Abstract
Background. The objective of this study was to quantify human immunodeficiency virus (HIV) type 1 RNA decay and dolutegravir (DTG) concentrations in the semen of HIV-infected patients receiving DTG-based first-line therapy.
Methods. This was a prospective, single-arm, open-label study including 15 HIV-1–infected, antiretroviral therapy–naive men starting once-daily treatment with DTG (50 mg) plus abacavir-lamivudine (600/300 mg). HIV-1 RNA was measured in seminal plasma (SP) and blood plasma (BP) at baseline, on days 3, 7, and 14, and at weeks 4, 12, and 24. The HIV-1 RNA decay rate was assessed using nonlinear mixed-effects models. Total and free DTG concentrations were quantified 24 hours after the dose at weeks 4 and 24 by means of a validated liquid chromatography–tandem mass spectrometry method.
Results. Viral decay was faster in BP than in SP in the first decay phase (half-life, 4.5 vs 8.6 days; P = .001) with no statistically significant differences in the second phase. HIV-1 RNA suppression (<40 copies/mL) was reached earlier in SP (4 vs 12 weeks; P = .008) due to lower baseline HIV-1 RNA levels. The median total DTG 24 hours after the dose in SP was 119.1 ng/mL (range, 27.2–377 ng/mL), which represents 7.8% of BP exposure. The median DTG free-fraction in SP was 48% of the total drug. Seminal protein-unbound DTG concentrations exceeded the in vitro 50% inhibitory concentration (0.21 ng/mL) by a median of 214-fold.
Conclusions. DTG concentrations in SP are sufficient to contribute to rapid seminal HIV-1 RNA suppression.
Keywords: HIV-1; antiretroviral therapy; semen; male genital tract, HIV reservoirs; dolutegravir
(See the editorial commentary by Coombs and Krieger on pages 1473–4.)
Sexual transmission is the most common route of human immunodeficiency virus (HIV) acquisition in most regions of the world [1], and semen of HIV-infected men is a major vector for new HIV infections. Treatment of HIV-infected patients with currently available combined antiretroviral (ARV) therapy (ART) reduces the risk of HIV acquisition by their sexual partners [2, 3]. HIV transmission during unprotected sexual intercourse is associated with the presence of HIV in genital fluids, and the efficacy of ART in preventing new infection is based on the ability to reduce HIV viral load in these fluids [4]. In addition, the male genital tract is a separate reservoir for HIV [5] and may contribute to HIV shedding in seminal fluid, even in patients receiving ART [6, 7]. Thus, the ability of ARV drugs to penetrate into the male genital tract is a key factor for achieving HIV suppression in seminal fluid and preventing sexual transmission of the virus [8].
Dolutegravir (DTG) is a new integrase inhibitor (INI) with high antiviral potency and a high genetic barrier to resistance [9]. The pharmacokinetic profile of this drug allows once-daily administration in INI-naive patients. In large phase III-a randomized clinical trials, DTG (50 mg once daily) in combination with 2 nucleos(t)ide reverse-transcriptase inhibitors (NRTIs) has shown noninferiority compared with raltegravir and superiority to efavirenz or ritonavir-boosted darunavir as first-line therapy in treatment-naive HIV-1–infected patients [10–12].
However, DTG is highly bound to plasma proteins (>99%, mainly albumin and α acid glycoprotein) [13] and is a substrate for the efflux transporter P-glycoprotein and breast cancer resistance protein (BCRP) [14], which might limit its penetration into viral reservoirs, such as the genital tract. A recent study in healthy volunteers showed that DTG penetration in seminal fluid was <7% of DTG exposure in blood plasma (BP), and the median seminal concentration at the end of the dosing interval (C24h) was lower than the in vitro protein-adjusted (PA) 90% inhibitory concentration (IC90) for wild-type HIV-1 [15].
There is no information regarding DTG concentrations in the semen of HIV-1–infected patients or the antiviral activity of a DTG-based ARV combination in this compartment. The aim of the current study was to compare viral decay kinetics and DTG concentrations (total drug and unbound fraction) in the seminal plasma (SP) and BP in a group of treatment-naive HIV-1–infected patients starting DTG plus abacavir (ABC) and lamivudine (3TC) once daily.
METHODS
Study Design and Population
A prospective, single-arm, open-label, 24-week pilot study including 15 HIV-1–infected patients was conducted at the HIV outpatient clinic of the Bellvitge University Hospital in Barcelona, Spain, between February and September 2014. Eligible participants were male adults (≥18 years old) with chronic HIV-1 infection who had not been previously exposed to ART and had a screening plasma viral load (HIV-1 RNA) >5000 copies/mL, and negative status for the HLA-B*5701 allele. The exclusion criteria were evidence of primary viral resistance to the study drugs, concomitant sexually transmitted infection, hepatitis B, hepatitis C that might need treatment during the study, moderate or severe hepatic impairment, estimated glomerular filtration rate <60 mL/min, active opportunistic infections, or active malignant conditions. All participants received ART with DTG (50 mg) together with the NRTI combination of ABC (600 mg( plus 3TC (300 mg) in a fixed-dose tablet (Kivexa; ViiV Healthcare), all taken once daily.
