Abstract
Background
The aim of this study was to determine whether activation of mammalian target of rapamycin (mTOR) is a key epileptogenic mechanism in the development of alcohol-related seizure.
Material/Methods
C57BL/6 mice were administered 10% ethanol in drinking water for 9 weeks. Video-electroencephalography (EEG) monitoring was then used to assess seizure frequency after alcohol and rapamycin treatment. In addition, mouse neuroblastoma NG108-15 cells were treated ethanol for 3 days and subsequently treated with AKT inhibitor LY294002 for 2–12 h. The in vitro kinase assay was performed for determining mTOR activity. Western blot analysis was used to determine the expression of P-AKT, P-S6K, and P-S6.
Result
Long-term ethanol treatment markedly increased the seizure frequency of C57/BL6 mice over time. Moreover, ethanol treatment increased the expression level of P-S6 over time. Ethanol-induced seizure can be reversed by rapamycin. In addition, the in vitro kinase assay showed mTOR activity was activated by ethanol. Compared with NG108-15 cells treated without both ethanol and LY294002, ethanol increased the expression level of P-AKT, P-S6K, and P-S6, whereas LY294002 had opposite effects on expression levels of these proteins.
Conclusions
Our findings indicate that long-term alcohol intake increases the risk of epilepsy via activation of mTOR signaling. Moreover, ethanol-induced mTOR activation may be dependent on the AKT-mTOR signaling pathway. The key molecules involved in AKT-mTOR signaling pathway may serve as potential targets in the treatment of epilepsy.
MeSH Keywords: Epilepsy, Ethanol, TOR Serine-Threonine Kinases
Background
Epilepsy is one of the most common neurologic conditions, characterized by an enduring predisposition to generate seizures [1]. It is a cause of substantial morbidity and mortality [2]. Long-term drug treatments are always required for epilepsy; however, approximately 30% of the patients with epilepsy are observed to have drug resistance or serious adverse reactions [3]. Moreover, multiple risk factors, such as brain injury, stroke, and drug and alcohol abuse, can result in epilepsy, and the relationship between alcohol and epilepsy is reported to be especially strong [4]. However, the molecular mechanisms underlying alcohol-related seizure are largely unknown to date. Therefore, a better understanding of the pathogenic mechanism underlying epilepsy will foster the development of new therapeutic strategies.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that can control protein synthesis associated with cell growth and proliferation [5,6]. The mTOR signaling pathway has been shown to mediate mechanisms of epileptogenesis in a model of temporal lobe epilepsy, and may serve as a potential therapeutic target [7]. mTOR inhibition has significant effects on several major aspects of epilepsy pathophysiology, such as neuronal degeneration, mossy fibers sprouting, and recurrent seizure. In addition, inhibition of mTOR is reported to suppress or block the progression of epilepsy [8,9]. Inhibition of mTOR is regarded as a antiepileptogenic and antiseizure therapy for epilepsy treatment [10]. It can therefore be speculated that the mTOR pathway may be a key mechanism involved in progression of epilepsy. However, whether the mTOR pathway contributes to alcohol-related seizures has not been fully determined.
In the present study we used video-electroencephalography (EEG) monitoring to assess seizure frequency after long-term alcohol treatment, and sought to determine whether rapamycin could effectively treat alcohol-induced seizures. In addition, we used NG108-15 cells to explore in vitro whether alcohol-induced mTOR activation is the key mechanism in epilepsy progression. The objective of our study was to determine if the mTOR pathway is a key epileptogenic mechanism in the development of alcohol-related seizures.
Material and Methods
Animals and drug protocol
Wild-type C57/BL6 mice were housed singly in standard polypropylene cages with free access to food and tap water and a 12: 12-hour light–dark cycle with an ambient room temperature of 21°C. At age 4 weeks, one group of mice continued to receive the control liquid diet and the other group received diet water containing 10% ethanol for 9 weeks. To maintain similar caloric intakes between the groups, the mean of the previous day’s consumption by the ethanol group was detected and the mice in the control group were then offered an equal amount of diet. After 7 weeks of ethanol treatment, wild-type C57/BL6 mice were injected with rapamycin (3 mg/kg/day, 5 days/week, LC Labs, Woburn, MA), an mTOR inhibitor, until subsequent analyses were completed at pre-determined time points. Notably, rapamycin was first dissolved in 100% ethanol and stored at −20ºC. Before injection, rapamycin was immediately diluted in a vehicle solution. Care and use of animals were conducted in accordance with an animal protocol approved by the local ethics committee and animal management committee.
