T-cell suppression by high-dose peptides is mediated via MEK/ERK signaling
Keywords: activation-induced cell death, cell cycle arrest, signal transduction, T-cell growth suppression
Abstract
Suppression of T-cell growth is an important mechanism for establishment of self-tolerance and prevention of unwanted prolonged immune responses that may cause tissue damage. Although negative selection of potentially self-reactive T cells in the thymus as well as in peripheral tissues has been extensively investigated and well documented, regulatory mechanisms to dampen proliferation of antigen-specific effector T cells in response to antigen stimulation remain largely unknown. Thus, in this work, we focus on the identification of growth suppression mechanisms of antigen-specific effector T cells. In order to address this issue, we investigated the cellular and molecular events in growth suppression of an ovalbumin (OVA)-specific T-cell clone after stimulation with a wide range of OVA-peptide concentrations. We observed that while an optimal dose of peptide leads to cell cycle progression and proliferation, higher doses of peptide reduced cell growth, a phenomenon that was previously termed high-dose suppression. Our analysis of this phenomenon indicated that high-dose suppression is a consequence of cell cycle arrest, but not Fas–Fas ligand-dependent apoptosis or T-cell anergy, and that this growth arrest occurs in S phase, accompanied by reduced expression of CDK2 and cyclin A. Importantly, inhibition of MEK/ERK activation eliminated this growth suppression and cell cycle arrest, while it reduced the proliferative response to optimal antigenic stimulation. These results suggest that cell cycle arrest is the major mechanism regulating antigen-specific effector T-cell expansion, and that the MEK/ERK signaling pathway has both positive and negative effects, depending on the strength of antigenic stimulation.
Introduction
During an immune response, an explosive proliferation of cognate T cells in response to their specific antigen is required for the rapid elimination of pathogens. However, because of their toxic effector functions, the expansion of these activated T cells has the potential to damage self-tissue. Thus, various mechanisms that negatively control the populations of activated T cells are vital for an appropriate immune response and prevention of autoimmune reactions. The strength of antigenic stimulation affects T-cell expansion: in response to optimal antigen stimulation, T cells enter cell cycle and proliferate, while strong stimulation suppresses their expansion. Strong TCR signal-mediated inhibition of thymocyte growth results in cell death, a process known as negative selection (1). In the periphery, studies of Fas and Fas ligand (FasL) mutant (Faslpr/lpr and Faslgld/gld) mice have demonstrated that cell death triggered by Fas/FasL interaction is important for negative regulation of T-cell growth (2–4). Fas/FasL-dependent cell death has in most cases been shown to be important for maintenance of T-cell tolerance to self-antigens; however, it is still controversial whether cell death is the main mechanism for growth suppression of antigen-activated T cells after foreign antigen stimulation. For example, the involvement of cell cycle arrest and/or T-cell anergy in the impaired proliferation of activated T cells following excessive antigenic stimulation has also been reported (5–9). Therefore, mechanisms and factors responsible for this type of growth suppression in peripheral effector T cells in response to foreign antigen remain to be elucidated.
Upon TCR engagement, multiple signaling cascades are activated and transmit not only positive signals leading to proliferation, cell cycle progression and growth factor production, but also negative signals resulting in cell death, anergy and/or cell cycle arrest. For T-cell activation and cytokine production, it is known that transcription factors of the NF-κB and NFAT families play a crucial role by binding and activating target promoters. The molecular events that link TCR/CD28 engagement to the activation of these transcription factors have also been well studied (10–12). Furthermore, molecular mechanisms of cell cycle progression are known to involve several signaling pathways, including PI3K/Akt, MEK/ERK, JNK, NF-κB and NFAT. Following TCR/CD28 engagement, such signals have been reported to induce the up-regulation of cyclin/cyclin-dependent kinase (CDK) and the degradation of CDK inhibitors (13–17). Although these positive signals leading to T-cell expansion have been extensively investigated, the molecular mechanisms that suppress T-cell growth following excessive antigenic stimulation remain unclear. For the negative regulation of T-cell growth, activation of TCR-mediated Ca2+/NFAT and NF-κB signaling pathways has been shown to result in the up-regulation of FasL and Fas/FasL-dependent cell death (14, 18). Furthermore, under conditions of strong antigenic stimulation, a disproportionate over-activation of Ca2+/NFAT induces several anergy-related molecules, which dampen the activation of other signaling molecules downstream of TCR engagement (19). In another case, the MEK/ERK signaling pathway blocked IL-2-induced proliferation by induction of the CDK inhibitor p21cip1/waf1 (20) or by disturbing STAT5 activation (21). However, it is not well understood how these different processes and signaling pathway downstream of TCR ligation uniquely and/or coordinately induce T-cell growth suppression.
In order to shed some light on the cellular and molecular mechanisms of the impaired proliferation of antigen-experienced T cells, in vitro experiments using T-cell clones have been used. Previous studies using this approach revealed that stimulation of T cells with high concentrations of antigen suppressed their proliferation, a biphasic proliferative response termed high-dose suppression (22–24). In this report, using an antigen-specific T-cell clone that exhibits high-dose suppression (25), we found that cell cycle arrest in S phase, rather than Fas/FasL-dependent cell death or anergy, is crucial for the induction of high-dose suppression. Furthermore, we discovered that the activation of MEK/ERK signaling led to the cell cycle arrest mediated by inhibiting the expression of cyclin A and CDK2.
Methods
Preparation and culture of T-cell clones
Preparation of the ovalbumin (OVA)-peptide323–339 (OVA-p)-specific T-cell clones has been previously described (25). Briefly, inguinal and popliteal lymph node cells of BALB/c mice immunized in the footpad with 100 µg OVA-p in CFA were harvested 2 weeks after immunization and cultured with 3.9nM of OVA-p. Viable T cells were isolated and re-stimulated with irradiated (30 Gy) BALB/c whole spleen cells and the same concentration of OVA-p used in the initial culture. After several rounds of re-stimulation, T cells were cloned by limiting dilution. Each T-cell clone was then collected weekly and re-stimulated in the same way. Cells were maintained in complete medium consisting of RPMI 1640 supplemented with 10% fetal bovine serum, penicillin, streptomycin, non-essential amino acids, sodium pyruvate, 10mM HEPES (pH 7.55) and 50 µM 2ME.
Antibodies and reagents
Cyclosporine A (CsA), Akt inhibitor VIII, Rapamycin, p38 inhibitor SB203580, JNK inhibitor and MEK1/2 inhibitor U0126 were purchased from Calbiochem (Millipore). The following antibodies were purchased from Santa Cruz Biotechnology: anti-CDK4 (C-22), anti-Cyclin E (H-145), anti-CDK2 (D-12), anti-Cyclin A (H-432), anti-p21 (C-19) and anti-p16 (F-12). Anti-Cyclin D3 and anti-p27 antibodies were purchased from Cell Signaling Technology.
T-cell proliferation assays
T cells were cultured in triplicate at a density of 2×104 cells per well with titrated amounts of antigenic peptide and 2×103 bone marrow-derived dendritic cells (BMDCs) in 200 μl complete medium in 96-well flat-bottom plates. T cells were pulsed with [3H]TdR for the last 6h of total incubation.