The study protocol was approved by the ethics review committee of the Bellvitge University Hospital, in accordance with the principles of the 2008 Declaration of Helsinki and the Spanish regulatory authorities. Written informed consent was obtained from all participants before any study procedures were performed. This study was registered at the EU Clinical Trials Registry (EudraCT 2013-003243-36).
Procedures and Assessments
Study visits were scheduled at baseline, on days 3, 7, and 14, and at weeks 4, 12, and 24. At each study visit, paired blood and semen samples were obtained through peripheral venous puncture and self-masturbation, respectively, to assess HIV-1 RNA. At weeks 4 and 24, an additional set of blood and semen samples were collected 3 days apart for the analysis of DTG concentrations. These additional samples were planned in the study protocol to ensure that the quantity of seminal fluid would be enough to analyze both HIV-1 RNA and drug concentrations. Participants were advised to take ABC/3TC and DTG every day at same time in the morning. After confirmation that the last doses of ABC/3TC and DTG were taken correctly and on time, blood and semen samples were obtained at the end of the dosing interval (23–25 hours after the last dose and before the next). Participants were recommended to abstain from sexual activity for at least 72 hours before each semen sample was obtained.
In addition, CD4+ lymphocyte count, hematology, and chemistry (liver function, renal function, electrolytes and lipids) tests were performed at baseline and at weeks 4, 12, and 24. Clinical events and ART-related adverse events were recorded at each study visit.
Laboratory Methods
Sexually transmitted infections were ruled out in all participants before ART was started. Urinary leukocytes were measured by flow cytometry (negative at <6/µL), Chlamydia trachomatis and Neisseria gonorrhoeae were tested by polymerase chain reaction in urine samples (Xpert CT/NG; Cepheid), and syphilis was screened for by serologic testing in plasma.
Semen samples (collected at home or in a private room at hospital) were transported in sterile sample collection containers. Both blood and semen samples were processed within 2 hours after collection. Specimens were centrifuged to obtain BP and SP. HIV-1 RNA levels in BP and SP were measured using a real-time polymerase chain reaction assay (Abbott RealTime HIV-1) with a quantification limit of 40 copies/mL. The BP and SP samples used to measure DTG concentration were transferred to microvials and frozen at −80°C until analysis.
DTG concentrations were measured at the University of North Carolina Center for AIDS Research Clinical Pharmacology and Analytical Chemistry Core, using a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) method, as described elsewhere [15]. The calibration range was 20–20 000 ng/mL for BP and 1–1000 ng/mL for SP. All calibrators and quality control samples were within 15% of the nominal value for both within-day and between-day runs. Within-day and between-day analytical precision was <15%. Recovery of DTG and its internal standard with this method was approximately 100%. Protein-unbound DTG concentrations were analyzed in plasma and semen from 13 subjects using a modification of a method developed by Weller et al [16], with rapid equilibrium dialysis (Thermo Scientific) and LC/MS/MS analysis. To quantify both bound and unbound DTG concentrations, the extraction was slightly modified to allow for a calibration range of 0.25–5000 ng/mL. The removal of the final dilution step before analysis with an increased injection volume allowed for the new lower limit of quantification.
Statistical Methods
Nonparametric statistical tests were used in the between- and within-group comparisons (Mann–Whitney U and signed-rank tests, respectively) and to determine associations between variables (Spearman test). A log rank test and Cox regression model were used to assess the time to achieve HIV-1 RNA below the limit of detection. The ratio between log10 HIV RNA variances in BP and SP was evaluated using a paired version of the Levene test. Mean longitudinal changes in log10 HIV RNA in the paired samples were analyzed using nonlinear mixed-effects models, based on the equations suggested by Perelson et al [17] and Wu and Ding [18]. Each parameter was assumed to have a fixed effect, and, given the study design, 2-level nested random effects (individual|time point|compartment). Akaike and Bayesian information criteria were used for model selection. All analyses were carried out using the R (version 3.1.2) statistical package [19].
RESULTS
Of 16 patients screened, 15 who were eligible according to the study entry criteria were enrolled and completed the study. The participants' characteristics are summarized in Table 1. The median age (range) was 35 (22–60) years, the median CD4+ T-cell count at baseline was 504 (60–782) cells/µL, and the median BP and SP HIV-1 RNA levels were 5.03 (4.02–5.76) and 3.91 (2.97–4.82) log10 copies/mL, respectively. There were no differences in median BP or SP HIV-1 RNA levels according to B or non-B HIV-1 subtype (data not shown).
