Abstract
Background/Aim
Operating far from its equilibrium resting point, the thyroid gland requires stimulation via feedback-controlled pituitary thyrotropin (TSH) secretion to maintain adequate hormone supply. We explored and defined variations in the expression of control mechanisms and physiological responses across the euthyroid reference range.
Methods
We analyzed the relational equilibria between thyroid parameters defining thyroid production and thyroid conversion in a group of 271 thyroid-healthy subjects and 86 untreated patients with thyroid autoimmune disease.
Results
In the euthyroid controls, the FT3-FT4 (free triiodothyronine-free thyroxine) ratio was strongly associated with the FT4-TSH ratio (tau = −0.22, p < 0.001, even after correcting for spurious correlation), linking T4 to T3 conversion with TSH-standardized T4 production. Using a homeostatic model, we estimated both global deiodinase activity and maximum thyroid capacity. Both parameters were nonlinearly and inversely associated, trending in opposite directions across the euthyroid reference range. Within the panel of controls, the subgroup with a relatively lower thyroid capacity (<2.5 pmol/s) displayed lower FT4 levels, but maintained FT3 at the same concentrations as patients with higher functional and anatomical capacity. The relationships were preserved when extended to the subclinical range in the diseased sample.
Conclusion
The euthyroid panel does not follow a homogeneous pattern to produce random variation among thyroid hormones and TSH, but forms a heterogeneous group that progressively displays distinctly different levels of homeostatic control across the euthyroid range. This suggests a concept of relational stability with implications for definition of euthyroidism and disease classification.
Key Words: Thyrotropin, Thyroid hormones, Thyrotropin feedback control, Thyroid homeostasis
Introduction
Attainment of a physiologically adequate thyroidal state relies on the ability of the organism to (1) sufficiently raise the hormone output of the thyroid gland to a level appropriate for health and (2) supply the various tissues with the amount of hormone required for their proper functioning. The basal unstimulated production rate of the thyroid gland is insufficient by itself to maintain healthy concentrations of thyroid hormones. Only through appropriate glandular stimulation by the pituitary hormone thyrotropin (TSH) will the levels rise adequately to satisfy the needs of the body. To prevent overstimulation, the system is regulated tightly via multiple negative feedback loops that monitor hormone concentrations and adjust the required stimulatory response [1]. Therefore, as the thyroid gland operates far from the equilibrium position of its unstimulated resting point, there is always a certain degree of physiological pressure on the system. The human thyroid gland directly produces mainly thyroxine (T4) together with a smaller proportion of triiodothyronine (T3) [2]. However, the latter is the biologically more active thyroid hormone, and activation of T4 is therefore required during entry of the hormones into the cells involving enzymatic monodeiodination converting T4 into T3. Contrary to what has long been believed, tissue distribution and accumulation of intracellular T3 is not facilitated by passive diffusion, but depends on active transmembrane transport [3].
From these principles, it follows that a certain free T4 (FT4) level inherently reflects the degree of TSH stimulation that is exerted by the system on the thyroid gland. Thus, when measuring FT4 and TSH in the circulation by thyroid function tests, we are not determining parameters independent of each other, but instead measuring their equilibria which are homeostatically determined and strongly interdependent [1]. A similar relationship exists between free T3 (FT3) and FT4, which also maintain interdependent equilibrium positions. While allowing for greater flexibility in the physiological responses of individuals across the euthyroid reference range, this may demand significant variation in the expression of control mechanisms [1].
In the present study, we hypothesized that individual stability within the reference range of the circulating hormones may result from the expression of different homeostatic equilibria by healthy subjects. This may help balance potential influences that may disturb normal thyroidal function in different ways, depending on the subject's current placement within the range. We therefore explored the concept of relational stability whereby T3 levels are maintained by examining the various interrelationships in euthyroid subjects and patients with autoimmune thyroiditis.
Methods
Patients
The present study is a secondary analysis in two groups of patients: (1) thyroid-healthy and otherwise healthy controls (n = 271), who showed no clinical evidence of the presence of thyroid dysfunction based on patient history, symptoms, physical examination, antibody status, and thyroid imaging, and (2) untreated patients with thyroid autoimmune disease (n = 86), as evidenced by a positive test result for thyroid peroxidase antibodies (TPO Ab). Patients with potentially interfering comorbidities such as nonthyroidal illness, renal insufficiency, pituitary disease, and pregnancy were ineligible or excluded, as were hospitalized patients and patients on thyroid hormone replacement. Patient characteristics for the groups are reported in Results. Samples were collected as part of a previously published prospective trial (www.ClinicalTrials.gov, NCT 01969552) involving 1,912 consecutively seen adult patients [4]. The study was approved by the local ethics committee and all participants gave written informed consent. Available information included details on patient history, thyroid medication, other medication, thyroid associated symptoms, a thyroid-related physical examination, and standardized questionnaire documenting gender, age, height, weight, smoking habits (75% answered), prior surgery or radioiodine treatment, laboratory tests for FT3, FT4, TSH, and TPO Ab, plus in elected cases TSH-receptor antibodies (TSH-R Ab) and an ultrasound of the thyroid gland. Blood samples were drawn during the day from late morning to early afternoon.
