Abstract
Many anti-human immunodeficiency virus 1 nucleoside reverse-transcriptase inhibitors have low central nervous system (CNS) distribution due in part to active efflux transport at the blood-brain barrier. We have previously shown that zidovudine (AZT) and abacavir (ABC) are in vitro substrates for the efflux transport protein breast cancer resistance protein (Bcrp) 1. We evaluated the influence of Bcrp1 on plasma pharmacokinetics and brain penetration of zidovudine and abacavir in wild-type and Bcrp1-deficient (Bcrp1−/−) FVB mice. There was no difference in either area under the concentration-time profiles for plasma (AUCplasma) or brain (AUCbrain) for zidovudine between the wild-type and Bcrp1−/− mice. The AUCplasma of abacavir was 20% lower in the Bcrp1−/− mice, whereas the AUCbrain was 20% greater. This difference resulted in a 1.5-fold increase in abacavir brain exposure in the Bcrp1−/− mice. The effect of selective and nonselective transport inhibitors on the ABC brain/plasma ratio at a single time point was evaluated. 3-(6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionicacid tert-butyl ester (Ko143), N[4[2-(6, 7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]-5-methoxy-9-oxo-10H-acridine-4-carboxamide (GF120918), probenecid, and Pluronic P85 increased abacavir plasma concentrations in the wild-type mice. Abacavir plasma concentrations in Bcrp1−/− mice were increased by (R)-4-((1aR,6R,10bS)-1,2-difluoro-1,1a,6,10b-tetrahydrodibenzo(a,e)cyclopropa(c)cycloheptan-6-yl)-α-((5-quinoloyloxy)methyl)-1-piperazineethanol trihydrochloride (LY335979), GF120918, and probenecid, but not by Ko143. Brain/plasma concentration ratios in both the wild-type and Bcrp1−/− mice were increased by the P-glycoprotein inhibitors LY335979 and GF120918, but not by BCRP-selective inhibitors. These data indicate that deletion of Bcrp1 has little influence on the pharmacokinetics or brain penetration of AZT. However, for abacavir, deletion of Bcrp1 reduces plasma exposure and enhances brain penetration. These findings suggest that Bcrp1 does not play a significant role in limiting the CNS distribution of zidovudine and abacavir; however, brain penetration of abacavir is dependent on P-glycoprotein-mediated efflux.
Nucleoside analog reverse transcriptase inhibitors (NRTIs) continue to be a vital and effective component of combinatorial antiretroviral therapy (ART) for treating HIV infection (Dieterich, 2006). However, the CNS penetration of a number of NRTIs has been found to be low. The limited entry of these drugs into the brain has been attributed to the tight junctions and active efflux transport mechanisms at the blood-brain barrier (BBB) (Sawchuk and Yang, 1999). The specific mechanisms, i.e., BBB efflux transporters that contribute to the reduced CNS exposure of these anti-HIV agents need to be determined to yield insight into how one can improve their targeted bioavailability to the brain, an important pharmacological sanctuary site for the virus.
Efflux transport proteins, such as P-glycoprotein (P-gp) and the MRPs, have been identified as a major mechanism of multidrug resistance in chemotherapy-resistant cancers (Kvackajova-Kisucka et al., 2001). These efflux transport proteins have also been localized in various tissues, including physiological barriers such as the BBB (Cordon-Cardo et al., 1990; Nies et al., 2004). Polarized transport of these drugs by these efflux transporters at the microvessel endothelial cells of the BBB could be one such mechanism that influences the CNS drug penetration. A recent study reported the interaction between the NRTI abacavir (ABC) and P-gp (Shaik et al., 2007). Abacavir stimulated P-gp-mediated ATPase activity and showed decreased cellular accumulation as well as increased directional flux across MDR1-transfected MDCKII cells that overexpress human P-gp. Further in vivo examination of abacavir CNS distribution in the mdr1a-deficient mutant mouse model showed that abacavir brain exposure was increased 20-fold with a minor (2-fold) increase in plasma exposure compared with that in the wild-type mouse (Shaik et al., 2007). These observations indicate that the brain penetration of abacavir is influenced by P-gp-mediated efflux at the BBB.
BCRP/ABCG2 is another member of the ABC superfamily of efflux transport proteins that has been localized in various tissues and physiological barriers, including the BBB (Maliepaard et al., 2001; Cooray et al., 2002; Hori et al., 2004). A wide array of exogenous and endogenous compounds have been reported to be substrates for BCRP in vitro, with significant substrate overlap with P-gp and the MRPs (Krishnamurthy and Schuetz, 2006). Compounds from the major classes of ART have been shown to interact with BCRP in vitro. For example, decreased anti-HIV1 activity of AZT, 3TC, d4T, and ddI, as well as reduced accumulation of AZT and its metabolites, was observed in CD4+ T cells overexpressing BCRP (Wang et al., 2003). Treatment with the BCRP inhibitor, fumitremorgin C, reversed the effect of BCRP (Wang et al., 2004). Furthermore, anti-HIV1 protease inhibitors have been reported to be inhibitors, but not substrates, of BCRP-mediated efflux (Gupta et al., 2004). A number of anti-HIV drugs, including zidovudine and abacavir, have been reported to inhibit BCRP-mediated efflux of pheophorbide A (Weiss et al., 2007). Cells transfected with Bcrp1, the murine homolog of BCRP, exhibited polarized transport of zidovudine and abacavir that was abolished by BCRP inhibitors, GF120918 and Ko143 (Pan et al., 2007).
Currently, there are no reports examining the influence of BCRP on in vivo disposition of zidovudine or abacavir. A useful model available to study the influence of BCRP in vivo is the Bcrp1 knockout (Bcrp1−/−) mouse (Jonker et al., 2002). This transgenic knockout mouse model has been used to show the significance of Bcrp1 in drug absorption, distribution, metabolism, and excretion (ADME). For example, the absorption and disposition of the dietary carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), was determined by comparing hepatobiliary excretion and plasma AUCs after oral and i.v. administration in wild-type and Bcrp1−/− mice (van Herwaarden et al., 2003). The plasma AUCs of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in the Bcrp1−/− mice were 2.9- and 2.2-fold higher than that in wild-type mice after oral and i.v. administration, respectively. Recently, this model was used to demonstrate that the bioavailability and the brain penetration of phytoestrogens are limited by Bcrp1-mediated efflux (Enokizono et al., 2007). Increased systemic exposure and higher brain/plasma ratios were observed in Bcrp1−/− mice compared with the wild-type mice (Enokizono et al., 2007). These reports strongly suggest the importance of Bcrp1 in the ADME of its substrates and the possibility that it may be involved in substrate distribution to the CNS.
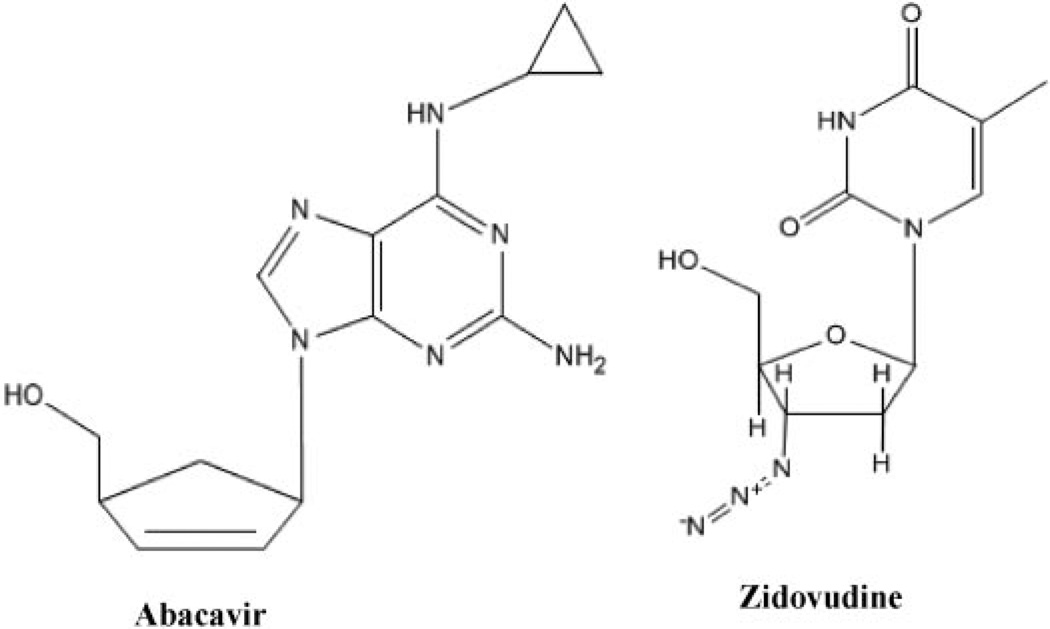
The objective of this study was to evaluate the in vivo contribution of murine Bcrp1 on the pharmacokinetics and brain exposure of Bcrp1 substrates, abacavir and zidovudine (Fig. 1), using Bcrp1−/− mice. We have also examined a series of NRTIs for their BCRP substrate status using Bcrp1-transfected cells. Furthermore, the effects of the inhibitors GF120918, LY335979, Ko143, probenecid. and Pluronic P85 on the brain penetration of abacavir were determined. If BCRP-mediated efflux transport is important in limiting the distribution of anti-HIV1 nucleosides to the brain, then improved efficacy against the virus in this sanctuary site may be realized through targeted inhibition of this mechanism.
