Abstract
BACKGROUND
A percutaneous approach with transcatheter aortic valve replacement (TAVR) and percutaneous coronary intervention (PCI) of the left main coronary artery (LM) is frequently used in high-risk patients with coexisting aortic stenosis and LM disease. Outcomes of TAVR plus LM PCI have not been previously reported.
OBJECTIVES
The primary objective of the TAVR-LM registry is to evaluate clinical outcomes in patients undergoing TAVR plus LM PCI.
METHODS
Clinical, echocardiographic, computed tomographic, and angiographic characteristics were retrospectively collected in 204 patients undergoing TAVR plus LM PCI. In total, 128 matched patient pairs were generated by performing 1:1 case-control matching between 167 patients with pre-existing LM stents undergoing TAVR and 1,188 control patients undergoing TAVR without LM revascularization.
RESULTS
One-year mortality (9.4% vs. 10.2%, p = 0.83) was similar between the TAVR plus LM PCI cohort and matched controls. One-year mortality after TAVR plus LM PCI was not different in patients with unprotected compared with protected LMs (7.8% vs. 8.1%, p = 0.88), those undergoing LM PCI within 3 months compared with those with LM PCI greater than 3 months before TAVR (7.4% vs. 8.6%, p = 0.61), and those with ostial versus nonostial LM stents (10.3% vs. 15.6%, p = 0.20). Unplanned LM PCI performed because of TAVR-related coronary complication, compared with planned LM PCI performed for pre-existing LM disease, resulted in increased 30-day (15.8% vs. 3.4%, p = 0.013) and 1-year (21.1% vs. 8.0%, p = 0.071) mortality.
CONCLUSIONS
Despite the anatomic proximity of the aortic annulus to the LM, TAVR plus LM PCI is safe and technically feasible, with short- and intermediate-term clinical outcomes comparable with those in patients undergoing TAVR alone. These results suggest that TAVR plus LM PCI is a reasonable option for patients who are at high risk for surgery.
Keywords: aortic valve stenosis, coronary artery disease, percutaneous coronary intervention, transcatheter aortic valve replacement
Patients being evaluated for transcatheter aortic valve replacement (TAVR) for severe aortic stenosis (AS) often have coexisting significant left main coronary artery (LM) disease (1,2). Concomitant surgical aortic valve replacement (SAVR) and coronary artery bypass grafting (CABG) is currently the standard of care in patients at low to intermediate surgical risk with coexisting AS and LM disease (3,4). The presence of significant untreated LM disease is an exclusion criterion in clinical trials of TAVR in intermediate-risk patients, including the PARTNER II (Placement of Aortic Transcatheter Valves) and SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) trials. However, patients undergoing TAVR at the present time, outside of clinical trials, are at high surgical risk. Because patients undergoing TAVR are already at high surgical risk for aortic valve replacement, the incremental risk resulting from CABG makes surgical treatment prohibitive for coexisting LM disease and severe AS. In the absence of concomitant SAVR plus CABG as a treatment option, a percutaneous approach with TAVR and percutaneous coronary intervention (PCI) of the LM is gaining increasing acceptance as a potentially feasible, less invasive alternative for such high-risk patients (5,6).
Because of the anatomic proximity of the LM ostium to the aortic annulus (Central Illustration), there is concern about LM stent impingement by the transcatheter aortic valve in patients with preexisting LM stents. In patients with coexisting LM disease and severe AS undergoing TAVR, there is concern for hemodynamic compromise. It is unclear whether the outcomes of TAVR plus LM PCI are influenced by the timing of LM PCI in relation to TAVR, the location of the LM stent, or the clinical indication for LM PCI (planned vs. unplanned). There is also a paucity of data on the feasibility and clinical outcomes of LM PCI in patients with pre-existing transcatheter aortic valves. This multicenter, multinational TAVR-LM registry was thus established to evaluate the clinical outcomes of TAVR plus LM PCI; the impact of unprotected versus protected LM, timing of LM PCI, and location of the LM stent on outcomes; and predictors of outcomes in patients undergoing TAVR plus LM PCI.
CENTRAL ILLUSTRATION. Transcatheter Aortic Valve Replacement and Left Main Coronary Artery Stenting.
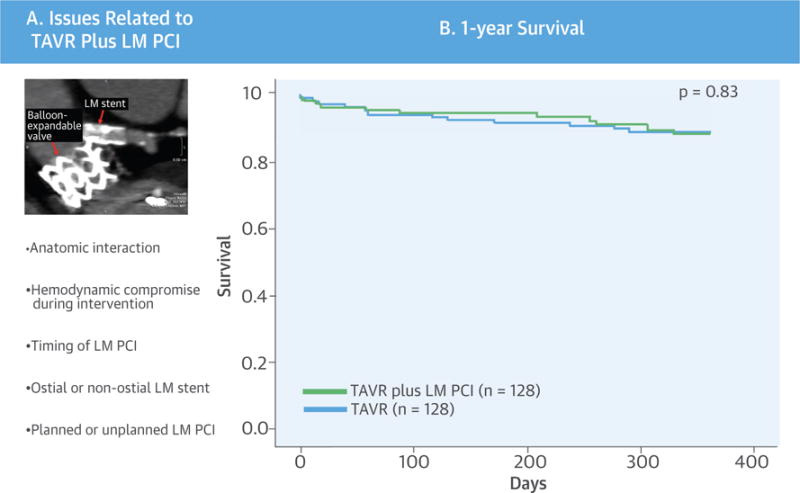
(A) Concept and technical aspects. (B) One-year mortality after transcatheter aortic valve replacement (TAVR) plus left main coronary artery (LM) percutaneous coronary intervention (PCI) versus matched control subjects. There was no difference in 1-year mortality in patients undergoing TAVR plus LM PCI compared with matched control subjects.
