Abstract
Purpose
Papillary thyroid microcarcinoma (MPTC) has an excellent prognosis. We aimed to evaluate the evolution of therapeutic strategies over time and the clinical outcome of MPTC.
Methods
In this retrospective multicenter observational study in a northwest Italian region, patients with intrathyroidal, unifocal tumor ≤1 cm in size, incidentally found at histology or preoperative cytology diagnosis, were included. Exclusion criteria were a previous head-and-neck irradiation and/or node metastases.
Results
From 1985 to 2012, 437 patients had an MPTC diagnosis, which was incidental in 85% and preoperative in 15%. Patients with a preoperative diagnosis were younger at the time of diagnosis (47.6 ± 12.7 years, p < 0.01) and had a larger tumor (7.0 ± 2.5 mm, p < 0.0001) than patients with an incidental diagnosis (age 52 ± 13.5 years, size 4.4 ± 2.8 mm), but there were no differences in clinical outcome between both groups. We observed a significant (p < 0.001) reduction in radioiodine remnant ablation during the years. TSH levels were: <0.1 mIU/l in 27.5%, 0.1-0.5 mlU/l in 33.7%, 0.5-2.5 mlU/l in 32.6%, 2.5-4.2 mlU/l in 3.9%, and >4.2 mlU/l in 2.3% of patients. Six patients (1.37%) had nodal recurrence; 5 of them were cured after therapy. MPTC-linked mortality was null.
Conclusions
We confirmed the favorable clinical outcome of MPTC. Despite the reduction in radioiodine ablation, overtreatment of MPTC is still observed.
Key Words: Clinical outcome, Papillary thyroid microcarcinoma, Prognosis, Therapeutic approach
Introduction
The incidence of papillary thyroid cancer (PTC) has almost doubled over the last three decades mainly due to the higher incidence of papillary thyroid microcarcinoma (MPTC) [1,2]. In Italy, a remarkably high PTC incidence has been reported [3]. MPTC is defined by the World Health Organization as a PTC with a maximum diameter of 10 mm [4]. In autopsy studies, the incidence of MPTC varies from 1 to 35.6% [5,6]. Most MPTCs are diagnosed incidentally after thyroid surgery. However, the widespread use of ultrasonography and fine-needle aspiration cytology (FNAC) markedly increased the rate of preoperative diagnosis [7]. It is still debated if preoperatively and incidentally diagnosed MPTC have different prognoses and should be managed differently [8]. Already in the 12th Annual Cancer Meeting in Porto, Portugal, on March 2003, in the document known as the ‘Porto Proposal’ [9], it was suggested that the term papillary ‘microcarcinoma’ could be replaced with papillary ‘microtumor’ only for a single focus ≤1 cm contained within the thyroid of an adult patient, found incidentally at thyroidectomy performed for other reasons. Despite the favorable outcome of MPTC, its management is still controversial and ranges from observation to total thyroidectomy [10]. MPTC mortality rate is very low, ranging from 0.2 to 2.2%. The 2015 American Thyroid Association Thyroid Cancer Management Guidelines [11] state that active surveillance ‘can be considered’ as an alternative to immediate surgery in very-low-risk patients. Nonsurgical observation has been suggested as an attractive alternative to surgery for asymptomatic MPTC [12] based on long-term follow-up studies [13,14,15,16,17]. Regarding radioactive iodine (RAI), there is agreement that patients with very-low-risk thyroid cancers (≤1 cm confined to the thyroid) do not require RAI. In addition, the TSH goal is a matter of debate [18]. According to the latest ATA guidelines [11], in patients free of disease, at low risk of recurrence, and without previous RAI, serum TSH should be kept within the low-normal range (0.5-2 mIU/l). In conclusion, single-focus intrathyroidal PTC ≤1 cm without prior head-and-neck irradiation or node metastases has an excellent prognosis and must be considered at very low risk, as clearly stated in the recent guidelines [11]. However, in clinical practice, overtreatment is still likely to occur.
The aim of the present retrospective, multicenter study was to evaluate the therapeutic strategies, their evolution over time, and clinical outcome of MPTC in a cohort of patients followed in five referral centers of Liguria, a northwest Italian region, from 1982 to December 2012.