Fig. 1.
Structures of nucleoside substrates AZT and abacavir ABC.
Materials and Methods
Chemicals
All radiolabeled nucleoside analogs used in the in vitro cell culture experiments ([14C]AZT, [3H]ABC, [3H]ddC, [3H]3TC, [3H]ddI, and [3H]d4T), were purchased from Moravek Biochemicals (Brea, CA). AZT, 3-azido 2,3-dideoxyuridine, and probenecid (4[(dipropylamino)sulfonyl]benzoic acid) were purchased from Sigma-Aldrich (St. Louis, MO). Abacavir powder was provided by the NIH AIDS Research and Reference Reagent program. GF120918 was a gift from GlaxoSmithKline (Research Triangle, NC). Ko143, a fumitremorgin C analog was kindly provided by Dr. Tetsuya Terasaki (Tohoku University, Sendai, Japan), and LY335979 was a gift from Eli Lilly & Co. (Indianapolis, IN). All other chemicals used were HPLC or reagent grade.
Cell Lines
In addition to our in vivo mouse studies, we also examined the substrate status of a series of nucleoside analogs using in vitro cell culture. MDCKII wild-type and Bcrp1-transfected cell lines (Jonker et al., 2000) were kindly provided by Dr. Alfred H. Schinkel, The Netherlands Cancer Institute (Amsterdam, The Netherlands). Cells used for all experiments were between passages 5 and 15. The cells were cultured in Dulbecco’s modified Eagle’s medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (SeraCare Life Sciences, Inc., Oceanside, CA), penicillin (100 U/ml), and streptomycin (100 µg/ml) (Sigma-Aldrich). All other cell culture materials were obtained from BD Biosciences (San Jose, CA)
Animals
Male wild-type and Bcrp1−/− knockout FVB mice were purchased from Taconic Farms (Germantown, NY). Animal handling was in compliance with the National Institutes of Health guidelines regarding the use of laboratory animals and was approved by The Institutional Animal Care and Use Committee of the University of Minnesota. The mice were acclimated for 1 week before being used for experiments and were maintained on a 12-h light/dark cycle with free access to food and water.
Intracellular Accumulation Studies
The wild-type and Bcrp1-transfected cells were seeded at a density of 2 × 105/well and were grown for 2 to 3 days on 12-well plates (TPP tissue culture plates) to form confluent epithelial monolayers. For the experiment, the growth medium was aspirated, and the cells in each well were washed twice with 2 ml of assay buffer, followed by preincubation for 30 min with 1 ml of assay buffer. The accumulation experiment involved incubation of these cells for 180 min at 37°C in 1 ml of assay buffer (122 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 10 mM HEPES, 3 mM KCl, 1.2 mM MgSO4 · 7H2O, 1.4 mM CaCl2 · H2O, and 0.4 mM K2HPO4) containing tracer concentrations of the radiolabeled nucleoside analogs (2.9 µM AZT, 1.6 µM ABC, 0.16 µM ddC, 0.16 µM 3TC, 0.16 µM ddI, and 0.14 µM d4T). Assay buffer containing radiolabeled drug was aspirated at the end of the incubation period, and the cells were washed three times with 1 ml of ice-cold phosphate-buffered saline (PBS). The cells were solubilized by adding 1 ml of 1% Triton X-100 (Sigma-Aldrich). Total protein concentration in each well was determined by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL), and the corresponding radioactivity was determined by liquid scintillation counting (LS-6500; Beckman Coulter, Fullerton, CA). Tracer accumulation in the two cell variants (wild-type and Bcrp1-transfected MDCK cells) was compared, and the results were expressed as a percentage of control (total amount of radioactivity accumulated per milligram of protein in the wild-type cell).
Drug Administration and Sampling Design
Zidovudine and Ziagen (abacavir sulfate oral solution, 20 mg/ml) were diluted in saline to yield concentrations of 7.5 and 5 mg/ml, respectively. Zidovudine (15 mg/kg) and abacavir (10 mg/kg) were administered to wild-type and Bcrp1−/− mice via tail vein injection. The animals were euthanized using CO2 inhalation at the specified time points postdose, and plasma and whole brain samples were collected at these predetermined time points. For zidovudine, samples were collected at 5, 15, 30, 45, and 60 min (n = 5–6 at each time point) and for abacavir; samples were collected at 5, 10, 20, and 40 min (n = 4–6 at each time point). Blood was collected by cardiac puncture and transferred to heparinized tubes, and plasma was isolated from the blood by centrifugation at 3000 rpm for 10 min at 4°C. Whole brain was immediately removed from the skull, rinsed with ice-cold PBS to remove extraneous blood, and flash-frozen in liquid nitrogen. All samples were stored at −80°C until further analysis by HPLC.
Effect of Inhibitors on the CNS Distribution of Abacavir
GF120918 (10 mg/kg) was suspended in a mixture of propylene glycol and water (3:2), LY335979 (25 mg/kg) was prepared in 20% ethanol in saline, probenecid (200 mg/kg) was prepared in 5% sodium bicarbonate solution, Ko143 (1 mg/kg) was prepared in 1% dimethyl sulfoxide, and Pluronic P85 (20 mg/kg) was prepared as a 0.1% solution (w/v) in saline. Mice were injected i.v. with inhibitor solutions either 30 min (GF120918, LY335979, Ko143, and probenecid) or 60 min (Pluronic P85) before the i.v. administration of abacavir to evaluate the influence of various inhibitors on abacavir brain distribution. The animals were euthanized using CO2 and plasma, and whole brain samples were collected 20 min postdose. Plasma and whole brain samples were collected as described earlier and stored at −80°C until further analysis by HPLC.
HPLC Analysis
The analysis was performed on a Hypersil-BDS column (C-18, 2.1 mm × 150 mm, 5 µM; Thermo Electron Corporation, Bellefonte, PA) maintained at 40°C using a Shimadzu column oven (CTO-10Avp). The HPLC system consisted of a Shimadzu pump (LC-10ATvp), flow control valve (FCV-10ALvp), degasser (DGU-20A5), autoinjector (SIL-10ADvp), system controller (SCL-10Avp), and detector (SPD-10Avp).
Determination of Zidovudine and Abacavir Concentrations in Plasma and Brain Homogenate
Plasma and brain homogenate samples were analyzed separately using spiked standards in blank plasma and brain homogenate. 3-Azido 2,3-dideoxyuridine and AZT were used as internal standards for zidovudine and abacavir, respectively. For plasma, 100-µl samples were spiked with 100 ng of internal standard. The samples were subjected to liquid-liquid extraction using 8 parts of ethyl acetate for 1 part of sample and vortexed vigorously for 5 min. The mixture was centrifuged at 14,000 rpm at room temperature for 10 min. The supernatant was transferred to clean glass tubes, dried under a flow of liquid nitrogen, and reconstituted in 100 µl of mobile phase, and 30 µl was injected onto the HPLC column. Whole brain was homogenized in 3 volumes of 5% bovine serum albumin in PBS using a Dounce homogenizer (7 ml; Kontes Glass, Vineland, NJ); 500 µl of brain homogenate was spiked with 50 ng of internal standard, and sample preparation was similar to that of plasma. The mobile phase for zidovudine analysis consisted of buffer (50 mM ammonium phosphate, 50 mM sodium citrate buffer, and 10 ppm sodium azide, pH 6.5) and methanol (82:18) at a flow rate of 0.2 ml/min, and UV absorbance was measured at 266 nM. The mobile phase for abacavir analysis consisted of buffer, acetonitrile, and methanol (89:10:1) at a flow rate of 0.25 ml/min, and UV absorbance was measured at 284 nM. The plasma and brain concentrations are reported as nanograms per milliliter and nanograms per gram of brain tissue, respectively. The brain homogenate concentrations were corrected for drug in the brain vascular space as determined previously by the i.v. administration of [3H]inulin to FVB mice (Dai et al., 2003).
