Laboratories should individually optimize procedures for blood culture (BC) collection, processing, analysis, and result reporting. Implementation of rapid BC diagnostics should be done with clinical decision support systems that maximize clinical benefits.
Keywords: blood culture, bacteremia
Abstract
Many strategies and technologies are available to improve blood culture (BC)–based diagnostics. The ideal approach to BCs varies between healthcare institutions. Institutions need to examine clinical needs and practices in order to optimize BC-based diagnostics for their site. Before laboratories consider offering rapid matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ToF MS) or expensive rapid panel-based molecular BC diagnostics, they should optimize preanalytical, analytical, and postanalytical processes and procedures surrounding BC systems. Several factors need to be considered, including local resistance rates, antibiotic prescribing patterns, patient- and provider-types, laboratory staffing, and personnel available to liaise with clinicians to optimize antibiotic use. While there is much excitement surrounding new high-technology diagnostics, cost-neutral benefits can be realized by optimizing existing strategies and using available tools in creative ways. Rapid BC diagnostics should be implemented in a manner that optimizes impact. Strategies to optimize these BC diagnostics in individual laboratories are presented here.
Automated continuous monitoring blood culture (BC) systems introduced in the last century, such as those from Becton, Dickinson and Company (New Jersey), bioMérieux (France), and Trek Diagnostic Systems (Ohio), have revolutionized BC diagnostics. Recently, new strategies and technologies have evolved for further improving BC-based diagnostics. The ideal approach, given the numerous available schemes, will vary between healthcare institutions; there is no universal strategy. It is challenging for laboratories to choose among numerous rapid BC diagnostics, including rapid panel-based diagnostics, that are currently available. In our opinion, attention should be devoted to optimizing processes and procedures to ensure quality BC practices prior to adopting new rapid BC-based diagnostics. BC approaches need to be individualized based on local resistance rates, antibiotic usage patterns, patient types, provider make-up, laboratory setups and routines, stewardship practices, and personnel available to liaise with clinicians to optimize antibiotic use and clinical outcomes. Although there is much excitement surrounding high-technology diagnostics, cost-neutral benefits may be realized by optimizing existing strategies using available tools. Several areas related to BC-based diagnostics are in need of clarification through future research.
Two tenets underscore BC-based diagnostics. The first is that early, effective treatment of bloodstream infection is ideal [1]. The second is that unnecessary antibiotic use (eg, treatment of contaminants or use of excessively broad-spectrum agents) results in excess cost [2] and/or potentially adverse outcomes, including toxicity, Clostridium difficile infection, and selection of resistance. Achieving these goals requires optimal BC use (Table 1). BCs should be performed on the right patient at the right time, ideally prior to administration of antibiotics. Many BCs are collected on patients who have received antibiotics [3]. It goes without saying that the sooner a BC is collected, the sooner positive results will be available. An adequate volume of blood needs to be collected. Ideally (for adults), that would be at least 40 mL divided into 2 sets [4], each via separate peripheral venipuncture (unless diagnosing catheter-associated bloodstream infection, in which case 1 peripheral and 1 catheter draw should be considered). Each set should include at least an aerobic and anaerobic bottle [5]. We have shown that collection of 2 aerobic and 1 anaerobic BC bottles per set (ie, 30 mL per set) results in improved yield compared with 2 bottles per set [5]. Single-set BCs should typically be avoided because they are insensitive and, if positive, do not allow contamination assessment, which may lead to downstream negative patient outcomes. In patients with positive BCs, follow-up BCs should only be performed as clinically indicated.
Table 1.
Pre- and Post-Analytical Areas for Optimization of Blood Cultures and Their Impact
| Category | Steps to be Optimized | Impact |
|---|---|---|
| BC collection | Collection of BCs prior to administration of antimicrobial agents and from patients for whom BCs are appropriate | Improves BC yield |
| Adequate blood volume per bottle | Improves BC yield | |
| Sufficient number of draws (2 or more from separate venipuncture sites); avoid single-draw BCs | Improves BC yield; allows discrimination of contamination | |
| Sufficient number of bottles (at least 1 aerobic and 1 anaerobic bottle) with each draw | Improves BC yield | |
| Transportation | Efficient transportation of BC bottles to the laboratory | Reduces time to positivity |
| Processing | Timely placement of BC bottles onto BC instrument | Reduces time to positivity |
| Prompt removal of positive BC bottles from the instrument and immediate workup of those bottles | Reduces time to organism identification | |
| Reporting | Prompt communication of positive results to clinicians | Reduces time to optimal therapy |
| Linking results to treatment guidance | Use of templated comments within BC result report (eg, NDM detected by polymerase chain reaction. This organism is resistant to carbapenems and other β-lactam antibiotics. Patient requires contact precautions if hospitalized. Consult infectious diseases.) | Reduces time to optimal therapy |
| Antimicrobial stewardship team notification for provision of treatment recommendations or direct microbiologist oversight of positive BCs with provision of treatment recommendations to clinical team by MD microbiologist | Reduces time to optimal therapy | |
| Use of electronic decision-support systems | Reduces time to optimal therapy | |
Abbreviations: BC, blood culture; NDM, New Delhi metallo-β-lactamase.
