Antibody-secreting cells survive in the respiratory tract in the absence of eosinophils
Keywords: epithelial cells, lung, nasal-associated lymphoid tissue, nearest neighbors, virus infection
Abstract
Antibody-secreting cells (ASCs) in respiratory tract tissues provide a first line of defense against invading pathogens. These cells often secrete IgA that is efficiently transcytosed across epithelial barriers into the airway lumen where pathogens can be blocked at their point of entry. Previous literature has reported that in the bone marrow, eosinophils are required for the maintenance of ASCs, and that eosinophils co-localize with ASCs as nearest neighbors. To determine if these rules similarly apply to the maintenance of ASCs in respiratory tract tissues, we evaluated virus-specific responses 1 month and 4 months following an intranasal virus infection of eosinophil-null (∆dblGATA-1) mice. Results showed that ASCs were fractionally reduced, but were nonetheless observed in respiratory tract tissues in the absence of eosinophils. Virus-specific antibodies were similarly observed in the airways of eosinophil-deficient mice. Respiratory tract ASCs were also present in mice lacking neutrophils (Mcl1∆M). The staining of tissue sections from the upper respiratory tract of wild-type mice following viral infections demonstrated that virus-specific ASCs were most frequently situated adjacent to epithelial cells rather than eosinophils or neutrophils. Taken together, these data emphasize that rules for cell maintenance are not absolute and that ASCs can survive in the respiratory tract without eosinophils or neutrophils as their nearest neighbors.
Introduction
The development of resident antibody-secreting cells (ASCs) following respiratory virus infection or vaccination is key to the establishment of durable immune protection against airway pathogens. Replication-competent vaccines can induce ASC responses that last for the lifetime of a host (1). After smallpox vaccination, for example, virus-specific ASCs persist in human bone marrow for decades (2, 3). After intranasal vaccinations, the majority of ASCs resides in the respiratory tract rather than the bone marrow (4) and can durably secrete IgA as a first line of defense against a pathogen challenge (5–7). Chu et al. (8, 9) previously examined the requirements for ASC persistence in the bone marrow and reported that eosinophils were required. Here, we ask if similar rules apply for the maintenance of ASCs in the respiratory tract.
Methods
Animals and infections
Gata1tm6Sho/J (∆dblGATA-1) mice and controls were purchased from Jackson Laboratories. Mice were anesthetized with avertin and infected intranasally with 250 plaque-forming units (pfu) Sendai virus (SeV; Enders strain) in 30 μl PBS. Neutrophil-deficient (Mcl1∆M, see details below) and control animals were gifts from Drs Peter Murray and Joseph Opferman, and were from a breeding colony at St. Jude Children’s Research Hospital (St. Jude). Tissues from C57BL/6 wild-type mice (Jackson Laboratories) were also sampled before and after infections with SeV. Animal work was conducted following Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines, and was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at St. Jude. All experiments were repeated to ensure reproducibility.
ASC enumeration and antibody measurements
To prepare tissues after animal sacrifice, lungs were perfused by clipping the dorsal aorta and injecting PBS through the right ventricle of the heart. Cervical lymph nodes (CLN) were removed for preparation of single-cell suspensions. To harvest nasal tissues, snouts were collected by discarding lower jaws, soft palates, muscles, cheek bones, skin and teeth. Snouts were separated into small pieces with forceps. The perfused lung was separated into small pieces using a scalpel blade. Nasal tissues and lungs were digested in 4mg/ml collagenase (Worthington Type II) in PBS for 30min with shaking at 37°C. Collagenase-treated cells were strained and fractionated on a percoll 40/75% discontinuous gradient spun 2500 r.p.m. in an IEC Centra 8B centrifuge for 30min. Cells at the interface were washed 2× in 1% BSA in PBS and suspended in RPMI 1640, gentamycin (50 µg/ml), 2mM glutamine, and 10% heat-inactivated fetal bovine serum (Atlanta Biological; supplemented RPMI is termed R10 medium below).
