Abstract
Background
Radiation thyroiditis caused by 131I therapy for Graves' hyperthyroidism is asymptomatic in most patients and is rarely associated with pain or fever. Currently, there are few reports on the ultrasonographic findings of radiation thyroiditis after 131I therapy for Graves' hyperthyroidism.
Case Report
We herein report 5 cases with painful radiation thyroiditis (including 2 febrile cases) after 131I therapy for Graves' hyperthyroidism. The cases included 4 women, aged 49, 50, 76, and 81 years, and 1 man, aged 60 years. Anterior neck pain developed 0-10 days after 131I administration (fixed dose of 481 MBq). Each patient visited our clinic 0-4 days after the development of anterior neck pain. The thyroid glands were noticeably enlarged (increasing from 18 g at 131I administration to 35 g after the development of anterior neck pain in 1 patient, and from 20 to 33 g, 21 to 39 g, 21 to 51 g, and 40 to 51 g in the other patients) and tender. The echogenicity of the thyroid parenchyma was increased, and the parenchyma was more heterogeneous. Granular hyperechoic lesions were scattered throughout the thyroid gland in the most severe case. The border between the thyroid gland and the surrounding tissue was blurred, and the surrounding tissue was hyperechoic.
Conclusion
Painful radiation thyroiditis should be reacknowledged as one of the complications of 131I therapy for Graves' hyperthyroidism. Ultrasonography demonstrated the characteristic changes caused by 131I-induced radiation thyroiditis.
Key Words: Radiation thyroiditis, Radioactive iodine, Graves’ disease, Hyperthyroidism
What Is Known about This Topic?
Anterior neck pain and fever, caused by radiation thyroiditis, is a rare occurrence after 131I therapy for Graves' hyperthyroidism. In addition, the ultrasonographic findings have not been well described.
What Does This Case Report Add?
Painful radiation thyroiditis after 131I therapy for Graves' hyperthyroidism is associated with characteristic findings in the thyroid parenchyma as well as in the surrounding tissue on ultrasonography.
Introduction
131I therapy is one of the main treatment options for Graves' hyperthyroidism. Although 131I therapy is generally safe, adverse events may occur [1]. Among the adverse events, radiation thyroiditis is an acute condition occurring within 2 weeks after 131I administration [2]. Radiation thyroiditis is asymptomatic in most patients, but painful inflammation of the thyroid gland occurs in 1% of patients with hyperthyroidism [3]. It is even rarer for radiation thyroiditis to be associated with fever. In addition, there are few reports on the ultrasonographic findings of radiation thyroiditis after 131I therapy for Graves' hyperthyroidism [4]. We herein report 5 cases with painful radiation thyroiditis (including 2 febrile cases) after 131I therapy for Graves' hyperthyroidism, with clinical features and serial ultrasound images.
Methods
The absorbed radiation dose of the thyroid gland was estimated by using the Quimby-Marinelli formula and thyroidal 99mTc uptake, as previously reported [5]. The patients underwent ultrasonographic examination within 10 days prior to 131I administration as well as after the development of painful radiation thyroiditis. Ultrasonographic evaluation of the thyroid and neck was performed with the Aplio XG, Aplio 400, or Aplio 500 systems (Toshiba Medical Systems Co., Tokyo). Intraglandular vascular flow was evaluated with Doppler color flow imaging. Thyroid weight was estimated through ultrasound as previously reported [6]. Thyroid scan and uptake were performed at 20 min after intravenous injection of 185 MBq 99mTc-pertechnetate prior to 131I administration on the same day. Serum levels of free thyroxine (FT4) and thyrotropin (TSH) were measured by using commercial immunoassays (Roche Diagnostics Inc., Tokyo). Serum TSH receptor antibody (TRAb) and anti-thyroid peroxidase (TPO) antibody levels were measured using commercial electrochemiluminescence immunoassays (Elecsys Anti-TSHR and Elecsys Anti-TPO; Roche Diagnostics Inc., Tokyo; reference ranges <2.0 IU/l and <16 IU/ml, respectively). In some patients, autoantibody levels to the thyroid microsomal antigen were measured by means of particle agglutination using commercial kits (Serodia-AMC; Fujirebio Inc., Tokyo; reference range less than ×100).