Flow cytometry
After 72h co-culture with OVA-p (0, 10, 1000nM) and BMDCs, T cells were washed once with FACS buffer (PBS plus 0.5% calf serum and 0.1% NaN3) and incubated with unlabeled anti-FcR (FcγII/IIIR, laboratory prepared) to block non-specific binding. Samples were stained with fluorescent dye-conjugated or biotin-conjugated antibodies to CD4, Fas (CD95) and FasL (CD95L). Biotinylated antibodies were detected with fluorescent dye-conjugated streptavidin. For Annexin V/propidium iodide (PI) staining, T cells were co-cultured with OVA-p (0, 10, 1000nM) and BMDCs for 72h, and then stained with FITC-labeled anti-Thy1.2 (30H12) antibody. After the cells were washed with Annexin V Binding Buffer [ABB; 10mM HEPES (pH 7.4), 140mM NaCl, 2.5mM CaCl2], samples were incubated with 100 µl ABB, Annexin V-Alexa647 (BioLegend) and PI (25 μg ml−1) for 15min at room temperature. After treatment, cells were resuspended in 100 µl ABB. For cytometric analysis, we used a FACSCalibur and analyzed the data using FlowJo software.
Cell division analysis
Cells were washed twice with PBS and CFSE (1 µM in PBS) was added to the cell suspension at a 1:1 volume. Samples were mixed immediately and incubated for 2min at 37°C in the dark and then washed. These CFSE-labeled T cells were cultured at a density of 3×105 cells per well with titrated amounts of antigenic peptide (0, 10, 1000nM) and 5×106 T-cell-depleted splenocytes per well in 2ml complete medium in 24-well plates. After 48h, cells were washed with FACS buffer and then stained with Cy5-labeled anti-CD4 antibody. Samples were analyzed by flow cytometry (BD, FACSCalibur). Data were analyzed using FlowJo software.
Cell cycle analysis
For T-cell stimulation, 5×105 T cells were incubated with OVA-p (0, 10, 1000nM) and 5×104 BMDCs in 2ml complete medium in 24-well plates for 72h. Cells were washed with FACS buffer and then stained with FITC-labeled anti-Thy1.2 (30H12) antibody. After the cell surface staining, samples were fixed with 70% ethanol on ice for at least 30min. Fixed cells were incubated with RNase (0.1mg ml−1) for 30min at 37°C and then stained with PI (25 μg ml−1) for 20min on ice. After addition of 1ml 0.1% BSA, samples were analyzed by flow cytometry (BD, FACSCalibur). Data were analyzed using FlowJo software. Cells were gated on Thy1.2+ cells, excluding any sub-G1 population.
Western blotting
For T-cell stimulation, T cells were cultured with OVA-p and BMDCs in complete medium in 24-well plates for 72h. After stimulation, cells were harvested and washed in cold PBS. Total cells (2×106 cells) were lysed in 150 µl SDS–PAGE sample buffer. Samples were subjected to SDS–PAGE and transferred to polyvinylidene difluoride (PVDF) membranes, which were immunoblotted with the indicated primary antibody followed by the appropriate HRP-conjugated anti-mouse Ig, anti-rabbit Ig or anti-goat Ig secondary antibodies. Immunodetection was performed by ECL (PerkinElmer) and results were analyzed with an LAS-3000 image analyzer.
Gene expression analysis
T cells and BMDCs were co-cultured at a ratio of 10:1 with 0, 15.6 or 1000nM of OVA-p. Cells were harvested at 4 or 24h, CD4+ T cells were sorted using a BD FACSAria II and then total RNA was extracted with TRI reagent (Sigma–Aldrich). DNA microarray was performed using the SurePrint G3 Mouse Gene Expression 8×60K Chip (Agilent Technologies). Raw data were normalized by a quantile normalization method using GeneSpring (Agilent Technologies) and analyzed with GenePattern (26).
Statistical analysis
Analyses used an unpaired two-tailed Student’s t-test, and values of P < 0.05 were considered to indicate statistical significance.
Results
The impaired proliferation of hyperstimulated antigen-specific effector T cells is not mediated by Fas/FasL- or caspase-dependent cell death
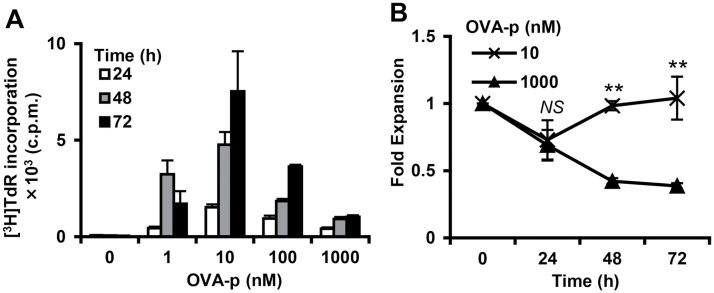
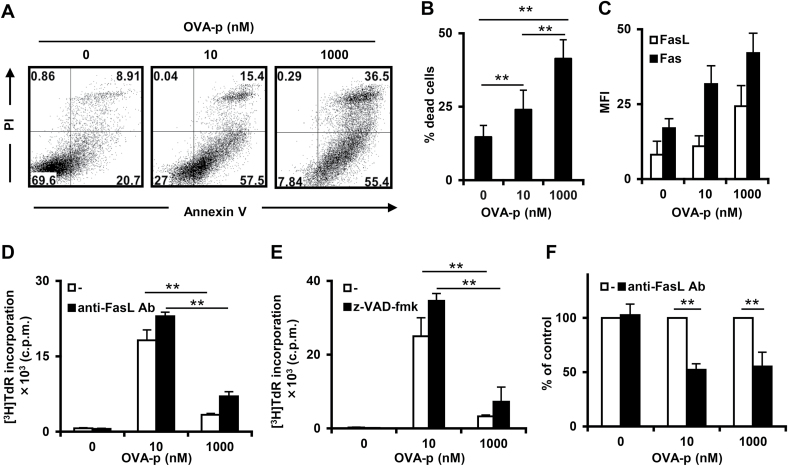
We have previously generated a panel of OVA-peptide323–339 (OVA-p)-specific CD4+ T-cell clones that require different concentrations of peptide for optimal proliferation (25). A representative T-cell clone that had been maintained with a low optimal dose of peptide displayed a bell-shaped proliferation pattern in which stimulation with high (suppressive) dose OVA-p inhibited [3H]TdR incorporation (Fig. 1A). We also examined viable cell numbers in a time course experiment. Early after stimulation (24h), the number of cells was decreased at both optimal and suppressive peptide dose. However, at 48–72h, the T cells stimulated with the optimal dose recovered, whereas cell numbers continued to decline with the suppressive dose (Fig. 1B). Since many studies have reported increased cell death with high-dose antigen stimulation (5, 8, 27–30), we investigated cell death involvement in the impaired proliferation of our T-cell clones at the suppressive dose. Consistent with other reports, the percentage of dead cells identified by Annexin V/PI staining increased in a dose-dependent manner (Fig. 2A and B). This type of T-cell death following excessive antigenic stimulation, which is called activation-induced cell death (AICD), has generally been considered to be triggered by Fas/FasL interactions (2, 31). In order to investigate a possible role for Fas/FasL-dependent AICD in the high-dose growth suppression, we first examined the cell surface expression of Fas and FasL on the effector T-cell clone following stimulation with each dose of peptide. Although both molecules were up-regulated in a peptide dose-dependent manner (Fig. 2C), such increases in mean fluorescence intensity (MFI) do not necessarily equate to functional outcomes. Therefore, we evaluated the influence of an antagonistic FasL antibody that blocks Fas and FasL interactions on the proliferative response. The antibody treatment had no effect on the growth suppression observed with excessive antigenic stimulation, thus clearly indicating that the AICD observed here is independent of Fas/FasL (Fig. 2D). To confirm this result and examine the involvement of other Fas/FasL-independent pathways, such as TNFα AICD, in the growth suppression, we used the caspase inhibitor z-VAD-fmk to block activation of the caspase cascade, which is vital for both Fas/FasL-dependent and -independent AICD (32). Similar to the results observed with FasL antibody treatment, the caspase inhibitor had no effect on high-dose suppression (Fig. 2E), indicating that caspase-mediated AICD is also not involved in this type of growth suppression.
