Abstract
Acyl-coenzymeA:cholesterol acyltransferase 2 (ACAT2) is abundantly expressed in intestine and fetal liver of healthy human. Our previous studies have shown that in monocytic cells the low-level expression of human ACAT2 gene with specific CpG-hypomethylated promoter is regulated by the CCAAT/enhancer binding protein (C/EBP) transcription factors. In this study, we further report that the ACAT2 gene expression is attributable to the C/EBPs in the human leukocytes and correlated with the excretion of fluorescent lipoproteins containing the ACAT2-catalyzed NBD22-steryl esters. Moreover, this lipoprotein excretion can be inhibited by the ACAT2 isoform-selective inhibitor pyripyropene A (PPPA) in a dose-dependent manner, and employed to determine the half maximum inhibitory concentration (IC50) values of PPPA. Significantly, it is found that the differentiation-inducing factor all-trans retinoic acid, but not the proinflammatory cytokine tumor necrosis factor-α, enhances this ACAT2-dependent lipoprotein excretion. These data demonstrate that the ACAT2 expression of human leukocytes is responsible for the excretion of lipoproteins containing cholesteryl/steryl esters (CE/SE), and suggest that the excretion of lipoproteins containing the ACAT2-catalyzed CS/SE may avoid cytotoxicity through decreasing the excess intracellular cholesterols/sterols (especially various oxysterols), which is essential for the action of the human leukocytes.
Keywords: ACAT2 expression, C/EBPs, human leukocytes, lipoprotein excretion, NBD22-steryl ester, ACAT2 isoform-selective inhibitor PPPA
Introduction
Acyl-coenzymeA:cholesterol acyltransferase (ACAT) catalyzes the formation of cholesteryl esters (CE) from cholesterol and long-chain fatty acyl-CoA, and is the exclusive intracellular enzyme in mammals [1]. At present, two ACAT isoforms, ACAT1 and ACAT2, have been identified [2,3]. In healthy human, ACAT2 is abundantly expressed and involved in secreting chylomicrons and very low density lipoproteins in the intestine and fetal liver, respectively [4–9]. In fact, both ACAT1 and ACAT2 exist in the cells of intestine and fetal liver. Moreover, the cholesteryl/steryl esters (CE/SE) catalyzed by ACAT2 can be incorporated into both lipoproteins for the secretion and lipid droplets for the storage of cells [6–8]. Very importantly, two isotype-specific ACAT inhibitors, K-604 [10] and pyripyropene A (PPPA) [11], have been characterized for human ACAT1 and ACAT2, respectively.
Current methods for assaying ACAT activity are laborious and time-consuming, involving the use of radioactive substrates in live cells or in incubations of cell homogenates or microsomes with the isolation of the radioactive CE products by thin-layer chromatography and subsequent quantification [12–14]. To facilitate the determination of ACAT activity, we have developed a more rapid and high-throughput cell-based assay using a fluorescent sterol, also an ACAT substrate, 22-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-23,24-bisnor-5-cholen-3-ol (NBD22-sterol) in which the NBD moiety replaces the terminal segment of the partial alkyl tail of cholesterol [15]. NBD22-sterol has been shown to mimic native cholesterol absorption in hamsters [16], cholesterol efflux in macrophages [17], intracellular lipid or sterol carrier protein transport [18–21], and esterification in vivo by cultured cells [16]. The weak or strong fluorescence of NBD22-sterol can be dependently measured when it is in a polar or nonpolar environment [22,23]. This property of NBD22-sterol serves to measure ACAT activity in live cells expressing ACAT1 and/or ACAT2 [24].
We have previously reported that a specific cholesterol metabolic pathway, involving induction of ACAT2 and esterification of excess oxysterols for excretion to avoid cytotoxicity, is established in a subset of hepatocellular carcinomas (HCCs) for tumor growth [25]. More recently, our studies showed that the low-level expression of human ACAT2 gene with specific CpG-hypomethylated promoter is regulated by the CCAAT/enhancer binding protein (C/EBP) transcription factors in monocytic cells [26]. However, the role of ACAT2 in the monocytic cells is still unknown, and the possibility that the increased expression of ACAT2 in the monocytic cells may be associated with some physiological/pathological role has not been examined.
In this study, we revealed that the ACAT2 expression attributable to the C/EBPs is responsible for the lipoprotein excretion of human leukocytes. Significantly, the differentiation-inducing factor all-trans retinoic acid (ATRA) enhances this ACAT2-dependent lipoprotein excretion.
Materials and Methods
Reagents
RPMI 1640 and fetal bovine serum (FBS) were from Gibco-BRL (Grand Island, USA). Ficoll-Paque Plus was from GE Healthcare Life Sciences (Piscataway, USA). The phorbol 12-myristate 13-acetate (PMA) and NBD22-sterol were from Sigma-Aldrich (St Louis, USA). Human Pan T Cell Isolation Kit and Human Pan B Cell Isolation Kit were from Miltenyi Biotech (Auburn, USA). ACAT2 isoform-selective inhibitor PPPA was from ALEXIS Biochemicals (Lausen, Switzerland).