For the enumeration of virus-specific ASCs, 96-well ELISPOT plates (MAIPS4510, Millipore) were pre-coated with disrupted sucrose-gradient purified SeV, 10 μg/ml in PBS, incubated overnight (ON) at 4°C. Plates were washed 3× with PBS and blocked with R10 medium. Cells were plated at 105 cells/well in 200 μl and incubated for 3h at 37°C, 5% CO2. After plates were washed 3× with PBS and 3× with 0.1% Tween-20 in PBS, wells were incubated with 100 μl goat anti-mouse IgA or IgG conjugated to alkaline phosphatase (Southern Biotech Assoc. Cat#1040-04 and 1030-04, respectively) diluted 1:1000 in 1% BSA in PBS for ON incubation at 4°C. After another 6–7× plate washes with PBS, wells were developed with 100 μl bromo-chloro-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT, Sigma, Cat# B5655, 1 tablet/10ml H2O). The reaction was stopped by washing with H2O. Plates were then air dried and spots were counted using a Nikon dissecting microscope. Background virus-specific ASC values with tissues from unvaccinated mice were routinely <5 ASC/100000 cells. The testing of total rather than virus-specific ASCs was performed as above except that the plate was pre-coated with 100 µl/well goat anti-mouse IgG H+L (2 µg/ml, Southern Biotech Cat#1031-01) rather than with virus.
For the measurement of antibodies from respiratory tract secretions, bronchoalveolar lavage (BAL) samples were collected prior to tissue collections. Washes were obtained by clipping the mouse trachea and flushing the lower respiratory tract with 1ml PBS with a plastic gavage needle. For the conduct of ELISAs, 96-well flat-bottomed plates were coated with disrupted SeV at 10 μg/ml (50 μl/well) for ON incubation at 4°C. Wells were washed 3× with PBS and blocked with 100 μl 3% BSA in PBS ON at 4°C. Test and control samples were serially diluted (1:10) in 3% BSA and 0.1% Tween-20 in PBS and added to wells in 50 μl for a 1h incubation at 37°C. After 7× well washes with 0.1% Tween-20 in PBS, AP-conjugated goat anti-mouse IgA (Southern Biotech Assoc., 1:1000 dilution, 50 μl) was added for a 1h incubation at room temperature. After 7× well washes with 0.1% Tween-20 in PBS, p-nitrophenyl phosphate (pNPP, Sigma–Aldrich) in 1mg/ml diethanolamine buffer was added to wells (50 μl). After 30min, readings at OD 405nm were recorded. Antibody titers were calculated using nonlinear regression software (GraphPad Prism). Samples from unprimed mice typically yielded titers of less than 50.
Phenotyping of test mice by flow cytometry
To phenotype ∆dblGATA-1 mice, blood was collected in 10 µl 0.5M EDTA (IBI Scientific) as anti-coagulant. Cells were allowed to settle and the buffy coat was collected followed by lysis of remaining RBCs. Cells were stained with anti-CD11b and anti-Siglec antibodies (anti-CD11b PE Cat#553311 1:200 BD Pharmingen, anti-Siglec F Biotin Cat # BAF1706 1:100 R&D Systems followed by APC Streptavidin Cat#554067 1:500 BD Pharmingen) and examined on a FACS Calibur. To confirm the phenotype of Mcl1∆M and control littermates, blood was collected in 2% dextran (from Leuconostoc spp. Sigma–Aldrich Cat#31392-10G) in PBS and cells were stained with anti-CD11b (anti CD11b PerCPCy5.5 1:100 Cat # 550993 BD Pharmingen) and anti-Gr1 (anti-Ly6-G-APC 1:100 Cat# 563129 BD Pharmingen) antibodies for FACS analyses. Dot plots were evaluated using FlowJo software.