Clinical and Laboratory Standards Institute guidelines state that BC bottles should be sent to the laboratory within 2 hours of collection [4]; in our opinion, they should be placed onto BC instruments within this timeframe. A solution for laboratories that receive bottles from several hospitals is to place satellite, small- to medium-sized BC systems in respective hospitals. Quality improvement projects surrounding BC processes, from collection to reporting, can identify areas for improvement to enable more rapid reporting of positive BCs [6]. Using such an approach, quality improvement teams follow trajectories of BC bottles, from collection through transportation to the laboratory, from arrival in the laboratory through placement onto BC instruments, and finally, from removal from instruments, when positive, to reporting of positive results; a journey that involves multiple hand-offs and different personnel types (Table 1) [6]. By examining these processes and finding opportunities for improvement, the time between BC collection and results reporting can be decreased (by almost 3 hours, in our study). Such interventions are cost neutral, with effects maintained as the process changes that are executed become routine practice [6]. Of course, collection of BCs at off-site locations and timing of such collections as well as numbers of transports per day from off-site locations to centralized laboratories affect time from BC collection to placement of bottles onto instruments, as do laboratory work hours [7]. If trained microbiology laboratory staff is unavailable, systems that enable BCs to be loaded onto instruments, including use of nonmicrobiology staff or robotic loading, such as that available with the BacT/ALERT VIRTUO BC system (bioMérieux), may be helpful. Although these issues seem mundane, failure to optimize them can negatively impact conventional and new BC diagnostics and should be addressed as part of routine quality systems.
There may be differences in time to detection of positivity and organism detection rates with different BC bottle types and instruments. We showed that for anaerobic bacteria, BACTEC Lytic (Becton, Dickinson and Company) bottles had shorter detection times and better detection rates compared with BACTEC-Plus (Becton, Dickinson and Company) and BacT/ALERT-FN and -FN Plus (bioMérieux) bottles [8]. Similarly, we showed that in the presence of antifungals, the number of bottles positive for Candida species was higher using BacT/Alert FA compared with BACTEC Mycosis IC/F bottles (Becton, Dickinson and Company) [9]. Recently, we demonstrated that overall time to detection was approximately 20% shorter for bottles incubated on the BacT/ALERT VIRTUO BC system compared with the BacT/ALERT 3D system, although overall detection rates did not differ [10]. Future enhancements in BC bottle media and positivity detection mechanisms are likely to yield improvements. Current systems are based on detection of carbon dioxide production; by the time bottles flag positive, there is a relatively high concentration of organisms present. Given this, time to positivity can likely be decreased by detection of growth ahead of when bottles signal positive today. That this is possible is evidenced by a study we performed showing that the FilmArray Blood Culture Identification (BCID) Panel (BioFire Diagnostics, LLC) can detect bacteria in BC bottles before they signal positive [11]. Since it would be logistically challenging to interrogate bottles before they signal positive and would potentially risk the introduction of contaminants and since most bottles are negative, new systems that allow earlier “in-bottle” detection should be a priority for development. Such novel approaches may detect organisms ahead of Gram stain positivity.
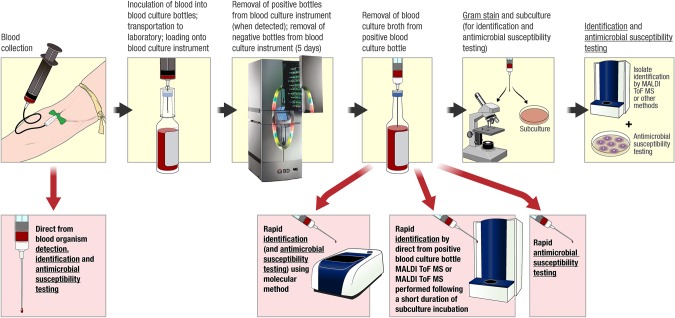
Once bottles signal positive on a BC instrument, they must be removed from the instrument, worked up, and results reported (Figure 1); faster results will impact patient care. The College of American pathologists requires twice daily examination of bottles during the first 48 hours of incubation and daily monitoring thereafter. This may seem insufficient but is affected by hours that staff is in the laboratory. Some laboratories remove and work up positive BC bottles in real time (ie, as they become positive), others do so at regular intervals (eg, hourly), and yet others vary practices depending on times of the day. There may be no removal of positive BC bottles at night or on weekends. Laboratories should examine strategies to optimize workflow in order to decrease turnaround time to reporting positive BCs. If this is done, systems need to be in place to handle associated results. We have shown that frequent, scheduled monitoring of BC instruments decreases turnaround time to reporting positive BCs [6]. Work-up of new positive bottles should be prioritized, and perhaps, accrediting agencies should revisit requirements for frequency of monitoring of BC instruments. In addition, staffing limitations present an opportunity to develop new technologies that automatically detect and report results of positive BC bottles.