Thyroid Ultrasonography and Volumetry
Thyroid size, nodularity, and echogenicity were assessed in all subjects by ultrasonography (10-MHz transducer). Thyroid volume was estimated according to the ellipsoid formula (longitudinal diameter × width × depth × 0.5 cm3) for each lobe and addition of the lobe volumes. Reference values are <18 ml for female and <25 ml for male subjects.
Laboratory Methods
Thyroid function tests were performed at a single accredited institution, the Institute of Laboratory Medicine of Klinikum Luedenscheid. Standard laboratory quality procedures were routinely employed, and regular participation in interlaboratory tests were part of the quality management strategy [5]. The laboratory-evaluated reference intervals used for routine diagnostics were as follows: 0.4-4 mIU/l for TSH, 3.1-6.8 pmol/l for FT3, 10-23 pmol/l for FT4, <60 IU/ml for TPO Ab, and for <2 IU/l TSH-R Ab.
TSH was measured with an automated direct chemiluminescence method (third generation, TSH3-Ultra, ADVIA Centaur XP; Siemens Healthcare Diagnostics, Erlangen, Germany). Briefly, the standard curve was calibrated with the 3rd International Standard of the World Health Organization for Human TSH (IRP 81/565). It showed a functional sensitivity of 0.008 mIU/l. Intra-assay coefficients of variation (CVs) in pooled serum samples in the range from 0.52 mIU/l to 132.8 mIU/l (n = 20) were 1.4-2.4%. Interassay imprecision measured in duplicate over 10 consecutive days was 0.9-2.9%. At a TSH value of 0.52 mIU/l, the intra-assay CV was 1.4% and the interassay CV was 2.2%, but at the functional sensitivity (TSH of 0.008 mIU/l) the interassay CV rose to 14.1% [5].
FT3 and FT4 were measured with an automated competitive chemiluminescence method (FT4, FT3, ADVIA Centaur XP; Siemens Healthcare Diagnostics). Serum samples with FT3 concentrations ranging from 2.9 to 14.2 pmol/l showed intra-assay CVs from 2.4 to 3.1% and interassay CVs from 2.3 to 3.9%. Serum samples with FT4 concentrations in the range from 9.3 to 38.8 pmol/l showed intra-assay CVs from 2.2 to 3.3% and interassay CVs from 2.5 to 4.0% [5].
TPO Abs were measured with an automated competitive chemiluminescence method (Anti-TPO, ADVIA Centaur XP; Siemens Healthcare Diagnostics). TSH-R Abs were measured with a competitive ELISA (Anti-TSH-Receptor; EUROIMMUN AG, Luebeck, Germany).
Interrelational Measures
As a simple estimate of the conversion of T4 to T3, we used the FT3-FT4 ratio of the molar serum concentrations of the hormones. We divided FT4 by TSH (FT4-TSH ratio) to get a standardized estimate of T4 production per unit TSH.
In addition to these crude ratios, more refined measures on global deiodinase activity (SPINA-GD) and maximum thyroid capacity (SPINA-GT) were derived according to a mathematical model of thyroid hormone homeostasis, as has been previously described [6,7] and recently reviewed [8]. The SPINA parameters have been validated in a number of studies in different populations comprising together more than 10,000 subjects [6,7,8,9,10,11]. They can be calculated with free open source software (SPINA Thyr 4.0), available from sourceforge.net (RRID:SCR_014352, http://spina.sf.net).
Briefly, SPINA-GD estimates the maximum global activity of peripheral deiodinases per unit of time (termed deiodinase activity, nmol/s) from equilibrium levels of FT3, FT4, and estimated constants for plasma protein binding, distribution, and elimination with
SPINA-GT delivers the maximum secretory capacity of the thyroid gland, i.e. the stimulated amount of T4 released per unit of time (termed ‘thyroid capacity’, pmol/s), based on equilibrium levels of TSH and FT4 as follows:
Constants for hormone kinetics and plasma protein binding used in these equations have been reported [6,11]. The specific GT refers to SPINA-GT divided by thyroid volume (pmol/s/ml).
For further background information and additional methodological details including a Monte Carlo evaluation in comparison with conventional hormone measurements, we refer to recent review articles [6,8]. As for biological variation, when remeasuring the parameters after an average of 8 months in 108 euthyroid subjects, the intraindividual coefficient of variation for GT was only 16%, compared to 20% for TSH in the same subjects.
Statistical Methods
Descriptive data are shown as means (SD) or medians (interquartile range). Non-normally distributed TSH values were natural logarithmically transformed (lnTSH). Between-group comparisons for continuous variables were based on Welch's t test or, if normality could not be assumed, Wilcoxon's rank-sum test. A χ2 test with Yates' correction for continuity was used for categorical variables. Variables were considered explanatory and therefore no adjustments were made for multiple comparisons. In the control group, we obtained a reference interval for each single parameter, based on the 95% CI after prior removal of outliers by Horn's method. While the Pearson product-moment correlation was used for simple correlations, nonlinear relationships were tested using Kendall's tau rank correlation. Differences in slopes were tested for significance by the paired correlation test. A generalized linear model fitted by maximum likelihood was used to analyze linear and nonlinear (third-degree polynomial) relationships between two variables.