Fig. 1.
The proliferative response of a T-cell clone at different doses of antigenic stimulation. (A) The T-cell clone was cultured with BMDCs and different concentrations of OVA-p (0–1000nM) for the indicated times and pulsed with [3H]TdR for the last 6h. Bars represent group means ± SD. Similar results were obtained for more than three additional experiments. (B) The T-cell clone was stimulated for the indicated times and fold expansion was calculated. Data are the mean ± SD from three independent experiments. **P < 0.01.
Fig. 2.
Induction of high-dose suppression independently of Fas or caspase-mediated AICD. The T-cell clone was stimulated with BMDCs and the indicated OVA-p concentration (0, 10, 1000nM). (A) At 72h after stimulation, dead cells were detected by Annexin V and PI staining. Cells were gated on the Thy1.2+ population. One representative of more than three independent experiments is shown. (B) The percentage of dead cells (Annexin V+/PI+) was determined by flow cytometry as shown in (A). Data are the mean ± SD from more than three independent experiments. (C) After T cells were stimulated for 24h, the expression of Fas (black bars) and FasL (white bars) was detected by flow cytometry. Cells were gated on the CD4+ population. Data are the mean ± SD from three independent experiments. (D) The T-cell clone was cultured for 72h in the absence or presence of FasL antibody (1.25 μg ml−1). Proliferation was analyzed by [3H]TdR incorporation. Data are shown as the mean ± SD of triplicate cultures and similar results were obtained in more than three additional experiments. (E) The T-cell clone was stimulated with the caspase inhibitor z-VAD-fmk (0, 10 μM). After 72h, the proliferative response was analyzed by [3H]TdR incorporation. Data are shown as the mean of triplicate cultures and similar results were obtained in more than three additional experiments. (F) T cells were cultured under the same conditions as in (D) and dead cells were detected by Annexin V/PI staining. Cells were gated on the Thy1.2+ population. Data are the mean ± SD from more than three independent experiments. **P < 0.01.
Many studies have previously reported that excessive antigenic stimulation induces Fas/FasL-dependent AICD (2, 31), and we also observed the increase of dead cells in an antigen dose-dependent manner correlated with Fas/FasL expression levels (Fig. 2A and B). Thus, to investigate the role of Fas signal inhibition in observed cell death, we measured dead cells after treatment with the FasL antibody. As shown in Fig. 2(F), this treatment significantly decreased cell death at the suppressive dose, but a similar level of inhibition of cell death occurred at the optimal antigen dose. This result indicates that antigen stimulation induces Fas/FasL-dependent AICD to the same extent regardless of the antigen dose and is consistent with the proliferative response measured by [3H]TdR incorporation.
Cell cycle arrest, but not T-cell anergy, is a major mechanism for the impaired proliferation with excessive antigenic stimulation
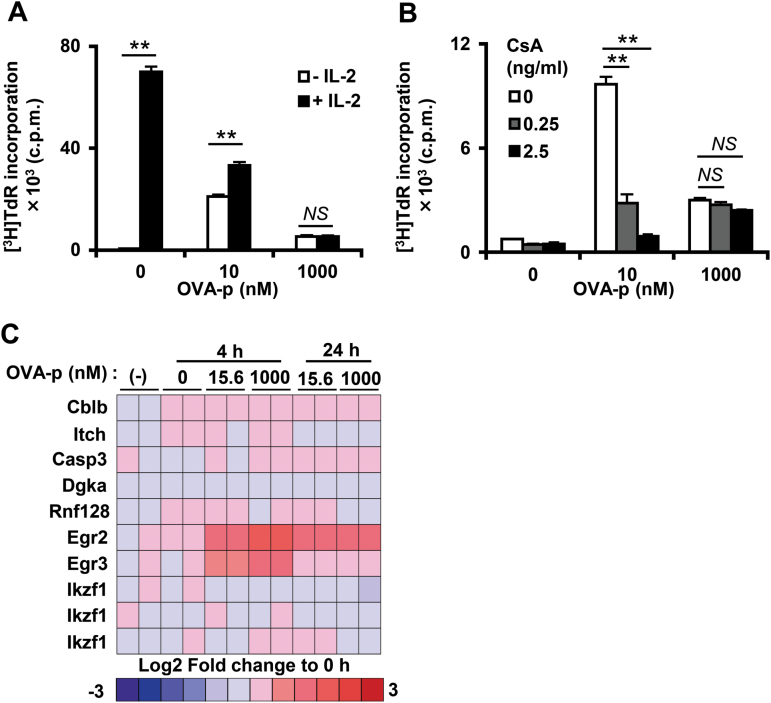
Since anergy induction is one of the important mechanisms for T-cell growth suppression, we next assessed this mechanism in our system. It has been reported that this type of growth suppression can be reversed with additional IL-2 (33–35) or by inhibiting calcineurin with CsA (9, 36). Thus, we examined whether addition of IL-2 or CsA could rescue high-dose suppression and found that neither treatment was effective (Fig. 3A and B). Previous studies identified several anergy-related genes, such as E3 ubiquitin ligases and transcriptional repressors (19); therefore, we compared expression of a panel of these genes at suppressive and optimal doses of peptide. The expression of almost all anergic genes, except for Egr2 and Egr3, was not significantly altered by peptide stimulation (Fig. 3C). However, the expression level of these two genes was nearly identical at the suppressive and optimal doses, suggesting that these gene products are not involved in the high-dose growth suppression. Taken together, these observations indicate that the growth suppression caused by excessive antigenic stimulation is not caused by T-cell anergy induction.
Fig. 3.
T cells stimulated with high-dose peptide are not anergic. (A) At 72h after stimulation in the presence (black bars) or absence (white bars) of exogenous IL-2 (25U ml−1), the proliferative response of the T-cell clone was analyzed by [3H]TdR incorporation. **P < 0.01. (B) The T-cell clone was cultured with BMDCs and different doses of OVA-p (0, 10, 1000nM) without (white bars) or with CsA, 0.25 or 2.5ng ml−1, gray and black bars, respectively. At 72h, proliferation was determined by [3H]TdR incorporation. **P < 0.01. Data are shown as the mean ± SD of triplicate cultures and similar results were obtained in more than three additional experiments. (C) Anergy-related genes (19) were extracted from our microarray data. The heatmap shows the fold change of the expression levels relative to 0h.
