Abstract
Background: As global initiatives increase patient access to surgical treatments, there remains a need to understand the adverse effects of surgery and define appropriate levels of perioperative care.
Methods: We designed a prospective international 7-day cohort study of outcomes following elective adult inpatient surgery in 27 countries. The primary outcome was in-hospital complications. Secondary outcomes were death following a complication (failure to rescue) and death in hospital. Process measures were admission to critical care immediately after surgery or to treat a complication and duration of hospital stay. A single definition of critical care was used for all countries.
Results: A total of 474 hospitals in 19 high-, 7 middle- and 1 low-income country were included in the primary analysis. Data included 44 814 patients with a median hospital stay of 4 (range 2–7) days. A total of 7508 patients (16.8%) developed one or more postoperative complication and 207 died (0.5%). The overall mortality among patients who developed complications was 2.8%. Mortality following complications ranged from 2.4% for pulmonary embolism to 43.9% for cardiac arrest. A total of 4360 (9.7%) patients were admitted to a critical care unit as routine immediately after surgery, of whom 2198 (50.4%) developed a complication, with 105 (2.4%) deaths. A total of 1233 patients (16.4%) were admitted to a critical care unit to treat complications, with 119 (9.7%) deaths. Despite lower baseline risk, outcomes were similar in low- and middle-income compared with high-income countries.
Conclusions: Poor patient outcomes are common after inpatient surgery. Global initiatives to increase access to surgical treatments should also address the need for safe perioperative care.
Study registration: ISRCTN51817007
Keywords: cohort studies, critical care/utilisation, operative/mortality, postoperative care/methods, postoperative care/statistics and numerical data, surgery, surgical procedures
Editor’s key points
As global access to surgical procedures increases, it is important to understand differences in outcomes depending on economic development and access to perioperative care.
The International Surgical Outcomes Study evaluated incidence and risk factors for complications and death after inpatient elective surgery in 27 countries of varied economic status.
Adverse outcomes were common after inpatient surgery and were similar in low- and middle-income compared with high-income countries despite lower baseline risk of the former.
About 310 million patients undergo surgery worldwide each year, with more procedures taking place in high-income countries.1 2 Findings from epidemiological studies suggest that 4.8 billion people are unable to access safe surgical treatments,3 and that at least 143 million additional procedures are required each year, primarily in low- and middle-income countries.4 5 However, as health care systems develop to improve access to surgical treatments, the number of patients who suffer postoperative complications will also increase.3 4
Postoperative complications increase treatment costs6 and reduce both life expectancy and quality of life.7 8 Nonetheless, our global understanding of outcomes after surgery remains limited. Estimates from high-income countries suggest postoperative complications occur in up to 20% of patients,9 10 and short-term mortality varies from 1 to 4%.11–18 While effective perioperative care is considered essential to the safe provision of surgical treatments,8 the optimal level of such care has not been defined. Admission to a critical care unit is often considered necessary to prevent or treat life-threatening complications. However, this level of patient care is very expensive and there is little or no evidence to confirm the critical care resource provision needed for a safe surgical service.
As we seek to ensure the global availability of surgical treatments to all patients, we need to understand how often patients develop complications after surgery, the severity of harm that results, and how hospital systems should be configured to safely respond. We performed the International Surgical Outcomes Study (ISOS) to evaluate the global incidence and risk factors for complications and death after inpatient elective surgery and to describe current standards of postoperative care.
Methods
Project organisation
ISOS was a 7-day international cohort study. Regulatory requirements differed between countries, with some requiring research ethics approval and some requiring only data governance approval. In the UK, the study was approved by the Yorkshire & Humber Research Ethics Committee (reference: 13/YH/0371). The inclusion criteria were all adult patients (age ≥18 years) undergoing elective surgery with a planned overnight stay in the hospital. Each participating country selected a single data collection week between April and August 2014. Patients undergoing emergency surgery, day-case surgery or radiological procedures were excluded. Patient data included only that recorded as part of routine care. In some countries, patient consent was sought to allow the collection of supplementary data for prespecified substudies. In each country we approached individuals to act as national coordinators using contacts in national and international specialist societies in surgery and anaesthesia. Individual participating hospitals were then identified through a global online recruitment campaign led by the study management group and through the direct approach of the national coordinators. Nominations for participation were then confirmed as appropriate through discussion with national coordinators. The study website provided all study documentation and guidance on study procedures (www.isos.org.uk/documents). ISOS was registered prospectively with an international trial registry (ISRCTN51817007).
Data collection
Data describing perioperative care facilities were collected for each hospital at the beginning of the study. Data describing consecutive patients were collected until hospital discharge on paper case record forms (Supplementary file). Complications were assessed according to predefined criteria and graded as mild, moderate, or severe.19 Data were censored at 30 days following surgery for patients who remained in the hospital. Data were anonymised before entry onto a secure Internet-based electronic case record form designed specifically for ISOS, which incorporated automated checks for plausibility, consistency and completeness.
Outcome measures
The primary outcome measure was in-hospital postoperative complications. Secondary outcomes were death following a postoperative complication (failure to rescue) and in-hospital mortality. Process measures were admission directly to critical care after surgery, admission to critical care for treatment of a postoperative complication, and duration of hospital stay. A single prospective definition of critical care was used for all countries (a facility routinely capable of admitting patients who require invasive ventilation overnight).
