Abstract
Background
Hypoxia is an important sign that can result from body injuries or a special condition such as being at a high altitude or deep water diving. In the current studies, hypoxic preconditioning (HPC) plays a key role in reducing hypoxia-induced apoptosis. We aimed to study the pharmacologic preconditioning effects of salidroside versus those of HPC in hypoxia-/anoxia-induced apoptosis in PC12 cells (pheochromocytoma).
Material/Methods
PC12 cells were treated by different experimental conditions: control condition, hypoxia condition, HPC condition, low-/middle-/high-dose condition of salidroside, cyclosporine A (CsA), and oratractyloside (ATR). The cell viability, lactate dehydrogenase (LDH) activity, apoptosis, mitochondrial membrane potential (MMP), intracellular Ca2+, caspase-3 activity, and expression of Bcl-2 were detected in PC12 cells after the hypoxia treatment. Salidroside, extracted from the traditional Chinese herb Rhodiola rosea L, plays an essential role in reducing hypoxia-induced apoptosis in PC12 cells by the mitochondrial pathway.
Results
Salidroside decreased the apoptosis and increased the viability of hypoxia-induced PC12 cells more effectively than HPC Moreover, salidroside markedly stabilized MMP and intracellular Ca2+, reduced or inhibited LDH and caspase-3 activity, and up-regulated Bcl-2; CsA and ATR showed corresponding function.
Conclusions
Salidroside administration restrains apoptosis induced by hypoxia in PC12 cells. The protective effects are mediated by preservation of mitochondrial integrity and MMP to inhibit the excessive Ca2+ influx and caspase-3 activity and to promote the Bcl-2 expression, providing a potential clinical and effective therapeutic mechanism to reduce deaths from ischemic or hypoxic injury.
MeSH Keywords: Anoxia; Apoptosis; Medicine, Chinese Traditional
Background
Because of the increasing numbers of outdoor high-altitude and deep water−diving tourist attractions are associated with certain brain and cardiovascular diseases, it is necessary to study how to alleviate the physical damage caused by hypoxia. From 1986, sublethal and temperate ischemia or hypoxic preconditioning (HPC) has been gradually confirmed as effective against subsequent serious ischemia and hypoxia [1,2]. In various experimental models, HPC triggered autonomous endogenous protective mechanisms, including hypoxia-inducible factor 1 [3],mitochondrial metabolism, to resist severe anoxic injury and reduce hypoxia- or ischemia-induced apoptosis. The mitochondria, unique oxygen-consumption organelles, are inextricably linked to hypoxia injuries [4], as will be shown in the following study.
Rhodiola, growing in the cold and hypoxia-inducing mountainous regions of Asia and Europe, is known as an adaptogen in traditional Chinese and Tibetan medicine and is popularly used to prevent acute mountain sickness and extend human life [5]. The active ingredient of Rhodiola, salidroside, is a compound with the chemical structure of phenol glycosides: p-hydroxyphenethyl-b-D-glucoside; C14H20O7. Salidroside has displayed many pharmacologic properties [5,6], such as antifatigue, anti-inflammation, anticancer, antioxidation, and antihypoxia/anti-ischemia. Previous studies have shown that salidroside plays a more significant role in disease models than in models involving various drug effects or animals with induced hypoxia. It increased the survival time of experimental mice under hypoxic conditions [7], improved high-altitude pulmonary edema (HAPE) induced in hypoxic mice [6], and attenuated ischemic brain injury [8]. The pharmacologic properties of salidroside were found to contain the positive regulators of survival signal, including BcL-2, BaX proportion, Akt phosphorylation, and mitochondrial integrity protection [8,9]. Whether used in traditional Chinese and Tibetan medicine or researched in experimental animal models, salidroside has shown the ability to protect the body or cells against hypoxia; however, the underlying mechanism of action against simulated and hypoxia conditions is unclear. On this basis, we speculate that with respect to antihypoxia, salidroside may be comparable to HPC, by maintaining the integrity of the mitochondria [4,10,11]. Therefore, in this study, we aim to explore the antihypoxia mechanism of salidroside compared with HPC in PC12 cells and also expect to provide experimental data for its further clinical application.
Material and Methods
Drugs and cell culture
Salidroside was provided by the National Institutes for Food and Drug Control (batch number 110818-201206). Using the Spearman-Karber method, half maximal inhibitory concentration (IC50) of the salidroside was calculated in the PC12 cells of 96-well microculture plates.
PC12 cells were obtained from Shanghai Cell Bank of Chinese Academy of Sciences and were cultured in 5% CO2 at 37°C with RPMI 1640 (Sigma) and 10% FBS (Hyclone).