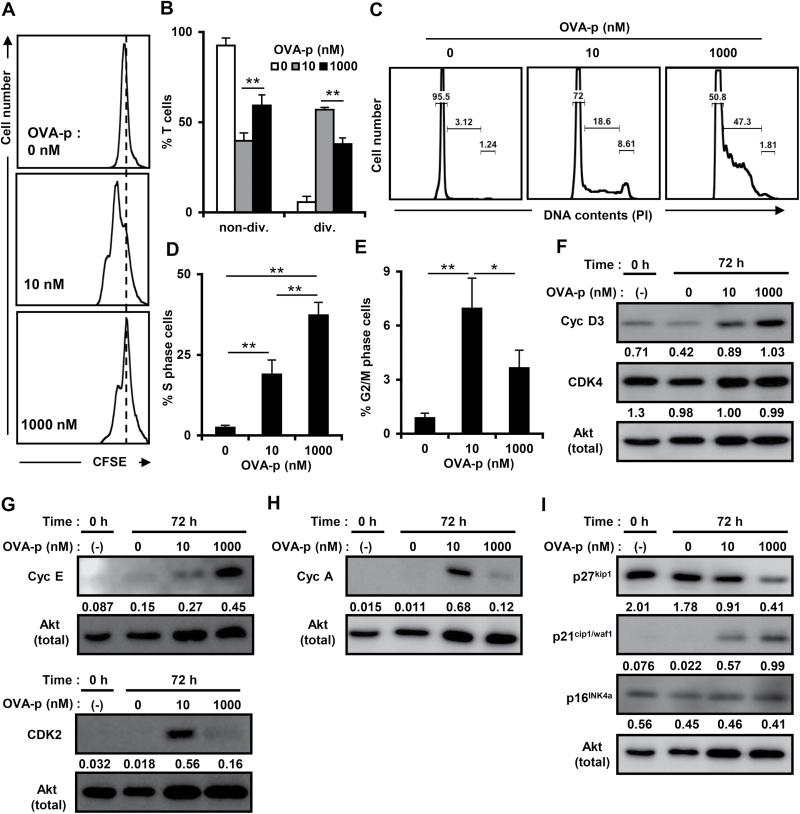
In order to clarify the proliferative pattern at a single-cell level, we analyzed the division of CFSE-labeled T cells at each dose of peptide. At the suppressive dose, a major population of the T-cell clone had not divided (Fig. 4A and B), indicating that the growth suppression of effector T cells stimulated with high-dose antigen was caused by impaired cell division.
Fig. 4.
Cell cycle arrest is induced with a high dose of peptide. (A and B) Cells were labeled with CFSE and stimulated with each dose of peptide and T-cell-depleted splenocytes for 48h. Cells were analyzed by flow cytometry, gated on the PI−CD4+ population. The percentages of non-divided or divided T cells (B) are shown as the mean ± SD of more than three independent experiments. (C) The T-cell clone was cultured with BMDCs and stimulated with different doses of OVA-p (0, 10, 1000nM) for 72h. Cell cycle progression of viable cells was assessed by flow cytometry of PI-labeled cells, excluding the sub-G1 population. Results are representative of at least three independent experiments. (D and E) The T-cell clone was stimulated as in Fig. 4(D). The percentages of T cells in S phase (D) or G2/M phase (E) are shown as the mean ± SD of more than three independent experiments. *P < 0.05, **P < 0.01. (F–I) Cell lysates immunoblotted with cyclin D3, CDK4, cyclin E, CDK2, cyclin A, p16INK4a, p21cip1/waf1 and p27kip1 antibodies. The expression levels of each molecule are shown as the fold differences compared with the same control protein (Akt). Similar results were obtained in at least three independent experiments.
Next, to investigate whether cell cycle arrest, which leads to impaired cell division, occurs during growth suppression, the cell cycle profile at both optimal and suppressive antigenic doses was determined (Fig. 4C). The transition from G1 to S phase was observed at both doses, and the percentage of T cells in S phase increased in a peptide dose-dependent manner (Fig. 4D). By contrast, the percentage of cells in the G2/M phase was significantly diminished at the suppressive dose (Fig. 4E), indicating that entry into the G2/M phase was inhibited. Taken together, these data indicate that cell cycle arrest in S phase has a pivotal role in the growth suppression of antigen-specific effector T cells.
Excessive antigenic stimulation resulted in lower expression of CDK2/cyclin A and up-regulation of p21cip1/waf1
In order to characterize the nature of the cell cycle arrest induced at the suppressive dose, we examined the expression of several CDK complexes and cyclins, which regulate cell cycle progression in different phases of the cell cycle (37). The expression of cyclin D3, a G1 phase cyclin, was enhanced at both optimal and suppressive doses (Fig. 4F, top panel and Supplementary Figure 1A, available at International Immunology Online). CDK4, an early G1 phase CDK, was expressed before stimulation and its level was unaffected by antigenic stimulation (Fig. 4F, second panel and Supplementary Figure 1B, available at International Immunology Online). Dose-dependent differences were observed in the expression of CDK2, a late G1/S phase CDK, and cyclin E, which binds and activates CDK2. At the suppressive dose, CDK2 expression was lower than at the optimal dose, while cyclin E was up-regulated (Fig. 4G and Supplementary Figure 1C and D, available at International Immunology Online). Similar to the CDK2 expression pattern, the expression of cyclin A, which binds CDK2 and is generally expressed in the S/G2 phase, was also lower at the suppressive dose (Fig. 4H and Supplementary Figure 1E, available at International Immunology Online). These results indicate that although T cells stimulated at the suppressive dose of antigen are able to enter cell cycle, the transition from the late G1/S to the G2 phase was disturbed.
CDK and cyclin inhibitors (CKI) also play an important role in cell cycle regulation by binding CDK/cyclin complexes (38); therefore, we examined the expression of p21cip1/waf1 and p27kip1, which suppress the activity of many CDK/cyclin complexes, and p16INK4a, which mostly targets CDK4/6. The expression of p21cip1/waf1 was increased in a dose-dependent manner (Fig. 4I, second panel and Supplementary Figure 1F, available at International Immunology Online). It is conceivable that up-regulation of p21cip1/waf1 at the suppressive dose inhibited the activity of CDK4 by binding CDK4/cyclin D3 complexes. On the other hand, the expression of p27kip1 was observed before stimulation and decreased in a dose-dependent manner (Fig. 4I, top panel and Supplementary Figure 1G, available at International Immunology Online). A high basal expression of p16INK4a was observed before stimulation (Fig. 4I, third panel and Supplementary Figure 1H, available at International Immunology Online), suggesting that this inhibitor is not critical for suppressing T-cell growth. Taken together, these data suggest that down-regulation of CDK2/cyclin A is involved in cell cycle arrest at the suppressive dose.