Statistical analysis
We aimed to recruit as many hospitals and countries as possible and asked investigators in those hospitals to enrol all eligible patients. No formal sample size calculation was performed. Only hospitals returning valid data describing ≥10 patients and countries with ≥10 participating hospitals were included in the primary analysis.
Association between surgical procedure category and patient outcomes
We assessed the association between surgical procedure category and complications or mortality both before and after adjustment for potential confounding factors. The unadjusted analysis was performed using a logistic regression model with the surgical procedure category included as a fixed factor. The adjusted analysis was performed using a three-level mixed-effects logistic regression model. Patients were entered at the first level, hospitals at the second level, and countries at the third level. This model accounted for correlation between patients in the same hospital or country. The following variables were included as fixed factors in the model: age, current smoker, American Society of Anesthesiologists (ASA) physical status score, severity of surgery, surgical procedure category, and presence of ischaemic heart disease, heart failure, diabetes mellitus, chronic obstructive pulmonary disease/asthma, cirrhosis, stroke, or other comorbid diseases. Factors were selected for biological plausibility, scientific rationale, and a low rate of missing data. We used restricted cubic splines to account for a potential non-linear association between age and outcome.20 To assess the effect of predefined exclusions on our findings, we repeated our analyses for all patients in the database. For both the unadjusted and adjusted analyses, Hosmer–Lemeshow goodness-of-fit statistics were used to test model calibration and multicollinearity was assessed using the variance inflation factor. The ability of the model to discriminate cases from non-cases was assessed using the area under the receiver operating characteristic curve (AUROC). Data are presented as mean (sd) and median [interquartile range (IQR)] for continuous data, number (%) for binary data, or odds ratios (ORs) with 95% confidence intervals (CIs). Analyses were performed using Stata 14 (StataCorp, College Station, TX, USA).
Results
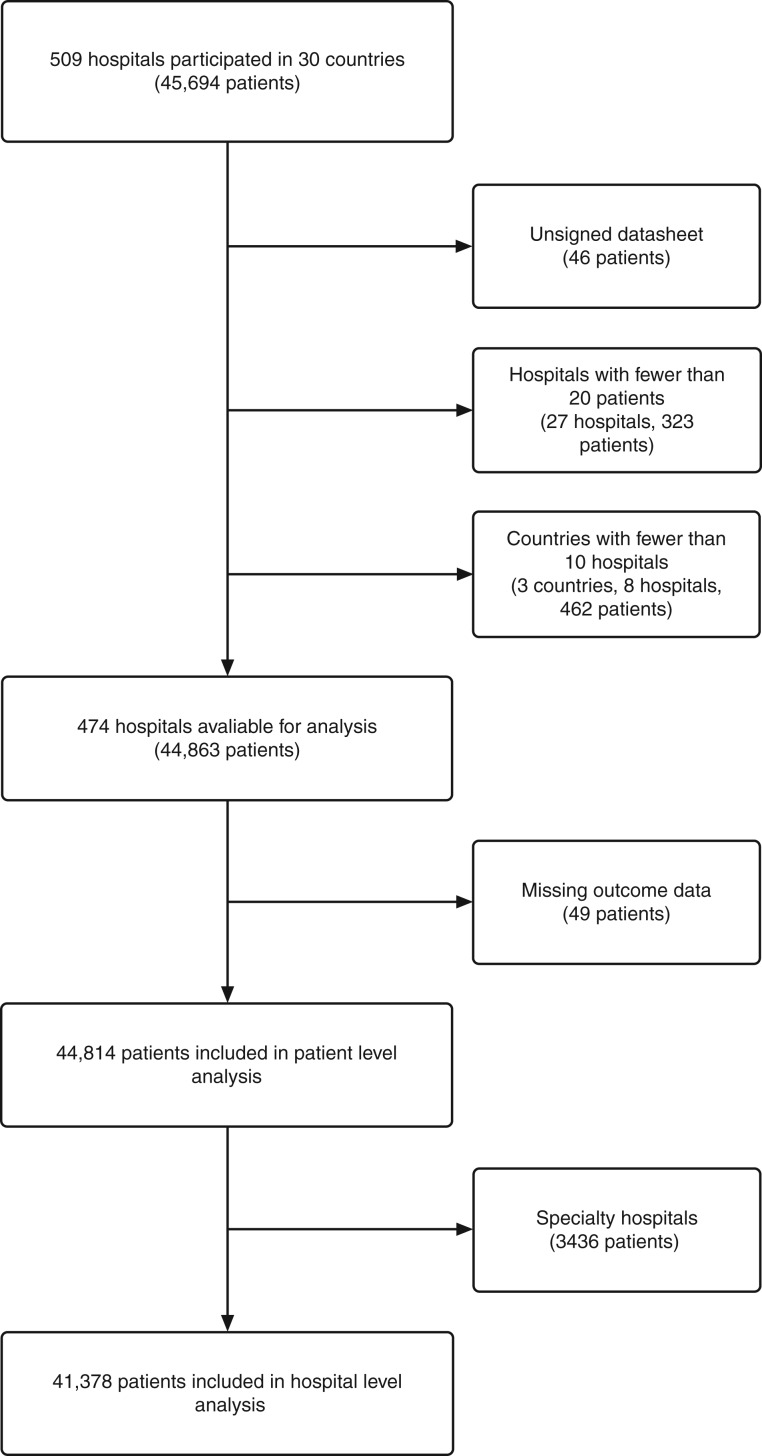
Data describing 44 814 patients were collected from 474 hospitals in the following countries and regions: Australia, Austria, Belgium, Brazil, Canada, China, Denmark, France, Germany, Greece, Hong Kong, Indonesia, Italy, Malaysia, The Netherlands, New Zealand, Nigeria, Portugal, Romania, Russia, South Africa, Spain, Sweden, Switzerland, Uganda, UK, and USA (Fig. 1). Fewer than 10 hospitals participated in India, Iraq, and Mexico, and in accordance with the prospective statistical analysis plan, patients recruited in these countries were excluded from the primary analysis (Fig. 2). Seven countries were classed as middle income and one as low income, with 134 participating hospitals between them.21 Hospitals had a median of 550 (range 329–850) ward beds and 21 (range 10–38) critical care beds. The median critical care capacity (ratio of critical care beds to total hospital beds) was 4% (IQR 2–6). A total of 310 hospitals (66%) were affiliated with a university. Seventy-seven percent of hospitals provided only government funded health care, 3% only privately funded health care, and 21% were funded by both sources. Baseline patient data are presented in Table 1.