Table 1.
Patient Characteristics (n = 15)
| Characteristic | Value |
|---|---|
| Age, median (range), y | 35 (22–60) |
| HIV risk factor, No. (%) | |
| MSM | 12 (80) |
| Heterosexual | 3 (20) |
| HIV subtype, No. (%) | |
| B | 11 (73) |
| Non-Ba | 4 (27) |
| AIDS, No. (%) | 0 |
| CD4+ cell count at baseline, median (range), cells/µL | 504 (60–782) |
| HIV-1 RNA at baseline, median (range), log10 copies/mL | |
| Blood plasma | 5.03 (4.02–5.76) |
| Seminal plasma | 3.91 (2.97–4.82) |
Abbreviations: HIV, human immunodeficiency virus; MSM, men who have sex with men.
a Non-B subtypes include CRF02_AG (n = 2), CRF01_AE (n = 1), and G (n = 1).
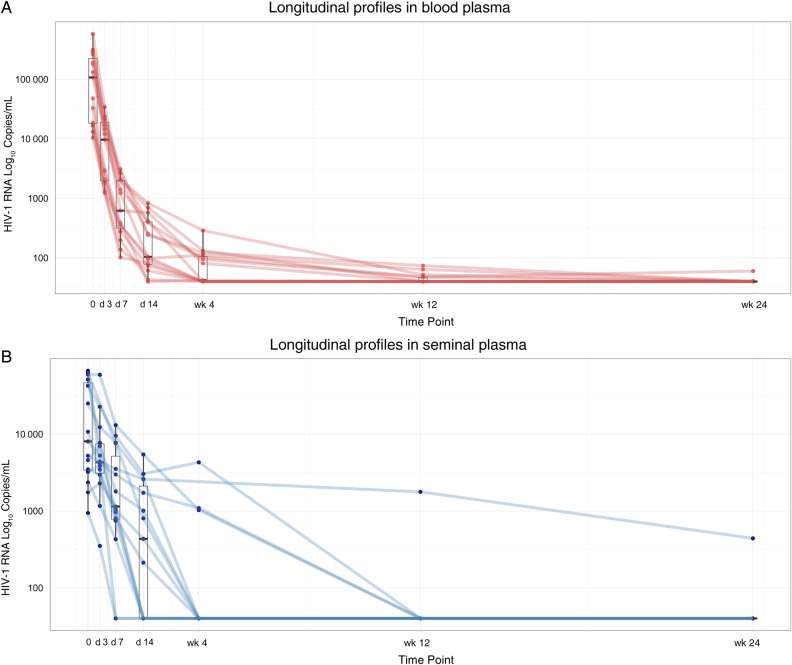
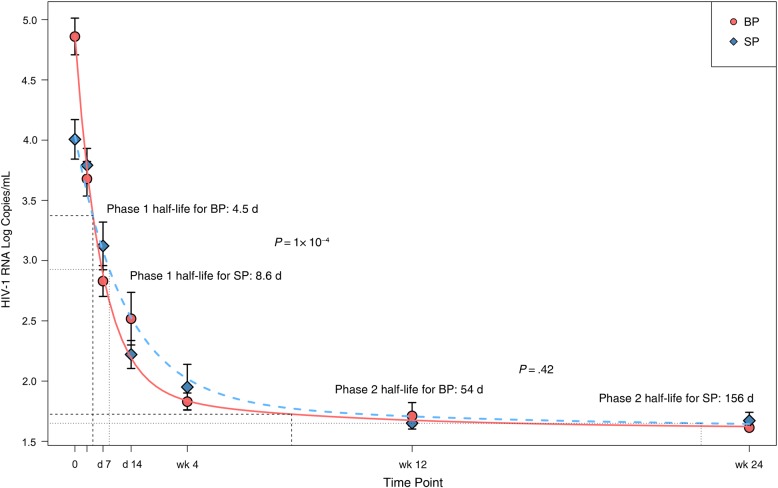
A significant correlation between HIV-1 RNA levels in BP and SP was observed at baseline (Spearman ρ = 0.575; P = .03). The HIV-1 RNA decrease at each study time point is shown in Figure 1 and Table 2. The median HIV-1 RNA decrease from baseline was significantly smaller in SP than in BP samples at all time points. Furthermore, the Levene test showed higher heterogeneity of variance in SP than in BP samples (data not shown). These findings are consistent with differing decay dynamics in the 2 compartments. To further evaluate the dynamics of viral decay after ART initiation based on HIV-1 RNA decreases in BP and SP samples, the model that best fit our data was 2-phase decay kinetics followed by a plateau (Figure 2 and Supplementary Table). Viral decay was significantly faster in BP than in SP in the first decay phase (half-life, 4.5 vs 8.6 days; P = .001). Although decay in the second phase was also faster in BP, the difference was not statistically significant (half-life, 54 vs 156 days; P = .43).