METHODS
The TAVR-LM registry retrospectively collected data on 204 consecutive patients undergoing TAVR plus LM PCI from 11 medical centers across North America, Europe, and Canada (January 2007 to December 2014) (Figure 1). The study protocol was approved by the Institutional Review Boards or ethics committees of the individual collaborating sites. Data were collected on baseline clinical, echocardiographic, computed tomographic (CT), angiographic, and procedural characteristics; as well as procedural, 30-day, and 1-year outcomes. Baseline coronary angiograms of LM PCI and baseline CT scans were available for core laboratory analysis from 5 of 9 sites. An experienced interventional cardiologist (R.S.) and an experienced cardiac CT reader (N.T.) performed core laboratory analysis of the available coronary angiograms and CT scans, respectively. Data on the entire population undergoing TAVR at each center, including the total number of patients, vascular approach, mean age, and mean surgical risk scores (Society of Thoracic Surgeons score), were also collected.
FIGURE 1. Study Methodology.
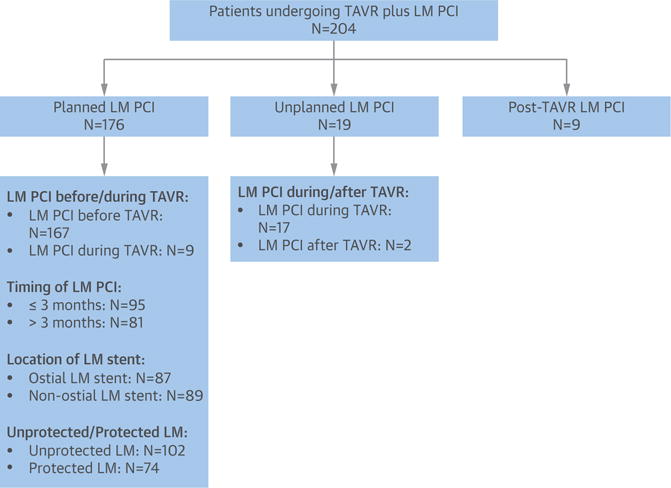
Flowchart of the total number of study patients, subcategorized into those undergoing planned, unplanned, or post-transcatheter aortic valve replacement (TAVR) left main coronary artery (LM) percutaneous coronary intervention (PCI). Outcomes in patients undergoing planned LM PCI were further evaluated on the basis of timing of LM PCI, location of the LM stent, and the presence of protected or unprotected LM.
Patients were divided into 3 groups: planned LM PCI, unplanned LM PCI, and post-TAVR LM PCI. Planned LM PCI was defined as LM PCI performed before or during TAVR for pre-existing LM disease in patients undergoing TAVR. Unplanned LM PCI was defined as LM PCI performed during or within 24 h of TAVR because of TAVR-related coronary complications. Post-TAVR LM PCI was defined as LM PCI performed at least 24 h after TAVR for LM stenosis not related to the TAVR stent frame. Planned LM PCI for pre-existing LM disease was performed in 176 patients, including 167 patients undergoing LM PCI prior to TAVR, as well as in 9 patients undergoing elective planned LM PCI during TAVR. Unplanned LM PCI due to TAVR-related coronary complications was performed in 19 patients, including 17 patients undergoing LM PCI during TAVR and 2 patients undergoing LM PCI within 24 h after TAVR. Post-TAVR LM PCI was performed in 9 patients.
DEFINITIONS
TAVR-related procedural and clinical endpoints were defined according to the Valve Academic Research Consortium 2 criteria (7). Target lesion revascularization was defined as any repeat intervention of the LM stent (including LM bifurcation, if stented) or within 5 mm of the stented segment. Target vessel revascularization (TVR) was defined as repeat intervention of the treated vessel, including any segment of the left anterior descending and left circumflex coronary arteries. Unprotected LM stenosis was defined as the presence of LM stenosis in the absence of history of CABG or with occluded bypass grafts (including arterial and venous grafts) to the left coronary circulation. Protected LM stenosis was defined as LM stenosis in the presence of at least 1 patent bypass graft to the left coronary circulation.
STATISTICAL ANALYSIS
Continuous variables are expressed as mean ± SD or median (interquartile range) and were compared using 2-sided Student t tests or Wilcoxon rank sum tests, as appropriate. Categorical variables are expressed as number (percentage) and were compared using the chi-square test or Fisher exact test, as appropriate. Survival curves were constructed using Kaplan-Meier analysis and compared using log-rank tests. To evaluate the outcomes of TAVR in patients with pre-existing LM stents, 1:1 case-control matching was performed between 167 patients with pre-existing LM stents undergoing TAVR and 1,188 control patients undergoing TAVR without LM revascularization at Cedars-Sinai Medical Center from December 2007 to December 2013. The clinical variables used for matching included age (±10 years), weight (±15 kg), Society of Thoracic Surgeons score (±5%), mean transaortic gradient (≤30 vs. >30 mm Hg), ejection fraction (≤30% vs. >30%), vascular approach (transarterial [transfemoral or subclavian] vs. alternative access [transapical or transaortic]), and unprotected LM and valve size (23 to 25 mm vs. 26 to 27 mm vs. 29 to 31 mm). We generated 128 matched pairs of patients; the remaining 39 patients with pre-existing LM stents undergoing TAVR could not be matched with control patients. Categorical variables in the matched cohort were compared using McNemar tests, and continuous variables were compared using paired Student t tests. Outcomes in patients undergoing planned LM PCI were further compared according to the timing of LM PCI and location of the LM stent. We evaluated the intraclass correlations to determine whether patients within each site were more similar than patients from other sites and found no evidence of clustering; thus, simple univariate Cox proportional hazards modeling was used to evaluate predictors of outcomes in patients with pre-existing LM stents undergoing TAVR. The proportional hazards assumption, as assessed using Schoenfeld residuals, was met for all variables. All statistical analyses were conducted using SPSS version 22.0 (IBM, Armonk, New York).
RESULTS
Of 6,405 patients undergoing TAVR between January 2007 and December 2014 at participating institutions, 204 patients (3.2%) undergoing TAVR plus LM PCI (performed before, during, or after TAVR) were included in the study. Baseline clinical, echocardiographic, CT, and TAVR procedural characteristics, as well as LM PCI procedural characteristics, of the study population are summarized in Tables 1 and 2.