Cell culture
The human monocytic cell line THP-1, the T lymphocyte cell lines Jurkat and MOLT4, the B lymphocyte cell lines BJAB and RAJI, the myeloid cell line U937 and the promyeloblast cell line HL-60 were maintained in RPMI 1640 supplemented with 10% FBS. For differentiating into macrophages, THP-1 cells were cultured in 0.1 μM PMA for 48 h. All cell lines were maintained in 100 μg/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Human peripheral blood mononuclear cells (PBMCs) and granulocytes were isolated from 200 ml of blood from each healthy donor (Shanghai Blood Service Center, Shanghai, China) by density gradient centrifugation with Ficoll-Paque Plus. Human blood monocytes were isolated from PBMCs as previously reported [27] and cultured in RPMI 1640 with 7% human AB serum. Human blood monocytes were differentiated into macrophages as described previously [28]. Human blood T and B lymphocytes were isolated from PBMCs by using Human Pan T and B Cell Isolation Kits, respectively.
Bisulfite genomic DNA sequencing
Bisulfite genomic DNA sequencing (BGS) was performed as described previously [25]. Briefly, the genomic DNAs were treated by using the EZ DNA Methylation-Gold kit (ZYMO Research, Shanghai, China) according to the manufacturer's instructions. Then, by using the above treated DNAs, the −742 to −307 region or −291 to +297 region of human ACAT2 gene promoter was amplified by polymerase chain reaction (PCR) (94°C for 5 min; 35 cycles of 94°C for 30 s, 58°C for 45 s, and 72°C for 45 s; 72°C for 5 min) with primer sets BGS-F1/BGS-R1 or BGS-F2/BGS-R2 listed in Table 1. PCR products were inserted into pGEM-T easy vector (Promega, Madison, USA), and individual plasmids from 10 cloned colonies were sequenced by Sangon Company (Shanghai, China).
Table 1.
Sequences of all primer sets used for amplifying human ACAT2 gene promoter regions
| Primer set | Sequence (5′→3′) |
|---|---|
| BGS-F1/R1 | TTTTATTTGGATAATTTTATTTTGAGT/AACTAAAAATCAAAAAAAAATCAAAAATA |
| BGS-F2/R2 | TGGGAGGTTAGTTATGTTAGGTGAT/CTTCCTTAACCTCTCTAAACAACAA |
RT-PCR analysis
Total RNA was freshly prepared using the Absolutely RNA® RNA Miniprep Kit (Stratagene, Cedar Creek, USA). An aliquot of the total RNA was reverse-transcribed to obtain cDNAs. Quantitative RT-PCR was carried out on an Mx3005PTM instrument (Stratagene) using a hot-start SYBR-green-based method followed by melting curve analysis to verify the specificity of the products. The cycle number at threshold (Ct) was used to calculate the relative amount of mRNA. The Ct value of each determined RNA sample was normalized by subtracting that of control GAPDH mRNA to obtain the ΔCt value, and the relative mRNA level was calculated as 2-ΔCt. All primer sets for quantitative RT-PCR were individually listed in Table 2.
Table 2.
Sequences of all primer sets used for RT-PCR analysis
| mRNA | Primer set | Sequence (5′→3′) |
|---|---|---|
| ACAT2 | ACAT2-F/R | CATGCTGCTGCTCATCTTCT/ACTGCGGAGACCAGGAACA |
| C/EBPα | C/EBPα-F/R | TGTATACCCCTGGTGGGAGA/TCATAACTCCGGTCCCTCTG |
| C/EBPβ | C/EBPβ-F/R | GACAAGCACAGCGACGAGTA/AGCTGCTCCACCTTCTTCTG |
| C/EBPε | C/EBPε-F/R | CTCTGCGCGTTCTCAAGG/GCCGGTACTCAAGGCTATCTT |
| Cdx2 | Cdx2-F/R | AGCCAAGTGAAAACCAGGAC/CAGAACCCCAGGGACAGA |
| HNF1α | HNF1α-F/R | TCATCATGGCCTCACTTC/CCATTGCTGGAGTCTGAG |
| GAPDH | GAPDH-F/R | ACCCACTCCTCCACCTTTG/CTGTAGCCAAATTCGTTGTCAT |
Fluorescence assay
The fluorescence assay was performed as described previously [15]. Briefly, the cells were incubated with 0.5 μg/ml NBD22-sterol and the ACAT2 isoform-selective inhibitor PPPA at different concentrations (ranging from 0.1 to 10 μM). After incubation, the fluorescence intensity (FI) of lipoproteins containing the ACAT2-catalyzed NBD22-steryl esters in cultured media was measured using the Envision Multilabel Reader (Perkin Elmer, Waltham, USA) by setting the excitation and emission wavelength to 488 nm and 535 nm, respectively. The FI of lipoproteins in cultured media without cells used as the blank control was subtracted. The FI of lipoproteins in cultured media of cells incubated with DMSO and 10 μM ACAT2 isoform-selective inhibitor PPPA was used as the controls of no inhibition (NI) and total inhibition (TI), respectively, and then, the following formula was used for the calculation:
The IC50 value of PPPA for ACAT2 was obtained through the non-linear fitting of concentration-dependent curve by using Graphpad Prism 5.