Video-EEG monitoring
C57/BL6 mice underwent weekly video-EEG monitoring initiating at 4 weeks of age to assess seizure frequency. Video-EEG monitoring was performed as described previously [11]. In brief, mice under isoflurane anesthesia were surgically implanted with 4 epidural screw electrodes and allowed to recover from surgery for more than 24 h before recording. Then, once a week, 48-h epochs of continuous EEG data from each mouse were detected using Grass P-100 AC amplifiers (Astro-Med, West Warwick, RI, USA), analyzed by Axon Digidata A–D converters and saved digitally using Axoscope software (Molecular Devices, Sunnyvale, CA, USA). Simultaneously, digital video was recorded with a Sanyo Day-Night camera and a Darim MG-100 MPEG video capture card (Darim Vision Corp., Pleasanton, CA, USA) to analyze the behavioral correlate of electrographic seizures. On video analysis, electrographic seizures were identified when a characteristic pattern of discrete periods of rhythmic spike discharges appeared. Rhythmic spike discharges were characterized by frequency and amplitude lasting for more than 10 s, ended with repetitive burst discharges, and followed by severe voltage suppression. The behavioral correlate to these seizures was typically characterized by head bobbing, rearing with forelimb clonus, and occasional generalized convulsive activity. Seizure frequencies were finally calculated from each 48-h epoch.
Cell culture and treatment
Mouse neuroblastoma NG108-15 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) containing 10% fetal bovine serum in a humidified incubator at 37°C with 5% CO2. The cells were treated with 100 mM ethanol for 3 days and subsequently treated with 10 uM LY294002, an inhibitor of AKT, for 2–12 h.
In vitro kinase assay for mTOR activity
The in vitro kinase assay was performed for determining mTOR activity as described previously. In brief, NG108-15 cells treated with ethanol were first lysed in lysis buffer with protease inhibitor. Cell lysate was then incubated with anti-mTOR antibody and 120 μl of Protein A agarose beads at 4°C for 3 h. Afterwards, beads were washed 3 times with lysis buffer and twice with kinase buffer. Then beads were divided into equal aliquots and were assayed for determining kinase activity. The kinase activity for mTOR activity was finally determined by monitoring the phosphorylation levels of S6.
Western blot analysis
Western blot analysis was performed to assay the expression levels of P-AKT, P-S6K, and P-S6 by standard methods. Briefly, neocortex of mice were dissected and sonicated with Cell Lysis Buffer supplement with protease inhibitors (BestBio, Shanghai, China) to extract total protein. Similarly, NG108-15 cells were also lysed with Cell Lysis Buffer on ice for 1 h. Total protein concentration was determined with the Bradford method (Pierce, Rockford, IL, USA). Equal amounts of protein extract were separated on 10% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). After blocking, the membrane was incubated with appropriate primary antibody at 4°C overnight. Antibodies to P-AKT, P-S6K, and P-S6 were from Cell Signaling Technology with a diluted concentration of 1:1000, and antibody to GAPDH (1:1000) was from Genetex. After washing 3 times, the membranes were then incubated with appropriate horseradish-peroxidase-labeled anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, 1:5000) at 25°C for 1 h. The bands were finally developed by enhanced chemiluminescence kit (Millipore) and the expression level of these proteins was normalized to GAPDH.
Statistics analysis
All statistical analyses were performed using SPSS13.0 software. The data are expressed as mean ±SD and the differences between groups were compared using Student’s t-test or one-way ANOVA, as appropriate. Statistically significant difference was accepted at a value of p<0.05.
Results
The effect of ethanol on seizure frequency and mTOR activation
Video-EEG monitoring displayed the effect of ethanol on seizure frequency in C57/BL6 mice (Figure 1A). The results showed that long-term ethanol treatment markedly increased the seizure frequency of C57/BL6 mice, and seizure frequency increased with longer duration of ethanol treatment (from 4 to 7 weeks), especially at 7 weeks (p<0.05), compared with control group.