Patients and Methods
Patients
This retrospective multicenter study involved five Ligurian referral centers of endocrinology, nuclear medicine, and internal medicine with endocrinology degree, covering a catchment area of 878,000 inhabitants. According to the Porto Proposal [10], histological and clinical inclusion criteria were: tumor size ≤1 cm, limited to the thyroid, unifocal tumor, no prior head-and-neck irradiation, absence of cervical node metastases, and incidental diagnosis after surgery for benign thyroid disease or diagnosis on specimens from surgery performed for suspicious cytology. We identified 437 patients with MPTC, classified as stage 1 according to American Joint Committee on Cancer classification, fulfilling the above inclusion criteria. We excluded patients with differentiated thyroid carcinoma <1 cm which was multifocal (pT1m) or patients with cervical node (N1) or distant metastasis (M1) at the time of diagnosis. Three hundred fifty-eight patients were female (mean age 51 ± 14 years, range 14-82 years), and 79 were male (mean age 51 ± 14 years, range 15-75 years). Mean age at diagnosis was 51 ± 13.7 years.
Protocol and Assays
From medical records, the following parameters were evaluated: diagnosis (incidental or after FNAC), extent of primary neck surgery (total/near-total thyroidectomy or lobectomy), possible RAI, serum TSH at the time of the last control follow-up, survival and recurrence rates throughout ultrasonography, and thyroglobulin (Tg) and anti-Tg autoantibody levels. In RAI-treated patients, Tg levels after recombinant human TSH (rhTSH) treatment (two consecutive daily doses of 0.9 mg i.m.; Genzyme Co., Cambridge, Mass., USA) were recorded 1 year after primary treatment. Since this is a multicenter study which has been extended over a wide range of years, different standardized and certified methods were used to assay TSH and Tg. For TSH, 0.3-4.2 mIU/l was assumed as the normal range. Concerning Tg assays, the large majority of Tg values were obtained before the introduction of ‘ultrasensitive’ methods. Based on the functional sensitivities of the methods used, we selected 0.5 ng/dl as the cutoff value discriminating undetectable from detectable basal Tg. Tg antibodies were assessed by quantitative immunometric assays and defined as ‘negative’ when not exceeding the positive cutoff value currently used in each single laboratory.
Statistical Analysis
SPSS for Windows 20.0 (SPSS Inc., Chicago, Ill., USA) and GraphPad Prism 5 (San Diego, Calif., USA) were used for statistical analyses. Results are expressed as means ± SD [or medians (ranges)] for quantitative variables and as percentages for qualitative variables. Differences between frequencies were evaluated by χ2 test. Comparisons between means were computed by t test (two tailed). Possible differences in outcome between the subgroups of incidental MPTC and a preoperative diagnosis of MPTC were evaluated by Kaplan-Meier analysis. Differences were regarded as statistically significant at p < 0.05. Informed consent for their clinical data management was obtained from all individual patients included in the study.
Results
Histological, Clinical, and Biochemical Features
We identified 437 MPTC. Average size was 5.1 ± 2.9 mm (range 1-10 mm). Based on histology, 383 (87.6%) patients were classified as papillary carcinoma with ‘classical’ features, 50 (11.4%) patients as follicular variant of papillary carcinoma, and 4 (1%) patients as PTC with uncertain potential of malignancy.
