Abstract
Aims
Previously we have demonstrated altered microglia P2X4R expression in response to alcohol and pharmacological blockade with a selective P2X4R antagonist can reverse the action, suggesting that P2X4R play a role in mediating alcohol-induced effects on microglia. In the present study, we investigated the underlying signaling mediators, which may play a role in modulating P2X4R expression in microglia cells in response to alcohol.
Methods
Embryonic stem cell-derived microglia (ESdM) cells were used to investigate the potential mechanisms involved in the regulation of P2X4R in response to alcohol. Selective P2X4R antagonist and kinase inhibitors were used to further corroborate the signal transduction pathway through which alcohol modulates P2X4R expression in microglia.
Results
Alcohol (100 mM) suppressed phosphorylated AKT and ERK cascades in native ESdM cells. This alcohol-induced suppression was confirmed to be P2X4R-dependent through the use of a selective P2X4R antagonist and knockdown of P2XR4 by siRNA. Alcohol increased transcriptional activity of CREB. P2X4R antagonist blocked alcohol-induced effects on CREB, suggesting a P2X4R-mediated effect.
Conclusion
These findings provide important clues to the underlying mechanism of purinoceptors in alcohol-induced microglia immune suppression.
INTRODUCTION
Microglial cells, the resident immune cells of the central nervous system (CNS), are important mediators of immune response (Graeber et al., 2011), and maintenance of the CNS (Perry and Teeling, 2013). Microglial surface receptors are important sensory tools for a myriad of microglial functions. A growing body of evidence has indicated that purinergic receptors are important mediators of microglia signaling including phagocytosis, proliferation, migration and immune response leading to the release of chemokines and cytokines (Inoue, 2008; Kettenmann et al., 2011). Purinergic receptors are also vital sensory receptors responsible for microglial function and downstream activation (Burnstock, 2015) and play a pivotal role in regulation of neuro-inflammatory and neurodegenerative processes. Purinergic receptors regulate microglial activation via ATP, which is a common pathological indicator of insult or injury (Verkhratsky et al., 2009).
Alcohol is known to alter the neuropathology of the brain and attenuate many microglial functions including phagocytosis, migration and proliferation (Rimland and Hand, 1980; Aroor and Baker, 1998; Suk, 2007; Nixon et al., 2008; Karavitis et al., 2012). Recent evidence demonstrates that P2X4R is an alcohol-sensitive receptor (Ostrovskaya et al., 2011) and is an important mediator of alcohol-induced effects. We recently demonstrated that alcohol increases mRNA and protein expression of P2X4R in microglia (Gofman et al., 2014). Furthermore we have shown that alcohol-induced P2X4R expression affects microglial function including migration and phagocytosis which can be reversed via the P2X4R selective antagonist, 5-BDBD (Gofman et al., 2014). Studying alcohol-induced microglial dysfunction through purinergic receptor X4 could shed light on the microglial function, since P2X4R has been shown to be an alcohol-sensitive receptor (Ostrovskaya et al., 2011).
Emerging evidence highlights a central role for the mitogen-activated protein kinase (MAPK) family in alcohol use (Gonzaga et al., 2014). The MAPK signaling cascade plays an essential role in the initiation of cellular processes such as proliferation, differentiation, development, apoptosis, stress and inflammatory responses (Kim and Choi, 2010). Activation of intracellular signaling pathways involving phosphatidylinositol 3-kinase (PI3K)-Akt and mitogen-activated protein kinase kinase (MAPK kinase, MEK), extracellular signal-regulated kinase (ERK), and has been shown to have distinct roles in the up-regulation of P2X4R expression in microglia (Tsuda et al., 2009). Transcriptional activity such as CREB, NFAT and NFκB have been linked with regulation of purinergic receptor expression (Brautigam et al., 2005).
In the current study, we sought to determine the signal transduction pathway through which alcohol modulates P2X4R expression in microglia. Downstream signaling of purinergic receptors has been extensively studied; evidence shows their role in trophic events such as proliferation (Monif et al., 2010; Ohtani et al., 2011), immune response (Stokes and Surprenant, 2009), and activation through mechanisms associated with MAPK (Markou et al., 2003; Katz et al., 2008) and transcription factors (Inoue, 2006). Our data indicates that alcohol decreases phosphorylation of key regulatory proteins and increases CREB transcriptional activity in a P2X4R-dependent manner. Elucidating alcohol-induced P2X4R cell signaling mechanisms may address the importance of purinergic receptors in microglial immune functions and potentially other neuroprotective/neurodegenerative functions linked to microglia activity.
MATERIALS AND METHODS
Cell culture
Embryonic stem cell-derived microglia (ESdM) were a generous gift from Dr Harald Neumann (University of Bonn Germany; Bonn, Germany), and were prepared according to the method described previously (Napoli et al., 2009; Beutner et al., 2010). In brief, ESdM cells were cultured in DMEM/F12 50:50 (Cellgro, Manassas, VA, USA) containing N2 supplement (Invitrogen, Carlsbad, CA, USA) with 0.048 mM L-glutamine (Cellgro), 1% D-glucose (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin/streptomycin (Cellgro) (Gofman et al., 2014).