Pharmacokinetic Analysis
Noncompartmental analysis was performed using WinNonlin 5.2 (Pharsight, Mountain View, CA) to obtain pharmacokinetic parameters from concentration-time data in plasma and brain. The noncompartmental analysis module provides special methods to analyze concentration data from sparse sampling (one sample from each mouse). We used this built-in capability of noncompartmental analysis that uses an extension of Bailer’s (1988) approach (Nedelman and Jia, 1998). The terminal rate constants for plasma and brain were determined from the last three data points of the respective concentration-time profiles. The areas under the concentration-time profiles for plasma (AUCplasma) and brain (AUCbrain) from time 0 to infinity were calculated using the linear trapezoidal method. The AUC from the last measured time point to infinity was estimated by dividing the last measured concentration by the respective terminal rate constant. For plasma, the AUC from time 0 to the first measured time point was obtained by back-extrapolation of the first two data points to time 0. The enhancement in brain exposure of either AZT or abacavir in the Bcrp1-deficient mice compared with wild-type mice was represented by the drug targeting index (DTI), calculated as
| (1) |
This metric represents the change in the tissue “partition coefficient” for a specific tissue or organ, in this case the brain, when there has been a genetic deletion (gene knockout). It is useful to describe differences in tissue targeting, because in each genotype effects on the driving force concentration for distribution, the plasma concentration, are accounted for by normalizing ratios. In essence, the drug targeting index allows for correction when changes in systemic clearance would in turn influence distribution.
Statistical Analysis
Statistical analysis was performed using SigmaStat 3.1 (Systat Software, Inc., San Jose, CA). Groups were compared using simple one-way analysis of variance, and the Holm-Sidak method was used for post hoc multiple comparison. Destructive sampling was used in the collection of in vivo data; that is, each animal contributed a single observation and the area under the concentration-time curve was constructed from the mean plasma concentration from multiple mice at each time point. Therefore, the variance of AUC0–∞ was estimated using the method described by Yuan (1993). This method allows for the determination of the variability in the AUC estimate from the variability about the mean concentration at each time point, assuming that the mean at each time point is independent and the terminal rate constant is the same for each animal. It is reasonable to assume that the sample means are independent of each other, and given that the coefficient of variation in the terminal phase of the curve is low, this method was used to statistically compare AUCs. The AUCs were statistically compared using the Z test (at a significance level of p < 0.05) as described by Bailer (1988) for testing statistical significance in experiments involving destructive sampling.
Results
Intracellular Accumulation
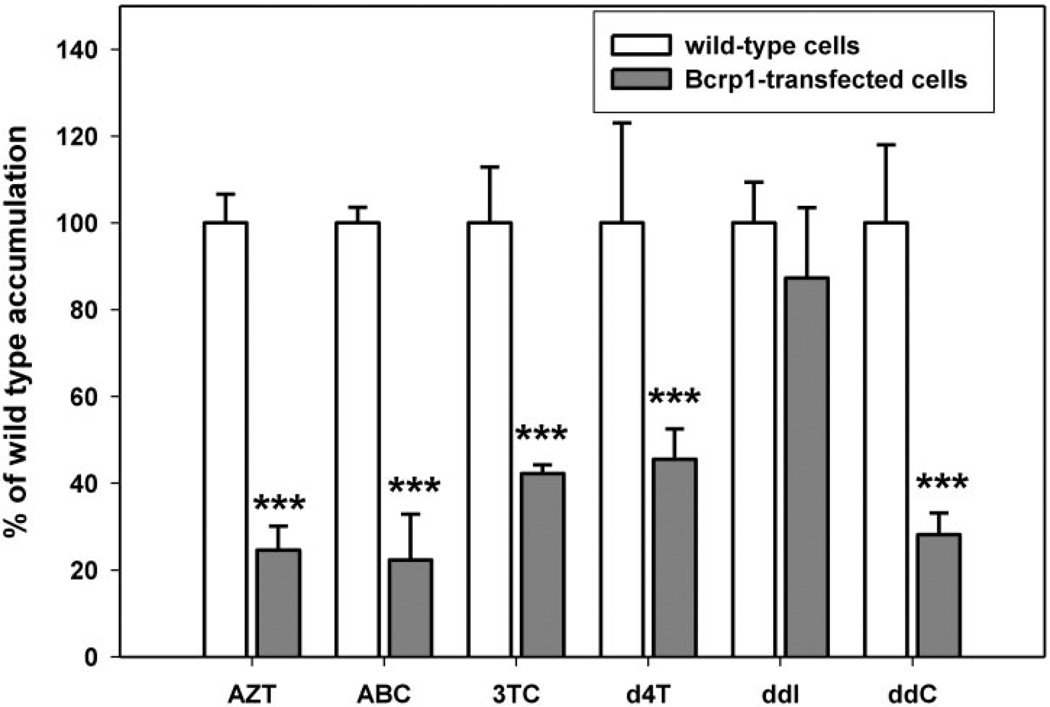
Intracellular accumulations of [14C]-AZT, [3H]ABC, [3H]ddC, [3H]3TC, [3H]ddI, and [3H]d4T were determined by measuring the total intracellular radioactivity (disintegrations per minute) in the Bcrp1-transfected or the wild-type cells normalized to the respective total protein content. The intracellular accumulations of [14C]AZT, [3H]ABC, [3H]ddC, [3H]3TC, and [3H]-d4T in the Bcrp1-transfected cells (Fig. 2) were approximately 75, 80, 60, 55, and 70% lower than the observed wild-type control levels, respectively (p < 0.001). There was no statistically significant difference in the intracellular accumulation of [3H]ddI between the two cell types (Fig. 2).
Fig. 2.
Accumulation of [14C]AZT, [3H]ABC, [3H]3TC, [3H]d4T, [3H]ddI, and [3H]ddC in the wild-type (□) and Bcrp1-transfected ( ) MDCKII cells. Results are expressed as means ± S.D.; n = 3. ***, p < 0.001, compared with the wild-type control group.
) MDCKII cells. Results are expressed as means ± S.D.; n = 3. ***, p < 0.001, compared with the wild-type control group.
Pharmacokinetics and Brain Distribution of Zidovudine in the Wild-Type and Bcrp1−/− Mice
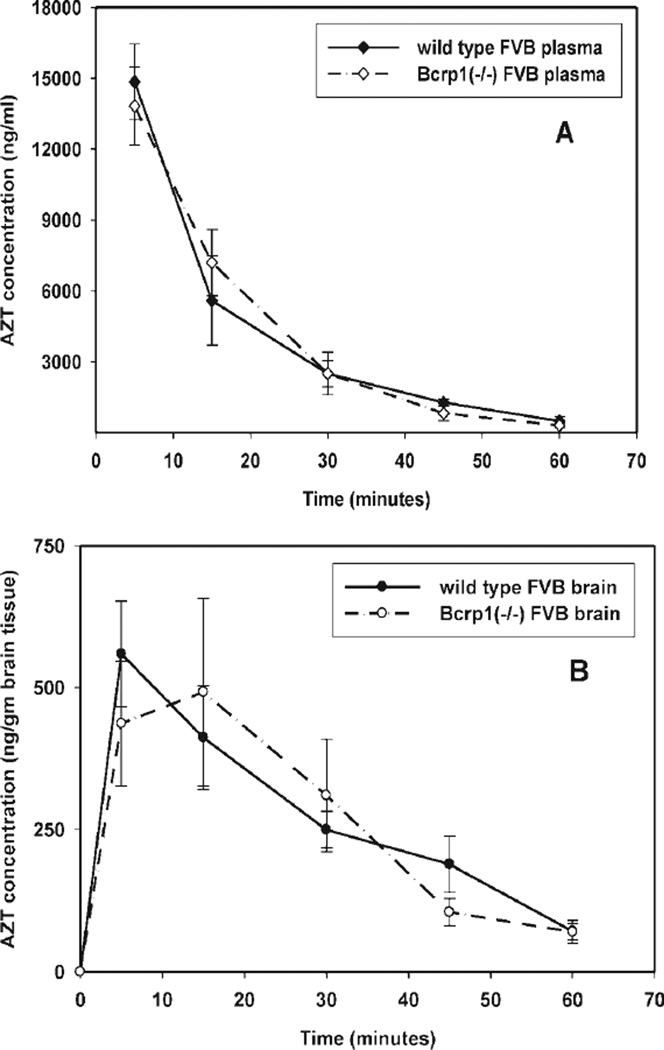
Plasma and brain concentration-time profiles after an i.v. bolus dose of AZT were determined in wild-type and Bcrp1−/− mice. Zidovudine concentrations in the brain were significantly lower (p < 0.05) than those in the plasma at all sampled time points (5, 15, 30, 45, and 60 min) for both the wild-type and Bcrp1−/− mice (Fig. 3, A and B). The brain/plasma concentration ratio (Cb/Cb) in wild-type mice was low and not significantly different from the Cb/Cb ratio in Bcrp1−/− mice (Fig. 4A). Noncompartmental analysis of plasma and brain data yielded terminal half-lives of 13 and 17 min in the plasma and brain of the wild-type mice, respectively (Table 1). In the Bcrp1−/− mice, the half-life was 10 min in plasma and 14 min in the brain. The total body clearance in wild-type mice was 48 ml/min/kg, whereas it was 50 ml/min/kg in Bcrp1−/− mice. The volumes of distribution at steady state, based on the plasma concentration-time profiles, were 0.9 and 0.7 l/kg in the wild-type and Bcrp1−/− mice, respectively. Neither the plasma nor the brain AUCs were significantly different between the wild-type and Bcrp1−/− mice. In the wild-type mice, the AUCplasma was 311 ± 13 µg · min/ml (mean ± S.D) and AUCbrain was 18.2 ± 0.8 µg · min/ml, and in the Bcrp1−/− mice, the AUCplasma was 298 ± 12 µg · min/ml and AUCbrain was 17.6 ± 1.3 µg · min/ml. The AUCbrain/AUCplasma ratio was 0.06 for both wild-type and Bcrp1−/− mice. Therefore, the resultant drug targeting index (see eq. 1 for brain exposure of zidovudine attributable to the deletion of Bcrp1) in the mice was equal to unity, indicating that BCRP did not influence the CNS distribution of zidovudine.
