ABSTRACT
In air-breathing vertebrates at high altitude, fine-tuned adjustments in hemoglobin (Hb)–O2 affinity provide an energetically efficient means of mitigating the effects of arterial hypoxemia. However, it is not always clear whether an increased or decreased Hb–O2 affinity should be expected to improve tissue O2 delivery under different degrees of hypoxia, due to the inherent trade-off between arterial O2 loading and peripheral O2 unloading. Theoretical results indicate that the optimal Hb–O2 affinity varies as a non-linear function of environmental O2 availability, and the threshold elevation at which an increased Hb–O2 affinity becomes advantageous depends on the magnitude of diffusion limitation (the extent to which O2 equilibration at the blood–gas interface is limited by the kinetics of O2 exchange). This body of theory provides a framework for interpreting the possible adaptive significance of evolved changes in Hb–O2 affinity in vertebrates that have colonized high-altitude environments. To evaluate the evidence for an empirical generalization and to test theoretical predictions, I synthesized comparative data in a phylogenetic framework to assess the strength of the relationship between Hb–O2 affinity and native elevation in mammals and birds. Evidence for a general trend in mammals is equivocal, but there is a remarkably strong positive relationship between Hb–O2 affinity and native elevation in birds. Evolved changes in Hb function in high-altitude birds provide one of the most compelling examples of convergent biochemical adaptation in vertebrates.
KEY WORDS: Biochemical adaptation, Blood oxygen transport, Hemoglobin, High-altitude adaptation, Hypoxia, Physiological adaptation
Summary: Evolved changes in hemoglobin–oxygen affinity in high-altitude birds and mammals provide striking examples of convergent biochemical adaptation.
Introduction
An adaptive trend in phenotypic evolution is indicated when genetically based trait variation is consistently associated with the same environmental factors in multiple taxa, and when the pattern of co-variation exhibits a regularity that cannot be explained by chance alone. Such generalizations or ‘ecogeographic rules’ are instructive about relationships between form and function, and they motivate the development of mathematical models to describe particular features that organisms might be expected to possess in particular environments. These models can be used to explain evolved trait differences among organisms that inhabit different environments, or to predict features of organisms based on information about their habitats.
In comparative physiology, a fairly well-accepted empirical generalization is that vertebrate taxa that are native to high-altitude environments tend to have elevated hemoglobin (Hb)–O2 affinities in comparison with lowland relatives (Hall et al., 1936; Bullard, 1972; Lenfant, 1973; Bunn, 1980; Monge and León-Velarde, 1991; Weber, 1995, 2007; Storz, 2007; Powell and Hopkins, 2010; Storz et al., 2010b). However, this putative trend is based on a relatively small number of case studies, and comparative data have not always been interpreted in a well-informed phylogenetic framework. There are also reasons to question whether an elevational trend should generally be expected, as theory predicts that the optimal Hb–O2 affinity is a non-monotonic function of the ambient partial pressure of O2 (PO2). Thus, if a given lowland species colonizes a high-altitude environment, it is not always clear whether an increased or decreased Hb–O2 affinity should be expected to improve tissue O2 delivery, and this has been the subject of considerable debate (Barcroft et al., 1923; Aste-Salazar and Hurtado, 1944; Lenfant et al., 1968, 1969, 1971; Eaton et al., 1969; Torrance et al., 1970/71; Bullard, 1972; Turek et al., 1973; Eaton et al., 1974; Dempsey et al., 1975; Frisancho, 1975; West and Wagner, 1980; Bencowitz et al., 1982; Willford et al., 1982; Samaja et al., 1986, 2003; Mairbäurl, 1994).
The purpose of this Review is to evaluate the evidence for an empirical generalization about the relationship between Hb–O2 affinity and native elevation in terrestrial vertebrates. I start by providing an overview of Hb function and allosteric regulatory control (see Glossary). I then review theoretical and experimental results that demonstrate how the optimal Hb–O2 affinity varies as a function of environmental O2 availability. Finally, I synthesize comparative data in a phylogenetic framework to assess the strength of the relationship between Hb–O2 affinity and native elevation in mammals and birds, the vertebrate groups for which the most data are available.
Challenges to respiratory gas transport under hypoxia
In order for air-breathing vertebrates to cope with the low ambient PO2 at high altitude, blood O2 transport capacity must be increased to sustain O2 flux to the tissue mitochondria in support of aerobic ATP synthesis (Mairbäurl, 1994; Samaja et al., 2003; Storz et al., 2010b; Scott, 2011; Mairbäurl and Weber, 2012). Such changes complement physiological adjustments in other convective and diffusive steps in the O2 transport pathway (Bouverot, 1985; Scott and Milsom, 2006). Although an increased Hb–O2 affinity helps to safeguard arterial O2 saturation under environmental hypoxia, it can hinder O2 unloading in the systemic circulation. For this reason, vertebrates at high altitude face the physiological challenge of optimizing the trade-off between O2 loading in the pulmonary capillaries and O2 unloading in the tissue capillaries.
The oxygenation properties of blood reflect inherent properties of the Hb protein as well as the physicochemical operating conditions for Hb in the red blood cell. Below, I briefly describe the intrinsic O2-binding properties of the Hb protein, and I then explain how these properties are modulated by metabolically induced changes in the erythrocytic microenvironment.
List of symbols and abbreviations.
- βbO2
blood O2 capacitance coefficient
- CaO2
arterial O2 content
- CvO2
venous O2 content
- DPG
2,3-diphosphoglycerate
- Hb
hemoglobin
- IHP
inositol hexaphosphate
- IPP
inositol pentaphosphate
- n
Hill coefficient
- P50
partial pressure of O2 at which Hb is half-saturated
- PO2
partial pressure of O2
- PaO2
arterial O2 pressure
- PvO2
venous O2 pressure
cardiac output
- SO2
fraction of O2-saturated Hb relative to total Hb (unsaturated+saturated) in the blood
- SaO2
arterial O2 saturation
- SvO2
venous O2 saturation
- V̇O2
rate of O2 consumption
Hb function and allosteric regulatory control
The Hbs of jawed vertebrates are heterotetramers, composed of two α-type and two β-type subunits. Each of the subunit polypeptides contains a covalently bound heme group (see Glossary) that can reversibly bind a single O2 molecule when the iron atom is in the ferrous (Fe2+) state; thus tetrameric Hb binds up to four O2 molecules. The Hb tetramer, α2β2, consists of paired, semi-rigid αβ dimers that undergo a symmetrical rotation during the oxygenation-linked transition in quaternary structure between the high-affinity (oxygenated) ‘R’ state and the low-affinity (deoxygenated) ‘T’ state (Fig. 1A). The oxygenation-linked shift in the T↔R conformational equilibrium is central to homotropic allostery (cooperative O2-binding that stems from subunit–subunit interaction) and heterotropic allostery (regulation of heme reactivity by ligands that bind at sites remote from the heme pocket) (Perutz, 1970; Baldwin and Chothia, 1979).
Glossary.
Allosteric regulation
Regulation of protein activity by binding a cofactor molecule at a site other than the protein's active site; the binding of allosteric cofactors typically induces a change in protein conformation.
Bohr effect
The modulation of Hb–O2 affinity by changes in pH and CO2 content. In the physiological range, the O2 affinity of vertebrate Hb is inversely related to the acidity and CO2 concentration of the blood.
Donnan equilibrium
The ionic equilibrium attained in an electrolyte solution when diffusible and non-diffusible ions are separated by a semi-permeable membrane.
Heme group
A porphyrin ring that coordinates an iron atom at the centre; it serves as a prosthetic group for Hb and other hemoproteins.
Homotropic allostery
Modulation of protein activity by binding a ligand that serves as a substrate for the protein (O2 in the case of Hb), but which also serves as a regulatory molecule. For example, heme–O2 binding at one Hb subunit alters the heme reactivity of other subunits in the same tetrameric assembly.
Heterotropic allostery
Modulation of protein activity by binding a cofactor that is not a substrate for the protein; hydrogen ions, chloride ions and organic phosphates are examples of heterotropic regulators of Hb function.
Hypoxemia
Reduced PO2 in arterial blood.
Fig. 1.
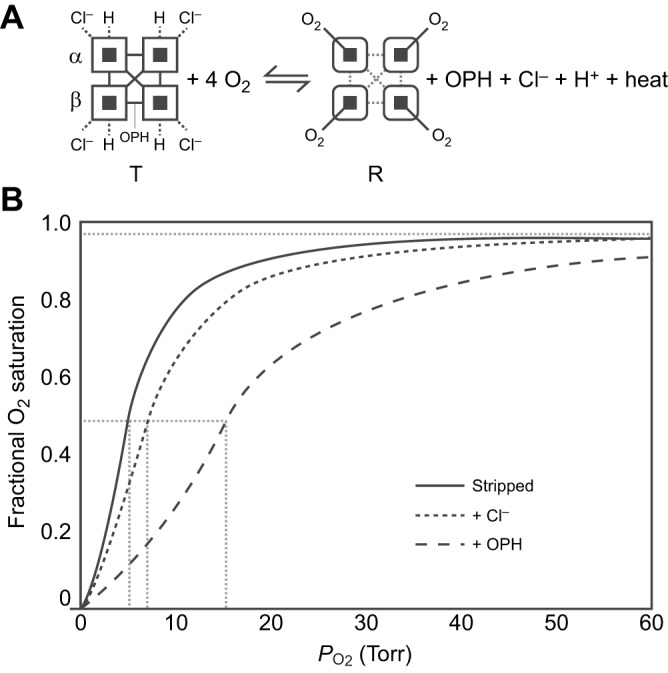
The allosteric regulation of hemoglobin (Hb)–O2 affinity. (A) The oxygenation reaction of tetrameric Hb (α2β2) involves an allosteric transition in quaternary structure from the low-affinity T-state to the high-affinity R-state. The oxygenation-induced T→R transition entails a breakage of salt bridges and hydrogen bonds within and between subunits (open squares), dissociation of allosterically bound organic phosphates (OPHs), Cl− ions and protons, and the release of heat (heme oxygenation is an exothermic reaction). Oxygenation-linked proton binding occurs at multiple residues in the α- and β-chains, Cl− binding mainly occurs at the N-terminal α-amino groups of the α- and β-chains in addition to other residues in both chains, and phosphate binding occurs between the β-chains in the central cavity of the Hb tetramer. (B) O2 equilibrium curves for purified Hb in the absence of allosteric effectors (Stripped) and in the presence of chloride ions (+Cl−) and organic phosphates (+OPH). The preferential binding of allosteric effectors to deoxyHb stabilizes the T-state, thereby shifting the allosteric equilibrium in favour of the low-affinity quaternary structure. The O2 equilibrium curves are therefore right-shifted (Hb–O2 affinity is reduced) in the presence of such effectors. Hb–O2 affinity is indexed by the P50 value (dashed grey lines) – the PO2 at which Hb is half-saturated. The sigmoidal shape of the O2 equilibrium curves reflects cooperative O2 binding, involving a PO2-dependent shift from low- to high-affinity conformations.
