Abstract
Omega-3 polyunsaturated fatty acids (n-3 PUFAs) found in fish protect against cardiovascular morbidity and mortality; however, many individuals avoid fish consumption due to concerns about pollutants. We tested the hypothesis that n-3 PUFAs would prevent vascular dysfunction induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). C57Bl/6 male mice were fed a chow or n-3 PUFA diet for 10 weeks and were exposed to vehicle or 300 ng/kg/d TCDD during the final 2 weeks on each diet. Aortic vasoconstriction mediated by arachidonic acid (AA) ± SKF525 (P450 inhibitor) or SQ29548 (thromboxane/prostanoid [TP] receptor antagonist) was assessed. RBC fatty acids and expression of n-3 and n-6 PUFA metabolites were analyzed. Cytochrome P4501A1 (CYP1A1), CYP1B1, and aryl hydrocarbon receptor (AHR) expression was measured. TCDD significantly increased AA-mediated vasoconstriction on a chow diet by increasing the contribution of P450s and TP receptor to the constriction response. In contrast, the n-3 PUFA diet prevented the TCDD-induced increase in AA vasoconstriction and normalized the contribution of P450s and TP receptor. Although TCDD increased the levels of AA vasoconstrictors on the chow diet, this increase was prevent by the n-3 PUFA diet. Additionally, the n-3 PUFA diet significantly increased the levels of n-3 PUFA-derived vasodilators and TCDD increased these levels further. Interestingly, the n-3 PUFA diet significantly attenuated CYP1A1 induction by TCDD without a significant effect on AHR expression. These data suggest that n-3 PUFAs can prevent TCDD-induced vascular dysfunction by decreasing vasoconstrictors, increasing vasodilators, and attenuating CYP1A1 induction, which has been shown previously to contribute to TCDD-induced vascular dysfunction.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; aryl hydrocarbon receptor; omega-3 polyunsaturated fatty acids; cytochrome P4501A1; vasoconstriction; arachidonic acid; 20-HETE
Both prospective cohort studies and randomized clinical trials have shown that omega-3 polyunsaturated fatty acids (n-3 PUFAs), specifically eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), protect against cardiovascular morbidity and mortality, including reducing cardiac events (Kris-Etherton et al., 2002), decreasing the progression of atherosclerosis, and reducing hypertension (Morris et al., 1993; Toft et al., 1995). As a result of this evidence the American Heart Association recommends that all individuals consume an average of 0.5–1 g of EPA + DHA daily (Kris-Etherton et al., 2002). Despite these recommendations, intake of EPA + DHA in the U.S. averages 0.1–0.2 g/d (Kris-Etherton et al., 2000).
Dietary n-3 PUFA intake in the United States is low, in part, due to concern about environmental pollutants accumulated in fish. In general, consumer’s awareness of the potential risk posed by pollutants is higher than their awareness of the benefits of fish consumption (Verbeke et al., 2005). All 50 U.S. states as well as the U.S. Environmental Protection Agency, and Food and Drug Administration issue separate advisories on fish consumption due to the presence of these pollutants. These advisories are often complex and conflicting, leading to confusion of the public and health care providers. This confusion is further compounded by a limited number of studies that have investigated health risks versus benefits of fish consumption. Although a few studies have attempted to estimate a risk-benefit ratio using probabilistic models (Sioen et al., 2008a,b,c), no studies have experimentally investigated the interaction of n-3 PUFAs and environmental pollutants on a defined clinical health outcome.
Halogenated aromatic hydrocarbons, exemplified by polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls, represent one class of persistent environmental contaminants that accumulates in fish. Our previous research demonstrates that exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), the most potent and prototypical halogenated aromatic hydrocarbon, induces vascular dysfunction, hypertension, and cardiac hypertrophy via induction of cytochrome P4501A1 (CYP1A1) (Kopf et al., 2010a). Specifically, we show that the TCDD-induced vascular dysfunction is mediated by a loss of vasodilation via increased oxidative stress. Recently, however, it has been shown that TCDD also increases cytochrome P450-dependent metabolites of arachidonic acid (AA, 20:4n-6) that lead to vasoconstriction (Bui et al., 2012; Hankinson 2016; Yang et al., 2013). The potential impact of these vasoconstrictors to TCDD-induced vascular dysfunction has not been investigated.
This study was designed to investigate the degree to which TCDD induces vascular dysfunction via AA-generated vasoconstrictors and elucidate the ability of n-3 PUFAs to prevent this toxic response. Although studies have suggested that n-3 PUFAs might protect against toxicity of halogenated aromatic hydrocarbon pollutants in cultured endothelial cells and hepatocytes (Majkova et al., 2011; Turkez et al., 2011b), no studies have investigated the potential for n-3 PUFAs to protect against pollutant-induced cardiovascular disease in vivo. Thus, we tested the hypothesis that a diet enriched with n-3 PUFAs would prevent TCDD-induced vascular dysfunction.
MATERIALS AND METHODS
Chemicals
AA, SKS525, SQ29548, and 20-hydroxyeicosatetraenoic acid (20-HETE) were purchased from Cayman Chemical (Ann Arbor, Michigan). Potassium chloride (KCl), acetylcholine, phenylephrine and all ingredients of physiological saline and homogenation buffer were purchased from Sigma‐Aldrich (St Louis, Missouri). TCDD was a gift from Dr Richard E. Peterson (University of Wisconsin-Madison).
Diets
Standard mouse chow (2020×, Teklad Diets, Harlan Laboratories, Madison, Wisconsin) was prepared using soybean oil (65 g oil/kg diet), while the n-3 PUFA diet was prepared using menhaden fish oil (TD.110516; 150 g oil/kg diet, Teklad Diets). The nutrient and fatty acid composition of each diet is shown in Table 1. As expected n-3 PUFA fatty acids were highly enriched in the n-3 PUFA diet (36% of total fatty acids vs 8% in chow diet) and the n-6 PUFA fatty acids were greatly reduced (4.1% vs 53% in chow diet). In addition, the n-3 PUFA diet contained more total fat by weight, a higher percentage of saturated fatty acids, and slightly higher Kcal/g than the standard chow diet. The protein and carbohydrate composition of the 2 diets were similar.
TABLE 1.
Nutrient and Fatty Acid Composition, and Calories of Chow and n-3 PUFA-Enriched Diets
| Composition | Standard Chow (2020×) | n-3 PUFA Diet (TD.110516) |
|---|---|---|
| Proteina | 19.1 | 18.6 |
| Carbohydratesa | 47.0 | 52.2 |
| Total fata | 6.5 | 15.2 |
| Fatty acidsb | ||
| Saturated | 15.0 | 28.1 |
| Monounsaturated | 23.4 | 22.7 |
| n-3 PUFA | ||
| ALA (18:3) | 8.0 | 1.8 |
| Stearidonic acid (SDA; 18:4) | 0 | 3.5 |
| Eicosapentaenoic acid (EPA; 20:5) | 0 | 16.0 |
| Docosapentaenoic acid (DPA; 22:5) | 0 | 3.9 |
| Docosahexaenoic acid (DHA; 22:6) | 0 | 10.8 |
| n-6 PUFA | ||
| Linolenic acid (LA; 18:2) | 53.0 | 1.8 |
| AA (20:4) | 0 | 2.3 |
| Kcal/g | 3.1 | 4.2 |
aPercent by weight.
bPercent of total fatty acids.
Animals
C57BL/6J male mice were housed in a temperature-controlled environment and fed standard mouse chow. At 3 months of age, mice were continued on standard mouse chow or were fed an n-3 PUFA-enriched chow diet for ten weeks as described previously in Agbor et al. (2014). After 8 weeks on the respective diets, mice were randomly assigned to receive dough pills (Bio-Serv, Frenchtown, New Jersey) containing vehicle (1,4-p-dioxane) or 300 ng/kg/d TCDD for 2 weeks, as described previously in Walker et al. (2012). When tissues were harvested, mice were anesthetized with a single intraperitoneal injection of ketamine/xylazine (80/4 mg/kg) and euthanized by exsanguination. Packed RBCs were collected for analysis of fatty acid composition (OmegaQuant, Souix Falls, South Dakota). Tissues were weighed and flash frozen. All animal protocols were approved by the University of New Mexico Animal Care and Use Committee (no. 100849) and the investigations conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85‐23, revised 1996).