Fig. 3.
Concentration-time profile of AZT in Bcrp1−/− and wild-type FVB mice after i.v. dosing (15 mg/kg). A, concentration-time profiles of AZT in plasma of Bcrp1−/− (◊) and wild-type (♦) FVB mice. B, concentration-time profiles in brain tissue of Bcrp1−/− (○) and wild-type (●) FVB mice. Data are represented as means ± S.D.
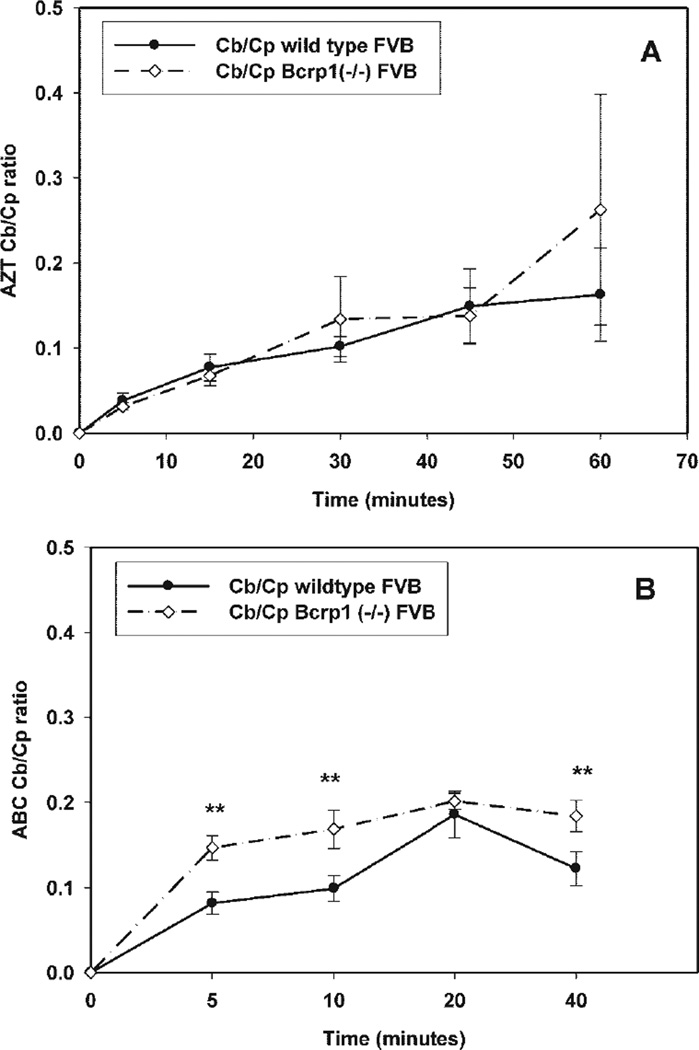
Fig. 4.
Comparison of the Cb/Cp ratios between wild-type and Bcrp1-deficient FVB mice at different sampling time points. A, the Cb/Cp ratio for AZT in the Bcrp1-deficient mice was not different from that in the wild-type mice at any time points. n = 5–6 per time point. B, the Cb/Cp ratio for ABC in the Bcrp1-deficient mice was significantly higher than that in the wild-type mice at 5, 10, and 40 min postdose. **, p < 0.01. n = 4–5 per time point. The results are expressed as means ± S.D.
TABLE 1.
Plasma and brain pharmacokinetic parameters determined by noncompartmental analysis after the administration of a single i.v. bolus dose of zidovudine in wild-type FVB and Bcrp1−/− FVB mice
| Pharmacokinetic Parameters | Wild-Type FVB Mouse | Bcrp1−/− FVB Mouse | ||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| λz (min−1) | 0.05 | 0.04 | 0.07 | 0.05 |
| Half-life (min) | 13 | 17 | 10 | 14 |
| Clearance (ml/min/kg) | 48 | 50 | ||
| Volume of distribution (l/kg) | 0.9 | 0.7 | ||
| AUC(0–∞) (µg · min/ml)a | 311 ± 13 | 18.2 ± 0.8 | 298 ± 12 | 17.6 ± 1.3 |
| AUCbrain/AUCplasma | 0.06 | 0.06 | ||
| Drug targeting index | 1 | |||
Mean ± S.D.
Pharmacokinetics and Brain Distribution of Abacavir in Wild-Type and Bcrp1−/− Mice
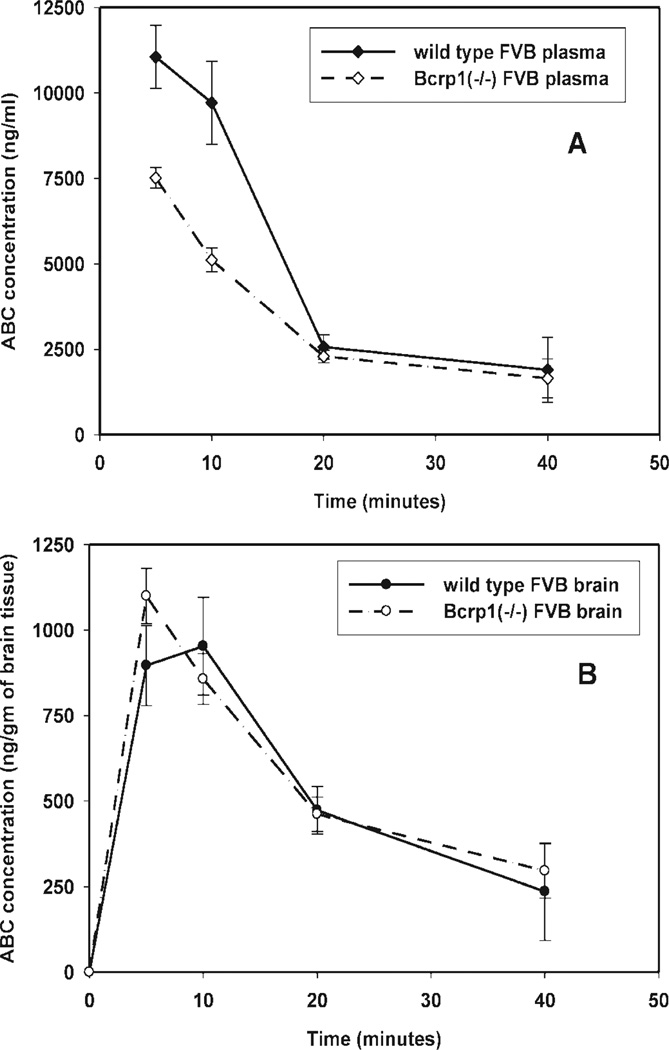
Plasma and brain concentration-time profiles of abacavir, after an intravenous bolus dose, were determined in wild-type and Bcrp1−/− mice. As seen with zidovudine, the measured brain abacavir concentrations were significantly lower (p < 0.05) than those in the plasma at all sampled time points (5, 10, 20, and 40 min) for both the wild-type and the Bcrp1−/− mice (Fig. 5, A and B). The measured plasma concentrations of abacavir at early time points (5- and 10-min sampling) in the Bcrp1−/− mice were lower than those in the wild-type mice (Fig. 5A). Thus, the Cb/Cp ratio in the Bcrp1−/− mice was greater (p < 0.01) than that in the wild-type mice at 5, 10, and 40 min postdose (Fig. 4B). Noncompartmental analysis of plasma and brain data gave terminal half-lives of 14 and 15 min in the plasma and brain of wild-type mice, respectively (Table 2). In the Bcrp1−/− mice, the terminal half-life was 20 min in both plasma and brain. The total body clearance was 39 ml/min/kg in wild-type mice and 47 ml/min/kg in the Bcrp1−/− mice. The volumes of distribution at steady state, based on the plasma concentration-time profiles, were 0.8 and 1.4 l/kg in the wild-type and Bcrp1−/− mice, respectively. The AUCplasma in the Bcrp1−/− mice was significantly lower (p < 0.05) than that for the wild-type mice [202 ± 10.2 versus 256 ± 17.5 µg · min/ml, respectively (mean ± S.D)], whereas the AUCbrain was not statistically different (31.6 ± 1.5 versus 26.3 ± 2.3 µg · min/ml, Bcrp1−/− versus wild-type mice, respectively). The AUCbrain/AUCplasma ratios in the Bcrp1−/− and wild-type mice were 0.15 and 0.1, respectively. Given these AUC ratios, the resultant drug targeting index (see eq. 1 for the enhancement in brain exposure of abacavir in the Bcrp1−/− mice compared with that in the wild-type mice) was equal to 1.5. This distribution enhancement is essentially due to differences in the plasma concentrations at early time points, without a concomitant decrease in brain concentrations.