In some tests (e.g. in the correlation between SPINA-GT and SPINA-GD) FT4 is part of both the dependent and the independent expression. In order to correct for spurious correlations in these cases, FT4 values were randomly permuted in the dataset, thus delivering physiologically decoupled test values and spurious correlations only. Subsequently, associations based on true and permuted values were compared with a paired correlation test based on Fisher's z. All tests were two-sided with p < 0.05 denoting statistical significance. We used the R statistical software base package (version 3.2.3 for Mac) together with JGR 1.1-18, Deducer 0.7-9, and psych 1.5-8 [12,13,14].
Results
Patient characteristics for the control group (n = 271) and untreated disease sample (n = 86) are shown in table 1. The 95% confidence limits for the various parameters in the control group after removing three outliers representing reference intervals are also given in the table.
Table 1.
Characteristics of the control group and untreated patients with thyroid autoimmune disease
| Parameter | Control group |
TAD |
p value2 | |
|---|---|---|---|---|
| mean (SD) or median [IQR]1 (n = 271) | 95% CI1 | mean (SD) or median [IQR] (n = 86) | ||
| Gender (female/male), n | 201 (75%)/67 (25%) | – | 76 (88%)/10 (12%) | 0.01 |
| Age, years | 46.5 (16.7) | – | 44.6 (16.8) | 0.35 |
| BMI, kg/m2 | 26.1 (4.55) | – | 26.2 (5.15) | 0.82 |
| Thyroid volume, ml | 12.1 (4.30) | – | 12.1 (6.20) | 0.96 |
| FT4, pmol/l | 14.2 (1.59) | 11.06–17.31 | 13.7 (2.04) | 0.04 |
| FT3, pmol/l | 5.04 (0.54) | 4.14–5.94 | 4.95 (0.62) | 0.25 |
| TSH, mIU/l | 1.49 [1.00; 2.05] | 0.47–3.77 | 2.97 [1.72; 4.48] | <0.001 |
| lnTSH, mIU/l | 0.37 (0.52) | – | 1.06 (0.70) | <0.001 |
| TPO Ab, IU/l | – | 728 [106; 1,300] | – | |
| FT3-FT4 ratio | 0.36 (0.05) | 0.27–0.45 | 0.37 (0.06) | 0.20 |
| Deiodinase activity, nmol/s | 33.1 (4.58) | 24.6–41.9 | 34.0 (5.48) | 0.20 |
| FT4-TSH ratio | 9.31 [6.87; 14.3] | 3.4–30.9 | 4.81 [2.97; 7.62] | <0.001 |
| Thyroid capacity, pmol/s Specific thyroid capacity, | 3.43 (1.37) | 1.67–7.51 | 2.34 (1.20) | <0.001 |
| pmol/s/ml volume | 0.31 (0.15) | 0.13–0.66 | 0.23 (0.14) | <0.001 |
IQR = Interquartile range; – = not applicable; TAD = thyroid autoimmune disease.
After removal of three outliers.
Compared to the control group.
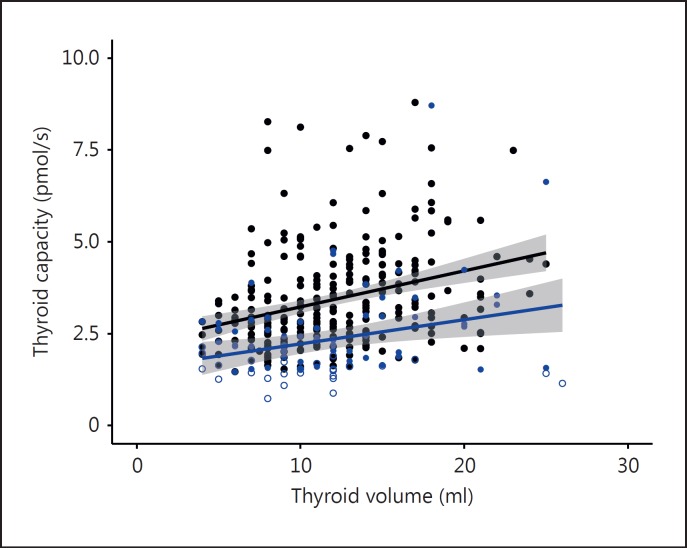
The FT4-TSH ratio was significantly associated with thyroid volume in the control group (tau = 0.23, p < 0.001, n = 268). Similarly, thyroid capacity was significantly correlated with the anatomical thyroid volume determined by ultrasound (fig. 1). Interestingly, the functional versus anatomical relationship was preserved in patients with autoimmune thyroiditis, but at any given volume thyroid capacity was lower in the diseased sample compared to the control group (fig. 1). Thyroid capacity in controls was positively associated with age (tau = 0.13, p = 0.002, n = 268) and negatively with BMI (tau = −0.09, p = 0.04). It was negatively associated with deiodinase activity (tau = −0.26, p < 0.001), but remained unrelated to gender (p = 0.20) and FT3 concentrations (tau = −0.02, p = 0.71). The relationships with the specific thyroid capacity per thyroid volume (pmol/s/ml) are as follows: gender (0.10 higher in females, p < 0.001), age (tau = 0.025, p = 0.55), BMI (tau = −0.20, p < 0.001), deiodinase activity (tau = −0.19, p < 0.001), and FT3 (tau = −0.06, p = 0.14). Deiodinase activity in controls, in turn, was influenced by gender (2.1 nmol/s on average higher in men, p = 0.001), age (tau = −0.21, p < 0.001), and TSH levels (lnTSH, tau = 0.12, p = 0.004), but not by BMI (tau = 0.01, p = 0.74) and thyroid volume (tau = −0.04, p = 0.32).