Figure 1.
The effect of ethanol on seizure frequency and mTOR activation. (A) Video-EEG monitoring displayed the effect of ethanol on seizure frequency in C57/BL6 mice. (B) Western bolt analysis showing the effect of ethanol on the expression of p-S6. * Indicates significant difference compared with control group (P<0.05).
To verify whether ethanol induced seizure frequency via activation of mTOR signaling, the expression level of P-S6 in neocortex of mice was determined by Western blot analysis (Figure 1B). As expected, results showed that ethanol gradually increased the expression level of P-S6 with longer duration of ethanol treatment, indicating the mTOR activation in neocortex of mice continuously increased with longer duration of ethanol treatment.
The effect of rapamycin on ethanol-induced seizure
To determine whether rapamycin could improve the ethanol-induced seizures, rapamycin was injected into C57/BL6 mice to observe its effect after 7 weeks of ethanol treatment. As shown in Figure 2, compared with the mice only treated with ethanol, the seizure frequency of mice treated with both ethanol and rapamycin gradually decreased with the increase of treatment time, suggesting that ethanol-induced seizures can be reversed by rapamycin.
Figure 2.
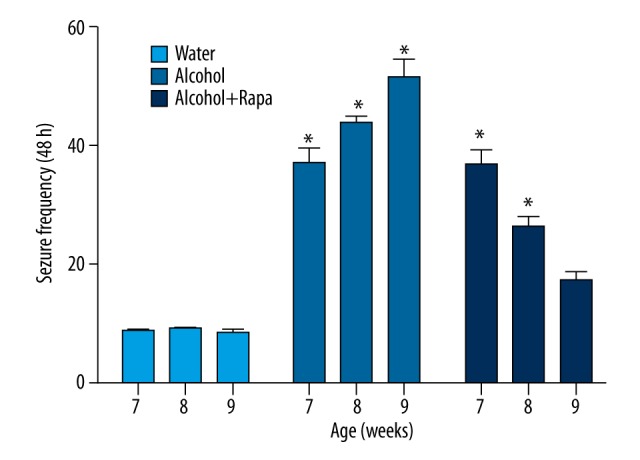
The effect of rapamycin on ethanol-induced seizure. The results showed compared with the mice only treated with ethanol, the seizure frequency of mice treated both ethanol and rapamycin gradually decreased with increase of treatment time. * Indicates significant difference compared with control group (P<0.05).
The effect of ethanol on mTOR activation in NG108-15 cells
NG108-15 cells were used to explore the mechanism of ethanol-induced mTOR activation in vitro. The in vitro kinase assay also showed that mTOR activity was activated by ethanol. Figure 3 shows that the relative kinase activity gradually increased with longer duration of ethanol treatment.
Figure 3.

The in vitro kinase assay also showed that mTOR activity was activated by ethanol.
In addition, western blot analysis showed that, compared with NG108-15 cells treated without ethanol and an AKT inhibitor LY294002, ethanol treatment increased the expression level of P-AKT, P-S6K, and P-S6 in cells only treated with ethanol, whereas LY294002 had the opposite effect on expression levels of these proteins in cells treated with both ethanol and LY294002 (Figure 4), suggesting that ethanol-induced mTOR activation is dependent on the AKT-mTOR signaling pathway.
Figure 4.
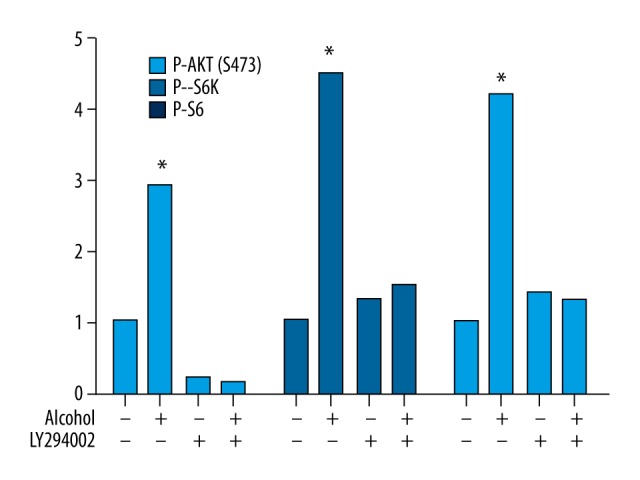
Western blotting analysis showed the effect of both ethanol and an AKT inhibitor LY294002 on mTOR activation. The results show that, compared with NG108-15 cells treated without both ethanol and an AKT inhibitor LY294002, ethanol increased the expression levels of P-AKT, P-S6K, and P-S6 in cells only treated with ethanol, whereas LY294002 had opposite effects on expression levels of these proteins in cells treated with both ethanol and LY294002. * Indicates significant difference compared with control group (P<0.05).