Tissue staining
Tissues from SeV-infected C57BL/6 wild-type mice were sampled before and after infections with SeV. After euthanasia, mice were perfused with 10% formalin (Thomas Scientific). Tissues were post-fixed for an additional two weeks with 10% buffered formalin. Samples were decalcified in formic acid (TBD-2 decalcification; Thermo Scientific) for 2 days. Tissues were embedded in paraffin and 4 µm sections were cut. Deparaffinization was with xylene (2×3min), 100% ethanol (2×3min), 95% ethanol (3min), 70% ethanol (3min), 50% ethanol (3min), and distilled water (Gibco). Heat-Induced Epitope Retrieval (HIER) followed by incubation of slides with 0.01M Tris, 1mM EDTA, 0.05% Tween-20, pH 9.0 at 100°C for 20min. Staining was then conducted in humidified chambers. Cooled slides were blocked with 3% bovine serum albumin (Sigma)-DPBS for 1h at room temperature. IgA staining was with goat anti-mouse IgA-Biotin (Southern Biotech Assoc, 1:100) in 3% BSA ON at 4°C, followed by a DPBS wash and incubation with streptavidin-alkaline phosphatase (1:100) in 3% BSA for 3h at room temperature. After a final wash with DPBS, slides were stained with Fast Red TR/Napthol AS-MX (Sigma). To stain slides for eosinophils, slides were incubated with rat anti-mouse murine eosinophil major basic protein (mMBP) MT-14.7.3 (Lee Lab, Mayo Clinic, 1:1000) in 3% BSA ON at 4°C. Slides were washed with DPBS and incubated with goat anti-rat IgG (H+L) peroxidase (Invitrogen Cat#A10549, 1:100) at room temperature for 3h. Another wash with DPBS was followed by stain with 3,3′-diaminobenzidine tetrahydrochloride (DAB, Sigma). To stain for neutrophils, slides were incubated with rat anti-mouse neutrophil NIMP-R14 (Santa Cruz Biotechnology, 1:100) in 3% BSA ON at 4°C, washed with DPBS, and incubated with goat anti-rat IgG (H+L) peroxidase (Invitrogen, 1:100) at room temperature for 3h. A DPBS wash was followed by incubation with DAB (Sigma). Slides were counterstained with hematoxylin solution (Sigma). Slides were washed with water, layered with Clear-Mount (Electron Microscopy Sciences), dried, and layered with Permount (Fisher) for coverslip mounting.
Results and discussion
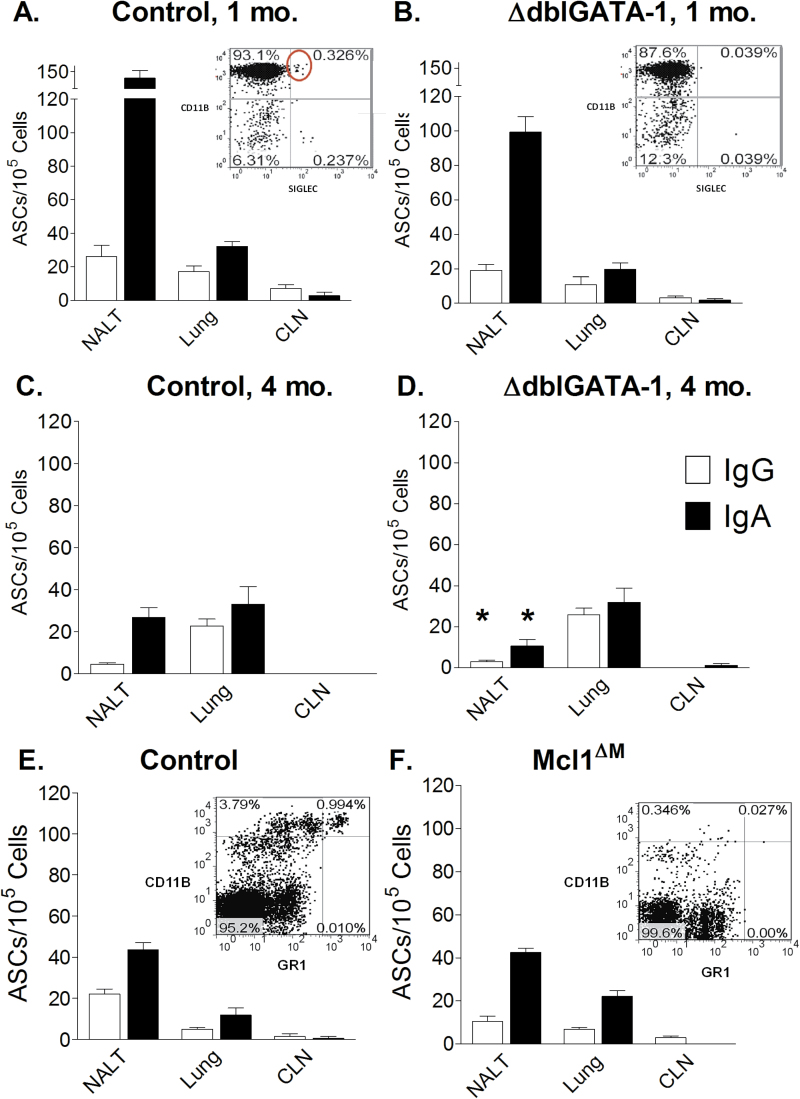
To test for virus-specific ASCs in the absence of eosinophils, we employed the Gata1tm6Sho/J mouse model, also named ∆dblGATA-1. This mouse strain fails to express the transcription factor GATA-1, and is therefore deficient in mature eosinophils as demonstrated by a lack of CD11bHI Siglec+ blood cells (see panel inserts of Fig. 1A and B) (8, 10). Control and ∆dblGATA-1 mice were infected intranasally with 250 EID50 SeV (30 μl per animal). Virus-specific IgG and IgA ASCs from nasal tissues, lungs, and CLN were then measured 1 month after infection. Tissues were tested individually or combined from two to five animals per group to characterize and compare ASCs. As shown in Fig. 1(A and B), virus-specific ASCs were present in nasal tissues and lungs of both ∆dblGATA-1 mice and controls (background levels <5 ASCs/100000 cells). ASCs were rare in the CLN of both mouse sets at 1 month post-infection, as expected based on previous studies showing an acute rise and fall of ASCs in draining lymph nodes immediately following a respiratory