TABLE 1.
Baseline Characteristics of Patients Undergoing Transcatheter Aortic Valve Replacement Plus Left Main Coronary Artery Percutaneous Coronary Intervention (n = 204)
| Age, yrs | 80.9 ± 8.4 |
|
| |
| Hypertension | 178 (87.3) |
|
| |
| Dyslipidemia | 139 (68.1) |
|
| |
| Smoker | 45 (22.1) |
|
| |
| Diabetes | 58 (28.4) |
|
| |
| Insulin-requiring diabetes | 21 (10.3) |
|
| |
| Prior aortic valve surgery | 8 (3.9) |
|
| |
| Peripheral vascular disease | 68 (33.3) |
|
| |
| SYNTAX score pre-TAVR | 4.1 ± 6.3 |
|
| |
| STS score | 8.3 ± 5.6 |
|
| |
| BMI, kg/m2 | 26.2 ± 4.6 |
|
| |
| NYHA functional class | |
| I | 5 (2.5) |
| II | 31 (15.2) |
| III | 113 (55.4) |
| IV | 40 (19.6) |
|
| |
| Timing of PCI | |
| Before TAVR | 167 (81.9) |
| During TAVR | 26 (12.7) |
| Post-TAVR | 11 (5.4) |
|
| |
| Vascular approach | |
| Transfemoral | 149 (73.0) |
| Transapical | 38 (18.6) |
| Subclavian | 5 (2.5) |
| Transaortic | 12 (5.9) |
|
| |
| TAVR prosthesis size, mm | |
| 23–25 | 54 (26.5) |
| 26–27 | 95 (46.6) |
| 29–31 | 55 (27.0) |
| Valve-in-valve | 4 (2.0) |
|
| |
| Valve type | |
| Edwards | 132 (64.7) |
| CoreValve | 68 (33.3) |
| Direct Flow | 4 (2.0) |
|
| |
| Echocardiographic characteristics | |
| EF | 51.5 ± 14.4 |
| Mean gradient, mm Hg | 46.8 ± 18.3 |
| Aortic valve area, cm2 | 0.67 ± 0.20 |
|
| |
| Cross-sectional CT characteristics | |
| Mean annular diameter, mm | 24.0 ± 3.4 |
| Annular area, mm2 | 482.3 ± 92.7 |
| Sinotubular junction, mm | 22.6 ± 10.8 |
| Sinus of Valsalva diameter, mm | 26.7 ± 12.7 |
| center coronary artery height, mm | 11.4 ± 6.1 |
| Right coronary artery height, mm | 15.9 ± 3.6 |
Values are mean ± SD or n (%).
BMI = body mass index; CT = computed tomographic; EF = ejection fraction; LM = Left main coronary artery; NYHA = New York Heart Association; PCI = percutaneous coronary intervention; STS = Society of Thoracic Surgeons; TAVR = transcatheter aortic valve replacement.
TABLE 2.
Procedural Characteristics of Left Main Coronary Artery Percutaneous Coronary Intervention
| Location of LM stenosis | |
| LM only | 43 (22.9) |
| LM and 1 vessel | 54 (28.7) |
| LM and 2 vessels | 53 (28.2) |
| LM and 3 vessels | 38 (20.2) |
|
| |
| RCA disease | 69 (33.8) |
|
| |
| Location of LM stent | |
| Ostial | 110 (53.9) |
| Nonostial | 94 (46.1) |
|
| |
| LM bifurcation stenting | 103 (50.5) |
|
| |
| Treatment of RCA | 29 (14.2) |
|
| |
| Stent type | |
| BMS | 29 (14.2) |
| DES | 149 (73.0) |
| Both DES and BMS | 2 (1.0) |
|
| |
| Percentage stenosis | 67.4 ± 20.8 |
|
| |
| Lesion length, mm | 12.1 ± 8.2 |
|
| |
| Stent length, mm | 18.8 ± 7.5 |
|
| |
| Stent diameter, mm | 3.6 ± 1.0 |
|
| |
| Number of lesions stented | 1.6 ± 1.3 |
|
| |
| Number of vessels stented | 1.7 ± 0.9 |
|
| |
| Number of stents per lesion | 1.1 ± 0.3 |
Values are n (%) or mean ± SD.
BMS = bare-metal stent; DES = drug-eluting stent; LM = Left main coronary artery; RCA = right coronary artery.
OUTCOMES OF TAVR PLUS LM PCI VERSUS TAVR ALONE IN MATCHED PATIENTS
Baseline characteristics of the TAVR plus LM PCI cohort (n = 128) and matched control subjects (n = 128) are summarized in Table 3. There was no difference in 30-day mortality (3.1% vs. 2.3%; hazard ratio [HR]: 1.38; 95% confidence interval [CI]: 0.31 to 6.16; p = 0.67) or 1-year mortality (9.4% vs. 10.2%; HR: 1.09; 95% CI: 0.50 to 2.39; p = 0.83) between the TAVR plus LM PCI cohort and matched control subjects. The Kaplan-Meier survival curve is shown in the Central Illustration. The procedural, 30-day, and 1-year outcomes were similar between the TAVR plus LM PCI and matched control groups, except for a trend toward increased TVR rates at 1 year in the TAVR plus LM PCI group (5.5% vs. 1.6%, p = 0.06) (Table 4).
TABLE 3.