Fig. 5.
Concentration-time profile of ABC in Bcrp1−/− and wild-type FVB mice after i.v. dosing (10 mg/kg). A, concentration-time profiles of ABC in plasma of Bcrp1−/− (◊) and wild-type (♦) FVB mice. B, concentration-time profiles in brain tissue of Bcrp1−/− (○) and wild-type (●) FVB mice. Data are represented as means ± S.D.
TABLE 2.
Plasma and brain pharmacokinetic parameters determined by noncompartmental analysis after the administration of a single i.v. bolus dose of abacavir in wild-type FVB and Bcrp1−/− FVB mice
| Pharmacokinetic Parameters | Wild-Type FVB Mouse | Bcrp1−/− FVB Mouse | ||
|---|---|---|---|---|
| Plasma | Brain | Plasma | Brain | |
| λz (min−1) | 0.05 | 0.05 | 0.04 | 0.03 |
| Half-life (min) | 14 | 15 | 20 | 21 |
| Clearance (ml/min/kg) | 39 | 50 | ||
| Volume of distribution (l/kg) | 0.8 | 1.43 | ||
| AUC(0–∞) (µg · min/ml)a | 256 ± 17.5 | 26.3 ± 2.3 | 202 ± 10.2* | 31.6 ± 1.5 |
| AUCbrain/AUCplasma | 0.10 | 0.15 | ||
| Drug targeting index | 1.5 | |||
P < 0.05 for mutant versus wild-type AUCplasma.
Mean ± S.D.
Effect of Inhibitors on the Distribution of ABC in the Wild-Type Mouse
Wild-type mice were dosed i.v. with LY335979, Ko143, GF120918, and probenecid 30 min before administration of abacavir, whereas Pluronic P85 was administered 60 min before abacavir administration. The brain and plasma concentrations of abacavir were determined 20 min postdose as described previously.
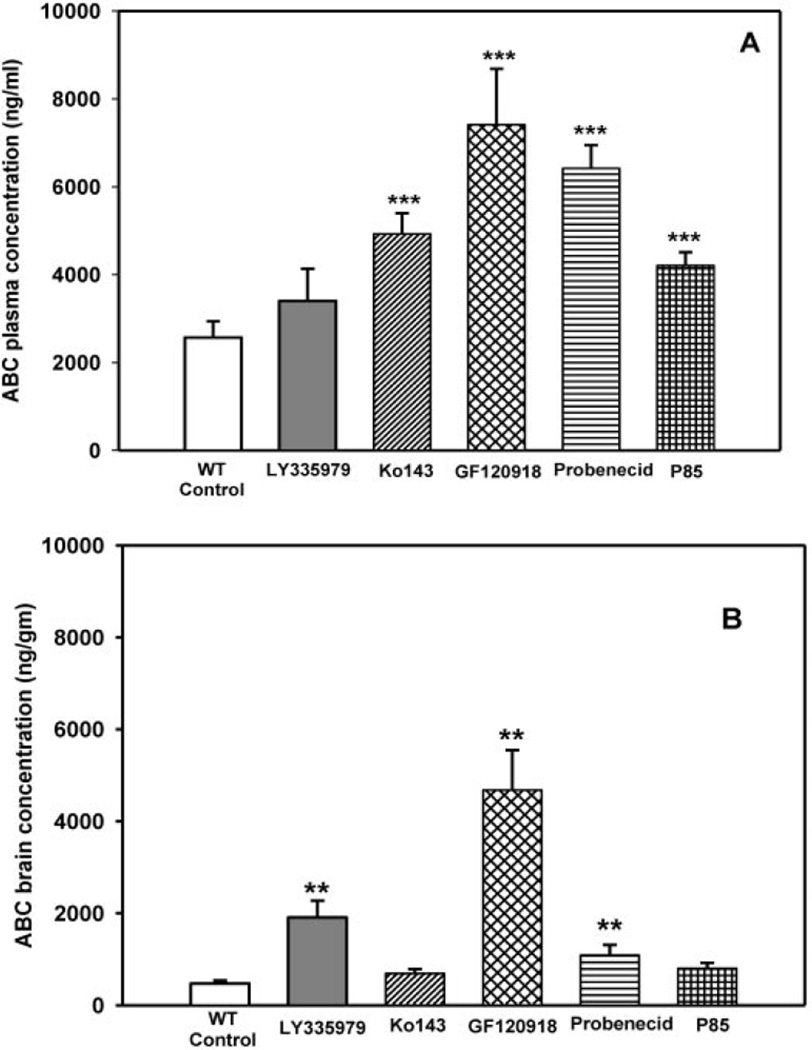
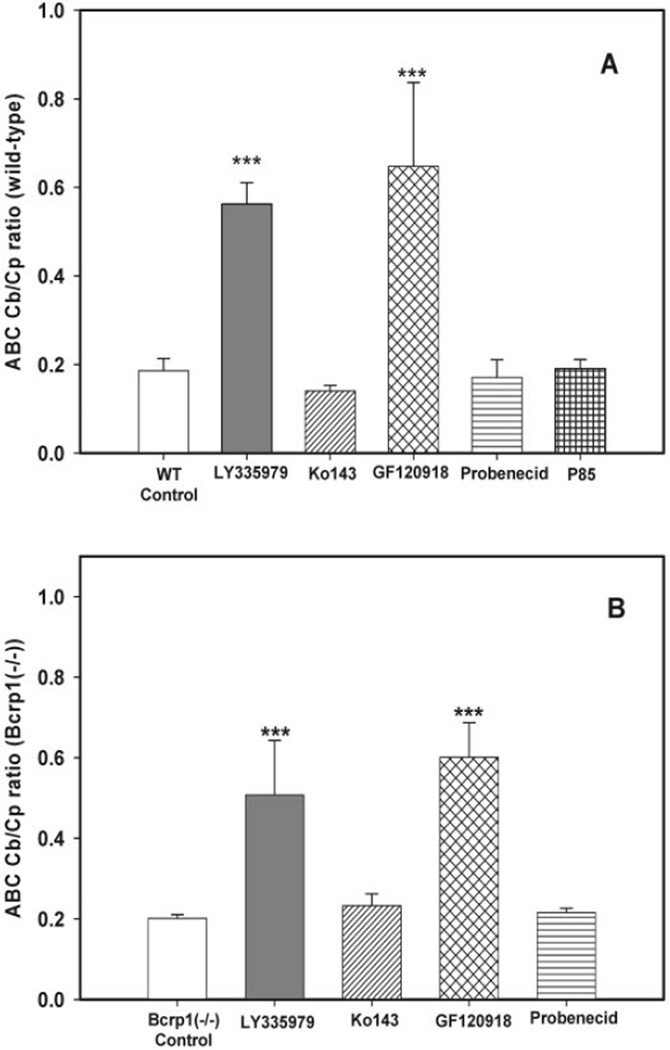
In the wild-type control mice (no transport inhibitors), the observed plasma and brain concentrations were 2568 ± 368 ng/ml and 47 3 ± 69 ng/g, respectively (mean ± S.D.). Coadministration of Ko143, GF120918, probenecid, and Pluronic P85 significantly increased (p < 0.001) abacavir plasma concentrations in wild-type mice (4924 ± 471, 7411 ± 1274, 6419 ± 528, and 4203 ± 306 ng/ml, respectively) (Fig. 6A). Coadministration of LY335979 did not have a significant effect on the plasma concentration of abacavir (3402 ± 731 ng/ml) (Fig. 6A). Coadministration of LY335979, GF120918, and probenecid significantly increased (p < 0.01) the brain concentrations of abacavir in the wild-type mice (1903 ± 366, 4675 ± 833, and 1090 ± 223 ng/g, respectively) (Fig. 6B). Coadministration of Ko143 and Pluronic P85 did not have a significant effect on the brain concentrations of abacavir (692 ± 99 and 803 ± 120 ng/g, respectively) (Fig. 6B). Cb/Cp ratios for the wild-type mice were significantly increased (p < 0.001) only by the coadministration of LY335979 and GF120918 (0.56 ± 0.05 and 0.65 ± 0.2, respectively) (Fig. 7A) compared with the Cb/Cp ratio in the controls (0.19 ± 0.03) (Fig. 7A). Coadministration of Ko143, probenecid, and Pluronic P85 did not significantly affect Cb/Cp ratios of abacavir in the wild-type mice (0.14 ± 0.01, 0.17 ± 0.04, and 0.19 ± 0.02, respectively) (Fig. 7A).