virus infection (11). As expected based on ASC measurements, virus-specific IgA was present in respiratory tract washes in test and control animals 1 month after infection, and responses were not diminished in ∆dblGATA-1 mice compared with controls. In one experiment for example, virus-specific IgA antibody titers in the BAL of five ∆dblGATA-1 mice were 109, 94, 109, 75, and 75, and antibody titers in control mice were 78, 76, 56, 70, and 62. Antibody results for test and control mice were not significantly different.
Fig. 1.
ASCs in nasal tissues of mice deficient in eosinophils or neutrophils. (A) Virus-specific ASCs are shown 1 month post-infection for nasal tissues, lung, and CLN of control mice. In the insert panel, a typical FACS profile of anti-CD11b and anti-Siglec antibody-stained blood cells is shown. CD11bHI Siglec+ cells are circled. (B) Virus-specific ASCs are shown 1 month post infection for nasal tissues, lung, and CLN of ∆dblGATA-1 mice. As in A, a typical FACS profile is shown for stained blood cells from ∆dblGATA-1 mice. (C) Virus specific ASCs are shown 4 months post-infection in control animals. (D) Virus-specific ASCS are shown 4 months post-infection in ∆dblGATA-1 mice. (E) Total ASCS are shown in control littermates (for the Mcl1∆M mouse model). A typical FACS profile for control blood stained with anti-CD11b and anti-Gr1 anti bodies is shown in the panel insert. (F) Total ASCs are shown in Mcl1∆M mice. Similar to panel E, a typical FACS profile of stained blood cells from Mcl1∆M mice is shown in the panel insert. Significant and reproducible reductions in values for test mice compared to controls are indicated by asterisks (Student’s t test).
Animals were also tested 4 months after vaccination. Again, ∆dblGATA-1 mice exhibited ASCs in respiratory tract tissues (Fig. 1C and D). Statistical analyses compared results between ∆dblGATA-1 mice and controls at the 1 and 4 month time points, showing that only IgG and IgA ASCs in nasal tissues were significantly and reproducibly lower in ∆dblGATA-1 mice compared with controls at the 4 month time point (Fig. 1D). Apparently, eosinophils were not strictly required for virus-specific ASC residence or persistence in the respiratory tract.
Because neutrophils have also been identified as supporting ASC residence (12, 13), we next examined ASCs in the respiratory tract of neutrophil-deficient mice. In this case, we employed Mcl1∆M animals. These mice express Cre recombinase under the control of the lysozyme M promoter (LysM-Cre) causing a conditional deletion of mcl-1 in the myeloid lineage, and a profound deficiency in neutrophils (14–16). The neutrophil deficiency in test animals from a St. Jude breeding colony was confirmed by flow cytometry, demonstrating a lack of CD11b+Gr1 (Ly6G)Hi blood cells (Fig. 1E and F) (14). Because neutrophil-deficient mice were frail and unlikely to survive the anesthesia and virus infection processes, we sacrificed animals and examined only total ASCs rather than virus-specific ASCs in respiratory tract tissues. Results for control and Mcl1∆M-deficient animals are shown in Fig. 1(E and F). We observed no significant, reproducible reduction in ASCs in the respiratory tracts of Mcl1∆M mice compared with controls.