Statistical analysis
Experimental differences were analyzed as indicated in figure legends. Statistical analysis were performed by using the Student's t-test. P values of <0.05 were considered of statistically significant difference.
Results
ACAT2 gene expression of the human blood leukocytes attributable to the C/EBP transcription factors
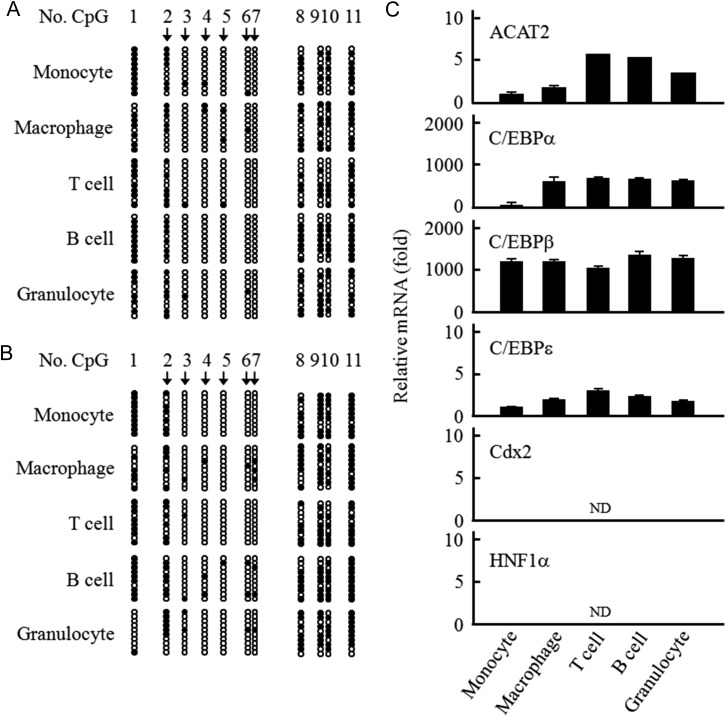
Our previous studies have shown that in the monocytic cells the low-level expression of human ACAT2 gene with specific CpG-hypomethylated promoter is regulated by the C/EBP transcription factors [26]. Here, by investigating the human blood leukocytes from two healthy donors, we observed that the same specific CpG sites (No. 2–7, arrow labeled) of ACAT2 gene promoter were hypomethylated in all the leukocytes including the monocytic cells (Fig. 1A,B). The quantitative RT-PCR results showed that all the leukocytes expressed ACAT2, which was attributable to the C/EBPs but not to Cdx2 and HNF1α, and the ACAT2 mRNA levels of monocytic cells were obviously lower than those of other leukocytes (Fig. 1C). The above results demonstrate that the hypomethylated status of ACAT2 gene promoter is correlated with its expression which is attributable to the C/EBP transcription factors in all the human blood leukocytes.
Figure 1.
Promoter-CpG-methylation status and expression of ACAT2 gene in the human blood leukocytes (A,B) Promoter-CpG-methylation status of ACTA2 gene in the human blood leukocytes from two healthy donors was individually measured by bisulfite genomic sequencing. Bisulfite genomic sequencing was performed according to the procedures described in the ‘Materials and Methods’. Each row of circles represents a single cloned allele, and each circle indicates a single CpG site. The filled and open circles represent methylated and unmethylated CpG sites, respectively. (C) Quantitative RT-PCR analysis of ACAT2, C/EBPs, Cdx2, and HNF1α mRNAs in the human blood leukocytes from two healthy donors. Quantitative RT-PCR was performed according to the procedures described in the ‘Materials and Methods’. The relative mRNA level of ACAT2 was expressed as fold to that in the monocytes, and the relative mRNA level of each transcription factor (C/EBPs, Cdx2, or HNF1α) in different cells was expressed as fold to that of C/EBPε in the monocytes. Results shown were representative of three independent experiments and data were shown as the mean ± SD of triplicates. ND: not detected.