Figure 1.
Decline in human immunodeficiency virus (HIV) type 1 RNA decline through week 24; times points denote days (d) and weeks (wk) after baseline. A, Blood plasma. B, Seminal plasma.
Table 2.
HIV-1 RNA Decrease in BP and SP
| Time Point | HIV-1 RNA, Median (Range) Log10 Copies/mL |
HIV-1 RNA Below Limit of Quantification, No. of Samples/Total No.a |
HIV-1 RNA Decrease From Baseline, Median (Range) Log10 Copies/mL |
P Valueb | |||
|---|---|---|---|---|---|---|---|
| BP | SP | BP | SP | BP | SP | ||
| Baseline | 5.03 (4.02–5.76) | 3.91 (2.97–4.82) | 0/15 | 0/15 | |||
| Day 3 | 3.99 (3.09–4.54) | 3.63 (2.55–4.77) | 0/15 | 0/15 | −1.05 (−0.76 to −1.48) | −0.23 (+0.18 to −1.18) | .001 |
| Day 7 | 2.79 (2.01–3.49) | 3.06 (1.59–4.12) | 0/15 | 2/15 | −2.01 (−1.46 to −2.46) | −0.74 (−0.19 to −1.92) | .001 |
| Day 14 | 2.02 (1.59–2.92) | 2.64 (1.59–3.73) | 1/15 | 6/15 | −2.51 (−2.04 to −3.47) | −1.41 (−0.48 to −2.44) | .001 |
| Week 4 | 1.62 (1.59–2.46) | 1.59 (1.59–3.63) | 7/15 | 11/15 | −3.05 (−2.42 to −3.75) | −1.95 (−0.68 to −3.21) | .002 |
| Week 12 | 1.59 (1.49–1.87) | 1.59 (1.59–3.25) | 10/15 | 14/15 | −3.36 (−2.42 to −4.08) | −2.13 (−1.38 to −3.21) | .001 |
| Week 24 | 1.59 (1.59–1.78) | 1.59 (1.59–2.64) | 13/15 | 14/15 | −3.44 (−2.42 to −3.98) | −2.18 (−1.38 to −3.21) | .001 |
Abbreviations: BP, blood plasma; HIV, human immunodeficiency virus; SP, seminal plasma.
a The limit of detection for the quantitative real-time HIV-1 polymerase chain reaction RNA assay is 1.59 log10 copies/mL.
b Spearman P value for comparison of HIV-1 RNA decrease from baseline in BP vs SP samples.
Figure 2.
Human immunodeficiency virus (HIV) type 1 RNA decay dynamics in blood plasma (BP) and seminal plasma (SP).
The median time to HIV-1 RNA <40 copies/mL was significantly shorter in SP than in BP samples (4 vs 12 weeks; P = .008). However, in a Cox regression model adjusted by HIV-1 RNA higher or lower than 5 log at baseline, no differences were found between the 2 compartments (P = .88). HIV-1 RNA suppression at week 4 occurred in SP in 11 individuals and in BP in 7. BP and SP viral loads at baseline did not differ significantly between patients with seminal HIV-1 RNA persistence or suppression at week 4 (4.39 vs 4.25 and 3.96 vs 3.66 log10 copies/mL, respectively; P = .24 and P = .79).
Two patients had detectable HIV-1 RNA in BP at week 24 (60 and 41 copies/mL, respectively), whereas HIV-1 RNA in SP of these patients had already fallen to <40 copies/mL at weeks 2 and 4, respectively. Both patients achieved BP HIV-1 RNA <40 copies/mL 8 weeks later. One other patient had detectable seminal HIV-1 RNA (441 copies/mL) at week 24, whereas the BP viral load had been <40 copies/mL since week 2. This patient, who remained on the same ART regimen, consented to provide a new semen sample 19 weeks after completion of the study, when the HIV-1 RNA level was <40 copies/mL.
A significant correlation was observed between DTG concentrations in the paired BP and SP samples (Spearman P < .001). The median (range) total DTG concentrations in BP at the end of the dosing interval (C24h) at weeks 4 and 24 were 1200 (124–2290) and 1420 (353–3100) ng/mL (Table 3), which exceed 18.7- and 22.2-fold, respectively, the in vitro PA-IC90 for wild-type HIV-1 (64 ng/mL). The median total DTG concentrations in SP at weeks 4 and 24 were 92.2 (15.7–331) and 129 (38.8–423) ng/mL, respectively (Table 3). Overall, the median SP-to-BP ratio was 7.83% (3.65%–20.54%). The total DTG C24h was below the in vitro PA-IC90 in 5 patients at week 4 and also at week 24 in 2. Those with the lowest seminal DTG C24h also had the lowest BP DTG C24h.