Antibodies and reagents
Antibodies were purchased from the following sources: pMEK, MEK, pERK, ERK, pAKT, and AKT (Cell Signaling, Danvers, MA, USA), inhibitors for signaling experiments U0126 and PD9895 (Cell Signaling), β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Alcohol 200 proof (Pharmaco-Aaper, Brookfield, CT, USA), lipopolysaccharide was purchased from Sigma-Aldrich, 5-BDBD (dissolved in DMSO) was purchased from Tocris (Bristol, UK). BCA protein assay reagent, Precast SDS protein gels 4–20%, Super Signal West Femto, and Pico Chemiluminescent Substrate were purchased from Thermo Scientific, Hudson, NH, USA), CelLytic-M Reagent (Sigma-Aldrich), 6x SDS loading buffer (Morganville Scientific, Morganville, NJ, USA), BSA (US Biologicals, Pittsburgh, PA, USA) TransAM™ CREB, and the nuclear extract kit were purchased from Active Motif (Carlsbad, CA, USA).
Knockdown of P2X4R via siRNA
P2X4R mRNA and protein expression in ESdM cells was silenced by siRNA transfection for 48 h. P2rx4 Trilencer-27 Mouse siRNA, 5 nM P2X4R siRNA or 5 nM Scrambled siRNA (OriGene Technologies; Rockville, MD, USA), along with jetPRIME transfection reagent (Polypus transfection™; Bioparc, France) were used to transfect ESdM cells to obtain sufficient knockdown. Briefly, ESdM cells were seeded at a density of 1 × 105 in T25 flasks. Transfection reaction mixture contained, 10 nM P2X4R siRNA or scrambled siRNA, 400 µl jetPRIME buffer, and 8 µl jetPRIME transfection reagent was incubated together to form duplexes (according to manufacturers recommendations). Cells were transfected for 24 h, following a media change for 48 h, cells were treated with alcohol (100 mM) for 48 h and used to evaluate downstream signaling studies.
RNA extraction and real-time qPCR
Total RNA was isolated using the Quick-RNA™ MiniPrep as per manufacturer's instructions (Zymo Research; Irvine, CA, USA). RNA purity and concentration was determined using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). Conversion to cDNA was performed by reverse transcription using [1 µg of total RNA] the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems; Foster City, CA, USA). qPCR was performed on an Applied Biosystems StepOnePlus Real-Time PCR System (Applied Biosystems). P2X4R-specific siRNA knockdown was confirmed by, qPCR primers against P2X4R, purchased from Life Technologies (Cat # Mm00501787_m1). The PCR conditions consisted of an initial melting cycle at 95°C for 15 min, followed by 40 cycles of amplification at 95°C for 15 s (denaturation), 60°C for 30 s (annealing), and 72°C for 30 s (extension). Raw data were analyzed using the ΔΔCt method (relative quantification). Results were expressed in relative gene expression levels (fold change) compared with the untreated control.
Western blotting
ESdM cells (250,000 cells/flask) were treated with alcohol (100 mM) for 5, 10, 15, 30, 45 and 60 min prior to cell lysis. Several studies have also demonstrated the use of 100 mM alcohol for protein work and its physiological relevance (Sanna et al., 2002; Gofman et al., 2014). For kinase experiments, U0126 (10 µM) was add to the cells for 30 min prior to alcohol treatment, and PD98095 (20 µM) was added for 1 h prior to alcohol treatment; for antagonist work, cells were pretreated with P2X4R antagonist 5-BDBD dissolved in DMSO (Tocris, 10 µM) for 30 min prior to alcohol treatment. For signaling studies, ESdM whole cell lysates were prepared using the CelLytic-M reagent (Sigma-Aldrich) with 1X dilution of a broad specific protease/phosphatase inhibitor cocktail (Thermo Scientific). Cell lysates were vortexed and centrifuged at 4°C for 13,000 rpm, 15 min. Protein concentrations were estimated using the BCA method (Thermo Scientific) (Ramirez et al., 2013). Lysates were loaded at 20 µg mixed with 6X loading buffer containing β-ME which were boiled for 10 min at 95°C. Samples (20 µg) were loaded and resolved by precast SDS-PAGE 4–20% gradient gels (Thermo Scientific). Proteins were transferred to a nitrocellulose membranes by electrophoretic transfer, then blocked in 5% BSA in 1X TBS/0.1% Tween 20 for 1 h. The primary antibodies were diluted in 5% BSA in 1X TBS/0.1% Tween 20 and incubated overnight with polyclonal antibodies anti pMEK (1:500), MEK (1:1000), pERK (1:1000), ERK (1:1000), pAKT (1:1000), AKT (1:1000), or β-actin (1:2000) protein loading control (Santa Cruz Biotechnology) were used to detect target proteins. All primary antibodies were incubated with the membranes overnight at 4°C with gentle shaking. Species-specific peroxidase-conjugated secondary antibodies (diluted 1:1000) (Thermo Scientific) were incubated with the membranes for 1 h at room temperature. Species-specific secondary antibodies conjugated to HRP (Thermo Scientific) were then added and incubated for 1 h at room temperature, and detected using Supersignal West Femto or Pico chemiluminescence substrate (Thermo Scientific). Chemiluminescence signal detection was performed with the gel documentation system G:Box Chemi HR16 (Syngene). Densitometry ratiometrics for phosphorylated protein were normalized to total protein and then normalized to actin for each time point. Densitometry analysis was performed with the GeneTools software package (Syngene, Cambridge, UK).