Fig 1.
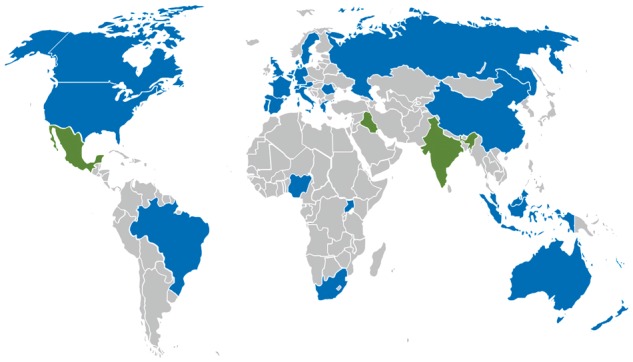
Countries participating in the International Surgical Outcomes Study. Blue: countries included in the primary analysis. Green: countries with <10 participating hospitals included in the secondary analysis.
Fig 2.
Patients, hospitals, and countries excluded from the study.
Table 1.
Baseline patient characteristics. All data presented as n (%) unless otherwise noted. ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease
| All patients(n = 44 814) | Patients with complications(n = 7508) | Patients with nocomplications (n = 37 306) | Patients whodied (n = 207) | Patients who survived(n = 44 607) | |
|---|---|---|---|---|---|
| Age, years, mean (sd) | 55.3 (17.1) | 61.8 (16.0) | 54.1 (17.0) | 69.1 (13.3) | 55.3 (17.1) |
| Age, years, median (range) | 57 (18–102) | 64 (18–100) | 55 (18–102) | 73 (28–93) | 57 (18–102) |
| Male | 20 458 (45.7) | 3968 (19.4) | 16 490 (80.6) | 121 (0.6) | 20 337 (99.4) |
| Smoker | 7913 (17.8) | 1305 (16.5) | 6608 (83.5) | 47 (0.6) | 7866 (99.4) |
| ASA score | |||||
| I | 11 227 (25.1) | 848 (7.6) | 10 379 (92.5) | 1 (0.1) | 11 226 (99.9) |
| II | 22 265 (49.8) | 3005 (13.5) | 19 260 (86.5) | 38 (0.2) | 22 227 (99.8) |
| III | 10 193 (22.8) | 3090 (30.3) | 7103 (69.7) | 115 (1.1) | 10 078 (98.9) |
| IV | 1038 (2.3) | 554 (53.4) | 484 (46.6) | 53 (5.1) | 985 (94.9) |
| Severity of surgery | |||||
| Minor | 8411 (18.8) | 672 (8.0) | 7739 (92.0) | 14 (0.2) | 8397 (99.8) |
| Intermediate | 20 203 (45.1) | 2494 (12.3) | 17 709 (87.7) | 56 (0.3) | 20 147 (99.7) |
| Major | 16 175 (36.1) | 4336 (26.8) | 11 839 (73.2) | 137 (0.9) | 16 038 (99.1) |
| Surgical procedure | |||||
| Orthopaedic | 9459 (21.1) | 1556 (16.5) | 7893 (83.5) | 25 (0.3) | 9434 (99.7) |
| Breast | 1538 (3.4) | 128 (8.3) | 1410 (91.7) | 2 (0.1) | 1536 (99.9) |
| Obstetrics and gynaecology | 5674 (12.7) | 554 (9.8) | 5120 (90.2) | 6 (0.1) | 5668 (99.9) |
| Urology and kidney | 4871 (10.9) | 720 (14.8) | 4151 (85.2) | 10 (0.2) | 4861 (99.8) |
| Upper gastrointestinal | 1986 (4.4) | 485 (24.4) | 1501 (75.6) | 29 (1.5) | 1957 (98.5) |
| Lower gastrointestinal | 3073 (6.9) | 748 (24.3) | 2325 (75.7) | 32 (1.0) | 3041 (99.0) |
| Hepatobiliary | 2282 (5.1) | 366 (16.0) | 1916 (83) | 14 (0.6) | 2268 (99.4) |
| Vascular | 1599 (3.6) | 410 (25.6) | 1189 (74.4) | 15 (0.9) | 1584 (99.0) |
| Head and neck | 6510 (14.5) | 674 (10.4) | 5836 (89.6) | 12 (0.2) | 6498 (99.8) |
| Plastics and cutaneous | 1670 (3.7) | 244 (14.6) | 1426 (85.4) | 5 (0.3) | 1665 (99.7) |
| Cardiac | 1716 (3.8) | 979 (57.0) | 737 (43.0) | 40 (2.3) | 1676 (97.7) |
| Thoracic | 1157 (2.6) | 305 (26.4) | 852 (73.6) | 10 (0.9) | 1147 (99.1) |
| Other | 3270 (7.3) | 328 (10.0) | 2942 (90.0) | 7 (0.2) | 3263 (99.8) |
| Comorbid disease | |||||
| Ischaemic heart disease | 4588 (10.3) | 1525 (33.2) | 3063 (66.8) | 67 (1.5) | 4521 (98.5) |
| Heart failure | 1882 (4.2) | 775 (41.2) | 1107 (58.8) | 49 (2.6) | 1833 (97.4) |
| Diabetes mellitus | 5171 (11.6) | 1319 (25.5) | 3852 (74.5) | 58 (1.1) | 5113 (98.9) |