Analysis of Aortic Vasoreactivity
Aortas were cleaned in ice cold physiological saline (130 mM NaCl, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4, 14.9 mM NaHCO3, 5.5 mM glucose, 26 µM CaNa2EDTA, 1.8 mM CaCl2, pH 7.4), cut into 3 mm segments and rings mounted in a wire myograph (Radnoti Glass Technology Inc., Monrovia, California) attached to a force transducer (Grass Technologies, West Warwick, Rhode Island). Vessel viability was confirmed by constriction to 100 mM KCl. After a 30 min wash and phenylephrine (10−5 M) constriction, endothelial viability was confirmed by minimum of 50% acetylcholine-mediated dilation (10−5 M). To assess responses to AA, dose-dependent vasoconstriction (10−5–10−9 M) was recorded prior to and after 30 min incubation with either SKF525 (25 µM) or SQ29548 (100 nM). To assess responses to 20-HETE, constriction to a single dose (10−5.4 M) was recorded prior to and after 30 min incubation with SQ29548 (100 nM).
Analysis of Gene Expression
Total RNA was isolated from the liver, kidney, aorta, heart, and lung using RNeasy kit (Qiagen, GmbH, Germany). cDNA was synthesized using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California) with the supplied random primers and 250 ng RNA. PCR amplification was performed using an iCycler (Bio-Rad Laboratories) with a reaction mixture comprised of iQ SYBR Green Supermix (Bio-Rad Laboratories) with 500 nM CYP1A1, CYP1B1, CYP4a12, prostaglandin-endoperoxide synthase 2 (PTGS2, cyclooxygenase 2) or RNA polymerase II (Pol2, reference) primers (Sigma-Aldrich, KiCqStart SYBR Green Primers, Supplementary Table 1) and 250 pg cDNA/µl. Cycle threshold data for both the target gene and reference gene were used to calculate mean normalized expression as previously described in Lund et al. (2003).
Analysis of Aryl Hydrocarbon Receptor and CYP1A1Protein Expression
Heart and lung tissue were homogenized either in lysis buffer (20mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% triton X-100, Complete Mini Protease Inhibitor Cocktail Tablets [Roche, Mannheim, Germany]) for microsomal protein analysis or in a modified lysis buffer (25 mM HEPES, pH 7.4, 20mM NaMoO4, 5mM EGTA, 3 mM MgCl2, 10% glycerol, 50 μM leupeptin, 20 μg/ml aprotonin) for whole protein analysis. Protein concentration was determined by Micro BCA Protein Assay Kit (Thermo Scientific, Rockford, Illinois), and microsomal- and total protein were analyzed for CYP1A1 and aryl hydrocarbon receptor (AHR), respectively, by Western blot. Protein samples were denatured by heating at 95ºC in SDS loading buffer for 5 min, resolved by electrophoresis on a 10% SDS-polyacrylamide gel, and transferred to a PVDF membrane. Membranes were probed using goat polyclonal anti-CYP1A1 (1:500, Santa Cruz Biotech, Dallas, Texas) or anti-AHR (Novus Biologicals, Littleton, Colorado), followed by donkey anti-goat IgG-HRP secondary antibody (1:20 000, Santa Cruz Biotech). Detection was performed using ECL reagent (Western Lighting-ECL kit, Perkin Elmer) and imaged on a ProteinSimple FluorChem system (ProteinSimple, Santa Clara, California). Membranes were then stripped using a mild stripping buffer, probed using a rabbit polyclonal anti-GAPDH primary antibody in heart homogenates, anti-β-actin in lung homogenates and anti-cytochrome P450 reductase (CYPOR) in microsomes (1:500 dilution) and a goat anti-rabbit IgG-HRP secondary antibody (1:2000 dilution). Band intensity was quantified using AlphaView SA digital image software.
Analysis of Cytochrome P450 Eicosanoids and Fatty Acids
Cytochrome P450-generated eicosanoids, including epoxyeicosatrienoic acids (EETs) from AA, and epoxyeicosatetraenoic acids (EEQs) and epoxydocosapentaenoic acids (EDPs) from EPA and DHA, respectively, were determined from liver and plasma of control and TCDD-exposed mice fed a chow or n-3 PUFA-enriched diet (n = 8/treatment/diet) as described previously in Arnold et al. (2010b). Briefly, plasma (100 µl) or homogenized tissue samples (30 mg aliquots) were mixed with internal standards (10 ng of each 20-HETE-d6; 14,15-EET-d8; 14,15-dihydroxyeicosatrienoic acid-d11; and 15-HETE-d8 (Cayman Chemical) and subjected to alkaline hydrolysis. The metabolites were extracted by solid-phase extraction and quantified by liquid chromatography tandem mass spectrometry (LC-MS/MS). Fatty acid analysis was conducted by LC-MS; details are described in Supplementary Materials and in Supplementary Tables 2 and 3.
Statistical Analysis
Data are expressed as mean ± SEM. Dose-response data and area under the AA-mediated constriction curves ± inhibitors were analyzed by repeated measures, 2-way analysis of variance (ANOVA) with post hoc Holm-Sidak comparisons. Treatment- and diet-related changes were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons. P < .05 was considered statistically significant.
RESULTS
TCDD Increased Liver Weight and the N-3 PUFA DietAttenuated This Increase
We first sought to determine if TCDD had any toxic effects reflected by changes in body or organ weights, or liver oxidative stress markers, and whether these effects were altered by the n-3 PUFA diet. Body weight was not altered by treatment or diet (Table 2). In contrast, TCDD significantly increased liver weight by 13 ± 2.4% on the chow diet. Although TCDD increased liver weight by 8.4 ± 1.7% on the n-3 PUFA diet, this increase was not statistically significant. Liver oxidative stress, as assessed by thiobarbituric acid reactive substances and the ratio of reduced to oxidized glutathione, was not altered by treatment or diet (data not shown). Last, the n-3 PUFA diet significantly increased kidney weight and kidney-to-body weight ratio in both control and TCDD treatment groups, compared with the chow diet.
TABLE 2.
Body and Organ Weights of 5.5 Month-Old Mice Fed a Chow or n-3 PUFA-Enriched Diet for 10 Weeks and Exposed to Control or TCDD for the Final 2 Weeks on Each Diet
| Weight | Chow Dieta |
n-3 PUFA Diet |
||
|---|---|---|---|---|
| Control | TCDD | Control | TCDD | |
| Body (g) | 27.9 ± 1.0 | 27.3 ± 0.2 | 29.1 ± 0.9 | 29.3 ± 0.7 |
| Heart (mg) | 111 ± 3 (0.39 ± 0.01)b | 113 ± 3 (0.41 ± 0.01) | 116 ± 3 (0.40 ± 0.01) | 108 ± 3 (0.37 ± 0.01) |
| Liver (mg) | 1,413 ± 57 (5.1 ± 0.4) | 1,598 ± 57* (5.8 ± 0.1) | 1,510 ± 57 (5.2 ± 0.1) | 1,628 ± 57 (5.6 ± 0.1) |
| Kidney (mg) | 295 ± 8 (1.06 ± 0.03) | 297 ± 8 (1.08 ± 0.02) | 339 ± 8** (1.17 ± 0.02)** | 328 ± 8** (1.12 ± 0.02)** |
| Lung (mg) | 148 ± 3 (0.53 ± 0.01) | 144 ± 3 (0.52 ± 0.01) | 150 ± 3 (0.52 ± 0.01) | 150 ± 3 (0.51 ± 0.01) |
aValues are expressed as mean ± SEM, n = 8. Data were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons.
b(Organ weight (g)/body weight (g)) × 100.
*P < .05 versus chow control.
**P < .05 versus same treatment group fed the chow diet.
TCDD and an N-3 PUFA Diet Differentially Affect RBCFatty Acid Composition
To determine the effects of diet and TCDD exposure on fatty acid levels, the fatty acid composition of RBCs from control and TCDD-exposed mice fed a chow or n-3 PUFA diet were analyzed. Unsaturated and monounsaturated fatty acids varied with diet, but there were no effects of TCDD treatment (Supplementary Figure 1). In contrast, mice fed an n-3 PUFA diet exhibited significantly higher percentages of the n-3 PUFAs, EPA, and DHA, and significantly lower percentages of n-6 PUFAs, linoleic acid (LA, 18:2n-6) and AA, compared with mice fed a chow diet (Table 3). Since the n-3 PUFA diet contained less of the n-3 PUFA precursor, α-linolenic acid (ALA, 18:3n-3), than the chow diet, mice fed the n-3 PUFA diet exhibited significantly lower percentages of ALA.