Table 3.
DTG Concentrations in BP and SPa
| Patient | DTG in BP, ng/mL |
DTG Free Fraction in BP, % | DTG in SP, ng/mL |
DTG Free Fraction in SP, % | SP/BP Ratio, % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 4 | Week 24 | Mean | Week 4 | Week 24 | Mean | Week 4 | Week 24 | Mean | |||
| 1 | 2290 | 924 | 1607 | 0.441 | 171 | 67.2 | 119.1 | 52.8 | 7.47 | 7.27 | 7.37 |
| 2 | 124 | 353 | 238.5 | NA | 15.7 | 38.8 | 27.25 | 34.7 | 12.66 | 10.99 | 11.83 |
| 3 | 1710 | 2230 | 1970 | 0.557 | 137 | 152 | 144.5 | 50.8 | 8.02 | 6.81 | 7.41 |
| 4 | 1330 | 2000 | 1665 | 0.457 | 141 | 207 | 174 | 42.6 | 10.60 | 10.35 | 10.47 |
| 5 | 1460 | 1750 | 1605 | 0.412 | 92.6 | 164 | 128.3 | 34.2 | 6.342 | 9.37 | 7.86 |
| 6 | 1340 | 3100 | 2220 | NA | 92.5 | 264 | 178.25 | NA | 6.90 | 8.51 | 7.71 |
| 7 | 1200 | 1480 | 1340 | NA | 92.2 | 129 | 110.6 | NA | 7.68 | 8.71 | 8.19 |
| 8 | 1390 | 2450 | 1920 | 0.415 | 331 | 423 | 377 | 48.0 | 23.81 | 17.26 | 20.54 |
| 9 | 864 | 1420 | 1142 | 0.439 | 23.9 | 64.5 | 44.2 | 42.4 | 2.77 | 4.54 | 3.65 |
| 10 | 1100 | 829 | 964.5 | 0.470 | 160 | 182 | 171 | 51.9 | 14.5 | 21.95 | 18.25 |
| 11 | 1050 | 1990 | 1520 | 0.447 | 88.5 | 233 | 160.75 | 48.6 | 8.43 | 11.71 | 10.07 |
| 12 | 838 | 1290 | 1064 | 0.455 | 32.1 | 111 | 71.55 | 51.5 | 3.83 | 8.60 | 6.22 |
| 13 | 677 | 627 | 652 | 0.481 | 33 | 47 | 40 | 59.7 | 4.87 | 7.49 | 6.18 |
| 14 | 805 | 1010 | 907.5 | 0.387 | 60.9 | 72.9 | 66.9 | 38.6 | 7.56 | 7.22 | 7.39 |
| 15 | 1200 | 1060 | 1130 | 0.459 | 70.2 | 104 | 87.1 | 47.1 | 5.85 | 9.81 | 7.83 |
| Median | 1200 | 1420 | 1340 | 0.455 | 92.2 | 129 | 119.1 | 48 | 7.56 | 8.71 | 7.83 |
Abbreviations: BP, blood plasma; DTG, dolutegravir; NA, not available; SP, seminal plasma.
a DTG concentrations at the end of the dosing interval (24 hours).
Unbound DTG concentrations in paired BP and SP samples could be analyzed in 13 of the 15 participants (including those with the lowest total DTG concentrations). The median (range) unbound DTG fractions in BP and SP were 0.45% (0.39%–0.56%) and 48% (34.2%–59.7%) of total drug concentration, respectively (Table 3). Extrapolating these percentages of DTG free concentrations to the total concentrations measured at weeks 4 and 24, the estimated median (range) unbound DTG concentrations in BP were 5.17 (3.11–10.01) and 6.23 (3.02–12.42) ng/mL, respectively. The estimated unbound DTG concentrations in SP were 34 (5.4–158.8) and 56 (13.4–203) ng/mL, which exceed 170- and 280-fold the in vitro unbound 50% inhibitory concentration (IC50) for wild type HIV-1 (0.21 ng/mL). Thus, the estimated average free DTG C24h in SP (45.05 ng/mL) was 214-fold higher than the in vitro unbound IC50, and the patient with the lowest total DTG C24h observed in SP would have free seminal DTG C24h 26-fold the in vitro unbound IC50 (Table 3).
The patients with detectable HIV-1 RNA in BP or SP samples at week 24 had total DTG concentrations above the in vitro PA-IC90 in both compartments. Interestingly, the patient with detectable seminal HIV-1 RNA at week 24 had the highest seminal DTG concentrations at both 4 and 24 weeks. No significant correlations were found between DTG concentrations at day 28 and time to HIV-1 RNA <40 copies/mL (Spearman P = .84).