Fig. 1.
Correlation between estimated functional thyroid capacity and thyroid volume determined by ultrasound in controls (black symbols, r = 0.31, p < 0.001, n = 268) and untreated patients with thyroid autoimmune disease (blue symbols, r = 0.21, p = 0.057, n = 86). The between group difference of the relationships was significant (p < 0.001). The shaded area indicates the 95% confidence limits for the regression line. For calculation of functional capacity see Methods. Unfilled symbols indicate the presence of subclinical hypothyroidism.
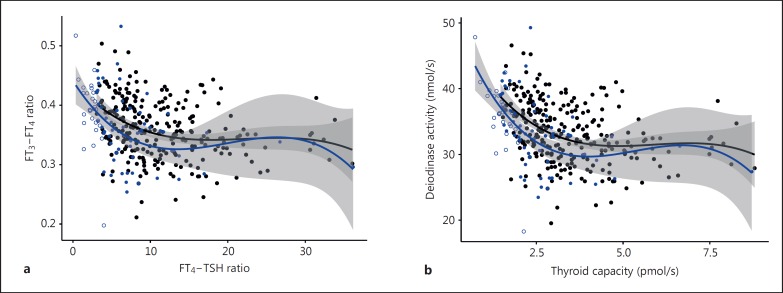
In the euthyroid control panel, the FT3-FT4 ratio was positively associated with lnTSH (tau = 0.12, p = 0.004) and inversely with the FT4-TSH ratio (tau = −0.22, p < 0.001), linking T3 conversion with TSH-standardised T4 production (fig. 2a). Similarly, using more refined measures of conversion and thyroid production, deiodinase activity showed an inverse association (tau = −0.26, p < 0.001) and a nonlinear relationship (fig. 2b) with thyroid capacity in the euthyroid range of the control group. The significant association between both the FT3-FT4 and FT4-TSH ratios or thyroid capacity and deiodinase activity was not the result of a spurious correlation since it was significantly stronger in real values than in a sample based on randomly permuted FT4 concentrations (p = 0.01, p = 0.02). The relationship was approximated by a third-degree polynomial regression, which provided a superior fit to a linear regression (model comparison by ANOVA, p < 0.001) (fig. 2b).
Fig. 2.
Relationship between the FT3-FT4 and FT4-TSH ratios (a) and global deiodinase activity and thyroid capacity (b) in euthyroid controls and untreated patients with thyroid autoimmune disease. The curves were fitted by a third-degree polynomial. The shaded area indicates the 95% confidence limits for the fitted line. Black symbols represent controls, and blue symbols represent subclinical hypothyroid patients with thyroid autoimmune disease. Closed circles refer to TSH values within the euthyroid range, while open circles indicate subclinical hypothyroidism.
The relationship between conversion and capacity extended to the subclinical hypothyroid range in untreated patients with thyroid autoimmune disease (fig. 2a, b). While thyroid capacity was reduced in subclinical hypothyroid (TSH >4 mIU/l) compared to euthyroid patients with thyroid autoimmune disease [1.53 (1.34; 1.69) vs. 2.45 (2.01; 2.97), p < 0.001], deiodinase activity was increased [36.3 (34.2; 38.9), n = 29 vs. 32.8 (29.6; 36.2), n = 57, p = 0.005], thereby maintaining FT3 levels [5.00 (4.70; 5.26) vs. 4.90 (4.58; 5.10), p = 0.23].