Figure 1.
Overview of the process of blood collection for culture, inoculation of blood into blood culture (BC) bottles, loading of bottles onto a BC instrument, removal of positive bottles from the instrument when the bottle signals positive, and work-up of positive bottles with Gram stain, subculture for identification and antimicrobial susceptibility testing, and rapid BC-based diagnostic testing. Rapid methods are highlighted with red arrows and background toward the bottom of the figure. Underlined text indicates clinical reporting opportunities.
Once a positive BC bottle is removed from the instrument, the contents are gram stained and the clinician notified of results. Historically, further information would not be forthcoming until a day or more later because the bottle would be subcultured with growth worked up for identification and antimicrobial susceptibility testing (Figure 1). Today, instead of just receiving a Gram stain morphology report when a BC bottle signals positive and having to wait a day or more for identification of the organism(s) involved, microbial identification and detection of selected antibacterial resistance genes can be reported in a 1- to 4-hour time frame (Figure 1). However, not all such new approaches are cleared by the US Food and Drug Administration (FDA).
Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-ToF MS) is available in many clinical microbiology laboratories and is FDA cleared for microorganism identification from colonies on plates (Figure 1). Although BC bottles may be tested “directly” using MALDI-ToF MS (Figure 1), this strategy is not FDA cleared. Also, because the bottles contain macromolecules from blood and growth media, testing requires preparatory processing that involves the use of differential centrifugation and washings, selective lysis of blood cells, serum separator tubes, or filtration. Commercial processing is available using the Sepsityper (Bruker Daltonics, Germany). Although results are valid when obtained, yield is generally not as good from BC bottles compared with direct colony testing, with more gram-negative than gram-positive organisms typically identified [12]. In addition, not all organisms present in polymicrobial infections will be detected. MALDI-ToF MS performed directly on positive BC bottles is rapid (approximately 30–45 minutes) but, due to required processing steps, is not as fast as conventional direct colony testing. Furthermore, such testing is typically batched, impacting turnaround time. For this reason and because it involves less work for the laboratory, the strategy of subculture to solid media with testing of early growth after a short period of incubation (ie, before distinct colony formation) is becoming popular (Figure 1), although again, not FDA cleared. A current limitation of MALDI-ToF MS is that it does not provide antimicrobial susceptibility data. To overcome this, many laboratories are using or exploring strategies to more rapidly provide phenotypic susceptibility testing through the use of available technology (Figure 1). Examples include going straight from positive BC bottles into currently available automated susceptibility instruments that can also provide identification (a non–FDA-cleared approach) [13] or placing antibiotic disks directly onto subculture plates from positive BC bottles alongside MALDI-ToF MS–based identification performed on subculture plates after a short duration of incubation [14].
Another strategy is to apply molecular methods to positive bottles (Figure 1). Two broad molecular panels are FDA cleared for testing positive bottles (Table 2), the BCID, which includes gram-positive and gram-negative bacteria as well as yeast in a single panel, and the Verigene Gram-Positive Blood Culture Test (BC-GP) and Gram-Negative Blood Culture Test (BC-GN) (Nanosphere, Inc.), which are separate panels for gram-positive and gram-negative bacteria. Cepheid (California) offers an assay, Xpert MRSA/SA Blood Culture, for rapid detection of methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MRSA) in positive BC bottles. Peptide nucleic acid fluorescence in situ hybridization (FISH) assays from AdvanDx, Inc. (Massachusetts) allow detection of a limited number of organism types as well as mecA. Prove-it Sepsis (Mobidiag, Finland) and the Unyvero BCU Application (Curetis, Germany) are broad panels available in Europe. These assays provide identification and detect select antibacterial resistance genes in 1 to 4 hours. They are easy to use but costly. Clinician ordering of these molecular tests is not practical because of associated delays, so laboratories must decide whether and when to use these “add-on” tests. For example, if they were used, only the first positive bottle from a patient in a certain period would warrant testing.