Evaluation of transcription factor activity
ESdM cells were seeded at a density of 600,000 cells and allowed to reach confluence over 4 days. ESdM cells were treated with alcohol (100 mM) over the course of 24 h to determine the maximum transcriptional activity for CREB (Supplementary Fig. S4). Nuclear extracts were isolated using the nuclear extract kit as per manufacturer's instructions (Active Motif). Transcription activity was measured by the TransAM™ CREB protein assay (Active Motif), according to the manufacturer's protocol and analyzed using a Molecular Devices (Sunnyvale, CA, USA) microplate reader M5 at an absorbance of 450 nm. Data are presented as fold change.
Brain-derived neurotrophic factor ELISA
Brain-derived neurotrophic factor (BDNF) in supernatant culture fluid was estimated using a custom Meso Scale Discovery assays (MSD) (Rockville, MD, USA). Briefly, supernatants were collected, centrifuged to remove cellular debris, and concentrated using the Amicon 3K Centrifugal Filter Units (EMD Millipore; Darmstadt, Germany). Two hundred microliters of samples and standards diluted in PBS containing 0.03% Triton were added and incubated overnight at room temperature. Detection antibody solution was added to the wells, followed by further incubation for 1.5 h with vigorous shaking at room temperature. Plate were washed 3X with PBS+ 0.05% Tween-20 and 150 µl of 2X Read Buffer was added to each well. Cytokine levels were estimated using provided standards and calculated by the SECTOR®Imager 2400A and MSD reader software.
Statistical analysis
Data were analyzed using either the Student's t-test for independent means or a one-way analysis of variance (ANOVA) followed by post hoc Student–Newman–Keuls test to determine which conditions were significantly different from each other, and a Dunnett's post-test for multiple comparisons. Results are expressed as mean values (±SEM), with standard errors deemed statistically significant when P < 0.05 (marked in the figures as *P < 0.05; **P < 0.01; ***P < 0.002).
RESULTS
Alcohol decreases pAKT and pERK in microglial cells
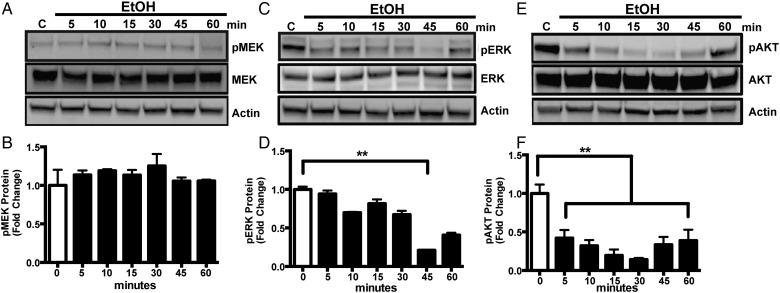
P2X4R signaling is crucially important for microglial function. P2X4R act as pathological sensors and regulate microglial phagocytosis (Stokes and Surprenant, 2009), migration (Ohsawa and Kohsaka, 2011), and proliferation (Nixon et al., 2008). Several signaling pathways, including MAPK/ERK and AKT, are coupled to P2 receptors, which are important for microglial function. Since our data showed that alcohol increases expression of P2X4R in microglia (Gofman et al., 2014), we sought to determine the signaling mediators involved in alcohol-induced P2X4R expression in microglial cells. Thus, we first tested the effect of alcohol on the levels of phosphorylated MEK, ERK and AKT via western blotting. Changes in protein levels were analyzed by one-way ANOVA and a Bonferroni post hoc test for multiple comparisons. ESdM cells were treated with alcohol (100 mM) for 5, 10, 15, 30, 45 and 60 min, and then whole cell lysates were used to assess phosphorylated and total protein levels for MEK, ERK, and AKT. Our data are similar to previous experiments that have also demonstrated alcohol's ability to decrease ERK and AKT phosphorylation (Sanna et al., 2002). Alcohol (100 mM) decreased pERK protein level in a time-dependent manner (Fig. 1C and D) compared with control. Similarly alcohol treatment dramatically decreased pAKT in a time-dependent manner when compared with the control (Fig. 1E and F), however interestingly protein level of pMEK (Fig. 1A and B) remained unchanged. Taken together, these data suggest that alcohol regulates phosphorylation of ERK and AKT protein levels.
Fig. 1.
Alcohol decreases pERK and pAKT in microglial cells. (A, C and E) Representative immunoblot images of pMEK and MEK, pERK and ERK or pAKT and AKT proteins in whole cell lysates from ESdM cells treated with alcohol (100 mM) for 5–60 min, subjected to western blot analysis and probed with anti-pMEK, anti-MEK, or anti-pERK, anti-ERK or, anti-pAKT, anti-AKT, and then anti-β-actin antibody. (B, D and F) Graphical representation of fold change ratio of pMEK, pERK, and pAKT to total MEK, ERK, and AKT, respectively, normalized to β-actin loading control ± SEM. Results are expressed as the ratio of phosphorylated MEK, ERK or AKT to their respective total proteins, normalized to the internal standard actin. Note: actin was used as a loading control. Data were analyzed by one-way ANOVA; data points represent the mean ± SEM (n = 3), *P < 0.05, **P < 0.01, ***P < 0.002.