| Cirrhosis | 342 (0.8) | 113 (33.0) | 229 (67.0) | 10 (2.9) | 332 (97.1) |
| Metastatic cancer | 1706 (3.8) | 508 (29.8) | 1198 (70.2) | 36 (2.1) | 1670 (97.9) |
| Stroke | 1492 (3.3) | 451 (30.2) | 1041 (69.8) | 38 (2.6) | 1454 (97.4) |
| COPD/asthma | 4094 (9.2) | 1012 (24.7) | 3082 (75.3) | 40 (1.0) | 4054 (99.0) |
| Other | 18 607 (41.6) | 4134 (22.2) | 14464 (77.8) | 134 (0.7) | 18473 (99.3) |
| Other measures | |||||
| Laparoscopic surgery | 7087 (15.8) | 905 (12.8) | 6182 (87.2) | 16 (0.2) | 7071 (99.8) |
| Cancer surgery | 9006 (20.3) | 2005 (22.2) | 7001 (77.7) | 70 (0.8) | 8936 (99.2) |
Data validation
There was high concordance in a random 1% data sample selected for duplicate entry (95% for categorical variables, 92% for continuous variables), with very high concordance for clinical outcomes (99.7%). Investigators were granted immediate access to their uncleaned data once this was locked following entry and were encouraged to review it for accuracy and completeness. All national coordinators confirmed the face validity of the baseline and crude outcome data for their countries. Only a small proportion of patients [451/44 814 (1%)] were missing data for at least one of the factors included in the model. Due to the low proportion of missing data, we performed a complete case analysis where patients with missing data were excluded from the analysis (Supplementary Table 1). Hosmer–Lemeshow goodness-of-fit statistics indicated that the models were well calibrated, with a good match between observed and expected outcomes. Discrimination of the model was good, with an AUROC of 0.80 (95% CI 0.80–0.81). Residuals showed that the assumptions for regression analyses were met. All variables had a variance inflation factor of <5.
Clinical outcomes
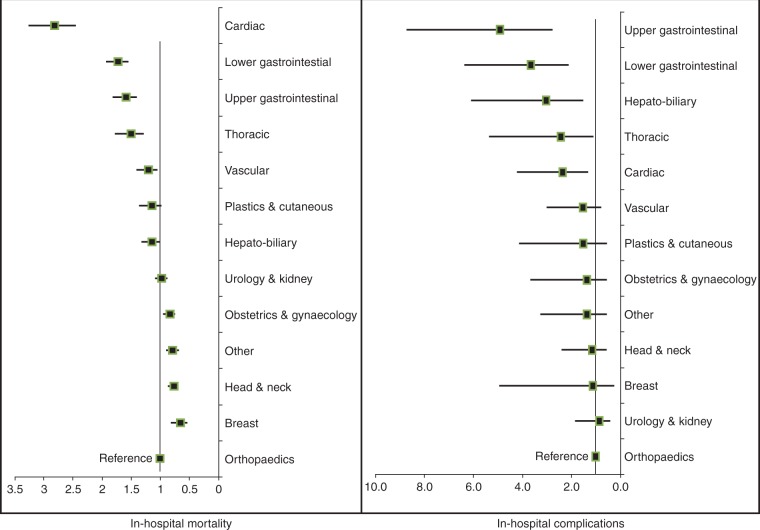
A total of 7508 (16.8%) patients developed complications in the hospital and 207 died before hospital discharge (0.5%), indicating an overall mortality among patients who developed complications (failure to rescue) of 2.8%. A total of 5254 (11.7%) patients developed a single postoperative complication, while another 2254 (5.0%) patients developed two or more complications. The breakdown of complications is presented in Table 2. Infectious complications were the most frequent, in particular superficial surgical site infections. A total of 2925 patients developed an unspecified complication (‘other’ category). There were significant variations in complications and mortality across surgical procedure categories and countries (Fig. 3, Supplementary Tables 2 and 3). Outcomes for patients according to planned admission to critical care immediately after surgery are presented in Table 3. A total of 1233 patients (16.4%) were admitted to a critical care unit to treat complications, of whom 119 (9.7%) died. Fifty-eight (28.0%) patients who died were not admitted to critical care at any stage during their admission, either immediately after surgery or for treatment of a complication. The clinical outcomes for all patients included in the database are presented in Supplementary Table 4.