Table 2.
Molecular Tests for Positive Blood Culture Bottles
| Gram-Positive Bacteria | Gram-Negative Bacteria | Candida Species |
|---|---|---|
| Xpert MRSA/SA Blood Culture (Cepheid, Sunnyvale, California) | ||
| Staphylococcus aureus | ||
| Resistance Gene mecA |
||
| FilmArray Blood Culture Identification (BCID) Panel (BioFire Diagnostics, LLC, Salt Lake City, Utah) | ||
|
Staphylococcus species Staphylococcus aureus Streptococcus species Streptococcus agalactiae Streptococcus pyogenes Streptococcus pneumoniae Enterococcus species Listeria monocytogenes |
Klebsiella oxytoca Klebsiella pneumoniae Serratia species Proteus species Acinetobacter baumannii Haemophilus influenzae Neisseria meningitidis Pseudomonas aeruginosa Enterobacteriaceae Escherichia coli Enterobacter cloacae complex |
Candida albicans Candida glabrata Candida krusei Candida parapsilosis Candida tropicalis |
| Resistance Genes mecA vanA/vanB |
Resistance Gene blaKPC |
|
| Verigene (Nanosphere, Inc, Northbrook, Illinois) | ||
| Gram-Positive Blood Culture Test (BC-GP) | Gram-Negative Blood Culture Test (BC-GN) | |
|
Staphylococcus aureus Staphylococcus epidermidis Staphylococcus lugdunensis Streptococcus anginosus group Streptococcus agalactiae Streptococcus pneumoniae Streptococcus pyogenes Enterococcus faecalis Staphylococcus species Streptococcus species Listeria species |
Escherichia coli Klebsiella pneumoniae Klebsiella oxytoca Pseudomonas aeruginosa Acinetobacter species Citrobacter species Enterobacter species Proteus species |
|
| Resistance Genes mecA vanA vanB |
Resistance Genes blaNDM blaKPC blaOXA blaVIM blaCTX-M |
|
| QuickFISH (AdvanDx, Inc., Woburn, Massachusetts) | ||
|
Staphylococcus QuickFISH Staphylococcus aureus Coagulase negative Staphylococcus species |
Gram-Negative QuickFISH Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa |
Candida QuickFISH Candida albicans Candida parapsilosis Candida glabrata |
|
Enterococcus QuickFISH Enterococcus faecalis Enterococcus faecium or other non-faecalis Enterococcus |
||
| Resistance Gene mecA XpressFISH mecA |
||
| Prove-it Sepsis (Mobidiag, Helsinki, Finland)a | ||
|
Clostridium perfringens Enterococcus casseliflavus Enterococcus faecalis Enterococcus faecium Enterococcus gallinarum Listeria monocytogenes Propionibacterium acnes Staphylococcus aureus Staphylococcus epidermidis Streptococcus agalactiae Streptococcus dysgalactiae subspecies equisimilis Streptococcus pneumoniae Streptococcus pyogenes Coagulase negative Staphylococcus |
Acinetobacter baumannii Enterobacter aerogenes Enterobacter cloacae Escherichia coli Haemophilus influenzae Kingella kingae Klebsiella oxytoca Klebsiella pneumoniae Neisseria meningitidis Proteus mirabilis Proteus vulgaris Pseudomonas aeruginosa Salmonella enterica subspecies enterica Serratia marcescens Stenotrophomonas maltophilia Bacteroides fragilis group Campylobacter jejuni/coli Enterobacteriaceae Neisseria sp. non-meningitidis |
Candida albicans Candida dubliniensis Candida glabrata Candida guilliermondii Candida krusei Candida lusitaniae Candida parapsilosis Candida tropicalis Pan-yeast |
| Resistance Genes mecA vanA vanB |
||
| Unyvero BCU Application (Curetis, Holzgerlingen, Germany)a | ||
|
Staphylococcus aureus Coagulase negative staphylococci Streptococcus species Streptococcus agalactiae Streptococcus pneumoniae Streptococcus pyogenes/dysgalactiae Enterococcus species Enterococcus faecalis Listeria monocytogenes Corynebacterium species Propionibacterium acnes Mycobacterium species |
Citrobacter freundii/koseri Escherichia coli Enterobacter cloacae complex Enterobacter aerogenes Klebsiella oxytoca Klebsiella pneumoniae Klebsiella variicola Proteus species Serratia marcescens Acinetobacter baumannii complex Pseudomonas aeruginosa Stenotrophomonas maltophilia Haemophilus influenzae Neisseria meningitidis |
Aspergillus species Candida species Candida albicans Candida dubliniensis Candida glabrata Candida krusei Candida parapsilosis Candida tropicalis |
| Resistance Genes vanA vanB mecA mecC ermA |
Resistance Genes aac(6′)aph(2″) aacA4 blaNDM blaKPC blaVIM blaCTX-M blaIMP blaOXA-23 blaOXA-24/40 blaOXA-48 blaOXA-58 |
|
a Available in Europe but not US Food and Drug Administration cleared.