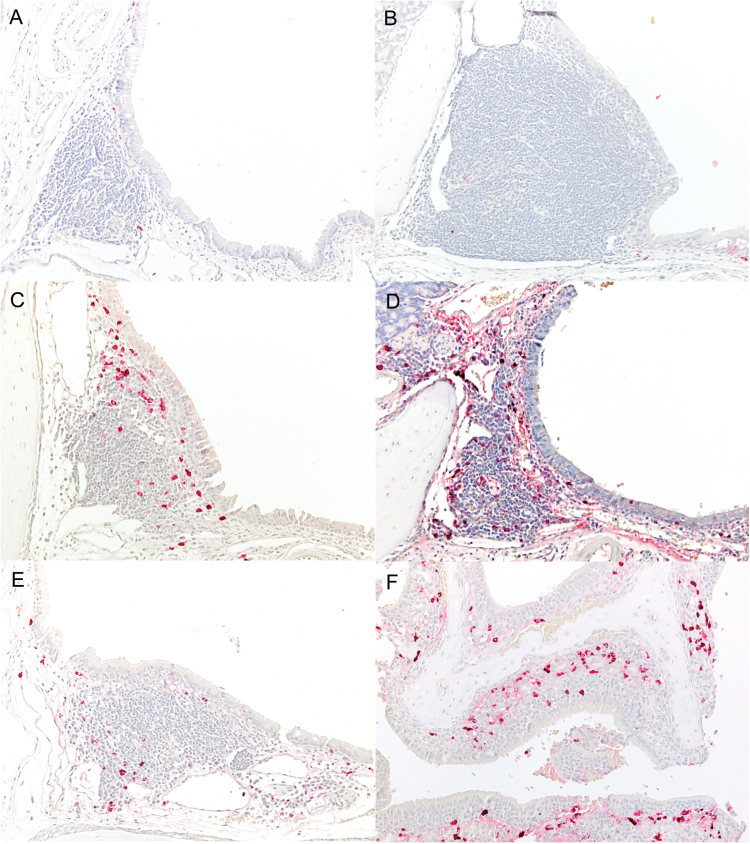
Finally, we sectioned upper respiratory tract tissues from wild-type animals at various stages after a SeV infection to define the precise location and nearest neighbors of IgA-producing ASCs. First, we co-stained for IgA (red) and eosinophils (brown) as shown in Fig. 2. On day 0 (pre-infection, panel A) and day 3 post-infection (panel B), IgA-producing ASCs in upper respiratory tract tissues were rare and their distribution was limited to the periphery of nasal associated lymphoid tissues (NALT). By day 6 post-infection (panel C), the IgA-producing ASCs had increased markedly in numbers but were still limited to the NALT. By day 10 post-infection (panel D) numerous IgA-producing cells were extending out from the NALT into surrounding epithelial tissues, and on day 25, although the numbers of ASCs within the NALT had decreased from peak levels (panel E), abundant IgA-producing ASCs were diffusely scattered throughout the respiratory and olfactory mucosa (panel F), and were most often situated adjacent to or between epithelial cells in these locations.
Fig. 2.
IgA-producing ASCs reside in nasal tissues without eosinophils as nearest neighbors. In panels A–F, immunohistochemical labeling of IgA-producing ASCs (red) and eosinophils (brown) in nasal tissues shows that epithelial cells, and not eosinophils, are nearest neighbors for ASCs in URT tissues. (A) Day 0: rare IgA-positive ASCs are located at the periphery of NALT and eosinophils are not present. ×20. (B) Day 3 post-infection: IgA-positive ASCs are still rare and located at the periphery of NALT and adjacent submucosa. There is a diffuse expansion of reactive NALT. ×20. (C) Day 6 post-infection: there are increased numbers of IgA-positive ASCs within the NALT, with the most concentrated ASCs adjacent to the epithelium. No eosinophils are collocated with the ASCs. ×20. (D) Day 10 post-infection: there is a diffuse increase in IgA-positive ASCs within the NALT and extending into adjacent tissues. Again, no eosinophils are present. ×20. (E) Day 25 post-infection: the number of IgA-positive ASCs within NALT has decreased, but eosinophils are not present in the NALT. (F) Day 25 post-infection: There is a massive increase in the numbers of IgA-positive ASCs below and within the respiratory and olfactory neuroepithelium covering nasal turbinates, but eosinophils are rarely found in these locations. ×20.