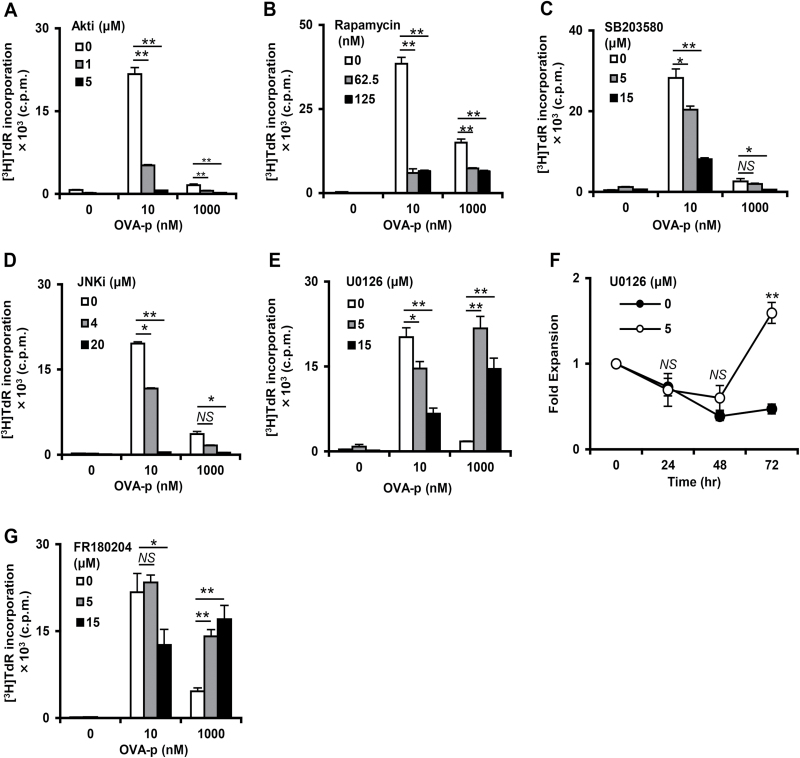
MEK/ERK is the key signaling pathway inducing the growth suppression of effector T cells
The MEK/ERK, PI3K/Akt/mTOR, p38 MAPK and JNK signaling pathways, which are all activated in response to TCR engagement, play important roles in enhancing cell cycle progression and the proliferative response of T cells (13, 15–17). On the other hand, it has also been reported that strong stimulation through mitogen-activated pathways drives cell cycle arrest in both lymphoid and non-lymphoid cells (39). To identify the key signaling pathway for the impaired proliferation following excessive antigenic stimulation, we examined the effect of pathway-specific inhibitors on T-cell proliferation. The Akt inhibitor (Akti 1/2), mTORC inhibitor (Rapamycin), JNK inhibitor (JNK inhibitor II) and p38 inhibitor (SB203580) all suppressed T-cell proliferative responses at both optimal and suppressive doses (Fig. 5A–D), indicating that these signaling pathways are essential for promoting T-cell proliferation regardless of antigenic peptide dose. Strikingly, however, the MEK inhibitor U0126 strongly enhanced [3H]TdR incorporation after stimulation with the suppressive dose of peptide (Fig. 5E). Consistent with increased [3H]TdR incorporation, the number of T cells recovered at 72h was also increased (Fig. 5F). Interestingly, the same treatment significantly inhibited the proliferation of T cells stimulated with the optimal dose (Fig. 5E). These results indicate that MEK/ERK activation after excessive antigenic stimulation induced growth suppression, while enhancing cell cycle progression with optimal stimulation. To further confirm this interpretation, we used an ERK-specific inhibitor FR180204. Similar to the result using the MEK inhibitor, FR180204 also facilitated the proliferative response at the suppressive dose, while suppressing it at the optimal dose (Fig. 5G). Taken together, these data suggest that the MEK/ERK signaling pathway has both positive and negative effects on T-cell clonal expansion, depending on the strength of antigenic stimulation, and plays an important role in induction of growth suppression following excessive antigen stimulation.
Fig. 5.
Inhibition of MEK/ERK signaling restores proliferation at high dose peptide. (A–E, G) The T-cell clone was cultured with BMDCs and OVA-p (0, 10, 1000nM) for 72h with Akti 1/2 (A), Rapamycin (B), SB203580 (C), JNK inhibitor II (D), U0126 (E) and FR180204 (G). Cultures were pulsed with [3H]TdR for the last 6h of the 72h incubation. Data are showed as the mean ± SD of triplicates and similar result were obtained in more than three additional experiments. (F) The T-cell clone was stimulated with a high dose of OVA-p (1000nM) with U0126 (5 μM), and fold expansion was calculated. Data are the mean ± SD of three independent experiments. *P < 0.05, **P < 0.01.
To generalize this phenomenon, we used short-term activated DO11.10 TCR-Tg CD4+ T cells, which are a more heterogeneous effector T-cell population. The activated T cells were generated by repeated (1–3 times) stimulation with low-dose OVA-p. Consistent with the findings using cloned T cells, activated DO11.10 TCR-Tg CD4+ T cells showed a biphasic growth curve, and blockade of the Fas/FasL interaction had no effect on the impaired proliferative response (Supplementary Figure 2A, available at International Immunology Online). Next, we assessed cell division of the activated T cells at each dose of peptide. As shown in Supplementary Figure 2B, available at International Immunology Online, the division of DO11.10 TCR-Tg activated T cells at the suppressive dose was impaired compared with that of T cells stimulated with the optimal dose. Finally, we measured the effect of U0126 treatment on the proliferative response of the DO11.10 TCR-Tg activated T cells. Similar to the result using T-cell clones, the proliferative response at the suppressive dose was restored by MEK/ERK inhibition but at the optimal dose it was inhibited (Supplementary Figure 2C, available at International Immunology Online). Taken together, these data indicate that the growth suppression mediated by MEK/ERK activation is not a phenomenon limited to cloned T cells.
The inhibition of MEK/ERK activation alleviates cell cycle arrest induced by excessive antigenic stimulation
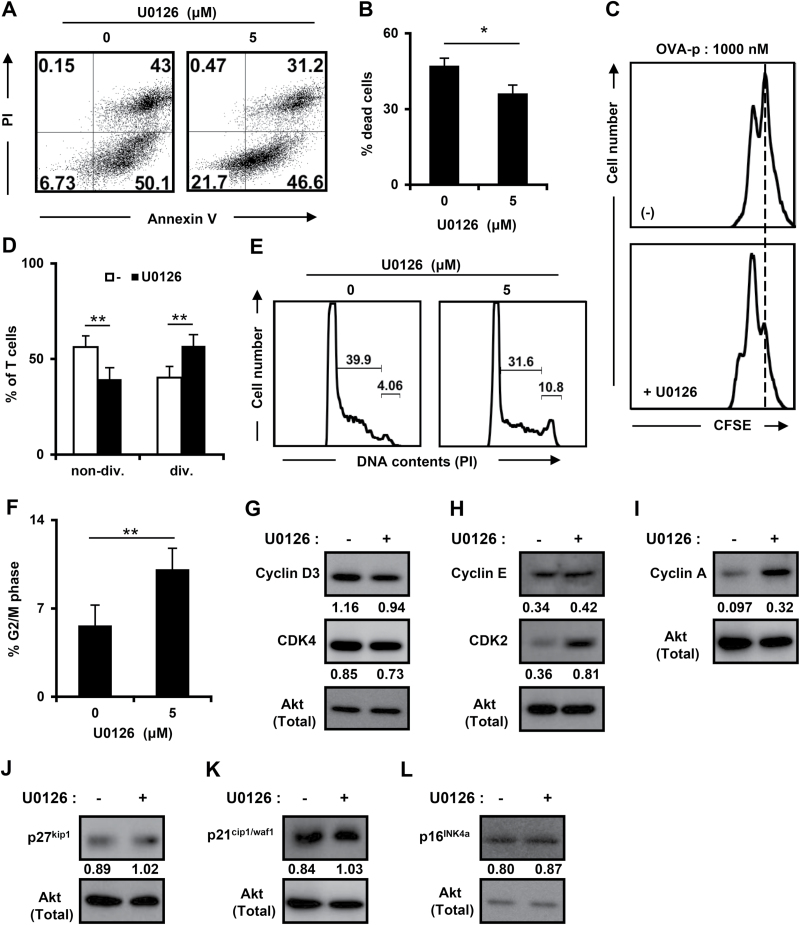
Since our results indicated that cell cycle arrest and cell death occurred at the suppressive antigen dose (Figs 2 and 4), we also examined whether the MEK/ERK signaling pathway is involved in these processes. As shown in Fig. 6(A and B), the percentage of dead cells at the suppressive dose was decreased by U0126 treatment, indicating that the activation of MEK/ERK signaling partially induced the cell death with excessive antigenic stimulation. Next, we assessed cell division at the suppressive dose of OVA-p by using a CFSE-labeled T-cell clone. Consistent with the result of [3H]TdR incorporation in Fig. 5(E), U0126 treatment of T cells stimulated with a suppressive dose of peptide restored cell division (Fig. 6C and D).