Lipoprotein excretion associated with the ACAT2 expression of the human leukocytic cell lines
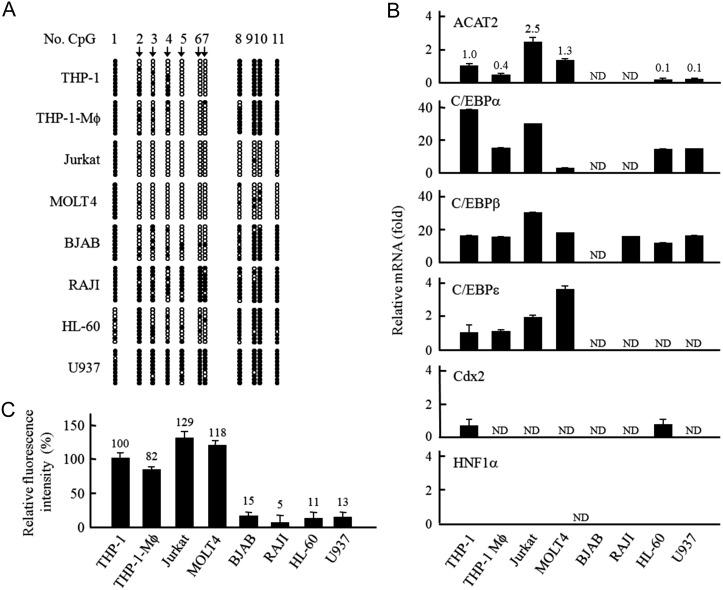
To detect the role of ACAT2 in human leukocytes, we used available human leukocytic cell lines to examine the promoter-CpG-methylation status of ACAT2 gene and its expression. It was observed that in four human leukocytic cell lines, the promoter-hypomethylated status of ACAT2 gene was correlated with its expression, which was attributable to the transcription factors C/EBPs, but not Cdx2 and HNF1α (top or left four cell lines in Fig. 2A,B). Generally, the CS/SE esters catalyzed by ACAT2 are incorporated into various lipoproteins for the secretion of different tissue cells [4–9]. Thus, we performed an assay with the fluorescent NBD22-sterol, also an ACAT substrate, for the extracellularly excreted lipoproteins containing ACAT2-catalyzed NBD22-steryl esters. It was found that mainly the above four human leukocytic cell lines expressing ACAT2 excreted fluorescent lipoproteins into the cultured media (left four cell lines in Fig. 2C). These results indicate that the lipoprotein excretion is associated with the ACAT2 expression of the human leukocytic cell lines.
Figure 2.
Promoter-CpG-methylation status, expression, and role of ACAT2 in the human leukocytic cell lines (A) Promoter-CpG-methylation status of ACTA2 gene in different human leukocytic cell lines was examined as described in Fig. 1A. (B) Quantitative RT-PCR analysis of ACAT2, C/EBPs, Cdx2, and HNF1α mRNAs in different human leukocytic cell lines. Quantitative RT-PCR was performed as described in Fig. 1B. The relative mRNA level of ACAT2 was expressed as fold to that in THP-1 cells, and the relative mRNA level of each transcription factor (C/EBPs, Cdx2, or HNF1α) in different cells was expressed as fold to that of C/EBPε in THP-1 cells. The data were obtained as indicated in Fig. 1B. (C) The fluorescence assay for the extracellularly excreted lipoproteins containing ACAT2-catalyzed NBD22-steryl esters. It was performed according to the procedures described in the ‘Materials and Methods’. The relative FI was expressed as percentage (%) to that in THP-1 cells. Results shown were representative of two independent experiments and data were shown as the mean ± SD of triplicates.
Inhibition of the lipoprotein excretion with the ACAT2 isoform-selective inhibitor PPPA in a dose-dependent manner
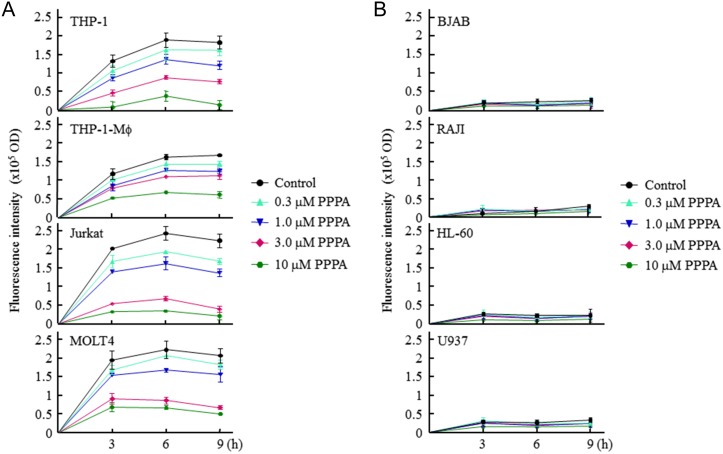
To demonstrate that NBD22-sterol was esterified by ACAT2, the human leukocytic cell lines were incubated with 0.5 μg/ml NBD22-sterol and the ACAT2 isoform-selective inhibitor PPPA at different concentrations (ranging from 0.3 to 10 μM). After the 3, 6, or 9 h of incubation, the FI of lipoproteins in the cultured media was measured. Figure 3A showed that in the four ACAT2-expressed leukocytic cell lines, the FI of lipoproteins in the cultured media was significantly decreased with the increase of PPPA concentration at each time-point, indicating that PPPA inhibits ACAT2-dependent excretion of lipoproteins containing ACAT2-catalyzed NBD22-steryl esters in a dose-dependent manner. Because the other four cell lines did not obviously express ACAT2 mRNA or excrete lipoproteins (right four cell lines in Fig. 2B,C), no inhibitory effect of PPPA was observed (Fig. 3B). These results demonstrate that the ACAT2 expression of human leukocytes is responsible for the excretion of lipoproteins containing CS/SE, and also the FI of lipoproteins containing NBD22-steryl esters specifically inhibited by PPPA is an indicator of the cellular ACAT2 activity.