DISCUSSION
HIV-1 RNA suppression in seminal fluid might be limited by ARV penetration into the male genital tract and compartmentalized infection in this HIV reservoir. The antiviral activity of a DTG-based ARV regimen in the genital tract of HIV-1–infected male patients has not been assessed to date. Furthermore, determination of HIV-1 decay kinetics in seminal fluid might help to better understand the role of DTG-based regimens for preventing HIV-1 transmission. In the current study, we found that HIV-1 RNA decay dynamics in HIV-1–infected patients starting DTG plus ABC/3TC as first-line ART differ in the 2 compartments, being faster in BP than in SP, although statistically significant differences were observed only in the first decay phase.
We also found that the DTG concentration in SP was 7.8% that seen in BP, similar to the findings reported in healthy volunteers [15], with the DTG C24h lower than the in vitro PA-IC90 in some patients. Nevertheless, all except 1 of the 15 patients studied achieved rapid HIV-1 RNA suppression in SP, with an even shorter median time to suppression than in BP.
In addition to other physicochemical properties, such as molecular weight or lipophilicity, ARV penetration into the genital tract is thought to be determined mainly by BP protein binding [20]. Thus, the low penetration of DTG into the male genital tract may be explained mainly by the drug's high BP protein binding (>99%). However, total DTG seminal exposure was higher than the free drug concentration in BP, suggesting that drug uptake transporters in addition to passive diffusion of unbound drug may be implicated in DTG penetration in the male genital tract [21].
Because of lower drug binding protein concentrations in SP than in BP [20, 22], protein binding of certain ARV drugs has been found to be lower in SP [23]. Unbound DTG C24h was measured in 13 of 15 participants. The median free DTG fraction in BP was 0.45% of the total drug, concordant with previously published data [13], whereas the median free DTG fraction in SP was 48% of the total drug. Therefore, we could estimate that the seminal unbound DTG C24h was a median of 214-fold higher than the in vitro unbound IC50 (0.21 ng/mL). The subject with the lowest observed total DTG C24h in SP had an unbound SP concentration 26-fold above the in vitro unbound IC50. Of note, 4 of the 5 patients with the lowest total DTG concentrations experienced rapid suppression of seminal HIV-1 RNA, even before that of BP.
Although ABC and 3TC achieve high seminal concentrations [20, 24, 25] and the activity of this NRTI backbone must also be taken also into account, the high free DTG concentrations in SP supports the contribution of DTG to HIV-1 suppression in this compartment. The only patient with detectable HIV-1 RNA at week 24 had the highest seminal DTG concentrations and the highest DTG SP/BP ratio. Resistance-associated mutations to INIs or NRTI were not observed with baseline BP genotypic resistance testing. We attempted to perform this testing on a stored baseline seminal remnant sample, but it was not technically possible. Further testing showed that this patient achieved HIV-1 RNA suppression after the study period. In this case, compartmentalized HIV replication in the genital tract might have been the obstacle preventing earlier HIV suppression rather than insufficient ARV levels in this reservoir.
Consistent with these findings, no significant correlation was observed between DTG concentrations and the time to achieve HIV-1 RNA below the detection limit in each compartment. Interestingly, a good correlation between BP DTG concentration and HIV-1 RNA decline after 10 days of DTG monotherapy has been observed in a previous phase IIa trial [26]. This differing pharmacodynamic behavior highlights the multiple factors that can influence HIV suppression in seminal fluid, including drug penetration, compartmentalized and autonomous HIV replication, and the possible interindividual variability related to these aspects.
A significant correlation between HIV-1 RNA in BP and SP was observed at baseline. Median HIV-1 RNA in SP at baseline was 1 log lower compared to median HIV-1 RNA in BP that is concordant with other previously published studies [4]. At week 24, all but 2 patients had HIV-1 RNA <40 copies/mL in BP although it is worthy of note that these 2 patients had a favorable HIV-1 RNA decrease (41 and 60 copies/mL). Only 1 patient did not have a favorable virologic response in SP, with the HIV-1 RNA level remaining at 441 copies/mL while undetectable in BP; that finding is concordant with compartmentalized HIV-1 RNA dynamics in the genital tract, as has also been previously described [5–7].
Significantly smaller decreases in HIV-1 RNA in SP compared with BP were observed at each time point, as well as greater heterogeneity in HIV-1 RNA decline over time. This heterogeneity is also concordant with the compartmentalized dynamics of HIV infection in the genital tract and the role of different organs within the male genital tract as the source of virions and/or infected cells for semen, such as the epididymis, seminal vesicles, prostate, and urethra [27].