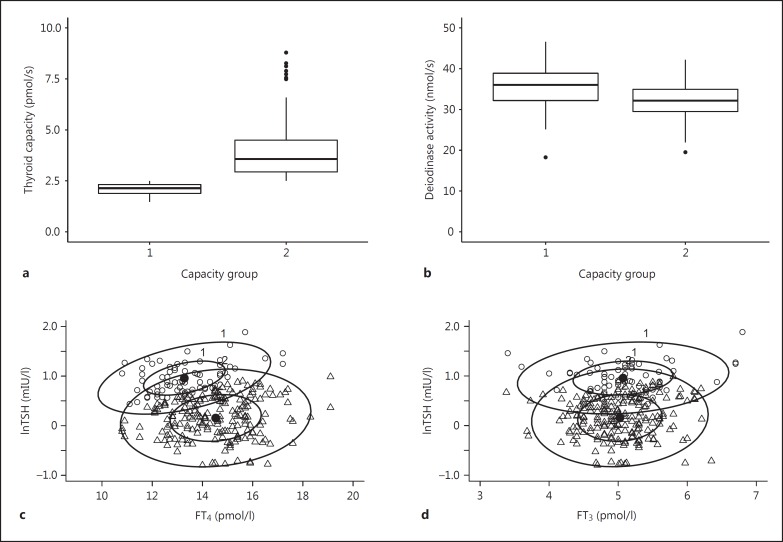
Within the panel of the controls, the subgroup with a low thyroid capacity (cutoff 2.5 pmol/s) displayed lower FT4 levels, but maintained FT3 levels at the same level as patients with higher functional and anatomical capacity (table 2). These patients also displayed a higher level of TSH stimulation, and their deiodinase activity was significantly elevated, compared to the subjects with greater thyroid capacity (table 2). Figure 3 illustrates the opposing changes in thyroid capacity and deiodinase activity. The bivariate 95% confidence ellipses for FT4 versus lnTSH stratified by thyroid capacity show an adjusted expression of the controlling relationships within the euthyroid range while FT3 concentrations are maintained (fig. 3c, d).
Table 2.
Thyroid parameters in controls with relatively low vs. high thyroid capacity (cutoff 2.5 pmol/s)
| Parameter | Low capacity (n = 71) | High capacity (n = 197) | p value |
|---|---|---|---|
| Gender (female/male), n | 51 (72%)/20 (28%) | 150 (76%)/47 (24%) | 0.56 |
| Age, years | 41.0 [29.0; 52.0] | 47.0 [37.0; 61.0] | 0.016 |
| BMI, kg/m2 | 26.6 [22.9; 30.6] | 25.2 [22.5; 28.4] | 0.11 |
| Thyroid volume, ml | 10.0 [8.00; 13.0] | 12.0 [10.0; 16.0] | <0.001 |
| FT4, pmol/l | 13.2 [12.4; 14.0] | 14.6 [13.4; 15.6] | <0.001 |
| FT3, pmol/l | 5.00 [4.75; 5.40] | 5.00 [4.70; 5.30] | 0.71 |
| TSH, mIU/l | 2.46 [2.06; 3.20] | 1.19 [0.88; 1.64] | <0.001 |
| lnTSH, mIU/l | 0.96 (0.29) | 0.16 (0.40) | <0.001 |
| FT3-FT4 ratio | 0.38 (0.05) | 0.35 (0.04) | <0.001 |
| Deiodinase activity, nmol/s | 35.6 (4.85) | 32.3 (4.16) | <0.001 |
| FT4-TSH ratio | 5.37 [4.22; 6.48] | 12.1 [8.71; 16.1] | <0.001 |
| Thyroid capacity, pmol/s Specific thyroid capacity, | 2.14 [1.89; 2.32] | 3.57 [2.94; 4.49] | <0.001 |
| pmol/s/ml volume | 0.21 [0.16; 0.26] | 0.31 [0.24; 0.40] | <0.001 |
Mean (SD) and p value by Welch t test are reported or, in case of non-normally distributed parameters, median [interquartile range] and p value based on Wilcoxon's test.
Fig. 3.
Thyroid capacity (a), deiodinase activity (b), confidence ellipses at 50% plus 95% of the bivariate distribution of FT4 and logarithmic TSH (c), and FT3 and logarithmic TSH (d) in controls with relatively low (n = 71) versus high (n = 197) thyroid capacity. The upper panel shows the opposing behavior of thyroid capacity and deiodinase activity. The lower panel shows a change in the lnTSH-FT4 relationship, accompanied by a FT4 shift but stable FT3 positions between the capacity groups (cutoff 2.5 pmol/s).
Discussion
The human thyroid glandoperates under physiological conditions far from its basal resting point, thus requiring continuous and adapted stimulation via feedback-controlled pituitary TSH secretion to maintain adequate thyroid hormone supply [1]. The system can thereby provide relational balance through conditional interrelationships aimed at T3 stability, the biologically most relevant hormone. In the present study, we show that there is significant variation in the physiological responses of individuals across the euthyroid reference range and in the expression of control mechanisms. This seems to be related especially to the positioning of an individual within the FT3 range. The three parameters describing thyroid function (TSH, FT4, and FT3) are classically defined in euthyroid humans by their respective reference ranges [15,16]. This has encouraged a tacit assumption that across the ranges individuals show similarly poised homeostatic responses to potential disturbances, e.g. subclinical or overt hypothyroidism. The present study and earlier findings question this assumption, suggesting significant variation in the expression of control mechanisms in euthyroid subjects.
From our results, the euthyroid panel does not present itself as a homogeneous group with a random multivariate distribution of independent parameters, but appears to be subject to subtle homeostatic control shifts and early fine-tuning at the individual level. The present results extend our previous findings in LT4-treated patients to more physiological conditions [4]. This may have important implications as the relationship between TSH and thyroid hormones is fundamentally different in treated patients and in euthyroid individuals [4,9,16,17,18,19,20]. The present findings suggest an additional concept of relational stability. They undermine the assumption that TSH alone may be sufficient for diagnosis of treatment adequacy, rather demanding a multivariable homeostatically rooted approach for defining true euthyroidism.