Case Reports
Table 1 gives a detailed description of the clinical characteristics of all 5 patients at 131I therapy and after radiation thyroiditis.
Table 1.
Clinical characteristics of the present patients
| Case No. | Age, years | At 131I therapy |
After radiation thyroiditis |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dose, MBq | Abs D, Gy | thyroid, g | Tc-U, % | FT4, ng/dl | TSH, mIU/l | TRAb, IU/l | anti-TPO, IU/ml | onset, day | fever | exam, day | thyroid, g | FT4, ng/dl | WBC, /µl | CRP, mg/dl | ||
| 1 | 50 | 481 | 145 | 40 | 5.3 | 1.52 | n.a. | >40.00 | 561 | 0 | – | 1 | 51 | n.a. | n.a. | n.a. |
| 2 | 49 | 481 | 258 | 20 | 4.7 | 1.38 | <0.005 | 15.69 | ×400a | 9 | + | 12 | 33 | 1.93 | 6,700 | 2.53 |
| 3 | 81 | 481 | 257 | 21 | 9.5 | 0.55 | 34.1 | 32.41 | >600 | 3 | + | 7 | 51 | 1.25 | 4,000 | 4.56 |
| 4 | 76 | 481 | 279 | 18 | 4.1 | 0.76 | 0.58 | 4.43 | 224 | 3 | – | 4 | 35 | n.a. | n.a. | n.a. |
| 5 | 60 | 481 | 246 | 21 | 5.0 | 1.37 | <0.005 | 4.71 | 64 | 10 | – | 10 | 39 | 1.22 | n.a. | 3.37 |
Dose: administered dose of 131I. Abs D: estimated absorbed radiation dose to thyroid gland from 131I. Tc-U: 99mTc uptake (20 min). Onset: onset after 131I administration. Exam: examination of thyroid and inflammatory reaction after 131I administration. TRAb = TSH receptor antibody; anti-TPO = anti-thyroid peroxidase antibody; WBC = white blood cell; CRP = C-reactive protein; n.a. = not available.
Anti-thyroid microsomal particle agglutination assay.
Case 1
A 50-year-old woman underwent 131I therapy for intractable Graves' hyperthyroidism. In spite of instructions to cease methimazole (MMI) prior to 131I administration, she had taken MMI 10 mg daily until the day of 131I administration. She developed anterior neck pain at night on the same day of 131I administration. The goiter was enlarged and tender. Radiation thyroiditis was treated with prednisolone 15 mg/day for 4 days. She resumed taking MMI 10 mg daily 2 days after 131I administration.
Case 2
A 49-year-old woman underwent 131I therapy for recurrent Graves' hyperthyroidism. MMI 10 mg daily was withdrawn 2 days prior to 131I administration, and she began to take potassium iodide (KI) 50 mg daily to avoid exacerbation of hyperthyroidism 2 days after 131I administration. Nine days after 131I administration, she developed anterior neck pain and high fever. When she visited our clinic 3 days after the onset, the goiter was enlarged and tender. Radiation thyroiditis was treated with prednisolone 15 mg/day for 10 days.
Case 3
An 81-year-old woman underwent 131I therapy for intractable Graves' hyperthyroidism (unstable thyroid function). When she underwent 131I therapy, she was hypothyroid due to overtreatment with MMI. She stopped taking MMI 5 mg daily 2 days prior to 131I administration and resumed MMI 5 mg every other day 2 days after 131I administration. Three days after 131I administration, she developed anterior neck pain and high fever. Since the anterior neck pain and high fever continued, she visited our clinic 4 days after the onset. The goiter was visibly enlarged and tender. She took prednisolone 15 mg/day for only 2 days, although it was prescribed for 7 days.