TABLE 3.
n-3 and n-6 PUFAs, Expressed as a Percent of Total Fatty Acids or Metabolite-to-Precursor Ratio, in RBCs of 5.5-Month-Old Mice Fed a Chow or n-3 PUFA-Enriched Diet for 10 Weeks and Exposed to Control or TCDD for the Final 2 Weeks on Each Diet
| Fatty Acida | Chow Dietb |
n-3 PUFA Diet |
||
|---|---|---|---|---|
| Control | TCDD | Control | TCDD | |
| n-3 PUFA | ||||
| ALA (%) | 0.096 ± 0.003 | 0.104 ± 0.003* | 0.034 ± 0.003** | 0.039 ± 0.003*,** |
| EPA (%) | 0.227 ± 0.057 | 0.223 ± 0.057 | 10.3 ± 0.057** | 10.6 ± 0.057*,** |
| DHA (%) | 7.06 ± 0.085 | 6.62 ± 0.085* | 13.9 ± 0.085** | 14.0 ± 0.085** |
| EPA+DHA/ALA | 76.5 ± 3.3 | 66.9 ± 3.4* | 710 ± 22** | 621 ± 15* ,** |
| n-6 PUFA | ||||
| LA (%) | 12.6 ± 0.2 | 12.8 ± 0.2 | 2.6 ± 0.2** | 2.6 ± 0.2** |
| AA (%) | 17.6 ± 0.2 | 16.5 ± 0.2* | 8.5 ± 0.2** | 8.5 ± 0.2** |
| AA/LA | 1.40 ± 0.03 | 1.29 ± 0.02* | 3.24 ± 0.08** | 3.28 ± 0.04** |
aAbbreviations: α-linolenic acid (ALA, 18:3n-3); eicosapentanoic acid (EPA, 20:5n-4); docosahexanoic acid (DHA, 22:6n-3); linoleic acid (LA, 18:2n-6); and AA (20:4n-6).
bValues are expressed as mean ± SEM, n = 8. Data were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons.
*P < .05 versus control group fed the same diet.
**P < .05 versus same treatment group fed the chow diet.
TCDD significantly increased the percentage of ALA in mice on both diets, compared with control mice, which might suggest an inhibition of the desaturase and elongase enzymes that convert the precursor ALA to it longer chain metabolites, EPA and DHA. In support of this, we found that TCDD significantly reduced the percentage of DHA on a chow diet and reduced the metabolite/precursor ratio of EPA + DHA/ALA on both diets. Since the same enzymes convert the n-6 precursor LA to its longer chain metabolite AA, we also assessed the AA/LA ratio. Although TCDD did not alter the percentage of the n-6 precursor LA, it reduced the percentage of AA on a chow diet and reduced the AA/LA ratio on a chow diet. Overall, these data suggest that TCDD may hinder the long chain PUFA biosynthesis pathway.
TCDD Enhanced AA-Mediated Vasoconstriction and N-3 PUFAs Prevented This Enhancement
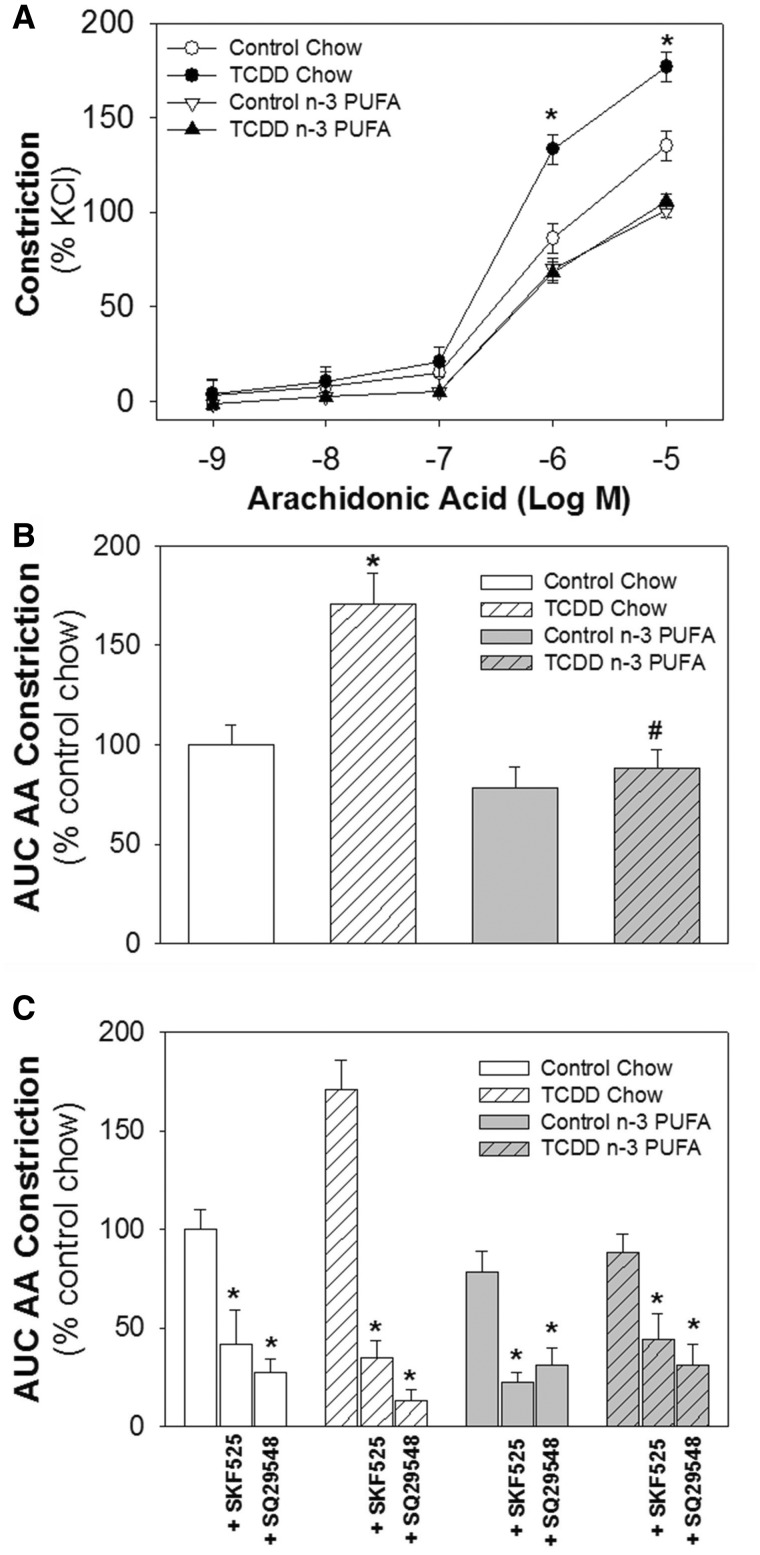
Since AA mediates vasoconstriction by P450-dependent metabolites (Escalante et al., 1989) and TCDD significantly induces a number of P450s, AA-mediated vasoconstriction in aortas from control and TCDD-exposed mice fed a chow or n-3 PUFA diet was analyzed. We found that TCDD significantly increased the magnitude of AA constriction in mice on a chow diet and this increase was prevented in mice fed an n-3 PUFA diet, as observed from the AA dose-response curves (Figure 1A) and area under the curve (AUC) analysis of AA constriction (Figure 1B).
FIG. 1.
Effects of TCDD and an n-3 PUFA diet on vasoconstriction responses to AA. A, Dose–response for AA-mediated constriction in aortic rings from control and TCDD-exposed mice fed a chow or n-3 PUFA-enriched diet. B, AUC analysis of the dose-response curves shown in panel A. C, AUC analysis of AA-mediated constriction in the presence of a P450 inhibitor (SKF525, 25 µM) or TP receptor antagonist (SQ29548, 100 nM). Data are shown as mean ± SEM, n = 6–7/group and were analyzed by 2 way, repeated measures ANOVA with post hoc Holm-Sidak comparisons. Panels A and B: *P < .05 compared with chow control; #P < .05 compared with chow TCDD; Panel C: *P < .05 compared with AA-constriction in the absence of inhibitor.
TCDD Enhanced the P450 and Thromboxane/Prostanoid Receptor Contribution to AA Vasoconstriction and n-3 PUFA Diet Normalized These Contributions
To further dissect the contribution of P450s and the thromboxane/prostanoid (TP) receptor to constriction mediated by the metabolism of exogenously applied AA, aortic rings from control and TCDD-exposed mice fed a chow or n-3 PUFA diet were incubated with a global P450 inhibitor (SKF525) or a TP receptor antagonist (SQ29548), and the AA dose-response repeated. Both P450 inhibition and TP receptor antagonism significantly reduced AA-mediated constriction in control and TCDD treatment groups on both diets, but the greatest reduction was observed in TCDD-exposed mice on a chow diet (Figure 1C; Table 4). In mice exposed to TCDD on a chow diet, nearly 80% of AA constriction was P450 dependent and 92% was mediated via the TP receptor, which was significantly higher than 55 and 69%, respectively, in control mice fed a chow diet. In contrast, in mice fed an n-3 PUFA diet and then exposed to TCDD, the percent contribution of P450s and TP receptor agonism to AA-mediated vasoconstriction was 65 and 71%, respectively. These values were significantly lower than TCDD-exposed mice on a chow diet, but were not different than control mice on the n-3 PUFA diet.