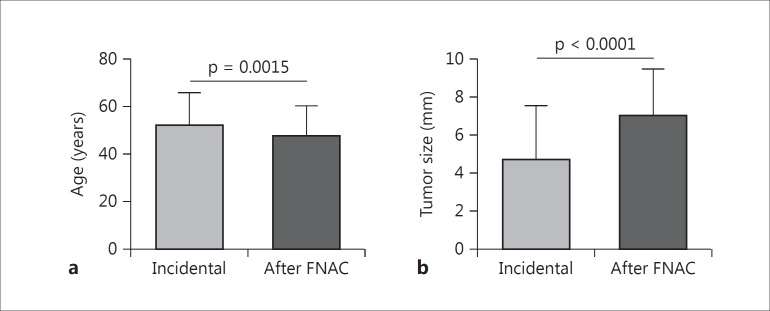
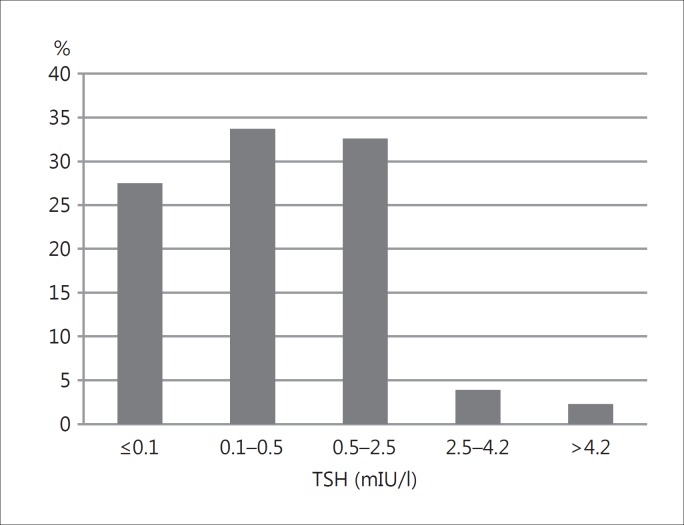
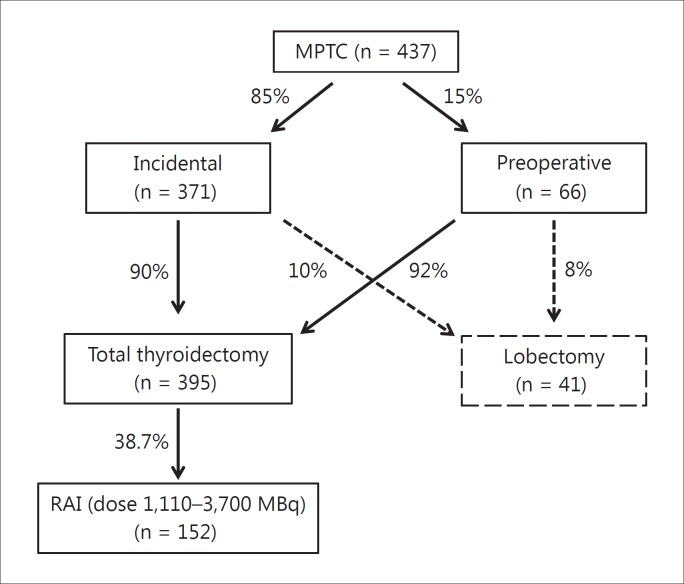
Women constituted the majority of the study group (n = 358/437, 81.9%). Among the 437 patients, 396 (90.6%) underwent total/near-total thyroidectomy and 41 (9.4%) lobectomy. The diagnosis was incidental in 85% of the patients (n = 371) after surgery for symptomatic benign disease (multinodular goiter or Graves’ disease); mean age at diagnosis was 52 ± 13.5 years, and tumor size was 4.7± 2.8 mm. Ninety percent of these patients (n = 335) underwent total/near-total thyroidectomy and 10% (n = 36) lobectomy. Sixty-six (15%) patients had a preoperative cytology diagnosis suspicious for malignancy; mean age at diagnosis was 47.6 ± 12.7 years, and tumor size was 7.0 ± 2.5 mm. Sixty-one (92%) of them underwent total/near-total thyroidectomy, and 5 (8%) lobectomy. We found a significant difference between both patient subgroups in terms of age at diagnosis (p = 0.0015) and tumor size (p < 0.0001) (fig. 1). At the last evaluation, levothyroxine dosage was 830.6 ± 202.3 μg/week (range 125-1,500 μg/week) and TSH was 1.07 ± 4.9 mIU/l (range 0.05-85 mIU/l). We also subdivided the patients according to their TSH levels at the time of the last visit recorded: in 27.5% (n = 120), we found ‘suppressed’ TSH levels (≤0.1 mIU/l), in 33.7% (n = 146) ‘semi-suppressed’ TSH levels (0.1 mIU/l < TSH ≤ 0.5 mIU/l); in 32.6% (n = 142), we found 0.5 mIU/l < TSH ≤2.5 mIU/l; in 3.9% (n = 17), we found 2.5 < TSH ≤ 4.2 mIU/l, and, finally, 2.3% (n = 12) presented TSH levels above the upper limit of normal (TSH >4.2 mIU/l) (fig. 2).
Fig. 1.
Differences in terms of age at diagnosis (a) and tumor size (b) between patients with the incidental diagnosis performed for benign thyroid disease and the preoperative diagnosis after FNAC.