We observed a faster HIV-1 RNA decay in BP than in SP, although statistically significant differences were only observed in the first decay phase. Although the absence of significant differences in the second decay phase could be explained by the limited sample size and the low HIV-1 RNA values in this second phase, a similar pattern of initially slower HIV-1 RNA decay in seminal fluid than in plasma, but no difference in the second-phase decay rate has been also observed in patients receiving efavirenz-based ART [28].
However, the median time to HIV-1 RNA <40 copies/mL was significantly shorter in SP than in BP samples, that can be explained by the lower seminal viral load at baseline. DTG and other INIs have shown earlier plasma HIV-1 suppression than either efavirenz or protease inhibitors [11, 12, 29, 30]. Although patients starting ART have a significantly lower risk of HIV transmission than untreated patients [3], a very rapid HIV-RNA decline and suppression in seminal fluid might result in an even lower probability of transmission immediately after starting therapy, which could be of interest in certain high-risk individuals. Interestingly, a recent study in serodiscordant couples has demonstrated high efficacy in preventing HIV transmission when preexposure prophylaxis is used by the HIV-negative partners of HIV-1–infected patients before and during the first 6 months after starting ART [31]. The early seminal HIV-1 suppression achieved by DTG-based regimens, and perhaps other INIs, could reduce the time needed for preexposure prophylaxis in serodiscordant couples with a high risk of HIV transmission.
Our study has some limitations. As in other studies assessing pharmacokinetics in these viral reservoirs, our sample size is small, which makes the results more susceptible to influence by interindividual and intraindividual variability. Nevertheless, we performed 2 separate determinations of seminal and BP DTG concentrations in each patient, and our results are similar to those observed in healthy volunteers. On the other hand, we only measured HIV-1 RNA in SP; therefore, the role of ongoing HIV-1 RNA production from long-lived infected cells in semen was not considered in our model.
In summary, this study showed that despite an initially slower HIV-1 RNA decline in SP than in BP samples, rapid HIV-1 RNA suppression in seminal fluid is achieved in most patients starting first-line ART with DTG plus ABC/3TC. These findings could be of interest for reducing the HIV transmission risk after ART initiation. Although the seminal DTG concentration is only 7.8% of the concentration in BP, it is sufficient to contribute to suppress HIV-1 replication in this compartment.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We are grateful to all the patients who participated in this study. We thank Antonio Navarro for assistance in samples processing and Celine Cavallo for English language support.
Author contributions. A. I. and D. P. designed the study; A. I., E. F., and D. P. recruited participants; A. I., E. F., and A. V. conducted the study visits; J. N. performed the microbiologic procedures; A. D. M. K. and C. S. performed LC-MS/MS to measure Dolutegravir concentrations in plasma and seminal fluid; N. R. and L. A. assisted in data collection and study coordination; D. O. and J. C. performed the statistical analysis; A. I., J. M.-P., and D. P. analyzed and interpreted the results; A. I. drafted the manuscript and J. M.-P., A. D. M. K., and D. P. reviewed it. All authors revised the manuscript for important intellectual content and contributed to the final version.
Disclaimer. ViiV Healthcare was given the opportunity to review a preliminary version of this manuscript for factual accuracy. The authors are solely responsible for the study design and the final content and interpretation.
Financial support. This work was provided by ViiV Healthcare. The study was also partially supported by the RD12/0017/0013 project as part of the Plan Nacional R + D + I and co-financed by Instituto de Salud Carlos III- Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER), the University of North Carolina Center for AIDS Research (grant P30 AI050410 to A. D. M. K. and C. S.), Collaboratory of AIDS Researchers for Eradication (grant U19 AI096113 to A. D. M. K. and C. S.), and the National Institutes of Health (grant R01 AI111891 to A. D. M. K. and C. S.).
Potential conflicts of interest. A. I. has received financial compensation for lectures, consultancies, and educational activities or funds for research from Bristol-Myers Squibb (BMS), Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. J. M.-P. has received financial compensation for consultancies, lectures, and educational activities and research support from Merck Sharp & Dohme, BMS, ViiV Healthcare, and Gilead. J. N. has received financial compensation for lectures and research from Abbott Molecular. A. D. M. K. has received financial compensation for a consultancy from Merck and grant support from Gilead, GlaxoSmithKline, and Merck. D. P. has received research grants and/or honoraria for advisories and/or conferences from Boehringer Ingelheim, GlaxoSmithKline, ViiV Healthcare, Pfizer, BMS, Abbott, Gilead, Janssen, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UN Joint Programme on HIV/AIDS (UNAIDS). The gap report, 2014. http://www.refworld.org/docid/53f1e1604.html. Accessed 8 April 2015.