Homotropic allostery
The cooperativity of Hb–O2 binding stems from an interaction between subunits of the tetrameric protein. The binding of O2 to the heme iron of each subunit produces a localized change in tertiary structure that is transmitted to each of the other unliganded heme-bearing subunits, triggering the T→R shift in quaternary structure [Perutz, 1970, 1979; Baldwin and Chothia, 1979; Gelin et al., 1983; Perutz et al., 1987; Liddington et al., 1988; extensions of this two-state allosteric mechanism are discussed by Yonetani and Tsuneshige (2003) and Eaton et al. (2007)]. The binding of O2 to each heme therefore increases the O2 affinity of the remaining unliganded hemes in the same Hb tetramer and, conversely, O2 released by each heme reduces the O2 affinity of the remaining liganded hemes. The physiological significance of cooperativity is that it permits efficient O2 unloading over a relatively narrow range of blood PO2. This property is manifest in the sigmoidal shape of the O2 equilibrium curve (Fig. 1), which describes how the fractional saturation of Hb varies as a function of PO2. This relationship is quantified by the Hill equation (Hill, 1910):
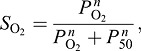 |
(1) |
where SO2 is the fractional saturation, PO2 is the partial pressure of O2 in Torr, P50 is the PO2 at which Hb is 50% saturated, and n is the cooperativity coefficient. The Hill equation is extended by the more complex Adair equation (Adair, 1925), which expresses SO2 as a function of PO2 and association constants for binding each of the four O2 molecules per Hb tetramer.
Heterotropic allostery
Heterotropic mechanisms of regulating Hb–O2 affinity involve the oxygenation-linked binding of non-heme ligands such as H+, Cl−, CO2, lactate and organic phosphates. Binding sites for these allosteric effectors are mainly located at the N- and C-termini of the globin subunits and in the positively charged central cavity of the Hb tetramer (Arnone, 1972; Arnone and Perutz, 1974; O'Donnell et al., 1979; Nigen et al., 1980). Different organic phosphates serve as allosteric effectors in the definitive red blood cells of different groups of terrestrial vertebrates: 2,3-diphosphoglycerate (2,3-DPG) in mammals, inositol pentaphosphate (IPP) in birds, adenosine triphosphate (ATP) and guanosine triphosphate (GTP) in non-avian reptiles, and ATP and DPG in amphibians (Rapoport and Guest, 1941; Benesch and Benesch, 1967; Bartlett, 1980; Hazard and Hutchison, 1982; Weber, 1995; Weber and Fago, 2004). These polyphosphate molecules electrostatically bind to a constellation of cationic residues lining the cleft between the β-chains of deoxyHb, thereby stabilizing the T-state through the formation of salt bridges within and between the α- and β-chain subunits (Arnone, 1972; Arnone and Perutz, 1974). Phosphate binding has the effect of reducing Hb–O2 affinity by shifting the conformational equilibrium in favour of the low-affinity T-state; this allosteric transition in quaternary structure promotes O2 unloading to the cells of respiring tissues. As organic phosphates are non-diffusible anions, changes in their intracellular concentration also exert an indirect effect on O2 affinity by perturbing the Donnan equilibrium (see Glossary) of protons across the red cell membrane, as changes in cellular pH modulate Hb–O2 binding via the Bohr effect (see Glossary).
Whereas cooperativity accounts for the shape of the O2 equilibrium curve, the O2 affinity of Hb determines the position of the curve along the x-axis. As shown in Fig. 1B, the curve is shifted to the right [corresponding to a reduction in Hb–O2 affinity (increased P50)] or to the left [corresponding to an increased Hb–O2 affinity (decreased P50)] in response to changes in temperature, pH and erythrocytic concentrations of Cl− ions and organic phosphates.
Changes in Hb–O2 affinity
Evolutionary changes in Hb−O2 affinity
Evolutionary changes in Hb–O2 affinity can involve changes in the intrinsic O2 affinity of Hb and/or changes in the responsiveness to allosteric effectors. The former mechanism can involve changes in the equilibrium constants of heme–O2 binding in the R- or T-state, or changes in the allosteric equilibrium constants for the R↔T transition. These genetically based modifications of Hb function are attributable to amino acid replacements in the α- and/or β-type subunits (Weber, 1995, 2007; Bellelli et al., 2006; Storz and Moriyama, 2008).
Reversible changes in Hb–O2 affinity
Reversible changes in Hb–O2 affinity can be achieved by modulating intraerythrocytic pH and/or the concentration of organic phosphates or other allosteric effectors (Nikinmaa, 2001; Jensen, 2004, 2009). These changes in the chemical milieu of the red blood cell alter the operating conditions for Hb, but are not associated with structural changes in the Hb protein itself.
In principle, reversible changes can also be produced by cellular changes in Hb isoform composition. All vertebrates possess multiple α- and β-type globin genes, and therefore express multiple, structurally distinct Hb isoforms during different stages of prenatal development and postnatal life (Hoffmann et al., 2010, 2012; Storz et al., 2011a, 2013; Storz, 2016b). In this Review, I focus on postnatally expressed Hb isoforms and blood O2 transport during adulthood [see Brittain (2002) for a discussion of prenatally expressed Hbs and their functional properties]. Whereas adult mammals typically express a single Hb isoform or multiple isoforms that have very similar functional properties, other terrestrial vertebrates generally express two or more Hb isoforms that are functionally distinct (Weber and Jensen, 1988; Weber, 1990; Storz, 2016b). For example, birds typically express two main Hb isoforms in definitive red blood cells – a major isoform, HbA, which incorporates α-type products of the αA-globin gene, and a minor isoform, HbD, which incorporates α-type products of the αD-globin gene; the two isoforms share the same β-type subunits (Hoffmann and Storz, 2007; Grispo et al., 2012; Opazo et al., 2015). In all bird species that have been examined to date, the minor HbD isoform has an appreciably higher O2 affinity than the major HbA isoform in the presence of allosteric effectors (Grispo et al., 2012; Projecto-Garcia et al., 2013; Cheviron et al., 2014; Galen et al., 2015; Natarajan et al., 2015b, 2016). Other sauropsid taxa also tend to co-express multiple, structurally distinct Hb isoforms in definitive erythrocytes (Weber and Jensen, 1988; Storz et al., 2011b, 2015b; Damsgaard et al., 2013). The Hb multiplicity that has been documented in birds and non-avian reptiles suggests a potential mechanism for modulating blood–O2 affinity via changes in the relative abundance of distinct Hb isoforms with different O2-binding properties (Hiebl et al., 1988; Weber et al., 1988a; Grispo et al., 2012; Opazo et al., 2015).
In summary, reversible modulation of Hb–O2 affinity via metabolically induced changes in the red cell microenvironment and regulatory changes in Hb isoform expression represent potential mechanisms of phenotypic plasticity. These mechanisms could complement genetically based changes in Hb function, or they could obviate the need for such changes in the first place.
Is it physiologically advantageous to have an increased Hb–O2 affinity at high altitude?
Having explored functional mechanisms for altering Hb–O2 affinity, let us now address the question of whether the optimal Hb–O2 affinity varies as a function of atmospheric PO2. This is central to the question of whether we should generally expect natural selection to favour different Hb–O2 affinities in populations or species that inhabit different elevational zones.
Theoretical results
At high altitude, the reduced PO2 of inspired air generally results in a concomitant reduction in the PO2 of arterial blood (PaO2). Under such conditions, changes in the oxygenation properties of red blood cells can limit the reduction in O2 flux while simultaneously preserving an adequate pressure gradient for O2 diffusion from the capillary blood to the cells of perfused tissue.
At a given PaO2, tissue O2 supply is enhanced by increasing cardiac output ( ) and/or the blood capacitance coefficient (βbO2; Fig. 2A). This latter parameter is defined as the slope of the line joining the arterial and venous points on the blood O2 equilibrium curve:
) and/or the blood capacitance coefficient (βbO2; Fig. 2A). This latter parameter is defined as the slope of the line joining the arterial and venous points on the blood O2 equilibrium curve:
| (2) |
Fig. 2.
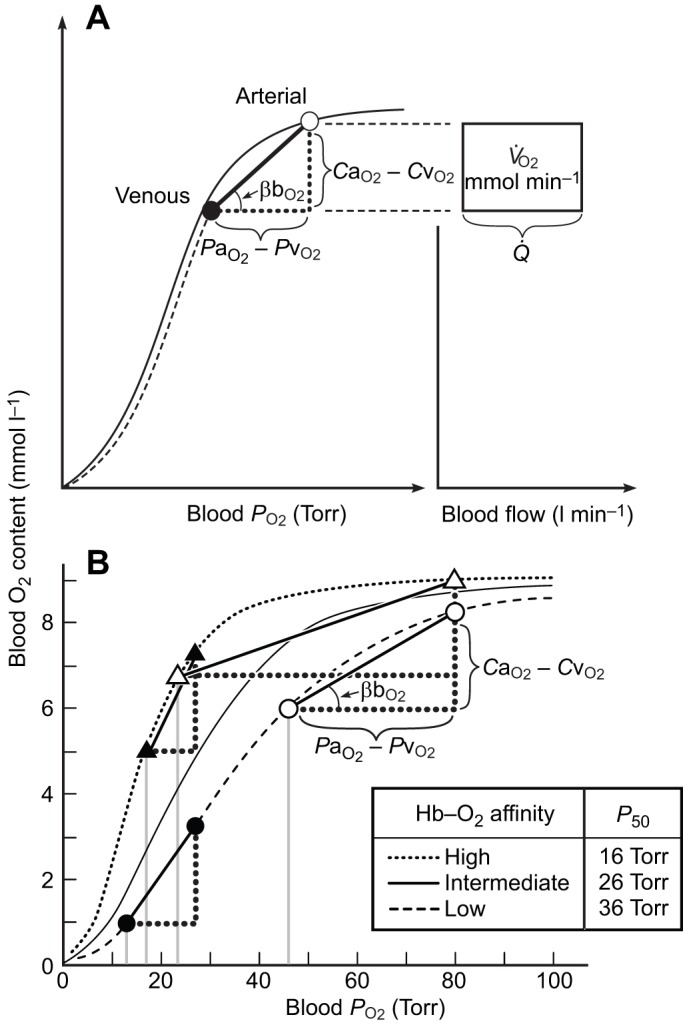
Schematic illustration of blood O2 transport. (A) An O2 equilibrium curve under the physicochemical conditions prevailing in arterial blood (a, continuous curve, open symbol) and venous blood (v, dashed curve, closed symbol). The curve is a plot of blood O2 content (y-axis) versus PO2 (x-axis), with paired values for arterial and venous blood connected by a continuous line. CaO2−CvO2 denotes the arterial–venous difference in blood O2 content, PaO2−PvO2 denotes the corresponding difference in PO2, βbO2 denotes the blood O2 capacitance coefficient (see text for details), 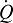 denotes cardiac output, and
denotes cardiac output, and 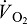 denotes the rate of O2 consumption. On the right-hand side of the graph, the area of the rectangle is proportional to total O2 consumption, which can be enhanced by increasing
denotes the rate of O2 consumption. On the right-hand side of the graph, the area of the rectangle is proportional to total O2 consumption, which can be enhanced by increasing 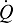 and/or by increasing βbO2. Increases in βbO2 produce a corresponding increase in CaO2−CvO2 through shifts in the shape or position of the O2 equilibrium curve. (B) O2 equilibrium curves showing the effect of changes in Hb–O2 affinity on tissue O2 delivery under conditions of moderate hypoxia (open symbols) and severe hypoxia (filled symbols). For each pair of arterial and venous points, the PO2 for venous blood (PvO2) is marked by a vertical grey line that extends to the x-axis. The sigmoid O2 equilibrium curves are shown for high, intermediate and low Hb–O2 affinities; P50, the PO2 at which Hb is 50% saturated. Each change in Hb–O2 affinity produces a shift in PvO2, but the PO2 of arterial blood (PaO2) is assumed to remain constant. Note that under conditions of moderate hypoxia the right-shifted curve maximizes βbO2 and preserves a higher PvO2 (an overall index of tissue oxygenation). Under severe hypoxia, by contrast, the left-shifted curve maximizes βbO2 and preserves a higher PvO2 relative to the right-shifted curve. When the kinetics of O2 transfer across the alveolar gas–blood barrier is a limiting step (diffusion limitation), a left-shifted O2 equilibrium curve may also be advantageous under less severe hypoxia (Bencowitz et al., 1982).