Deregulations of the constituent kinases play a role in alcohol-induced suppression of ERK phosphorylation
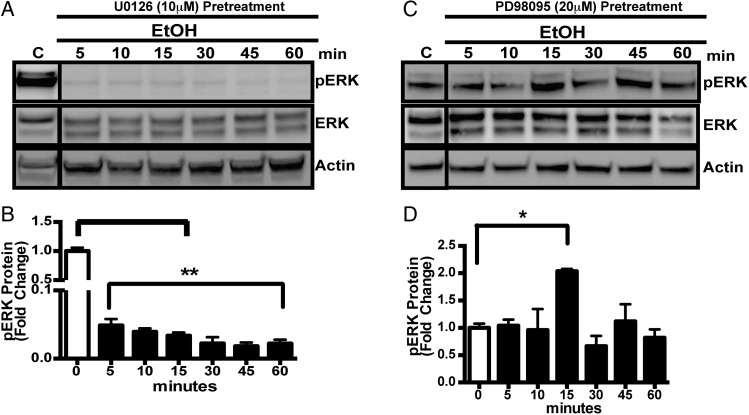
Dual-specific kinases, MEK-1/2 upon activation phosphorylate their best-known substrates, Erk1/2. However, since MEK isoforms are differentially regulated (Schaeffer and Weber, 1999) and may mediate different biological responses we examined the role of MEK1 and MEK2 isoforms on ERK phosphorylation in ESdM cells. ESdM cell were treated with U0126 (10 µM; Cell Signaling Technologies) a highly selective inhibitor of both MEK1 and MEK2 (Tsuda et al., 2009) for 30 min or PD98059 (20 µM; Cell signaling Technologies) that inhibits MEK1 more potently than MEK2 for 1 h prior to alcohol treatment (100 mM) for 5, 10, 15, 30, 45 and 60 min. U0126 pretreatment inhibited the alcohol-induced phosphorylation of ERK completely (Fig. 2A and B). However, PD98095 was not able to inhibit pERK in the presence of alcohol suggesting that MEK 2 may play a role in the pERK in the presence of alcohol. Relative protein levels are presented as a ratio of phosphorylated ERK and AKT to their respective total proteins normalized to an actin internal standard. The results are shown as the average regulation from three different experiments. Taken together, these data suggest that alcohol probably differentially regulates ERK phosphorylation through a MEK 2 dependent pathway.
Fig. 2.
(A) Representative western blotting images of pERK and total ERK protein level in whole cell lysates from ESdM cells pretreated with U0126 (10 µM) for 30 min prior to treatment with alcohol (100 mM) for 5–60 min, subjected to western blotting analysis and probed with anti-ERK, anti-ERK, then anti-β-actin antibody. (B) Graphical representation of fold change of ERK level when compared with total ERK control normalized to β-actin loading control ± SEM. (C) Representative western blot images of pERK and total ERK protein in whole cell lysates from ESdM cells pretreated with PD98095 (20 µM) for 1 h prior to alcohol (100 mM) treatment for 5–60 min, subjected to western blot analysis and probed with anti-ERK, anti-ERK, then anti-β-actin antibody. (D) Graphical representation of fold change of pERK expression as compared with total ERK normalized to β-actin loading control ± SEM. Data were analyzed by one-way ANOVA; data points represent mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.002.
P2X4R receptor antagonist reverses the effect of alcohol on AKT and ERK
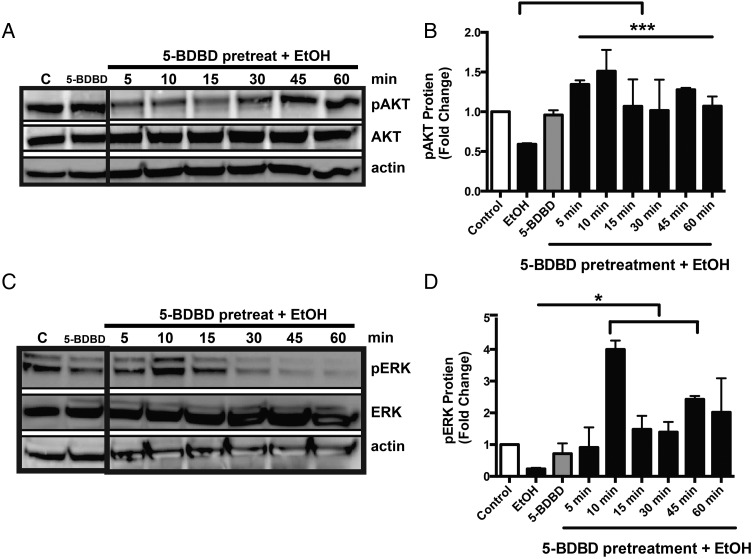
To determine the role of P2X4R in alcohol-induced decrease of ERK and AKT phosphorylated proteins, we utilized a P2X4R selective antagonist, 5-BDBD (Gofman et al., 2014). P2X4R is an alcohol-sensitive receptor (Ostrovskaya et al., 2011) and previously we have demonstrated its efficacy to reverse alcohol's effect on microglial function including migration and phagocytosis (Gofman et al., 2014). Microglial cells were pretreated with 5-BDBD (10 µM) for 30 min prior to alcohol treatment (100 mM). Our results demonstrate microglia pretreatment with 5-BDBD prior to alcohol treatment was able to block the effect alcohol has on phosphorylated ERK (Fig. 3C and D). The expression of phosphorylated AKT was also evaluated; pretreatment with 5-BDBD increased the expression of phosphorylated AKT, and prevented the decreased expression as with alcohol alone (Fig. 3A and B). These data demonstrate alcohol's critical role in modulating downstream signaling targets, specifically pERK and pAKT via P2X4R. 5-BDBD, a P2X4R selective antagonist, was able to block the effect of alcohol on phosphorylated protein expression, suggesting that P2X4R regulates alcohol-induced effects of downstream ERK-MAPK and AKT pathways. These data demonstrate the critical role of alcohol in modulating downstream signaling targets, specifically pERK and pAKT via P2X4R.