Fig. 6.
Effect of inhibitors on the plasma (A) and brain (B) concentrations of ABC in wild-type FVB mice after i.v. dosing. □, control (10 mg/kg);  , 25 mg/kg LY335979; ▨, 1 mg/kg Ko413; is ▩, 10 mg/kg GF120918; ▤, 200 mg/kg probenecid; ▦, 20 mg/kg Pluronic P85. Results are expressed as means ± S.D.; n = 4. ***, p < 0.001; **, p < 0.01 compared with the respective wild-type controls.
, 25 mg/kg LY335979; ▨, 1 mg/kg Ko413; is ▩, 10 mg/kg GF120918; ▤, 200 mg/kg probenecid; ▦, 20 mg/kg Pluronic P85. Results are expressed as means ± S.D.; n = 4. ***, p < 0.001; **, p < 0.01 compared with the respective wild-type controls.
Fig. 7.
Effect of inhibitors on the Cb/Cp ratios 20 min postdose in wild-type (A) and Bcrp1−/− (B) FVB mice after i.v. □, control (10 mg/kg);  , 25 mg/kg LY335979; ▨, 1 mg/kg Ko413; ▩, 10 mg/kg GF120918; ▤, 200 mg/kg probenecid; ▦, 20 mg/kg Pluronic P85. Results are expressed as means ± S.D.; n = 4. ***, p < 0.001. compared with the respective wild-type and Bcrp1−/− controls.
, 25 mg/kg LY335979; ▨, 1 mg/kg Ko413; ▩, 10 mg/kg GF120918; ▤, 200 mg/kg probenecid; ▦, 20 mg/kg Pluronic P85. Results are expressed as means ± S.D.; n = 4. ***, p < 0.001. compared with the respective wild-type and Bcrp1−/− controls.
Effect of Inhibitors on the Distribution of ABC in Bcrp1−/− Mice
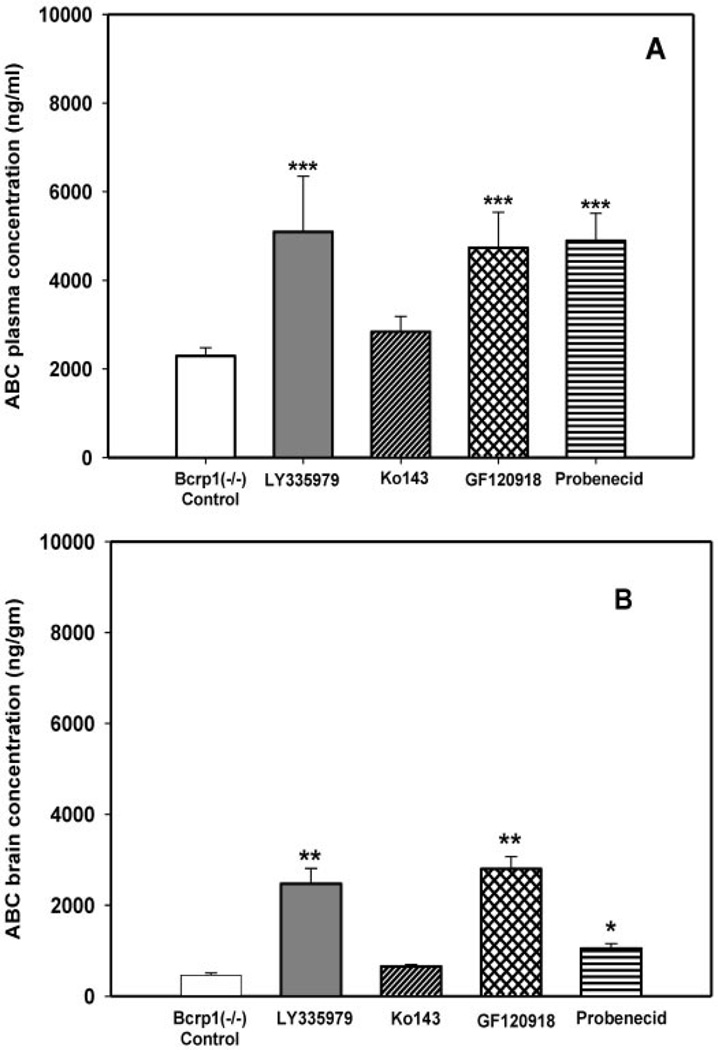
Bcrp1−/− mice were dosed i.v. with LY335979, Ko143, GF120918, and probenecid 30 min before the i.v. administration of abacavir. The brain and plasma concentrations of abacavir were determined 20 min postdose. Plasma and brain concentrations of abacavir in the Bcrp1−/− control mice (no transport inhibitors) were 2289 ± 189 ng/ml and 462 ± 50 ng/g, respectively (mean ± S.D.). Plasma abacavir concentrations in the Bcrp1−/− mice increased significantly (p < 0.001) with the coadministration of LY335979, GF120918, and probenecid (5094 ± 1256, 4732 ± 802, and 4890 ± 621 ng/ml, respectively) (Fig. 8A). Coadministration of Ko143 did not have a significant effect on the plasma concentration in the Bcrp1−/− mice (2840 ± 342 ng/ml) (Fig. 8A). Coadministration of LY335979, GF120918, and probenecid significantly increased the abacavir brain concentrations in the Bcrp1−/− mice (2468 ± 340, 2796 ± 271, and 1050 ± 106 ng/g, respectively) (p < 0.01 for LY335979 and GF120918; p < 0.05 for probenecid) (Fig. 8B). Coadministration of Ko143 did not have a significant effect on the brain concentration of abacavir (655 ± 41 ng/g) (Fig. 8B). Cb/Cp ratios in the Bcrp1−/− mice were significantly increased by the coadministration of LY335979 and GF120918 (0.51 ± 0.13 and 0.6 ± 0.1, respectively) compared with the control (0.2 ± 0.01). Ko143 and probenecid did not have any effect on the Cb/Cp ratios of abacavir in the Bcrp1−/− mice (0.23 ± 0.03 and 0.21 ± 0.01, respectively) (Fig. 7B).
Fig. 8.
Effect of inhibitors on the plasma (A) and brain (B) concentrations of ABC in Bcrp1−/− FVB mice after i.v. dosing. □, control (10 mg/kg);  , 25 mg/kg LY335979; ▨, 1 mg/kg Ko413; ▩, 10 mg/kg GF120918; ▤, 200 mg/kg probenecid; ▦, 20 mg/kg Pluronic P85. Results are expressed as means ± S.D.; n = 4. ***, p < 0.001; **, p < 0.01 compared with the respective Bcrp1−/− controls.
, 25 mg/kg LY335979; ▨, 1 mg/kg Ko413; ▩, 10 mg/kg GF120918; ▤, 200 mg/kg probenecid; ▦, 20 mg/kg Pluronic P85. Results are expressed as means ± S.D.; n = 4. ***, p < 0.001; **, p < 0.01 compared with the respective Bcrp1−/− controls.
Discussion
The penetration of anti-HIV nucleosides to the CNS is limited (Sawchuk and Yang, 1999). Efflux transport systems such as P-gp and the MRPs at the brain capillary endothelial cells may play a role in limiting the passage of therapeutic agents across the blood-brain barrier. BCRP is an efflux transport system that was discovered recently and is expressed in a number of normal tissues, such as the BBB (Doyle and Ross, 2003). Previously, we have reported that the in vitro transport of nucleoside analogs zidovudine and abacavir is mediated by the efflux transport protein Bcrp1, the murine ortholog of human BCRP (Pan et al., 2007). The abilities of Ko143 and GF120918 (a selective BCRP inhibitor and a dual P-gp-BCRP inhibitor, respectively) to completely abolish this Bcrp1-mediated transport confirmed that both nucleoside analogs were substrates for BCRP (Pan et al., 2007).
After the identification of the in vitro BCRP substrate status of the anti-HIV1 nucleoside analogs, it is valuable to ascertain the role of Bcrp1 on the nucleoside ADME processes in vivo. We used the Bcrp1 knockout mouse model (Bcrp1−/−) to examine the influence of BCRP-mediated efflux transport on the brain penetration of zidovudine and abacavir. The Bcrp1 knockout mouse model Bcrp1−/− was created by the deletion of Abcg2, the gene encoding murine Bcrp1 (Jonker et al., 2002). The rationale behind using this model is that differences in ADME that are observed in these mice, compared with those in the FVB wild-type mouse, will be related to the action of the Bcrp1 transport system.