and/or by increasing βbO2. Increases in βbO2 produce a corresponding increase in CaO2−CvO2 through shifts in the shape or position of the O2 equilibrium curve. (B) O2 equilibrium curves showing the effect of changes in Hb–O2 affinity on tissue O2 delivery under conditions of moderate hypoxia (open symbols) and severe hypoxia (filled symbols). For each pair of arterial and venous points, the PO2 for venous blood (PvO2) is marked by a vertical grey line that extends to the x-axis. The sigmoid O2 equilibrium curves are shown for high, intermediate and low Hb–O2 affinities; P50, the PO2 at which Hb is 50% saturated. Each change in Hb–O2 affinity produces a shift in PvO2, but the PO2 of arterial blood (PaO2) is assumed to remain constant. Note that under conditions of moderate hypoxia the right-shifted curve maximizes βbO2 and preserves a higher PvO2 (an overall index of tissue oxygenation). Under severe hypoxia, by contrast, the left-shifted curve maximizes βbO2 and preserves a higher PvO2 relative to the right-shifted curve. When the kinetics of O2 transfer across the alveolar gas–blood barrier is a limiting step (diffusion limitation), a left-shifted O2 equilibrium curve may also be advantageous under less severe hypoxia (Bencowitz et al., 1982).
where CaO2−CvO2 is the arterial–venous difference in O2 content and PaO2−PvO2 is the arterial–venous difference in PO2 (Dejours et al., 1970) (Fig. 2A). The capacitance coefficient therefore quantifies the amount of O2 unloaded to the tissues for a given arterial–venous difference in PO2.
With regard to the maintenance of an adequate pressure gradient for O2 diffusion to the cells of perfused tissue, the venous PO2 can be expressed as:
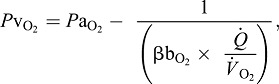 |
(3) |
where V̇O2 is the rate of O2 consumption and the product βbO2 × (Q̇/V̇O2) is the specific blood O2 conductance (Dejours et al., 1970; Bouverot, 1985).
With the above relationships in mind, we can see that under conditions of moderate hypoxia (PaO2 >45 Torr), a reduced Hb–O2 affinity (right-shifted curve) maximizes βbO2 (Fig. 2B). That is, it produces the largest increase in the slope of the line connecting the arterial and venous points, thereby maximizing tissue O2 delivery (CaO2−CvO2) for a given difference in PO2 between the sites of O2 loading in the pulmonary capillaries and the sites of O2 unloading in the systemic circulation (PaO2−PvO2). By contrast, under severe hypoxia, an increased Hb–O2 affinity (left-shifted curve) produces the largest increase in βbO2 because the arterial–venous difference in PO2 spans a steeper portion of the curve (Fig. 2B). Figure 2B also shows that a left-shifted curve preserves a higher PvO2 under such conditions (Woodson, 1988). Under hypoxia, an increased circulatory O2 conductance can also be achieved via increases in cardiac output, but increasing βbO2 via fine-tuned adjustments in Hb–O2 affinity is far more energetically efficient (Mairbäurl, 1994; Samaja et al., 2003).
In summary, this body of theory predicts that a reduced Hb–O2 affinity is generally beneficial under moderate hypoxia, whereas an increased Hb–O2 affinity is beneficial under severe hypoxia. This is consistent with theoretical investigations of tissue O2 delivery at rest and during exercise (Turek et al., 1973; West and Wagner, 1980; Bencowitz et al., 1982; Willford et al., 1982; Samaja et al., 1986, 2003; Scott and Milsom, 2006).
Several theoretical treatments have calculated optimal values of P50 that maximize the arterial–venous difference in O2 content or O2 saturation for a given arterial–venous difference in PO2 (Bencowitz et al., 1982; Willford et al., 1982; Brauner and Wang, 1997). The arterial–venous difference in O2 saturation can be expressed as:
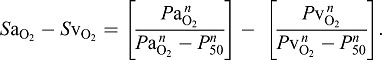 |
(4) |
Taking the first derivative of the maximum arterial–venous saturation difference with respect to P50 indicates that the optimal P50 (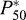 ) can be expressed as:
) can be expressed as:
| (5) |
Eqn 4 can also be solved for PvO2, but the same conditions that maximize SaO2−SvO2 also maximize PvO2 (Willford et al., 1982).
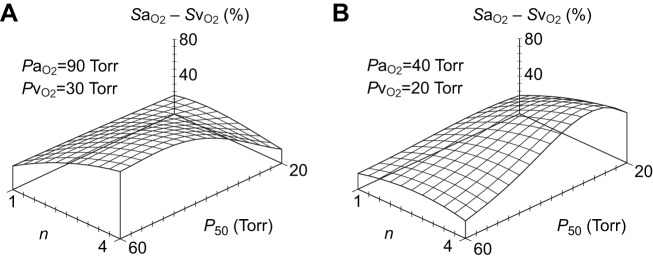
Under normoxia (PaO2=90 Torr, PvO2=30 Torr), and assuming that pH, partial pressure of CO2 (PCO2), cardiac output and Hb concentration remain constant, Eqn 4 predicts that tissue O2 delivery increases as n increases and as P50 increases to its optimum (Fig. 3A). According to Eqn 5, the optimal P50 under these conditions is (90×30)0.5=52.0 Torr. This is the point on the plot where the slope with respect to P50 is zero. By contrast, under severe hypoxia (PaO2=40 Torr, PvO2=20 Torr), with other assumptions as above, the optimal P50 is predicted to be (40×20)0.5=28.3 Torr, indicating that tissue O2 delivery is maximized at a far higher Hb–O2 affinity (Fig. 3B). In both cases, tissue O2 delivery generally increases as a positive function of n, demonstrating the adaptive significance of cooperative O2 binding. The only exception occurs under extremely severe hypoxia, when the venous point on the O2 equilibrium curve drops down to the lower curvilinear asymptote.
Fig. 3.
Blood P50 and Hill's cooperativity coefficient, n, influence blood O2 transport (indexed by the difference in arterial and venous O2 saturation). (A) Normoxia; (B) hypoxia. In these three-dimensional plots, the difference in arterial and venous O2 saturation (SaO2−SvO2) is indicated by the height of the projection above the reference plane. The higher the projection, the greater the difference in O2 saturation.
Figure 4 illustrates the relationships between P50, PaO2 and PvO2, while keeping P50 constant. Assuming 50% tissue O2 extraction (as might occur during exercise), the figure shows that a higher P50 maintains a higher PvO2 under normoxia (PaO2=∼90 Torr), resulting in improved tissue oxygenation as discussed above. By contrast, a lower P50 maintains a higher PvO2 under severe hypoxia (PaO2=∼40 Torr). If the ‘critical PvO2’ for tissue oxygenation is, for example, 10 Torr, then a blood P50 of 20 Torr would allow PaO2 to fall as low as 25 Torr, whereas a blood P50 of 50 Torr would not allow PaO2 to fall below 52 Torr. Results are qualitatively similar under the assumption of 25% tissue O2 extraction, corresponding to the situation at rest (Willford et al., 1982).
Fig. 4.
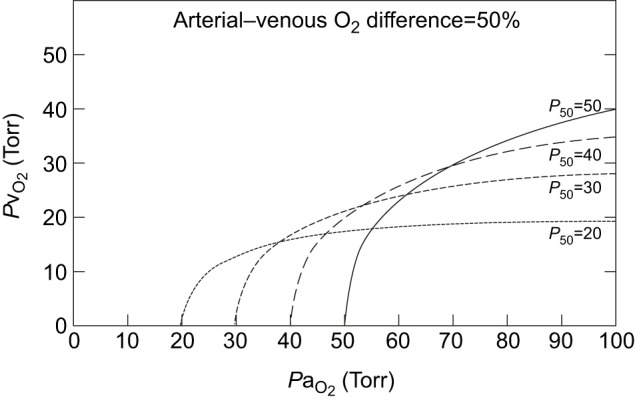
Isobars showing predicted values of venous PO2 (an index of tissue oxygenation) as a function of arterial PO2 at different values of blood P50, assuming 50% tissue O2 extraction and constant cardiac output. At normal or moderately reduced PaO2, a higher P50 results in a higher PvO2 (and hence improved tissue oxygenation). Under more severe hypoxemia, by contrast, a lower P50 results in a higher PvO2 while still maintaining constant O2 extraction.
Experimental results
The most direct means of testing theoretical predictions about how the optimal Hb–O2 affinity varies in relation to ambient PO2 is to experimentally evaluate how titrated changes in blood P50 affect relevant measures of physiological performance under normoxia and varying degrees of hypoxia. Experiments on rats with pharmacologically manipulated Hb–O2 affinities have confirmed theoretical predictions that an increased P50 improves tissue O2 delivery under normoxia and moderate hypoxia, and that a reduced P50 is beneficial under severe hypoxia (Turek et al., 1978a,b). Similarly, reciprocal-transplant experiments involving wild-derived strains of deer mice (Peromyscus maniculatus) revealed that high-altitude natives with high Hb–O2 affinities have higher capacities for thermogenesis and aerobic exercise under severe hypoxia, whereas lowland natives with lower Hb–O2 affinities exhibit superior performance under normoxia (Chappell and Snyder, 1984). Experiments involving other mammals have reported qualitatively similar findings, suggesting that a reduced Hb–O2 affinity improves tissue O2 delivery under normoxia or moderate hypoxia, whereas an increased O2 affinity provides the greatest improvement under more severe hypoxia (Dawson and Evans, 1966; Banchero and Grover, 1972).
