Abstract
The tilt-translation ambiguity occurs because acceleration due to translation cannot be differentiated from gravitational acceleration. Head tilt can occur independent of body tilt which further complicates the problem. The tilt-translation ambiguity is examined for fore-aft (surge) translation with head and/or body orientations that are tilted in pitch 10° forward or backward. Eleven human subjects (6 female), mean age 40 years participated. Conditions included no tilt (NT), head and body tilt (HBT), head only tilt (HOT), and body only tilt (BOT). The fore-aft stimulus consisted of a 2s (0.5 Hz) sine wave in acceleration which a maximum peak velocity of 10 cm/s. After each stimulus the subject reported the direction of motion as forward or backward. Subsequent stimuli were adjusted to determine the point at which subjects were equally likely to report motion in either direction. During the HBT responses were biased such that upward pitch caused a neutral stimulus to be more likely to be perceived as forward and downward pitch caused the stimulus to be more likely to be perceived as backward. The difference in the point of subjective equality based on the direction of tilt was 3.3 cm/s. During the BOT condition the bias with respect to the direction of body tilt was in a similar direction with a difference in PSE 1.6 cm/s. During HOT and NT there was no significant bias on fore-aft perception. These findings demonstrate that body tilt shifts the PSE of fore-aft direction discrimination while head tilt has no influence.
Keywords: Tilt, Translation, Vestibular, Perception, Otolith, Human
Introduction
The integration of multiple sensory inputs is key to perception and behavior during many common tasks. One of the most basic tasks is maintaining equilibrium during ambulation where changes in acceleration due to both translation and tilt as well as changes in the position of the head relative to the body. Linear acceleration can either represent gravity (i.e. tilt) or linear acceleration due to translation(Graybiel et al. 1979; Paige and Tomko 1991; Seidman et al. 1998; Angelaki et al. 2004; Zhou et al. 2006; Green and Angelaki 2007; Angelaki and Cullen 2008; Osler and Reynolds 2012). This tilt-translation ambiguity is in large part due to the laws of physics which do not permit any accelerometer to differentiate acceleration due to gravity from acceleration due to translation. But, to maintain orientation in space and appropriately interpret sensory information, tilt and translation must be disambiguated. There is a rich neuroscience literature on how this ambiguity is resolved for eye movement, head orientation, and translation perception (Graybiel et al. 1979; Curthoys 1996; Seidman et al. 1998; Mittelstaedt 1999; Clement et al. 2001; Groen and Bles 2004; Shaikh et al. 2004; Merfeld et al. 2005b; Merfeld et al. 2005a; Vingerhoets et al. 2006; Au Yong et al. 2007; Osler and Reynolds 2012). Multiple factors have been implicated including involvement of other sensory systems and the frequency of the motion, but the influence of neck proprioception and body somatosensation has largely been absent from the prior literature.
A further challenge facing the vestibular system is estimating movement of the body using head fixed reference frame(Cullen and Minor 2002). Neck proprioception is one possible mechanism by which the differences between head and body reference frames may be disambiguated. Neurons in the vestibular nucleus neurons are influenced by static neck position in mice(Medrea and Cullen 2013) and a similar behavior has been shown in cats(Boyle and Pompeiano 1981; Anastasopoulos and Mergner 1982; Wilson et al. 1990). Neck proprioception has previously been shown to influence perception of head and body rotation in humans(Mergner et al. 1991; Mergner et al. 1992). Although effects of neck position on translation perception have not previously been directly studied, the underlying physiology suggests neck proprioception may have a key role.
The current paper examines how tilt and translation are disambiguated for human perception for fore-aft translation. Prior work has noted tilt and translation are disambiguated based on a combination of canal and otolith signals arising in the inner ear alone(Merfeld et al. 1999; Green and Angelaki 2003; Zupan et al. 2004). It has also been shown that somatic sensation in the body provides cues to tilt(Clement et al. 2001) and translation(Gianna et al. 1996). The current paper examines how tilt and translation are disambiguated by systematically varying the angle of the head relative to the body and measuring the point of subjective equality (PSE) between fore and aft translation. To the author’s knowledge, prior studies have not looked at the tilt-translation ambiguity from this perspective in humans.
Methods
Equipment
Fore-aft motion stimuli were delivered using a 6-degree-of-freedom motion platform (Moog model 6DOF2000E) as previously described in the current laboratory for fore-aft translation perception(Crane 2012a; Roditi and Crane 2012a; Crane 2013). Subjects were seated in a platform mounted racing seat with the body held in place by four-point harness. The head was stabilized using a rubber pad that approximated the contour of the head. During movement platform sounds were masked using a while noise stimulus as previously described(Roditi and Crane 2012a).
Head and platform movements were monitored in all six-degrees of freedom using a flux-gate magnetometer (trakSTAR, Ascension Technologies, Burlington, VT) using two model 800 position sensors, one on the subject’s head and other on the chair. Positional accuracy of the sensors was verified as <1 mm and <0.1° over the range of motion.
Prior to each experiment the subject positioned such the line between the inferior orbital rim and the top of the tragus was parallel with earth-horizontal. With the subject in this position platform completed a movement in a pattern starting at the center position, then moving 10 cm to the right, then 10 cm forward of center. This allowed the coordinate system of the platform to be precisely aligned with the coordinate system using rotation matrices. It also allowed the position of the sensors to be calibrated to this reference plane as previously described(Crane and Demer 1997).
Each stimulus was delivered after a subject pressed a button to indicate they were ready. After each stimulus subject pressed one of two buttons to identify the perceived direction of motion.
Stimuli
The stimulus consisted of a 2s (0.5 Hz) sine wave in acceleration. The maximum displacement was 10 cm (peak velocity 10cm/s, peak acceleration 16cm/s/s) but the stimulus was adjusted based on the subject’s responses. This motion profile contains no discontinuities in acceleration, velocity, or position, as has been previously described (Roditi and Crane 2012a). A small amount of side-to-side mechanical oscillation was delivered to every stimulus as previously described(Crane 2012a) to create a small amount of noise and vibration to minimize non-vestibular cues. This oscillation consisted of a 6 Hz sine wave. The wave was multiplied by the first cycle of a 0.25 Hz sine wave so that the intensity was largest near the peak velocity of the movement with a maximum amplitude of 0.6 mm. This mechanical vibration likely only had a significant effect for small movements (< 5 mm) and its purpose was to provide vibration so that absence of vibration could not be used as a cue to no motion occurring. The control condition in which fore-aft translation occurred with the head fixed with no tilt (NT) was previously reported in many of the current subjects in the current laboratory in two recent studies(Crane 2012a; Crane 2013) but was also repeated in the current study.