Table 2.
Postoperative complications and mortality for 44 814 patients undergoing elective surgery. Data presented as n (%). ARDS, acute respiratory distress syndrome; N/A, category not applicable for this complication. Some patients may have developed more than one complication and consequently, in some cases, the denominator is the number of complications while in the left-most column the denominator is the number of patients. The cell at the bottom of the far right column represents the number of deaths divided by the number of patients with at least one complication
| Severity of complications |
Mortality for patients whodeveloped complications | ||||
|---|---|---|---|---|---|
| N = 44 814 | Mild | Moderate | Severe | N = 207 | |
| Infectious complications | |||||
| Superficial surgical site | 1320 (2.9) | 681/1320 (51.6) | 517/1320 (39.2) | 122/1320 (9.2) | 17/1320 (1.3) |
| Deep surgical site | 566 (1.3) | 120/566 (21.2) | 250/566 (44.2) | 196/566 (34.6) | 28/566 (4.9) |
| Body cavity | 340 (0.8) | 97/340 (28.5) | 136/340 (40.0) | 107/340 (31.5) | 24/340 (7.0) |
| Pneumonia | 708 (1.6) | 240/708 (33.9) | 325/708 (45.9) | 143/708 (20.2) | 55/708 (7.8) |
| Urinary tract | 681 (1.5) | 294/681 (43.2) | 333/681 (48.9) | 54/681 (7.9) | 13/681 (1.9) |
| Bloodstream | 417 (0.9) | 140/417 (33.6) | 162/417 (38.8) | 115/417 (27.6) | 48/417 (11.5) |
| Total infectious complications | 4032 | 1572/4032 (39.0) | 1723/4032 (42.7) | 737/4032 (18.3) | 104/4032 (2.6) |
| Cardiovascular complications | |||||
| Myocardial infarction | 139 (0.3) | 45/139 (32.4) | 43/139 (30.9) | 51/139 (36.7) | 26/139 (18.7) |
| Arrhythmia | 1222 (2.7) | 468/1222 (38.3) | 568/1222 (46.5) | 186/1222 (15.2) | 74/1222 (6.1) |
| Pulmonary oedema | 330 (0.7) | 127/330 (38.4) | 141/330 (42.8) | 62/330 (18.8) | 34/330 (10.3) |
| Pulmonary embolism | 78 (0.2) | 17/78 (21.8) | 33/78 (42.3) | 28/78 (35.9) | 5/78 (6.4) |
| Stroke | 111 (0.2) | 31/111 (27.9) | 28/111 (25.2) | 52/111 (46.9) | 18/111 (16.2) |
| Cardiac arrest | 153 (0.3) | N/A | N/A | 153/153 (100.0) | 91/153 (59.5) |
| Total cardiovascular complications | 2033 | 688/2033 (33.8) | 813/2033 (40.0) | 532/2033 (26.2) | 141/2033 (6.9) |
| Other complications | |||||
| Gastrointestinal bleed | 201 (0.4) | 95/201 (47.3) | 66/201 (32.8) | 40/201 (19.9) | 24/201 (11.9) |
| Acute kidney injury | 778 (1.7) | 423/778 (54.4) | 203/778 (26.1) | 152/778 (19.5) | 76/778 (9.8) |
| Postoperative bleed | 1362 (3.0) | N/A | 1147/1362 (84.2) | 215/1362 (15.8) | 55/1362 (4.0) |
| ARDS | 142 (0.3) | 46/142 (32.4) | 41/142 (28.9) | 55/142 (38.7) | 34/142 (23.9) |
| Anastomotic leak | 208 (0.5) | 52/208 (25.0) | 62/208 (29.8) | 94/208 (45.2) | 21/208 (10.1) |
| All others | 2934 (6.5) | 1342/2925 (45.9) | 1200/2925 (41.0) | 392/2925 (13.4) | 83/2925 (2.8) |
| Total other complications | 5625 | 1958/5625 (34.8) | 2719/5625 (48.3) | 948/5625 (16.9) | 158/5625 (2.8) |
| Total number of complications | 11 690 | 4218/11 690 (36.1) | 5255/11 690 (45.0) | 2217/11 690 (19.0) | 207/7508 (2.8) |
Fig 3.
Adjusted risk (odds ratio) of complications with 95% confidence intervals and in-hospital mortality in different surgical procedure categories.
Table 3.