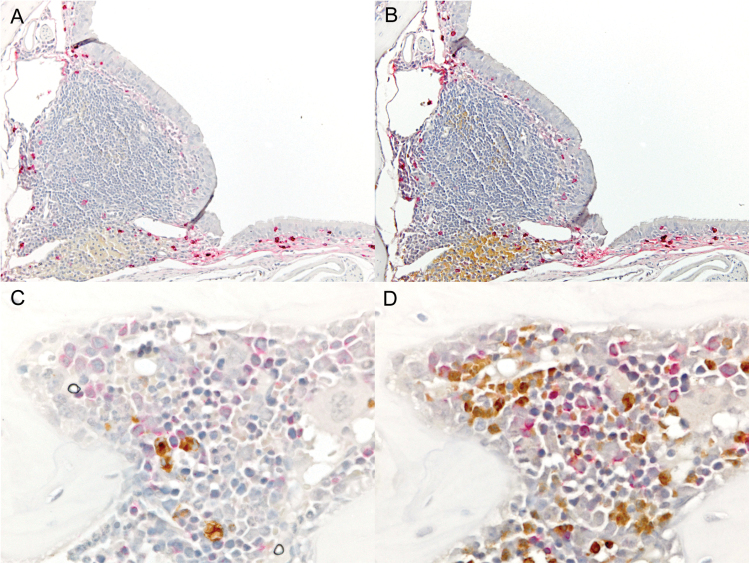
It was noteworthy that eosinophils were almost never located in the vicinity of ASCs within the NALT or other areas of the respiratory epithelium at any of the time points examined (Fig. 2A–F). When we co-stained sections for IgA (red) and either eosinophils or neutrophils (brown), we observed the IgA-producing ASCs in respiratory tract tissues were present in largest numbers where both eosinophils (Fig. 3A) and neutrophils (Fig. 3B) were absent. Virus-specific ASC are relatively rare in the bone marrow after an intranasal virus infection compared to a peripheral vaccination (4), but when ASCs were found in the bone marrow, they were often present in the vicinity of both eosinophils (Fig. 3C) and neutrophils (Fig. 3D). Taken together, these results indicate that ASCs resident in respiratory tract tissues do not require eosinophils or neutrophils as nearest neighbors for their development or persistence.
Fig. 3.
IgA-producing ASCs reside in nasal tissues without eosinophils or neutrophils as nearest neighbors, but often reside near eosinophils and neutrophils in bone marrow. Tissues were stained for IgA (red) and eosinophils (brown, panels A and C) or neutrophils (brown, panels B and D). NALT (panels A and B) or bone marrow (panels C and D, day 10, ×60) are shown.
If support by eosinophils or neutrophils is not required, how do ASCs persist in murine respiratory tract tissues? We and others have previously demonstrated that epithelial cells express a battery of cytokines, either constitutively or in response to activation by bacterial or viral pathogens. As an example, we showed that an epithelial cell line derived from the respiratory tract produced both IL-6 and GMCSF, cytokines that together enhanced IgA production by splenic B cells in vitro (17). In humans, it was shown that epithelial cells expressed a proliferation-inducing ligand (APRIL), another cytokine known to support both T cell dependent and independent IgA production by ASCs (9, 12, 18, 19). Specifically, Huard et al. (12) illustrated that ASCs and epithelial cells were in close contact in sectioned human tonsils, and ASCs accumulated in APRIL-rich niches in the subepithelial zone of infected crypts. Neutrophils infiltrated epithelia acutely following infection and were avid short-term producers of APRIL, but epithelial cells also expressed basal levels of APRIL in the absence or presence of an overt infection, and had the potential to upregulate APRIL in response to toll-like receptor stimulation (12). On the basis of these results, we suggest that cytokine-producing granulocytes and epithelial cells might function in unison to support ASC residence.
While granulocytes and epithelial cells may each provide support for ASC residence, it is possible that neither is strictly required. Even in the bone marrow, the ASC dependence on eosinophils is not absolute (8). The numerous distinct cell populations that may accompany ASCs in systemic and mucosal niches (e.g. T cells, macrophages, dendritic cells, granulocytes, and epithelial cells) might each play important, but nonetheless redundant roles in support of tissue-resident ASCs.