This adds a homeostatic perspective to the ongoing controversy on thyroid reference intervals [21,22,23,24]. Technically, the interrelationships can be approximately represented by the T3-T4 ratio reflecting conversion efficiency and the FT4-TSH ratio estimating the standardized amount of FT4 released per unit of TSH. The equilibrated levels measured can also be mathematically deconstructed into estimates of the maximum thyroidal capacity (SPINA-GT or abbreviated GT, see Methods) and the pituitary response curve using a validated model of thyroid hormone homeostasis [6,7,8,9,10,11]. In this study we confirmed a strong correlation between functional and sonographically determined anatomical capacity. GT was influenced by age and body weight, but not gender. Interestingly, the ratio of FT3 to FT4 and the ratio of FT4 to TSH were inversely associated, as thyroid capacity and deiodinase activity trended in opposite directions across the euthyroid range. In particular, global deiodinase activity increased with declining thyroid capacity within the euthyroid panel, involving both FT4 and TSH levels and maintaining T3 stability. This suggests a subtle homeostatically driven balance between these two main physiological processes. Consequently, the euthyroid panel does not follow a homogeneous pattern to produce random variation among thyroid hormones and TSH. Instead, it forms a heterogeneous group that displays distinctly different levels of homeostatic control at the individual level.
We cannot determine whether genetic factors may possibly have contributed to the observed heterogeneity in our controls as this study did not include any genetic testing [25,26,27,28]. Our estimated conversion rate may reflect global tissue demand and thyroid hormone utilization. However, it does not discriminate between the relative contributions by the three types of deiodinase, which are known to respond differently to T4 and T3 [29,30]. Global conversion efficiency appears to be centrally controlled via TSH, while allowing for conditional tissue adjustments to meet varying local demands [17,31,32]. This allows the various tissues to temporarily borrow from each other, thus making the total system more efficient. Only the brain would always retain highest priority due to its predominant expression of type 2 deiodinase [29].
The theoretical background including methodological aspects and reliability of the calculated parameters has recently been reviewed [6,8]. Measurement of thyroid parameters is affected by several types of variation including assay performance, high interindividual variation compared to other laboratory parameters, considerable biological (intraindividual) variation, and variation from circadian and circannual rhythms [33,34]. The latter is apparent for TSH and also to a lesser extent FT3, which follows the nightly surge in TSH with some delay, but is less pronounced in FT4[35]. Circadian variability was a minor influence in our study because all blood samples were taken during the day from late morning to early afternoon, thus avoiding greater TSH or FT3 deviations that may occur during late or nightly hours [34]. It is also expected to be a random influence in a larger sample. As these thyroid parameters are strongly interrelated, their joint interpretation appears appropriate, and may also reduce variation on theoretical grounds. Indeed, GT showed a lower intrasubject variability (intraindividual coefficient of variation) compared to TSH in this study (16 vs. 20%), confirming earlier reports [8].
Concerns have been raised about the impact of unrecognized thyroid autoimmune disease on the normal reference range [36,37]. As contamination of the control group by hidden pathologies cannot be ruled out, we have compared the relevant relationships in the controls to a group of patients with untreated thyroid autoimmune disease. While the functional capacity relative to the patients' thyroid volume was on average lower in the pathological sample than in the control group - as may be expected in a condition characterized by structural tissue damage - the relationships between conversion and capacity were generally preserved and extended to the subclinical range. The latter aspect questions in particular the definition of the subclinical group, who are currently classified as diseased solely based on an abnormal TSH measurement [16]. We have recently shown that a substantial portion of subclinical diseases were reclassified differently by using a multivariate reference system [38]. Both the FT4-TSH ratio and estimated functional thyroid capacity constitute more standardized measures of the thyroid production capability than circulatory FT4 concentration, as the latter is inherently dependent on the degree of TSH stimulation. Any imbalance could reveal capacity stress at a very early stage where a compensatory increase in conversion efficiency is still able to maintain the euthyroid state. Its potential as an early indicator of a progressively failing thyroid gland in conditions such as autoimmune thyroiditis invites further prospective studies.
Deriving euthyroid reference levels therefore becomes a combined task of defining the healthy equilibrium state of all parameters rather than a univariate statistical distribution of a single parameter [1]. Accordingly, these findings raise the possibility of a predisease definition of euthyroid individuals using a FT3 and FT4 measurement in addition to TSH, thereby laying down archival markers unique to each subject. This would allow assessment of treatment protocols based on prior knowledge of the subject's homeostatic set point in the event of glandular dysfunction arising in the future.
In conclusion, the homeostatic balances between the parameters defining thyroid function appear to be individually expressed, whether in euthyroidism or in dysfunction. Relational stability is achieved through conditional interrelationships and variation in the expression of control mechanisms. This emphasizes the need to consider the patient as an individual with a unique physiological makeup and argues against the statistical approach of simply consigning subjects to particular diagnoses based only on whether their results lie within or without the respective reference ranges.