The impact of rapid BC diagnostics (eg, MALDI-ToF MS, molecular diagnostics) on clinical and economic outcomes has been difficult to demonstrate, with mixed results reported (Supplementary Table 1). Clinical impact depends on multiple institution-specific factors, including local pathogen resistance rates, patient populations, antimicrobial prescribing practices, and antimicrobial stewardship program characteristics. Several studies have demonstrated decreased time to optimal therapy with rapid diagnostics (Supplementary Table 1). While some have reported rapid diagnostics to be associated with decreased length of stay [15–22], lower mortality [15, 16, 21–25], and reduced cost [15, 17, 21, 22, 24, 26, 27], others, including the only randomized controlled trial [28], have reported no such benefit compared with conventional subculture and susceptibility testing [29–31]. Study design is an important consideration as use of historical controls may introduce bias.
Finding ways to integrate rapid diagnostics into clinical care and link results to treatment is a challenge to be addressed by individual institutions. Methods to communicate results to clinicians include telephone, email, text page, and electronic medical record–associated provider notification. Linking rapid test results to treatment guidance can occur through a variety of clinical decision-support strategies, including placing prescribing recommendations within the microbiology report, using audit and feedback by antimicrobial stewardship teams, and using more sophisticated computerized systems within electronic health records platforms that integrate clinical data, medication orders, and microbiology results to suggest appropriate antiinfective therapy or alert clinicians to modify suboptimal therapy [32–34] (Supplementary Table 1).
Evidence, largely from observational studies, suggests that rapid testing together with stewardship interventions provide more favorable outcomes than rapid testing alone [13, 15, 16, 18, 21, 23, 24, 26, 27, 29, 35–38] (Supplementary Table 1). In the only prospective randomized controlled trial to evaluate methods of test implementation, we found that antibiotic escalation was faster when rapid testing was implemented with templated comments in the microbiology report or with antimicrobial stewardship guidance [28]. However, antibiotic de-escalation was only enhanced when rapid testing was done together with antimicrobial stewardship intervention [28]. Our opinion is that reporting comments with prescribing guidance can enhance appropriate antibiotic use for straightforward scenarios, such as addition of vancomycin when a blood isolate is identified as MRSA or discontinuation of antibiotics when Staphylococcus epidermidis is detected and deemed a likely contaminant. In contrast, stewardship involvement is helpful for more complicated scenarios, such as antibiotic de-escalation in critically ill or immunocompromised patients. Stewardship teams can also help interpret reports of resistance gene detection, which may be unfamiliar to clinicians. It is not clear how best to coordinate rapid BC diagnostic testing and result reporting with antimicrobial stewardship activities. This depends on institutional stewardship practices. Some stewardship programs are notified of all positive BCs and perform audit and feedback on all patients with bacteremia. Programs with minimal funding or personnel may only be able to review select patients with bacteremia, such as those who have S. aureus bacteremia [39]. Decentralized stewardship is another possibility, especially to cover smaller hospitals that are part of a large healthcare system. Another strategy, common in Europe, is to have clinical microbiologists with a medical degree directly involved in antimicrobial stewardship, notifying clinicians of positive results as they are identified, while simultaneously providing treatment guidance. An important consideration is how to implement rapid diagnostic testing during off-hours and on weekends when stewardship interventionists may be unavailable. Automated, electronic clinical decision-support systems that provide clinicians with rapid test result interpretation and treatment guidance may be especially useful during off-hours. Although few studies are performed in such settings, rapid, easy-to-use, panel-based diagnostics may be particularly useful for small hospitals that lack expert microbiology staff and that may struggle with reading Gram stains from positive BC bottles.