Case 4
A 76-year-old woman underwent 131I therapy for recurrent Graves' hyperthyroidism. MMI 10 mg daily was withdrawn 2 days prior to 131I administration, and she resumed MMI 5 mg daily 2 days after 131I administration. Three days after 131I administration, she developed anterior neck pain. When she visited our clinic the day following the onset, the goiter was enlarged and tender. Radiation thyroiditis was treated with prednisolone 15 mg/day for 7 days.
Case 5
A 60-year-old man underwent 131I therapy for Graves' hyperthyroidism. Because of MMI-induced liver injury, he had been switched to KI. KI 50 mg daily was withdrawn 5 days prior to 131I administration, and he resumed KI 50 mg daily 2 days after 131I administration. Ten days after 131I administration, he developed anterior neck pain and swelling. When he visited our clinic on the same day of the onset, the goiter was enlarged and tender. Because of his diabetes and relatively mild neck pain, radiation thyroiditis was treated with acetaminophen.
The present cases included 4 women and 1 man, and all were nonsmokers who had been treated with 481 MBq of 131I. At the time of 131I administration, the patients' estimated thyroid gland weights were 18-40 g, and thyroidal 99mTc uptake was increased to 4.1-9.5% (reference range 0.5-4.0%). One patient (case 3) had overt hypothyroidism. TRAb and anti-TPO antibody were all positive. Anterior neck pain developed 0-10 days after 131I administration. Two patients developed high fever along with the anterior neck pain. The clinical examinations were conducted 0-4 days after the development of anterior neck pain. Goiters were enlarged 1.3- to 2.4-fold and were tender. Serum thyroid hormone levels were increased from those seen just prior to 131I administration in cases 2 and 3. Serum C-reactive protein levels (reference range <0.5 mg/dl) were elevated in all 3 patients who underwent examination. All 5 patients were diagnosed with 131I-induced radiation thyroiditis based on the clinical course, thyroid tenderness, and ultrasonographic changes of the thyroid. Four were treated with prednisolone, and the administration periods were determined by each attending physician. The patients' anterior neck pain and fever were eliminated within 1 or 2 days after taking prednisolone. Their goiters shrank relatively rapidly, and they all eventually developed hypothyroidism, after which replacement of L-thyroxine was initiated.
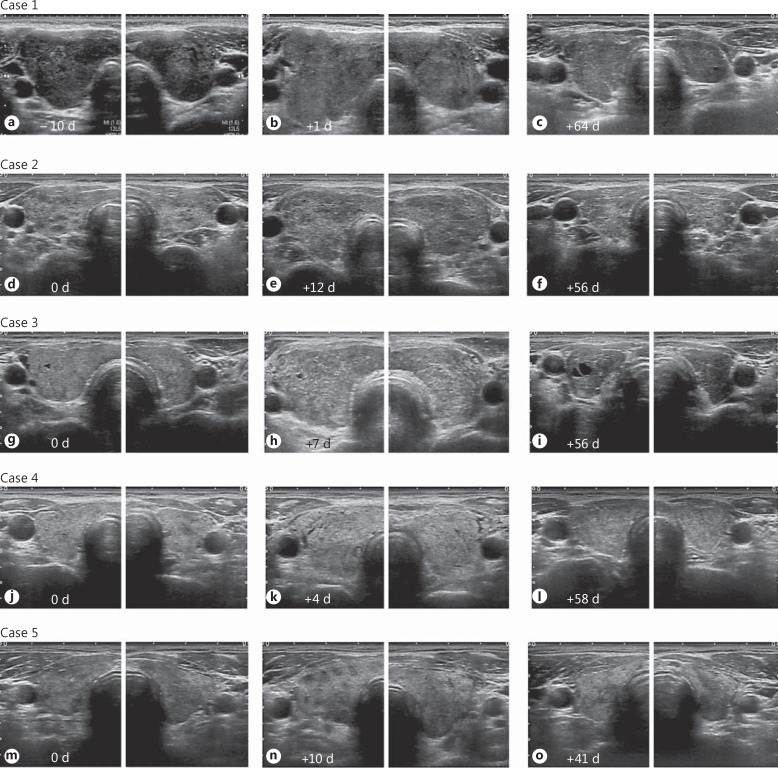
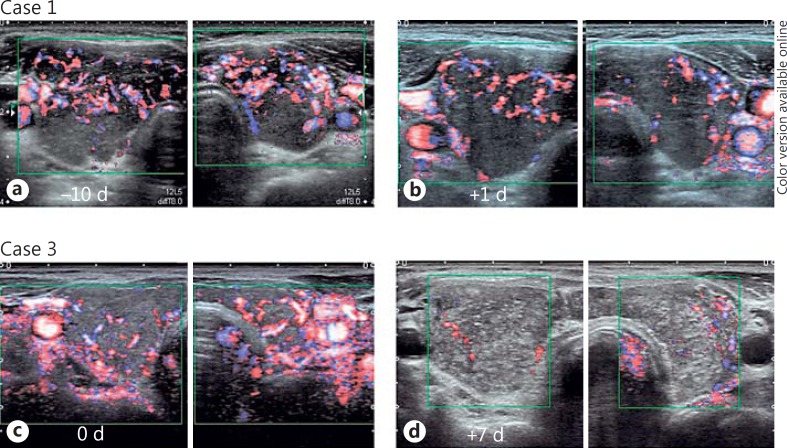