Experiments involving rats with pharmacologically manipulated Hb–O2 affinities have also demonstrated that reductions in blood P50 significantly increase the survival of animals subjected to acute, severe hypoxia (Eaton et al., 1974; Penney and Thomas, 1975). Similar results were reported in studies of physiological performance under hypoxia in other mammals with naturally occurring variation in Hb–O2 affinity (Dawson and Evans, 1966; Hall, 1966; Hebbel et al., 1978). In addition to studies of survival and whole-animal physiological performance, ex vivo studies of microvascular O2 transport and tissue perfusion have also demonstrated that an increased Hb–O2 affinity enhances O2 delivery under severe hypoxia (Bakker et al., 1976; Stein and Ellsworth, 1993; Yalcin and Cabrales, 2012).
Threshold altitude
The theoretical and experimental results reviewed above indicate that the optimal Hb–O2 affinity varies according to ambient PO2. The relationship is non-linear and depends critically on the magnitude of diffusion limitation (Bencowitz et al., 1982). For any given species, theory predicts that there must be some threshold altitude at which it becomes beneficial to have an increased Hb–O2 affinity. However, the threshold altitude at which this becomes beneficial varies from one species to the next due to variation in PaO2 (mainly determined by ventilation) and numerous other diffusive and convective steps in the O2 transport pathway (Bencowitz et al., 1982; Scott and Milsom, 2006). The key question is whether a given species inhabits elevations above that critical threshold. In a comparison between sister species with contrasting elevational ranges, the species with the higher elevational range limit would not necessarily be expected to have evolved a higher Hb–O2 affinity unless an appreciable fraction of its native range exceeded the upper critical threshold.
In the case of humans living at high altitude, modelling results suggest that an increased Hb–O2 affinity only confers a benefit to tissue O2 delivery at elevations >5000–5400 m (Samaja et al., 1986, 2003). At 5400 m above sea level, which is roughly the elevation of the South Everest base camp in Nepal, the standard barometric pressure is 53 kPa (399 Torr), meaning that the ambient PO2 is 52% of that at sea level. The highest human settlements in the Himalayas and the Andes are generally at elevations of <4900 m (the Peruvian mining town, La Rinconada, is situated 5100 m above sea level, and most mine workers have homes at lower elevation). The highest permanent settlements in the Ethiopian highlands are <3500 m above sea level. The fact that humans do not live at elevations above the theoretically predicted 5000–5400 m threshold provides a possible explanation for why increased Hb–O2 affinities have not evolved in indigenous mountain dwellers. Whether the same may be true of non-human mammals and birds at high altitude is an open question.
Insights from comparative studies
Since natural selection is ‘the ultimate arbiter of what constitutes an adaptation’ (Snyder, 1982, p. 92), a systematic survey of altitude-related changes in Hb–O2 affinity – as revealed by comparisons among extant species – can provide insights into the possible adaptive significance of such changes. If high-altitude taxa have generally evolved increased Hb–O2 affinities relative to lowland sister taxa – and if the elevational pattern is too consistent to be ascribed to chance (i.e. genetic drift) – this would be consistent with the hypothesis that the elevational differences reflect a history of natural selection. Ideally, comparative studies that exploit the outcomes of natural experiments (e.g. the independent colonization of high-altitude environments by multiple species) complement insights derived from controlled laboratory experiments. For example, comparative studies have documented that high-altitude rodents often have higher Hb and/or blood O2 affinities than their lowland relatives (Hall et al., 1936; Bullard et al., 1966; Ostojic et al., 2002; Storz, 2007; Storz et al., 2009, 2010a; Natarajan et al., 2013, 2015a; Jensen et al., 2016), and these observations complement the results of experiments demonstrating that increases in blood-O2 affinity enhance tissue O2 delivery and measures of physiological performance in rodents subjected to environmental hypoxia (Eaton et al., 1974; Turek et al., 1978a,b; Chappell and Snyder, 1984). Such consilience of evidence from comparative and experimental studies can greatly strengthen conclusions about the adaptive significance of evolutionary changes in Hb–O2 affinity.
The importance of accounting for phylogenetic history
In comparative analyses of phenotypic variation it is important to account for the fact that trait values from different species are not statistically independent because the sampled species did not evolve independently of one another; the phylogenetic history of any set of species is represented by a hierarchically nested pattern of relationships (Garland et al., 2005). If a phylogeny is available for a given set of species, then phylogenetically independent contrasts (PIC) (Felsenstein, 1985) can be used to test for a relationship between native elevation and Hb–O2 affinity. The PIC approach uses phylogenetic information and a model of trait evolution (typically a stochastic, Brownian motion-like model) to transform the data for the set of surveyed species into values that are statistically independent and identically distributed. This approach was used to document a strong positive correlation between Hb–O2 affinity and native elevation in Andean hummingbirds (Projecto-Garcia et al., 2013).
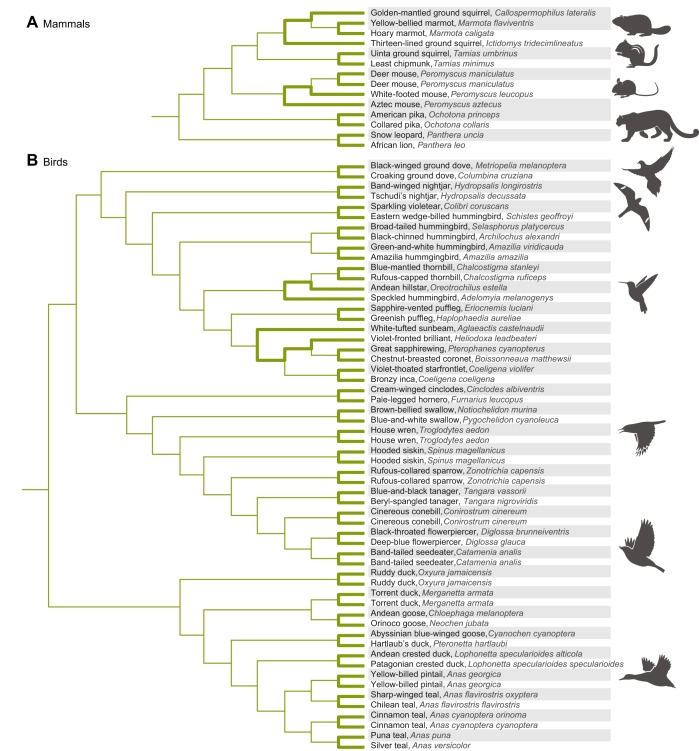
An alternative to using PIC is the paired-lineage test, which restricts comparisons to phylogenetically replicated pairs of taxa that are chosen so that there is no overlap in evolutionary paths of descent (see Fig. 5). A non-random association between Hb–O2 affinity and native elevation can then be assessed using a sign test (a non-parametric test that contrasts matched pairs of samples with respect to a continuous outcome). If the comparative analysis includes a phylogenetically diverse range of taxa, an advantage of the paired-lineage test is that comparisons can be restricted to closely related species by excluding pairs with long paths between them. To determine whether there is a relationship between Hb–O2 affinity and native elevation, we want to make comparisons between close relatives so that we can minimize the number of potentially confounding differences in other aspects of their biology. For example, a comparison of Hb–O2 affinity between a pair of high- and low-altitude hummingbirds is more physiologically informative than a comparison involving a high-altitude hummingbird and a low-altitude duck. In the latter case, an evolved difference in Hb function may be related to the difference in native elevation, but it may also be related to differences in metabolic rate or any number of other physiological differences between the two taxa.
Fig. 5.
Phylogenetic relationships of 14 mammalian taxa and 58 avian taxa used in comparative analyses of Hb function. Rows corresponding to high-altitude taxa are shaded. (A) In the mammalian phylogeny, branches in bold connect pairs of high- and low-altitude taxa that were used to test for a relationship between Hb–O2 affinity and native elevation. As there are no overlaps in the paths of descent connecting each designated pair of high- and low-altitude taxa, the seven pairwise comparisons are statistically independent. For information regarding elevational ranges, see Storz et al. (2009, 2010a), Revsbech et al. (2013), Janecka et al. (2015), Natarajan et al. (2015a) and Tufts et al. (2015). (B) In the avian phylogeny, branches in bold connect pairs of high- and low-altitude taxa that were used to test for a relationship between Hb–O2 affinity and native elevation. As in the case with the mammals, the 29 pairwise comparisons are phylogenetically independent. For information regarding elevational ranges, see Projecto-Garcia et al. (2013), Cheviron et al. (2014), Galen et al. (2015) and Natarajan et al. (2015b, 2016).
There are two additional issues to consider in comparative studies of Hb function in relation to native elevation. The first relates to the effect of environmentally induced variation, which can obscure phylogenetic signal in trait values (Garland et al., 2005; Storz et al., 2015a). Measurements of an environmentally labile trait (like the O2 affinity of whole blood, which is influenced by red cell metabolism and acid–base status) may prevent an accurate assessment of the extent to which phenotypic similarity between a given pair of species is attributable to shared phylogenetic heritage versus a shared, plastic response to similar environmental conditions (i.e. exposure to hypoxia). This problem can be avoided if the trait is measured under common-garden conditions to control for environmentally induced variation, or if measurements are restricted to genetically based trait variation (e.g. O2 affinity of purified Hb rather than O2 affinity of blood).
Another issue concerns genealogical discordance between the phylogeny of the examined species and the phylogenies of the genes that underlie the measured trait (Hahn and Nakhleh, 2016; Storz, 2016a). In comparative studies of Hb evolution involving orthologous genes from a diversity of species, it may often be the case that phylogenies of the α- and β-globin genes are not congruent with one another or with the assumed species tree (Storz et al., 2007; Hoffmann et al., 2008a,b; Opazo et al., 2008a,b, 2009; Runck et al., 2009, 2010; Gaudry et al., 2014; Natarajan et al., 2015a). This genealogical discordance can have multiple biological causes, including ectopic gene conversion (a form of non-reciprocal recombinational exchange between duplicated genes), introgressive hybridization (incorporation of allelic variants from one species into the gene pool of another species by means of hybridization and repeated back-crossing) and incomplete lineage sorting (the retention of ancestral polymorphism from one split between populations to the next, followed by stochastic sorting of allelic lineages among the descendant species). A given amino acid substitution may have occurred a single time on an internal branch of the gene tree, but it can present the appearance of having occurred twice independently when mapped onto a discordant species tree, resulting in spurious inferences. Hahn and Nakhleh (2016) discuss possible solutions to the problem of species tree/gene tree discordance in comparative studies of trait evolution.
Considerations of zoogeographic history
In addition to making comparisons between high- and low-altitude taxa that are as closely related as possible, it is also important to consider the evolutionary histories of study species with regard to their current elevational distributions. Many alpine and subalpine natives may have predominantly lowland ancestries, possibly reflecting post-glacial range shifts. Alternatively, residence at high altitude may represent the ancestral condition for members of groups that diversified in mountainous regions. For example, Andean hummingbirds in the Brilliants/Coquettes clade diversified during a period of rapid uplift of the Andean massif in the period between ∼10 and ∼6 million years ago (McGuire et al., 2014). Within this group, many species with lowland distributions may have descended from highland ancestors. In such cases, it is important to consider that any altitude-related species differences in Hb–O2 affinity could be attributable to derived increases in O2 affinity in highland species and/or secondarily derived reductions in O2 affinity in lowland species (Projecto-Garcia et al., 2013).
