Whether it is due to changing epidemiology or increased recognition, hyperferritinemic sepsis is becoming increasingly diagnosed in critically-ill children. An acute rise in ferritin is part of the normal host response to infection and recent in vivo and in vitro studies have demonstrated the immunomodulatory effects of ferritin and its individual subunits, light chain ferritin (L-ferritin) and heavy chain ferritin (H-ferritin).1–3 In Brazilian children with severe sepsis and septic shock, lack of an increased ferritin response (ferritin level < 200 ng/mL) is associated with 23% mortality, an increased ferritin response (ferritin level 200–500 ng/mL) is associated with best outcome 9% mortality, and a hyperferritinemic response (ferritin level > 500 ng/mL) is associated with 58% mortality.4 This suggests that an increased ferritin level is protective during sepsis but that too high of a response is not, either because ferritin at higher concentrations is injurious or hyperferritinemia is a marker of injurious hyper-inflammation. In a study of all children admitted to Seattle Children’s Hospital tested for serum ferritin, patients with ferritin ≥ 1,000 ng/mL and ≥ 3,000 ng/mL have a stepwise increased risk of intensive care admission and death over the next five years.5 Although patients with cancer, hemoglobinopathy, or autoimmune disease were more likely to have elevated serum ferritin, the increased risk of PICU admission and death was present even after controlling for these underlying diagnoses.
When hyperferritinemic sepsis is associated with five of eight criteria reflective of hyperinflammation (Ferritin > 500 ng/mL, two line cytopenia, organomegaly, hypertriglyceridemia, hypofibrinogenemia, elevated sCD25, absent NK cytotoxic activity, and hemophagocytosis) it might be called multiple organ dysfunction syndrome (MODS) by intensivists, macrophage activation syndrome (MAS) by rheumatologists, and hemophagocytic lymphohistiocytosis histiocytosis (HLH) by oncologists.6 Unfortunately, the distinction between these syndromes is so important that children cannot afford to have diagnoses given according to specialty practice. We therefore believe it is paramount for intensivists to understand the pathobiology and risk factors for these three clinical syndromes and guide their clinical practice accordingly.
MAS has a mortality rate < 10%7. Hyperinflammation in this syndrome is related to heterozygous mutations or epigenetically reduced T-regulatory (Treg) cell, Natural Killer (NK) cell, and Cytoxic T Lymphocyte (CTL) cell function in rheumatologic diseases or mutations in inflammasome proteins such as in cryopyrin-associated periodic syndromes (CAPS).8 Importantly, immune cell function is reduced, but not absent, in these patients. In the normal host, Treg cells dampen inflammation, NK and CTLs kill cancer cells and viral infected cells and induce activated immune cells (e.g. macrophages) to undergo programmed cell death. Patients with macrophage activation syndrome cannot adequately turn off inflammation, to the point that it becomes self-injurious. Therapies for these patients are meant to reduce inflammation without preventing them from fighting infection. Such therapies include methylprednisolone (less immune suppressive than dexamethasone), recombinant IL-1ra (anakinra), and plasma exchange.9
By contrast, mortality in HLH is quite high at 50% with survivors requiring bone marrow transplantation to correct their immune deficiency.10 HLH is a very severe disease in which NK cells and CTL cells have absent function due to homozygous mutations in genes that affect granzyme and perforin signaling. Theirs is a qualitative defect as their NK and CTL numbers are normal, but they do not function properly. Not uncommonly these children have lymphoproliferative diseases, either B-cell driven in the case of EBV virus or T-cell driven by other triggers. Anti-inflammatory therapies alone do not work for these patients. Instead, lympholytics are used for lymphoproliferation such as rituximab for B-cell lymphoproliferation, Campath for T-cell proliferation, and Etoposide for macrophage proliferation. Not surprisingly death in these patients is most often related to severe sepsis and multi-organ dysfunction, as infections are unchecked in the face of HLH treatments that attack the existing immune system. In this regard, perforin knockout animals develop HLH when given an otherwise innocuous viral challenge, however, they do not have an increased susceptibility to death when subjected to cecal ligation and puncture bacterial sepsis. Hence deaths from sepsis in this population of children likely occur not from defects in the perforin/granzyme signaling system, but rather from the immune suppressant effects of dexamethasone and chemotherapy.