Fig. 3.
P2X4R receptor antagonist reverses the effect of alcohol on AKT and ERK. In vitro protein levels of phosphorylated ERK and AKT were assessed after pretreatment with 5-BDBD, a P2X4R selective antagonist, before alcohol treatment. ESdM cells (5 × 105) were pretreated with P2X4R antagonist (10 µM) then treated with alcohol (100 mM) for 5–60 min. Whole cell lysates were used to assess phosphorylated and total ERK and AKT. (A) Treatment of P2X4R antagonist reversed the alcohol effect on AKT phosphorylation at 5 min. (B) Fold change of pAKT expression when compared with total AKT normalized to β-actin loading control ± SEM. (C) Treatment with 5-BDBD was able to reverse the alcohol effect on ERK phosphorylation. (D) Fold change of pERK when compared with total ERK normalized to β-actin loading control ± SEM. Data were analyzed by one-way ANOVA; data represent mean ± SEM (n = 3).
P2X4R siRNA confirms 5-BDBD pharmacological studies
To confirm the role of P2X4R on alcohol induced on ERK phosphorylation and substantiate pharmacological studies we knockdown P2X4R expression in ESdM cells by siRNA. P2X4R-specific siRNA selectively knocked down the target as assessed by qPCR (Supplementary Fig. S1). P2X4R gene expression was silenced for about 48 h continued with alcohol treatment for up to 48 h. ERK phosphorylation in cells transfected with P2X4R siRNA significantly (P = 0.05) reversed alcohol effect pERK when compared with cells treated with alcohol alone (Supplementary Fig. S2A and B), suggesting that P2X4R regulates alcohol-induced effects on ERK phosphorylation.
P2X4R antagonist selectively decreases alcohol-induced transcription activity
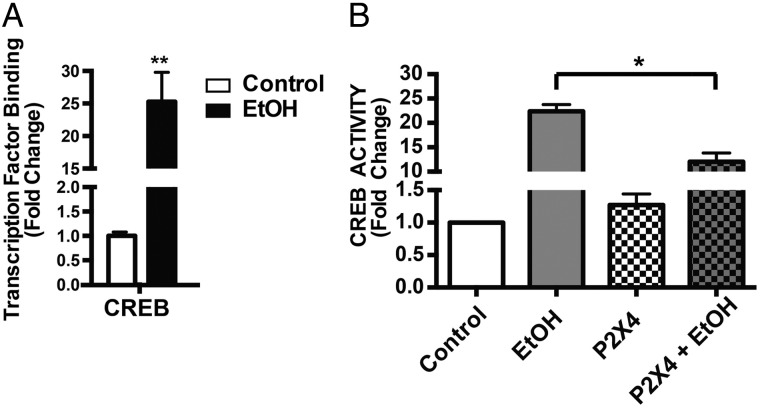
In our previous study, we demonstrated alcohol up-regulated P2X4R protein expression in microglial cells (Gofman et al., 2014). To determine the downstream signaling mechanism by which this occurs we investigated alcohol effects on MAPK/ERK signaling cascade, which demonstrated the partaking of P2X4R (Fig. 3). These cellular signaling mechanisms positively regulate transcription factors promoting P2X4R receptor expression. Purinergic receptors are linked to many downstream cellular mechanisms regulating proliferation, migration and inflammatory responses induced by transcription factors (Armstrong et al., 2007). In particular we focused our study on the effects of alcohol on CREB based on the involvement of MAPK/ERK, and their regulation through purinergic receptors (Inoue, 2006; Tsuda et al., 2009). Activity of transcription factor was determined using Trans AM transcription assay. Preliminary experiments performed to determine time kinetics (0.5, 1, 2, 4, 6, 8, 12, 16, 18 and 24 h) showed, microglia cells were treated with alcohol (100 mM) for 2 h showed maximum peak activity and was time depended as the activity reached back to control levels after 12 h (Supplementary Fig. S4). ESdM after 2 h of alcohol (100 mM) treatment had 24-fold higher CREB binding when compared with control (Fig. 4A), suggesting alcohol's role in modulating CREB activity in ESdM cells.
Fig. 4.
P2X4R antagonist selectively decreases alcohol-induced transcription activity. (A) Alcohol promotes transcriptional activity of CREB. ESdM cells (6 × 105) were grown for 4 days to confluence and treated with alcohol (100 mM) for 2 h. Nuclear protein extracts were harvested CREB activity were measured by TransAM™ CREB protein assay. Microglial cells treated with alcohol for 2 h showed a statistically significant increase in CREB (*P < 0.001) when compared with control. Data are represented as fold change compared with untreated control. Data were analyzed using a two-tailed t-test. Data points represent mean ± SEM (n = 3). (B) Treatment with 5-BDBD significantly blocked CREB activity compared with alcohol treatment alone (***P < 0.0001). Data are represented as fold change compared with control. Results were determined using a two-way ANOVA with Bonferroni post hoc for multiple comparisons. Data points represent mean ± SEM (n = 3).