Fig. 2.
Percentage distribution of TSH levels at the last evaluation.
RAI Remnant Ablation and Tg Levels
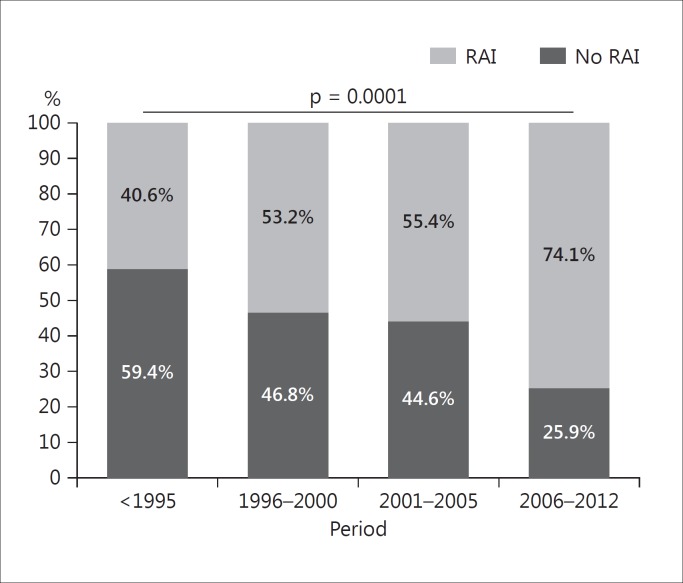
Among 396 patients who underwent total/near-total thyroidectomy, 152 (38.7%) underwent RAI, with 131 activities ranging from: 1,110 to 3,700 MBq. We stratified them according to the year of treatment: 59.4% (19/32) of patients underwent RAI before 1995, 46.8% (29/62) between 1996 and 2000, 44.6% (62/139) between 2001 and 2005, and 25.9% (42/162) between 2006 and 2012. The reduction over time was significant (p = 0.0001), notably when comparing those treated before 1995 with the most recent ones (fig. 3). Figure 4 depicts a schedule of the MPTC therapeutic approach. As expected, we found significantly (p < 0.0001) more serum elevated Tg levels in untreated patients (2.1 ± 7.15 ng/ml) than in patients treated with RAI (Tg 0.5 ± 0.74 ng/ml). Among patients who underwent RAI, 104 had assessment of serum Tg and whole-body scan (WBS) after rhTSH stimulation at different years from diagnosis. In 98 patients, peak serum Tg after rhTSH stimulation was <1 ng/ml and diagnostic WBS showed no pathological uptake. In 5 patients, Tg levels had a rise >1 ng/ml after rhTSH, but WBS and neck ultrasonography were negative. One of them (table 1; patient 1) was further treated with RAI 4 years after diagnosis based on significantly raised Tg levels after rhTSH (14.3 ng/ml). According to rhTSH stimulation performed 1 year after the second RAI, this patient has been considered cured. In another patient (table 1; patient 6), rhTSH stimulation performed 5 years after diagnosis showed peak Tg levels of 3.7 ng/ml, with negative WBS and an ultrasonographically suspicious node, which was 18FDG-PET positive. He underwent surgery, but was subsequently lost to follow-up.
Fig. 3.
Treatment with RAI remnant ablation among different time periods.
Fig. 4.
Schedule of the MPTC therapeutic approach.
Table 1.
Characteristics of the patients with recurrence
| Pt | Surgery | Age at Dx, years | Way of Dx | Size, mm | RAI remnant ablation | Recurrence |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| years after Dx | side | treatment | Tg after rhTSH, ng/ml | likely cured | ||||||
| 1 | Tx tot | 15 | incidental | 4 | 1,110 MBq | 4 | not founda | RAI (3,700 MBq) | 0.3 | yes |
| 2 | Tx tot | 36 | incidental | 6 | no RAI | 4 | local | RAI (2,960 MBq) | 0.5 | yes |
| 3 | Tx tot | 75 | incidental | <10 | no RAI | 5 | node mts | surgery + RAI | n.a. | yes |
| 4 | Tx tot | 55 | after FNAC | 10 | 2,960 MBq | 8 | node mts | surgery + RAI | 0.7 | yes |
| 5 | Tx tot | 76 | incidental | <10 | no RAI | 1 | local | RAI (3,700 MBq) | 0.1 | yes |
| 6b | Tx tot | 75 | incidental | 9 | 2,960 MBq 5,550 MBq | 5 | node mts PET pos. WBS neg. | surgery | 3.7 | n.a. |
Pt = Patient; Tx tot = total thyroidectomy; Dx = diagnosis; mts = metastasis; PET = 18FDG-PET; n.a. = data not available.
