In a randomized phase II study of sorafenib plus hepatic arterial infusion chemotherapy with cisplatin in comparison with sorafenib alone in patients with advanced hepatocellular carcinoma, it yielded favorable overall survival when compared with sorafenib alone. This is the first report of its effectiveness in relation to the overall survival in comparison with that of sorafenib alone in patients with advanced hepatocellular carcinoma.
Keywords: cisplatin, hepatic arterial infusion chemotherapy, hepatocellular carcinoma, sorafenib, randomized phase II trial
Abstract
Background
Sorafenib (Sor) is acknowledged as a standard therapy for advanced hepatocellular carcinoma (HCC). This trial was conducted to evaluate the effect of addition of hepatic arterial infusion chemotherapy with cisplatin (SorCDDP) to Sor for the treatment of advanced HCC.
Patients and methods
We conducted a multicenter open-labeled randomized phase II trial in chemo-naïve patients with advanced HCC with Child-Pugh scores of 5–7. Eligible patients were randomly assigned 2:1 to receive SorCDDP (sorafenib: 400 mg bid; cisplatin: 65 mg/m2, day 1, every 4–6 weeks) or Sor (400 mg bid). The primary end point was overall survival.
Results
A total of 108 patients were randomized (Sor, n = 42; SorCDDP, n = 66). The median survival in the Sor and SorCDDP arms were 8.7 and 10.6 months, respectively [stratified hazard ratio (95% confidence interval), 0.60 (0.38–0.96), P = 0.031]. The median time to progression and the response rate were, respectively, 2.8 months and 7.3% in the Sor arm and 3.1 months and 21.7% in the SorCDDP arm. The adverse events were more frequent in the SorCDDP arm than in the Sor arm, but well-tolerated.
Conclusion
SorCDDP yielded favorable overall survival when compared with Sor in patients with advanced HCC.
Clinical Trial registration
UMIN-CTR (http://www.umin.ac.jp/ctr/index-j.htm), identification number: UMIN000005703.
introduction
Sorafenib is currently acknowledged as a standard therapy for advanced hepatocellular carcinoma (HCC), and is available worldwide [1]. After the introduction of sorafenib, a number of phase III trials of various molecular-targeted agents versus sorafenib as first-line chemotherapy have been conducted, but none of the agents examined so far has shown superior survival benefit to sorafenib [1].
Hepatic arterial infusion chemotherapy (HAIC) is employed to treat patients with advanced HCC [2, 3]. This treatment modality is associated with increased local concentrations of the anticancer agents in the tumor and reduced systemic distribution of the drugs, and a stronger antitumor effect and lower incidence of systemic adverse reactions may be expected when compared with systemic chemotherapy. In fact, high response rates, favorable long-term outcomes, and acceptable toxicities with some chemotherapeutic regimens of HAIC have been reported [2, 3]. However, no consensus has been reached as to its place as a standard treatment of advanced HCC. Among HAI regimens, cisplatin alone can be easily administered using the Seldinger technique, without the need for an indwelling reservoir system [4]. In addition, sorafenib has been shown to interact with platinum transporter proteins [5], and to exert a synergistic anticancer effect with cisplatin in preclinical research [6]. Clinical trials of sorafenib used in combination with cisplatin have been carried out for various cancers [7–9], and favorable outcomes have been reported. Herein, we report the results of a randomized phase II trial of sorafenib plus HAIC with cisplatin (SorCDDP) versus sorafenib alone (Sor). The primary end point was the overall survival, while the secondary end points were the time to progression, response rate, and adverse events.
methods-patients and methods
patient eligibility
The patient inclusion criteria were as follows: advanced HCC confirmed histologically or by typical findings of hypervascular tumor on computed tomography (CT) or angiography and elevated serum alpha-fetoprotein (AFP), or protein induced by vitamin K absence or antagonist-II level; unsuitable for surgical resection, liver transplantation, local ablative therapy or transarterial chemoembolization (TACE); no prior history of chemotherapy; age 20–79 years old; presence of intrahepatic tumors affecting the prognosis irrespective of the presence of extrahepatic tumors; Eastern Cooperative Oncology Group Performance Status 0–1; adequate organ function [neutrophil count ≥1500 /mm3, hemoglobin ≥8.5 g/dl, platelet count ≥60 000 /mm3, serum total bilirubin ≤2.0 mg/dl, serum albumin ≥2.8 g/dl, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤5 times the upper limits of normal, serum creatinine ≤1.2 mg/dl, creatinine clearance ≥60 ml/min]; Child-Pugh score 5–7; HAIC technically feasible; written informed consent.