Clinical microbiology laboratories need to select BC diagnostics based not only on test accuracy, turn-around-time, and work flow implications, but also on local resistance rates and mechanisms. Resistance mechanisms that are represented on molecular platforms must be fairly prevalent for such tests to be useful. For example, in regions of Sweden with extremely low MRSA rates, mecA detection would not add to clinical management of patients more so than rapid S. aureus identification, without susceptibility information. In contrast, in regions with high MRSA prevalence, rapid mecA detection can impact antimicrobial prescribing. In our prospective randomized controlled trial we found that use of the BCID panel led to more judicious antimicrobial use for treatment of gram-positive bacteremia but did not impact antimicrobial management of patients with gram-negative bacteremia. The sole gram-negative resistance determinant interrogated, blaKPC, was not identified in our study [28]. Current molecular platforms detect a limited number of resistance genes. Thus, for bloodstream infection with gram-negative bacteria, which can harbor multiple resistance mechanisms, these tests are helpful for escalating therapy when resistance is detected, but may not provide sufficient information to “rule out” resistance enabling antibiotic de-escalation. In institutions where broad-spectrum agents are commonly prescribed as empiric therapy for sepsis, antibiotic de-escalation is unlikely to be enhanced if available molecular platforms are used without additional stewardship interventions. Even so, due to potential resistance not identified by these platforms (eg, extended-spectrum β-lactamase or plasmid-mediated AmpC production with a concomitant porin mutation conferring carbapenem resistance), there may be reluctance to de-escalate. In this regard, rapid phenotypic susceptibility methods currently in development may have more clinical utility. In contrast, for gram-positive bacteria, currently available molecular platforms detect common resistance determinants such as mecA, vanA, and vanB and generally provide sufficient information to support antibiotic escalation or de-escalation. Technologies that provide early identification of organisms but do not provide antimicrobial susceptibility results (eg, MALDI-ToF MS) may not significantly impact antimicrobial prescribing, except when species identification predicts antimicrobial susceptibility (eg, Streptococcus pyogenes, Listeria monocytogenes) or possible contamination (eg, coagulase-negative Staphylococcus species), resistance rates are low (eg, low MRSA rates in Sweden), or patients are already known to harbor pathogens with specific susceptibility profiles (eg, in urine) that are subsequently detected in blood.
Several novel approaches are under development for BC-based diagnostics. A technology using a growth sensing strategy that detects volatiles outgassed by growing organisms, with the goal of not only earlier detection of positive results but “in-bottle” microbial identification, is being developed [40]. Several companies are developing automated systems for rapid phenotypic susceptibility testing that rapidly assess growth with and without antimicrobial agents using innovative strategies such as high-resolution imaging [41], individual cell mass measurement, and other sophisticated cell-counting methods. Given that BCs are typically incubated for 5 days before being resulted as negative, another strategy is to deploy methods for faster finalization of negative BCs. Finally, direct detection of bacteria in blood is under development, with no method currently FDA cleared. Future studies are needed to assess not only accuracy but also clinical usefulness of these approaches.
Semantics are a challenge with new diagnostics. As new techniques are adopted alongside conventional methods, clinicians may see a mixture of old and new tests being applied to the same patient to identify and perform susceptibility testing on a pathogen that is causing their infection, especially if the patient has multiple positive cultures (eg, blood and urine “isolates”). There may be differences in nomenclature between systems, resulting in the same organism being identified differently (eg, Proteus species vs Proteus mirabilis), and antimicrobial susceptibility panels may vary between systems.
In conclusion, novel platforms for rapid pathogen identification and antimicrobial susceptibility testing from BCs are available or in development and have potential to improve treatment and outcomes of bacteremia. However, before costly new diagnostics are routinely used, their impact on clinical outcomes and cost of care must be evaluated and strategies to optimally integrate testing into clinical practice developed. The optimal diagnostic test or implementation strategy needs to be individualized for each institution, based on unique microbiology, local prevalence of resistance mechanisms, antimicrobial prescribing patterns, and antimicrobial stewardship activities. Before implementing costly add-on tests, institutions can put in place process improvements to idealize collection of blood for culture, more rapidly place bottles on BC instruments, and more rapidly remove positive bottles from instruments, work them up and report results to clinicians. In our opinion, given that there is no single standard or universal strategy, it is imperative that healthcare institutions evaluate and optimize the systems that they have in place.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. The authors thank the Antimicrobial Resistance Leadership Group of the National Institutes of Health (UM1 AI104681) and the Mayo Clinic–Karolinska Institutet Collaboration.