- 2.Donnell D, Baeten JM, Kiarie J et al. ; Partners in Prevention HSV/HIV Transmission Study Team. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375:2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M et al. ; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Kahle E, Lingappa JR et al. ; Partners in Prevention HSV/HIV Transmission Study Team. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011; 3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JA, Ping LH, Dibben O et al. . Center for HIV/AIDS vaccine immunology. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog 2010; 6:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang H, Dornadula G, Beumont M et al. . Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med 1998; 339:1803–9. [DOI] [PubMed] [Google Scholar]
- 7.Lambert-Niclot S, Tubiana R, Beaudoux C et al. . Detection of HIV-1 RNA in seminal plasma samples from treated patients with undetectable HIV-1 RNA in blood plasma on a 2002–2011 survey. AIDS 2012; 26:971–5. [DOI] [PubMed] [Google Scholar]
- 8.Taylor S, Davies S. Antiretroviral drug concentrations in the male and female genital tract: implications for the sexual transmission of HIV. Curr Opin HIV AIDS 2010; 5:335–43. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M, Yoshinaga T, Seki T et al. . In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother 2011; 55:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raffi F, Rachlis A, Stellbrink HJ et al. ; SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013; 381:735–43. [DOI] [PubMed] [Google Scholar]
- 11.Walmsley SL, Antela A, Clumeck N et al. ; SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 12.Clotet B, Feinberg J, van Lunzen J et al. ; ING114915 Study Team. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
- 13.Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet 2013; 52:981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reese MJ, Savina PM, Generaux GT et al. . In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos 2013; 41:353–61. [DOI] [PubMed] [Google Scholar]
- 15.Greener BN, Patterson KB, Prince HM et al. . Dolutegravir pharmacokinetics in the genital tract and colorectum of HIV-negative men after single and multiple dosing. J Acquir Immune Defic Syndr 2013; 64:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weller S, Borland J, Chen S et al. . Pharmacokinetics of dolutegravir in HIV-seronegative subjects with severe renal impairment. Eur J Clin Pharmacol 2014; 70:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 1996; 271:1582–6. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Ding AA. Population HIV1 dynamics in vivo: applicable models and inferential tools for virological data from AIDS clinical trials. Biometrics 1999; 55: 410–8. [DOI] [PubMed] [Google Scholar]
- 19.Gentleman R, Ihaka R, Bates D. The R project for statistical computing. 2009. http://www.rproject.org/254. Accessed 17 February 2015.
- 20.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther 2011; 16:1149–67. [DOI] [PubMed] [Google Scholar]
- 21.Klein DM, Wright SH, Cherrington NJ. Xenobiotic transporter expression along the male genital tract. Reprod Toxicol 2014; 47:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lizana J, Blad E. Immunonephelometry of specific proteins in human seminal plasma. Clin Chem 1983; 29:618–23. [PubMed] [Google Scholar]
- 23.Brown KC, Patterson KB, Jennings SH et al. . Single and multiple dose pharmacokinetics of darunavir plus ritonavir and etravirine in semen and rectal tissue of HIV-negative men. J Acquir Immune Defic Syndr 2012; 61:138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira AS, Kashuba AD, Fiscus SA et al. . Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J Infect Dis 1999; 180:2039–43. [DOI] [PubMed] [Google Scholar]
- 25.van Praag RM, van Heeswijk RP, Jurriaans S, Lange JM, Hoetelmans RM, Prins JM. Penetration of the nucleoside analogue abacavir into the genital tract of men infected with human immunodeficiency virus type 1. Clin Infect Dis 2001; 33:e91–2. [DOI] [PubMed] [Google Scholar]
- 26.Min S, Sloan L, DeJesus E et al. . Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS 2011; 25:1737–45. [DOI] [PubMed] [Google Scholar]
- 27.Houzet L, Matusali G, Dejucq-Rainsford N. Origins of HIV-infected leukocytes and virions in semen. J Infect Dis 2014; 210(suppl 3):S622–30. [DOI] [PubMed] [Google Scholar]
- 28.Graham SM, Holte SE, Dragavon JA et al. . HIV-1 RNA may decline more slowly in semen than in blood following initiation of efavirenz-based antiretroviral therapy. PLoS One 2012; 7:e43086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lennox JL, DeJesus E, Lazzarin A et al. ; STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naïve patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009; 374:796–806. [DOI] [PubMed] [Google Scholar]
- 30.DeJesus E, Rockstroh JK, Henry K et al. ; GS-236-0103 Study Team. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate versus ritonavir-boosted atazanavir plus co-formulated emtricitabine and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet 2012; 379:2429–38. [DOI] [PubMed] [Google Scholar]
- 31.Baeten J, Heffron R, Kidoguchi L et al. . Near elimination of HIV transmission in a demonstration project of PrEP and ART. In: Conference on Retroviruses and Opportunistic Infections (CROI) 2015 23–26 February 2015; Seattle, Washington Abstract 24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.