Experimental protocols
Control condition (C/Con)
PC12 cells were maintained in 5% CO2 and normoxic conditions for 24 h.
Hypoxia condition (H)
PC12 cells were maintained in 2.5% O2 [12] and 5% CO2 for 24 h.
HPC condition (PH)
PC12 cells were maintained in 2.5% O2 and 5% CO2 for 2 h, then in normoxic conditions for 12 h and maintained in 2.5% O2 and 5% CO2 for 24 h.
Low-dose condition of salidroside (LH)
PC12 cells were maintained in 5 μM salidroside and 5% CO2 at 37°C for 2 h, then in normoxic conditions for 12 h and maintained in 2.5% O2 and 5% CO2 for 24 h.
Middle-dose condition of salidroside (MH)
the concentration of salidroside pretreatment was 10 μM.
High-dose condition of salidroside (HH)
the concentration of salidroside pretreatment was 15 μM. The dose was less than the value of IC50 (40 μM).
Cyclosporine A (CsA) condition
as a positive control group, PC12 cells were preincubated with 0.2 μmol/L CsA (Novartis, Camberley, UK) for 30 min before the hypoxia, HPC, and salidroside pretreatment.
Atractyloside (ATR) condition
as a negative control group, PC12 cells were preincubated with 20 μmol/L ATR (Sigma-Aldrich, St Louis, MO, USA) for 30 min before the hypoxia, HPC, and salidroside pretreatment.
Cell viability and lactate dehydrogenase (LDH) activity assay
By the density of 4 to 5×103 cells/well, the PC12 cells were seeded in 96-well plates. According to the aforementioned experimental protocols (2:1, 1-6 groups), after being cultured, the cells were incubated with 5 mg/mL 3-(4, 5-dimethyl-thiazol- -2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) (Sigma-Aldrich, USA). Next, the cells were maintained in the dark for 4 h; afterward, the intensity of cells was measured with microplate reader at an absorption wavelength of 570 nm.
The activity of LDH in the culture fluid was measured by a spectrophotometry test kit (Beyotime, Beijing, China).
Apoptosis assay
For the apoptosis assay, the cells were harvested and washed once with phosphate buffered saline (PBS), then resuspended and incubated in PI-ANXA5 solution (Key GEN Biotech, KGA108). Flow cytometry was performed with a FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA, USA).
Mitochondrial membrane potential analysis
The CsA and ATR were respectively added to experimental group cells as the comparison before the hypoxia, according to the mitochondria-specific dye tetra methyl rhodamine ethyl ester (TMRE) method as fluorescent probes was used to monitor the mitochondrial membrane potential (MMP) of PC12 cells and the fluorescent dye as a potentially dependent aggregation in the mitochondria with the red emission to reflect the change of MMP. The fluorescence intensity of TMRE was monitored at 582 nm. The stable MMP could be conducive to maintaining the mitochondrial permeability transition pore (mPTP).
Intracellular Ca2+ assay
For the intracellular calcium-assays, we used the FluoFort calcium assay kit from Enzo Life Science (Farmingdale, NY, USA). The assay was performed according to the manual. In brief, PC12 cells were resuspended in FluoFort dye-loading solution (Hanks Balanced Salt Solution, dye efflux inhibitor, FluoFort dye). Cells were plated using 2.5×105 cells per 96-well plates at a plating volume of 100 μL/well and incubated for 45 min at 37°C. Nerve growth factor (100 ng/mL) and nimodipine (2 μM) were added directly before measurement. Positive control was induced by adding 150 μM ATP, and DMSO was added to the baseline control. The fluorescence values were recorded with a time resolution of 3 s using a Fluoroskan Ascent from Labsystems (Helsinki, Finland; Ex=485 nm/Em=530 nm). Recording was started 5 s after adding the stimulus reagents.
Caspase-3 activity assay
Caspases activities were measured using Caspase Activity Kit (Beyotime, Beijing, China) according to the manufacturer’s instructions. Briefly, cells were washed with cold PBS, re-suspended in lysis buffer and left on ice for 15 min. The lysate was centrifuged at 16,000 g at 4°C for 15 min. Activities of caspase-3 was measured using substrate peptides Ac-DEVD-pNA. The release of p-nitroanilide (pNA) was quantitated by determining the absorbance with Multiskan Spectrum (Thermo) at 405 nm.
