Abstract
We describe a case of biochemical neonatal thyrotoxicosis caused by biotin supplementation. Biotin may interact with thyroid function testing to imitate thyrotoxicosis with low thyroid-stimulating hormone and elevated triiodothyronine and thyroxine levels.
Key Words: Biotin, Assay interaction, Neonatal hyperthyroidism
Introduction
Neonatal hyperthyroidism is a rare disease. Most cases are due to placental transferal of thyroid-stimulating hormone (TSH) receptor autoantibodies (TRAb) from mothers with Graves' disease to their fetuses. Even rarer causes are sporadic Graves' disease and genomic activating mutations in the TSH receptor of the neonate [1].
Despite the clinical thyrotoxicosis of the patient, thyroid function tests are important tools in diagnosing hyperthyroidism. The results of the tests are normally easy to interpret. There are, however, some caveats in the measurement and judgement [2], one of which is interference with the assays used in the analyses.
Biotin is a water-soluble vitamin present in a range of natural dietary products and also produced by bacteria in the gut. Moreover, internal recycling plays an important role in the maintenance of the pool of biotin in the body [3]. A daily intake of 30 µg of biotin is recommended for adults [4]. The major biological function of biotin is to be a cofactor responsible for carbon dioxid transfer in a number of important carboxylases, whereby the enzymes are activated [5]. The majority of biotin, both in food and during internal recycling is covalently bound to the amino acid lysin as biotinyl. Free biotin is released from biotinyl by action of the enzyme biotinidase. In the disorder biotinidase deficiency, biotinyl cannot be cleaved, and biotin deficiency develops, which may lead to cutaneous abnormalities, irreversible neurological disorders and other diseases after the age of 7 weeks. The treatment is supplementation with free biotin as early as possible [3].
We describe a newborn baby with biochemical hyperthyroidism caused by assay interaction from biotin intake.
Case Report
Here, the case of a newborn girl born at 37 weeks of gestation by caesarean section after an uncomplicated pregnancy is discussed. Her birth weight was 2,960 g, and her Apgar score was 10/10. Two hours after birth, pediatric consult recommended biotin supplement 5 mg per day based on her family history.
The infant was the second child of healthy parents. The 22- months-older brother was diagnosed with biotinidase deficiency upon neonatal routine screening. He had received a biotin supplement of 5 mg per day since he was 18 days old and completely healthy. Because of the brother's disease, the mother had taken biotin 10 mg per day from the 20th gestational week of the present pregnancy and until delivery.
Two days old, the newborn girl was transferred to the Neonatal Department as she was lethargic, not thriving and refused to suck. She was described as ‘not reacting to anything and with a pale color’. Although she was afebrile and her infection parameters were normal, sepsis could not be ruled out, and treatment with antibiotics, placement in an incubator and nasogastric feeding were initiated. After 1-2 days of treatment, she recovered without a final diagnosis having been reached.
Subsequently, results of thyroid function tests were available and pointed in the direction of neonatal hyperthyroidism with a suppressed serum TSH and triiodothyronine (T3) and thyroxine (T4) levels just below the upper limit of the age-specific reference range [6] (table 1). However, the infant was clinically euthyroid and was thus released from the hospital.
Table 1.
Thyroid parameters of the neonate and the mother
| Neonate |
Mother |
||||
|---|---|---|---|---|---|
| Days after birth | 4 | 10 | 13 | 14 | 10 |
| Biotin supplementation | yes | yes | no | no | no |
| TSH, mU/l | 0.10 | 0.04 | 4.3 | 2.5 | 1.4 |
| T3, nM | >10 | >10 | 2.5 | 2.3 | 1. 9 |
| T4, nM | 304 | 242 | 117 | 130 | 143 |
| TRAb, IU/l | – | – | negative | – | negative |
TSH, T3 and T4 were measured in the neonate 4, 10, 13 and 14 and in the mother 10 days after birth. TRAb was only measured once in both the neonate and the mother. The mother stopped biotin intake immediately after the delivery and the neonate 12 days after birth. The TRAb assay used in the present study was not biotinylated.