Outcomes for patients according to planned admission to critical care immediately after surgery. Data presented as n (%)
| All patients(n = 44 814) | Patients admittedto critical care immediatelyafter surgery (n = 4360) | Patients not admittedto critical care immediatelyafter surgery (n = 39 935) | |
|---|---|---|---|
| Mortality | 207/44 814 (0.5) | 105/4360 (2.4) | 99/39 935 (0.2) |
| Complication(s) | 7508/44 814 (16.8) | 2198/4360 (50.4) | 5270/39 935 (13.2) |
| Critical care admission to treat complication(s) | 1233/7508 (16.4) | 857/2198 (39.0) | 365/5270 (6.9) |
| Death following a complication (failure to rescue) | 207/7508 (2.8) | 105/2198 (4.8) | 99/5270 (1.9) |
Process measures
The median stay in a post-anaesthetic care unit was 1 (IQR 0–2) h. A total of 4360 (9.7%) patients were admitted to a critical care unit as routine immediately after surgery. The median length of time spent in critical care for those with a planned admission directly after surgery was 1 (IQR 1–3) day. Of these patients, 2198 (50.4%) developed a complication, with 105 (2.4%) deaths. A total of 1233 (4.9%) patients were admitted to a critical care unit to treat complications, of whom 119 (9.7%) died. The median length of time spent in critical care for patients admitted to treat a complication was 3 (IQR 1–6) days. The median overall hospital stay was 4 (IQR 2–7) days, increasing to 8 (IQR 5–14) days among those patients who developed complications.
Outcomes in low-, middle-, and high-income countries
Patient outcomes and process measures according to low- and middle- or high-income country status are presented in Table 4. One country in the low- and middle-income groups, which returned a large patient sample, experienced much lower complication rates than other participating nations. Patients in low- and middle-income countries tended to be younger with lower ASA scores. Crude complication rates were lower, but mortality rates overall, and for patients developing complications, were similar to those in high-income countries, suggesting care for patients who develop complications may be less effective. There was a much lower rate of planned admission to critical care immediately after surgery in low- and middle-income countries.
Table 4.
Hospital resources, process measures and patient outcomes in low-, middle-, and high-income countries
| Low- and middle-income countries (n = 8) | High-income countries (n = 19) | ||
|---|---|---|---|
| Number of hospitals | 126 | 348 | |
| Number of patients | 15 806 | 29 008 | |
| Hospital characteristics, median (IQR) | |||
| Total beds per hospital | 825 (412–1318) | 570 (361–835) | |
| Critical care beds per hospital | 25 (12–45) | 20 (11–37) | |
| Critical care capacity per hospital | 2.8% (1.5–4.8) | 3.6% (2.4–5.9) | |
| Patient characteristics | |||
| Age, years, mean (sd) | 50.8 (16.0) | 57.8 (17.2) | |
| ASA I and II, n (%) | 13 766 (87.2) | 19 726 (68.2) | |
| ASA III and IV, n (%) | 2029 (12.8) | 9202 (31.8) | |
| Comorbid disease (any), n (%) | 6488 (41.2) | 19 590 (67.6) | |
| Metastatic cancer, n (%) | 297 (1.9) | 1409 (4.9) | |
| Process measures | |||
| Post-anaesthetic care unit stay (h), median (IQR) | 1 (0–1) | 1 (1–2) | |
| Length of hospital stay (days), median (IQR) | 5 (3–8) | 3 (1–6) | |
| Planned critical care admission, n (%) | 1051/15 299 (6.9) | 3309/28 996 (11.4) | |
| Critical care to treat complication(s) , n (%) | 317/15 806 (2.0) | 916/28 905 (3.2) | |
| Patient outcomes, n (%) | |||
| Complication(s) | 1760/15 806 (11.1) | 5748/29 008 (19.8) | |
| Mortality | 58/15 806 (0.4) | 149/29 008 (0.5) | |
| Mortality following complications | 58/1760 (3.3) | 149/5748 (2.6) | |
Discussion
This international prospective cohort study has provided detailed outcome data on a population of >44 000 consecutive patients undergoing elective inpatient surgery in 27 low-, middle-, and high-income countries worldwide. The principal finding was that 1 in 6 patients experienced a complication before hospital discharge and 1 in 35 patients who experienced a complication subsequently died without leaving the hospital. The mortality among patients who developed complications (failure to rescue) of 2.8% indicates the continued need for a more effective treatment response for patients who develop postoperative complications. Despite lower baseline risk, crude patient outcomes were broadly similar in low- and middle-income compared with high-income countries.