Evaluating evidence for an empirical generalization regarding the relationship between native altitude and Hb–O2 affinity
The theoretical and experimental results reviewed above suggest that it is generally beneficial to have an increased Hb–O2 affinity under conditions of severe hypoxia. An obvious prediction is that derived increases in Hb–O2 affinity will have evolved repeatedly in disparate vertebrate taxa that have independently colonized extreme altitudes (provided that their range limits exceed the elevational threshold at which an increased Hb–O2 affinity becomes beneficial). Let us now test this prediction using available comparative data for mammals and birds (Fig. 5).
I restrict the analysis to data based on standardized measurements of purified Hbs, so the variation in P50 values is purely genetic, reflecting evolved changes in the amino acid sequences of the α- and/or β-chain subunits. This focus on purified Hbs avoids problems associated with the confounding effects of environmentally induced variation. However, an analysis based on in vitro measures of protein function involves its own interpretative challenges because evolved changes in the inherent properties of Hb are physiologically relevant to circulatory O2 transport only to the extent that such changes affect the oxygenation properties of blood (Berenbrink, 2006). I have opted to focus on data for purified Hbs while recognizing that species differences in Hb–O2 affinity may not perfectly reflect in vivo differences in blood–O2 affinity.
In the case of mammals, I have summarized data from 14 taxa representing seven high- versus low-altitude pairwise comparisons (Fig. 5A). These comparisons include rodents (marmotine ground squirrels and Peromyscus mice), lagomorphs (pikas) and carnivores (Storz et al., 2009, 2010a; Revsbech et al., 2013; Janecka et al., 2015; Natarajan et al., 2015a; Tufts et al., 2015). Six of the comparisons involve closely related species with contrasting elevational ranges, and one comparison involves high- and low-altitude populations of the broadly distributed deer mouse, P. maniculatus. Each of these pairwise comparisons involves a pronounced elevational contrast between an alpine or subalpine taxon and a closely related lowland taxon. For example, the high-altitude ground squirrels, deer mice and pikas occur at elevations >4300 m (the highest elevations that occur within the limits of their geographical distributions in North America). Since an increased Hb–O2 affinity is only expected to be physiologically beneficial above a given threshold elevation, potentially adaptive differences in Hb–O2 affinity can only be detected if the high- and low-altitude members of each taxon pair have range limits on opposite sides of that threshold.
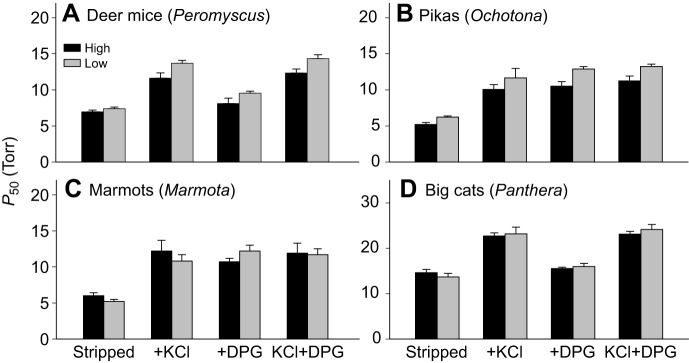
In each taxon, O2 affinities of purified Hbs were measured in the presence and absence of Cl− ions (added as KCl) and DPG (see Fig. 6 legend for experimental details). The ‘KCl+DPG’ treatment is most relevant to in vivo conditions in mammalian red blood cells, and I therefore focus primarily on measures of P50(KCl+DPG). However, measurements under each of the experimental treatments are valuable because they can provide insights into the functional mechanism responsible for observed differences in Hb–O2 affinity.
Fig. 6.
O2 affinities of purified Hbs from representative pairs of high- and low-altitude mammals. O2 equilibria were measured at pH 7.40, 37°C, in the presence and absence of allosteric effectors ([Cl−], 0.10 mol l−1; [Hepes], 0.1 mol l−1; DPG:tetrameric Hb ratio, 2.0: [heme], 0.2–0.3 mmol l−1). For each taxon, P50 values (±s.e.m.) are reported for stripped Hbs in the absence of added anions, in the presence of Cl− alone (added as KCl), in the presence of DPG alone, and in the presence of both anions combined. This latter ‘KCl+DPG’ treatment is most relevant to in vivo conditions in mammalian red blood cells, but measurements of O2 affinity under each of the four standardized treatments can provide insights into the functional mechanism responsible for observed differences in P50(KCl+DPG). (A) Comparison between Hb variants of high- and low-altitude deer mice, Peromyscus maniculatus, from the Rocky Mountains and Great Plains, respectively [data from Natarajan et al., 2015a; for additional details, see Storz et al. (2009, 2010a); Jensen et al. (2016)]. (B) Comparison between Hbs of the high-altitude American pika (Ochotona princeps) and the low-altitude collared pika (O. collaris) (data from Tufts et al., 2015). (C) Comparison between Hbs of the high-altitude yellow-bellied marmot (Marmota flaviventris) and the low-altitude hoary marmot (M. caligata) (data from Revsbech et al., 2013). (D) Comparison between Hbs of the high-altitude snow leopard (Panthera uncia) and the low-altitude African lion (P. leo) (data from Janecka et al., 2015). For both cat species, P50 is shown as the mean value for two co-expressed isoforms, HbA and HbB, which are present at roughly equimolar concentrations (Janecka et al., 2015).
In the presence of anionic effectors, high-altitude taxa have higher Hb–O2 affinities than their lowland counterparts in some cases (e.g. deer mice and pikas; Fig. 6A,B), but in other cases there are no appreciable differences (e.g. marmots and big cats; Fig. 6C,D). In comparisons involving deer mice, pikas and some ground squirrels, integrated analyses of Hb function and sequence divergence revealed that the high-altitude member of each pair evolved a derived increase in Hb–O2 affinity (i.e. the phenotype of the lowland taxon represents the ancestral condition). In the comparisons between conspecific populations of deer mice and between the golden-mantled ground squirrel (Callospermophilus lateralis) and thirteen-lined ground squirrel (Ictidomys tridecemlineatus), the evolved changes in Hb function involved an increase in intrinsic O2 affinity in combination with a suppressed sensitivity to anionic effectors (Storz et al., 2009, 2010a; Natarajan et al., 2013; Revsbech et al., 2013; Natarajan et al., 2015a). In the case of the deer mice, this is indicated by the fact that the high-altitude Hb variant exhibits a slightly lower P50 in the absence of anions (‘stripped’) and the P50 difference is further augmented in the presence of Cl− and DPG (Fig. 6A). By contrast, in the comparison between the two pika species (Ochotona princeps and O. collaris), the difference in Hb function was exclusively attributable to an evolved change in intrinsic O2 affinity (Tufts et al., 2015) (Fig. 6B).
Phylogenetically independent comparisons involving the full set of mammalian taxa revealed no significant association between Hb–O2 affinity and elevation (Wilcoxon's signed-rank test, W=6.5, P>0.05, N=7; Fig. 7). Previous studies involving experimental measurements on whole blood indicate that high-altitude mammals have lower P50 values than their lowland relatives in some cases (Chiodi, 1970/71; León-Velarde et al., 1996; Ostojic et al., 2002) but not in others (Lechner, 1976). Unless measures of blood–O2 affinities are integrated with measurements on purified Hbs (Petschow et al., 1977; Campbell et al., 2010), components of environmental and genetic variation are confounded and it is unclear to what extent the measured differences represent evolved changes in Hb function and/or red cell metabolism. Overall, evidence for an altitudinal trend in Hb–O2 affinity in mammals is equivocal; data from additional taxa may eventually reveal a clearer relationship.
Fig. 7.
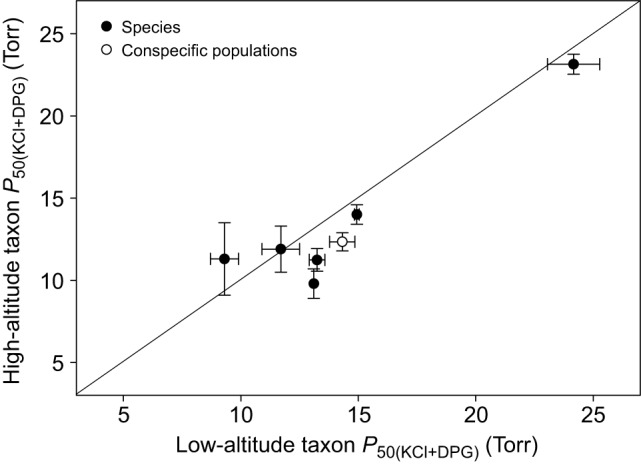
There is no evidence for a significant elevational trend in the Hb–O2 affinities of mammals. The plot shows measures of Hb–O2 affinity in the presence of anionic effectors [P50(KCl+IHP) (±s.e.m.)] for seven matched pairs of high- and low-altitude taxa. Data points that fall below the diagonal (x=y) denote cases in which the high-altitude member of a given taxon pair possesses a higher Hb–O2 affinity (lower P50). The paired-lineage design ensures that all data points are statistically independent (see text for details). Filled symbols denote comparisons between species, whereas the open symbol denotes a comparison between high- versus low-altitude populations of the same species (P. maniculatus).
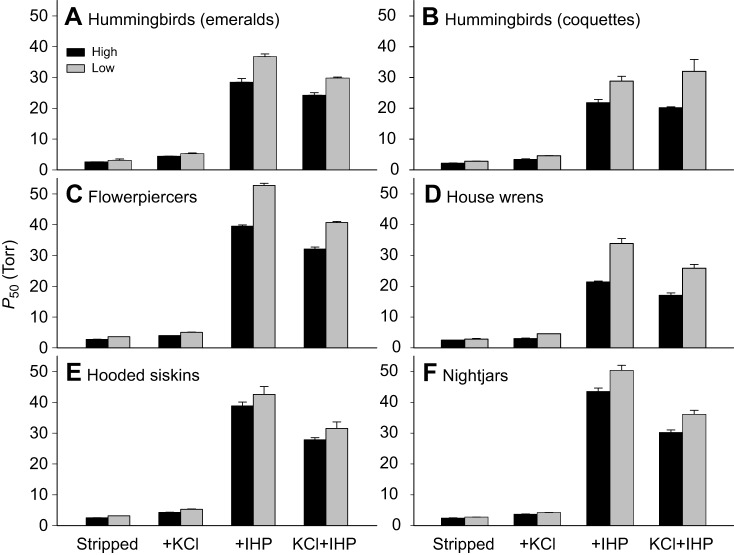
In the case of birds, I have summarized data from 58 taxa representing 29 matched pairs of high- versus low-altitude taxa (Fig. 5B). These taxa include ground doves, nightjars, hummingbirds, passerines and waterfowl (Projecto-Garcia et al., 2013; Cheviron et al., 2014; Galen et al., 2015; Natarajan et al., 2015b, 2016). In the case of the passerines and waterfowl, we can make comparisons between closely related species as well as conspecific populations. All pairwise comparisons involved dramatic elevational contrasts; high-altitude taxa native to very high elevations (3500–5000 m above sea level) were paired with close relatives that typically occur at or near sea level. As with the analysis of mammalian Hbs, O2 affinities of purified avian Hbs were measured under standardized conditions in the presence and absence of anionic effectors (see legend for Fig. 8). However, to obtain measurements that are physiologically relevant to in vivo conditions in avian red cells, inositol hexaphosphate (IHP; a chemical analogue of IPP) was used instead of DPG. In species that expressed both HbA and HbD, the O2-binding properties of isolated isoforms were measured separately.