Disclosure Statement
J.W.D. received funding and personal fees from Sanofi-Henning, Hexal AG, and Pfizer, and is co-owner of the intellectual property rights for the patent ‘System and Method for Deriving Parameters for Homeostatic Feedback Control of an Individual’ (Singapore Institute for Clinical Sciences, Biomedical Sciences Institutes, Application No. 201208940-5, WIPO No. WO/2014/ 088516). All of the other authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgement
The authors wish to thank Hans Günther Wahl, Institute of Laboratory Medicine, Klinikum Luedenscheid, for measurement of thyroid hormones.
References
- 1.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Homeostatic control of the thyroid-pituitary axis: perspectives for diagnosis and treatment. Front Endocrinol. 2015;6:1–17. doi: 10.3389/fendo.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. Am J Physiol Endocrinol Metab. 1990;258:E715–E726. doi: 10.1152/ajpendo.1990.258.4.E715. [DOI] [PubMed] [Google Scholar]
- 3.Visser WE, Friesema ECH, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Mol Endocrinol. 2011;25:1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoermann R, Midgley JEM, Giacobino A, Eckl WA, Wahl HG, Dietrich JW, Larisch R. Homeostatic equilibria between free thyroid hormones and pituitary thyrotropin are modulated by various influences including age, body mass index and treatment. Clin Endocrinol (Oxf) 2014;81:907–915. doi: 10.1111/cen.12527. [DOI] [PubMed] [Google Scholar]
- 5.Larisch R, Giacobino A, Eckl WA, Wahl HG, Midgley JEM, Hoermann R. Reference range for thyrotropin. Post hoc assessment. Nuklearmedizin. 2015;54:112–117. doi: 10.3413/Nukmed-0671-14-06. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich JW, Landgrafe G, Fotiadou EH. TSH and thyrotropic agonists: key actors in thyroid homeostasis. J Thyroid Res. 2012;2012:1–29. doi: 10.1155/2012/351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich JW, Tesche A, Pickardt CR. Thyrotropic feedback control: evidence for an additional ultrashort feedback loop from fractal analysis. Cybern Syst. 2004;35:315–331. [Google Scholar]
- 8.Dietrich JW, Landgrafe-Mende G, Wiora E, Chatzitomaris A, Klein HH, Midgley JEM, Hoermann R. Calculated parameters of thyroid homeostasis: emerging tools for differential diagnosis and clinical research. Front Endocrinol. 2016;7:57. doi: 10.3389/fendo.2016.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Is pituitary TSH an adequate measure of thyroid hormone-controlled homoeostasis during thyroxine treatment? Eur J Endocrinol. 2013;168:271–280. doi: 10.1530/EJE-12-0819. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich JW. Der Hypophysen-Schilddrüsen-Regelkreis. Berlin: Logos; 2002. [Google Scholar]
- 11.Dietrich JW, Stachon A, Antic B, Klein HH, Hering S. The AQUA-FONTIS Study: protocol of a multidisciplinary, cross-sectional and prospective longitudinal study for developing standardized diagnostics and classification of non-thyroidal illness syndrome. BMC Endocr Disord. 2008;8:13. doi: 10.1186/1472-6823-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Core Team R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing 2015. http://www.R-project.org/
- 13.Fellows I. Deducer: a data analysis GUI for R. J Stat Soft. 2012;49:1–15. [Google Scholar]
- 14.Revelle W: psych, Version 1.5.8 2015: procedures for personality and psychological research. Evanston: Northwestern University; http://CRAN.R-project.org/package=psych. [Google Scholar]
- 15.Baloch ZW, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, LiVosli VA, Nicoli-Sire P, Rhys J, Rut J, Smyth PPA, Spencer CA, Stockight JR. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 16.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RB, Rosenthal MS, Sawka AM. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Integration of peripheral and glandular regulation of triiodothyronine production by thyrotropin in untreated and thyroxine-treated subjects. Horm Metab Res. 2015;47:674–680. doi: 10.1055/s-0034-1398616. [DOI] [PubMed] [Google Scholar]
- 18.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito M, Miyauchi A, Morita S, Kudo T, Nishihara E, Kihara M, Takamura Y, Ito Y, Kobayashi K, Miya A, Kubota S, Amino N. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol. 2012;167:373–378. doi: 10.1530/EJE-11-1029. [DOI] [PubMed] [Google Scholar]
- 20.Ito M, Miyauchi A, Kang S, Hisakado M, Yoshioka W, Ide A, Kudo T, Nishihara E, Kahara M, Ito Y, Kobayashi K, Miya A, Fukata S, Nakamura H, Amino N. Effect of the presence of remnant thyroid tissue on the serum thyroid hormone balance in thyroidectomized patients. Eur J Endocrinol. 2015;173:1–178. doi: 10.1530/EJE-15-0138. [DOI] [PubMed] [Google Scholar]