To determine the role of P2X4R in alcohol-induced increased transcription factor activity, we pretreated ESdM cells with a P2X4R selective antagonist, 5-BDBD (5 µM), prior to alcohol treatment. Two-way ANOVA revealed significant decrease in CREB binding [interaction: F(1,6) = 38.94, P < 0.0008) increase; alcohol: F(1,6) = 355.2, P = 0.0001; antagonist: F(1,6) = 35.14, P = 0.001] (Fig. 4B). These data suggest a possible role for CREB transcriptional activity, and regulating alcohol-induced increase of P2X4R receptors expression in microglial cells, since CREB is known to promote P2X4R expression (Impey et al., 2004).
5-BDBD. blocks effects of alcohol on BDNF
The current literature demonstrate acute and chronic ethanol exposure have been shown to modulate function of the activity-dependent gene transcription factor, cAMP-responsive element binding (CREB) (Moonat et al., 2010). Other studies have identified several important CREB-related genes, such as neuropeptide Y, brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein, and corticotrophin-releasing factor, that may play a crucial role molecular changes in the brain (Moonat et al., 2010). We have demonstrated that alcohol treatment in ESdM cells increases the expression of BDNF in cell culture media Supplementary Fig. S3. To determine whether P2X4R play a role in the increased expression of BDNF, we utilized 5-BDBD. Pretreatment with 5-BDBD prior to alcohol treatment significantly blocked the effects of alcohol on BDNF expression in ESdM cells (P = 0.03).
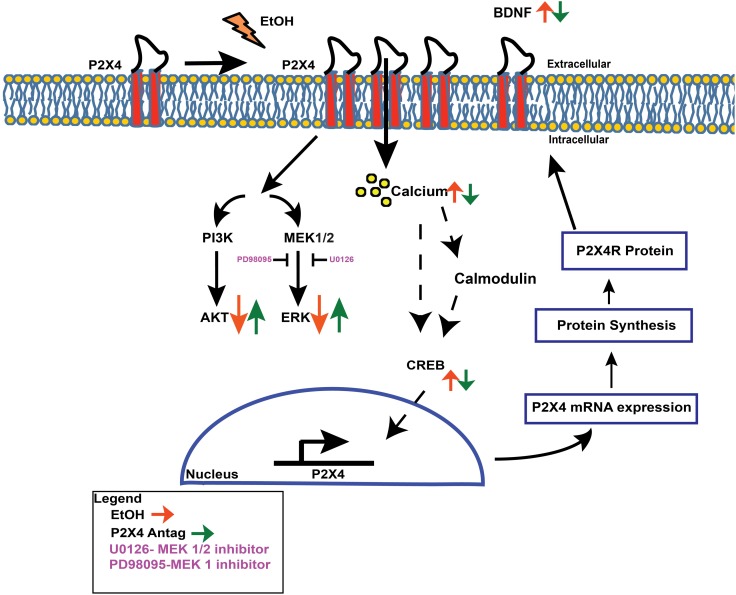
Model: proposed alcohol-induced P2X4R regulation in microglia
In Fig. 5, we propose a model for the effect of alcohol on P2X4R expression. Activation of the gene transcription factors, CREB has been strongly associated with alcohol use and abuse. The activation of downstream transcription factors such as CREB increases BDNF. One possible mechanism by which alcohol increases P2X4R expression, could involve CREB. Expression of BDNF in microglia after alcohol exposure was elevated when compared with control (P = 0.05) (Supplementary Fig. S3). To determine if P2X4R is involved in increased levels of BDNF we utilized the P2X4R antagonist, 5-BDBD prior to alcohol exposure and evaluated the expression of BDNF. Treatment with 5-BDBD prior to alcohol treatment altered the level of secreted BDNF, when compared with alcohol treatment alone (P = 0.03). These data suggests the importance of P2X4R as an important modulator of alcohol-mediated microglial function.
Fig. 5.
Schematic representation: potential mechanism for alcohol-induced P2X4R receptor regulation in microglia. Alcohol treatment increases P2X4R receptor expression in microglial cells. To determine the possible mechanisms involved in P2X4R expression, the effects of alcohol on calcium and pERK, pAKT and NT factors, including BDNF were evaluated. Alcohol increased calcium mobilization, decreased pERK and pAKT and increased BDNF levels. Alcohol did not affect total MEK expression and inhibitors U0126 and PD98095 completely blocked the alcohol effect and partially blocked pERK expression, respectively. To determine the role of P2X4R in regulating alcohol-induced effects, we utilized a P2X4R selective antagonist, 5-BDBD, prior to alcohol treatment. Pretreatment with 5-BDBD reversed the effects of alcohol. As with purinergic receptor activity, alcohol was able to mobilize calcium by modulating CREB transcription factor binding, where CREB is able to translocate to the nucleus of the cell and bind to regions that promote P2X4R gene expression, which may be responsible for the increase of P2X4R upon alcohol treatment.
DISCUSSION
Increasing evidence demonstrates the importance of microglial cells in CNS pathology; more important are the purinergic receptors that regulate multiple aspects of cell activity and function including proliferation, migration, phagocytosis, activation, release of cytokines and chemokine (Burnstock, 2015). Ionotropic P2X receptor subtype P2X4R has emerged as a key regulator of microglial functions and has been implicated in neurodegenerative and neuroimmune disorders. Alcohol abuse hinders microglial immune response and dampens microglial function. P2X4R receptors have been shown to be an alcohol-sensitive receptor (Popova et al., 2013). Moreover, our group has demonstrated that alcohol modulates P2X4R expression which play a role in regulating alcohol-induced responses in microglia (Gofman et al., 2014).