The current study revealed that the distribution to the brain, depicted by the AUCbrain/AUCplasma ratio (a distributional tissue partition coefficient), for both zidovudine (6%) and abacavir (10%) in the wild-type mice (FVB strain) was found to be low (Tables 1 and 2). Similar observations of low brain penetration in mice has been reported previously for both AZT [approximately 6% versus plasma, NIH-Swiss strain (Doshi et al., 1989)] and ABC [approximately 17%, CF1 strain (Shaik et al., 2007)].
Contrary to the expected result suggested by our in vitro observation, the deletion of Bcrp1 in the Bcrp1−/− mice did not produce notable changes to either the brain penetration or plasma pharmacokinetics of zidovudine (Table 1). This finding is in contrast to the results of Enokizono et al. (2008), who recently reported that the brain/plasma concentration ratios (brain/plasma “Kp” values at one time point) for the phytoestrogens daidzein, genistein, and coumestrol were significantly increased in Bcrp1−/− mice compared with wild-type mice. However, it has also been reported that these Bcrp1−/− single-knockout mice do not show any enhanced BBB permeability of the Bcrp1 substrates dehydroepiandrosterone sulfate, mitoxantrone, imatinib, and topotecan to the brain (Lee et al., 2005; Bihorel et al., 2007; de Vries et al., 2007). It is important that all of the abovementioned compounds are known substrates for P-gp-mediated efflux transport. Interestingly, it was recently reported that the AUCbrain of topotecan (a dual substrate) was enhanced in P-gp/Bcrp1 double-knockout mice as well as P-gp or Bcrp1 single knockouts (de Vries et al., 2007). However, because of a significant increase in topotecan plasma concentrations in the Bcrp1−/− mice, the brain/plasma AUC ratio was increased only in the P-gp/Bcrp1 knockouts and the P-gp knockouts compared with the wild-type genotype alone. From the above findings, we can conclude that substrate overlap of Bcrp1 with P-gp or other efflux transport proteins may confound the role of BCRP in limiting brain penetration through its action at the BBB and hinder clear experimental evidence for the in vivo action of BCRP in the BBB. The use of a specific BCRP substrate may be required to fully elucidate the role of BCRP at the BBB in an in vivo model (Ohtsuki and Terasaki, 2007).
Enokizono et al. (2008) examined the role of BCRP on the brain and testicular distribution of BCRP substrates in Bcrp1−/− mice. Under steady-state conditions, they observed that the brain/plasma concentration ratio (Kp values) of Bcrp1 substrates was significantly greater in the Bcrp1−/− mice compared with that in the wild-type mice. Interestingly, this increase in Kp values for specific Bcrp1 substrates was greater than the increase observed for compounds that were also substrates for P-gp (Enokizono et al., 2008). These observations indicate that for compounds that are substrates for both P-gp and Bcrp1, the involvement of P-gp at the BBB could reduce the impact of deletion of Bcrp1 at the BBB (Enokizono et al., 2008).
In addition, it has been reported that a probenecid-sensitive transport system influences zidovudine efflux in rats and rabbits (Takasawa et al., 1997; Wang et al., 1997). This system is not expected to be Bcrp1, because we have shown that probenecid does not inhibit Bcrp1-mediated transport of either zidovudine or abacavir (Pan et al., 2007). This yet unidentified efflux transport system could also mask the role of Bcrp1 in zidovudine BBB efflux in the mouse. Examining the influence of BCRP on brain penetration and pharmacokinetics of zidovudine in other preclinical models, such as rats, dogs, and primates, by using selective inhibitors of BCRP may reveal additional information about its role in vivo.
The drug targeting index for abacavir due to the deletion of Bcrp1 in the mouse was 1.5 (Table 2), indicating an enhanced distribution of abacavir in the absence of Bcrp1. However, this increase in abacavir penetration was moderate compared with the increase in abacavir brain penetration due to deletion of P-gp (Shaik et al., 2007) and primarily due to decreased plasma concentrations at early time points in the Bcrp1−/− mouse. As mentioned previously, abacavir is an in vitro substrate for both P-gp and BCRP (Pan et al., 2007; Shaik et al., 2007) and other purine nucleoside transporters, such as Mrp4 (Takenaka et al., 2007) may transport abacavir. Changes in the expression levels of other efflux transport proteins in the Bcrp1−/− mice that could transport abacavir or zidovudine, may explain the lack of effect attributable to Bcrp1 deletion. In another study, we examined the expression levels of mRNA that encodes various efflux transporters in the brain microvessel endothelial cells of both wild-type and Bcrp1−/− mice by quantitative real-time polymerase chain reaction. There was up-regulation of Mrp1, 2, 3, and 5 mRNA in the Bcrp1−/− mice, but there were no notable changes in the expression of P-gp and Mrp4 message (G. Pan, N. Giri, N. Shaik, L. R. Drewes, W. F. Elmquist, manuscript in preparation). Takenaka et al. (2007) also reported no changes in the protein expression of Mrp4 in Bcrp1−/− mice. It will be necessary to use more exacting measurements of the expression of the transport proteins at the BBB to truly understand the functional differences between different genotypes (e.g., knockout versus wild-type), different mouse strains (e.g., FVB versus CF-1), and different species. The use of highly sensitive liquid chromatography-tandem mass spectrometry proteomic methods is an example of new technology to more carefully quantify the comparative expression of drug transporters in the BBB (Kamiie et al., 2008).
The ability of efflux transport inhibitors to improve the targeted bioavailability of zidovudine and abacavir to viral sanctuaries, such as the brain, could be valuable to enhance efficacy at these sites. In this study, we evaluated the effect of in vivo modulation of efflux transport, by known inhibitors, on the plasma and brain concentrations of one of the study drugs, abacavir. We examined the effect of potent transporter inhibitors LY335979, Ko143, and GF120918 (selective P-gp, BCRP, and dual BCRP/P-gp inhibition, respectively) in the wild-type and Bcrp1−/− mice. The effect of probenecid, a broad-spectrum organic anion transport system inhibitor that has been shown to improve the CNS penetration of NRTIs, such as AZT, ddC, and ddI (Takasawa et al., 1997; Wang et al., 1997; Gibbs and Thomas, 2002), on the plasma concentration and brain penetration of abacavir in the wild-type and Bcrp1−/− mice, was also determined. In addition, we examined the effect of Pluronic P85, a P-gp inhibitor (Batrakova et al., 2001), on abacavir disposition in wild-type mice.
All inhibitors, except LY335979, increased the plasma abacavir concentrations in the wild-type mice. GF120918, a P-gp/BCRP dual inhibitor, showed the greatest effect with an approximately 3-fold increase in plasma concentration. It is very possible that P-gp does not contribute as much as BCRP to the overall systemic clearance of abacavir, whereas P-gp has a significant influence on brain distribution. LY335979, GF120918, and probenecid increased the measured brain concentrations in both the wild-type and Bcrp1−/− mice. However, the observed brain penetration (Cb/Cp ratio) in both wild-type and Bcrp1−/− mice was enhanced only in the presence of the potent P-gp inhibitors LY335979 and GF120918. Inhibition of P-gp alone increased the CNS penetration of abacavir. This finding confirms previous reports that the brain penetration of abacavir is influenced by P-gp (Shaik et al., 2007) and suggests that P-gp may be the dominant transporter limiting the CNS entry of abacavir. However, the increase in the Cb/Cp ratio was much less than the increase observed in CF-1 mutant mice naturally deficient in P-gp (Shaik et al., 2007). One could speculate that an unidentified efflux transport system that is distinct from either a P-gp-, BCRP-, or probenecid-sensitive system may also limit the brain penetration of abacavir in mice. In addition, this finding also raises interesting questions about genetic differences between FVB strain knockout mice and P-gp-deficient CF-1 mutant mice, both commonly used to examine P-gp effects on ADME.
Delivery of anti-HIV agents to viral sanctuary sites, such as the CNS, is crucial in viral eradication and for prevention of emergent drug-resistant strains. Identification of the mechanism by which anti-HIV agents are excluded from sanctuary sites will be valuable in devising effective therapy. There is strong in vitro evidence that the NRTIs, zidovudine, abacavir, 3TC, d4T, and ddC, are substrates for the efflux transporter Bcrp1. Our in vivo results show that deletion of Bcrp1 has little influence on the brain penetration or overall disposition of AZT and only a moderate effect on abacavir. The current results also confirm previous observations that P-gp plays a significant role in the brain penetration of abacavir. Studies with the aim of identifying the specific mechanism of zidovudine efflux and additional mechanisms of abacavir efflux at the BBB are warranted and should be valuable in extrapolation to other NRTIs to improve CNS delivery. Further elucidation of the role BCRP plays in the drug-drug interactions that may occur by the coadministration of substrates and/or inhibitors, such as NRTIs and protease inhibitors (Gupta et al., 2004) in ART cocktail therapy, will be extremely valuable.
Acknowledgments
The project was supported by National Institutes of Health Grant NS42549 (to W.F.E.).