Patient 1 was treated on the basis of raised Tg levels after rhTSH stimulation (14.3 ng/ml).
Patient 6 had two RAI treatments.
Long-Term Follow-Up: Recurrence and Mortality Rates
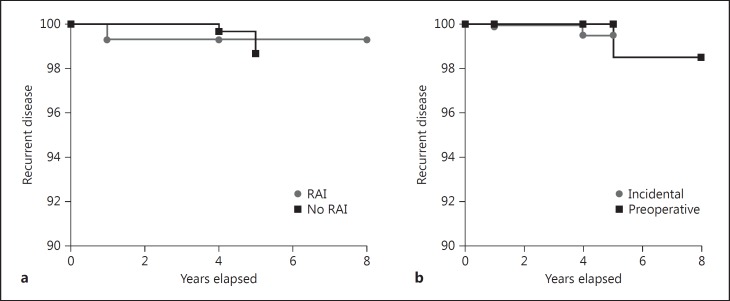
Follow-up ranged from 2 to 24 years (mean 5.8 ± 4.8 years); 34 patients were lost to follow-up 1 year after primary treatments. Neck ultrasonography performed during follow-up was negative in all but 9 patients: in 2 patients who underwent total thyroidectomy not followed by RAI, round suspicious nodes <1 cm size were described: cytology with Tg measurement in the needle washout was negative for recurrence; in 1 patient who underwent lobectomy, a nodule sized 1 cm in the residual gland was described: cytology revealed a benign lesion (Thy 2); 6 female patients (prevalence 1.37%) had recurrence: in 1 patient (table 1; patient 5), ultrasonography 1 year after primary therapy revealed recurrent disease; the others developed recurrence several years after diagnosis. Five patients can currently be considered cured after therapy. Some characteristics of this subgroup of patients are resumed in table 1. At the time of the last visit, all the patients but 1 were alive, who died at the age of 86 years for causes not related to MPTC. No significant differences were found in clinical and biochemical outcome between subgroups of incidental and preoperative MPTC and between subgroups of patients treated with or without RAI (fig. 5).
Fig. 5.
Kaplan-Meyer curves between subgroups of patients: incidental vs. preoperative diagnosis of MPTC (b) and with RAI versus without RAI treatment (a). No significant difference was found.
Discussion
In this retrospective study, we investigate the clinical presentation (incidental or after FNAC), therapeutic approach, follow-up, and clinical outcome of MPTC over a quite long period. Follow-up ranged from 1 to 20 years: during this time period, the recommendations regarding the therapeutic approach of MPTC have evolved [11,19,20]. The data on the MPTC cohort studied have been collected in referral centers of Liguria, with a strong prevalence of the metropolitan area of Genoa. In our study, MPTC diagnosis was predominantly incidental after surgery performed for symptomatic benign disease; only in a minority of cases, diagnosis resulted from preoperative cytological diagnosis, in agreement with 2010 guidelines [21], recommending FNAC in nodules ≤1 cm only with ultrasound findings suspicious for malignancy. At variance, 2015 ATA guidelines [11] strongly discourage FNAC of asymptomatic subcentimeter nodules, even if ultrasonographically suspicious, endorsing ultrasonography follow-up with cytology only if there is evidence of disease progression.
There is agreement that MPTC do not need RAI [22]. We observed a significant reduction in MPTC in patients undergoing RAI remnant ablation during time: from 59.4% before 1995 to 26.2% in the last period (2006-2012). However, about a quarter of the patients are still overtreated with RAI up to recent years. Concerning levothyroxine treatment, current guidelines [11] recommend in patients clinically free of disease not undergoing RAI remnant ablation, notably those at low risk, target TSH values within the low-normal range (0.5-2 mIU/l), avoiding TSH suppression (<0.1 mIU/l), to prevent long-term adverse effects especially on the heart [23] and bones [24]. However, in our population, we observed levothyroxine overtreatment: as many as 27.5% had suppressed TSH and only 32.6% had TSH within the low-normal range at the time of the last visit.