- 21.Dickey RA, Wartofsky L, Feld S. Optimal thyrotropin level: normal ranges and reference intervals are not equivalent. Thyroid. 2005;15:1035–1039. doi: 10.1089/thy.2005.15.1035. [DOI] [PubMed] [Google Scholar]
- 22.Wartofsky L, Dickey RA. The evidence for a narrower thyrotropin reference range is compelling. J Clin Endocrinol Metab. 2005;90:5483–5488. doi: 10.1210/jc.2005-0455. [DOI] [PubMed] [Google Scholar]
- 23.Brabant G, Beck-Peccoz P, Jarzab B, Laurberg P, Orgiazzi J, Szabolcs I, Weetman AP, Wieringa WM. Is there a need to redefine the upper normal limit of TSH? Eur J Endocrinol. 2006;154:633–637. doi: 10.1530/eje.1.02136. [DOI] [PubMed] [Google Scholar]
- 24.Laurberg P, Andersen S, Carlé A, Karmisholt J, Knudsen N, Pedersen IB. The TSH upper reference limit: where are we at? Nat Rev Endocrinol. 2011;7:232–239. doi: 10.1038/nrendo.2011.13. [DOI] [PubMed] [Google Scholar]
- 25.Panicker V, Cluett C, Shields B, Murray A, Parnell KS, Perry JRB, Weedon MN, Singleton A, Hernandez D, Evans J, Durant C, Ferrucci L, Melzer D, Saravanan P, Visser TJ, Ceresini G, Hattersley AT, Vaisya B, Dayan CM, Frayling TM. A common variation in deiodinase 1 gene DIO1 is associated with the relative levels of free thyroxine and triiodothyronine. J Clin Endocrinol Metab. 2008;93:3075–3081. doi: 10.1210/jc.2008-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor PN, Panicker V, Sayers A, Shields B, Iqbal A, Bremner AP, Beilby JP, Leedman PJ, Hattersley AT, Vaisya B, Frayling T, Evans J, Tobias JH, Timpson NJ, Walsh JP, Dayan CM. A meta-analysis of the associations between common variation in the PDE8B gene and thyroid hormone parameters, including assessment of longitudinal stability of associations over time and effect of thyroid hormone replacement. Eur J Endocrinol. 2011;164:773–780. doi: 10.1530/EJE-10-0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoftijzer HC, Heemstra KA, Visser TJ, le Cessie S, Peeters RP, Corssmit EPM, et al. The type 2 deiodinase ORFa-Gly3Asp polymorphism (rs12885300) influences the set point of the hypothalamus-pituitary-thyroid axis in patients treated for differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2011;96:E1527–E1533. doi: 10.1210/jc.2011-0235. [DOI] [PubMed] [Google Scholar]
- 28.McAninch EA, Jo S, Preite NZ, Farkas E, Mohácsik P, Fekete C, Egri P, Gereben B, Li Y, Deng Y, Patti ME, Zevenbergen C, Peeters RP, Mash DC, Bianco AC. Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes. J Clin Endocrinol Metab. 2015;100:920–933. doi: 10.1210/jc.2014-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- 30.Gereben B, McAninch EA, Ribeiro MO, Bianco AC. Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol. 2015;11:642–652. doi: 10.1038/nrendo.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonseca TL, Correa-Medina M, Campos MPO, Wittmann G, Werneck-de-Castro JP, Arrojo e Drigo R, Mora-Garzon M, Ueta CB, Caicedo A, Fekete C, Gereben B, Lechan RM, Bianco AC. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest. 2013;123:1492–1500. doi: 10.1172/JCI61231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla SM, Wittmann G, Lechan RM, Gerber B, Bianco AC. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest. 2015;125:769–781. doi: 10.1172/JCI77588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069–1078. doi: 10.1089/105072503770867237. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenkranz J, Bach PR, Snow GL, Schneider A, Lee JL, Ilstrup S, Bennet ST, Benvenga S. Circadian and circannual rhythms in thyroid hormones: determining the TSH and free T4 reference intervals based upon time of day, age, and sex. Thyroid. 2015;25:954–961. doi: 10.1089/thy.2014.0589. [DOI] [PubMed] [Google Scholar]
- 35.Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, Ross RJ. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008;93:2300–2306. doi: 10.1210/jc.2007-2674. [DOI] [PubMed] [Google Scholar]
- 36.Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007;92:4236–4240. doi: 10.1210/jc.2007-0287. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton TE, Davis S, Onstad L, Kopecky KJ. Thyrotropin levels in a population with no clinical, autoantibody, or ultrasonographic evidence of thyroid disease: implications for the diagnosis of subclinical hypothyroidism. J Clin Endocrinol Metab. 2008;93:1224–1230. doi: 10.1210/jc.2006-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoermann R, Larisch R, Dietrich JW, Midgley JEM. Derivation of a multivariate reference range for pituitary thyrotropin and thyroid hormones: diagnostic efficiency compared to conventional single reference method. Eur J Endocrinol. 2016;174:735–743. doi: 10.1530/EJE-16-0031. [DOI] [PubMed] [Google Scholar]