The pitch angles of the head and body were systematically varied by ±10°. This angle was chosen because it could be easily and comfortably delivered by our apparatus. Additionally it is within the range that occurs during physiologic movement such as ambulation(Crane and Demer 1997). Each test condition: No tilt (NT), Head only tilt (HOT), Head body tilt (HBT), and Body only tilt (BOT) were performed in separate blocks of trials. However the direction of any tilt and direction of motion were randomly interleaved within each trial block. The order in which the trial blocks were presented was varied between subjects.
Prior to each translation the head and platform tilt were oriented to the correct pitch angle (±10°) for the next trial. The platform automatically assumed the correct body tilt for the next stimulus at the same time it moved back to the center position. In the HBT and NT conditions no independent head positioning was required because the head was fixed to the platform and always remained in the same position relative to the body. In the HOT and BOT trial blocks head position was set after the platform was in position for the next trial. This was accomplished by having subjects voluntarily move their head with real-time feedback on the desired orientation. On an LCD screen in front of the subject the current head position was represented as a cross. The intended position was represented as a box centered on the intended position with the sides at ±1°. The subjects were instructed to move their head such that the cross was centered in the box then stabilize their head against the head-rest in preparation for the subsequent stimulus. Most subjects could accurately reposition their head within a few seconds. Once the stimulus started the previous visible box disappeared and no further position feedback was provided although the head position was monitored and recorded (Fig. 1).
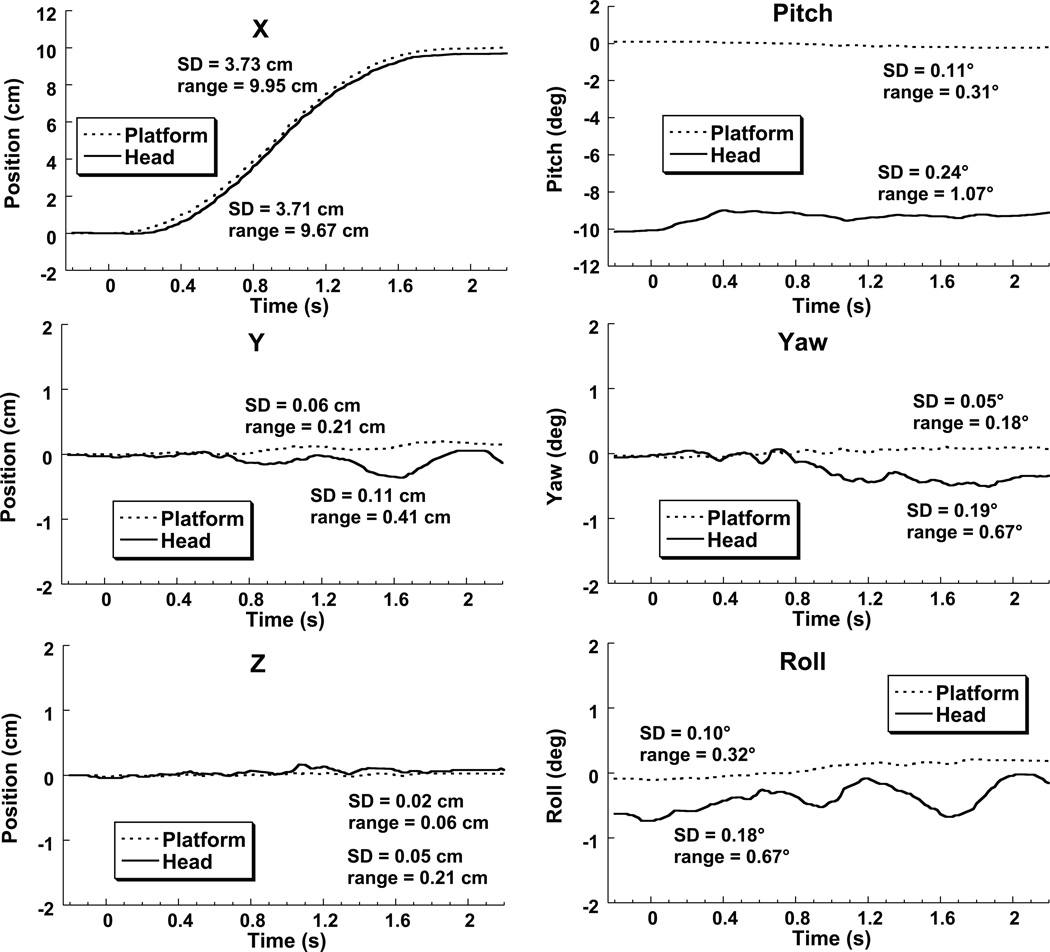
Figure 1.
Sample of motion data from a typical subject during 10 cm of forward translation.
A staircase was used to determine which fore-aft translation stimuli to present next based on previous responses(Crane 2012a; Crane 2012b; Crane 2013). The staircases were designed to start with 10 cm/s stimuli because these were likely to be unambiguously perceived, and work toward smaller stimuli. Interleaved independent staircases were used, one staircase started with forward 10 cm/s translation and the other with backward 10 cm/s. This was done to eliminate a potential artifacts based on the initial test stimulus, and minimize the ability of subjects to identify patterns in the stimulus presentation. Additionally, this paradigm minimized the potential for aftereffects which have previously been described for fore-aft translation(Crane 2012a). Each staircase contained 25 stimulus presentations. For each response in the direction of the staircase the stimulus velocity was moved in the opposite direction. The test stimulus velocity was varied on a continuum such that each staircase could step through zero. Thus, the staircases tended to deliver most stimuli in the range where subjects where equally likely to perceive a movement in either direction. With each reversal in response direction the step size decreased by half to a minimum of 0.4 cm/s. The level was changed in a 1-up, 1-down manner – i.e. a backward response causes the next stimulus to be delivered in a more forward direction and vice versa. If the subject did not respond with a perceived direction within 2 seconds no response was recorded and the stimulus was re-presented when that staircase was active again. These types of lapses were rare and occurred in <1% of stimulus presentations.