Discussion
Alcohol can cause seizures in a variety of ways; however, the molecular mechanisms underlying alcohol-related seizure remain elusive. In the current study, we explored the potential epileptogenic mechanism of alcohol in vivo and in vitro. The results showed that long-term alcohol treatment increased the seizure frequency of C57/BL6 mice via activation of mTOR signaling. Moreover, ethanol also induced mTOR activation in NG108-15 cells in vitro, and ethanol-induced mTOR activation was shown to be dependent on the AKT-mTOR signaling pathway. All these findings indicate that the AKT-mTOR signaling pathway is likely to be the key mechanism of ethanol-induced epilepsy.
The relationship between alcohol and seizures is reported to be complex [12] and many studies have been carried out to explore alcohol-related or withdrawal seizures [13,14]. Long-term alcohol abuse can change the seizure threshold with alcohol levels and increase the risks of prolonged or sustained seizure activity [15]. Moreover, alcohol withdrawal is shown to increase the risk of sudden unexpected death in epilepsy (SUDEP) [15,16]. Welch and Derry also suggested that alcohol misuse might underpin the association between mild traumatic brain injury and epilepsy [17]. Sabino et al. demonstrated that mTOR activation was required for the anti-alcohol effect of ketamine in alcohol-preferring rats [18]. mTOR signaling can regulate neuronal development and synaptic plasticity in the brain [19,20]. Moreover, the mTOR signaling pathway is reported to be activated during status epilepticus (SE) in a model of temporal lobe epilepsy, as evident by an increase in P-S6 expression [7]. In our study, video-EEG monitoring showed that ethanol induced increased seizure frequency in C57/BL6 mice. Moreover, we observed the increased expression level of P-S6 in ethanol-treated C57/BL6 and NG108-15 cells. Therefore, we speculate that ethanol could induce epilepsy via activating mTOR signaling.
Increased expression of P-S6 is evidence of mTOR activation [7]. S6 ribosomal protein is phosphorylated by S6 kinase, which is one of the downstream effectors of the AKT pathways [21]. To further explore the mechanism of ethanol-induced mTOR activation, NG108-15 cells were treated with both ethanol and an AKT inhibitor LY294002. The results showed that ethanol increased the expression level of P-AKT, P-S6K, and P-S6, whereas LY294002 had opposite effects on expression levels of these proteins, suggesting that ethanol-induced mTOR activation is dependent on the AKT-mTOR signaling pathway. Moderate alcohol consumption has been shown to induce sustained cardiac protection through activating AKT [22]. Phosphoinositide-3-kinase/AKT signaling cascade is considered to be a cellular signaling mechanism in the process of liver defects associated with chronic alcohol consumption [23]. In addition, rapamycin/RAD001 is shown to have benefit in the treatment of tuberous sclerosis (TSC) via having effects on mTORC1 and AKT signaling, and TSC in the brain is likely to cause epilepsy, suggesting that mTORC1 and AKT signaling may be a key mechanism involved in epilepsy [24]. Antiepileptic drugs lead to decreased concentrations of the active forms of AKT, suggesting that AKT may be a potential target in antiepileptic therapy [25]. Therefore, our results are in line with previous studies and suggest that the AKT-mTOR signaling pathway may contribute to the development of ethanol-induced epilepsy.