Figure 3.
Effect of the isoform-selective inhibitor PPPA on the role of ACAT2 in the human leukocytic cell lines (A,B) The FI of lipoproteins in the cultured media of different human leukocytic cell lines incubated with PPPA. The fluorescence assay was performed as described in the ‘Materials and Methods’. Results shown were representative of two independent experiments and data were shown as the mean ± SD of triplicates.
IC50 values of PPPA for ACAT2 of the human leukocytic cell lines
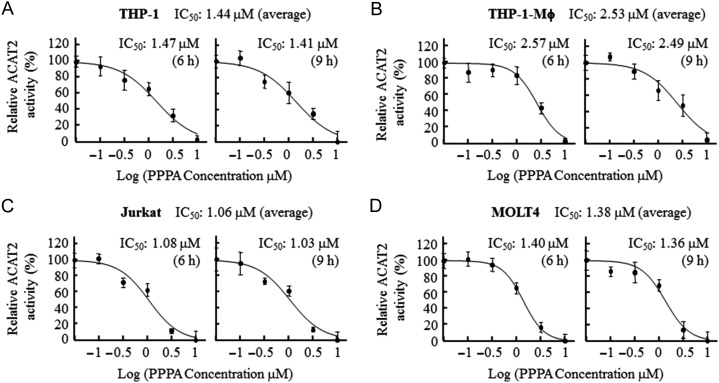
In addition, we further performed the fluorescence assay to measure the FI of lipoproteins containing ACAT2-catalyzed NBD22-steryl esters selectively inhibited by PPPA for calculating its half maximum inhibitory concentration (IC50) value. The results shown in Fig. 4 indicated that the IC50 values of PPPA with the 6 and 9 h of incubation from the same cell line were similar, but exhibited difference (consistent with that of ACAT2 mRNA level, Fig. 2B) among the individual cell lines. The average IC50 values of PPPA with the 6 and 9 h of incubation from the leukocytic cell lines THP-1 (1.44 μM) and MOLT4 (1.38 μM) were rarely different, but smaller than that from the macrophage THP-1-Mϕ (2.53 μM) and larger than that from the leukocytic cell line Jurkat (1.06 μM). These results confirm that the excretion of lipoproteins containing CS/SE depends on the ACAT2 activity of human leukocytes.
Figure 4.
IC50 values of PPPA for ACAT2 of the human leukocytic cell lines (A–D) The IC50 values of PPPA for ACAT2 of the four human leukocytic cell lines. The fluorescence assay was performed as described in the ‘Materials and Methods’. And the IC50 values of PPPA was obtained through non-linear fitting of the concentration-dependent curve by using Graphpad Prism 5 as described in the ‘Materials and Methods’. Results shown were representative of two independent experiments and data were shown as the mean ± SD of triplicates.
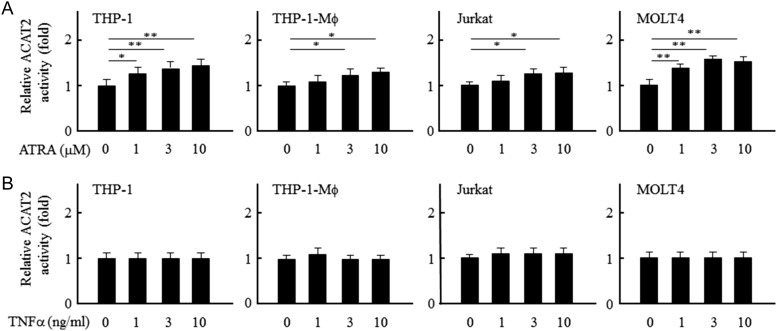
Effect of ATRA and tumor necrosis factor-α (TNFα) on the ACAT2-dependent lipoprotein excretion of the human leukocytic cell lines
With the ATRA-induced partial differentiation of THP-1 cells [29], we found that the expressions of ACAT2 and C/EBPs (α, β, and ε) were all evidently increased [26]. Therefore, we measured the FI of lipoproteins containing NBD22-steryl esters selectively inhibited by PPPA for the ACAT2-dependent lipoprotein excretion. The results showed that this lipoprotein excretion was evidently increased with the ATRA treatment, whereas it was not affected with the tumor necrosis factor-α (TNFα) treatment in the four ACAT2-expressing leukocytic cell lines (Fig. 5). Significantly, these results demonstrate that the differentiation-induced factor ATRA, but not the proinflammatory cytokine TNF-α [30], enhances the ACAT2-dependent lipoprotein excretion of human leukocytes or increases the ACAT2 activity.
Figure 5.