Subjects
A total of 11 normal naïve human subjects (6 female, mean age 40, range 20–66) participated. Neither the author nor others employed in the lab participated as subjects. They were told that each stimulus would consistent of either forward or backward motion and that their task was to identify the direction. They were also given instruction on how to orient their head between stimuli. This was practiced a few times without motion to ensure they could do it accurately. Trial blocks were usually completed over multiple days and testing sessions. When trial blocks were completed the same day there were breaks between blocks of trials. Subjects were screened prior to participation to confirm normal peripheral vestibular function.
Informed written consent was obtained from all participants. The protocol was approved by the University of Rochester Research Science Review Board.
Analysis
The percentage of backward responses for each stimulus level was plotted as a function of the translation stimulus (Fig. 2). A cumulative Gaussian function as previously described(Wichmann and Hill 2001a; Wichmann and Hill 2001b), and used in the current laboratory(Crane 2012a; Crane 2012b; Roditi and Crane 2012b; Roditi and Crane 2012a). Data from each subject was resampled and fit 2,000 times so that multiple estimates of the mean could be generated and 95% confidence intervals determined (Fig. 2C). The level of significance in the difference in two distributions as determined as previously described (Crane 2012a). This technique was effective for measuring the PSE (the mean of the cumulative distribution function also known as bias) and the threshold (sigma or width of the cumulative distribution function) for individual subjects. It could also be applied to data combined across subjects to measure an overall mean with confidence intervals. This method was not appropriate for measuring the threshold of combined data because the biases varied between subjects and such fitting of combined data greatly overestimated sigma. Thus the average sigma was reported as an average of individually determined values. The repeated measures analysis of variance (ANOVA) was used to compare the bias between subjects and test conditions. Paired T-tests were used to compare data across subjects.
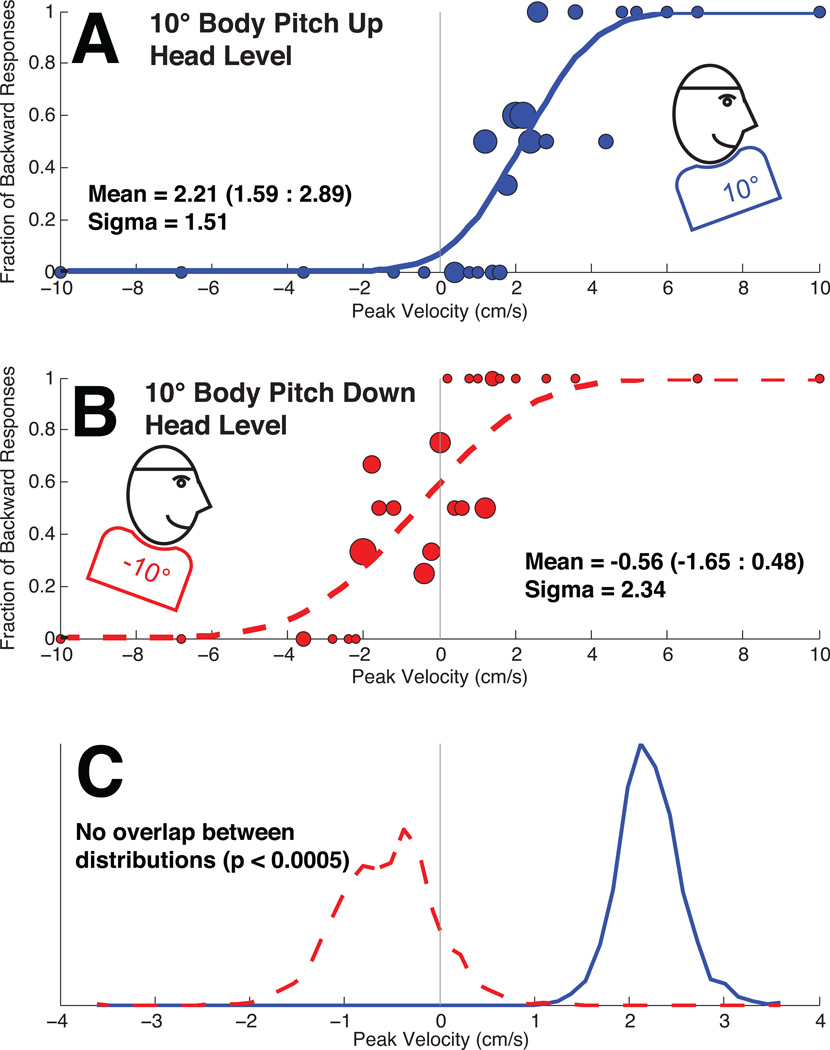
Figure 2.
A block of trains demonstrating the influence of body only tilt (BOT) in a sample subject (#1). The circles in panels A&B are sized proportionally to the number of responses they represent with the smallest circles representing a single stimulus presentation. Panel A: Best fit cumulative distribution function (CDF) to trials in which the body was tilted backward while the head remained level in space. The CDF is shifted to the right indicating that a neutral (0 cm/s) translation would likely be perceived as forward motion. Panel B: The CDF to trials in which the body was tilted forwards while the head remained level. This demonstrates a shift of the CDF to the left relative to the pitch-up condition indicating a neutral translation would be more likely to be perceived as backward motion. Panel C: The CDF was fit to the data in panels A&B after being randomly resampled 2,000×. The mean was calculated for each iteration. The histograms of these means demonstrate a significant difference and no overlap between the two distributions.