There are few large datasets of complication rates after surgery, and none we are aware of which provide data at an international level, although the findings of a recent study of almost 11 000 patients undergoing emergency abdominal surgery in 58 low-, middle-, and high-income countries indicate high mortality following such procedures.22 Caution should be exercised when comparing between country-level datasets because of international differences in patterns of surgical disease and genetic backgrounds, as well as in health care systems. A variable degree of selection bias is also likely to result in important differences between reports that are few in number. While overall complication rates in the current data were slightly lower than those previously reported in the USA,9 23 this might simply be due to differences in patient risk factors and the surgical procedures included. In particular, ISOS only included patients undergoing elective surgery. Previous mortality estimates for unselected patient populations undergoing inpatient surgery vary between 1 and 4%.15–18 A recent study of postoperative mortality in Europe suggested in-hospital mortality of 3% for elective inpatient surgery,11 similar to overall mortality rates in reports from the USA.9 16 23
These data provide detailed insights into patterns of critical care admission after surgery. This is an expensive resource, and rates of admission in low- and middle-income countries appear to be much lower than in high-income countries. The value of routine admission of high-risk patients to a critical care unit after surgery remains uncertain and allocation of this resource appears inconsistent. For example, admission to critical care after cardiac surgery is routine in most countries, while high-risk patients undergoing non-cardiac surgery may not be provided with this level of care despite a much higher mortality rate.12–14 The findings of two recent health care registry studies in the UK suggest that provision of critical care can improve survival for surgical patients, although the effect may be subtle.24 25 Meanwhile, a study of Medicare registry data in the USA failed to identify any benefit of critical care admission.26 27 Comparison of failure to rescue (rate of death after postoperative complications) between hospitals and health care systems could help us to understand the impact of postoperative critical care on patient outcomes. While it seems unlikely that we could ever reduce mortality from postoperative complications to zero, failure to rescue has provided a useful metric of the quality of postoperative care for surgical patients in high-income countries.9 28–31 We could argue that in a well-resourced system, very few patients should die after elective surgery without being admitted to a critical care unit. The current data confirm there is an important rate of failure to rescue at a global level, which is placed in context by the rates of use of critical care facilities. Global strategies to improve access to surgical treatments should take account of the increased demand for perioperative care services, in particular critical care, for patients who develop complications.3 4 While the surgical population is very large, few countries have any reliable system to monitor the volume of activity and clinical outcomes. Understanding the safety and effectiveness of surgical treatments is therefore limited and the need remains for robust audit and public reporting of outcomes after all surgery worldwide.8 Data-driven improvement in the quality of perioperative care might be possible even in resource-limited environments.32
A strengths of this study is the large number of consecutive patients enrolled worldwide. Importantly, critical care beds were classified according to a standard definition in participating hospitals. We also distinguished between planned admission to critical care immediately after surgery as a part of routine postoperative management and unplanned admission to critical care to treat a life-threatening complication. By developing a very simple dataset consisting primarily of categorical variables, we were able to minimise the amount of missing data. Patient-level variables were selected on the basis that they were objective, routinely collected for clinical reasons, could be transcribed with a high level of accuracy, subject to a low rate of missing data, and relevant to a risk adjustment model that included a wide variety of surgical procedures. The online data entry system was designed specifically for ISOS and included a variety of internal error checks while avoiding the redundant functionality of generic software designed for complex trials.
The study also has a number of weaknesses. Despite the large sample size, we cannot consider this study as representative of current practice in all countries. ISOS was a pragmatic study and only a small proportion of hospitals took part in a small number of countries. While we are pleased to have recruited hospitals in 30 countries, only 27 of these reached the predefined number of participating hospitals. We discussed participation with potential investigators in a number of countries who did not feel they had adequate resources to take part. This affected the participation of low-, middle-, and high-income countries. Many patients were enrolled in university hospitals while smaller, low-volume centres are underrepresented. This effect was greater in the low- and middle-income countries that took part. The risk adjustment methods used might not fully account for high mortality rates in hospitals specialising in more complex surgery. After risk adjustment there were differences in postoperative outcomes between countries, but there are likely to be differences in case mix that are not fully represented in our baseline data.12 We note that crude complication and mortality rates were lower in one high-volume country, reducing the overall event rate. Given the pragmatic nature of this study, it was only possible to follow patients until hospital discharge. In countries where the availability of hospital beds is more limited, early hospital discharge of patients could have resulted in a lower measured complication rate. Although we planned to enrol every eligible patient undergoing surgery during the study period, we cannot be sure of the exact proportion of eligible patients included. Despite these limitations, assuming the volume of surgery during the cohort week is typical of the participating hospitals, these centres perform >3 million inpatient surgical procedures each year, ∼1% of the estimated volume of surgery taking place worldwide.12
Conclusions
The findings of this international cohort study indicate that a large number of patients develop complications after elective inpatient surgery. Global strategies to improve access to surgical treatments should take account of the increased demand placed on perioperative care services.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Acknowledgements
The ISOS investigators would like to thank our patient representative, Naomi Pritchard, for her guidance and support throughout this project.
Funding
This was an investigator initiated study funded by Nestle Health Sciences through an unrestricted research grant, by a National Institute for Health Research Professorship held by R.P., and sponsored by Queen Mary University of London.
Declaration of interests
R.P. has given lectures and/or performed consultancy work for Nestle Health Sciences, Medtronic, Edwards Lifesciences, and Massimo and is a member of the associate editorial board of the British Journal of Anaesthesia. P.H. is a National Institute for Health Research clinician scientist. D.W. is supported in part by a New Investigator Award from the Canadian Institutes of Health Research and a Merit Award from the Department of Anesthesia at the University of Toronto.
Author contributors
ISOS investigators were entirely responsible for the study design, conduct, and data analysis.
Members of the writing committee had full data access and were solely responsible for data interpretation, drafting and revision of the manuscript, and the decision to submit for publication.
Nestle Health Sciences had no data access and no role in study design, conduct, analysis, or drafting this report.
R.P. conceived the study and designed it together with all members of the writing committee and steering committees. Patient recruitment and data collection were performed by the members of the ISOS study group (see supplementary file).
T.A. and B.K. performed the data analysis with input from all members of the writing committee.
The manuscript was drafted by R.P. and revised following critical review by all members of the writing committee and steering committees.