Figure 1 shows ultrasonographic images from the present patients before and after 131I administration. At 131I administration, the thyroid glands were diffusely enlarged with a heterogeneous and coarse echotexture; 1-4 days after the development of anterior neck pain, the thyroid glands were clearly enlarged. The echogenicity of the thyroid parenchyma was increased, and the parenchyma was more heterogeneous. Especially in case 3, granular hyperechoic lesions were scattered throughout the thyroid gland. The border between the thyroid gland and the surrounding tissue was blurred and irregular (cases 1-5), and the surrounding tissue was hyperechoic (cases 3-5). Hypervascularity of the thyroid glands diminished shortly after the development of painful radiation thyroiditis (fig. 2). The thyroid glands also became hypoechoic as they shrank over a period of a few months. The border between the thyroid gland and the surrounding tissue became clear, and the echogenicity of the surrounding tissue normalized. The estimated weights of the thyroid decreased to less than 10 g within 6 months during the follow-up.
Fig. 1.
Ultrasonographic images from each case before and after 131I administration. d = Days. a-c Case 1: ultrasonographic images 10 days before (a), 1 day after (b), and 64 days after 131I administration (c). One day after neck pain developed (b), the thyroid gland was clearly enlarged and the echogenicity of the thyroid parenchyma was increased. d-f Case 2: ultrasonographic images at the same day (d), 12 days after (e), and 56 days after 131I administration (f). Three days after neck pain developed (e), a diffusely enlarged goiter with more heterogeneous internal echoes was observed. g-i Case 3: ultrasonographic images on the day (g), 7 days after (h), and 56 days after 131I administration (i). Four days after neck pain developed (h), a diffusely enlarged goiter with relatively hyperechoic internal echoes was observed. Granular hyperechoic lesions were scattered throughout the thyroid gland. j-l Case 4: ultrasonographic images on the day (j), 4 days after (k), and 58 days after 131I administration (l). One day after neck pain developed (k), a diffusely enlarged goiter was observed. m-o Case 5: ultrasonographic images at the same day (m), 10 days after (n), and 41 days after 131I administration (o). On the day the neck pain developed (n), the thyroid gland was enlarged and the echogenicity of the thyroid parenchyma was increased. The border between the thyroid gland and the surrounding tissue became blurred and irregular (cases 1-5), and the surrounding tissue was hyperechoic (cases 3-5) within several days of neck pain development (b, e, h, k, n).