Immunoblot for Bcl-2
Cell samples were lysed with 50 mM Tris-base, 1.0 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate (Sigma-Aldrich, D6750-25G), and 1 mM phenyl methane sulfonyl fluoride (Sigma-Aldrich, 78830-5G). Proteins (20 to 60 μg) were size-fractionated by 12% SDS-PAGE, and then transferred to PVDF (polyvinyl difluoride) membranes (Millipore, ISEQ00010). After blocking, the membranes were incubated with primary antibodies: β-actin (1:1000, sc-69879, Santa Cruz), Bcl-2 (1:400, BA0412, Boster). After washing with Tris-buffered saline containing 0.1% Tween 20 (TBST; Beyotime, ST825), the blots were probed with secondary antibodies (ZB-2305 and ZB-2301, ZSGB-Bio, Beijing, China) for 1 h at room temperature. Target proteins were examined using enhanced chemiluminescence reagents (Millipore, WBKLS0500). Band intensity was determined by densitometry using Image J software. The relative intensity of each band was standardized to the corresponding internal control. For relative quantification, the intensity of each band was standardized to the corresponding internal control and normalized to the intensity of the band of control group.
Statistical analysis
All the experiments were repeated 3 times with 5 replicated samples each time. Data were expressed as mean plus or minus standard deviation. Statistical significance was determined by 1-way analysis of variance and subsequent Bartlett test. Differences were considered significant at P<0.05.
Results
Effects of salidroside pretreatment and HPC on anoxia-induced decrease of cell viability in PC12 cells
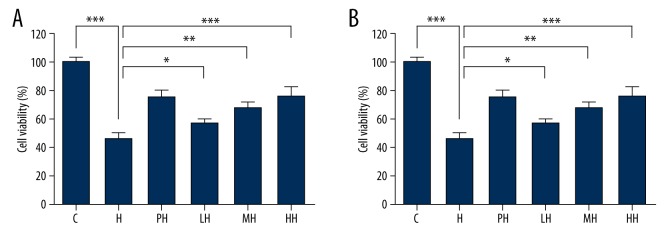
To investigate the damage of anoxia on PC12 cells, we exposed the cells to anoxic environment (2.5% O2 and 5% CO2) for 24 h; compared with the control group, cell viability (Figure 1A) was significantly decreased (48±1.36%). The preincubation of different concentrations of salidroside and HPC protected the cells from anoxic-induced damage and restored the cell viability to 73.73±2.08% or 69.15±1.28%, respectively. Salidroside at high concentration (15 μM) was more effective than HPC to protect PC12 cells in hypoxic conditions.
Figure 1.
PC12 cell viability determined using the conventional MTT assay and LDH release assay. (A) Effects of salidroside on the cell viability after cultured in hypoxia; (B) Effects of salidroside on LDH release of PC12 cells after cultured in hypoxia.(* P< 0.05, ** P<0.01 and *** P<0.001.)
LDH leakage (Figure 1B), as an indication of the number of damaged PC12 cells, was detected and analyzed. LDH leakage rate showed the visible statistical differences among the various experimental programs. The hypoxic condition significantly increased LDH release of PC12 cells from 11.17±1.32% to 36.77±2.45%. The HPC and salidroside groups exhibited resistance to hypoxia, and the most pronounced protective effect of salidroside occurred at 5 μM (LH), which led to the decrease in LDH release to 23.6±7.56%; and at 15 μM (HH), which led to the decrease in LDH release to 28.86%±8.94% – less than the hypoxia group (H, 36.78±6.78%). According to either MTT or LDH assay, salidroside pretreatment resulted in less cell viability survival and less LDH release in anoxic PC12 cells. Compared with HPC, which was recognized as beneficial to cellular resistance to anoxia, the results consistently indicated that salidroside pretreatment can lessen hypoxia-induced cell viability loss and increase LDH release in PC12 cells.