TABLE 4.
Percent Contribution of P450s and the TP Receptor to AA-Mediated Aortic Constriction in Control and TCDD-Exposed Mice Fed Chow or n-3 PUFA-Enriched Diet
| Diet and Treatment | P450a | TP Receptorb |
|---|---|---|
| Chow | ||
| Control | 55 ± 8 | 69 ± 4 |
| TCDD | 79 ± 8* | 92 ± 4* |
| n-3 PUFA | ||
| Control | 71 ± 3 | 60 ± 6 |
| TCDD | 65 ± 5** | 71 ± 6** |
aPercent inhibition by P450 inhibitor, SKF525, mean ± SEM, n = 7–8. Data were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons.
bPercent inhibition by TP receptor antagonist, SQ29548, mean ± SEM, n = 7–8. Data were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons.
*P < .05 compared with control group fed a chow diet.
**P < .05 compared with TCDD chow diet.
TCDD Increased AA-Derived Vasoconstrictors and n-3 PUFA Diet Attenuated this Increase
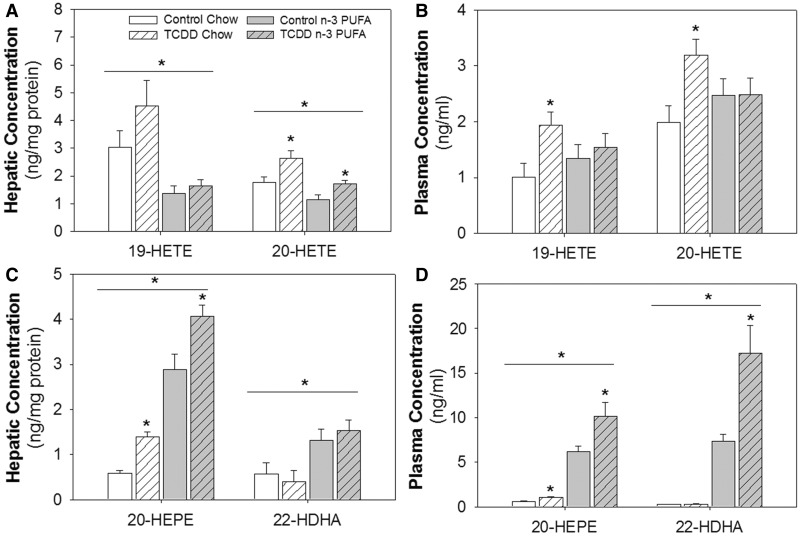
AA mediates vasoconstriction via the P450 hydroxylase metabolite 20-HETE (Escalante et al., 1989; Imig et al., 1996; Toth et al., 2013), although the P450 hydroxylase metabolite 19-HETE has also been reported to cause vasoconstriction (Escalante et al., 1989). Thus, the expression of these 2 metabolites in liver and plasma were analyzed. TCDD significantly increased 20-HETE in the liver of mice on both diets (Figure 2A), while levels in control and TCDD groups were significantly attenuated on the n-3 PUFA diet. TCDD also significantly increased 19- and 20-HETE in plasma of mice on a chow diet, and this increase was prevented by the n-3 PUFA diet (Figure 2B). These data suggest that TCDD increases AA-derived vasoconstrictors and that the n-3 PUFA diet prevents or attenuates these increases.
FIG. 2.
Effects of TCDD and an n-3 PUFA diet on AA-, EPA-, and DHA-derived monohydroxy metabolites commonly generated by CYP4A12 determined by LC-MS/MS from liver and plasma. A,B, Hepatic and plasma concentrations of 19-HETE and 20-HETE from control and TCDD-exposed mice fed a chow or n-3 PUFA-enriched diet. C,D, Hepatic and plasma levels of 20-HEPE and 22-HDHA from control and TCDD-exposed mice fed a chow or n-3 PUFA-enriched diet. Data are shown as mean ± SEM, n = 8/group and were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons. *Above a horizontal line, indicates a statistically significant difference between chow and n-3 diet, P < .05. *Above a vertical bar indicates a significant difference between control and TCDD exposure groups, P < .05. 20-HEPE, 20-hydroxyeicosapentaenoic acid; 19- and 20-HETE, 19- and 20-hydroxyeicosatrienoic acid; 22-HDHA, 22-hydroxydocosahexaenoic acid.
Since CYP4A12 is the major P450 isoform that generates 20-HETE in male mice (Muller et al., 2007), we further investigated whether TCDD also increased the levels of CYP4A12-generated hydroxylase metabolites of EPA and DHA, 20-hydroxyeicosapentaenoic acid (20-HEPE) and 22-hydroxydocosahexaenoic acid (22-HDHA). We found that TCDD significantly increased 20-HEPE in liver and plasma of mice on both diets and 22-HDHA only in plasma of mice fed the n-3 PUFA diet (Figs. 2C and D).
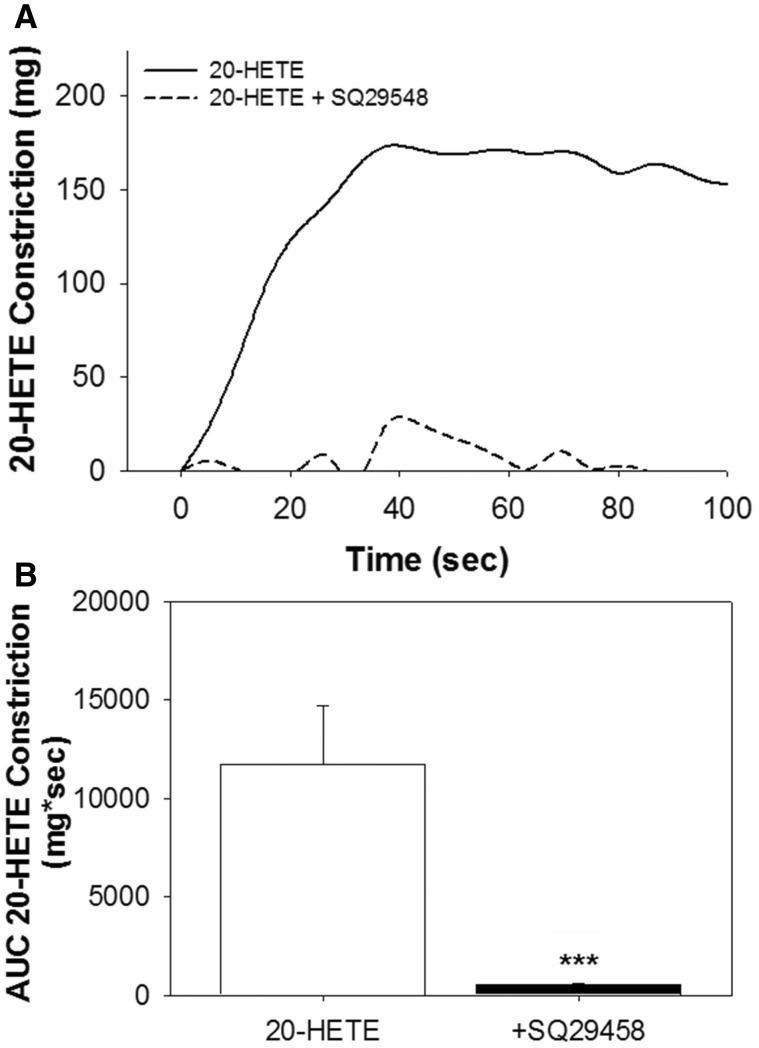
20-HETE Induced Constriction via TP Receptor in Mouse Aorta
Although 20-HETE has been shown to induce vasoconstriction in various vascular beds, its ability to constrict the mouse aorta has never been reported. Thus, to confirm that 20-HETE could induce constriction in the mouse aorta and the degree to which this was mediated by the TP receptor, the effects of 20-HETE ± TP receptor antagonist in aortic rings of control mice on a chow diet were assessed. We found that 20-HETE significantly induced constriction of the mouse aorta and this response was blocked by a TP receptor antagonist (Figs. 3A and B).
FIG. 3.