Fig. 2.
Doppler color flow images of case 1 (a, b) and case 3 (c, d) before (a, c) and after (b, d) 131I administration. d = Days. Hypervascularity of the thyroid glands diminished after the development of painful radiation thyroiditis.
Discussion
During the last 5 years in which the 5 present patients were treated, we administered 131I therapy to a total of 927 patients with Graves' hyperthyroidism. Therefore, the incidence of painful radiation thyroiditis after 131I therapy for Graves' hyperthyroidism was estimated to be 0.5% in our clinic. However, since some patients may not have reported relatively mild neck pain, the actual incidence of painful radiation thyroiditis is presumed to be higher. The manifestation of 131I-induced radiation thyroiditis typically begins within a few days, generally in the first 2 weeks, after 131I administration [7]. Consistent with this, the present patients developed anterior neck pain due to radiation thyroiditis 0-10 days after 131I therapy. Two patients were febrile. Variations in the onset and severity of 131I-induced radiation thyroiditis suggest that there are interindividual differences in the inflammatory response against radiation-induced tissue damage as well as in radiosensitivity of the thyroid gland.
Radiation thyroiditis sometimes leads to a temporary exacerbation of thyrotoxicosis in patients who had hyperthyroidism at 131I administration [3,8]. Serum thyroid hormone levels increased from those at 131I administration in cases 2 and 3. This may be due to the release of thyroid hormones from follicular cells destroyed by 131I as well as the increased production of thyroid hormone during the cessation of MMI. When the patients visited our clinic after 131I administration, aggravation of thyrotoxicosis was not apparent. The stores of glandular hormones may have been depleted owing to the administration of MMI for more than several months prior to 131I administration. In addition, the patients may have undergone thyroid function testing before the maximum release of thyroid hormones from the destroyed follicular cells. Painful radiation thyroiditis can be treated with nonsteroidal anti-inflammatory drugs [3]. We used an oral glucocorticoid to treat 4 patients because of its definite effect on reducing radiation-induced inflammation. Short-term administration of oral glucocorticoid was highly effective in relieving their symptoms.
In radiation thyroiditis after 131I therapy for Graves' hyperthyroidism, the absorbed radiation dose to the thyroid gland appears to be the main precipitating factor [2]. The administered dose of 131I, thyroidal 131I uptake, and thyroid volume all contribute to the absorbed radiation dose to the thyroid gland. With regard to the administered dose, we adopted a high-dose 131I therapy protocol (fixed dose of 481 MBq) to avoid persistent hyperthyroidism following 131I therapy. In terms of the thyroidal uptake of 131I, we found elevated TSH as well as elevated TRAb-simulated 131I uptake in case 3. Since she was 81 years old, we intended to render her mildly hypothyroid before 131I therapy to minimize the risk of exacerbating the preexisting hyperthyroidism during 131I therapy. However, the serum TSH level was pretty high at the time of 131I administration. Lastly, with regard to thyroid volume, a larger goiter decreases the absorbed radiation dose to the thyroid gland from 131I. The goiters in the present 4 patients (cases 2-5) were not very large. Thus, radiation thyroiditis following 131I therapy is of particular concern in patients with active Graves' hyperthyroidism who are treated with a larger dose of 131I for a relatively small goiter.
In fact, the absorbed radiation dose to the thyroid gland was estimated to be more than 200 Gy in the 4 cases with a smaller goiter (cases 2-5) but was around 150 Gy in the case with the largest goiter (case 1). The absorbed radiation dose to the thyroid gland was not necessarily very high. Histological variation of the thyroid parenchyma and the perithyroid tissue may also contribute to the development of painful radiation thyroiditis. Risk factors for painful radiation thyroiditis apart from the absorbed radiation dose to the thyroid gland have yet to be elucidated.