Fig. 8.
Comparison of oxygenation properties of the major Hb isoform (HbA) between pairs of high- and low-altitude birds in the Andes. O2 equilibria were measured at pH 7.40, 37°C in the presence and absence of allosteric effectors ([Cl−], 0.10 mol l−1; [Hepes], 0.1 mol l−1; IHP:tetrameric Hb ratio, 2.0: [heme], 0.3 mmol l−1). For each taxon, P50 values (±s.e.m.) are reported for stripped Hbs in the absence of added anions, in the presence of Cl− alone (added as KCl), in the presence of IHP alone, and in the presence of both anions combined. As explained in the main text, the ‘KCl+IHP’ treatment is most relevant to in vivo conditions in avian red blood cells, but measurements of O2 affinity under each of the four standardized treatments provide insights into the functional mechanism responsible for observed differences in P50(KCl+IHP). In each pairwise comparison shown here, slight differences in intrinsic Hb–O2 affinity (reflected by stripped P50 values) become more pronounced in the presence of IHP. (A) Comparison of HbA O2 affinities between high- and low-altitude hummingbirds in the Emeralds clade (Trochilidae: Apodiformes): the green-and-white hummingbird, Amazilia viridicauda, and the amazilia hummingbird, A. amazilia, respectively. (B) Comparison of HbA O2 affinities between high- and low-altitude hummingbirds in the Coquettes clade (Trochilidae: Apodiformes): the Andean hillstar, Oreotrochilus estella, and the speckled hummingbird, Adelomyia melanogenys, respectively. (C) Comparison of HbA O2 affinities between high- and low-altitude flowerpiercers (Thraupidae: Passeriformes): the black-throated flowerpiercer, Diglossa brunneiventris, and deep-blue flowerpiercer, D. glauca, respectively. (D) Comparison of HbA O2 affinities between high- and low-altitude populations of the house wren, Troglodytes aedon (Troglodytidae: Passeriformes). (E) Comparison of HbA O2 affinities between high- and low-altitude populations of the hooded siskin, Spinus magellanica (Fringillidae: Passeriformes). (F) Comparison of HbA O2 affinities between high- and low-altitude nightjars (Caprimulgidae: Caprimulgiformes): the band-winged nightjar, Hydropsalis longirostris, and Tschudi's nightjar, H. decussata, respectively. Data from Projecto-Garcia et al. (2013), Galen et al. (2015), and Natarajan et al. (2016).
In the overwhelming majority of pairwise comparisons, the high-altitude taxon exhibited a higher Hb–O2 affinity across all treatments, as illustrated by representative examples for the HbA isoform (Fig. 8). Phylogenetically independent comparisons revealed that highland natives generally have an increased Hb–O2 affinity relative to their lowland counterparts, a pattern consistent for both HbA (Wilcoxon's signed-rank test, Z=−4.314, P<0.0001, N=29; Fig. 9A) and HbD (Z=−2.798, P=0.0051, N=21; Fig. 9B). In all pairwise comparisons in which the high-altitude taxa exhibited significantly higher Hb–O2 affinities relative to the lowland taxa (N=23 taxon pairs for HbA, N=15 for HbD), the measured differences were almost entirely attributable to differences in intrinsic O2 affinity rather than differences in sensitivity to Cl− ions or IHP (Natarajan et al., 2015b, 2016). Comparisons between the high-flying bar-headed goose (Anser indicus) and lowland congeners based on O2 affinity measurements of whole blood or purified hemolysates (Petschow et al., 1977; Black and Tenney, 1980; Jessen et al., 1991) are also consistent with the relationship shown in Fig. 9A,B.
Fig. 9.
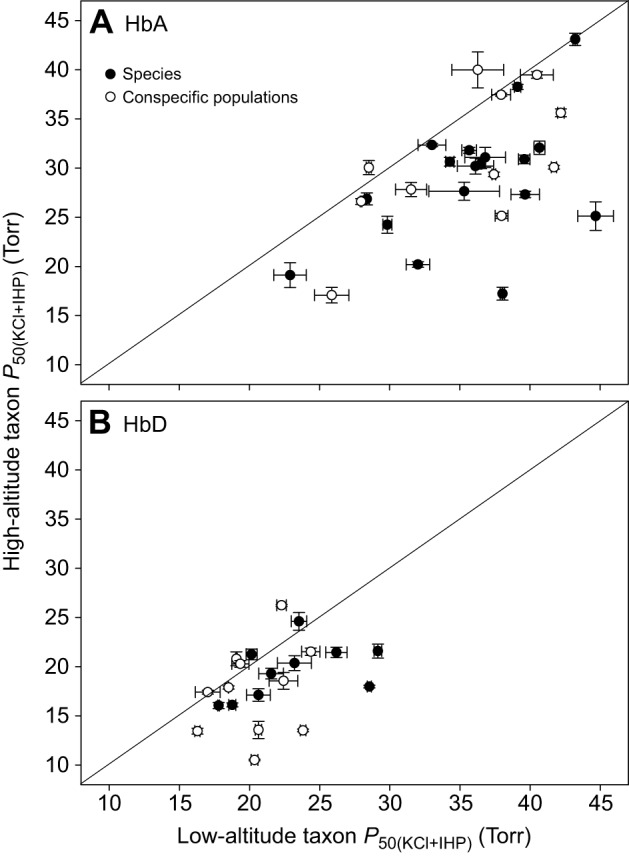
Convergent increases in Hb–O2 affinity in high-altitude Andean birds. (A) Plot of P50(KCl+IHP) (±s.e.m.) for HbA in 29 matched pairs of high- and low-altitude taxa. Data points that fall below the diagonal (x=y) denote cases in which the high-altitude member of a given taxon pair possesses a higher Hb–O2 affinity (lower P50). Comparisons involve phylogenetically replicated pairs of taxa, so all data points are statistically independent. (B) Plot of P50(KCl+IHP) (±s.e.m.) for the minor HbD isoform in a subset of the same taxon pairs in which both members of the pair express HbD. Sample sizes are larger for HbA than for HbD because the two ground dove species (Metriopelia melanoptera and Columbina cruziana) expressed no trace of HbD, and several hummingbird species expressed HbD at exceedingly low levels (Projecto-Garcia et al., 2013; Natarajan et al., 2016). In such cases, sufficient quantities of HbD could not be purified for measures of O2 equilibria. Filled symbols denote comparisons between species, whereas open symbols denote comparisons between high- versus low-altitude populations of the same species. Data from Natarajan et al. (2015b, 2016).
The role of Hb isoform switching in hypoxia adaptation
In principle, regulatory changes in the expression of Hb isoforms with different oxygenation properties could provide an effective means of reversibly modulating blood–O2 affinity in response to changes in O2 availability or metabolic demand. This regulatory mechanism could potentially complement genetically based changes in the O2 affinity of individual isoforms. Due to differences in the nature of isoform differentiation in birds and mammals, I will discuss relevant data for each of these taxa in turn.
In birds, consistent differences in O2 affinity between HbA and HbD suggest that an increased blood–O2 affinity could be achieved by up-regulating HbD (Hiebl et al., 1988; Weber et al., 1988a; Grispo et al., 2012; Opazo et al., 2015). A comparison involving 26 closely related pairs of high- and low-altitude taxa revealed that regulatory changes in Hb isoform abundance do not represent an important general mechanism of adaptation to chronic hypoxia (Fig. 10) (Natarajan et al., 2016). It remains to be seen whether isoform switching plays a role in the seasonal acclimatization to acute hypoxia, for example in species that undergo trans-Himalayan migratory flights like bar-headed geese, ruddy shelducks (Tadoma ferruginea) and demoiselle cranes (Anthropoides virgo).
Fig. 10.
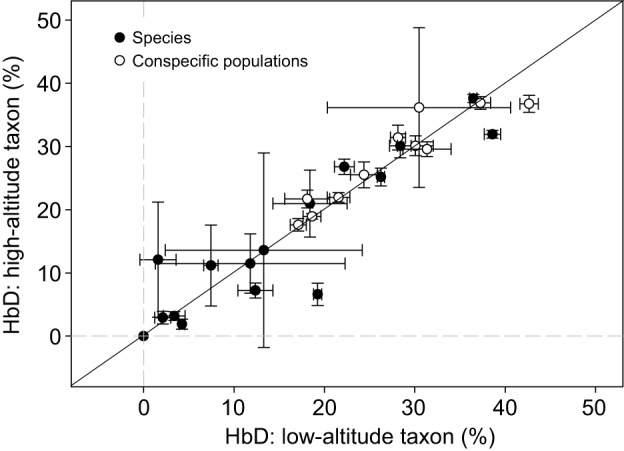
There is no evidence for altitude-related differences in the relative abundance of HbA and HbD isoforms in Andean birds. Phylogenetically independent comparisons involving 26 pairs of high- and low-altitude taxa revealed no systematic difference in the relative expression level of the minor HbD isoform (Wilcoxon signed-ranks test, Z=−0.775, P=0.441). The diagonal represents the line of equality (x=y). Filled symbols denote comparisons between species, whereas open symbols denote comparisons between high- versus low-altitude populations of the same species. Data from Natarajan et al. (2016).
In contrast to birds and other sauropsid taxa, most mammals do not co-express functionally distinct Hb isoforms during postnatal life. Since prenatally expressed Hb isoforms often have higher O2 affinities than normal adult Hbs, it is possible that the continued expression of such isoforms into postnatal life could contribute to an enhanced blood–O2 affinity in response to environmental hypoxia (a form of biochemical paedomorphosis). Among eutherian mammals, stage-specific expression of fetal Hb isoforms evolved independently in simian primates (New World monkeys, Old World monkeys, apes and humans) and in bovid artiodactyls (cattle, antelope, sheep and goats) (Storz, 2016b). In humans, retention of fetal Hb expression into adulthood is known to ameliorate the pathological effects of sickle-cell anaemia (Akinsheye et al., 2011; Pack-Mabien and Imran, 2013), but there is no evidence for the existence of a similar expression pattern in indigenous high-altitude populations. In yaks, high-affinity fetal Hb isoforms are expressed at high levels in one-month-old calves (Weber et al., 1988b), but it is not known whether high expression is retained into adulthood. There is one dubious report claiming that fetal Hb accounts for 55% of total Hb in adult alpacas (Vicugna pacos) that were acclimatized at an elevation of 4200 m (Reynafarje et al., 1975). In this study, the putative fetal Hb isoform was identified as such only because it exhibited a resistance to alkaline denaturation similar to that of human fetal Hb (HbF). Thus, identification of the isoform as ‘fetal Hb’ was based on functional analogy rather than true homology. Moreover, there is no evidence that camelids express a true fetal Hb isoform in the first place. Comparative genomic data indicate that alpacas and other camelids do not share orthologues of the fetally expressed β-type globin gene in bovid artiodactyls [the bovid-specific duplication event that gave rise to the fetally expressed β-globin gene occurred long after the ancestors of bovids split from the ancestors of camelids (Gaudry et al., 2014)].