Vasoconstriction effects of 20-HETE in the absence or presence of TP receptor antagonist. A, Representative tracing of 20-HETE (10−5.4 M) constriction of mouse aortic ring ± TP receptor antagonist (SQ29548, 100 nM). B, AUC analysis of 20-HETE constriction ± TP receptor antagonist (SQ29548). Data are shown as mean ± SEM, n = 3/group and were analyzed by t-test. *** P < .0001. 20-HETE, 20-hydroxyeicosatrienoic acid.
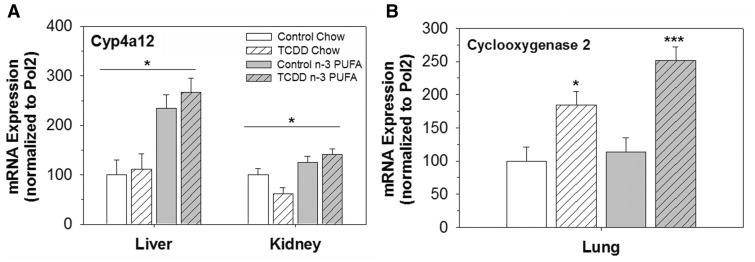
TCDD Induced Cyclooxygenase 2 in Lung but not Cyp4a12in Liver or Kidney
As noted earlier 20-HETE is predominately generated by CYP4A12 in male mice and in some vascular beds vasoconstriction by 20-HETE is dependent on subsequent cyclooxygenase metabolism to a TP receptor agonist (Escalante et al., 1989; Muller et al., 2007; Schwartzman et al., 1989; Toth et al., 2011). Thus, the mRNA expression of Cyp4a12 in the liver and kidney, and prostaglandin-endoperoxide synthase 2 (aka cyclooxygenase 2) was analyzed in the lung, representing the tissues where they are predominately expressed. Cyp4a12 mRNA was significantly higher in tissues of mice fed an n-3 PUFA diet, but its expression was not altered by TCDD (Figure 4A). In contrast, cyclooxygenase 2 mRNA was significantly induced by TCDD, but its expression was not altered by diet (Figure 4B).
FIG. 4.
Effects of TCDD and an n-3 PUFA diet on mRNA expression of Cyp4a12 and prostaglandin endoperoxide synthase 2 (aka cyclooxygenase 2). A, Cyp4a12 mRNA expression in liver and kidney, normalized to the housekeeping gene, RNA polymerase 2 (Pol2). B, Cyclooxygenase 2 mRNA expression in lung, normalized to Pol2. Data are shown as mean ± SEM, n = 6-8/group and were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons. *Above a horizontal line, indicates a statistically significant difference between chow and n-3 diet, P < .05. *Above a vertical bar indicates a significant difference between control and TCDD exposure groups, * P < .05, *** P < .001.
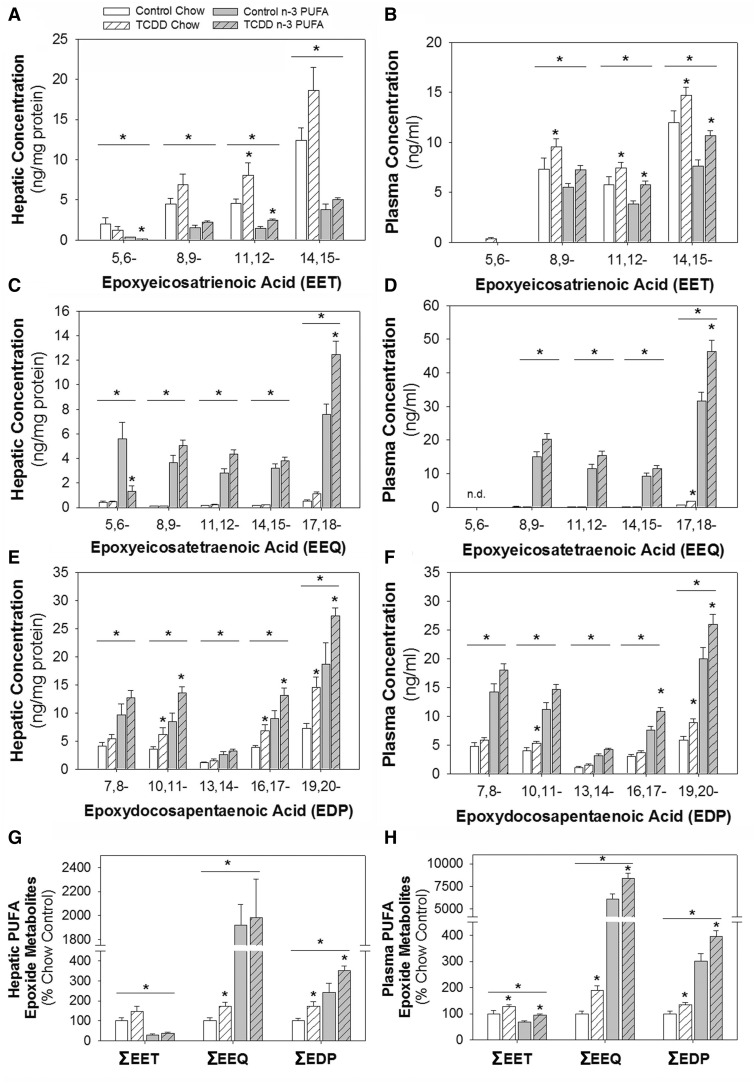
TCDD Selectively Increased CYP Epoxygenase Metabolitesof n-6 and n-3 PUFAs
In addition to focusing on the generation of- and response to- vasoconstrictors, we sought to determine if vasoprotective effects of the n-3 PUFA diet were associated with increases in P450-generated vasodilators. Thus, the levels of the vasodilatory epoxide metabolites from AA, EPA, and DHA were assessed. The epoxide metabolites of AA (EETs) were higher on the chow diet than the n-3 PUFA diet and TCDD increased 11,12-EET in the liver and 8,9-, 11-12-, and 14,15-EET in plasma (Figs. 5A and B). The epoxide metabolites of EPA and DHA (EEQs and EDPs) were all higher on the n-3 PUFA diet than the chow diet, except 5,6-EEQ which was not detected in plasma, and TCDD significantly increased 4 of the 10 metabolites in both liver and plasma; 17,18-EEQ, and 10,11-, 16,17- and 19,20-EDP (Figs. 5C–F). We also compared the sum (Σ) of EETs, EEQs, and EDPs, based on diet and treatment. In both liver and plasma we found that ΣEET derived from AA was significantly higher on the chow diet, while the ΣEEQ and ΣEDP derived from EPA and DHA, respectively, were significantly higher on the n-3 PUFA diet (Figs. 5G and H). In particular, ΣEEQs and ΣEDPs were 20–80× and 2–4× higher, respectively, in liver and plasma on the n-3 PUFA diet, compared with the chow diet. Further, TCDD induced significant increases in the ΣEEQs and ΣEDPs in the liver and all metabolites in plasma.
FIG. 5.
Effects of TCDD and an n-3 PUFA diet on the profile of AA-, EPA-, and DHA-derived monoepoxide metabolites determined by LC-MS/MS from liver and plasma. A,B, AA-derived EETs; C,D, EPA-derived EEQs; and E,F, DHA-derived EDPs. G,H, Sum (Σ) of EETs, EEQ, and EDPs from liver and plasma. Data were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons. *Above a horizontal line, indicates a statistically significant difference between chow and n-3 diet, P < .05. *Above a vertical bar indicates a significant difference between control and TCDD exposure groups, * P < .05. EET, epoxyeicosatrienoic acid; EEQ, epoxyeicosatetraenoic acid; EDP, epoxydocosapentaenoic acid.
These epoxide metabolites are subsequently converted to less active fatty acid diols by soluble epoxide hydrolase and this conversion, as assessed by the ratio of the epoxide/diol product, was largely unaffected by diet or TCDD treatment (Supplementary Figure 2). Additionally, the products of PUFA autoxidation and metabolism by lipoxygenase 5, 12, and 15 were analyzed. We found that the n-3 PUFA products were higher on the n-3 PUFA diet and the n-6 PUFA products were high on the chow diet, and that 6 of the 23 products (11-HETE, 5-HEPE, 15-HEPE, 11-HDHA, 17-HDHA, and 20-HDHA) were significantly increased by TCDD (Supplementary Figs. 3–6).