Eight days old, the neonate was seen in the outpatient clinic. According to the parents, she had loose stools, and they feared a salmonella infection, as the brother had suffered from salmonella septicemia when he was 5 days old. Another possibility was loose stools due to hyperthyroidism.
The neonate had stool cultures performed and they were all negative. A new blood test confirmed a suppressed TSH and increasing values of T3 and T4 (table 1). The thyroid function of the mother was tested and turned out to be normal, and, above all, she was TRAb negative (table 1).
Due to the blood tests indicating neonatal hyperthyroidism, the girl was hospitalized when she was 10 days old. At that time, she was thriving, and there were no clinical signs of hyperthyroidism. An ultrasound of the thyroid gland was performed, and apart from a 2-mm microadenoma the gland appeared normal with a volume of 0.5 ml.
The results of the biotinidase screening became finally available and revealed that the baby was only a carrier of biotinidase deficiency. Therefore, biotin supplementation was ceased. Subsequent thyroid function testing showed test results within the reference ranges (table 1). Thus, biotin withdrawal was followed by disappearance of ‘biochemical thyrotoxicosis’ in the child.
Discussion
In the present case report, a newborn child might have been misclassified with thyrotoxicosis due to assay interference secondary to biotin intake of both the mother during pregnancy and the child during the first postpartum days.
The clinical thyroid state of the patient is important when thyroid function tests are evaluated, and it is especially important to be open-minded if the clinical picture and biochemical results do not fit together. In the present case, the pitfall was assay interference caused by a high level of biotin in the patient sample. Other causes of the discrepancy between blood tests and the clinical picture may be considered, such as pregnancy, medication including hormone therapy, compliance if treated with thyroid medication, non-thyroid illness, age and more rare conditions such as hormone resistance, presence of macro-TSH and heterophile autoantibodies [2,7].
Biotinidase deficiency is an autosomal recessive disease, and more than 165 different mutations have been identified [8]. In the present case, the brother of the newborn was homozygotic for biotinidase deficiency and harboring two different mutations, one from each parent as both parents were heterozygotic carriers of the disease.
In an Australian study from 2012 [9], one of the authors ingested 30 mg of biotin, and blood samples were collected before intake and after 1, 2, 4, 8 and 25 h. An increase in the measured concentrations of both free T3 and free T4 was present. Both concentrations peaked around 2 h after ingestion, and the increase lasted for at least 24 h.
Biotin is a small molecule found in every cell. Avidin, also called streptavidin, is a much larger protein that binds biotin with a very high affinity. When these two molecules are in the same solution, they will bind with such a high affinity that the binding is essentially irreversible. This fact is used in a number of immunoassays for measuring among others TSH, T3, T4 and TRAb.