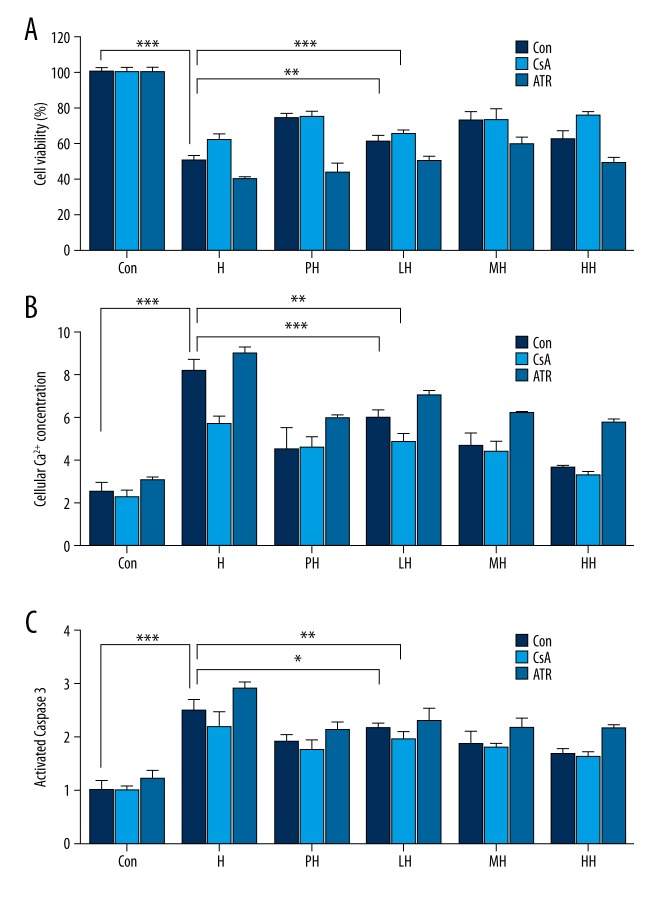
CsA was a strong inhibitor of mPTP, and the positive control was used to investigate the protection principle of HPC or salidroside. In the data, both the HPC and different-concentration salidroside pretreatment plus CsA (Figure 2A) can display a preferable efficiency in attenuating the hypoxia-mediated cellular damage. Contrarily, the ATR was also used and showed the more severe cell viability loss (Figure 2A). CsA and ATR suggested that mPTP was related to the hypoxia-induced loss of cell viability; maybe salidroside or HPC could inhibit mPTP opening, and enhance the resistance of PC12 cells to hypoxia.
Figure 2.
PC12 cell viability, cellular Ca2+ concentration and caspase- 3 activity were determined after hypoxia stimulation for 24 h with the preincubation of CsA and ATR. (A) Effects of salidroside on cell viability of hypoxia-induced PC12 cells with the preincubation of CsA and ATR; (B) Effects of salidroside on cellular Ca2+ concentration of hypoxia-induced PC12 cells with the preincubation of CsA and ATR; (C) Effects of salidroside on c caspase 3 activity of hypoxia-induced PC12 cells with the preincubation of CsA and ATR. (* P<0.05, ** P<0.01 and *** P<0.001.)
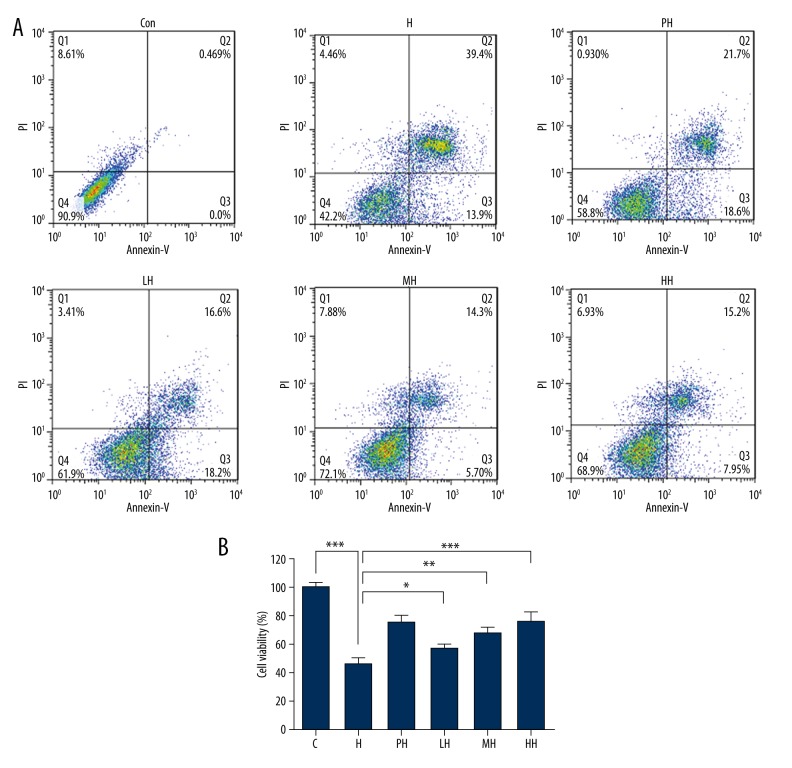
Effects of salidroside pretreatment and HCP on anoxia-induced apoptosis of PC12 cells (Figures 3 and 4)
Figure 3.
Flow cytometric analyses with annexin-V-FITC and PI label of PC12 cells in different incubation conditions. (A) Apoptosis determined by staining with annexin-V+PI. (B) The percentage of apoptotic PC12 cells in each group. (* P<0.05, ** P<0.01 and *** P<0.001 vs. con.)