Results
Subjects positioned their own head in two of the three conditions (HOT and BOT). Monitoring of head position indicated they were able to do this accurately with head stability maintained during motion. Head stability for subject #1 is shown (Fig. 1) during the largest possible translation (10 cm). In the HBT condition in which the head and body were both fixed to the platform during the average trial the absolute value of the head pitch relative to the intended position (±10°) was within 0.10 ± 0.10° (mean ± SD) at the start of the trial and peak to peak head pitch during motion averaged 0.51 ± 0.28°. In the BOT condition the starting head position in pitch averaged 0.14° ± 0.13°, with the peak-to-peak pitch angle of 0.63 ± 0.26°. For the HOT condition the average starting head pitch error relative to ±10° was 0.10 ± 0.08° and the peak-to-peak head pitch during motion was 0.93 ±0.97°. Thus subjects were able to accurately position their heads and maintain their head position during translation.
All subjects were able to accurately and reliably identify the direction of the stimulus during the largest excursions that occurred early in the trial. Sample psychometric data for a block of trials in subject #1 is shown in Fig. 2.
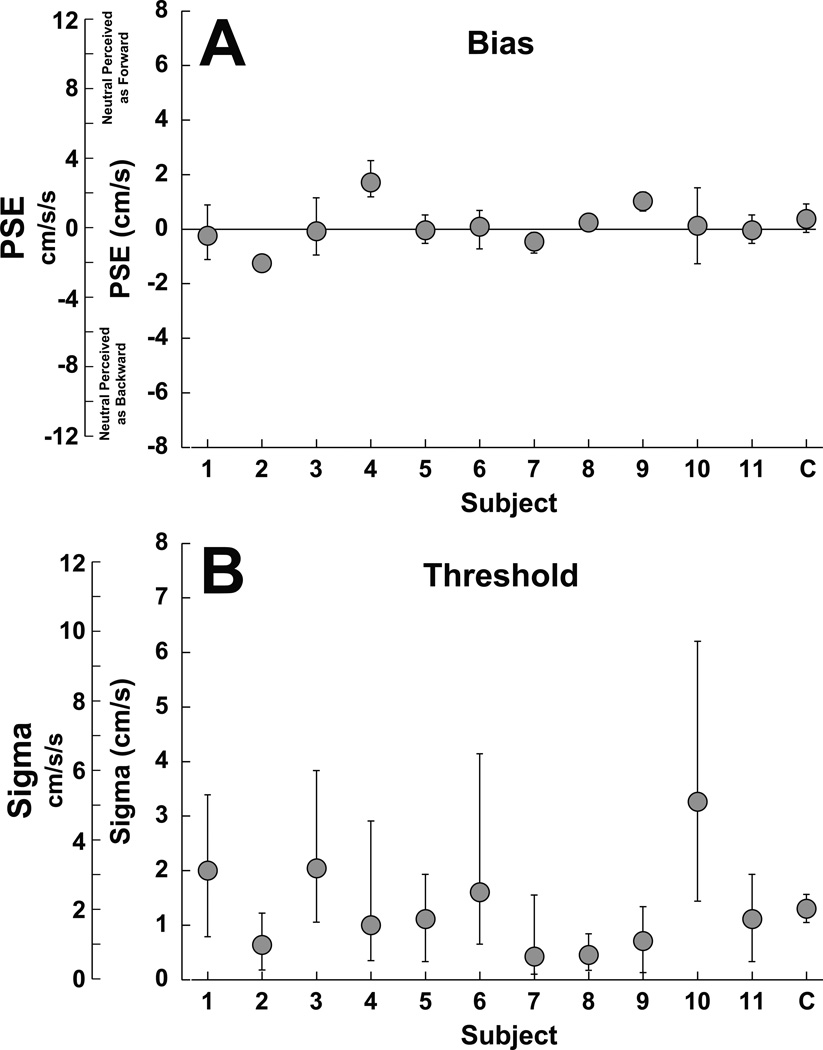
Control data was collected in which there was no tilt (NT, Fig. 3). Although some subjects demonstrated a small bias, in all cases in was below 2 cm/s. In the NT condition thresholds averaged 1.3 ± 0.2 cm/s (2.1 ± 0.4 cm/s/s). Tilting the head and body together (HBT) produced a bias in the point of subjective equality (PSE) such that in all subjects 10° upward pitch caused a neutral motion to be more likely to be perceived as forward relative to a 10° backward (Fig. 4A). During pitch down, movement with a peak velocity 1.9 ± 0.5 (mean ± SE) cm/s (or peak acceleration 3.0 ± 0.83 cm/s/s) backwards was most likely to be perceived as stationary. Similar during pitch up movement translation with a peak velocity of 1.4 ± 0.7 cm/s (2.2 ± 1.2 cm/s/s) forwards was most likely to be perceived as stationary. These PSEs were significantly different from each other (paired T-test, p < 0.001).
Figure 3.
Fore-aft perception with no tilt (NT). The furthest right column on each panel (labeled C) represents fits from all data combined across subjects. Panel A represents the bias which was near zero in most subjects. Error bars represent the 95% confidence interval (CI) of the PSE. Data were combined by fitting a psychometric function to the data points from all subjects. Panel B represents the threshold (sigma). For individual subjects error bars represent the mean ± 95% CI. The combined data is the mean ± SE of the individual values.
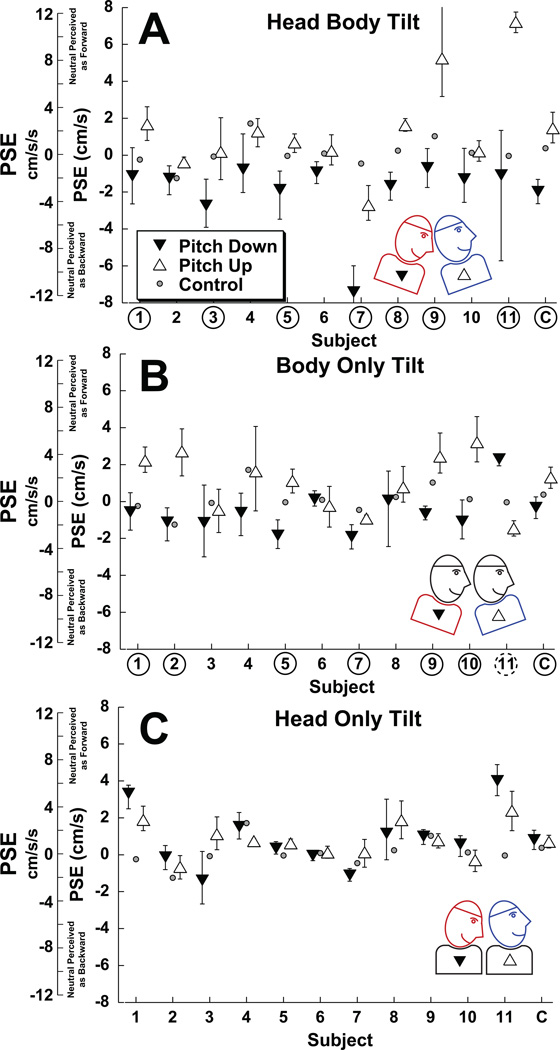
Figure 4.