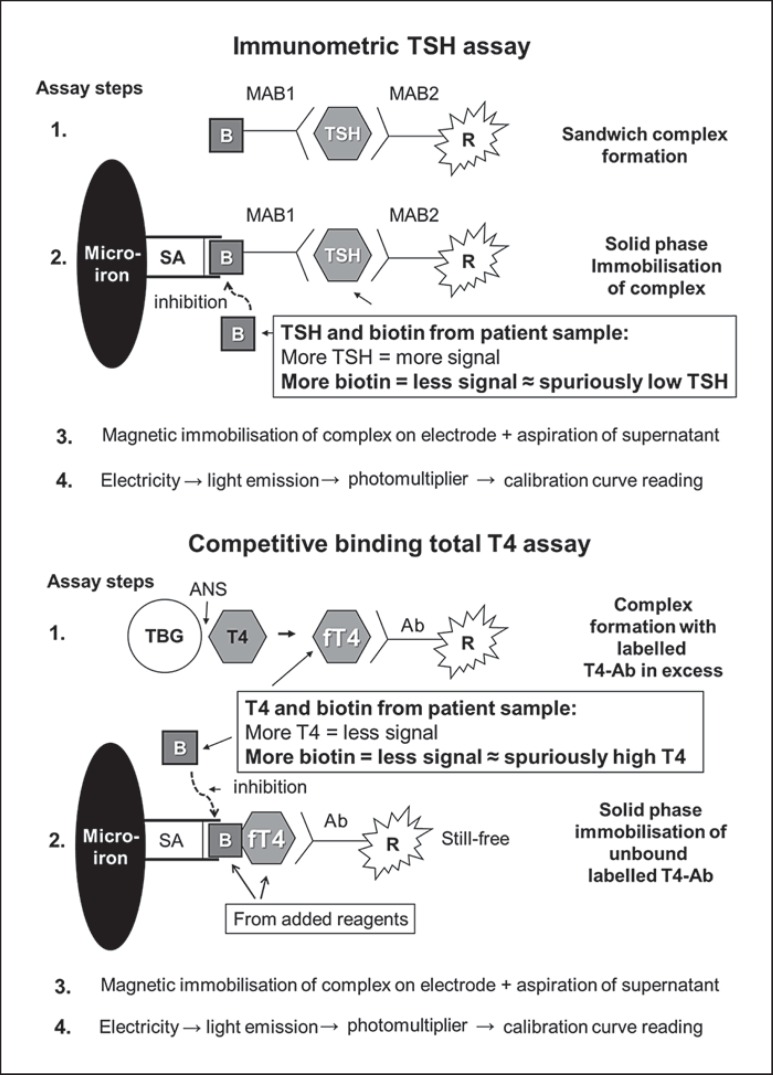
Figure 1 illustrates the use of biotin-avidin binding in the TSH, the T4 and T3 assays (Roche Modular System) used to test thyroid function in the newborn and how biotin in the sample may interfere.
Fig. 1.
Principles of the immunometric assay used for TSH measurement (upper panel), and the competitive binding assay used for the measurement of T3 and T4 (lower panel). In the immunometric assay, the interference of biotin from the patient sample results in a spuriously low concentration of TSH, whereas the opposite is seen with the competitive binding assay measuring a spuriously high concentration of T4 or T3. B = Biotin; R = ruthenium; SA = streptavidin; MAB = monoclonal antibody; Ab = antibody; ANS = 8-anilino-1-naphthalene sulphonic acid (to dissociate T4 from binding proteins); TBG = thyroxine-binding globulin; fT4 = free T4.
The TSH assay illustrated is an immunometric assay. TSH from the patient sample, a biotinylated monoclonal TSH-specific antibody and a monoclonal TSH-specific antibody labeled with a ruthenium complex react and form a sandwich complex (fig. 1). In the second incubation, streptavidin-coated microparticles are added, and due to the high affinity of streptavidin and biotin, the complexes are bound to the solid phase. The microparticles are magnetically captured on the electrode, and the supernatant is aspirated. Application of a voltage to the electrode then induces light emission, which is measured by a photomultiplier. Results are determined via a calibration curve. High concentrations of biotin in the patient sample compete with the sandwich complexes in binding to the streptavidin-coated microparticles, resulting in less sandwich complexes bound and thus a lower signal and a low TSH measurement result, suggesting hyperthyroidism.
The T4 and T3 assays were based on the competitive binding principle, and these assays also took advantage of the biotin-streptavidin reaction (fig. 1). Monoclonal T4- (or T3-)specific antibody in excess labeled with a ruthenium complex is incubated with the patient sample. Biotinylated T4- (or T3-)specific antibody and streptavidin-coated microparticles are then added, and the still free binding sites of the labeled antibody become occupied. The complexes are bound to the solid phase via interaction of biotin and streptavidin and the supernatant aspirated. The next two steps are comparable with the TSH assay (fig. 1). As the TSH assay, more T4 (or T3) in the patient sample will give a smaller amount of ruthenium-labeled antibody bound in the solid phase and a lower signal, which in a competitive binding assay is read as a high hormone concentration. With a high concentration of biotin in the patient sample, this biotin will compete with the biotin from the assay, resulting in less ruthenium-labeled antibody complexes bound and therefore a lower signal than if the concentration of T4 (or T3) was high.