There is controversy as to whether incidental and nonincidental MPTC present different outcomes and require different treatment. A recent meta-analysis [8] of 3,523 MPTC showed that incidental MPTC had different clinical features and a much lower recurrence rate than nonincidental MPTC. However, this meta-analysis also included MPTC with characteristics less stringent than those suggested by the Porto Proposal. In our series, patients with a preoperative diagnosis of MPTC had larger tumors (7.0 ± 2.5 mm) than those with an incidental diagnosis (4.7± 2.8 mm). Besides, patients with an incidental diagnosis after surgery were older at the time of diagnosis (52 ± 13.5 years) than those with a preoperative diagnosis (47.6 ± 12.7 years). This is expected, as the incidence rates of goiter and its complications, which could lead to surgery, increase with age. Despite the difference in size and age at diagnosis, we did not observe any differences in clinical outcome and recurrence rates between the two subgroups (fig. 5). Therefore, size ≤1 cm, in the absence of metastasis or multifocality, according to the inclusion criteria of this study, seems to be the best prognostic factor. The concomitant presence of multinodular goiter or preoperative cytology suspicious for malignancy seems to be irrelevant in term of prognosis. In summary, the differences in the inclusion criteria might explain the differences between our results and the meta-analysis of Mehanna et al. [8]. According to the literature, our study confirmed the excellent prognosis of MPTC, with no mortality rate and 1.37% recurrence. In several studies [25,26], microcarcinomas with BRAF V600E mutation were associated with aggressive behavior. A recent multicenter study [27] suggested that BRAF V600E may represent a surrogate marker for an increased clinical risk. However, the rarity of MPTC recurrence and progression, which was also observed in our study, raises doubts about the usefulness of molecular biology screening aimed at identifying potentially aggressive forms of microcarcinoma. Current guidelines, in fact, classified intrathyroidal papillary microcarcinoma harboring the V600E mutation with no other worrisome features as low-risk tumors [11]. The observation of rare recurrences years after diagnosis suggests a long-term and low-cost follow-up (annual neck ultrasound and thyroglobulin levels) to early detect an increasing trend.
In conclusion, we studied a large cohort of MPTC and observed overtreatment up to recent years, which should be avoided considering the excellent prognosis of MPTC. Reversal of this trend should be encouraged to avoid adverse effects of unnecessary therapies and reduce health care costs.
Disclosure Statement
The authors have no conflict of interest.
References
- 1.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980-2008. Thyroid. 2013;23:103–110. doi: 10.1089/thy.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 3.Dal Maso L, Lise M, Zambon P, Falcini F, Crocetti E, Serraino D, Cirilli C, Zanetti R, Vercelli M, Ferretti S, Stracci F, De Lisi V, Busco S, Tagliabue G, Budroni M, Tumino R, Giacomin A, Franceschi S, AIRTUM Working Group Incidence of thyroid cancer in Italy, 1991-2005: time trends and age-period-cohort effects. Ann Oncol. 2011;22:957–963. doi: 10.1093/annonc/mdq467. [DOI] [PubMed] [Google Scholar]
- 4.Livolsi VA, Saavedra JA. Papillary carcinoma. In: De Lellis RA, Lloyd RV, Heitz PU, Eng C, editors. World Health Organization. Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon: IARC Press; 2004. pp. 57–60. [Google Scholar]
- 5.Bramley MD, Harrison BJ. Papillary microcarcinoma of the thyroid gland. Br J Surg. 1996;83:1674–1683. doi: 10.1002/bjs.1800831206. [DOI] [PubMed] [Google Scholar]
- 6.Hay ID, Hutchinson ME, Gonzalez-Losada T, Mclver B, Reinalda ME, Grant CS, Thompson GB, Sebo TJ, Goellner JR. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008;144:980–988. doi: 10.1016/j.surg.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid. 2015;25:1127–1136. doi: 10.1089/thy.2015.0116. [DOI] [PubMed] [Google Scholar]