In summary, there is currently no compelling evidence to suggest that Hb isoform switching represents an important mechanism of physiological acclimatization to hypoxia in mammals or birds (Storz, 2016b). However, aside from recent studies of Andean birds (Natarajan et al., 2015b, 2016), it is also true that hypoxia-induced isoform switching has not been systematically investigated as a mechanism of phenotypic plasticity in terrestrial vertebrates.
Maladaptive plasticity and hypoxia acclimatization
I have reviewed theoretical and experimental evidence suggesting that an increased Hb–O2 affinity is beneficial under severe hypoxia, and I have synthesized available comparative data which suggest that natural selection has favoured increased Hb–O2 affinities in numerous high-altitude mammals and birds. It therefore seems paradoxical that the acclimatization response to environmental hypoxia in humans and other lowland mammals typically involves a reduction in blood–O2 affinity (Mairbäurl et al., 1993; Mairbäurl, 1994; Samaja et al., 2003; Storz et al., 2010b). This is largely attributable to an increase in red cell DPG concentration (Torrance et al., 1970/71; Lenfant et al., 1971; Mairbäurl et al., 1986, 1993; Mairbäurl, 1994), or – more specifically – an increase in the relative concentration of Hb liganded with DPG and other anions that make smaller contributions. The increased concentration of DPG-liganded Hb at high altitude is largely attributable to a hypoxia-induced increase in ventilation; the resultant respiratory alkalosis stimulates red cell glycolytic activity which, in turn, increases DPG synthesis (Rapoport et al., 1977). At the whole-blood level, the hypoxia-induced increase in [DPG] is also attributable to the stimulation of erythropoiesis because this produces a downward shift in the mean age of circulating red blood cells, and newly produced red cells have higher [DPG] than older cells (Mairbäurl, 1994; Samaja et al., 2003). A number of previous authors interpreted the hypoxia-induced increase in red cell [DPG] (and the associated reduction in blood O2 affinity) as an adaptive response (Aste-Salazar and Hurtado, 1944; Lenfant et al., 1968, 1969, 1971; Eaton et al., 1969; Frisancho, 1975). The theoretical and empirical results reviewed above suggest that such a response may be beneficial under moderate hypoxia. However, in humans and other mammals, the hypoxia-induced increase in red cell [DPG] continues at elevations well above the threshold at which further reductions in Hb–O2 affinity become counterproductive due to arterial desaturation (Winslow et al., 1984; Samaja et al., 2003). Even when all Hb is fully liganded with DPG (at a DPG:Hb ratio of ≥2–3), further increases in [DPG] continue to indirectly reduce Hb–O2 affinity because the increased erythrocytic concentration of non-diffusible anions reduces cellular pH, thereby reducing Hb–O2 affinity via the Bohr effect (Duhm, 1971; Samaja and Winslow, 1979; Mairbäurl, 1994). Consequently, the increase in plasma pH caused by respiratory alkalosis has offsetting effects in mammalian red cells: the Bohr effect promotes an increased Hb–O2 affinity, but this is counterbalanced by the increase in intracellular [DPG] (Winslow et al., 1984; Samaja et al., 1997).
In mammals that have acclimatized to chronic hypoxia, the seemingly maladaptive increase in red cell [DPG] may represent a miscued response to environmental hypoxia in species whose ancestors evolved in a lowland environment (Storz et al., 2010b; Tufts et al., 2013). In contrast to mammals, teleost fishes typically respond to environmental hypoxia by reducing red cell concentrations of nucleotide triphosphates such as ATP and GTP, thereby increasing Hb–O2 affinity to enhance O2 uptake in the gills (Weber, 1996; Jensen et al., 1998; Val, 2000; Nikinmaa, 2001). Whereas the enucleated red cells of mammals rely on a metabolite of anaerobic glycolysis to regulate Hb–O2 affinity, the aerobically metabolizing red cells of fish contain high levels of ATP, so the synthesis of allosteric effectors that reduce Hb–O2 affinity is directly dependent on blood PO2, providing a means of positive feedback control. In mammalian red cells, DPG is a far more potent allosteric regulator of Hb–O2 affinity than ATP, partly because ATP is ∼90% complexed with Mg2+ (Bunn, 1971; Mairbäurl, 1994). It remains to be seen whether birds and other terrestrial vertebrates with nucleated red cells acclimatize to environmental hypoxia via changes in red cell organic phosphate concentrations, but available evidence indicates that [IPP] in avian red cells is highly constant and is unresponsive to changes in temperature or PO2 (Jaeger and McGrath, 1974; Lutz, 1980).
Conclusions
Theoretical results indicate that it is generally beneficial to have an increased Hb–O2 affinity under conditions of severe hypoxia. This prediction is supported by experimental studies of survival and whole-animal physiological performance and by ex vivo studies of microvascular O2 transport and tissue perfusion. A number of detailed case studies involving mammals and birds have provided evidence for adaptive increases in Hb–O2 affinity in high-altitude natives. Evolutionary changes in Hb–O2 affinity involve a variety of functional mechanisms. In mammals, evolved increases in Hb–O2 affinity in high-altitude populations or species involve changes in the intrinsic O2 affinity of Hb and, in some cases, suppressed sensitivities to anionic effectors. In birds, evolved increases in Hb–O2 affinity are consistently attributable to changes in intrinsic affinity that do not compromise allosteric regulatory capacity (Natarajan et al., 2015b, 2016). Available evidence suggests that regulatory changes in Hb isoform composition do not play a general role in adaptation to high-altitude hypoxia in birds or mammals.
In mammals, the evidence for a positive relationship between Hb–O2 affinity and native elevation is equivocal. In birds, by contrast, there is a remarkably strong positive relationship between Hb–O2 affinity and native elevation. In fact, the data for high-altitude birds provide one of the most compelling examples of convergent biochemical adaptation in vertebrates. An important question for future work concerns the reason for this apparent difference between mammals and birds, two amniote lineages that independently evolved endothermy and which diversified in parallel during periods of increasing atmospheric O2 in the late Mesozoic Era. In comparisons within and among different vertebrate groups, it will also be important to determine whether it is possible to predict the threshold values of PaO2 at which it becomes beneficial to have an increased or decreased Hb–O2 affinity.
Acknowledgements
The data summarized in this review were generated in close collaboration with numerous colleagues, in particular: A. Fago, C. Natarajan, R. E. Weber and C. C. Witt. I thank M. Berenbrink, K. L. Campbell, A. Fago, G. R. Scott, R. E. Weber and two anonymous reviewers for helpful comments and discussion.
Footnotes
Competing interests
The author declares no competing or financial interests.
Funding
My work on hemoglobin evolution is funded by grants from the National Heart, Lung, and Blood Institute (R01 HL087216) and the National Science Foundation (IOS-0949931, IOS-1354390 and MCB-1517636). Deposited in PMC for release after 12 months.
References
- Adair G. S. (1925). The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J. Biol. Chem. 63, 529-545. [Google Scholar]
- Akinsheye I., Alsultan A., Solovieff N., Ngo D., Baldwin C. T., Sebastiani P., Chui D. H. K. and Steinberg M. H. (2011). Fetal hemoglobin in sickle cell anemia. Blood 118, 19-27. 10.1182/blood-2011-03-325258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone A. (1972). X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237, 146-149. 10.1038/237146a0 [DOI] [PubMed] [Google Scholar]
- Arnone A. and Perutz M. F. (1974). Structure of inositol hexaphosphate–human deoxyhaemoglobin complex. Nature 249, 34-36. 10.1038/249034a0 [DOI] [PubMed] [Google Scholar]
- Aste-Salazar H. and Hurtado A. (1944). The affinity of hemoglobin for oxygen at sea level and at high altitudes. Am. J. Physiol. 142, 733-743. [Google Scholar]
- Bakker J. C., Gortmaker G. C., Vrolijk A. C. M. and Offerijns F. G. J. (1976). The influence of the position of the oxygen dissociation curve on oxygen-dependent functions of the isolated perfused rat liver. 1. Studies at different levels of hypoxic hypoxia. Pflugers Arch. 362, 21-31. 10.1007/BF00588677 [DOI] [PubMed] [Google Scholar]
- Baldwin J. and Chothia C. (1979). Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J. Mol. Biol. 129, 175-220. 10.1016/0022-2836(79)90277-8 [DOI] [PubMed] [Google Scholar]
- Banchero N. and Grover R. F. (1972). Effect of different levels of simulated altitude on O2 transport in llama and sheep. Am. J. Physiol. 222, 1239-1245. [DOI] [PubMed] [Google Scholar]
- Barcroft J., Binger C. A., Bock A. V., Doggart J. H., Forbes H. S., Harrop G., Meakins J. C., Redfield A. C., Davies H. W., Scott J. M. D. et al. (1923). Observations upon the effect of high altitude on the physiological processes of the human body, carried out in the Peruvian Andes, chiefly at Cerro de Pasco. Philos. Trans. R. Soc. B Biol. Sci. 211, 351-480. 10.1098/rstb.1923.0008 [DOI] [Google Scholar]
- Bartlett G. R. (1980). Phosphate compounds in vertebrate red blood cells. Am. Zool. 20, 103-114. 10.1093/icb/20.1.103 [DOI] [Google Scholar]
- Bellelli A., Brunori M., Miele A. E., Panetta G. and Vallone B. (2006). The allosteric properties of hemoglobin: insights from natural and site directed mutants. Curr. Protein Pept. Sci. 7, 17-45. 10.2174/138920306775474121 [DOI] [PubMed] [Google Scholar]
- Bencowitz H. Z., Wagner P. D. and West J. B. (1982). Effect of change in P50 on exercise tolerance at high-altitude - a theoretical study. J. Appl. Physiol. 53, 1487-1495. [DOI] [PubMed] [Google Scholar]
- Benesch R. and Benesch R. E. (1967). The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biophys. Res. Commun. 26, 162-167. 10.1016/0006-291X(67)90228-8 [DOI] [PubMed] [Google Scholar]
- Berenbrink M. (2006). Evolution of vertebrate haemoglobins: histidine side chains, specific buffer value and Bohr effect. Respir. Physiol. Neurobiol. 154, 165-184. 10.1016/j.resp.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Black C. P. and Tenney S. M. (1980). Oxygen transport during progressive hypoxia in high-altitude and sea-level waterfowl. Respir. Physiol. 39, 217-239. 10.1016/0034-5687(80)90046-8 [DOI] [PubMed] [Google Scholar]