Baseline Characteristics of Matched Transcatheter Aortic Valve Replacement Plus Left Main Coronary Artery Percutaneous Coronary Intervention and Control Patients
| TAVR-LM Registry (n = 128) |
Matched Control Subjects (n = 128) |
p Value | |
|---|---|---|---|
| Age, yrs | 81.7 ± 6.8 | 81.0 ± 7.9 | 0.41 |
|
| |||
| Female | 47 (36.7) | 40 (31.3) | 0.31 |
|
| |||
| Diabetes | 39 (30.5) | 46 (35.9) | 0.43 |
|
| |||
| Insulin-dependent diabetes mellitus | 14 (10.9) | 12 (9.4) | 0.82 |
|
| |||
| Hypertension | 113 (88.3) | 116 (90.6) | 0.68 |
|
| |||
| Peripheral arterial disease | 44 (34.4) | 50 (41.4) | 0.49 |
|
| |||
| Valve-in-valve | 1 (0.8) | 0 (0.0) | >0.99 |
|
| |||
| History of aortic valve surgery | 1 (0.8) | 1 (0.8) | >0.99 |
|
| |||
| Mean gradient ≤30 mm Hg | 10 (7.8) | 10 (7.8) | >0.99 |
|
| |||
| EF ≤30% | 9 (7.0) | 9 (7.0) | >0.99 |
|
| |||
| Vascular approach | >0.99 | ||
| Transfemoral/subclavian | 103 (80.5) | 91 (71.1) | |
| Alternative access | 25 (19.5) | 19 (14.8) | |
|
| |||
| Weight, kg | 72.5 ± 13.2 | 73.3 ± 12.9 | 0.58 |
|
| |||
| BMI, kg/m2 | 25.8 ± 5.5 | 25.8 ± 4.5 | 0.96 |
|
| |||
| STS score | 7.8 ± 4.9 | 8.0 ± 4.5 | 0.58 |
|
| |||
| Ejection fraction, % | 53.5 ± 12.4 | 55.5 ± 13.6 | 0.10 |
|
| |||
| Mean gradient, mm Hg | 47.9 ± 16.5 | 45.7 ± 12.3 | 0.17 |
|
| |||
| Aortic valve area, cm2 | 0.7 ± 0.2 | 0.6 ± 0.2 | <0.01 |
Values are mean ± SD or n (%).
Abbreviations as in Table 1.
TABLE 4.
Clinical Outcomes in Matched Transcatheter Aortic Valve Replacement Plus Left Main Coronary Artery Percutaneous Coronary Intervention and Control Patients
| TAVR-LM Registry (n = 128) |
Matched Control Subjects (n = 128) |
p Value | |
|---|---|---|---|
| Procedural outcomes | |||
| LM obstruction | 2 (1.6) | 0 (0.0) | 0.50 |
| Need for second valve | 5 (3.9) | 8 (6.3) | 0.58 |
| Cardiac tamponade | 0 (0.0) | 1 (0.8) | >0.99 |
| Intraprocedural death | 0 (0.0) | 1 (0.8) | >0.99 |
| Major or life-threatening bleeding | 22 (17.2) | 33 (25.8) | 0.14 |
| Major vascular complications | 21 (16.4) | 5 (3.9) | <0.01 |
| Acute kidney injury | 6 (4.7) | 7 (5.4) | >0.99 |
| Permanent pacemaker | 34 (26.6) | 18 (14.1) | 0.02 |
| CoreValve | 46.1 | 42.8 | |
| Direct Flow | 25.0 | 0.0 | |
| Edwards | 12.5 | 11.3 | |
| Valve embolization | 0 (0.0) | 2 (1.6) | 0.50 |
| Cardiogenic shock | 5 (3.9) | 2 (1.6) | 0.45 |
|
| |||
| 30-day outcomes | |||
| Mortality | 4 (3.1) | 3 (2.3) | 0.67 |
| Target vessel revascularization | 0 (0.0) | 0 (0.0) | NA |
| Myocardial infarction | 0 (0.0) | 0 (0.0) | NA |
| Stroke | 1 (0.8) | 2 (1.6) | 0.57 |
|
| |||
| 1-yr mortality | |||
| Mortality | 12 (9.4) | 13 (10.2) | 0.83 |
| Target vessel revascularization | 7 (5.5) | 2 (1.6) | 0.06 |
| Myocardial infarction | 3 (2.4) | 1 (0.8) | 0.25 |
| Stroke | 1 (0.8) | 3 (2.3) | 0.38 |
Values are n (%).
NA = not applicable; other abbreviations as in Table 1.
Because 39 patients with pre-existing LM stents undergoing TAVR could not be adequately matched by case-control matching, we performed sensitivity analysis to evaluate the impact of the unmatched cohort on outcomes after TAVR plus LM PCI. One-year mortality was not significantly different between the matched cohort (n = 128) and the unmatched cohort (n = 39) in the TAVR-LM registry (HR: 0.239; 95% CI: 0.031 to 1.84; p = 0.17). Using a model that was adjusted for baseline covariate differences using inverse propensity score weighting, the 1-year mortality between patients undergoing TAVR plus LM PCI (n = 167) was not significantly different compared with those undergoing TAVR alone (n = 1,188) (HR: 0.61; 95% CI: 0.32 to 1.18; p = 0.14).
OUTCOMES OF TAVR PLUS LM PCI IN UNPROTECTED VERSUS PROTECTED LM
TAVR plus LM PCI was performed in 102 patients with unprotected LMs and in 74 patients with protected LMs. There was no difference in 1-year mortality after TAVR plus LM PCI between patients with unprotected compared with protected LM disease (7.8% vs. 8.1%; HR: 0.92; 95% CI: 0.32 to 2.66; p = 0.88) (Online Figure 1). The rates of stroke (3.0% vs. 0.0%, p = 0.15), myocardial infarction (MI) (3.0% vs. 1.4%, p = 0.47), and TVR (5.9% vs. 1.4%, p = 0.13) at 1 year were not significantly different between the 2 groups, although the rates were numerically greater in patients with unprotected LMs.