Before 131I administration, the ultrasonographic presentation was compatible with the underlying autoimmune disease, in this case Graves' disease. The ultrasonographic findings of the thyroid parenchyma and the surrounding tissue were noticeably changed after the development of 131I-induced radiation thyroiditis. To the best of our knowledge, this is the first report on the ultrasonographic changes of 131I-induced radiation thyroiditis before and after onset. Case 3 revealed the most severe pattern and may demonstrate the peak of severe 131I-induced radiation thyroiditis. The echogenicity of the thyroid parenchyma was increased, and granular hyperechoic lesions were scattered throughout the thyroid gland. Consistent with these findings, Sekizawa et al. [4] also reported that ultrasonography revealed several hyperechoic lesions within the thyroid gland in a patient with painful radiation thyroiditis after 131I therapy for Graves' hyperthyroidism.
The differences in ultrasonographic patterns among the patients may partly reflect the time course of 131I-induced radiation thyroiditis. A large dose of ionizing radiation initially causes injury to vessels, necrosis of the follicular cells, and breakdown of some follicles [9]. Consequently, edema and hemorrhage appear, followed by fibrosis. In addition, the β-rays emitted by 131I travel a few millimeters within the tissue. Therefore, 131I can cause damage to the perithyroid tissues. Ultrasonography revealed that neck pain due to 131I-induced radiation thyroiditis is ascribed not only to the 131I-induced injury of the thyroid parenchyma but also to associated lesions of the capsule and surrounding tissues.
In conclusion, the development of painful radiation thyroiditis should be considered for patients with Graves' hyperthyroidism after 131I therapy. 131I-induced radiation thyroiditis is associated with characteristic findings on ultrasonography.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33:920–980. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- 2.Maxon HR, Thomas SR, Saenger EL, Buncher CR, Kereiakes JG. Ionizing irradiation and the induction of clinically significant disease in the human thyroid gland. Am J Med. 1977;63:967–978. doi: 10.1016/0002-9343(77)90552-6. [DOI] [PubMed] [Google Scholar]
- 3.Ross DS. Radioiodine therapy for hyperthyroidism. N Engl J Med. 2011;364:542–550. doi: 10.1056/NEJMct1007101. [DOI] [PubMed] [Google Scholar]
- 4.Sekizawa D, Nagasaka S, Takahashi N, Osuga J, Ishibashi S. A patient with Basedow's disease displaying transient exacerbation of thyrotoxicosis with fever and inflammatory response after radioiodine therapy (in Japanese, abstract in English) Jichi Med Univ J. 2013;36:101–105. [Google Scholar]
- 5.Nakatake N, Fukata S, Tajiri J. Prediction of post-treatment hypothyroidism using changes in thyroid volume after radioactive iodine therapy in adolescent patients with Graves' disease. Int J Pediatr Endocrinol. 2011;2011:14. doi: 10.1186/1687-9856-2011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tajiri J. Radioactive iodine therapy for goitrous Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2006;91:4497–4500. doi: 10.1210/jc.2006-1163. [DOI] [PubMed] [Google Scholar]
- 7.Shah KK, Tarasova V, Davidian M, Anderson RJ. Painful acute radiation thyroiditis induced by 131I treatment of Graves' disease. BMJ Case Rep. doi: 10.1136/bcr-2014-207670. DOI: 10.1136/bcr-2014-207670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyer SL, Newbold K, Harmer CL. Early and late toxicity of radioiodine therapy: detection and management. Endocr Pract. 2010;16:1064–1070. doi: 10.4158/EP10170.RA. [DOI] [PubMed] [Google Scholar]
- 9.Baloch ZW, Livolsi VA. Pathology and cytopathology. In: Braverman LE, Cooper DS, editors. Werner and Ingbar's The Thyroid: A Fundamental and Clinical Text. ed 10. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 326–353. [Google Scholar]