- 8.Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, McCabe C, Boelaert K, Franklyn JA. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab. 2014;99:2834–2843. doi: 10.1210/jc.2013-2118. [DOI] [PubMed] [Google Scholar]
- 9.Rosai J, LiVolsi VA, Sobrinho-Simoes M, Williams ED. Renaming papillary microcarcinoma of the thyroid gland: the Porto Proposal. Int J Surg Pathol. 2003;11:249–251. doi: 10.1177/106689690301100401. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Huang T. Papillary thyroid microcarcinoma: an over-treated malignancy? World J Surg. 2016;40:764–765. doi: 10.1007/s00268-015-3244-9. [DOI] [PubMed] [Google Scholar]
- 11.Haugen BR, Alexander EK, Bible KC, Doherty G, Mandel SJ, Nikiforov YE, Pacini F, Randolph G, Sawka A, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward D, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang BH, Wong CK. A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur J Endocrinol. 2015;173:367–375. doi: 10.1530/EJE-15-0454. [DOI] [PubMed] [Google Scholar]
- 13.Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, Masuoka H, Yabuta T, Fukushima M, Higashiyama T, Kihara M, Kobayashi K, Miya A. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016;26:150–155. doi: 10.1089/thy.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugitani I, Fujimoto Y. Management of low-risk papillary thyroid carcinoma: unique conventional policy in Japan and our efforts to improve the level of evidence. Surg Today. 2010;40:199–215. doi: 10.1007/s00595-009-4034-5. [DOI] [PubMed] [Google Scholar]
- 15.Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010;34:1222–1231. doi: 10.1007/s00268-009-0359-x. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K, Miya A. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 17.Pacini F. Observation for newly diagnosed micro-papillary thyroid cancer: is now the time? J Endocrinol Invest. 2014;38:101–102. doi: 10.1007/s40618-014-0200-8. [DOI] [PubMed] [Google Scholar]
- 18.Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014;38:673–678. doi: 10.1007/s00268-013-2335-8. [DOI] [PubMed] [Google Scholar]
- 19.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 20.Pacini F. Thyroid microcarcinoma. Best Pract Res Clin Endocrinol Metab. 2012;26:381–389. doi: 10.1016/j.beem.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Gharib H, Papini E, Paschke R, Duick DS, Valcavi R, Hegedüs L, Vitti P. AACE/AME/ETA Task Force on Thyroid Nodules: American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16:1–43. doi: 10.4158/10024.GL. [DOI] [PubMed] [Google Scholar]
- 22.Lamartina L, Durante C, Filetti S, Cooper DS. Low-risk differentiated thyroid cancer and radioiodine remnant ablation: a systematic review of the literature. J Clin Endocrinol Metab. 2015;100:1748–1761. doi: 10.1210/jc.2014-3882. [DOI] [PubMed] [Google Scholar]
- 23.Faber J, Selmer C. Cardiovascular disease and thyroid function. Front Horm Res. 2014;43:45–56. doi: 10.1159/000360558. [DOI] [PubMed] [Google Scholar]
- 24.Wang LY, Smith AW, Palmer FL, Tuttle RM, Mahrous A, Nixon IJ, Patel SG, Ganly I, Fagin JA, Boucai L. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid. 2015;25:300–307. doi: 10.1089/thy.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virk RK, Van Dyke AL, Finkelstein Prasad A, Gibson J, Hui P, Theoharis CG, Carling T, Roman SA, Sosa JA, Udelsman R, Prasad ML. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype-phenotype correlation. Mod Pathol. 2013;26:62–70. doi: 10.1038/modpathol.2012.152. [DOI] [PubMed] [Google Scholar]
- 26.Bastos AU, Oler G, Nozima BH, Moysés RA, Cerutti JM. BRAF V600E and decreased NIS and TPO expression are associated with aggressiveness of a subgroup of papillary thyroid microcarcinoma. Eur J Endocrinol. 2015;173:525–540. doi: 10.1530/EJE-15-0254. [DOI] [PubMed] [Google Scholar]
- 27.Tallini G, de Biase D, Durante C, Acquaviva G, Bisceglia M, Bruno R, Bacchi Reggiani ML, Casadei GP, Costante G, Cremonini N, Lamartina L, Meringolo D, Nardi F, Pession A, Rhoden KJ, Ronga G, Torlontano M, Verrienti A, Visani M, Filetti S. BRAF V600E and risk stratification of thyroid microcarcinoma: a multicenter pathological and clinical study. Mod Pathol. 2015;28:1343–1359. doi: 10.1038/modpathol.2015.92. [DOI] [PubMed] [Google Scholar]