In conclusion, eosinophils have been reported to be required for the continued residence of ASCs in bone marrow, but we find that this requirement is not absolute, particularly for residence of ASCs in the respiratory tract. ASCs can also survive in the respiratory tract in the absence of neutrophils. A frequent nearest neighbor of the virus-specific, IgA-producing ASC in nasal tissue is the epithelial cell rather than the granulocyte. Altogether, our results emphasize that rules for ASC residence are flexible. Redundancy in the innate and adaptive immune system ensures the survival of virus-specific ASCs in the respiratory tract even when eosinophils or neutrophils are lacking.
Funding
This work was supported in part by funding from NIH NIAID R01 AI088729, NIH NCI CA21765, and the American Lebanese Syrian Associated Charities (ALSAC).
Acknowledgements
We thank Drs Jessica Haverkamp, Peter Murray, and Joseph Opferman for provision of Mcl1∆M and control littermates. We thank Victoria Frohlich and the Scientific Imaging Core at St. Jude for assistance with first analyses of tissue sections.
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Hyland L. Sangster M. Sealy R. and Coleclough C. 1994. Respiratory virus infection of mice provokes a permanent humoral immune response. J. Virol. 68:6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slifka M. K. Antia R. Whitmire J. K. and Ahmed R. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363. [DOI] [PubMed] [Google Scholar]
- 3. Crotty S. Felgner P. Davies H. Glidewell J. Villarreal L. and Ahmed R. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 171:4969. [DOI] [PubMed] [Google Scholar]
- 4. Sealy R. Webby R. J. Crumpton J. C. and Hurwitz J. L. 2013. Differential localization and function of antibody-forming cells responsive to inactivated or live-attenuated influenza virus vaccines. Int. Immunol. 25:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rudraraju R. Surman S. Jones B. Sealy R. Woodland D. L. and Hurwitz J. L. 2011. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology 410:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Surman S. L. Rudraraju R. Sealy R. Jones B. and Hurwitz J. L. 2012. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral Immunol. 25:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Surman S. L. Jones B. G. Sealy R. E. Rudraraju R. and Hurwitz J. L. 2014. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine 32:2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chu V. T., Frohlich A., Steinhauser G., et al. 2011. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12:151. [DOI] [PubMed] [Google Scholar]
- 9. Chu V. T. Beller A. Nguyen T. T. Steinhauser G. and Berek C. 2011. The long-term survival of plasma cells. Scand. J. Immunol. 73:508. [DOI] [PubMed] [Google Scholar]
- 10. Yu C. Cantor A. B. Yang H. Browne C. Wells R. A. Fujiwara Y. and Orkin S. H. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coleclough C. Sealy R. Surman S. Marshall D. R. and Hurwitz J. L. 2005. Respiratory vaccination of mice against influenza virus: dissection of T- and B-cell priming functions. Scand. J. Immunol. 62(Suppl. 1):73. [DOI] [PubMed] [Google Scholar]
- 12. Huard B., McKee T., Bosshard C., et al. 2008. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Invest. 118:2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida T. Mei H. Dorner T. Hiepe F. Radbruch A. Fillatreau S. and Hoyer B. F. 2010. Memory B and memory plasma cells. Immunol. Rev. 237:117. [DOI] [PubMed] [Google Scholar]
- 14. Steimer D. A. Boyd K. Takeuchi O. Fisher J. K. Zambetti G. P. and Opferman J. T. 2009. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood 113:2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dzhagalov I. St John A. and He Y. W. 2007. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood 109:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haverkamp J. M., Smith A. M., Weinlich R., et al. 2014. Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity 41:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rudraraju R. Jones B. G. Surman S. L. Sealy R. E. Thomas P. G. and Hurwitz J. L. 2014. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS One 9:e86554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He B., Xu W., Santini P. A., et al. 2007. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26:812. [DOI] [PubMed] [Google Scholar]
- 19. Belnoue E. Tougne C. Rochat A. F. Lambert P. H. Pinschewer D. D. and Siegrist C. A. 2012. Homing and adhesion patterns determine the cellular composition of the bone marrow plasma cell niche. J Immunol. 188:1283. [DOI] [PubMed] [Google Scholar]