Why is this important? Intake of biotin supplement is vital in the rare condition of biotinidase deficiency, but such intake is also common in the population. Many people seek information on the internet, where biotin supplements are recommended in many situations such as in pregnancy, aging, stress, bowel conditions and in relation to carbohydrate tolerance problems. Furthermore, biotin supplementation is praised as a remedy against skin and hair disorders, fatigue and stress and is brought forward as being important to maintain healthy kidney function and a good immune system. Thus, many people take biotin as a ‘health-improving supplement’.
During the preparation of this case report, assay interference in an adult patient with progressive multiple sclerosis taking biotin in megadoses was described [10].
In conclusion, biotin intake may cause interference in assays based on biotin-streptavidin reactions, which are commonly used for analyzing thyroid function tests. Because the types of assays used for measuring TSH and thyroid hormones are technically different, biotin interaction may imitate biochemical thyrotoxicosis. If blood tests suggest thyrotoxicosis and the clinical picture does not fit, ask for biotin intake and repeat sampling after at least 1-2 days without biotin supplement, depending on the dose.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Führer D, Wonerow P, Willgerodt H, Paschke R. Identification of a new thyrotropin receptor germline mutation (Leu629Phe) in a family with neonatal onset of autosomal dominant nonautoimmune hyperthyroidism. J Clin Endocrinol Metab. 1997;82:4234–4238. doi: 10.1210/jcem.82.12.4405. [DOI] [PubMed] [Google Scholar]
- 2.Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27:745–762. doi: 10.1016/j.beem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf B. Biotinidase deficiency: ‘if you have to have an inherited metabolic disease, this is the one to have’. Genet Med. 2012;14:565–575. doi: 10.1038/gim.2011.6. [DOI] [PubMed] [Google Scholar]
- 4.Zempleni J, Mock DM. Biotin biochemistry and human requirements. J Nutr Biochem. 1999;10:128–138. doi: 10.1016/s0955-2863(98)00095-3. [DOI] [PubMed] [Google Scholar]
- 5.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr. 2002;22:221–239. doi: 10.1146/annurev.nutr.22.121101.112819. [DOI] [PubMed] [Google Scholar]
- 6.Fisher DA, Nelson JC, Carlton EI, Wilcox RB. Maturation of human hypothalamic-pituitary-thyroid function and control. Thyroid. 2000;10:229–234. doi: 10.1089/thy.2000.10.229. [DOI] [PubMed] [Google Scholar]
- 7.Rix M, Laurberg P, Porzig C, Kristensen SR. Elevated thyroid-stimulating hormone level in a euthyroid neonate caused by macro thyrotropin-IgG complex. Acta Paediatr. 2011;100:135–137. doi: 10.1111/j.1651-2227.2011.02212.x. [DOI] [PubMed] [Google Scholar]
- 8.Procter M, Wolf B, Crockett DK, Mao R. The biotinidase gene variants registry: a paradigm public database. G3 (Bethesda) 2013;3:727–731. doi: 10.1534/g3.113.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijeratne NG, Doery JC, Lu ZX. Positive and negative interference in immunoassays following biotin ingestion: a pharmacokinetic study. Pathology. 2012;44:674–675. doi: 10.1097/PAT.0b013e32835a3c17. [DOI] [PubMed] [Google Scholar]
- 10.Barbesino G. Misdiagnosis of Graves' disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid. 2016;26:860–863. doi: 10.1089/thy.2015.0664. [DOI] [PubMed] [Google Scholar]