The main exclusion criteria were as follows: refractory pleural effusion or ascites; hepatic encephalopathy; severe and active co-morbidity or concomitant malignancy; allergic reaction to iodine contrast medium precluding angiography; pregnant and lactating females; females of childbearing age unless using effective contraception; and unsatisfactory general condition. Patients with hepatitis B or C virus infection were eligible for enrollment in this trial, provided they fulfilled the eligibility criterion pertaining to hepatic reserve.
treatments
The enrolled patients were randomly assigned 2:1 to the SorCDDP arm or the Sor arm. Randomization was done centrally using a minimization method with biased-coin assignment [10]. The dynamic allocation factors were the presence of portal vein tumor thrombosis and extrahepatic metastasis. In patients of the SorCDDP arm, based on the results of a phase I trial [11], sorafenib (Nexavar®, Bayer Health Care Pharmaceuticals; West Haven, CT, USA) was administered orally at a dose of 400 mg bid, and cisplatin (IA call®, Nippon Kayaku Co., Ltd; Tokyo, Japan) was administered concurrently at 65 mg/m2/cycle via a catheter placed in the proper, right, or left hepatic artery, or another feeding artery, every 4–6 weeks. In patients of the Sor arm, sorafenib was administered orally at a dose of 400 mg bid. The sorafenib treatment in both arms was continued until tumor progression or unacceptable toxicity, and the HAIC with cisplatin was administered up to a maximum of six cycles until radiological or symptomatic tumor progression, unacceptable toxicity, or technical difficulty in repeating the HAIC. If the protocol therapies were discontinued, the patient was allowed to receive other anticancer treatment at the physician's discretion.
The occurrence of grade 4 hematological toxicity, grade 3 non-hematological toxicity was generally considered as indication for suspending the sorafenib administration. When the toxicities improved by at least one grade when compared with the suspension criteria, the treatment was resumed at a reduced dose of 400 mg daily. If additional dose reduction was required, the dose was reduced further to a single administration of 400 mg every other day.
The criteria for administering HAIC with cisplatin were as follows: neutrophil count ≥1200/mm3, platelet count ≥50 000/mm3, serum total bilirubin ≤3.0 mg/dl, serum AST or ALT levels ≤5 times the upper limit of normal, and a serum creatinine level ≤1.5 mg/dl. If the above parameters did not fall within the starting criteria, the HAIC with cisplatin was postponed until the criteria were fulfilled.
response and toxicity assessment
Evaluation of the tumor response by dynamic CT or MRI was carried out every 6 weeks using the modified Response Evaluation Criteria in Solid Tumors (RECIST) [12]. The responses were evaluated centrally by three independent reviewers. Overall survival was measured from the date of enrollment to the date of death or the date of the last follow-up. Time to progression was defined as the time from the date of enrollment to the first documentation of disease progression or death. Assessment of adverse events was based on the National Cancer Institute Common Toxicity Criteria, version 4.0.
statistical analysis
This was a multicenter open-labeled randomized phase II trial. The primary end point was overall survival stratified by the allocation factors, including the presence/absence of portal vein tumor thrombosis and extrahepatic metastases. If the median survival associated with Sor were assumed as 7.0 months and that of SorCDDP as 9.5 months, the hazard ratio (HR) was 0.74. SorCDDP would be judged as being favorable if the HR is 0.74 or lower. A total of 105 patients were needed to estimate the 1-year survival rate with an accuracy of ±10%. This study did not have sufficient statistical power to permit formal statistical comparison between the two arms.
The differences in the categorical data between the two groups were analyzed by Wilcoxon's test. The overall survival time and time to progression were estimated by using the Kaplan–Meier method and the curves were compared using the log-rank test. HRs of the treatment effects were estimated using a Cox regression model, and stratified results by dynamic allocation factors, including the presence/absence of portal vein tumor thrombosis and extrahepatic metastasis, as well as unstratified results, were presented. This clinical trial was conducted with the approval of the review board of each participating institution and in accordance with the Declaration of Helsinki. This trial is registered with UMIN-CTR (http://www.umin.ac.jp/ctr/index-j.htm), identification number (UMIN000005703). Patient registration, random treatment allocation, and data collection were managed by the Japan Clinical Research Support Unit data center. The integrity of the data was ensured through careful review by the staff of the data center, the coordinating investigators (MI and SS), and the trial statistician (TS). All the data were fixed on 28 December 2014, and all the analyses of efficacy were carried out based on the full analysis set (FAS) by the TS using SAS 9.4 and JMP Pro 11.