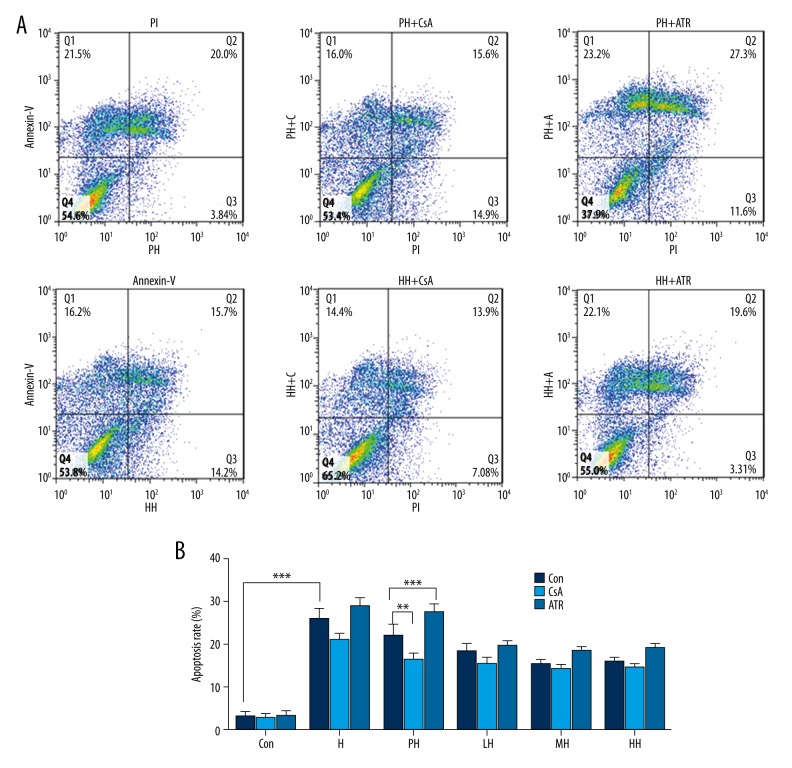
Figure 4.
Flow cytometric analyses with annexin-V-FITC and PI label of PC12 cells in different incubation conditions. (A) Apoptosis determined by staining with annexin-V+PI. (B) The percentage of apoptotic PC12 cells in each group. (*P< 0.05, **P<0.01 and ***P<0.001.).
Using flow cytometry, hypoxia-induced apoptosis of PC12 cells was consistently demonstrated, and the apoptotic cells were increased to 39.4% compared with control. Pretreatment with hypoxia and salidroside, in line with results of MTT and LDH release, reduced the cell damage effect of hypoxia on PC12 cells, respectively. The blocker and stimulant of mPTP (CsA and ATR) exhibited the appropriate pharmacologic characteristics to decrease and increase apoptosis, respectively.
Salidroside attenuates the hypoxia-induced decrease in MMP
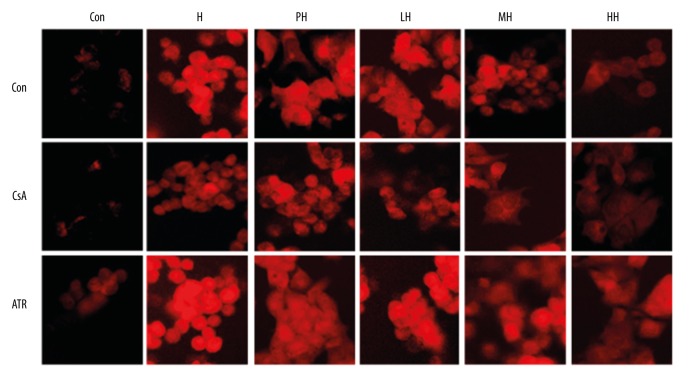
After the hypoxia, the depolarization of MMP appeared red by the fluorescent dye. Between the control group and CsA in the control group, the fluorescence intensity did not show obvious differences, but the ATR in the control group showed a slight increase. The results comparing the PH and H groups suggest HPC had no significant effect on MMP in hypoxia-induced PC12 cells. However, the fluorescence intensity of LH, MH, and HH was less than that in other groups, especially the HH group (Figure 5).
Figure 5.
The mitochondrial membrane potential (MMP) of PC12 cells was monitored by TMRE method, and the red emission reflected the change of MMP at 582 nm.