Fig. 6.
Recovery from cell cycle arrest at a high dose of peptide by U0126 treatment. The T-cell clone was stimulated with high dose of OVA-p (1000nM) for 72h with or without U0126 (5 μM). (A and B) Cell death was analyzed by Annexin V/PI staining as in Fig. 2(A). Cells were gated on the Thy1.2+ population. (C and D) T cells were stimulated as in Fig. 4(A) in the presence or absence of U0126. Cell division was determined by flow cytometry. The percentages (D) are shown as the mean ± SD of three independent experiments. (E) Cell cycle progression of viable cells was assessed by flow cytometric analysis of DNA content as in Fig. 3(A). (F) The percentages of T cells in G2/M phase are shown as the mean ± SD of more than three independent experiments. *P < 0.05. (G–L) Cell lysates were prepared at 72h of culture with or without U0126 (5 μM) and immunoblotted with cyclin D3, CDK4, cyclin E, CDK2, cyclin A, p16INK4a, p21cip1/waf1 and p27kip1 antibodies. The expression levels of each molecule are shown as the fold differences compared with the same control protein (Akt). Similar results were obtained in at least three independent experiments.
Next, we examined the effect of U0126 treatment on cell cycle progression. The percentage of T cells in G2/M phase was significantly elevated, while the accumulation in S phase was reduced, at the high-dose peptide by U0126 treatment (Fig. 6E and F), indicating that cell cycle arrest was alleviated by MEK/ERK inhibition. To further explore the regulation of the cell cycle by the MEK/ERK signaling pathway, the expression of cell cycle-related molecules, as described in Fig. 4, was examined under suppressive conditions in the absence or presence of U0126 (Fig. 6G–K and Supplementary Figure 3, available at International Immunology Online). While the expression of cyclin D3, E and CDK4 was unaffected, CDK2 and cyclin A were up-regulated by U0126 treatment (Fig. 6G–I and Supplementary Figure 3A–E, available at International Immunology Online). Furthermore, the expression of p27kip1, p21cip1/waf1 and p16INK4a was not significantly affected by U0126 treatment, indicating that the expression of these molecules is not regulated by MEK/ERK at the suppressive dose (Fig. 6J–L and Supplementary Figure 3F–H, available at International Immunology Online). Taken together, these results suggest that cell cycle progression and transition from the S to G2/M phase is blocked by the MEK/ERK signaling pathway by decreasing CDK2/cyclin A expression under conditions of strong antigenic stimulation. In addition, it is possible that at the suppressive dose, cell cycle progression is repressed by low level expression of CDK2 and cyclin A, but not elevated expression of p21cip1/waf1. These results suggest that MEK/ERK signaling pathway controls cell cycle arrest plays a dominant role in the impaired proliferation of effector T cells.
Discussion
The purpose of this study was to elucidate the cellular and molecular mechanisms underlying growth suppression of antigen-experienced activated T cells following excessive antigen stimulation. Here, we showed that this stimulation elicits cell cycle arrest in S phase, which is caused by the down-modulation of CDK2 and cyclin A (Fig. 4). In addition, we found a novel function of the MEK/ERK signaling pathway in controlling activated T-cell expansion; in response to optimal antigen stimulation, the MEK/ERK activation enhances cell cycle progression and proliferation, while it induces cell cycle arrest with excessive stimulation. We also showed that CDK2 and cyclin A are the down-regulated downstream targets of MEK/ERK signaling (Fig. 6). Taken together, cell cycle arrest mediated by strong MEK/ERK activation is a major mechanism for the growth suppression following excessive antigenic stimulation in antigen-experienced effector T cells.
Apoptosis caused by Fas/FasL interaction has been thought to be an important mechanism for the regulation of peripheral T-cell populations. Examples of defects in Fas/FasL-dependent cell death that lead to massive lymphoproliferation and spontaneous autoimmunity can be found in both mice (Faslpr/lpr and Faslgld/gld mice) and humans (ALPS, autoimmune lymphoproliferation syndrome), indicating that the Fas signaling pathway is critical for maintenance of self-tolerance and homeostasis of peripheral T cells (3, 40). These mice are also resistant to peptide-induced deletion in vivo (28). However, we showed that the blockade of cell death mediated by Fas/FasL interaction and caspase cascades did not eliminate the growth suppression of antigen-specific T-cell clones caused by excessive antigenic stimulation, indicating that this phenomenon is not caused by Fas/FasL-dependent AICD (Fig. 2D and E). It should be noted that treatment with the FasL antibody partially inhibited cell death after antigenic stimulation, but this effect was observed at both suppressive and optimal peptide doses. This result leads to the conclusion that Fas-mediated cell death is a general cellular event in effector T cells responding to a given antigen regardless of TCR signal strength. Singer and Abbas (28) showed that peripheral T cells from 2B4 TCRαβ transgenic mice carrying the lpr mutation are resistant to growth suppression, a finding inconsistent with our conclusion. This discrepancy may be explained by differences in the responding T cells; in their experimental system antigen-inexperienced T cells first respond to antigens under Fas-signal deficient conditions, whereas in our system we used antigen-specific cloned T cells maintained by repeated antigen stimulation. Another group has also shown that T-cell clones or pre-activated T cells from lpr mutant mice have a defect in suppression of proliferative response induced by a high dose of super antigen or anti-TCR antibody cross-linking (30). This may be in accord with the intrinsic abnormality of lpr T cells, because Davidson et al. (41) reported that lpr T cells showed spontaneous proliferation and hypersecretion of some cytokines, including IFN-γ and TNF-α.
Impairment of cell division and entry into G2/M phase clearly indicated that cell cycle arrest in S phase occurred in the response of cloned T cells to excessive antigenic stimulation (Fig. 4). Although little has been reported on cell cycle arrest of T cells, an in vitro experimental system using T-cell hybridomas, where a pivotal role of Fas mediated death signaling in AICD was identified, also provided evidence that, accompanied with apoptosis, cell cycle arrest occurred following TCR-mediated signaling. Ashwell et al. (6) showed that stimulation with appropriate peptide antigens resulted in a cell cycle block at the G1/S border regardless of antigen dose. Similarly, Miyatake et al. (42) reported that activation by TCR cross-linking with antibody induced the down-regulation of cyclin D3 expression and cyclin D-dependent kinase activity, leading to growth arrest in the G1 phase. Although our results obtained using T-cell clones and theirs with T-cell hybridomas reached the same conclusion, that cell cycle arrest is responsible for growth suppression of T cells, the phase of arrest was different. These differences may be due to the distinctive nature of T-cell hybridomas and T-cell clones; the former show constitutive proliferation and cell cycling, whereas the latter require antigenic stimulation to enter cell cycling. Furthermore, since T-cell hybridomas are prepared by fusing to a thymoma cell line, the consequences of TCR signaling may represent that of immature T cells rather than effector T cells.