Data sharing
The authors are happy to consider data sharing requests from bona fide researchers. Enquiries should be addressed to the chief investigator at: admin@isos.org.uk.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008; 372: 139–44 [DOI] [PubMed] [Google Scholar]
- 2.Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015; 385: S11. [DOI] [PubMed] [Google Scholar]
- 3.Alkire BC, Raykar NP, Shrime MG, et al. Global access to surgical care: a modelling study. Lancet Glob Health 2015; 3: e316–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Lancet Commission on Global Surgery. 2015. Available from www.globalsurgery.info (accessed 25th September 2016)
- 5.Rose J, Weiser TG, Hider P, Wilson L, Gruen RL, Bickler SW. Estimated need for surgery worldwide based on prevalence of diseases: a modelling strategy for the WHO Global Health Estimate. Lancet Glob Health 2015; 3: S13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scally CP, Thumma JR, Birkmeyer JD, Dimick JB. Impact of surgical quality improvement on payments in Medicare patients. Ann Surg 2014; 262: 249–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Head J, Ferrie JE, Alexanderson K, et al. Diagnosis-specific sickness absence as a predictor of mortality: the Whitehall II prospective cohort study. BMJ 2008; 337: a1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearse RM, Holt PJ, Grocott MP. Managing perioperative risk in patients undergoing elective non-cardiac surgery. BMJ 2011; 343: d5759. [DOI] [PubMed] [Google Scholar]
- 9.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med 2009; 361: 1368–75 [DOI] [PubMed] [Google Scholar]
- 10.Khuri SF, Daley J, Henderson W, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 1998; 228: 491–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012; 380: 1059–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Findlay G, Goodwin A, Protopappa K, Smith N, Mason M. Knowing the Risk: A Review of the Peri-operative Care of Surgical Patients London: National Confidential Enquiry into Patient Outcome and Death, 2011
- 13.Jhanji S, Thomas B, Ely A, Watson D, Hinds CJ, Pearse RM. Mortality and utilisation of critical care resources amongst high-risk surgical patients in a large NHS trust. Anaesthesia 2008; 63: 695–700 [DOI] [PubMed] [Google Scholar]
- 14.Pearse RM, Harrison DA, James P, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care 2006; 10: R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg 2012; 255: 696–702 [DOI] [PubMed] [Google Scholar]
- 16.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360: 1418–28 [DOI] [PubMed] [Google Scholar]
- 17.Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology 2010; 112: 1105–15 [DOI] [PubMed] [Google Scholar]
- 18.Yu PC, Calderaro D, Gualandro DM, et al. Non-cardiac surgery in developing countries: epidemiological aspects and economical opportunities—the case of Brazil. PLoS One 2010; 5: e10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015; 32: 88–105 [DOI] [PubMed] [Google Scholar]
- 20.Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol 2009; 62: 511–7.e1 [DOI] [PubMed] [Google Scholar]
- 21.World Bank open data. Available from http://data.worldbank.org/country (accessed 25th September 2016)
- 22.GlobalSurg Collaborative. Mortality of emergency abdominal surgery in high-, middle- and low-income countries. Br J Surg 2016; 103: 971–88 [DOI] [PubMed] [Google Scholar]
- 23.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005; 242: 326–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillies MA, Power GS, Harrison DA, et al. Regional variation in critical care provision and outcome after high-risk surgery. Intensive Care Med 2015; 41: 1809–16 [DOI] [PubMed] [Google Scholar]
- 25.Ozdemir BA, Sinha S, Karthikesalingam A, et al. Mortality of emergency general surgical patients and associations with hospital structures and processes. Br J Anaesth 2016; 116: 54–62 [DOI] [PubMed] [Google Scholar]
- 26.Wunsch H, Gershengorn HB, Cooke CR, et al. Use of intensive care services for Medicare beneficiaries undergoing major surgical procedures. Anesthesiology 2016; 124: 899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillies MA, Pearse RM. Intensive care after high-risk surgery: what’s in a name? Anesthesiology 2016; 124: 761–2 [DOI] [PubMed] [Google Scholar]
- 28.Silber JH, Rosenbaum PR, Schwartz JS, Ross RN, Williams SV. Evaluation of the complication rate as a measure of quality of care in coronary artery bypass graft surgery. JAMA 1995; 274: 317–23 [PubMed] [Google Scholar]
- 29.Silber JH, Rosenbaum PR, Williams SV, Ross RN, Schwartz JS. The relationship between choice of outcome measure and hospital rank in general surgical procedures: implications for quality assessment. Int J Qual Health Care 1997; 9: 193–200 [DOI] [PubMed] [Google Scholar]
- 30.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care 1992; 30: 615–29 [DOI] [PubMed] [Google Scholar]
- 31.Sinha S, Ata Ozdemir B, Khalid U, et al. Failure-to-rescue and interprovider comparisons after elective abdominal aortic aneurysm repair. Br J Surg 2014; 101: 1541–50 [DOI] [PubMed] [Google Scholar]
- 32.Jammer I, Ahmad T, Aldecoa C, et al. Point prevalence of surgical checklist use in Europe: relationship with hospital mortality. Br J Anaesth 2015; 114: 801–7 [DOI] [PubMed] [Google Scholar]