results
patient characteristics
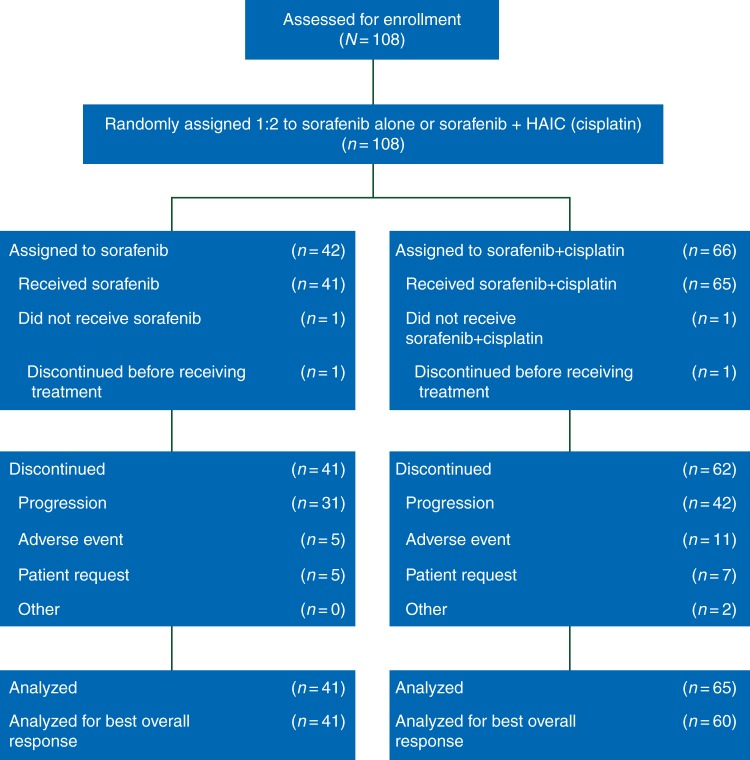
From June 2011 to December 2013, a total of 108 patients were enrolled and randomized into the two treatment arms (Figure 1). Forty-two patients were assigned to the Sor arm and 66 patients to the SorCDDP arm. While the planned random assignment was 2:1, the actual randomization ratio was 1.6:1, which was within random error. One patient from each of the arms could not receive the chemotherapy (development of paraplegia due to disease progression in one patient of the Sor arm, and withdrawal of informed consent in one patient of the SorCDDP arm). Therefore, the FAS included 41 patients in the Sor arm and 65 patients in the SorCDDP arm.
Figure 1.
Consort diagram.
The patient characteristics of the 106 patients of the FAS are presented in Table 1. Seropositivity for hepatitis C viral antibody was more frequent in the Sor arm (n = 20, 48.8%) than in the SorCDDP arm (n = 18, 27.7%), and portal vein tumor thrombosis was less frequent in the Sor arm (n = 17, 41.5%) than in the SorCDDP arm (n = 40, 61.5%). In terms of all other variables, the patient characteristics were well-balanced.
Table 1.