We thank Dr. Alfred H. Schinkel from The Netherlands Cancer Institute for generously providing Bcrp1-transfected MDCKII cells, Dr. Tetsuya Terasaki from Tohoku University for Ko143, Eli Lilly & Co. for kindly providing us with LY335979, and GlaxoSmithKline for kindly providing us with GF120918. Abacavir (catalog 4680) was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases.
ABBREVIATIONS
- NRTI
nucleoside analog reverse transcriptase inhibitor
- ART
antiretroviral therapy
- HIV
human immune deficiency virus
- CNS
central nervous system
- BBB
blood-brain barrier
- P-gp
P-glycoprotein
- MRP/Mrp
multidrug resistance-associated protein
- ABC
abacavir, (1S,4R)-4[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol)
- MDR/mdr
multidrug resistance
- MDCK
Madin-Darby canine kidney
- BCRP/Brcp
breast cancer resistance protein
- AZT
zidovudine, 3-azido-3-deoxythymidine
- 3TC
2′,3′-dideoxy-3′-thiacytidine
- d4T
stavudine, 2′,3′-didehydro-2′,3′-dideoxythymidine
- ddI
2′,3′-dideoxyinosine
- GF120918
N[4[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]-5-methoxy-9-oxo-10H-acridine-4-carboxamide)
- Ko143
3-(6-isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydropyrazino[1′,2′:1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester
- ADME
absorption, distribution, metabolism, and excretion
- AUCplasma
area under the concentration-time profile for plasma
- AUCbrain
area under the concentration-time profiles for brain
- LY33579
(R)-4-((1aR,6R,10bS)-1,2-difluoro-1,1a,6,10b-tetrahydrodibenzo(a,e)cyclopropa(c)cycloheptan-6-yl)-α-((5-quinoloyloxy)methyl)-1-piperazineethanol trihydrochloride
- ddC
2′,3′-dideoxycytidine
- NIH
National Institutes of Health
- HPLC
high-performance liquid chromatography
- PBS
phosphate-buffered saline
Footnotes
Portions of this work were presented in abstract form as Influence of Abcg2/Bcrp1 on the Brain Distribution of Zidovudine (AZT) (Giri N, Shaik N, Pan G, Elmquist WF) at the American Association of Pharmaceutical Scientists Annual Meeting and Exposition, 2007 Nov 11–15, San Diego, CA.
References
- Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16:303–309. doi: 10.1007/BF01062139. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Miller DW, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296:551–557. [PubMed] [Google Scholar]
- Bihorel S, Camenisch G, Lemaire M, Scherrmann JM. Modulation of the brain distribution of imatinib and its metabolites in mice by valspodar, zosuquidar and elacridar. Pharm Res. 2007;24:1720–1728. doi: 10.1007/s11095-007-9278-4. [DOI] [PubMed] [Google Scholar]
- Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38:1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Dai H, Marbach P, Lemaire M, Hayes M, Elmquist WF. Distribution of STI-571 to the brain is limited by P-glycoprotein-mediated efflux. J Pharmacol Exp Ther. 2003;304:1085–1092. doi: 10.1124/jpet.102.045260. [DOI] [PubMed] [Google Scholar]
- de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, van Tellingen O. P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res. 2007;13:6440–6449. doi: 10.1158/1078-0432.CCR-07-1335. [DOI] [PubMed] [Google Scholar]
- Dieterich DT. Disease management—constructing optimal NRTI-based combinations: past, present, and future. Med Gen Med. 2006;8:16. doi: 10.1186/1758-2652-8-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi KJ, Gallo JM, Boudinot FD, Schinazi RF, Chu CK. Comparative pharmacokinetics of 3′-azido-3′-deoxythymidine (AZT) and 3′-azido-2′,3′-dideoxyuridine (AZddU) in mice. Drug Metab Dispos. 1989;17:590–594. [PubMed] [Google Scholar]
- Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug Metab Dispos. 2008;36:995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Sugiyama Y. Effect of breast cancer resistance protein (Bcrp/Abcg2) on the disposition of phytoestrogens. Mol Pharmacol. 2007;72:967–975. doi: 10.1124/mol.107.034751. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Thomas SA. The distribution of the anti-HIV drug, 2′3′-dideoxycytidine (ddC), across the blood-brain and blood-cerebrospinal fluid barriers and the influence of organic anion transport inhibitors. J Neurochem. 2002;80:392–404. doi: 10.1046/j.0022-3042.2001.00711.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Zhang Y, Unadkat JD, Mao Q. HIV protease inhibitors are inhibitors but not substrates of the human breast cancer resistance protein (BCRP/ABCG2) J Pharmacol Exp Ther. 2004;310:334–341. doi: 10.1124/jpet.104.065342. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, Nakashima E, Terasaki T. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s) J Neurochem. 2004;90:526–536. doi: 10.1111/j.1471-4159.2004.02537.x. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JH, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, Sekine Y, Uchida Y, Ito S, Terasaki T. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res. 2008 doi: 10.1007/s11095-008-9532-4. in press. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- Kvackajova-Kisucka J, Barancik M, Breier A. Drug transporters and their role in multidrug resistance of neoplastic cells. Gen Physiol Biophys. 2001;20:215–237. [PubMed] [Google Scholar]
- Lee YJ, Kusuhara H, Jonker JW, Schinkel AH, Sugiyama Y. Investigation of efflux transport of dehydroepiandrosterone sulfate and mitoxantrone at the mouse blood-brain barrier: a minor role of breast cancer resistance protein. J Pharmacol Exp Ther. 2005;312:44–52. doi: 10.1124/jpet.104.073320. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–3464. [PubMed] [Google Scholar]
- Nedelman JR, Jia X. An extension of Satterthwaite’s approximation applied to pharmacokinetics. J Biopharm Stat. 1998;8:317–328. doi: 10.1080/10543409808835241. [DOI] [PubMed] [Google Scholar]
- Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, Keppler D. Expression and immunolocalization of the multidrug resistance proteins, MRP1–MRP6 (ABCC1–ABCC6), in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24:1745–1758. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- Pan G, Giri N, Elmquist WF. Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug Metab Dispos. 2007;35:1165–1173. doi: 10.1124/dmd.106.014274. [DOI] [PubMed] [Google Scholar]
- Sawchuk RJ, Yang Z. Investigation of distribution, transport and uptake of anti-HIV drugs to the central nervous system. Adv Drug Deliv Rev. 1999;39:5–31. doi: 10.1016/s0169-409x(99)00017-4. [DOI] [PubMed] [Google Scholar]
- Shaik N, Giri N, Pan G, Elmquist WF. P-glycoprotein-mediated active efflux of the anti-HIV1 nucleoside abacavir limits cellular accumulation and brain distribution. Drug Metab Dispos. 2007;35:2076–2085. doi: 10.1124/dmd.107.017723. [DOI] [PubMed] [Google Scholar]
- Takasawa K, Terasaki T, Suzuki H, Sugiyama Y. In vivo evidence for carrier-mediated efflux transport of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine across the blood-brain barrier via a probenecid-sensitive transport system. J Pharmacol Exp Ther. 1997;281:369–375. [PubMed] [Google Scholar]
- Takenaka K, Morgan JA, Scheffer GL, Adachi M, Stewart CF, Sun D, Leggas M, Ejendal KF, Hrycyna CA, Schuetz JD. Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007;67:6965–6972. doi: 10.1158/0008-5472.CAN-06-4720. [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Jonker JW, Wagenaar E, Brinkhuis RF, Schellens JH, Beijnen JH, Schinkel AH. The breast cancer resistance protein (Bcrp1/Abcg2) restricts exposure to the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. Cancer Res. 2003;63:6447–6452. [PubMed] [Google Scholar]
- Wang X, Furukawa T, Nitanda T, Okamoto M, Sugimoto Y, Akiyama S, Baba M. Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol. 2003;63:65–72. doi: 10.1124/mol.63.1.65. [DOI] [PubMed] [Google Scholar]
- Wang X, Nitanda T, Shi M, Okamoto M, Furukawa T, Sugimoto Y, Akiyama S, Baba M. Induction of cellular resistance to nucleoside reverse transcriptase inhibitors by the wild-type breast cancer resistance protein. Biochem Pharmacol. 2004;68:1363–1370. doi: 10.1016/j.bcp.2004.05.052. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wei Y, Sawchuk RJ. Zidovudine transport within the rabbit brain during intracerebroventricular administration and the effect of probenecid. J Pharm Sci. 1997;86:1484–1490. doi: 10.1021/js950330v. [DOI] [PubMed] [Google Scholar]
- Weiss J, Rose J, Storch CH, Ketabi-Kiyanvash N, Sauer A, Haefeli WE, Efferth T. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. J Antimicrob Chemother. 2007;59:238–245. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- Yuan J. Estimation of variance for AUC in animal studies. J Pharm Sci. 1993;82:761–763. doi: 10.1002/jps.2600820718. [DOI] [PubMed] [Google Scholar]