One of the most striking findings of our study was that the impairment of proliferation seen at high concentration of antigen could be reversed by MEK/ERK inhibition, which instead decreased proliferation at the optimal antigen dose. A previous report showed that strong MEK/ERK activation induced by high-dose CD3 mAb decreased IL-2-dependent proliferation (20). In these studies, MEK inhibition slightly improved the proliferative response and depressed the expression of p21cip1/waf1 (20), indicating that up-regulation of p21cip1/waf1 mediated the cell cycle arrest. Here, we used an antigen-specific effector T-cell clone, which has the same specificity and threshold, stimulated with varying antigen concentrations in the presence of APCs, and observed that despite the high level of p21cip1/waf1 expression, cell cycle progression and the expression of CDK2/cyclin A with strong antigenic stimulation was restored by MEK/ERK inhibition (Fig. 6). These results suggest that in antigen-specific effector T cells, cell cycle arrest induced by strong MEK/ERK activation preferentially depends on the expression level of CDK2/cyclin A rather than that of p21cip1/waf1. Although how the MEK/ERK signaling pathway regulates the expression of CDK2/cyclin A currently remains unclear, we observed that MEK inhibition enhanced the phosphorylation of Akt at a suppressive antigen dose 24h after stimulation (data not shown). Thus, it is possible that cell cycle arrest in S phase may be responsible for the inhibition of Akt activation mediated by strong MEK/ERK activation, because Akt activation is required for up-regulation of cyclin A and CDK2 (15). Furthermore, a recent study showed that the MEK/ERK signaling pathway can suppress Akt activation mediated by the translocation of PTEN to the membrane, which regulates the concentration of PIP3 (43). Therefore, it is conceivable that the same molecular mechanism, the MEK/ERK–PTEN axis, dampens Akt phosphorylation in our experimental system.
In our study, MEK/ERK activation had distinct effects on T-cell expansion, depending on the strength of antigenic stimulation (Fig. 5E and F). Although activation of the MEK/ERK signaling pathway mediates multiple functions, including cell expansion, apoptosis and cell cycle arrest (44), how MEK/ERK activation determines these functions in T cells is still controversial. Previously, it has been suggested that strong and sustained ERK activation can lead to cell cycle arrest, whereas transient and/or cyclic ERK activation induced cell cycle progression (45). Consistent with this idea, we observed that the phosphorylation of ERK at the suppressive dose was strong and sustained compared with that at optimal antigen dose (data not shown), suggesting the possibility that the different duration and strength of ERK signaling can result in distinct T-cell functions by serving as a sensor for the strength of antigenic stimulation to regulate cell cycle progression. Then, the important question arises: how can the sensing ability of MEK/ERK be controlled by the strength of antigenic stimulation? Following the initial T cell–APC contact, pMHC–TCR complexes rearrange to form microclusters and the immunological synapse (IS), where the molecules upstream of MEK/ERK, such as LAT (46) and PAK1 (47), are recruited and serve as a platform for TCR signal transduction (48). We observed a higher incidence of IS formation in T cell–APC conjugates at the suppressive dose at both 15min and 2h (data not shown), suggesting that the IS may have a longer functional half-life at the high antigen dose. Such persisting ISs could elicit excessive and sustained activation of signaling molecules upstream of MEK/ERK, causing the impaired proliferation of T cells. In addition, it is conceivable that the mode of microcluster formation or its composition is different between optimal and suppressive doses. Another possibility is that the differential MEK/ERK activation between the optimal and suppressive dose of peptide is caused by distinct signaling molecules upstream of MEK/ERK. Several signaling pathways, such as LAT-PLCγ, Bam32-PLCγ-PAK1 and Lck-PKCθ-RasGRP, have been shown to be responsible for TCR-dependent MEK/ERK activation (49), thus these pathways downstream of TCR ligation may uniquely and/or coordinately regulate the activation of MEK/ERK. Further studies are required to evaluate these possibilities.
What then is the biologic role of this phenomenon under physiological conditions? At a suppressive antigen dose, T cells produce higher amounts of inflammatory cytokines, such as IL-2 and IFN-γ, than at a dose optimal for proliferation (25). Therefore, during a normal immune response following pathogen infection, it is possible that the T-cell growth suppression induced by strong antigenic stimulation suppresses the harmful cytokine-dependent responses, thus helping minimization tissue destruction. Our observations gave rise to an additional question of why cell cycle arrest is necessary for the effector T-cell response at high-dose antigenic stimulation. It may be that rapid and complete deletion of activated T cells, such as by Fas/FasL-dependent AICD, may significantly dampen an otherwise protective T cell-dependent immune response. Since we observed that, despite cell cycle arrest, activated T cells stimulated with high-dose antigen were still able to produce abundant cytokines, these T cells may retain the ability to help in the clearance of pathogens. Thus, cell cycle arrest at the site of infection is one of the important mechanisms to balance removal of foreign antigens and inhibition of excessive immune responses to self-tissue.
Our findings provide new insight into the regulation of effector T-cell clonal expansion. Furthermore, we found that the impaired proliferation of antigen-experienced T cells following excessive antigenic stimulation is caused by MEK/ERK activation. Thus, by modulating the strength of MEK/ERK activation, it may be possible to not only control TCR-dependent effector T-cell expansion in a normal immune response but also to reverse undesired growth suppression of peripheral effector T cells in response to strong and/or chronic antigenic stimulation, such as in the microenvironment of tumors.
Supplementary data
Supplementary data are available at International Immunology Online.
Acknowledgements
We thank Dr Jonathan D. Ashwell (Center for Cancer Research, National Cancer Institute) and Dr Peter D. Burrows (University of Alabama at Birmingham) for helpful discussion.
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Palmer E. 2003. Negative selection–clearing out the bad apples from the T-cell repertoire. Nat. Rev. Immunol. 3:383. [DOI] [PubMed] [Google Scholar]
- 2. Snow A. L. Pandiyan P. Zheng L. Krummey S. M. and Lenardo M. J. 2010. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol. Rev. 236:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen P. L. and Eisenberg R. A. 1991. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9:243. [DOI] [PubMed] [Google Scholar]
- 4. Strasser A. Jost P. J. and Nagata S. 2009. The many roles of FAS receptor signaling in the immune system. Immunity 30:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wahl C. Miethke T. Heeg K. and Wagner H. 1993. Clonal deletion as direct consequence of an in vivo T cell response to bacterial superantigen. Eur. J. Immunol. 23:1197. [DOI] [PubMed] [Google Scholar]
- 6. Ashwell J. D. Cunningham R. E. Noguchi P. D. and Hernandez D. 1987. Cell growth cycle block of T cell hybridomas upon activation with antigen. J. Exp. Med. 165:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liblau R. S., Tisch R., Shokat K., et al. 1996. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc. Natl Acad. Sci. USA 93:3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sytwu H. K. Liblau R. S. and McDevitt H. O. 1996. The roles of Fas/APO-1 (CD95) and TNF in antigen-induced programmed cell death in T cell receptor transgenic mice. Immunity 5:17. [DOI] [PubMed] [Google Scholar]