Baseline patient characteristics
| Characteristics | Sorafenib alone (n = 41) |
Sorafenib + HAIC (cisplatin) (n = 65) |
||
|---|---|---|---|---|
| Number of patients | % | Number of patients | % | |
| Age, years | ||||
| Median | 64 | 66 | ||
| Range | 42–78 | 25–79 | ||
| Sex | ||||
| Male | 32 | 78.1 | 56 | 86.2 |
| Female | 9 | 22.0 | 9 | 13.8 |
| ECOG performance status | ||||
| 0 | 33 | 80.5 | 50 | 76.9 |
| 1 | 8 | 19.5 | 15 | 23.1 |
| Etiology | ||||
| Hepatitis B | 9 | 22.0 | 22 | 33.8 |
| Hepatitis C | 20 | 48.8 | 18 | 27.7 |
| Child-Pugh score | ||||
| 5 | 27 | 65.9 | 38 | 58.5 |
| 6 | 12 | 29.3 | 19 | 29.2 |
| 7 | 2 | 4.9 | 8 | 12.3 |
| Ascites | 4 | 9.8 | 10 | 15.4 |
| Previous therapy | 21 | 51.2 | 33 | 50.8 |
| Resection | 6 | 17 | ||
| PEI/RFA | 7 | 8 | ||
| TACE | 14 | 23 | ||
| Radiation | 1 | 1 | ||
| Other | 2 | 1 | ||
| BCLC stage | ||||
| B | 16 | 39.0 | 19 | 29.2 |
| C | 25 | 61.0 | 46 | 70.8 |
| Portal vein tumor thrombosis | 17 | 41.5 | 40 | 61.5 |
| Vp1 | 0 | 4 | 10.0 | |
| Vp2 | 4 | 23.5 | 9 | 22.5 |
| Vp3 | 7 | 41.4 | 14 | 35.0 |
| Vp4 | 6 | 35.3 | 13 | 32.5 |
| Extrahepatic spread | 13 | 31.7 | 19 | 29.2 |
| Lung | 6 | 8 | ||
| Bone | 3 | 1 | ||
| Lymph node | 6 | 10 | ||
| Adrenal | 1 | 1 | ||
| Other | 2 | 4 | ||
| Number of tumors | ||||
| 1 | 4 | 9.8 | 8 | 12.3 |
| 2 | 3 | 7.3 | 5 | 7.7 |
| 3 | 1 | 2.4 | 1 | 1.5 |
| 4 | 3 | 7.3 | 5 | 7.7 |
| ≥5 | 30 | 73.2 | 46 | 70.8 |
| Maximum tumor size, cm | ||||
| Median | 5.2 | 5.1 | ||
| Range | 1.1–17.5 | 1.0–20.0 | ||
| Serum α-fetoprotein, ng/ml | ||||
| Median | 188 | 223.5 | ||
| Range | 2–749 412 | 1.2–394 944 | ||
| PIVKA II, mAU/ml | ||||
| Median | 1790 | 1772 | ||
| Range | 9–1 410,000 | 10–261 920 | ||
ECOG, Eastern Cooperative Oncology Group; PEI/RFA, percutaneous ethanol injection/radiofrequency ablation; TACE, transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer Group; Vp1, tumor thrombosis distal to the second branches of the portal vein; Vp2, tumor thrombosis in the second branches of the portal vein; Vp3, tumor thrombosis in the first branches of the portal vein; Vp4, tumor thrombosis in the main trunk of the portal vein or the opposite side branch of the portal vein; PIVKA II, protein induced by vitamin K absence or antagonist-II.
treatments
By the data cutoff point, the protocol treatment had been discontinued in 41 patients of the Sor arm and 62 patients of the SorCDDP arm. The median number of cisplatin administrations and the median total dose of cisplatin in the SorCDDP arm were two times (range, 1–6 times) and 222 mg (range, 70–709 mg), respectively. The median dose intensity (range) was 488 mg/day (146–800 mg) in the Sor arm and 540 mg/day (193–800 mg) in the SorCDDP arm (P = 0.70). The proportion of patients in whom dose reduction of sorafenib was necessitated was 49.2% in the Sor arm and 63.4% in the SorCDDP arm. The median treatment duration (range) was 86 days (16–449 days) in the Sor arm and 75 days (4–881 days) in the SorCDDP arm (P = 0.58). After termination of the protocol treatment, 24 patients (59%) in the Sor arm and 40 patients (61.5%) in the SorCDDP arm received subsequent therapies, as follows: HAIC (8 and 19 patients, respectively), TACE (8 and 14 patients, respectively), local ablation (1 and 2 patients, respectively), other systemic chemotherapy (11 and 32 patients, respectively), palliative resection (2 and 5 patients, respectively), and radiotherapy (0 and 9 patients, respectively).