OUTCOMES OF TAVR PLUS LM PCI, ACCORDING TO THE TIMING OF LM PCI
LM PCI was performed within 3 months before TAVR in 95 patients and >3 months before TAVR in 81 patients. There was no statistically significant difference in 1-year mortality among patients undergoing LM PCI within 3 months before TAVR or >3 months before TAVR (7.4% vs. 8.6%; HR: 0.76; 95% CI: 0.27 to 2.17; p = 0.61) (Online Figure 2). Performing LM PCI within 3 months before TAVR was not associated with increased risk for acute kidney injury (AKI) (6.3% vs. 5.2%, p = 0.76), major vascular complications (13.7% vs. 12.3%, p = 0.79), or major or life-threatening bleeding (13.7% vs. 14.8%, p = 0.83), compared with those undergoing LM PCI >3 months before TAVR. One-year mortality (11.1% vs. 7.8%, p = 0.88) with TAVR plus LM PCI was not statistically different among patients undergoing planned LM PCI before or during TAVR.
OUTCOMES OF TAVR PLUS LM PCI, ACCORDING TO THE LOCATION OF LM STENT
Among those with pre-existing LM stents undergoing TAVR, ostial LM stents were present in 87 patients and nonostial LM stents in 89 patients. Two patients with pre-existing ostial LM stents undergoing TAVR with the Edwards SAPIEN (Edwards Lifesciences, Irvine, California) valve experienced LM stent impingement by the TAVR prosthesis. Both patients were hemodynamically stable during the procedure and underwent successful balloon angioplasty of the LM stent. There was no statistically significant difference in 1-year survival between patients with and those without ostial LM stents undergoing TAVR (10.3% vs. 15.6%; HR: 2.03; 95% CI: 0.68 to 6.05; p = 0.20) (Online Figure 3). There was no statistically significant difference between the 2 groups in the rates of TVR (2.4% vs. 5.7%, p = 0.44) and MI (1.2% vs. 3.4%, p = 0.42) at 1 year. The type of transcatheter heart valve (Edwards or Medtronic CoreValve [Medtronic, Minneapolis, Minnesota]) did not significantly influence 30-day or 1-year mortality.
OUTCOMES OF TAVR PLUS LM PCI, ACCORDING TO PLANNED VERSUS UNPLANNED LM PCI
Baseline characteristics and outcomes of patients undergoing planned and unplanned LM PCI are summarized in Tables 4 and 5, respectively. Unplanned LM PCI was due to TAVR stent-related coronary obstruction and LM dissection in 18 of 19 and 1 of 19 patients, respectively. The median number of days between LM PCI and TAVR in the planned LM PCI group was 64 (interquartile range: 25 to 206 days). Patients undergoing unplanned LM PCI more often had higher mean and peak gradients, had lower LM height, and were more likely to have multivessel coronary artery disease. Valve-in-valve procedures, TAVR with the balloon-expandable Edwards valve, and TAVR with larger valves was more often performed in patients undergoing unplanned LM PCI. One patient had obstruction of both the LM and right coronary artery.
TABLE 5.
Clinical Outcomes According to Planned and Unplanned Left Main Coronary Artery Percutaneous Coronary Intervention
| Planned LM PCI (n = 176) |
Unplanned LM PCI (n = 19) |
p Value | |
|---|---|---|---|
| Procedural outcomes | |||
| LM obstruction | 2 (1.1)* | 18 (94.7) | <0.01 |
| LM dissection | 0 (0.0) | 1 (5.3) | <0.01 |
| RCA obstruction | 0 (0.0) | 1 (5.3) | <0.01 |
| Need for cardiopulmonary resuscitation | 1 (0.6) | 3 (15.8) | <0.01 |
| Emergent open heart surgery | 0 (0.0) | 0 (0.0) | NA |
| Timing of LM obstruction | 0.93 | ||
| After BAV | 0 (0.0) | 1 (6.3) | |
| After valve implantation | 1 (100.0)* | 11 (68.8) | |
| After post-dilation | 0 (0.0) | 2 (12.5) | |
| Within 24 h of TAVR | 0 (0.0) | 2 (12.5) | |
| Intraprocedural death | 0 (0.0) | 0 (0.0) | NA |
| Valve embolization | 1 (0.6) | 0 (0.0) | 0.74 |
| Need for second valve | 8 (4.8) | 1 (5.3) | 0.93 |
| Cardiac tamponade | 0 (0.0) | 0 (0.0) | NA |
| Cardiogenic shock | 6 (3.4) | 4 (21.1) | <0.01 |
| New LBBB | 20 (11.4) | 2 (10.5) | 0.91 |
| Permanent pacemaker | 38 (21.6) | 2 (10.5) | 0.26 |
| Acute renal failure | 10 (5.8) | 5 (26.3) | <0.01 |
| Need for hemodialysis | 6 (3.5) | 2 (11.1) | 0.12 |
| Major vascular complication | 23 (13.1) | 2 (10.5) | 0.75 |
| Major or life-threatening bleeding | 25 (14.2) | 2 (10.5) | 0.66 |
| Duration of hospital stay (days) | 5.5 ± 7.1 | 10.9 ± 10.0 | 0.01 |
|
| |||
| 30-day outcomes | |||
| Mortality | 6 (3.4) | 3 (15.8) | 0.013 |
| Target vessel revascularization | 0 (0.0) | 0 (0.0) | NA |
| Myocardial infarction | 1 (0.6) | 0 (0.0) | 0.74 |
| Stroke | 2 (1.1) | 1 (5.3) | 0.16 |
|
| |||
| 1-yr mortality | |||
| Mortality | 14 (8.0) | 4 (21.1) | 0.07 |
| Target vessel revascularization | 7 (4.0) | 0 (0.0) | 0.40 |
| Myocardial infarction | 4 (2.3) | 0 (0.0) | 0.52 |
| Stroke | 3 (1.7) | 1 (5.3) | 0.29 |
Values are n (%).
Two patients with pre-existing LM stents undergoing TAVR experienced LM stent impingement by the transcatheter heart valve. This was successfully managed with balloon angioplasty of the LM stent.