In our current studies, we sought to investigate the signaling mechanisms by which alcohol increases P2X4R receptor expression in microglial cells. Several signaling pathways including MAPK/ERK and AKT are coupled to P2 receptors, which are important for microglial function (Brautigam et al., 2005). Downstream intracellular signaling pathways involving (PI3K)-Akt and MAPK-ERK cascade have a distinct role in regulation of P2X4R (Markou et al., 2003). Western blot analysis of microglial cells treated with alcohol (100 mM) demonstrated decrease in phosphorylated ERK and AKT, respectively (Fig. 1B and C) that returned to basal protein levels compared with control by 60 min suggesting that alcohol has a transient effect on both ERK and AKT protein targets. Our data are in line with previous studies that have demonstrated alcohol's ability to decrease ERK and AKT phosphorylation and changes in phosphorylation of these proteins depends on duration and dose of alcohol.
To explore the signaling mediator that could be involved in alcohol-induced suppression of ERK phosphorylation we adapted strategies to pharmacologically inhibit dual-specificity kinases (MEK 1/2) that mediate ERK1/2 activation. U0126, a highly selective inhibitor of both MEK1 and MEK2 prior to alcohol treatment completely blocked the phosphorylation of ERK protein expression (Fig. 2A and B) while PD98095, a potent inhibitor of MEK1 but a weak inhibitor of MEK2 had a modest effect on phosphorylated ERK1/2 (Fig. 2C and D) suggesting alcohol differentially regulates ERK phosphorylation through a MEK 2-dependent pathway. Contrary to this finding in Fig. 1A, interestingly phosphorylated MEK protein expression was not affected by alcohol. In line with these finding it is conceivable that the differentially regulation MEK-1 and MEK-2 heterodimer could come into play where negative regulation of one dual-specificity kinases might result in compensatory phosphorylation of the second (Catalanotti et al., 2009). Alternatively, On the other hand it is plausible that in addition to MEK several scaffolds and adaptors proteins that temporally and spatially regulate ERK pathway (Kolch, 2005) could play a role.
Earlier pharmacological blockade studies with a selective P2X4R antagonist demonstrated the specific role of P2X4R in mediating alcohol-induced effects on microglia (Gofman et al., 2014). A similar approach was used in the current study to determine the role of P2X4R in the alcohol-induced suppression of ERK and AKT phosphorylation. Treatment with a P2X4R selective antagonist, 5-BDBD delayed alcohol's effect on decreased phosphorylation of AKT (Fig. 3A and B) and ERK (Fig. 3C and D) indicating the role of P2X4R in alcohol-induced ERK and AKT levels. These findings were confirmed by siRNA knockdown studies (Supplementary Fig. S2A and B) further highlighting the role of P2X4R in the alcohol-induced suppression of ERK.
Several studies have reported activation of transcription factor CREB as consequence of exposure to alcohol (Yang et al., 1998; Acquaah-Mensah et al., 2006). Furthermore, studies have elucidated the involvement of transcription factor, CREB (Impey et al., 2004) in regulation of P2X4R (Brautigam et al., 2005). In the current study alcohol treated microglial cells compared with control demonstrated a robust increase in CREB (Fig. 4A) activity. Consistent with these reports, our studies show that CREB transcriptional activity is an important regulator of alcohol-induced P2X4R receptors expression in microglial cells. Pharmacological inhibition of P2X4R reversed the alcohol-induced effect on CREB activity (Fig. 4B).
Although, the precise role of CREB in regulation of P2X4R remains elusive, evidence of putative correlation between purinergic receptors and transcription factor, CREB has been reported (Potucek et al., 2006). Calcium, a major signaling molecule, activates many transcription factors including CREB (Sun et al., 1994; West et al., 2001). Notably, we have earlier demonstrated that alcohol-mediated calcium mobilization in microglial cells was P2X4R dependent (Gofman et al., 2014). In that context, CREB activation could be due in part due to alcohol's effect on calcium mobilization leading to activation of CREB.
In summary our study provided new mechanistic insight into the alcohol-induced alteration of P2X4 receptor expression and propose a model of transcriptional regulatory mechanisms underlying the role P2X4R has on microglial function in the context of alcohol-mediated immune dysfunction (Fig. 5).
SUPPLEMENTARY MATERIAL
Supplementary material is available at Alcohol and Alcoholism online.