The effect of head and body tilt on bias of fore-aft translation perception. In each panel filled downward pointing arrows represent 10° pitch down and open upward pointing arrows represent 10° pitch up. The error bars represent the 95% confidence interval of the PSE. The furthest right column on each panel (labeled C) represents fits performed on all the data combined across subjects. For individual subjects in which upward pitch caused a significant (p < 0.01) forward bias relative to downward pitch the subject number is circled with a solid line. For conditions in which the bias was significant in the opposite direction, the subject number is circled with a dashed line. Small gray circles represent the bias in the NT condition (also shown in Fig. 3A). The scale on the left includes both the peak velocity and peak acceleration of the stimulus. Panel A: Head body tilt (HBT). In every instance the pitch up tilt created a forward bias relative to the pitch down condition. Panel B: Body only tilt (BOT). In all but one subject (#11) the pitch up tilt led to a forward bias relative to the pitch down condition. Panel C: Head only tilt (HOT). No significant bias based on tilt direction were found.
During body only tilt (BOT), the direction of body tilt had a qualitatively similar influence on perception when compared with HBT (Fig 4B). In 10/11 subjects pitch up caused neutral motion to be more likely to be perceived as forward relative to the pitch down condition (Fig. 4B). The subject who perceived pitch up movements as more likely to be backward (#11), had a much smaller effect than she had in the HBT condition. During pitch up a movement with a peak velocity 1.0 ± 0.5 (mean ± SE) cm/s (peak acceleration 1.6 ± 0.8 cm/s/s) backwards was most likely to be perceived as stationary. Similar during pitch down movement translation with a peak velocity of 0.6 ± 0.3 cm/s (0.9 ± 0.5 cm/s/s) forwards was most likely to be perceived as stationary. These PSE differences were significant (paired T-test, p = 0.04).
Head only tilt (HOT) did not significantly influence perception in any of the subjects (Fig. 4C). Overall the average bias was small but tended towards forward movements at 0.8 ± 0.3 cm/s (1.3 ± 0.5 cm/s/s) to be perceived as neutral, but not significantly different between up and down pitch conditions (paired T-test, p=0.89).
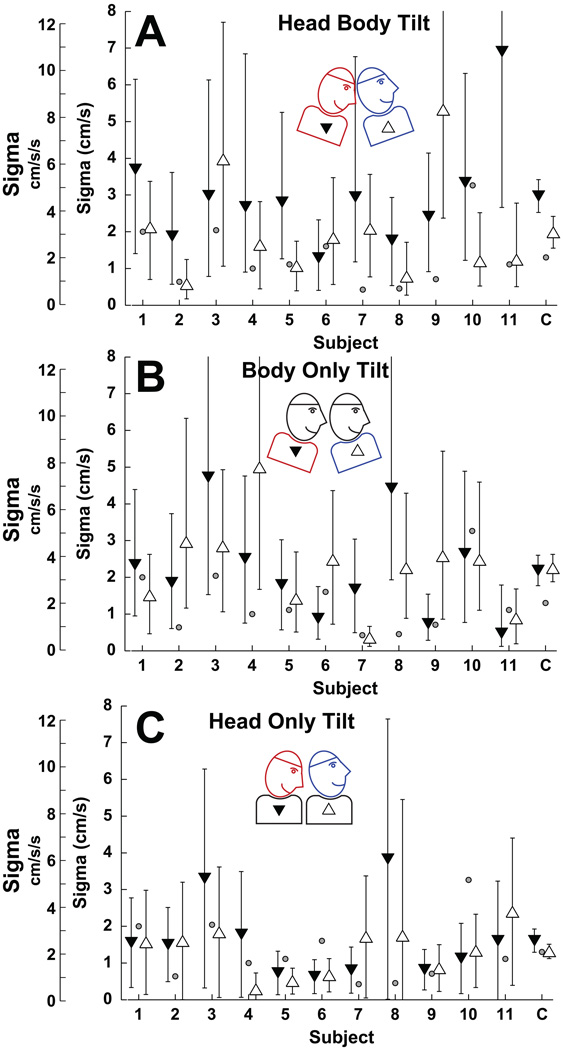
The threshold (width of the psychometric function) also significantly varied by tilt condition (Fig. 5, two-way repeated measures ANOVA, p=0.01, F[2,30]=4.9). But there was no significance of pitch (whether the tilt was up or down) within each condition(p=0.11, F[1,30]=2.6). For HBT, the threshold was 3.9 ± 0.7 cm/s/s (mean ± SE), for BOT it was 3.4 ± 0.6 cm/s/s, and for HOT it was 2.3 ± 0.5 cm/s/s. Thus conditions in which tilt contributed to the bias it also increased the threshold.
Figure 5.
The effect of head and body tilt on fore-aft translation thresholds. Filled downward pointing triangles represent pitch down and open upright triangles represent pitch up. For individual subjects error bars represent the 95% CI. Combined data (labeled C) is the mean ± SE across subjects. Small gray circles represent the threshold in the NT condition (also shown in Fig. 3B). The scale on the left includes both the peak velocity and peak acceleration.