Effect of ATRA and TNFα on the ACAT2-dependent lipoprotein excretion of human leukocytic cell lines Relative ACAT2 activity of different human leukocytic cell lines with or without the treatment of ATRA (A) or TNFα (B). The fluorescence assay was performed as described in Fig. 2. The data were obtained as indicated in Fig. 4. Relative ACAT2 activity was expressed as fold to that of each cell line without the ATRA or TNFα treatment. Statistical analyses were done with Student's t-test. *P < 0.05, **P < 0.01.
Discussion
It is believed that ACAT2 is restricted to intestine and fetal liver in healthy human [1]. However, our previous studies have shown that in the human monocytic cells the low-level expression of ACAT2 gene with specific CpG-hypomethylated promoter is regulated by the C/EBP transcription factors [26]. Here, by investigating the human blood leukocytes and leukocytic cell lines, we first observed that in the human blood leukocytes, the same specific CpG sites (No. 2–7) of ACAT2 gene promoter are hypomethylated (Fig. 1A,B), and the mRNAs of ACAT2 and C/EBPs (α and ε) are consistently expressed (Fig. 1C). Then, with the available leukocytic cell lines, it was found that in the four leukocytic cell lines (THP-1, THP-1-Mϕ, Jurkat, and MOLT4), the ACAT2 mRNA expression attributable to the C/EBPs is correlated with the promoter-hypomethylated status of its gene, whereas in the other four (BJAB, RAJI, HL-60, and U937) there is no obvious ACAT2 expression mainly due to the very low-level expression of C/EBPs or together with the hypermethylated promoter (RAJI and U937). More importantly, by using a cell-based fluorescence assay with the NBD22-sterol (also an ACAT substrate) for measuring ACAT2 activity of cells expressing ACAT1 and/or ACAT2 [15], the functional studies indicated that the lipoprotein excretion is associated with the ACAT2 expression in the four human leukocytic cell lines. Furthermore, this lipoprotein excretion can be inhibited by the ACAT2 isoform-selective inhibitor PPPA in a dose-dependent manner, and IC50 values of PPPA are determined. These data demonstrate that the ACAT2 expression attributable to the C/EBPs in human leukocytes is responsible for the excretion of lipoproteins containing ACAT2-catalyzed CS/SE.
Additionally, we have previously reported that ACAT1 regulates the dynamics of free cholesterols in plasma membrane, which leads to the amyloid precursor protein (APP)-α-processing alteration, and postulated a model for the regulatory dynamic of free cholesterols in plasma membrane by the exclusive intracellular cholesterol esterification enzyme ACAT1 [31]. Our recent work has found that inhibiting cholesterol esterification in T cells by genetic ablation or pharmacological inhibition of ACAT1 leads to the potentiated effector function and enhanced proliferation of CD8+ but not CD4+ T cells, and therefore ACAT1 is a potential target for cancer immunotherapy [32]. In this study, we further found that ACAT2 is responsible for the lipoprotein excretion of human leukocytes. Thus, we are very interested in investigating the mechanism(s) for the regulatory roles between ACAT1 and ACAT2 in the human leukocytes in the near future.
Human monocyte-derived macrophages can produce discoidal and vesicular lipoprotein particles following and during the enrichment of macrophages with cholesterol from acetylated low density lipoprotein or cholesterol crystals [33]. But there is rare report about the lipoprotein excretion of other human leukocytes except for macrophages. Here, we also found that ATRA enhances the ACAT2-dependent lipoprotein excretion of human leukocytes. However, ATRA is used in several clinical trials for the treatment of acute promyelocytic leukemia (APL), one subtype of acute myeloid leukemia, and it is reported that APL undergoes differentiation in response to the treatment of ATRA [34–36]. More possibly, ACAT2 might function in ATRA-induced differentiation of APL through decreasing the excess intracellular cholesterols/sterols. But, it has to be clarified whether the enhanced ACAT2-dependent lipoprotein excretion of human leukocytes, and which subtype of lipoproteins containing ACAT2-catalyzed steryl esters, is involved in this clinical ATRA treatment.
ACAT2 inhibitor or elimination of ACAT2 has been used in atherogenic mouse models to improve hypercholesterolemia, atherosclerosis, and nonalcoholic fatty liver disease [37–39]. We have previously shown that in a subset of HCCs, the high-level expression of human ACAT2 gene with the CpG hypomethylation of its whole promoter synergistically regulated by Cdx2 and HNF1α is involved in the esterification of excess oxysterols transported from all extrahepatic tissues for excretion to avoid cytotoxicity, and postulated that specifically blocking this HCC-established cholesterol metabolic pathway may have potential therapeutic applications for HCC patients [25]. Here, we further reported that in the leukocytes, the low-level expression of human ACAT2 gene with specific CpG-hypomethylated promoter attributable to the C/EBPs is responsible for the excretion of lipoproteins containing ACAT2-catalyzed CS/SE, which may avoid cytotoxicity through decreasing the excess intracellular cholesterols/sterols (especially various oxysterols), and is essential for the action of the human leukocytes. So, it is possible that the low-level expression of ACAT2 in the leukocytes may play an important physiological role in the immune system. It is worthy to be further studied.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 31271377 and 31470802 to B.L.) and the National Institutes of Health (No. AG 037609 to T.C. and C.C.).