Patients undergoing unplanned LM PCI, compared with planned LM PCI, had an increased incidence of cardiogenic shock (21.1% vs. 3.4%, p < 0.001), need for cardiopulmonary resuscitation (15.8% vs. 0.6%, p < 0.001), and AKI (26.3% vs. 5.8%, p = 0.002). Difficulty engaging the LM ostium after acute occlusion was experienced in 5 of 19 patients. Compared with planned LM PCI, unplanned LM PCI was associated with significantly increased 30-day (15.8% vs. 3.4%; HR: 4.91; 95% CI: 1.23 to 19.64; p = 0.013) and 1-year (21.1% vs. 8.0%; HR: 2.67; 95% CI: 0.88 to 8.12; p = 0.071) mortality (Figure 2). In 6 of 19 patients undergoing unplanned LM PCI, the LM was protected with a coronary guidewire, with or without a stent, because of the high risk for LM occlusion during TAVR. LM protection was more often performed in patients undergoing transcatheter valve-in-valve procedures or with ostial LM stenosis and preexisting ostial LM stents. There were 0 of 6 deaths at 1 year in patients undergoing unplanned LM PCI with coronary protection, compared with 4 of 13 deaths in patients undergoing unplanned LM PCI without coronary protection (log-rank p = 0.143).
FIGURE 2. Mortality in Patients Undergoing Planned Versus Unplanned Left Main Coronary Artery Percutaneous Coronary Intervention.
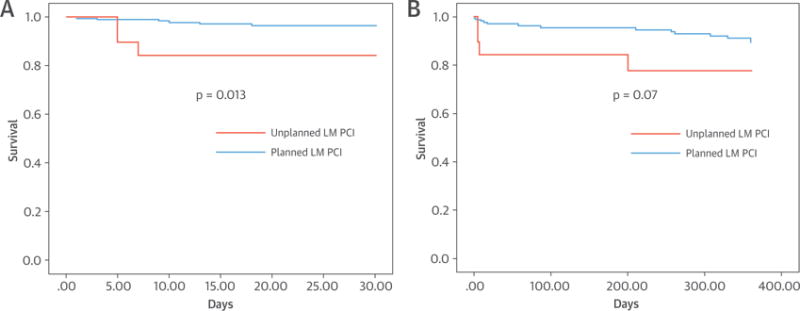
Increased 30-day (A) and 1-year mortality (B) in patients undergoing unplanned left main coronary artery (LM) percutaneous coronary intervention (PCI), in comparison with planned LM PCI.
PREDICTORS OF MORTALITY AFTER TAVR PLUS LM PCI
The univariate predictors of mortality in the 176 patients undergoing planned TAVR plus LM PCI are summarized in Table 6. Unplanned LM PCI (compared with planned LM PCI), need for second transcatheter aortic valve, AKI, and need for hemodialysis were univariate predictors of 30-day mortality after TAVR plus LM PCI. Unplanned LM PCI, need for a second valve, AKI, and low body weight were univariate predictors of 1-year mortality after TAVR plus LM PCI.
TABLE 6.
Univariate Predictors of 30-Day and 1-Year Mortality After Transcatheter Aortic Valve Replacement Plus Left Main Coronary Artery Percutaneous Coronary Intervention
| Hazard Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| 30-day mortality | |||
|
| |||
| Unplanned vs. planned | 4.91 | 1.23–19.64 | 0.025 |
| Need for second valve | 8.22 | 1.66–40.81 | 0.010 |
| Need for hemodialysis | 7.14 | 1.48–34.46 | 0.014 |
| Acute renal failure | 10.41 | 2.79–38.89 | <0.01 |
| Mean gradient | 1.03 | 1.00–1.06 | 0.079 |
|
| |||
| 1-year mortality | |||
|
| |||
| Unplanned vs. planned | 2.70 | 0.88–8.12 | 0.080 |
| Need for second valve | 3.86 | 0.87–17.0 | 0.075 |
| Acute renal failure | 3.26 | 1.07–9.93 | 0.037 |
| Weight | 0.96 | 0.92–1.00 | 0.027 |
POST-TAVR LM PCI
LM PCI was performed after TAVR in 9 patients. The median number of days between LM PCI and TAVR was 368 (interquartile range: 204 to 534 days). Four patients had the self-expanding Medtronic CoreValve, and 5 patients had the balloon-expandable Edwards valve. Seven of 9 patients presented with unstable angina or non–ST-segment elevation MI, and 2 patients presented with stable angina. All cases were related to progression of coronary artery disease in the LM, with no cases related to LM ST-segment elevation MI or LM thrombus. None of the cases of post-TAVR LM PCI were related to LM stent impingement by the TAVR valve. Difficulty engaging the LM ostium was not encountered in any patient undergoing LM PCI post-TAVR. One-year mortality in the post-TAVR LM PCI cohort was 11.1%.
DISCUSSION
Our study, the first to provide substantial evidence from a large cohort of patients with TAVR plus LM PCI, has 3 principal findings: 1) performing planned LM PCI before or during TAVR does not result in incremental risk compared with TAVR alone; 2) outcomes after TAVR plus LM PCI are not influenced by the presence of unprotected or protected LMs, the location of the stent (ostial vs. nonostial LM stent), or the timing of LM PCI; and 3) unplanned LM PCI for coronary occlusion occurring during TAVR results in increased 30-day and 1-year mortality.