Hyperferritinemic sepsis related MODS (which some call MAS or secondary HLH) can be induced in genetically normal animals by giving the microbial DNA mimicker CpG which is a potent Toll-like receptor 9 (TLR9) stimulant. In the face of this TLR 9 stimulation, an otherwise survivable sepsis insult induced by mild cecal ligation and puncture, low dose staphylococcal toxin, or low dose endotoxin becomes overwhelmingly lethal.11–13 Similar to patients with MAS or CAPS, treatment with anakinra rescues these animals from CpG facilitated sepsis and death. Importantly, anakinra has also been shown to improve survival in adult sepsis patients with features of MAS including thrombocytopenia, prolonged INR, increased bilirubin and increased SGPT.14 Rajasekaran et al. report in a case-series of patients with hyperferritinemia-associated sepsis/MODS/MAS/HLH that treatment with anakinra was associated with a significant reduction in C-reactive protein and serum ferritin and overall mortality of 12%.15,16 Demirkol et al. showed in a center specific cohort study that Turkish children with hyperferritinemic sepsis MODS/MAS/HLH who were treated with methylprednisolone, IVIG, and plasma exchange had 100% survival, whereas those treated with the HLH-94 protocol using chemotherapy and dexamethasone had only 50% survival with all deaths occurring from overwhelming sepsis.17 Why might this be? Well, children with severe sepsis have low NK activity because they have low NK and CTL cell numbers, not because their NK and CTL cells do not work (it is the opposite of the situation with HLH children). When the sepsis improves with appropriate antibiotics and source control, the NK and CTL cell numbers return to normal and, in turn, their activity turns off macrophage activation/inflammation as the patient recovers.18 If instead these patients are treated with agents such as etoposide and dexamethasone, the NK and CTL cell numbers are iatrogenically prevented from recovery if not further reduced. In today’s journal, another set of Turkish investigators demonstrate that a multimodal strategy of source removal and anti-inflammatory measures in children without HLH risk factors or genetic mutations is associated with better outcomes than use of the HLH-94 protocol with dexamethasone and chemotherapy in children with risk factors for HLH.19
So, given the current state of knowledge on immunophenotypes and the overlap between the syndromes of severe sepsis, MAS, and HLH, here is how we manage the hyperferritinemic patient with sepsis MODS/MAS/HLH (Figure 1). First, we assess for the presence of risk factors for familial HLH: age < 2 years, history of parental consanguinity, history of young family member dying from febrile illness, or presentation with central nervous system involvement. We send diagnostic tests, including genetic analysis related to primary immune deficiency syndromes and granzyme/perforin signaling. However, because we rarely receive these tests back in a timely fashion we guide our initial therapy based on the risk factor approach. If the child has one of the four risk factors for familial HLH, then we consult immunology and hematology/oncology to evaluate for HLH and treat accordingly, often using the updated HLH-2004 protocol. We follow C-reactive protein (as a bacterial infection biomarker) and ferritin levels (as a marker of macrophage activation). If ferritin levels go down but C-reactive protein goes up then we reduce immune suppression and treat for new bacterial or fungal infection.
Figure 1. Initial approach to the critically ill child with hyperferritinemic sepsis/MODS/MAS/HLH.
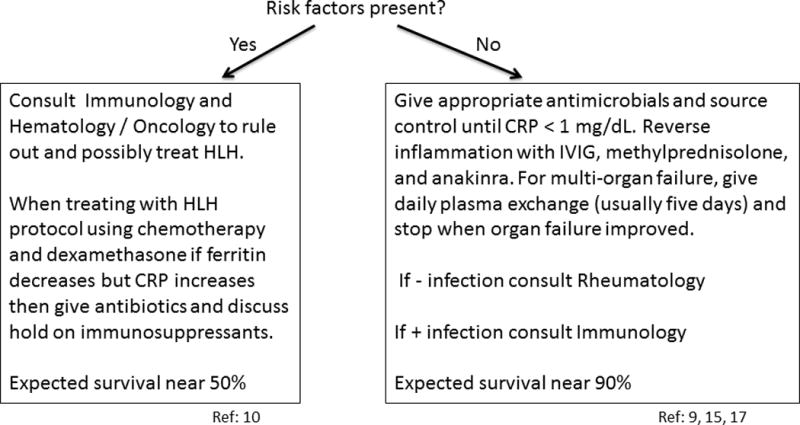
a) Send immunology, rheumatology, and hematology laboratory diagnostics.
b) Assess risk : Are risk factors for familial HLH present: age < 2 years, primary central nervous system presentation, consanguineous parents, or history of death of young family member with unexplained fever?