References
- 1.Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A:cholesterol acyltransferases. Am J Physiol Endocrinol Metab 2009, 297: E1–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang CC, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J Biol Chem 1993, 268: 20747–20755. [PubMed] [Google Scholar]
- 3.Song BL, Wang CH, Yao XM, Yang L, Zhang WJ, Wang ZZ, Zhao XN, et al. Human acyl-CoA:cholesterolacyl-transferase 2 gene expression in intestinal Caco-2 cells and in hepatocellular carcinoma. Biochem J 2006, 394: 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CC, Sakashita N, Ornvold K, Lee O, Chang ET, Dong R, Lin S, et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J Biol Chem 2000, 275: 28083–28092. [DOI] [PubMed] [Google Scholar]
- 5.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med 2000, 6: 1341–1347. [DOI] [PubMed] [Google Scholar]
- 6.Chang TY, Chang CC, Lin S, Yu C, Li BL, Miyazaki A. Roles of acyl-coenzyme A:cholesterol acyltransferase-1 and -2. Curr Opin Lipidol 2001, 12: 289–296. [DOI] [PubMed] [Google Scholar]
- 7.Repa JJ, Buhman KK, Farese RV Jr, Dietschy JM, Turley SD. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology 2004, 40: 1088–1097. [DOI] [PubMed] [Google Scholar]
- 8.Lee RG, Shah R, Sawyer JK, Hamilton RL, Parks JS, Rudel LL. ACAT2 contributes cholesteryl esters to newly secreted VLDL, whereas LCAT adds cholesteryl ester to LDL in mice. J Lipid Res 2005, 46: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 9.Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem 1998, 273: 26755–26764. [DOI] [PubMed] [Google Scholar]
- 10.Ikenoya M, Yoshinaka Y, Kobayashi H, Kawamine K, Shibuya K, Sato F, Sawanobori K, et al. A selective ACAT-1 inhibitor, K-604, suppresses fatty streak lesions in fat-fed hamsters without affecting plasma cholesterol levels. Atherosclerosis 2007, 191: 290–297. [DOI] [PubMed] [Google Scholar]
- 11.Ohshiro T, Rudel LL, Omura S, Tomoda H. Selectivity of microbial acyl-CoA:cholesterol acyltransferase inhibitors toward isozymes. J Antibiot 2007, 60: 43–51. [DOI] [PubMed] [Google Scholar]
- 12.Temel RE, Gebre AK, Parks JS, Rudel LL. Compared with Acyl-CoA:cholesterol O-acyltransferase (ACAT) 1 and lecithin:cholesterol acyltransferase, ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. J Biol Chem 2003, 278: 47594–47601. [DOI] [PubMed] [Google Scholar]
- 13.Bose C, Bhuvaneswaran C, Udupa KB. Age-related alteration in hepatic acyl-CoA:cholesterol acyltransferase and its relation to LDL receptor and MAPK. Mech Ageing Dev 2005, 126: 740–751. [DOI] [PubMed] [Google Scholar]
- 14.Lin S, Cheng D, Liu MS, Chen J, Chang TY. Human acyl-CoA:cholesterol acyltransferase-1 in the endoplasmic reticulum contains seven transmembrane domains. J Biol Chem 1999, 274: 23276–23285. [DOI] [PubMed] [Google Scholar]
- 15.Zhan Y, Zhang XW, Xiong Y, Li BL, Nan FJ. Design and synthesis of simple, yet potent and selective non-ring-A pyripyropene A-based inhibitors of acyl-coenzyme A:cholesterol acyltransferase 2 (ACAT2). Org Biomol Chem 2016, 14: 747–751. [DOI] [PubMed] [Google Scholar]
- 16.Sparrow CP, Patel S, Baffic J, Chao YS, Hernandez M, Lam MH, Montenegro J, et al. A fluorescent cholesterol analog traces cholesterol absorption in hamsters and is esterified in vivo and in vitro. J Lipid Res 1999, 40: 1747–1757. [PubMed] [Google Scholar]
- 17.Song W, Wang W, Wang Y, Dou L, Chen L, Yan X. Characterization of fluorescent NBD-cholesterol efflux in THP-1-derived macrophages. Mol Med Rep 2015, 12: 5989–5996. [DOI] [PubMed] [Google Scholar]
- 18.van Meer G, Stelzer EH, Wijnaendts-van-Resandt RW, Simons K. Sorting of sphingolipids in epithelial (Madin-Darby canine kidney) cells. J Cell Biol 1987, 105: 1623–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koval M, Pagano RE. Sorting of an internalized plasma membrane lipid between recycling and degradative pathways in normal and Niemann-Pick, type A fibroblasts. J Cell Biol 1990, 111: 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chattopadhyay A, London E. Spectroscopic and ionization properties of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids in model membranes. Biochim Biophys Acta 1988, 938: 24–34. [DOI] [PubMed] [Google Scholar]