Discussion
No accelerometer can differentiate between acceleration due to gravity and acceleration due to translation. This is known as the tilt-translation ambiguity. Tilting the head back shifts the gravity vector such that it now has a backward component in head coordinates, thus the acceleration experienced during backward tilt is similar to that experienced during forward translation. Likewise, tilting the head down could ambiguously be interpreted as backward translation. Yet, in our day-to-day experience we experience head tilt without becoming disoriented. The analogous situation in the current experiment was HOT which produced no consistent perceptual bias in translation perception(Fig. 4C), confirming the ambiguity was appropriately resolved. Furthermore during HOT the threshold was 2.3 ± 0.5 cm/s/s which was similar (p = 0.7, T-test) to the 2.1 ± 0.4 cm/s/s seen in the NT condition. Previously reported fore-aft (surge) thresholds measured without tilt was similar for subjects under age 50 at 2.5 cm/s/s using a different staircase procedure (2-down/1-up) but otherwise similar methods(Roditi and Crane 2012a). These findings are consistent with HOT not biasing how surge motion is perceived or changing the threshold of perception.
The current study used a 1-up/1-down method with variable step size which tends to focus stimuli close to the PSE or point at which there is a 50–50 chance of responding in either direction similar to other studies in the current laboratory(Crane 2012a; Crane 2012b; Crane 2013; Coniglio and Crane 2014). Similar methods have also been used by others(Watson and Pelli 1983; MacNeilage et al. 2010) and have potential advantages in terms of efficiency and stability over 1-up/2-down fixed step size methods(Kaernbach 1991), and have been argued to be ideal for PSE determination(Meese 1995). Although such methods more directly target the PSE, this also means that in a large fraction of stimulus presentations subjects are forced to guess. Guessing is susceptible to cognitive strategies; it has been shown that telling subjects to choose one response if uncertain or giving altered feedback can shift the PSE estimate(Morgan et al. 2012). Such bias shifts did not occur in their control condition, but it can be difficult to determine if such cognitive factors are at play. A three-response format has been proposed which adds an “undecided” option(Garcia-Perez and Alcala-Quintana 2013). This avoids guessing, but also assumes no lapses and that the uncertain responses are centered about the PSE. These are dangerous assumptions as failure to consider lapses can lead to significant errors in parameter estimates(Treutwein 1995; Wichmann and Hill 2001a), and vestibular thresholds have been shown to be frequently asymmetric(Roditi and Crane 2012a). It is unclear if such a method would offer advantages for the vestibular field. A simpler method to decrease guessing would be a 3-down/1-up staircase which targets a point at which performance is 79.4% correct(Leek 2001). Such methods focus stimulus presentations near the threshold and have been successful for vestibular threshold determination(Grabherr et al. 2008; Merfeld 2011; Valko et al. 2012; Chaudhuri and Merfeld 2013). It is unclear if such methods would work as well for PSE determination since data near the PSE is limited, and the PSE must be determined by making assumptions such as symmetric thresholds. Given what is known we felt that the method used to measure the PSE in these experiments is reasonable and accurate.
One of the major aims of the current experiments was to try to gain insight on how translation is correctly perceived during the HOT tilt condition despite changes in the gravity vector. This problem could be solved using temporal integration of the angular velocity signal that arises in the semicircular canals(Green and Angelaki 2003; Zhou et al. 2006). Inactivation of the semicircular canals leads to erroneous eye movements in response to translation(Angelaki et al. 1999) which supports this hypothesis. Studies which have shown an erroneous perception can be induced after prolonged rotations which activate the velocity storage mechanism which supports a role for the semicircular canals(Merfeld et al. 1999; Merfeld et al. 2001). Modeling of this type of data suggests that the tilt-translation ambiguity can be solved using canal and otolith signals alone(Zupan et al. 2004). Although it is clear from these studies that the semicircular canals play a key role in disambiguating tilt and translation, this hypothesis alone cannot explain the current findings. In fact conscious knowledge of the head tilt alone is not enough to resolve the tilt-translation ambiguity, because the 10° used in the HBT condition was well above threshold and during the HOT the subject tilted their own head. Also, in the HBT and HOT conditions the semicircular canals and otoliths experienced a similar motion in both conditions. In the HOT condition the tilt translation ambiguity was appropriately resolved, while in the HBT condition it seems that tilt was interpreted as translation. These observations are consistent with the observations of others that have suggested neurons encoding tilt are likely influenced by extra vestibular signals(Zhou et al. 2006).
Another possible mechanism is that the fore-aft translation was sensed by somatic sensation in the body rather than the labyrinth. Some have argued that translation thresholds of vestibular defective subjects is near normal(Gianna et al. 1996) supporting a major role for somatic sensation although others have shown the threshold of translation perception to be an order of magnitude greater in vestibular deficient individuals(Walsh 1961; Valko et al. 2012). In the current paradigm if body proprioception was the deciding factor in fore-aft motion discrimination head orientation would not influence the perception, and this is to a large extent true. However somatic sensation alone would likely have much larger thresholds than currently observed(Walsh 1961; Valko et al. 2012), suggesting that at least the translation was sensed by the otoliths even if tilt was based on somatosensation.
It is possible that neck proprioception is used an indicator of head tilt allowing acceleration sensed in the otoliths to be converted into body coordinates. This hypothesis is also consistent with head tilt not biasing fore-aft motion perception when the body remains upright as observed during the HOT condition (Fig. 4C). When proprioceptive signals indicate the head is upright relative to the body change in the gravity vector would be interpreted as translation. This is consistent with the observations during HBT (Fig. 4A). There is a plausible neurophysiologic mechanism by which neck proprioception can be used to solve the tilt-translation ambiguity. Vestibular-proprioceptive integration has previously been shown in cats(Boyle and Pompeiano 1981; Anastasopoulos and Mergner 1982; Wilson et al. 1990) as well as in monkeys(Gdowski et al. 2001; Sadeghi et al. 2009) and mice(Medrea and Cullen 2013). The current findings suggest static head position relatively to the body may influence vestibular perception in humans. It should be noted that this is clearly not the only mechanism at play as if the entire acceleration sensed during a 10° tilt were interpreted as translation the perceptual shifts would have been much larger than observed.