efficacy
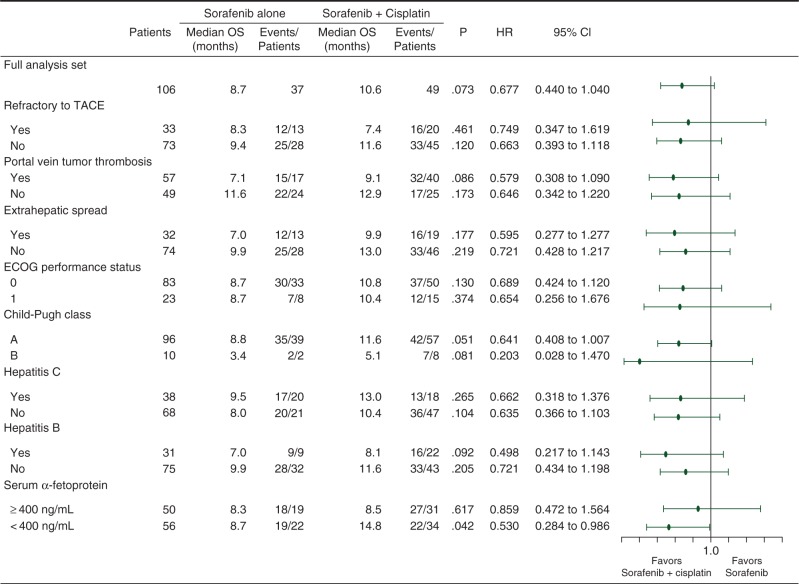
At the final analysis, 37 patients of the Sor arm and 49 patients of the SorCDDP arm had died. The median survivals in the Sor and SorCDDP arms were 8.7 and 10.6 months, respectively (Figure 2A). The HR stratified by the allocation factors, including the presence/absence of portal vein tumor thrombosis and extrahepatic metastases (95% CI), was 0.60 (0.38–0.96), and P-value was 0.031. The crude HR [95% confidence interval (CI)] was 0.68 (0.44–1.049) (P = 0.073). The forest plot showing the pre-specified subgroup analyses of overall survival is shown in Figure 3. The patient subgroup with serum AFP <400 ng/ml showed a better overall survival in the SorCDDP arm (median 14.8 months) than in the Sor arm (median 8.7 months) (P = 0.042). At the data cutoff point, disease progression was observed in 39 patients in the Sor arm and 61 patients in the SorCDDP arm. The median time to progression was 2.8 months in the Sor arm and 3.1 months in the SorCDDP arm (Figure 2B). The crude HR was 0.78 (95% CI, 0.52–1.16, P = 0.212) and the HR stratified by the allocation factors was 0.78 (95% CI, 0.50–1.21, P = 0.257).
Figure 2.
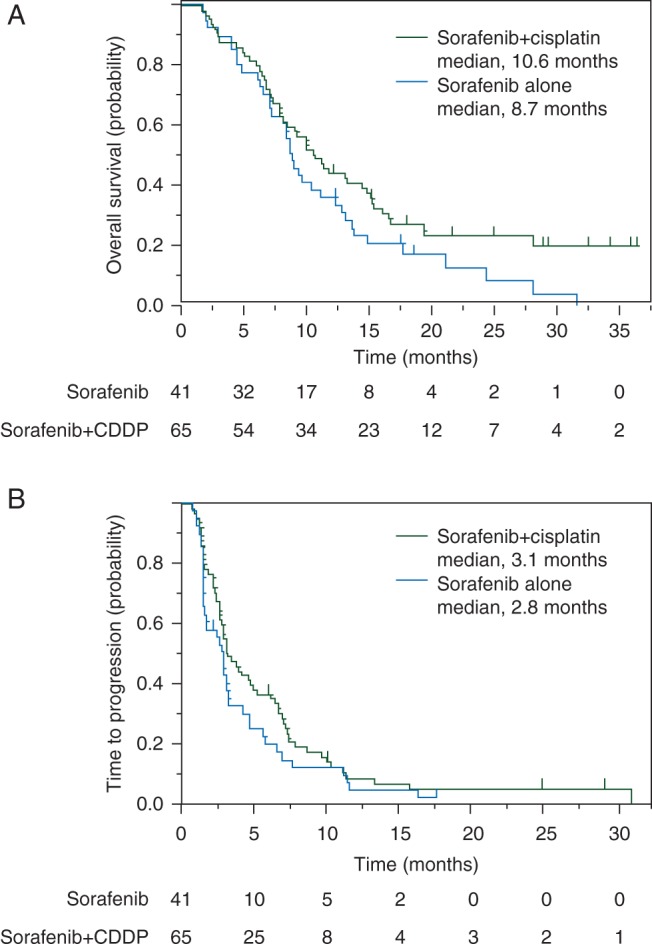
Kaplan–Meier curves of overall survival (A) and time to progression (B) in the sorafenib arm (blue line) and sorafenib plus hepatic arterial infusion chemotherapy with cisplatin arm (green line). The tick marks indicate censored cases.