FUNDING
This work was made possible by the support of NIDA-Supported Institutional Training Grant (T32 DA007237LG), NIH grant R01 DA031064, and Temple University Development Grant to R.P.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- Acquaah-Mensah GK, Misra V, Biswal S (2006) Ethanol sensitivity: a central role for CREB transcription regulation in the cerebellum. BMC Genomics 7:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S, Korcok J, Sims SM et al. (2007) Activation of transcription factors by extracellular nucleotides in immune and related cell types. Purinergic Signal 3:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroor AR, Baker RC (1998) Ethanol inhibition of phagocytosis and superoxide anion production by microglia. Alcohol 15:277–80. [DOI] [PubMed] [Google Scholar]
- Beutner C, Roy K, Linnartz B et al. (2010) Generation of microglial cells from mouse embryonic stem cells. Nat Protoc 5:1481–94. [DOI] [PubMed] [Google Scholar]
- Brautigam VM, Frasier C, Nikodemova M et al. (2005) Purinergic receptor modulation of BV-2 microglial cell activity: potential involvement of p38 MAP kinase and CREB. J Neuroimmunol 166:113–25. [DOI] [PubMed] [Google Scholar]
- Burnstock G. (2015) Physiopathological roles of P2x receptors in the central nervous system. Curr Med Chem 22:819–44. [DOI] [PubMed] [Google Scholar]
- Catalanotti F, Reyes G, Jesenberger V et al. (2009) A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat Struct Mol Biol 16:294–303. [DOI] [PubMed] [Google Scholar]
- Gofman L, Cenna JM, Potula R (2014) P2X4 receptor regulates alcohol-induced responses in microglia. J Neuroimmune Pharmacol 9:668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga NA, Callera GE, Yogi A et al. (2014) Acute ethanol intake induces mitogen-activated protein kinase activation, platelet-derived growth factor receptor phosphorylation, and oxidative stress in resistance arteries. J Physiol Biochem 70:509–23. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Li W, Rodriguez ML (2011) Role of microglia in CNS inflammation. FEBS Lett 585:3798–805. [DOI] [PubMed] [Google Scholar]
- Impey S, McCorkle SR, Cha-Molstad H et al. (2004) Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119:1041–54. [DOI] [PubMed] [Google Scholar]
- Inoue K. (2006) The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther 109:210–26. [DOI] [PubMed] [Google Scholar]
- Inoue K. (2008) Purinergic systems in microglia. Cell Mol Life Sci 65:3074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitis J, Murdoch EL, Deburghgraeve C et al. (2012) Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcgammaR-mediated phagocytosis. Cell Immunol 274:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S, Boland R, Santillan G (2008) Purinergic (ATP) signaling stimulates JNK1 but not JNK2 MAPK in osteoblast-like cells: contribution of intracellular Ca2+ release, stress activated and L-voltage-dependent calcium influx, PKC and Src kinases. Arch Biochem Biophys 477:244–52. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M et al. (2011) Physiology of microglia. Physiol Rev 91:461–553. [DOI] [PubMed] [Google Scholar]
- Kim EK, Choi EJ (2010) Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 1802:396–405. [DOI] [PubMed] [Google Scholar]
- Kolch W. (2005) Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6:827–37. [DOI] [PubMed] [Google Scholar]
- Markou T, Vassort G, Lazou A (2003) Regulation of MAPK pathways in response to purinergic stimulation of adult rat cardiac myocytes. Mol Cell Biochem 242:163–71. [PubMed] [Google Scholar]
- Monif M, Burnstock G, Williams DA (2010) Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol 42:1753–6. [DOI] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A et al. (2010) Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci 67:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Kierdorf K, Neumann H (2009) Microglial precursors derived from mouse embryonic stem cells. Glia 57:1660–71. [DOI] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN et al. (2008) Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis 31:218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa K, Kohsaka S (2011) Dynamic motility of microglia: purinergic modulation of microglial movement in the normal and pathological brain. Glia 59:1793–9. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Ohura K, Oka T (2011) Involvement of P2X receptors in the regulation of insulin secretion, proliferation and survival in mouse pancreatic beta-cells. Cell Physiol Biochem 28:355–66. [DOI] [PubMed] [Google Scholar]
- Ostrovskaya O, Asatryan L, Wyatt L et al. (2011) Ethanol is a fast channel inhibitor of P2X4 receptors. J Pharmacol Exp Ther 337:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Teeling J (2013) Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol 35:601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova M, Trudell J, Li K et al. (2013) Tryptophan 46 is a site for ethanol and ivermectin action in P2X4 receptors. Purinergic Signal 9:621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potucek YD, Crain JM, Watters JJ (2006) Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochem Int 49:204–14. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Fan S, Dykstra H et al. (2013) Inhibition of glycogen synthase kinase 3beta promotes tight junction stability in brain endothelial cells by half-life extension of occludin and claudin-5. PLoS One 8:e55972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimland D, Hand WL (1980) The effect of ethanol on adherence and phagocytosis by rabbit alveolar macrophages. J Lab Clin Med 95:918–26. [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R et al. (2002) ERK regulation in chronic ethanol exposure and withdrawal. Brain Res 948:186–91. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ (1999) Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 19:2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes L, Surprenant A (2009) Dynamic regulation of the P2X4 receptor in alveolar macrophages by phagocytosis and classical activation. Eur J Immunol 39:986–95. [DOI] [PubMed] [Google Scholar]
- Suk K. (2007) Microglial signal transduction as a target of alcohol action in the brain. Curr Neurovasc Res 4:131–42. [DOI] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS et al. (1994) Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev 8:2527–39. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Toyomitsu E, Kometani M et al. (2009) Mechanisms underlying fibronectin-induced up-regulation of P2X4R expression in microglia: distinct roles of PI3K-Akt and MEK-ERK signalling pathways. J Cell Mol Med 13:3251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Krishtal OA, Burnstock G (2009) Purinoceptors on neuroglia. Mol Neurobiol 39:190–208. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB et al. (2001) Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98:11024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Horn K, Wand GS (1998) Chronic ethanol exposure impairs phosphorylation of CREB and CRE-binding activity in rat striatum. Alcohol Clin Exp Res 22:382–90. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.