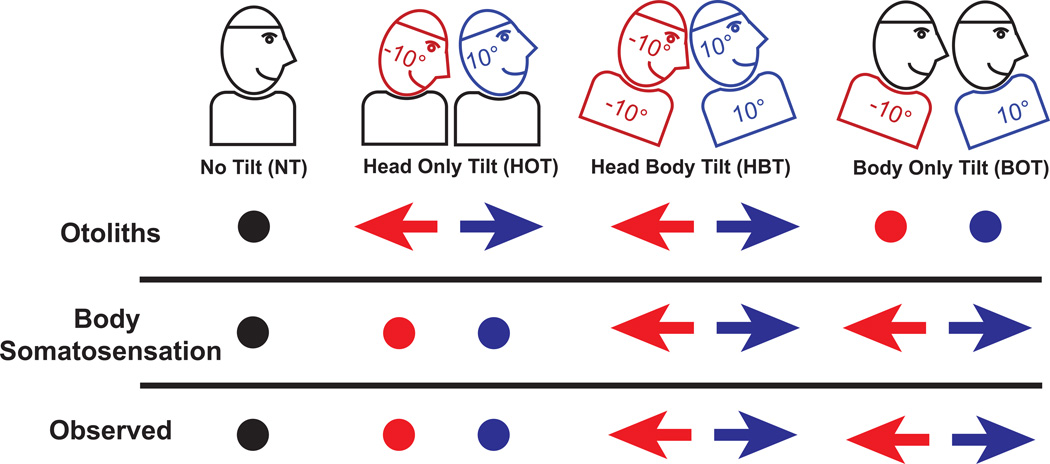
The current data demonstrate that the tilt-translation ambiguity is resolved in body-centered coordinates likely using a combination of neck proprioception and body somatosensation (Fig. 6). This is likely to be a reasonable assumption during most situations encountered during natural conditions, but as the current results demonstrate, is not sufficient to solve the tilt-translation ambiguity during situations in which the body is tilted.
Figure 6.
Predicted and observed bias in fore-aft translation perception of a neutral movement during each test condition. An arrow pointing to the left indicates a perception of backward movement, an arrow to the right indicates forward perception, and a dot indicates no shift in perception. The observations in this study indicated the perception was predicted by the orientation of the body.
Acknowledgments
This work was funded by a grant from the NIDCD (K23 DC011298) with additional support provided by a clinician-scientist grant from the Triological Society. Technical support was provided by Shawn Olmstead-Leahey.
Footnotes
Conflict of interest: The author has no conflict of interest
Bibliography
- Anastasopoulos D, Mergner T. Canal-neck interaction in vestibular nuclear neurons of the cat. Exp Brain Res. 1982;46:269–280. doi: 10.1007/BF00237185. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJ. Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci. 1999;19:316–327. doi: 10.1523/JNEUROSCI.19-01-00316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564. doi: 10.1038/nature02754. [DOI] [PubMed] [Google Scholar]
- Au Yong N, Paige GD, Seidman SH. Multiple sensory cues underlying the perception of translation and path. J Neurophysiol. 2007;97:1100–1113. doi: 10.1152/jn.00694.2006. [DOI] [PubMed] [Google Scholar]
- Boyle R, Pompeiano O. Responses of vestibulospinal neurons to neck and macular vestibular inputs in the presence or absence of the paleocerebellum. Ann N Y Acad Sci. 1981;374:373–394. doi: 10.1111/j.1749-6632.1981.tb30884.x. [DOI] [PubMed] [Google Scholar]
- Chaudhuri SE, Merfeld DM. Signal detection theory and vestibular perception: III. Estimating unbiased fit parameters for psychometric functions. Exp Brain Res. 2013;225:133–146. doi: 10.1007/s00221-012-3354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement G, Moore ST, Raphan T, Cohen B. Perception of tilt (somatogravic illusion) in response to sustained linear acceleration during space flight. Exp Brain Res. 2001;138:410–418. doi: 10.1007/s002210100706. [DOI] [PubMed] [Google Scholar]
- Coniglio AJ, Crane BT. Human Yaw Rotation Aftereffects with Brief Duration Rotations Are Inconsistent with Velocity Storage. J Assoc Res Otolaryngol. 2014;15:305–317. doi: 10.1007/s10162-013-0438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane BT. Fore-aft translation aftereffects. Exp Brain Res. 2012a;219:477–487. doi: 10.1007/s00221-012-3105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane BT. Roll aftereffects: influence of tilt and inter-stimulus interval. Exp Brain Res. 2012b;233:89–98. doi: 10.1007/s00221-012-3243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane BT. Limited interaction between translation and visual motion aftereffects in humans. Exp Brain Res. 2013;224:165–178. doi: 10.1007/s00221-012-3299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane BT, Demer JL. Human gaze stabilization during natural activities: translation, rotation, magnification, and target distance effects. J Neurophysiol. 1997;78:2129–2144. doi: 10.1152/jn.1997.78.4.2129. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Minor LB. Semicircular canal afferents similarly encode active and passive head-on-body rotations: implications for the role of vestibular efference. J Neurosci. 2002;22:RC226. doi: 10.1523/JNEUROSCI.22-11-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys IS. The delay of the oculogravic illusion. Brain Res Bull. 1996;40:407–410. doi: 10.1016/0361-9230(96)00134-7. discussion 410-402. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez MA, Alcala-Quintana R. Shifts of the psychometric function: distinguishing bias from perceptual effects. Q J Exp Psychol (Hove) 2013;66:319–337. doi: 10.1080/17470218.2012.708761. [DOI] [PubMed] [Google Scholar]
- Gdowski GT, Belton T, McCrea RA. The neurophysiological substrate for the cervico-ocular reflex in the squirrel monkey. Exp Brain Res. 2001;140:253–264. doi: 10.1007/s002210100776. [DOI] [PubMed] [Google Scholar]
- Gianna C, Heimbrand S, Gresty M. Thresholds for detection of motion direction during passive lateral whole-body acceleration in normal subjects and patients with bilateral loss of labyrinthine function. Brain Res Bull. 1996;40:443–447. doi: 10.1016/0361-9230(96)00140-2. discussion 448-449. [DOI] [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res. 2008;186:677–681. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- Graybiel A, Johnson WH, Money KE, Malcolm RE, Jennings GL. Oculogravic illusion in response to straight-ahead acceleration of CF-104 aircraft. Aviat Space Environ Med. 1979;50:382–386. [PubMed] [Google Scholar]
- Green AM, Angelaki DE. Resolution of sensory ambiguities for gaze stabilization requires a second neural integrator. J Neurosci. 2003;23:9265–9275. doi: 10.1523/JNEUROSCI.23-28-09265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AM, Angelaki DE. Coordinate transformations and sensory integration in the detection of spatial orientation and self-motion: from models to experiments. Prog Brain Res. 2007;165:155–180. doi: 10.1016/S0079-6123(06)65010-3. [DOI] [PubMed] [Google Scholar]