Potential conflicts of interest. R. P. reports grants from BioFire, Check-Points, Curetis, 3M, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, Allergan, and the Medicines Company. R. P. is a consultant to Curetis, Roche, Qvella, and Diaxonhit; monies are paid to Mayo Clinic. In addition, R. P. has a patent on Bordetella pertussis/parapertussis polymerase chain reaction issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an antibiofilm substance issued. R. P. serves on an Actelion data monitoring board. R. P. receives travel reimbursement and an editor's stipend from the American Society of Microbiology and the Infectious Diseases Society of America, and honoraria from the National Board of Medical Examiners, Up-to-Date, and the Infectious Diseases Board Review Course. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kumar A, Roberts D, Wood KE et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 2.Roberts RR, Hota B, Ahmad I et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin Infect Dis 2009; 49:1175–84. [DOI] [PubMed] [Google Scholar]
- 3.Zadroga R, Williams DN, Gottschall R et al. Comparison of 2 blood culture media shows significant differences in bacterial recovery for patients on antimicrobial therapy. Clin Infect Dis 2013; 56:790–7. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Principles and Procedures for Blood Cultures; Approved Guideline Wayne, PA, 2007. [Google Scholar]
- 5.Patel R, Vetter EA, Harmsen WS, Schleck CD, Fadel HJ, Cockerill FR III. Optimized pathogen detection with 30- compared to 20-milliliter blood culture draws. J Clin Microbiol 2011; 49:4047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contezac J, Monson Jobe K, Gebrehiwot S et al. Improving blood culture (BC) turnaround time—A quality improvement project In: American Society for Microbiology General Meeting. Denver, CO, 2013. [Google Scholar]
- 7.Ronnberg C, Mildh M, Ullberg M, Özenci V. Transport time for blood culture bottles: underlying factors and its consequences. Diagn Microbiol Infect Dis 2013; 76:286–90. [DOI] [PubMed] [Google Scholar]
- 8.Almuhayawi M, Altun O, Abdulmajeed AD, Ullberg M, Özenci V. The performance of the four anaerobic blood culture bottles BacT/ALERT-FN, -FN Plus, BACTEC-Plus and -Lytic in detection of anaerobic bacteria and identification by direct MALDI-TOF MS. PLoS One 2015; 10:e0142398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ericson EL, Klingspor L, Ullberg M, Özenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis 2012; 73:153–6. [DOI] [PubMed] [Google Scholar]
- 10.Altun O, Almuhayawi M, Luthje P, Taha R, Ullberg M, Özenci V. Controlled evaluation of the new BacT/Alert Virtuo blood culture system for detection and time to detection of bacteria and yeasts. J Clin Microbiol 2016; 54:1148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almuhayawi M, Altun O, Stralin K, Özenci V. Identification of microorganisms by FilmArray and matrix-assisted laser desorption ionization-time of flight mass spectrometry prior to positivity in the blood culture system. J Clin Microbiol 2014; 52:3230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saffert RT, Cunningham SA, Mandrekar J, Patel R. Comparison of three preparatory methods for detection of bacteremia by MALDI-TOF mass spectrometry. Diagn Microbiol Infect Dis 2012; 73:21–6. [DOI] [PubMed] [Google Scholar]
- 13.Perez KK, Olsen RJ, Musick WL et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant gram-negative bacteremia. J Infect 2014; 69:216–25. [DOI] [PubMed] [Google Scholar]
- 14.Altun O, Botero-Kleiven S, Carlsson S, Ullberg M, Özenci V. Rapid identification of bacteria from positive blood culture bottles by MALDI-TOF MS following short-term incubation on solid media. J Med Microbiol 2015; 64:1346–52. [DOI] [PubMed] [Google Scholar]
- 15.Bauer KA, West JE, Balada-Llasat JM, Pancholi P, Stevenson KB, Goff DA. An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis 2010; 51:1074–80. [DOI] [PubMed] [Google Scholar]
- 16.Huang AM, Newton D, Kunapuli A et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57:1237–45. [DOI] [PubMed] [Google Scholar]
- 17.Sango A, McCarter YS, Johnson D, Ferreira J, Guzman N, Jankowski CA. Stewardship approach for optimizing antimicrobial therapy through use of a rapid microarray assay on blood cultures positive for Enterococcus species. J Clin Microbiol 2013; 51:4008–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen DT, Yeh E, Perry S et al. Real-time PCR testing for mecA reduces vancomycin usage and length of hospitalization for patients infected with methicillin-sensitive staphylococci. J Clin Microbiol 2010; 48:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker T, Dumadag S, Lee CJ et al. Clinical impact of laboratory implementation of Verigene BC-GN microarray-based assay for detection of Gram-negative bacteria in positive blood cultures. J Clin Microbiol 2016; 54:1789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Box MJ, Sullivan EL, Ortwine KN et al. Outcomes of rapid identification for gram-positive bacteremia in combination with antibiotic stewardship at a community-based hospital system. Pharmacotherapy 2015; 35:269–76. [DOI] [PubMed] [Google Scholar]