Figure 3.
Forest plots showing subgroup analyses of the overall survival. TACE, transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; HR, hazard ratio; CI, confidence interval.
In the judgment by the central review, the number of patients evaluable by the modified RECIST criteria was 41 in the Sor arm and 60 patients in the SorCDDP arm. The response rate (95% CI) was 7.3% (1.5–19.9%) in the Sor arm and 21.7% (12.1–34.2%) in the SorCDDP arm (P = 0.09) (supplementary Figure 1, available at Annals of Oncology online).
adverse events
The adverse events in both the arms during the entire treatment period until the final analysis are presented in Table 2. Neutropenia, leukocytopenia, decreased hemoglobin, thrombocytopenia, hyponatremia, nausea, and hiccups of all grades were more frequent in the SorCDDP arm than in the Sor arm. There were two treatment-related deaths in this series: one developed liver failure 9 months after the initiation of SorCDDP therapy, and the other developed pulmonary infection 2 months after the initiation of Sor therapy.
Table 2.
Adverse events
| Sorafenib alone arm |
Sorafenib + HAIC (cisplatin) arm |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All grades |
Grade 3 |
Grade 4 |
All grades |
Grade 3 |
Grade 4 |
|||||||
| No. of pts | (%) | No. of pts | (%) | No. of pts | (%) | No. of pts | (%) | No. of pts | (%) | No. of pts | (%) | |
| WBC decreased | 18 | 43.9 | 0 | 0 | 0 | 0 | 49 | 75.4 | 12 | 18.5 | 0 | 0 |
| Neu decreased | 18 | 43.9 | 0 | 0 | 0 | 0 | 39 | 60 | 8 | 12.3 | 2 | 3.1 |
| Hb decreased | 30 | 73.2 | 2 | 4.9 | 1 | 2.4 | 58 | 89.2 | 6 | 9.2 | 2 | 3.1 |
| Plt decreased | 33 | 80.5 | 1 | 2.4 | 0 | 0 | 58 | 89.2 | 19 | 29.2 | 0 | 0 |
| Bilirubin increased | 29 | 70.7 | 5 | 12.2 | 0 | 0 | 48 | 73.8 | 6 | 9.2 | 2 | 3.1 |
| AST increased | 41 | 100 | 8 | 19.5 | 3 | 7.3 | 65 | 100 | 21 | 32.3 | 1 | 1.5 |
| ALT increased | 37 | 90.2 | 7 | 17.1 | 1 | 2.4 | 61 | 93.8 | 12 | 18.5 | 1 | 1.5 |
| γGTP increased | 37 | 90.2 | 14 | 34.1 | 2 | 4.9 | 63 | 96.9 | 21 | 32.3 | 3 | 4.6 |
| Hypoalbuminemia | 32 | 78 | 3 | 7.3 | 0 | 0 | 63 | 96.9 | 2 | 3.1 | 0 | 0 |
| Cr increased | 11 | 26.8 | 0 | 0 | 0 | 0 | 25 | 38.5 | 1 | 1.5 | 0 | 0 |
| Hyponatremia | 22 | 53.7 | 5 | 12.2 | 0 | 0 | 53 | 81.5 | 18 | 27.7 | 0 | 0 |
| Amylase increased | 21 | 52.5 | 0 | 0 | 0 | 0 | 41 | 64.1 | 10 | 15.6 | 1 | 1.6 |
| Fatigue | 16 | 39 | 1 | 2.4 | – | – | 28 | 43.1 | 7 | 10.8 | – | – |
| Malaise | 17 | 41.5 | – | – | – | – | 31 | 47.7 | – | – | – | – |
| Appetite loss | 22 | 53.7 | 1 | 2.4 | 0 | 0 | 45 | 69.2 | 4 | 6.2 | 2 | 3.1 |
| Nausea | 8 | 19.5 | 0 | 0 | – | – | 27 | 41.5 | 0 | 0 | – | – |
| Vomiting | 5 | 12.2 | 0 | 0 | 0 | 0 | 12 | 18.5 | 0 | 0 | 0 | 0 |
| Diarrhea | 17 | 41.5 | 1 | 2.4 | 0 | 0 | 23 | 35.4 | 4 | 6.2 | 0 | 0 |
| Hand–foot synd | 26 | 63.4 | 7 | 17.1 | – | – | 41 | 63.1 | 9 | 13.8 | – | – |
| Skin rash | 11 | 26.8 | 2 | 4.9 | 0 | 0 | 12 | 18.5 | 3 | 4.6 | 0 | 0 |
| Hypertension | 24 | 58.5 | 9 | 22 | 0 | 0 | 32 | 49.2 | 19 | 29.2 | 0 | 0 |
| Hiccups | 0 | 0 | 0 | 0 | – | – | 6 | 9.2 | 0 | 0 | – | – |
WBC, white blood count; Neu, neutrophils; Hb, hemoglobin; Plt, platelets; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGTP, γ-glutamyl transpeptidase; Cr, creatinine; synd, syndrome; pts, patients.