- Groen EL, Bles W. How to use body tilt for the simulation of linear self motion. J Vestib Res. 2004;14:375–385. [PubMed] [Google Scholar]
- Kaernbach C. Simple adaptive testing with the weighted up-down method. Percept Psychophys. 1991;49:227–229. doi: 10.3758/bf03214307. [DOI] [PubMed] [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys. 2001;63:1279–1292. doi: 10.3758/bf03194543. [DOI] [PubMed] [Google Scholar]
- MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE. Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci. 2010;30:9084–9094. doi: 10.1523/JNEUROSCI.1304-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrea I, Cullen KE. Multisensory integration in early vestibular processing in mice: the encoding of passive vs. active motion. J Neurophysiol. 2013;110:2704–2717. doi: 10.1152/jn.01037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meese TS. Using the standard staircase to measure the point of subjective equality: a guide based on computer simulations. Percept Psychophys. 1995;57:267–281. doi: 10.3758/bf03213053. [DOI] [PubMed] [Google Scholar]
- Merfeld DM. Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2011 doi: 10.1007/s00221-011-2557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during Translation and Tilt. J Neurophysiol. 2005a;94:186–198. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt&Translation. J Neurophysiol. 2005b;94:199–205. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Zupan L, Peterka RJ. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Zupan LH, Gifford CA. Neural processing of gravito-inertial cues in humans. II. Influence of the semicircular canals during eccentric rotation. J Neurophysiol. 2001;85:1648–1660. doi: 10.1152/jn.2001.85.4.1648. [DOI] [PubMed] [Google Scholar]
- Mergner T, Rottler G, Kimmig H, Becker W. Role of vestibular and neck inputs for the perception of object motion in space. Exp Brain Res. 1992;89:655–668. doi: 10.1007/BF00229890. [DOI] [PubMed] [Google Scholar]
- Mergner T, Siebold C, Schweigart G, Becker W. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res. 1991;85:389–404. doi: 10.1007/BF00229416. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt H. The role of the otoliths in perception of the vertical and in path integration. Ann N Y Acad Sci. 1999;871:334–344. doi: 10.1111/j.1749-6632.1999.tb09196.x. [DOI] [PubMed] [Google Scholar]
- Morgan M, Dillenburger B, Raphael S, Solomon JA. Observers can voluntarily shift their psychometric functions without losing sensitivity. Atten Percept Psychophys. 2012;74:185–193. doi: 10.3758/s13414-011-0222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler CJ, Reynolds RF. Dynamic transformation of vestibular signals for orientation. Exp Brain Res. 2012 doi: 10.1007/s00221-012-3250-1. [DOI] [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol. 1991;65:1170–1182. doi: 10.1152/jn.1991.65.5.1170. [DOI] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol. 2012a;13:381–401. doi: 10.1007/s10162-012-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Suprathreshold asymmetries in human motion perception. Exp Brain Res. 2012b;219:369–379. doi: 10.1007/s00221-012-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Mitchell DE, Cullen KE. Different neural strategies for multimodal integration: comparison of two macaque monkey species. Exp Brain Res. 2009;195:45–57. doi: 10.1007/s00221-009-1751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman SH, Telford L, Paige GD. Tilt perception during dynamic linear acceleration. Exp Brain Res. 1998;119:307–314. doi: 10.1007/s002210050346. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Meng H, Angelaki DE. Multiple reference frames for motion in the primate cerebellum. J Neurosci. 2004;24:4491–4497. doi: 10.1523/JNEUROSCI.0109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutwein B. Adaptive psychophysical procedures. Vision Res. 1995;35:2503–2522. [PubMed] [Google Scholar]
- Valko Y, Lewis RF, Priesol AJ, Merfeld DM. Vestibular labyrinth contributions to human whole-body motion discrimination. J Neurosci. 2012;32:13537–13542. doi: 10.1523/JNEUROSCI.2157-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets RA, Medendorp WP, Van Gisbergen JA. Time course and magnitude of illusory translation perception during off-vertical axis rotation. J Neurophysiol. 2006;95:1571–1587. doi: 10.1152/jn.00613.2005. [DOI] [PubMed] [Google Scholar]
- Walsh EG. Role of the vestibular apparatus in the perception of motion on a parallel swing. J Physiol. 1961;155:506–513. doi: 10.1113/jphysiol.1961.sp006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys. 2001a;63:1293–1313. doi: 10.3758/bf03194544. [DOI] [PubMed] [Google Scholar]
- Wichmann FA, Hill NJ. The psychometric function: II. Bootstrap-based confidence intervals and sampling. Percept Psychophys. 2001b;63:1314–1329. doi: 10.3758/bf03194545. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Yamagata Y, Yates BJ, Schor RH, Nonaka S. Response of vestibular neurons to head rotations in vertical planes. III. Response of vestibulocollic neurons to vestibular and neck stimulation. J Neurophysiol. 1990;64:1695–1703. doi: 10.1152/jn.1990.64.6.1695. [DOI] [PubMed] [Google Scholar]
- Zhou W, Tang BF, Newlands SD, King WM. Responses of monkey vestibular-only neurons to translation and angular rotation. J Neurophysiol. 2006;96:2915–2930. doi: 10.1152/jn.00013.2006. [DOI] [PubMed] [Google Scholar]
- Zupan LH, Park S, Merfeld DM. The nervous system uses internal models to achieve sensory integration. Conf Proc IEEE Eng Med Biol Soc. 2004;6:4487–4490. doi: 10.1109/IEMBS.2004.1404247. [DOI] [PubMed] [Google Scholar]