Salidroside inhibits anoxia-induced rise in intracellular Ca2+and caspase-3 activity
Intracellular Ca2+.as an important apoptotic trigger was monitored by the sensitive fluorescence probe FluoFort and spectrofluorometry. As illustrated in Figure 2B, anoxia induced a rise in Ca2+i level in the H group; certainly, salidroside more effectivre than HPC at inhibiting the rise in Ca2+i level in a dose-dependent manner.
Caspase-3 (Figure 2C) is a proapoptotic protein; its activation is an important step in apoptosis. The preincubation of salidroside and HPC partially attenuated the increase of the caspase-3 activity, also in a dose-dependent manner.
Effects of salidroside pretreatment on expression of Bcl-2 in anoxia-induced PC12 cells
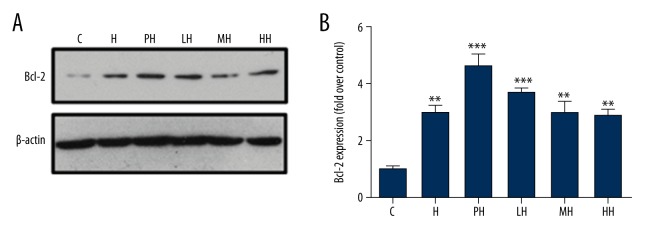
Immunoblot analysis (Figure 6) showed the expression of antiapoptotic protein Bcl-2 as in the mitochondrial fraction of the PC12 cells. The results demonstrated that Bcl-2 levels were up-regulated after anoxia culture by HPC or salidroside, however, the total amounts of Bcl-2 proteins of pretreatment with different concentrations of salidroside were not significantly different from HPC; even the MH group had the lesser expression amounts.
Figure 6.
Effects of salidroside on expression of apoptotic signaling proteins Bcl-2 of PC12 cells in different incubation conditions. (A) BcL-2 protein was analyzed by immunoblot; (B) The relative quantification of Bcl-2 in each group by corresponding internal control and the intensity of the band of control group. (* P<0.05, ** P<0.01 and *** P<0.001 vs. con).
Discussion
As the current study demonstrates, HPC can enhance the anoxic tolerance of some cultured cells and increase cell survival [13]. However, these findings are obviously difficult to apply to the need for day-to-day antihypoxia treatment, for example, for athletes, mountaineers, and highlands visitors. On the other hand, CsA and ATR, because of their special roles of mPTP and the effects for antidamage/prodamage by inducing hypoxia, served respectively as the positive control and the negative control in this study [14,15]. Although the action of CsA could be beneficial to suppress apoptosis, its function as a potent immunosuppressant carries undesirable adverse effects that seriously limit its application. There are also many restrictions for using HPC in the clinic (for unpredictable diseases such as cerebral hypoxia or ischemia) and in the activities of the highlands. Salidroside, a natural product extracted from the traditional Chinese and Tibetan medicine R rosea L, has shown some pharmacologic properties, including antagonism to drug-induced and simulated hypoxia or ischemia [9]. However, as an antihypoxic traditional Chinese and Tibetan medicine, whether and how salidroside affected PC12 cells with regard to hypoxia-induced apoptosis was unknown. The focus of this study, therefore, was to investigate that mechanism.
The cell viability and LDH leakage were detected after the hypoxic culture. The maintenance of cell viability and inhibition of LDH leakage showed the conservation of cell membrane integrity [9] and the loss of anoxia-induced cell death by salidroside. According to flow cytometry, it was just as predicted: the salidroside demonstrated suppression of hypoxia-caused apoptosis of PC12 cells. The consistent findings suggested that salidroside may protect PC12 cells from hypoxia injury; even in the middle- and high-dose groups, salidroside was more effective than HPC.
Mitochondria is a common pathway with a very important role in the regulation of cell necrosis and apoptosis; mitochondria also are known to be vulnerable targets of oxidative stress [4,16]. The hypoxia-induced mitochondrial dysfunction causes the opening of mPTP, followed by the depletion of ATP, the increase of reactive oxygen species; calcium overload; and release of proapoptotic proteins factors, such as apoptosis-inducing factor, cytochrome C, and various caspases [17]. This process plays a crucial role in the regulation of apoptosis, when cells have been exposed to hypoxia. Therefore, it is necessary to monitor and research the changes of mitochondrial function by mPTP, an electrical potential across the inner membrane created by H+ pumping during electron transfer and a key in mitochondrial bioenergetics [18]. The TMRE method was used for detecting MMP changes, which accumulate in the strongly negatively charged matrix of mitochondria in hypoxia-exposed cells and are shown by the red fluorescence. After 24 h of hypoxia, PC12 cells may initiate apoptosis/death-related procedures, with a rapid decrease of MMP, which was one of the primary signals in anoxia-induced cell apoptosis/death [4,19]. Consistent with this result, salidroside attenuated the decrease of MMP to some extent; salidroside plus CsA or ATR exhibit different and corresponding responses, this finding indicates that salidroside can prevent, at least in part, the deterioration of mitochondrial functions by stabilizing mitochondria and reducing MMP loss.