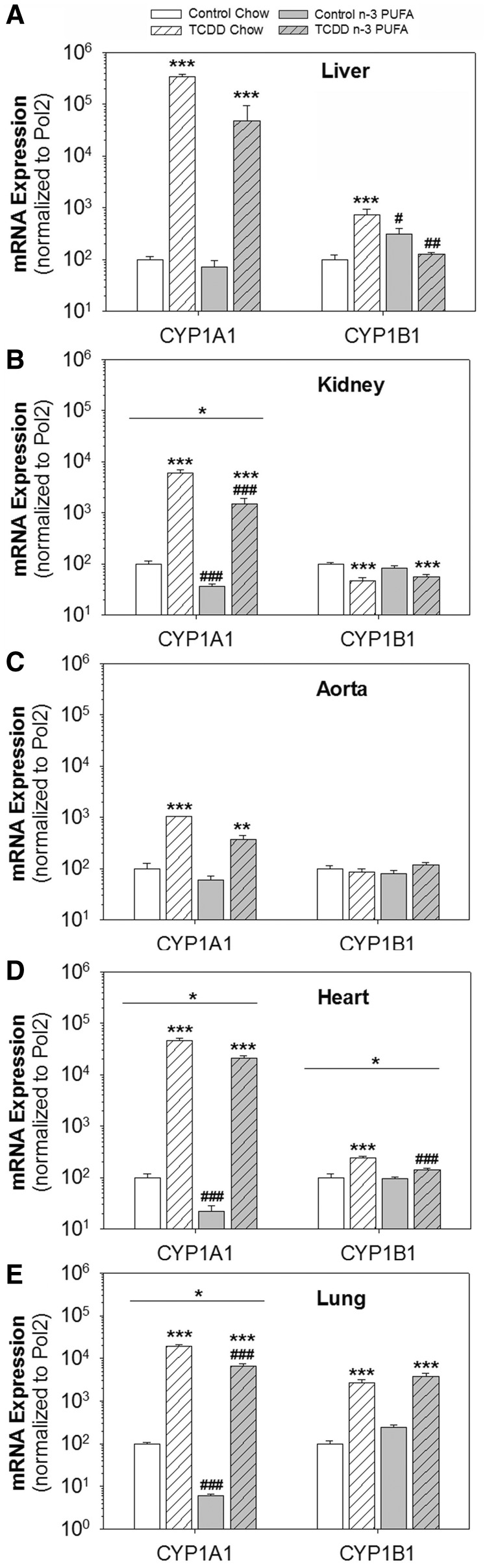
n-3 PUFA Diet Reduced TCDD-Induced Cyp1a1 and Cyp1b1 Expression in Selected Tissues
Since TCDD induces Cyp1a1 and Cyp1b1, which can metabolize AA, EPA, and DHA largely to vasodilatory products, we measured their mRNA expression in a number of tissues. As expected TCDD significantly induced Cyp1a1 in all tissues on both diets (Figs. 6A–E). Interestingly, however, the n-3 PUFA diet significantly reduced basal and TCDD-induced Cyp1a1 mRNA expression in the kidney, heart, and lung, compared with the chow diet. Similarly, TCDD induced Cyp1b1 mRNA in the liver and heart, and the n-3 PUFA diet significantly attenuated this induction. In contrast, TCDD induced Cyp1b1 in the lung, suppressed it in the kidney, and had no effect in aorta, and none of these levels was altered by diet.
FIG. 6.
Effects of TCDD and an n-3 PUFA diet on mRNA expression of Cyp1a1 and Cyp1b1. A–E, mRNA expression of Cyp1a1 and Cyp1b1, normalized to normalized to the housekeeping gene, RNA polymerase 2 (Pol2), in liver, kidney, aorta, heart, and lung. Data were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons. *Above a horizontal line, indicates a statistically significant difference between chow and n-3 diet, P < .05. *Above a vertical bar indicates a significant difference between control and TCDD treatment groups within the same diet, ** P < .01, *** P < .001. #Above a vertical bar indicates difference between chow and n-3 PUFA diets within the same treatment group, #P < .05, ##P < .01, ###P < .001.
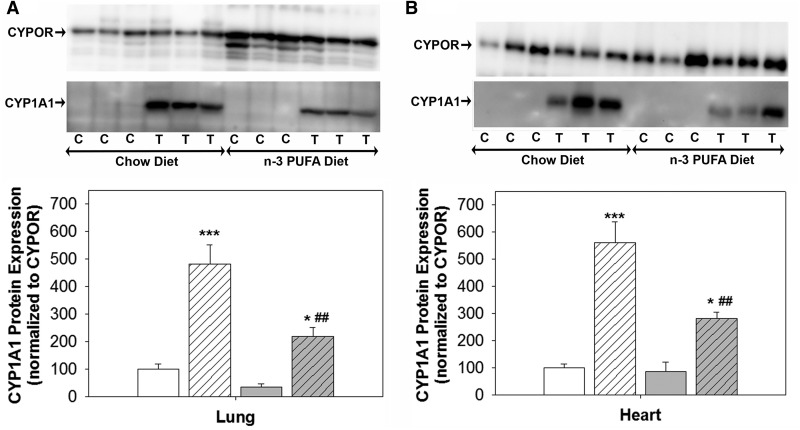
To determine whether the decrease in Cyp1a1 mRNA expression by the n-3 PUFA diet was associated with a decrease in protein expression as well as a decrease in AHR, the primary transcriptional regulator of Cyp1a1, CYP1A1, and AHR protein expression from heart and lung was measured by western blot. We found that the n-3 PUFA diet significantly attenuated TCDD-induced CYP1A1 protein expression, compared with level of TCDD induction on the chow diet (Figs. 7A–D). However, we did not observe any significant change in AHR expression (data not shown).
FIG. 7.
Effects of TCDD and an n-3 PUFA diet on protein expression of CYP1A1. A,B, Representative western blot of CYPOR and CYP1A1 protein, and quantification of CYP1A1 protein expression, normalized to CYPOR expression, in lung and heart microsomes, respectively. Data were analyzed by 2-way ANOVA with post hoc Holm-Sidak comparisons. * P < .05, *** P < .001 versus control within the same diet; ##P < .01 versus TCDD treatment on chow diet.
DISCUSSION
This work shows for the first time that a diet enriched with n-3 PUFAs can prevent vascular dysfunction induced by the persistent environmental pollutant, TCDD. This protection is associated with a decrease in P450-generated vasoconstrictors that agonize the TP receptor, including the AA metabolite, 20-HETE. Additionally, this protection is associated with an increase in P450-generated vasodilatory epoxide metabolites from EPA and DHA, which are further increased by TCDD exposure, potentially contributing to vasoprotective mechanisms afforded by the n-3 PUFA diet. Finally, we observed that n-3 PUFAs significantly reduce TCDD-induced CYP1A1 expression in a number of tissues.
Our previous work demonstrates that chronic TCDD exposure induces oxidative stress, reduces endothelial-dependent vasodilation, and increases blood pressure in male mice (Kopf et al., 2008). Further, we found that the reduction of endothelial-dependent vasodilation could be prevented by an antioxidant and both the oxidative stress and loss of endothelial-dependent vasodilation could be prevented by genetic deletion of Cyp1a1 (Kopf et al., 2010a). A major contribution of this study is that it further elucidates the mechanism underlying TCDD-induced vascular dysfunction. Our data demonstrate that TCDD significantly increases P450-dependent vasoconstriction, which is likely mediated by increases in the P450-generated hydroxylase metabolite of AA, 20-HETE. Vascular overproduction of 20-HETE decreases endothelial-dependent vasodilation, and increases oxidative stress, vasoconstriction and blood pressure (Wang et al., 2006). Furthermore, vasoconstriction by 20-HETE is mediated by activation of TP receptor in many vascular beds (Escalante et al., 1989; Muller et al., 2007; Schwartzman et al., 1989; Toth et al., 2011), including the mouse aorta as shown by our results. We also found that TCDD increases the contribution of the TP receptor to AA-mediated vasoconstriction, further implicating the potential contribution of 20-HETE to TCDD-induced vascular dysfunction. Finally, our results show that TCDD induces cyclooxygenase 2, consistent with reports by others (Vogel et al., 1998), which may contribute to 20-HETE metabolism to a TP receptor agonist.
Previously, it has been reported that TCDD significantly increases P450-generated metabolites of AA, 19- and 20-HETE, in the liver of male mice (Bui et al., 2012; Yang et al., 2013). Although TCDD induces CYP1A1, 1A2, and 1B1 and studies show they can produce 19-HETE, there is no evidence these P450s can produce 20-HETE (Choudhary et al., 2004; El-Sherbeni et al., 2014; Falck et al., 1990; Schwarz et al., 2004). CYP4A12 is the major P450 isoform that generates 20-HETE in male mice (Muller et al., 2007); however, it was not induced by TCDD in either the liver or kidney. It is possible that TCDD induces CYP4A12 in the vasculature, resulting in local increases in 20-HETE that act in a paracrine manner to cause oxidative stress and vasoconstriction. Nonetheless, the P450s and tissues responsible for the TCDD-induced increase in 20-HETE remain to be elucidated.
