 diabetes-induced endothelial dysfunction. American journal of physiology Heart and circulatory physiology. 2008;295:H1982–H1988. doi: 10.1152/ajpheart.01261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriwaki H, Kume N, Kataoka H, Murase T, Nishi E, Sawamura T, et al. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998;440:29–32. doi: 10.1016/s0014-5793(98)01414-8. [DOI] [PubMed] [Google Scholar]
- 28.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 29.Kappes A, Loffler G. Influences of ionomycin, dibutyryl-cycloAMP and tumour necrosis factor-alpha on intracellular amount and secretion of apM1 in differentiating primary human preadipocytes. Horm Metab Res. 2000;32:548–554. doi: 10.1055/s-2007-978684. [DOI] [PubMed] [Google Scholar]
- 30.Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. The Journal of biological chemistry. 2008;283:26850–26858. doi: 10.1074/jbc.M802787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 32.Li D, Patel AR, Klibanov AL, Kramer CM, Ruiz M, Kang BY, et al. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging. 2010;3:464–472. doi: 10.1161/CIRCIMAGING.109.896654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Violi F, Basili S, Nigro C, Pignatelli P. Role of NADPH oxidase in atherosclerosis. Future cardiology. 2009;5:83–92. doi: 10.2217/14796678.5.1.83. [DOI] [PubMed] [Google Scholar]
- 34.Rueckschloss U, Duerrschmidt N, Morawietz H. NADPH oxidase in endothelial cells: impact on atherosclerosis. Antioxidants & redox signaling. 2003;5:171–180. doi: 10.1089/152308603764816532. [DOI] [PubMed] [Google Scholar]
- 35.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen F, Haigh S, Barman S, Fulton DJ. From form to function: the role of Nox4 in the cardiovascular system. Frontiers in physiology. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Andresen BT, Hill M, Zhang J, Booth F, Zhang C. Role of Reactive Oxygen Species in Tumor Necrosis Factor-alpha Induced Endothelial Dysfunction. Current hypertension reviews. 2008;4:245–255. doi: 10.2174/157340208786241336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouedraogo R, Wu X, Xu SQ, Fuchsel L, Motoshima H, Mahadev K, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 39.Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler Thromb Vasc Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]