The balance of Ca2+I was directly related to the MMP and mitochondrial function [20], as an important target of apoptosis by leading to irreversible membrane, organelles, and chromatin damage [21,22]. In this study, our results showed that hypoxia evoked membrane depolarization, followed by influx of Ca2+ through Ca2+ channels [23] in PC12 cells, whereas salidroside pretreatment significantly blocked the elevation of Ca2+i. The antihypoxia protection of salidroside might be associated with the restoration of mitochondrial membrane Ca2+ pumps and inhibition of the calcium overload.
The caspase family plays a role in the initiation and execution of apoptosis. Although caspase-3 is the most critical apoptotic proteins in downstream of caspase cascade and elicits many nonapoptotic functions [24,25], caspace-3 is considered to be the common executor in the intrinsic and extrinsic apoptotic pathways in nucleated cells [5]. Salidroside pretreatment significantly antagonized the increase of caspase-3 activity, and CsA and ATR showed corresponding function. The salidroside could protect PC12 cells at the caspase-3 level or upstream of the activation of caspase cascade. The BcL-2 protein not only reduces mitochondrial membrane permeability, but also further inhibits release of cytochrome C, thereby inhibiting caspase-3 activity to reduce apoptosis [19,26]. As reported, we also found that HPC and salidroside increased antiapoptotic protein Bcl-2 expression in mitochondria. However, salidroside did not show a dose-dependent manner, while compared with H the total amount of Bcl-2 protein was unaffected in MH and HH group, regardless of pretreatment with or without salidroside. How Bcl-2blocked the degradation of MMP by two mechanisms: (I) directly or indirectly enhancing proton efflux, (II) preventing the apoptosis-associated permeability transition (PT) of the mitochondrial membrane [27]. The slight differences between Bcl-2 expression and antianoxia effects may be related to a number of factors such as cytokines, colony-stimulating factors, and mitogen [28].
The different pathways between salidroside and HCP will be the topic of one of our future studies. However, we cannot deny the possibility that under some pathologic conditions, such as application of salidroside at certain concentrations or for longer periods, salidroside protects PC12 cells against hypoxia-induced apoptosis by through regulation the Bcl-2 protein [29,30].
Rhodiola rosea L, as a traditional Chinese and Tibetan medicine, has been endorsed by our study results at the cellular level.
Conclusions
Our data indicate that salidroside administration could restrain the apoptosis induced by hypoxia in PC12 cells. The protective effects are mediated by the preservation of mitochondrial integrity, inhibiting the excessive Ca2+ influx and caspase-3 activity and promoting Bcl-2 overexpression. Compared with HPC, the protective pathway associated with salidroside provides a clinical potential therapeutic mechanism to reduce death following ischemic or hypoxic injuries.
Footnotes
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Source of support: This work was supported by grants from the National Natural Science Foundation of China (No. 81203000)
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Zhao T, Yu S, Ding A, et al. [Hypoxic preconditioning enhances hypoxic tolerance of hippocampal neurons and synaptic function of rat]. Acta physiologica Sinica. 2001;53(1):72–74. [in Chinese] [PubMed] [Google Scholar]
- 3.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813(7):1263–68. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol. 2004;287(2):H841–49. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 5.Qian EW, Ge DT, Kong S-K. Salidroside protects human erythrocytes against hydrogen peroxide-induced apoptosis. J Nat Prod. 2012;75(4):531–37. doi: 10.1021/np200555s. [DOI] [PubMed] [Google Scholar]
- 6.Lee S-Y, Li M-H, Shi L-S, et al. Rhodiola crenulata extract alleviates hypoxic pulmonary edema in rats. Evid Based Complement Alternat Med. 2013;2013:718739. doi: 10.1155/2013/718739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma H-P, Fan P-C, Jing L-L, et al. Anti-hypoxic activity at simulated high altitude was isolated in petroleum ether extract of Saussurea involucrata. J Ethnopharmacol. 2011;137(3):1510–15. doi: 10.1016/j.jep.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 8.Chen S-F, Tsai H-J, Hung T-H, et al. Salidroside improves behavioral and histological outcomes and reduces apoptosis via PI3K/Akt signaling after experimental traumatic brain injury. PLoS One. 2012;7(9):e45763. doi: 10.1371/journal.pone.0045763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Liu J, Gu X, Ding F. Salidroside attenuates glutamate-induced apoptotic cell death in primary cultured hippocampal neurons of rats. Brain Res. 2008;1238:189–98. doi: 10.1016/j.brainres.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: The essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75(3):530–35. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Sun Z, Sun S, et al. Effects of hypoxic preconditioning on synaptic ultrastructure in mice. Synapse. 2015;69(1):7–14. doi: 10.1002/syn.21777. [DOI] [PubMed] [Google Scholar]