- 9. Jenkins M. K. Chen C. A. Jung G. Mueller D. L. and Schwartz R. H. 1990. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J. Immunol. 144:16. [PubMed] [Google Scholar]
- 10. Müller M. R. and Rao A. 2010. NFAT, immunity and cancer: a transcription factor comes of age. Nat. Rev. Immunol. 10:645. [DOI] [PubMed] [Google Scholar]
- 11. Navarro M. N. and Cantrell D. A. 2014. Serine-threonine kinases in TCR signaling. Nat. Immunol. 15:808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vallabhapurapu S. and Karin M. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27:693. [DOI] [PubMed] [Google Scholar]
- 13. Appleman L. J. Chernova I. Li L. and Boussiotis V. A. 2006. CD28 costimulation mediates transcription of SKP2 and CKS1, the substrate recognition components of SCFSkp2 ubiquitin ligase that leads p27kip1 to degradation. Cell Cycle 5:2123. [DOI] [PubMed] [Google Scholar]
- 14. Mognol G. P. Carneiro F. R. Robbs B. K. Faget D. V. and Viola J. P. 2016. Cell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old player. Cell Death Dis. 7:e2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Appleman L. J. van Puijenbroek A. A. Shu K. M. Nadler L. M. and Boussiotis V. A. 2002. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 168:2729. [DOI] [PubMed] [Google Scholar]
- 16. Srivastava R. Burbach B. J. Mitchell J. S. Pagán A. J. and Shimizu Y. 2012. ADAP regulates cell cycle progression of T cells via control of cyclin E and Cdk2 expression through two distinct CARMA1-dependent signaling pathways. Mol. Cell. Biol. 32:1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charvet C., Canonigo A. J., Bécart S., et al. 2006. Vav1 promotes T cell cycle progression by linking TCR/CD28 costimulation to FOXO1 and p27kip1 expression. J. Immunol. 177:5024. [DOI] [PubMed] [Google Scholar]
- 18. Zhang J. Xu X. and Liu Y. 2004. Activation-induced cell death in T cells and autoimmunity. Cell. Mol. Immunol. 1:186. [PubMed] [Google Scholar]
- 19. Baine I. Abe B. T. and Macian F. 2009. Regulation of T-cell tolerance by calcium/NFAT signaling. Immunol. Rev. 231:225. [DOI] [PubMed] [Google Scholar]
- 20. Chen D. Heath V. O’Garra A. Johnston J. and McMahon M. 1999. Sustained activation of the raf-MEK-ERK pathway elicits cytokine unresponsiveness in T cells. J. Immunol. 163:5796. [PubMed] [Google Scholar]
- 21. Lee I. H. Li W. P. Hisert K. B. and Ivashkiv L. B. 1999. Inhibition of interleukin 2 signaling and signal transducer and activator of transcription (STAT)5 activation during T cell receptor-mediated feedback inhibition of T cell expansion. J. Exp. Med. 190:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki G. Kawase Y. Koyasu S. Yahara I. Kobayashi Y. and Schwartz R. H. 1988. Antigen-induced suppression of the proliferative response of T cell clones. J. Immunol. 140:1359. [PubMed] [Google Scholar]
- 23. Ceredig R. and Corradin G. 1986. High antigen concentration inhibits T cell proliferation but not interleukin 2 production: examination of limiting dilution microcultures and T cell clones. Eur. J. Immunol. 16:30. [DOI] [PubMed] [Google Scholar]
- 24. Lamb J. R. Skidmore B. J. Green N. Chiller J. M. and Feldmann M. 1983. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J. Exp. Med. 157:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohnuki K., Watanabe Y., Takahashi Y., et al. 2009. Antigen-specific CD4+ effector T cells: analysis of factors regulating clonal expansion and cytokine production: clonal expansion and cytokine production by CD4+ effector T cells. Biochem. Biophys. Res. Commun. 380:742. [DOI] [PubMed] [Google Scholar]
- 26. Reich M. Liefeld T. Gould J. Lerner J. Tamayo P. and Mesirov J. P. 2006. GenePattern 2.0. Nat. Genet. 38:500. [DOI] [PubMed] [Google Scholar]
- 27. Bossu P. Singer G. G. Andres P. Ettinger R. Marshak-Rothstein A. and Abbas A. K. 1993. Mature CD4+ T lymphocytes from MRL/lpr mice are resistant to receptor-mediated tolerance and apoptosis. J. Immunol. 151:7233. [PubMed] [Google Scholar]
- 28. Singer G. G. and Abbas A. K. 1994. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1:365. [DOI] [PubMed] [Google Scholar]
- 29. Critchfield J. M. Zúñiga-Pflücker J. C. and Lenardo M. J. 1995. Parameters controlling the programmed death of mature mouse T lymphocytes in high-dose suppression. Cell. Immunol. 160:71. [DOI] [PubMed] [Google Scholar]
- 30. Russell J. H. Rush B. Weaver C. and Wang R. 1993. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc. Natl Acad. Sci. USA 90:4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Green D. R. Droin N. and Pinkoski M. 2003. Activation-induced cell death in T cells. Immunol. Rev. 193:70. [DOI] [PubMed] [Google Scholar]
- 32. Ko S. C. Johnson V. L. and Chow S. C. 2000. Functional characterization of Jurkat T cells rescued from CD95/Fas-induced apoptosis through the inhibition of caspases. Biochem. Biophys. Res. Commun. 270:1009. [DOI] [PubMed] [Google Scholar]
- 33. Duré M. and Macian F. 2009. IL-2 signaling prevents T cell anergy by inhibiting the expression of anergy-inducing genes. Mol. Immunol. 46:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boussiotis V. A., Barber D. L., Nakarai T., et al. 1994. Prevention of T cell anergy by signaling through the gamma c chain of the IL-2 receptor. Science 266:1039. [DOI] [PubMed] [Google Scholar]
- 35. Beverly B. Kang S. M. Lenardo M. J. and Schwartz R. H. 1992. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int. Immunol. 4:661. [DOI] [PubMed] [Google Scholar]
- 36. Vanier L. E. and Prud’homme G. J. 1992. Cyclosporin A markedly enhances superantigen-induced peripheral T cell deletion and inhibits anergy induction. J. Exp. Med. 176:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Satyanarayana A. and Kaldis P. 2009. Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28:2925. [DOI] [PubMed] [Google Scholar]
- 38. Abukhdeir A. M. and Park B. H. 2008. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev. Mol. Med. 10:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blagosklonny M. V. 2003. Cell senescence and hypermitogenic arrest. EMBO Rep. 4:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bidère N. Su H. C. and Lenardo M. J. 2006. Genetic disorders of programmed cell death in the immune system. Annu. Rev. Immunol. 24:321. [DOI] [PubMed] [Google Scholar]
- 41. Davidson W. F. Calkins C. Hügins A. Giese T. and Holmes K. L. 1991. Cytokine secretion by C3H-lpr and -gld T cells. Hypersecretion of IFN-gamma and tumor necrosis factor-alpha by stimulated CD4+ T cells. J. Immunol. 146:4138. [PubMed] [Google Scholar]
- 42. Miyatake S., Nakano H., Park S. Y., et al. 1995. Induction of G1 arrest by down-regulation of cyclin D3 in T cell hybridomas. J. Exp. Med. 182:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zmajkovicova K. Jesenberger V. Catalanotti F. Baumgartner C. Reyes G. and Baccarini M. 2013. MEK1 is required for PTEN membrane recruitment, AKT regulation, and the maintenance of peripheral tolerance. Mol. Cell 50:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cagnol S. and Chambard J. C. 2010. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 277:2. [DOI] [PubMed] [Google Scholar]
- 45. Marshall C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179. [DOI] [PubMed] [Google Scholar]
- 46. Bonello G., Blanchard N., Montoya M. C., et al. 2004. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J. Cell Sci. 117(Pt 7):1009. [DOI] [PubMed] [Google Scholar]
- 47. Phee H. Abraham R. T. and Weiss A. 2005. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat. Immunol. 6:608. [DOI] [PubMed] [Google Scholar]
- 48. Yokosuka T. and Saito T. 2009. Dynamic regulation of T-cell costimulation through TCR-CD28 microclusters. Immunol. Rev. 229:27. [DOI] [PubMed] [Google Scholar]
- 49. Kortum R. L. Rouquette-Jazdanian A. K. and Samelson L. E. 2013. Ras and extracellular signal-regulated kinase signaling in thymocytes and T cells. Trends Immunol. 34:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.