Conclusions
Our findings indicate that long-term alcohol intake increases the risk of epilepsy via activation of mTOR signaling. Moreover, ethanol-induced mTOR activation may be dependent on the AKT-mTOR signaling pathway. The key molecules involved in the AKT-mTOR signaling pathway may serve as potential targets in the treatment of epilepsy.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Source of support: Departmental sources
References
- 1.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerjee PN, Filippi D, Hauser WA. The descriptive epidemiology of epilepsy – a review. Epilepsy Res. 2009;85:31–45. doi: 10.1016/j.eplepsyres.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verrotti A, Loiacono G, Rossi A, Zaccara G. Eslicarbazepine acetate: An update on efficacy and safety in epilepsy. Epilepsy Res. 2014;108:1–10. doi: 10.1016/j.eplepsyres.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Samokhvalov AV, Irving H, Mohapatra S, Rehm J. Alcohol consumption, unprovoked seizures, and epilepsy: A systematic review and meta-analysis. Epilepsia. 2010;51:1177–84. doi: 10.1111/j.1528-1167.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarbassov dD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Sandsmark D, Pelletier C, Weber J, Gutmann DH. Mammalian target of rapamycin: Master regulator of cell growth in the nervous system. Histol Histopathol. 2007;22:895–903. doi: 10.14670/HH-22.895. [DOI] [PubMed] [Google Scholar]
- 7.Zeng L-H, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neuroscience. 2009;29:6964–72. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunnen CN, Brewster AL, Lugo JN, et al. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52:2065–75. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–99. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryther RC, Wong M. Mammalian target of rapamycin (mTOR) inhibition: potential for antiseizure, antiepileptogenic, and epileptostatic therapy. Curr Neurol Neurosci Rep. 2012;12:410–18. doi: 10.1007/s11910-012-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng L-H, Rensing NR, Zhang B, et al. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;2:445–54. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj V, Vedi S, Govil S. Seizures in alcohol dependent patients. MJP Online Erly. 2011;20 MJP-01-02-11. [Google Scholar]
- 13.Rathlev NK, Ulrich AS, Delanty N, D’Onofrio G. Alcohol-related seizures. J Emerg Med. 2006;31:157–63. doi: 10.1016/j.jemermed.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Hughes JR. Alcohol withdrawal seizures. Epilepsy Behav. 2009;15:92–97. doi: 10.1016/j.yebeh.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Leach JP, Mohanraj R, Borland W. Alcohol and drugs in epilepsy: Pathophysiology, presentation, possibilities, and prevention. Epilepsia. 2012;53:48–57. doi: 10.1111/j.1528-1167.2012.03613.x. [DOI] [PubMed] [Google Scholar]
- 16.Téllez-Zenteno JF, Ronquillo LH, Wiebe S. Sudden unexpected death in epilepsy: Evidence-based analysis of incidence and risk factors. Epilepsy Res. 2005;65:101–15. doi: 10.1016/j.eplepsyres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Welch KA, Derry C. Mild traumatic brain injury and epilepsy: Alcohol misuse may underpin the association. J Neurol Neurosurg Psychiatry. 2014;85:593. doi: 10.1136/jnnp-2013-306267. [DOI] [PubMed] [Google Scholar]
- 18.Sabino V, Narayan AR, Zeric T, et al. mTOR activation is required for the anti-alcohol effect of ketamine, but not memantine, in alcohol-preferring rats. Behav Brain Res. 2013;247:9–16. doi: 10.1016/j.bbr.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang SJ, Reis G, Kang H, et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA. 2002;99:467–72. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V, Zhang M-X, Swank MW, et al. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–99. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60:293–300. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- 22.Zhou HZ, Karliner JS, Gray MO. Moderate alcohol consumption induces sustained cardiac protection by activating PKC-epsilon and Akt. Am J Physiol Heart Circ Physiol. 2002;283:H165–74. doi: 10.1152/ajpheart.00408.2001. [DOI] [PubMed] [Google Scholar]
- 23.Hoek JB, Pastorino JG. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis. 2004;24:257–72. doi: 10.1055/s-2004-832939. [DOI] [PubMed] [Google Scholar]
- 24.Meikle L, Pollizzi K, Egnor A, et al. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: Effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–32. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. In: Slikker W, Andrews RJ, Trembly B, editors. Neuroprotective Agents. New York: New York Acad Sciences; 2003. pp. 103–14. [DOI] [PubMed] [Google Scholar]