- 21.Perez KK, Olsen RJ, Musick WL et al. Integrating rapid pathogen identification and antimicrobial stewardship significantly decreases hospital costs. Arch Pathol Lab Med 2013; 137:1247–54. [DOI] [PubMed] [Google Scholar]
- 22.Pardo J, Klinker KP, Borgert SJ, Butler BM, Giglio PG, Rand KH. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn Microbiol Infect Dis 2016; 84:159–64. [DOI] [PubMed] [Google Scholar]
- 23.Forrest GN, Roghmann MC, Toombs LS et al. Peptide nucleic acid fluorescent in situ hybridization for hospital-acquired enterococcal bacteremia: delivering earlier effective antimicrobial therapy. Antimicrob Agents Chemother 2008; 52:3558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ly T, Gulia J, Pyrgos V, Waga M, Shoham S. Impact upon clinical outcomes of translation of PNA FISH-generated laboratory data from the clinical microbiology bench to bedside in real time. Ther Clin Risk Manag 2008; 4:637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki H, Hitomi S, Yaguchi Y et al. Prospective intervention study with a microarray-based, multiplexed, automated molecular diagnosis instrument (Verigene system) for the rapid diagnosis of bloodstream infections, and its impact on the clinical outcomes. J Infect Chemother 2015; 21:849–56. [DOI] [PubMed] [Google Scholar]
- 26.Forrest GN, Mankes K, Jabra-Rizk MA et al. Peptide nucleic acid fluorescence in situ hybridization-based identification of Candida albicans and its impact on mortality and antifungal therapy costs. J Clin Microbiol 2006; 44:3381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forrest GN, Mehta S, Weekes E, Lincalis DP, Johnson JK, Venezia RA. Impact of rapid in situ hybridization testing on coagulase-negative staphylococci positive blood cultures. J Antimicrob Chemother 2006; 58:154–8. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee R, Teng CB, Cunningham SA et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holtzman C, Whitney D, Barlam T, Miller NS. Assessment of impact of peptide nucleic acid fluorescence in situ hybridization for rapid identification of coagulase-negative staphylococci in the absence of antimicrobial stewardship intervention. J Clin Microbiol 2011; 49:1581–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malcolmson C, Ng K, Hughes S et al. Impact of matrix-assisted laser desorption and ionization time-of-flight and antimicrobial stewardship intervention on treatment of bloodstream infections in hospitalized children. J Pediatric Infect Dis Soc 2016; doi:10.1093/jpids/piw033. [DOI] [PubMed] [Google Scholar]
- 31.Beal SG, Thomas C, Dhiman N et al. Antibiotic utilization improvement with the Nanosphere Verigene gram-positive blood culture assay. Proc (Bayl Univ Med Cent) 2015; 28:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schurink CA, Lucas PJ, Hoepelman IM, Bonten MJ. Computer-assisted decision support for the diagnosis and treatment of infectious diseases in intensive care units. Lancet Infect Dis 2005; 5:305–12. [DOI] [PubMed] [Google Scholar]
- 33.Evans RS, Pestotnik SL, Classen DC et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med 1998; 338:232–8. [DOI] [PubMed] [Google Scholar]
- 34.Forrest GN, Van Schooneveld TC, Kullar R, Schulz LT, Duong P, Postelnick M. Use of electronic health records and clinical decision support systems for antimicrobial stewardship. Clin Infect Dis 2014; 59(suppl 3):S122–33. [DOI] [PubMed] [Google Scholar]
- 35.Barenfanger J, Drake C, Kacich G. Clinical and financial benefits of rapid bacterial identification and antimicrobial susceptibility testing. J Clin Microbiol 1999; 37:1415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carver PL, Lin SW, DePestel DD, Newton DW. Impact of mecA gene testing and intervention by infectious disease clinical pharmacists on time to optimal antimicrobial therapy for Staphylococcus aureus bacteremia at a university hospital. J Clin Microbiol 2008; 46:2381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clerc O, Prod'hom G, Vogne C, Bizzini A, Calandra T, Greub G. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with gram-negative bacteremia: a prospective observational study. Clin Infect Dis 2013; 56:1101–7. [DOI] [PubMed] [Google Scholar]
- 38.Frye AM, Baker CA, Rustvold DL et al. Clinical impact of a real-time PCR assay for rapid identification of staphylococcal bacteremia. J Clin Microbiol 2012; 50:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fowler VG Jr, Sanders LL, Sexton DJ et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–86. [DOI] [PubMed] [Google Scholar]
- 40.Lim SH, Mix S, Xu Z et al. Colorimetric sensor array allows fast detection and simultaneous identification of sepsis-causing bacteria in spiked blood culture. J Clin Microbiol 2014; 52:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price CS, Kon SE, Metzger S. Rapid antibiotic susceptibility phenotypic characterization of Staphylococcus aureus using automated microscopy of small numbers of cells. J Microbiol Methods 2014; 98:50–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.