A second major finding of our work is that dietary n-3 PUFAs can prevent the TCDD-induced vascular dysfunction in vivo. This is the first report that n-3 PUFAs can protect against in vivo cardiovascular toxicity of halogenated aromatic hydrocarbons. These results are consistent with studies showing that n-3 PUFAs can protect against the toxicity of TCDD and dioxin-like polychlorinated biphenyls in vitro and in vivo. EPA can reduce TCDD-induced oxidative stress, DNA damage, and cell death in cultured hepatocytes (Palanisamy et al., 2015; Turkez et al., 2011b) and that a higher ratio of n-3/n-6 PUFAs can reduce oxidative stress and inflammation induced by the dioxin-like polychlorinated biphenyl, PCB77, in cultured endothelial cells (Wang et al., 2008). Additionally, both EPA and DHA protect against TCDD-induced oxidative stress and hepatic and renal injury in rats in vivo (Palaniswamy et al., 2014; Turkez et al., 2011a). Notably, however, in our study TCDD did not increase oxidative stress markers or increase fatty acid autooxidation products in the liver. Although it is possible that increases in oxidative stress could have occurred in the vasculature, our results do not provide evidence that the vasoprotective benefits of the n-3 PUFA diet result from antioxidant activity.
Our results suggest that n-3 PUFAs likely protect against TCDD-induced vascular dysfunction by multiple mechanisms. First, the n-3 PUFA diet significantly decreases the percentage of AA and increases the percentage of EPA and DHA in cell membranes. We observed this in RBCs and this is consistent with results from human RBCs and various tissues in animal models in which diets are supplemented with n-3 PUFAs (Agbor et al., 2014; Arnold et al., 2010b; Fischer et al., 2014; Schebb et al., 2014). This shift in PUFA membrane composition subsequently shifts the P450-mediated omega-hydroxylase metabolic profile (Agbor et al., 2014; Arnold et al., 2010b; Fischer et al., 2014). Less of the vasoconstrictor, 20-HETE, is generated in the liver and more 20-HEPE and 22-HDHA are generated in liver and plasma, suggesting that the substrate for Cyp4a12 shifts away from AA and towards EPA and DHA. Although vascular studies have not been conducted with 20-HEPE or 22-HDHA, a decrease in levels of 20-HETE would be vasoprotective.
Second, the shift in PUFA membrane composition also shifts the P450-mediated epoxide metabolic profile; significantly decreasing the AA-derived EETs and significantly increasing the EPA- and DHA-derived EEQs and EDPs, respectively. Studies show that EEQs and EDPs are equipotent or more potent in causing vasodilation than EETs (Ye et al., 2002; Zhang et al., 2001), while our studies in mouse aorta show that EEQs and EDPs are significantly more potent vasodilators than EETs (unpublished data). Thus, the vasoprotective effects of n-3 PUFAs could also be mediated by significant increases in their vasodilatory epoxides. Additionally, the vasoprotective benefits of n-3 PUFAs may be enhanced further by TCDD, since TCDD significantly increases levels of 17,18-EEQ, and 10,11-, 16,17- and 19,20-EDP in the liver and plasma. The increases in 17,18-EEQ and 19,20-EDP likely result from induction of CYP1A1 and 1A2, since recombinant enzyme studies of CYP1A1 and 1A2 show that they generate these 2 epoxides with high efficiency and stereospecificity (Fer et al., 2008; Lucas et al., 2010; Schwarz et al., 2004). The P450s responsible for the increases of 10,11- and 16,17-EDP by TCDD are uncertain. Nonetheless, these increases are consistent with a previous study showing that a single high dose of TCDD (50 µg/kg) via intraperitoneal injection of mice on a chow diet significantly increases hepatic 14,15- and 17,18-EEQ, and 7,8-, 10,11-, 13,14-, 16,17-, and 19,20-EDP (Yang et al., 2013). Although the earlier study reported 5-20 fold increases in these epoxides 3 days after injection, we detected more modest increases in the range of 1.5- to 2.0-fold following 14 days of exposure to 300 ng/kg/d.
Third, reduced expression of CYP1A1 and increased substrate availability for CYP1A1 may also contribute to the vasoprotective benefits of n-3 PUFAs. As noted earlier, CYP1A1 is required for endothelial dysfunction and hypertension induced by TCDD (Kopf et al., 2010a,b). The n-3 PUFA diet significantly reduces TCDD-induced mRNA and protein expression of CYP1A1, which could reduce CYP1A1-mediated vascular injury. Additionally, the n-3 PUFA diet significantly increases EPA and DHA, highly preferred endogenous fatty acid substrates for CYP1A1 (Arnold et al., 2010a,b), which could also reduce non-specific CYP1A1 oxidation of other vascular fatty acid substrates. Others have reported that EPA reduces TCDD-induced CYP1A1 activity in vitro and in vivo (Palaniswamy et al., 2014, 2015). The mechanism by which n-3 PUFAs reduce TCDD-induced CYP1A1 expression and activity is not known, although we did not observe a decrease in AHR expression in mice on the n-3 PUFAs diet. Thus, future studies are needed to confirm these results and further investigate the ability of n-3 PUFAs to attenuate the AHR signaling pathway.
There are some limitations to the conclusions we can draw from this study regarding the risk-benefit ratio related to fish consumption. In our experimental model the n-3 PUFA diet preceded the TCDD exposure, rather than occurring simultaneously as would occur with the consumption of fish from the environment. Thus, the protect benefits of n-3 PUFAs following fish consumption would depend on the rate of n-3 PUFA accumulation versus the rate of pollutant accumulation. In contrast, our results more closely mimic the accumulation and protection of n-3 PUFAs resulting from dietary supplements of adequate quantity and quality prior to fish consumption and pollutant exposure. In addition, the potential protective benefits of n-3 PUFAs suggested in this study are most relevant to halogenated aromatic hydrocarbon pollutants, such as polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls, and cannot be extrapolated other accumulated pollutants such as mercury. Last, while the n-3 PUFA diet differed from the chow diet primarily in the percentage of n-3 PUFAs, the increased levels of saturated fatty acids could influence these results, although studies demonstrate that n-3 PUFAs can protect against vascular dysfunction caused by saturated fatty acids (Lamping et al., 2013).
Despite these limitations, our results demonstrate that a diet enriched in n-3 PUFAs can protect against in vivo cardiovascular toxicity of a prototypical halogenated aromatic hydrocarbon, TCDD, and this is mechanistically associated with a decrease in AA-generated vasoconstrictors, an increase in n-3 PUFA-generated vasodilators, and a decrease in CYP1A1 expression. In conclusion, a diet enriched in n-3 PUFAs has the potential to protect against toxicity associated with a class of environmental pollutants commonly accumulated in fish.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
ACKNOWLEDGMENTS
The authors thank Kayla Zehr and Amre Elmaoued for their helpful discussions and Avalon Abrums for her expert technical assistance.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences (1R15ES021896-01 to M.K.W.).