discussion
In this study, SorCDDP yielded favorable overall survival when compared with Sor in patients with advanced HCC. The pre-specified HR stratified by the allocation factors (95% CI) was 0.60 (0.38–0.96), and P-value was 0.031. Because we had set the condition that SorCDDP would be judged as favorable if the HR for overall survival was 0.74 or lower, the primary end point of this study was met. In the pre-specified subgroup analysis of overall survival, the SorCDDP arm showed more favorable overall survival than the Sor arm in all the subgroups, and the efficacy of SorCDDP can be anticipated in almost all subjects who are suitable candidates for sorafenib treatment. In this trial, patients with hepatitis C viral infection showed a more favorable overall survival following sorafenib treatment than those with hepatitis B viral infection. However, it remains unknown whether patients with hepatitis C viral infection actually benefitted more from this treatment or not, because of the small sample size of this study. Furthermore, the overall survival in the SorCDDP arm was better than that in the Sor arm among the patients with serum AFP <400 ng/ml [crude HR, 0.53 (95% CI, 0.28–0.99)], whereas no difference was observed between the SorCDDP arm and the Sor arm among the patients with serum AFP ≥400 ng/ml. AFP may be one of the predictive biomarkers in patients receiving SorCDDP therapy, although the reason remains unknown.
Recently, immuno-oncology agents, such as tremelimumab [13] and nivolumab [14], have been introduced as promising agents for advanced HCC. The characteristics of these agents are a high response rate and long-lasting antitumor efficacy. In our study also, the response rate in the SorCDDP arm (21.7%) was threefold higher than that in the Sor arm (7.3%), and some patients in the SorCDDP arm showed long-lasting survival over 2 years. With regard to time to progression, the stratified HR by the allocation factors was 0.78 (95% CI, 0.59–1.21), and it was slightly worse than that of overall survival. In some phase III trials conducted for HCC, significant difference was observed in the time to progression or progression-free survival, but not in the overall survival [1]. Eventually, a negative result was concluded. However, in this study, the results were completely opposite. The most important difference between this study and these aforementioned trials may be in the anticancer treatments used: in this study, sorafenib was combined with a cytotoxic agent, while in the aforementioned phase III trials, it was used in combination with other molecular-targeted agents. Among patients showing marked tumor shrinkage on account of the favorable tumor shrinkage effect of SorCDDP, even a slight increase in the tumor size could result in their being classified as showing disease progression, whereas these patients may also show a prolonged overall survival because of the smaller tumor burden. This might also be the reason for the more favorable improvement of the overall survival than the time to progression.
The frequencies of the adverse events in the SorCDDP arm, except for those of neutropenia, leukocytopenia, hypohemoglobinemia, thrombocytopenia, hyponatremia, nausea and hiccups, were similar to those in the Sor arm. These adverse events were not severe. HAIC with cisplatin had only a mild toxicity profile [4] and the toxicities were not overlapped with the adverse effects of sorafenib. Therefore, SorCDDP therapy was also considered to be well-tolerated.
Intra-arterial administration of cisplatin was generally thought to be troublesome, requiring the insertion of a catheter into the tumor-feeding arteries. Recently, a phase III trial of sorafenib plus intra-arterial cisplatin and 5-fluorouracil versus sorafenib alone demonstrated no survival benefit [15]. One of the reasons could be the difficulty in placing the indwelling reservoir system. However, cisplatin is easily administered without the need for an indwelling reservoir system. Furthermore, this combined treatment is medico-economically very viable, because the additional cost of the angiographic procedure and cisplatin is approximately $2000 per session, which is less than the cost of the recently administered molecular-targeted agents or immuno-oncology agents.