- 12.Chen JC, Huang J, Gao Y, et al. Effects of hypoxia preconditioning on intracelluar Ca2+ of PC12 cells. Medical Journal of National Defending Forces in Northwest China. 2008;29(4):250–53. [Google Scholar]
- 13.Wang J-a, Chen T-l, Jiang J, et al. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin. 2008;29(1):74–82. doi: 10.1111/j.1745-7254.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- 14.Alessandri B, Rice AC, Levasseur J, et al. Cyclosporin A improves brain tissue oxygen consumption and learning/memory performance after lateral fluid percussion injury in rats. J Neurotrauma. 2002;19(7):829–41. doi: 10.1089/08977150260190429. [DOI] [PubMed] [Google Scholar]
- 15.Halestrap A, Doran E, Gillespie J, O’Toole A. Mitochondria and cell death. Biochemical Society Transactions. 2000;28(1):11. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- 16.Kroemer G. Mitochondrial control of apoptosis: An introduction. Biochem Biophys Res Commun. 2003;304(3):433–35. doi: 10.1016/s0006-291x(03)00614-4. [DOI] [PubMed] [Google Scholar]
- 17.Schulz R, Cohen MV, Behrends M, et al. Signal transduction of ischemic preconditioning. Cardiovasc Res. 2001;52(2):181–98. doi: 10.1016/s0008-6363(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 18.Quarato G, D’Aprile A, Gavillet B, et al. The cyclophilin inhibitor alisporivir prevents hepatitis c virus – mediated mitochondrial dysfunction. Hepatology. 2012;55(5):1333–43. doi: 10.1002/hep.25514. [DOI] [PubMed] [Google Scholar]
- 19.Wu L-y, Ding A-s, Zhao T, et al. Involvement of increased stability of mitochondrial membrane potential and overexpression of Bcl-2 in enhanced anoxic tolerance induced by hypoxic preconditioning in cultured hypothalamic neurons. Brain Res. 2004;999(2):149–54. doi: 10.1016/j.brainres.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 20.Crestanello JA, Doliba NM, Babsky AM, et al. Opening of potassium channels protects mitochondrial function from calcium overload. J Surg Res. 2000;94(2):116–23. doi: 10.1006/jsre.2000.5979. [DOI] [PubMed] [Google Scholar]
- 21.Hawrysh PJ, Buck LT. Anoxia-mediated calcium release through the mitochondrial permeability transition pore silences NMDA receptor currents in turtle neurons. J Exp Biol. 2013;216(23):4375–87. doi: 10.1242/jeb.092650. [DOI] [PubMed] [Google Scholar]
- 22.Trump B, Berezesky I. Calcium-mediated cell injury and cell death. FASEB J. 1995;9(2):219–28. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- 23.Domenech RJ, Sánchez G, Donoso P, et al. Effect of tachycardia on myocardial sarcoplasmic reticulum and Ca2+ dynamics: a mechanism for preconditioning? J Mol Cell Cardiol. 2003;35(12):1429–37. doi: 10.1016/j.yjmcc.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22(53):8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 25.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Ann Rev Biochem. 1999;68(1):383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 26.Garcia I, Martinou I, Tsujimoto Y, Martinou J-C. Prevention of programmed cell death of sympathetic neurons by the bcl-2 proto-oncogene. Science. 1992;258(5080):302–4. doi: 10.1126/science.1411528. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu S, Eguchi Y, Kamiike W, et al. Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc Natl Acad Sci USA. 1998;95(4):1455–59. doi: 10.1073/pnas.95.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui WA, Ahad A, Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch Toxicol. 2015;89(3):289–317. doi: 10.1007/s00204-014-1448-7. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Yu H, Sun Y, et al. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564(1):18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 30.Huang X, Zou L, Yu X, et al. Salidroside attenuates chronic hypoxia-induced pulmonary hypertension via adenosine A2a receptor related mitochondria-dependent apoptosis pathway. J Mol Cell Cardiol. 2015;82:153–66. doi: 10.1016/j.yjmcc.2015.03.005. [DOI] [PubMed] [Google Scholar]