PLANNED LM PCI PROCEDURAL AND CLINICAL OUTCOMES
Despite the increasing acceptance of LM PCI for isolated LM disease in high-risk patients, the presence of coexisting severe AS has, until recently, been considered amenable only to surgical therapy. Percutaneous treatment of significant LM disease in the presence of severe AS is associated with a number of theoretical risks. Transient interruption of myocardial blood flow during balloon inflation for LM PCI in patients with severe AS can result in significant hemodynamic compromise, with rapid deterioration in cardiac function. Conversely, transcatheter aortic valve deployment, especially with rapid pacing, or balloon pre-dilation in preparation for TAVR, can compromise coronary flow in the presence of coexisting significant LM disease. There is increased risk for bleeding complications following TAVR, because of the need for uninterrupted dual-antiplatelet therapy after LM PCI. The risk for AKI after TAVR is increased because of the use of additional contrast for LM PCI. Even slight displacement of calcium nodules or native aortic leaflets or plaque shift toward the LM ostium can result in LM occlusion in the presence of pre-existing untreated ostial LM disease. In patients with pre-existing ostial LM stents, the transcatheter aortic valve frame can deform the LM stent, impairing coronary flow. In light of these concerns, our study provides reassuring evidence that the procedural, 30-day, and 1-year results of TAVR plus LM PCI are acceptable, with 0.0% intraprocedural mortality, 3.6% 30-day mortality, and 8.5% 1-year mortality; a 5.8% rate of AKI at 30 days; a 14.2% rate of major life-threatening bleeding complications at 30 days; and 0.0% conversion to SAVR. These results are comparable with those reported for TAVR in contemporary TAVR series (8–11). Moreover, procedural, 30-day, and 1-year outcomes in patients undergoing TAVR plus LM PCI were not statistically different from those in a matched cohort of 128 patients undergoing TAVR alone. The outcomes of TAVR plus LM PCI were not significantly affected by the presence of protected or unprotected LM disease.
TIMING OF LM PCI
The performance of LM PCI within 3 months before TAVR was not associated with increased risk for AKI, major vascular complications, major or life-threatening bleeding complications, or 1-year mortality compared with patients undergoing LM PCI >3 months before TAVR. This finding suggests that the TAVR procedure does not necessarily need to be deferred for a significant length of time after LM PCI. However, it may still be reasonable to stage the TAVR procedure in patients at increased risk for AKI, such as those with baseline renal dysfunction. Likewise, it may be reasonable to stage the TAVR procedure in patients requiring interruption of dual-antiplatelet therapy for TAVR to minimize the risk for stent thrombosis and bleeding complications, for instance, patients undergoing TAVR by alternative access (transapical or transaortic).
ANATOMIC INTERACTION BETWEEN LM STENT AND TRANSCATHETER AORTIC VALVE
Because the anatomic proximity of the aortic valve to the LM ostium predisposes patients with ostial LM stents to stent impingement by the transcatheter aortic valve, this condition warrants special attention. Cannulation of a potentially compromised ostial LM stent in the presence of a transcatheter aortic valve can be technically challenging. There were 4 cases of non-flow-limiting ostial LM stent impingement in our series. The LM had been pre-emptively protected with a coronary guidewire in all 4 cases before valve deployment. Successful balloon angioplasty of the LM stent was performed in all 4 cases without any technical difficulty. The clinical outcomes, at least up to intermediate-term follow-up, have not been different between ostial and nonostial LM stent cohorts. The clinical outcomes in patients with ostial LM stents were not influenced by the type of transcatheter aortic valve. Thus, although the presence of an ostial LM stent constitutes a high-risk feature for LM stent impingement after TAVR, the presence of ostial LM stent should not deter physicians from referring these patients for TAVR. Our study demonstrates that TAVR can be safely performed in such patients with careful patient selection and procedural planning.
UNPLANNED VERSUS PLANNED PCI
Ribeiro et al. (12) previously reported the incidence, predictive factors, and outcomes of coronary occlusion after TAVR. In the analysis by Ribeiro et al., coronary obstruction after TAVR resulted in increased 30-day mortality in patients unable to undergo successful PCI, compared with those who underwent successful PCI for coronary obstruction. However, it remained unclear from that study whether the outcomes in patients undergoing successful LM PCI for coronary obstruction (unplanned PCI in the TAVR-LM registry) were comparable with those undergoing planned LM PCI for pre-existing LM disease. Our study provides evidence that patients undergoing unplanned LM PCI (even if successful) continue to have significantly increased 30-day and 1-year mortality, compared with those undergoing successful planned LM PCI. Unplanned LM PCI, even if successful, is a predictor of 30-day mortality after TAVR. This result underscores the importance of careful procedural planning to identify patients at increased risk for coronary compromise. In our study, the 1-year mortality was 0% versus 69.2% in patients undergoing unplanned LM PCI with or without coronary protection. High-risk features for coronary obstruction, including ostial LM stenosis, pre-existing ostial LM stent, transcatheter valve-in-valve procedure (especially for stentless valves or valves with leaflets sutured on the outer side of the stent frame), and low LM height, should prompt the operator to exercise caution during the procedure and consider protecting the coronary arteries with placement of a guiding catheter, guidewire, angioplasty balloon, or undeployed coronary stent in the left coronary system to enable rapid PCI in the case of acute LM occlusion (13–15).
STUDY LIMITATIONS
Our registry data are retrospective. The reporting of cases and adjudication of complications are performed by the participating institutions on a voluntary basis, thereby introducing the possibility of reporting bias. The exact reason for performing TAVR plus LM PCI, instead of SAVR plus CABG, was not available. Although LM PCI after TAVR was performed with a 100% success rate in the 9 patients included in our study, the safety and technical feasibility of LM PCI in patients with pre-existing transcatheter aortic valves need to be verified in a larger cohort of patients. The majority of patients in the TAVR-LM registry underwent TAVR with the balloon-expandable Edwards valve and self-expanding Medtronic CoreValve; only 4 patients underwent TAVR with the Direct Flow Valve (Direct Flow Medical, Santa Rosa, California), and there were no cases performed with the other, newer generation transcatheter aortic valves. Thus, the study findings cannot be extrapolated to patients undergoing TAVR with the newer transcatheter aortic valves. The angiographic and CT data were available for core laboratory analysis from 4 of 9 collaborating centers; however, the availability of imaging studies did not significantly influence outcomes. The study did not have adequate statistical power to detect differences in small subgroup comparisons. Because of the limited data available, the impact of dual-antiplatelet therapy on the incidence of stent thrombosis in this patient population could not be evaluated.