In conclusion, this study demonstrated favorable overall survival in the SorCDDP arm when compared with that in the Sor arm in patients with advanced HCC, suggesting the effectiveness of HAIC against advanced HCC. However, since this study was only a randomized phase II trial, we could not arrive at any definitive conclusion with regard to the usefulness of sorafenib plus HAIC with cisplatin in the treatment of advanced HCC. A further phase III trial is being planned to confirm these results.
funding
This work was supported in part by the National Cancer Center Research and Development Fund (23-A-22).
disclosure
MI has received payment for lectures from Bayer Yakuhin and Nippon Kayaku; has been a consultant for Bayer Yakuhin; and has received research funding from Bayer Yakuhin. SS has received payment for lectures from Nippon Kayaku. YI has received payment for lectures from Bayer Yakuhin and Nippon Kayaku; and has received research funding from Bayer Yakuhin and Nippon Kayaku. AH has been on the speakers' bureau for Bayer Yakuhin. TO has received payment for lectures from Bayer Yakuhin. JF has received payment for lectures from Bayer Yakuhin and Nippon Kayaku; has been on advisory arrangements for Bayer Yakuhin; and received research funding from Bayer Yakuhin and Nippon Kayaku. TO has received research funding from Bayer Yakuhin and has received payment for lectures from Nippon Kayaku. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank all the patients and their families for their participation in this study. We thank the members of the Japan Clinical Research Support Unit Data Center for their support with the data management (Ms Youko Yoshimoto, Ms Rei Aida, and Mr Takashi Ando), the members of the study committee for safety and efficacy monitoring (Dr Yasuhiro Matsumura, Dr Kei Muro, and Dr Keigo Osuga), the members of the study committee for response evaluation (Dr Tatsushi Kobayashi, Dr Nobumasa Mizuno, and Dr Eiichiro Suzuki), and all the investigators of this trial.
references
- 1.Abou-Alfa GK, Venook AP. The antiangiogenic ceiling in hepatocellular carcinoma: does it exist and has it been reached? Lancet Oncol 2013; 14: e283–e288. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda M, Mitsunaga S, Shimizu S et al. . Current status of hepatocellular carcinoma in Japan. Chin Clin Oncol 2013; 240. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M. Treatment of advanced hepatocellular carcinoma with emphasis on hepatic arterial infusion chemotherapy and molecular targeted therapy. Liver Cancer 2012; 1: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshikawa M, Ono N, Yodono H et al. . Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res 2008; 38: 474–483. [DOI] [PubMed] [Google Scholar]
- 5.Heim M, Scharifi M, Zisowsky J et al. . The Raf kinase inhibitor BAY 43-9006 reduces cellular uptake of platinum compounds and cytotoxicity in human colorectal carcinoma cell lines. Anticancer Drugs 2005; 16: 129–136. [DOI] [PubMed] [Google Scholar]
- 6.Wei Y, Shen N, Wang Z et al. . Sorafenib sensitizes hepatocellular carcinoma cell to cisplatin via suppression of Wnt/beta-catenin signaling. Mol Cell Biochem 2013; 381: 139–144. [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Powell M, O'Dwyer PJ et al. . Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol 2010; 28: 2947–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Ares LG, Biesma B, Heigener D et al. . Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol 2012; 30: 3084–3092. [DOI] [PubMed] [Google Scholar]
- 9.Xue C, Huang Y, Huang PY et al. . Phase II study of sorafenib in combination with cisplatin and 5-fluorouracil to treat recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol 2013; 24: 1055–1061. [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975; 31: 103–115. [PubMed] [Google Scholar]
- 11.Hagihara A, Ikeda M, Ueno H et al. . Phase I study of combination chemotherapy using sorafenib and transcatheter arterial infusion with cisplatin for advanced hepatocellular carcinoma. Cancer Sci 2014; 105: 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010; 30: 52–60. [DOI] [PubMed] [Google Scholar]
- 13.Sangro B, Gomez-Martin C, de la Mata M et al. . A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013; 59: 81–88. [DOI] [PubMed] [Google Scholar]
- 14.El-Khoueiry AB, Melero I, Crocenzi TS et al. . Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol 2015;33 (suppl; abstr LBA101). [Google Scholar]
- 15.Ueshima KM, Yokosuka O et al. . Prospective randomized controlled phase III trial comparing the efficacy of sorafenib versus sorafenib in combination with low-dose cisplatin/fluorouracil hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma. J Hepatol 2016; 64: S209–S210 (Abstr LB04). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.