If the child has none of the four risk factors for HLH, then we treat infection with appropriate antibiotics and source control measures. In addition, we treat the hyper-inflammation related to unchecked immune activation with methylprednisone, IVIG, and anakinra. If MODS is also present, then we add daily plasma exchange, stopping when organ function improves (usually 5 days). We consider adequate infection source control to have occurred when C-reactive protein levels are normalized (< 1mg/dL). If we have not identified infection, then we consult rheumatology to help rule out rheumatologic disorders that may be associated with MAS or conditions such as CAPS. If we have identified infection we consult immunology to rule primary immune deficiency diseases such as chronic granulomatous disease, among others.
Acknowledgments
Dr. Carcillo received support for article research from the National Institutes of Health. His institution received grant support.
Footnotes
Copyright form disclosures:
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Ruddell RG, Hoang-Le D, Barwood JM, et al. Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology. 2009;49:887–900. doi: 10.1002/hep.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R, Luo C, Mines M, et al. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281:37616–27. doi: 10.1074/jbc.M607266200. [DOI] [PubMed] [Google Scholar]
- 3.Broxmeyer HE, Cooper S, Levi S, Arosio P. Mutated recombinant human heavy-chain ferritins and myelosuppression in vitro and in vivo: a link between ferritin ferroxidase activity and biological function. PNAS. 1991;88:770–4. doi: 10.1073/pnas.88.3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia PC, Longhi F, Branco RG, et al. Ferritin levels in children with severe sepsis and septic shock. Acta paediatrica. 2007;96:1829–31. doi: 10.1111/j.1651-2227.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett TD, Hayward KN, Farris RW, et al. Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients. Pediatr Crit Care Med. 2011;12:e233–6. doi: 10.1097/PCC.0b013e31820abca8. [DOI] [PubMed] [Google Scholar]
- 6.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387–92. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]
- 7.Stephan JL, Kone-Paut I, Galambrun C, et al. Reactive haemophagocytic syndrome in children with inflammatory disorders. A retrospective study of 24 patients. Rheumatology. 2001;40:1285–92. doi: 10.1093/rheumatology/40.11.1285. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman KM, Linghu B, Szustakowski JD, et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2014;66:3486–95. doi: 10.1002/art.38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minoia F, Davi S, Horne A, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66:3160–9. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 10.Trottestam H, Horne A, Arico M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118:4577–84. doi: 10.1182/blood-2011-06-356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens EM, Canna SW, Slade K, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121:2264–77. doi: 10.1172/JCI43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujimoto H, Ono S, Matsumoto A, et al. A critical role of CpG motifs in a murine peritonitis model by their binding to highly expressed toll-like receptor-9 on liver NKT cells. J Hepatol. 2006;45:836–43. doi: 10.1016/j.jhep.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Petrasek J, Dolganiuc A, Csak T, et al. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;140:697–708 e4. doi: 10.1053/j.gastro.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shakoory B, Carcillo J, Zhao H, et al. IL-1 receptor antagonism improves mortality in severe sepsis subset with hemophagocytic syndrome. Crit Care Med. 2013;41:A1105. [Google Scholar]
- 15.Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15:401–8. doi: 10.1097/PCC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 16.Simon DW, Aneja R, Carcillo JA, et al. Plasma exchange, methylprednisolone, IV immune globulin, and now anakinra support continued PICU equipoise in management of hyperferritinemia-associated sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome/secondary hemophagocytic lymphohistiocytosis syndrome. Pediatr Crit Care Med. 2014;15:486–8. doi: 10.1097/PCC.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demirkol D, Yildizdas D, Bayrakci B, et al. Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Critical Care. 2012;16:R52. doi: 10.1186/cc11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halstead ES, Carcillo JA, Schilling B, et al. Reduced frequency of CD56 dim CD16 pos natural killer cells in pediatric systemic inflammatory response syndrome/sepsis patients. Pediatr Res. 2013;74:427–32. doi: 10.1038/pr.2013.121. [DOI] [PubMed] [Google Scholar]
- 19.Kaya Z, B A, Albayrak M, et al. Prognostic factors and long term outcome in 52 Turkish children with hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000449. in press. [DOI] [PubMed] [Google Scholar]