CONCLUSIONS
Despite the anatomic proximity between the aortic annulus and LM, TAVR plus LM PCI is safe and technically feasible, with clinical outcomes comparable with those in patients undergoing TAVR alone. The presence of coexisting LM disease in patients with severe AS should not deter physicians from evaluating patients for TAVR. Future studies are required to compare percutaneous and surgical approaches for coexisting LM disease and severe AS.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS
Despite the anatomic proximity of the aortic valve annulus to the ostium of the LM, patients with symptomatic, severe AS, and LM disease who are poor candidates for an open surgical approach can be successfully managed with TAVR, even in the presence of ostial lesions, and TAVR can be performed before or after stenting of the LM.
TRANSLATIONAL OUTLOOK
Randomized trials are needed to compare the outcomes of percutaneous and surgical approaches for patients with coexisting LM disease and severe AS.
Acknowledgments
The authors thank Heidi Gransar, MS, of Cedars-Sinai Medical Center, Los Angeles, for help with the statistical analysis.
Dr. Lindman is supported by grant K23 HL116660 from the National Institutes of Health. Dr. Latib has served as a consultant for Medtronic and Direct Flow Medical. Dr. Jilaihawi has served as a consultant for Edwards Lifesciences, St. Jude Medical, and Venus Medtech. Dr. Tarantini has received a lecture fee from Edwards Lifesciences. Dr. Lefèvre was a proctor for Edwards Lifesciences; and has received minor fees from Medtronic and Direct Flow Medical. Dr. Lindman has received research support from and served on the scientific advisory board for Roche Diagnostics. Dr. Tuzcu is an Executive Committee member for the PARTNER trial; and principal investigator of the SALUS trial. Dr. Sievert has received study honoraria, travel expenses, and consulting fees (<$25,000) from Abbott, Access Closure, AGA, Angiomed, Aptus, Atrium, Avinger, Bard, Boston Scientific, Bridgepoint, Cardiac Dimensions, CardioKinetix, CardioMEMS, Coherex, Contego, Covidien, CSI, CVRx, EndoCross, ev3, FlowCardia, Gardia, Gore, Guided Delivery Systems, InSeal Medical, Lumen Biomedical, HLT, Lifetech, Lutonix, Maya Medical, Medtronic, NDC, Occlutech, Osprey, Ostial, PendraCare, pfm Medical, Recor, ResMed, Rox Medical, SentreHEART, Spectranetics, SquareOne, Svelte Medical Systems, Trireme, Trivascular, Venus Medical, Veryan, and Vessix; has received grant research support (<$25,000) from Cook and St. Jude Medical; and has stock options (<$25,000) with Cardiokinetix, Access Closure, Velocimed, Lumen Biomedical, Coherex, and SMT. Dr. Rodés-Cabau has received grant support from Boston Scientific and Edwards Lifesciences; and consulting fees and honoraria from AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo/Eli Lilly, GlaxoSmithKline, Janssen, Merck/Schering-Plough, and Regeneron. Dr. Colombo is a minor shareholder in Direct Flow Medical. Dr. Makkar is the principal investigator for the St. Jude Medical Portico trial; has received research grants from Edwards Lifesciences, St. Jude Medical, and Medtronic; and has received a consulting fee from Edwards Lifesciences.
ABBREVIATIONS AND ACRONYMS
- AKI
acute kidney injury
- AS
aortic stenosis
- CABG
coronary artery bypass grafting
- CI
confidence interval
- CT
computed tomographic
- HR
hazard ratio
- LM
left main coronary artery
- MI
myocardial infarction
- PCI
percutaneous coronary intervention
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
- TVR
target vessel revascularization
APPENDIX
For supplemental figures, please see the online version of this article.
Footnotes
Listen to this manuscript’s audio summary by JACC Editor-in-Chief Dr. Valentin Fuster.
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. John Bittl, MD, served as Guest Editor for this paper.
References
- 1.Andreini D, Pontone G, Mushtaq S, et al. Diagnostic accuracy of multidetector computed tomography coronary angiography in 325 consecutive patients referred for transcatheter aortic valve replacement. Am Heart J. 2014;168:332–9. doi: 10.1016/j.ahj.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Gautier M, Pepin M, Himbert D, et al. Impact of coronary artery disease on indications for transcatheter aortic valve implantation and on procedural outcomes. Eurointervention. 2011;7:549–55. doi: 10.4244/EIJV7I5A90. [DOI] [PubMed] [Google Scholar]
- 3.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2438–88. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe R, Finkelstein A, Lewis BS, et al. Stenting of the unprotected left main coronary artery in patients with severe aortic stenosis prior to percutaneous valve interventions. Cardiovasc Revasc Med. 2012;13:90–4. doi: 10.1016/j.carrev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Cevallos J, Andrea R, Falces C, et al. Transcatheter aortic valve implantation in patients with left main percutaneous coronary intervention. J Heart Valve Dis. 2013;22:874–7. [PubMed] [Google Scholar]
- 7.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–54. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Généreux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317–26. doi: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–69. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Smith CR, Leon MB, Mack MJ, et al. for the PARTNER Trial Investigators Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 11.Khatri PJ, Webb JG, Rodés-Cabau J, et al. Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann Intern Med. 2013;158:35–46. doi: 10.7326/0003-4819-158-1-201301010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol. 2013;62:1552–62. doi: 10.1016/j.jacc.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarty T, Jilaihawi H, Nakamura M, et al. Pre-emptive positioning of a coronary stent in the left anterior descending artery for left main protection: a prerequisite for transcatheter aortic valve-in-valve implantation for failing stentless bioprostheses? Catheter Cardiovasc Interv. 2013;82:E630–6. doi: 10.1002/ccd.25037. [DOI] [PubMed] [Google Scholar]
- 14.Dvir D, Leipsic J, Blanke P, et al. Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovasc Interv. 2015;8:e002079. doi: 10.1161/CIRCINTERVENTIONS.114.002079. [DOI] [PubMed] [Google Scholar]
- 15.Abramowitz Y, Chakravarty T, Jilaihawi H, et al. Clinical impact of coronary protection during transcatheter aortic valve implantation: first reported series of patients. EuroIntervention. 2015;11:572–81. doi: 10.4244/EIJV11I5A112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