REFERENCES
- Agbor L. N., Wiest E. F., Rothe M., Schunck W. H., Walker M. K. (2014). Role of CYP1A1 in modulating the vascular and blood pressure benefits of omega-3 polyunsaturated fatty acids. J. Pharmacol. Exp. Ther. 351, 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold C., Konkel A., Fischer R., Schunck W. H. (2010a). Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 62, 536–547. [DOI] [PubMed] [Google Scholar]
- Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F. C., Muller D. N., et al. (2010b). Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J Biol Chem 285, 32720–32733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui P., Solaimani P., Wu X., Hankinson O. (2012). 2,3,7,8-Tetrachlorodibenzo-p-dioxin treatment alters eicosanoid levels in several organs of the mouse in an aryl hydrocarbon receptor-dependent fashion. Toxicol. Appl. Pharmacol. 259, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D., Jansson I., Stoilov I., Sarfarazi M., Schenkman J. B. (2004). Metabolism of retinoids and arachidonic acid by human and mouse cytochrome p450 1b1. Drug Metab. Dispos. 32, 840–847. [DOI] [PubMed] [Google Scholar]
- El-Sherbeni A. A., El-Kadi A. O. (2014). Characterization of arachidonic acid metabolism by rat cytochrome P450 enzymes: The involvement of CYP1As. Drug Metab. Dispos. 42, 1498–1507. [DOI] [PubMed] [Google Scholar]
- Escalante B., Sessa W. C., Falck J. R., Yadagiri P., Schwartzman M. L. (1989). Vasoactivity of 20-hydroxyeicosatetraenoic acid is dependent on metabolism by cyclooxygenase. J. Pharmacol. Exp. Ther. 248, 229–232. [PubMed] [Google Scholar]
- Falck J. R., Lumin S., Blair I., Dishman E., Martin M. V., Waxman D. J., Guengerich F. P., Capdevila J. H. (1990). Cytochrome P-450-dependent oxidation of arachidonic acid to 16-, 17-, and 18-hydroxyeicosatetraenoic acids. J. Biol. Chem. 265, 10244–10249. [PubMed] [Google Scholar]
- Fer M., Dreano Y., Lucas D., Corcos L., Salaun J. P., Berthou F., Amet Y. (2008). Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch. Biochem. Biophys. 471, 116–125. [DOI] [PubMed] [Google Scholar]
- Fischer R., Konkel A., Mehling H., Blossey K., Gapelyuk A., Wessel N., von Schacky C., Dechend R., Muller D. N., Rothe M., et al. (2014). Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J. Lipid Res. 55, 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O. (2016). The role of AHR-inducible cytochrome P450s in metabolism of polyunsaturated fatty acids. Drug Metab. Rev. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig J. D., Zou A. P., Stec D. E., Harder D. R., Falck J. R., Roman R. J. (1996). Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am. J. Physiol. 270, R217–R227. [DOI] [PubMed] [Google Scholar]
- Kopf P. G., Huwe J. K., Walker M. K. (2008). Hypertension, cardiac hypertrophy, and impaired vascular relaxation induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are associated with increased superoxide. Cardiovasc. Toxicol. 8, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf P. G., Scott J. A., Agbor L. N., Boberg J. R., Elased K. M., Huwe J. K., Walker M. K. (2010a). Cytochrome P4501A1 is required for vascular dysfunction and hypertension induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 117, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf P. G., Walker M. K. (2010b). 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 245, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton P. M., Harris W. S., Appel L. J. (2002). Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106, 2747–2757. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton P. M., Taylor D. S., Yu-Poth S., Huth P., Moriarty K., Fishell V., Hargrove R. L., Zhao G., Etherton T. D. (2000). Polyunsaturated fatty acids in the food chain in the United States. Am. J. Clin. Nutr. 71, 179S–188S. [DOI] [PubMed] [Google Scholar]
- Lamping K. G., Nuno D. W., Coppey L. J., Holmes A. J., Hu S., Oltman C. L., Norris A. W., Yorek M. A. (2013). Modification of high saturated fat diet with n-3 polyunsaturated fat improves glucose intolerance and vascular dysfunction. Diabetes Obes. Metab. 15, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D., Goulitquer S., Marienhagen J., Fer M., Dreano Y., Schwaneberg U., Amet Y., Corcos L. (2010). Stereoselective epoxidation of the last double bond of polyunsaturated fatty acids by human cytochromes P450. J. Lipid Res. 51, 1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A. K., Goens M. B., Kanagy N. L., Walker M. K. (2003). Cardiac hypertrophy in aryl hydrocarbon receptor (AhR) null mice is correlated with elevated angiotensin II, endothelin-1 and mean arterial blood pressure. Toxicol. Appl. Pharmacol. 193, 177–187. [DOI] [PubMed] [Google Scholar]
- Majkova Z., Layne J., Sunkara M., Morris A. J., Toborek M., Hennig B. (2011). Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 251, 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. C., Sacks F., Rosner B. (1993). Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 88, 523–533. [DOI] [PubMed] [Google Scholar]
- Muller D. N., Schmidt C., Barbosa-Sicard E., Wellner M., Gross V., Hercule H., Markovic M., Honeck H., Luft F. C., Schunck W. H. (2007). Mouse Cyp4a isoforms: Enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem. J. 403, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy K., Krishnaswamy R., Paramasivan P., Chih-Yang H., Vishwanadha V. P. (2015). Eicosapentaenoic acid prevents TCDD-induced oxidative stress and inflammatory response by modulating MAP kinases and redox-sensitive transcription factors. Br. J. Pharmacol. 172, 4726–4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniswamy K. S., Vishwanadha V. P., Ramalingam Singaravelu S. (2014). Fish oil rich in eicosapentaenoic acid protects against oxidative stress-related renal dysfunction induced by TCDD in Wistar rats. Cell Stress Chaperones 19, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebb N. H., Ostermann A. I., Yang J., Hammock B. D., Hahn A., Schuchardt J. P. (2014). Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat. 113–115, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzman M. L., Falck J. R., Yadagiri P., Escalante B. (1989). Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygenase. Formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J. Biol. Chem. 264, 11658–11662. [PubMed] [Google Scholar]
- Schwarz D., Kisselev P., Ericksen S. S., Szklarz G. D., Chernogolov A., Honeck H., Schunck W. H., Roots I. (2004). Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: Highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem. Pharmacol. 67, 1445–1457. [DOI] [PubMed] [Google Scholar]
- Sioen I., Bilau M., Verdonck F., Verbeke W., Willems J. L., De Henauw S., Van Camp J. (2008a). Probabilistic intake assessment of polybrominated diphenyl ethers and omega-3 fatty acids through fish consumption. Mol. Nutr. Food. Res. 52, 250–257. [DOI] [PubMed] [Google Scholar]
- Sioen I., De Henauw S., Verbeke W., Verdonck F., Willems J. L., Van Camp J. (2008b). Fish consumption is a safe solution to increase the intake of long-chain n-3 fatty acids. Public Health Nutr. 11, 1107–1116. [DOI] [PubMed] [Google Scholar]
- Sioen I., Van Camp J., Verdonck F., Verbeke W., Vanhonacker F., Willems J., Henauw S. D. (2008c). Probabilistic intake assessment of multiple compounds as a tool to quantify the nutritional-toxicological conflict related to seafood consumption. Chemosphere 71, 1056–1066. [DOI] [PubMed] [Google Scholar]
- Toft I., Bonaa K. H., Ingebretsen O. C., Nordoy A., Jenssen T. (1995). Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann. Intern. Med. 123, 911–918. [DOI] [PubMed] [Google Scholar]
- Toth P., Csiszar A., Sosnowska D., Tucsek Z., Cseplo P., Springo Z., Tarantini S., Sonntag W. E., Ungvari Z., Koller A. (2013). Treatment with the cytochrome P450 omega-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats. Br. J. Pharmacol. 168, 1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P., Rozsa B., Springo Z., Doczi T., Koller A. (2011). Isolated human and rat cerebral arteries constrict to increases in flow: Role of 20-HETE and TP receptors. J. Cereb. Blood Flow Metab. 31, 2096–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H., Geyikoglu F., Mokhtar Y. (2011a). Ameliorative effect of docosahexaenoic acid on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced histological changes, oxidative stress, and DNA damage in rat liver. Toxicol. Ind. Health. 64, 15–25. [DOI] [PubMed] [Google Scholar]
- Turkez H., Geyikoglu F., Mokhtar Y. I., Togar B. (2011b). Eicosapentaenoic acid protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced hepatic toxicity in cultured rat hepatocytes. Cytotechnology 64, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke W., Sioen I., Pieniak Z., Van Camp J., De Henauw S. (2005). Consumer perception versus scientific evidence about health benefits and safety risks from fish consumption. Public Health Nutr. 8, 422–429. [DOI] [PubMed] [Google Scholar]
- Vogel C., Schuhmacher U. S., Degen G. H., Bolt H. M., Pineau T., Abel J. (1998). Modulation of prostaglandin H synthase-2 mRNA expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. Arch. Biochem. Biophys. 351, 265–271. [DOI] [PubMed] [Google Scholar]
- Walker M. K., Boberg J. R., Walsh M. T., Wolf V., Trujillo A., Duke M. S., Palme R., Felton L. A. (2012). A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol. Appl. Pharmacol. 260, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. S., Singh H., Zhang F., Ishizuka T., Deng H., Kemp R., Wolin M. S., Hintze T. H., Abraham N. G., Nasjletti A., and., et al. (2006). Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ. Res. 98, 962–969. [DOI] [PubMed] [Google Scholar]
- Wang L., Reiterer G., Toborek M., Hennig B. (2008). Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem. Biol. Interact. 172, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Solaimani P., Dong H., Hammock B., Hankinson O. (2013). Treatment of mice with 2,3,7,8-Tetrachlorodibenzo-p-dioxin markedly increases the levels of a number of cytochrome P450 metabolites of omega-3 polyunsaturated fatty acids in the liver and lung. J. Toxicol. Sci. 38, 833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D., Zhang D., Oltman C., Dellsperger K., Lee H. C., VanRollins M. (2002). Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 303, 768–776. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Oltman C. L., Lu T., Lee H. C., Dellsperger K. C., VanRollins M. (2001). EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am. J. Physiol. Heart Circ. Physiol. 280, H2430–H2440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.