- 21.Li NC, Fan J, Papadopoulos V. Sterol carrier protein-2, a nonspecific lipid-transfer protein, in intracellular cholesterol trafficking in testicular Leydig cells. PLoS One 2016, 11: e0149728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fery-Forgues S, Fayet JP, Lopez A. Drastic changes in the fluorescence properties of NBD probes with the polarity of the medium: involvement of a TICT state. J Photochem Photobiol B 1993, 70: 229–243. [Google Scholar]
- 23.Mukherjee S, Chattopadhyay A, Samanta A, Soujanya T. Dipole moment change of NBD group upon excitation using solvatochromic and quantum chemical approaches: implications in membrane research. J Phys Chem 1994, 98: 2809–2812. [Google Scholar]
- 24.Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J Lipid Res 2004, 45: 378–386. [DOI] [PubMed] [Google Scholar]
- 25.Lu M, Hu XH, Li Q, Xiong Y, Hu GJ, Xu JJ, Zhao XN, et al. A specific cholesterol metabolic pathway is established in a subset of HCCs for tumor growth. J Mol Cell Biol 2013, 5: 404–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo D, Lu M, Hu X, Xu J, Hu G, Zhu M, Zhang X, et al. Low-level expression of human ACAT2 gene in monocytic cells is regulated by the C/EBP transcription factors. Acta Biochim Biophys Sin 2016, 48: 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng W, Kvilekval KV, Abumrad NA. Dexamethasone enhances accumulation of cholesterylesters by human macrophages. Am J Physiol 1995, 269: E642–E648. [DOI] [PubMed] [Google Scholar]
- 28.Yang L, Yang JB, Chen J, Yu GY, Zhou P, Lei L, Wang ZZ, et al. Enhancement of human ACAT1 gene expression to promote the macrophage-derived foam cell formation by dexamethasone. Cell Res 2004, 14: 315–323. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi H, Breitman TR. Induction of functional differentiation of a human monocytic leukemia cell line (THP-1) by retinoic acid and cholera toxin. Jpn J Cancer Res 1985, 76: 345–351. [PubMed] [Google Scholar]
- 30.Zhu M, Lei L, Zhu Z, Li Q, Guo D, Xu J, Chen J, et al. Excess TNF-α in the blood activates monocytes with the potential to directly form cholesteryl ester-laden cells. Acta Biochim Biophys Sin 2015, 47: 899–907. [DOI] [PubMed] [Google Scholar]
- 31.Zhu M, Zhao X, Chen J, Xu J, Hu G, Guo D, Li Q, et al. ACAT1 regulates the dynamics of free cholesterols in plasma membrane which leads to the APP-α-processing alteration. Acta Biochim Biophys Sin 2015, 47: 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature 2016, 531: 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruth HS, Skarlatos SI, Gaynor PM, Gamble W. Production of cholesterol-enriched nascent high density lipoproteins by human monocyte-derived macrophages is a mechanism that contributes to macrophage cholesterol efflux. J Biol Chem 1994, 269: 24511–24518. [PubMed] [Google Scholar]
- 34.Kropf PL, Wang L, Zang Y, Redner RL, Johnson DE. Dasatinib promotes ATRA-induced differentiation of AML cells. Leukemia 2010, 24: 663–665. [DOI] [PubMed] [Google Scholar]
- 35.Ghavamzadeh A, Alimoghaddam K, Ghaffari SH, Rostami S, Jahani M, Hosseini R, Mossavi A, et al. Treatment of acute promyelocytic leukemia with arsenic trioxide without ATRA and/or chemotherapy. Ann Oncol 2006, 17: 131–134. [DOI] [PubMed] [Google Scholar]
- 36.Gu ZM, Wu YL, Zhou MY, Liu CX, Xu HZ, Yan H, Zhao Y, et al. Pharicin B stabilizes retinoic acid receptor-α and presents synergistic differentiation induction with ATRA in myeloid leukemic cells. Blood 2010, 116: 5289–5297. [DOI] [PubMed] [Google Scholar]
- 37.Ohshiro T, Matsuda D, Sakai K, Degirolamo C, Yagyu H, Rudel LL, Omura S, et al. Pyripyropene A, an acyl-coenzyme A:cholesterol acyltransferase 2-selective inhibitor, attenuates hypercholesterolemia and atherosclerosis in murine models of hyperlipidemia. Arterioscler Thromb Vasc Biol 2011, 31: 1108–1115. [DOI] [PubMed] [Google Scholar]
- 38.Ohshiro T, Tomoda H. Acyltransferase inhibitors: a patent review (2010-present). Expert Opin Ther Pat 2015, 25: 145–158. [DOI] [PubMed] [Google Scholar]
- 39.Lopez AM, Posey KS, Turley SD. Deletion of sterol O-acyltransferase 2 (SOAT2) function in mice deficient in lysosomal acid lipase (LAL) dramatically reduces esterified cholesterol sequestration in the small intestine and liver. Biochem Biophys Res Commun 2014, 454: 162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]