- Bouverot P. (1985). Adaptation to Altitude-Hypoxia in Vertebrates. Berlin: Springer-Verlag. [Google Scholar]
- Brauner C. J. and Wang T. (1997). The optimal oxygen equilibrium curve: A comparison between environmental hypoxia and anemia. Am. Zool. 37, 101-108. 10.1093/icb/37.1.101 [DOI] [Google Scholar]
- Brittain T. (2002). Molecular aspects of embryonic hemoglobin function. Mol. Aspects Med. 23, 293-342. 10.1016/S0098-2997(02)00004-3 [DOI] [PubMed] [Google Scholar]
- Bullard R. W. (1972). Vertebrates at altitude. In Physiological Adaptations: Desert and Mountain (ed. Yousef M. K., Horvath S. M. and Bullard R. W.), pp. 210-225. New York: Academic Press. [Google Scholar]
- Bullard R. W., Broumand C. and Meyer F. R. (1966). Blood characteristics and volume in two rodents native to high altitude. J. Appl. Physiol. 21, 994-998. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. (1971). Differences in the interaction of 2,3-diphosphoglycerate with certain mammalian hemoglobins. Science 172, 1049-1050. 10.1126/science.172.3987.1049 [DOI] [PubMed] [Google Scholar]
- Bunn H. F. (1980). Regulation of hemoglobin function in mammals. Am. Zool. 20, 199-211. 10.1093/icb/20.1.199 [DOI] [Google Scholar]
- Campbell K. L., Storz J. F., Signore A. V., Moriyama H., Catania K. C., Payson A. P., Bonaventura J., Stetefeld J. and Weber R. E. (2010). Molecular basis of a novel adaptation to hypoxic-hypercapnia in a strictly fossorial mole. BMC Evol. Biol. 10, 214 10.1186/1471-2148-10-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell M. A. and Snyder L. R. (1984). Biochemical and physiological correlates of deer mouse α-chain hemoglobin polymorphisms. Proc. Natl. Acad. Sci. USA 81, 5484-5488. 10.1073/pnas.81.17.5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheviron Z. A., Natarajan C., Projecto-Garcia J., Eddy D. K., Jones J., Carling M. D., Witt C. C., Moriyama H., Weber R. E., Fago A. et al. (2014). Integrating evolutionary and functional tests of adaptive hypotheses: a case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Mol. Biol. Evol. 31, 2948-2962. 10.1093/molbev/msu234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi H. (1970/71). Comparative study of the blood gas transport in high altitude and sea level camelidae and goats. Respir. Physiol. 11, 84-93. 10.1016/0034-5687(70)90104-0 [DOI] [PubMed] [Google Scholar]
- Damsgaard C., Storz J. F., Hoffmann F. G. and Fago A. (2013). Hemoglobin isoform differentiation and allosteric regulation of oxygen binding in the turtle, Trachemys scripta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R961-R967. 10.1152/ajpregu.00284.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. J. and Evans J. V. (1966). Effect of hypoxia on oxygen transport in sheep with different hemoglobin types. Am. J. Physiol. 210, 1021-1025. [DOI] [PubMed] [Google Scholar]
- Dejours P., Garey W. F. and Rahn H. (1970). Comparison of ventilatory and circulatory flow rates between animals in various physiological conditions. Respir. Physiol. 9, 108-117. 10.1016/0034-5687(70)90063-0 [DOI] [PubMed] [Google Scholar]
- Dempsey J. A., Thomson J. M., Forster H. V., Cerny F. C. and Chosy L. W. (1975). Hb-O2 dissociation in man during prolonged work in chronic hypoxia. J. Appl. Physiol. 38, 1022-1029. [DOI] [PubMed] [Google Scholar]
- Duhm J. (1971). Effects of 2,3-diphosphoglycerate and other organic phosphate compounds on oxygen affinity and intracellular pH of human erythrocytes. Pflugers Arch. 326, 341-356. 10.1007/BF00586998 [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Brewer G. J. and Groover R. F. (1969). Role of red cell 2,3-diphosphoglycerate in the adaptation of man to altitude. J. Lab. Clin. Med. 73, 603-609. [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D. and Berger E. (1974). Survival at extreme altitude: protective effect of increased hemoglobin-oxygen affinity. Science 183, 743-744. 10.1126/science.183.4126.743 [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Henry E. R., Hofrichter J., Bettati S., Viappiani C. and Mozzarelli A. (2007). Evolution of allosteric models for hemoglobin. IUBMB Life 59, 586-599. 10.1080/15216540701272380 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Phylogenies and the comparative method. Am. Nat. 125, 1-15. 10.1086/284325 [DOI] [Google Scholar]
- Frisancho A. R. (1975). Functional adaptation to high altitude hypoxia. Science 187, 313-319. 10.1126/science.1089311 [DOI] [PubMed] [Google Scholar]
- Galen S. C., Natarajan C., Moriyama H., Weber R. E., Fago A., Benham P. M., Chavez A. N., Cheviron Z. A., Storz J. F. and Witt C. C. (2015). Contribution of a mutational hot spot to hemoglobin adaptation in high-altitude Andean house wrens. Proc. Natl. Acad. Sci. USA 112, 13958-13963. 10.1073/pnas.1507300112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T. Jr., Bennett A. F. and Rezende E. L. (2005). Phylogenetic approaches in comparative physiology. J. Exp. Biol. 208, 3015-3035. 10.1242/jeb.01745 [DOI] [PubMed] [Google Scholar]
- Gaudry M. J., Storz J. F., Butts G. T., Campbell K. L. and Hoffmann F. G. (2014). Repeated evolution of chimeric fusion genes in the β-globin gene family of laurasiatherian mammals. Genome Biol. Evol. 6, 1219-1234. 10.1093/gbe/evu097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelin B. R., Lee A. W.-M. and Karplus M. (1983). Hemoglobin tertiary structural change on ligand binding its role in the co-operative mechanism. J. Mol. Biol. 171, 489-559. 10.1016/0022-2836(83)90042-6 [DOI] [PubMed] [Google Scholar]
- Grispo M. T., Natarajan C., Projecto-Garcia J., Moriyama H., Weber R. E. and Storz J. F. (2012). Gene duplication and the evolution of hemoglobin isoform differentiation in birds. J. Biol. Chem. 287, 37647-37658. 10.1074/jbc.M112.375600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. W. and Nakhleh L. (2016). Irrational exuberance for resolved species trees. Evolution 70, 7-17. 10.1111/evo.12832 [DOI] [PubMed] [Google Scholar]
- Hall F. G. (1966). Minimal utilizable oxygen and oxygen dissociation curve of blood of rodents. J. Appl. Physiol. 21, 375-378. [DOI] [PubMed] [Google Scholar]
- Hall F. G., Dill D. B. and Barron E. S. G. (1936). Comparative physiology in high altitudes. J. Cell. Comp. Physiol. 8, 301-313. 10.1002/jcp.1030080302 [DOI] [Google Scholar]
- Hazard E. S. and Hutchison V. H. (1982). Distribution of acid-soluble phosphates in the erythrocytes of selected species of amphibians. Comp. Biochem. Physiol. A Physiol. 73, 111-124. 10.1016/0300-9629(82)90101-3 [DOI] [Google Scholar]
- Hebbel R. P., Eaton J. W., Kronenberg R. S., Zanjani E. D., Moore L. G. and Berger E. M. (1978). Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J. Clin. Invest. 62, 593-600. 10.1172/JCI109165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebl I., Weber R. E., Schneeganss D., Kösters J. and Braunitzer G. (1988). High-altitude respiration of birds. Structural adaptations in the major and minor hemoglobin components of adult Rüppell's griffon (Gryps rueppellii, Aegypiinae): a new molecular pattern for hypoxic tolerance. Biol. Chem. Hoppe Seyler 369, 217-232. 10.1515/bchm3.1988.369.1.217 [DOI] [PubMed] [Google Scholar]
- Hill A. V. (1910). The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 40, iv-vii. [Google Scholar]
- Hoffmann F. G. and Storz J. F. (2007). The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol. Biol. Evol. 24, 1982-1990. 10.1093/molbev/msm127 [DOI] [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C. and Storz J. F. (2008a). Rapid rates of lineage-specific gene duplication and deletion in the α-globin gene family. Mol. Biol. Evol. 25, 591-602. 10.1093/molbev/msn004 [DOI] [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C. and Storz J. F. (2008b). New genes originated via multiple recombinational pathways in the β-globin gene family of rodents. Mol. Biol. Evol. 25, 2589-2600. 10.1093/molbev/msn200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F. G., Storz J. F., Gorr T. A. and Opazo J. C. (2010). Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol. Biol. Evol. 27, 1126-1138. 10.1093/molbev/msp325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F. G., Opazo J. C. and Storz J. F. (2012). Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol. Biol. Evol. 29, 303-312. 10.1093/molbev/msr207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. J. and McGrath J. J. (1974). Hematologic and biochemical effects of simulated high-altitude on Japanese quail. J. Appl. Physiol. 37, 357-361. [DOI] [PubMed] [Google Scholar]
- Janecka J. E., Nielsen S. S. E., Andersen S. D., Hoffmann F. G., Weber R. E., Anderson T., Storz J. F. and Fago A. (2015). Genetically based low oxygen affinities of felid hemoglobins: lack of biochemical adaptation to high-altitude hypoxia in the snow leopard. J. Exp. Biol. 218, 2402-2409. 10.1242/jeb.125369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen F. B. (2004). Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol. Scand. 182, 215-227. 10.1111/j.1365-201X.2004.01361.x [DOI] [PubMed] [Google Scholar]
- Jensen F. B. (2009). The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J. Exp. Biol. 212, 3387-3393. 10.1242/jeb.023697 [DOI] [PubMed] [Google Scholar]
- Jensen F. B., Fago A. and Weber R. E. (1998). Hemoglobin structure and function. In Fish Physiology, Vol. 17: Fish Respiration (ed. Perry S. F. and Tufts B. L.), pp. 1-40. San Diego: Academic Press. [Google Scholar]
- Jensen B., Storz J. F. and Fago A. (2016). Bohr effect and temperature sensitivity of hemoglobins from highland and lowland deer mice. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 195, 10-14. 10.1016/j.cbpa.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen T. H., Weber R. E., Fermi G., Tame J. and Braunitzer G. (1991). Adaptation of bird hemoglobins to high altitudes: demonstration of molecular mechanism by protein engineering. Proc. Natl. Acad. Sci. USA 88, 6519-6522. 10.1073/pnas.88.15.6519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner A. J. (1976). Respiratory adaptations in burrowing pocket gophers from sea-level and high-altitude. J. Appl. Physiol. 41, 168-173. [DOI] [PubMed] [Google Scholar]
- Lenfant C. (1973). High altitude adaptation in mammals. Am. Zool. 13, 447-456. 10.1093/icb/13.2.447 [DOI] [Google Scholar]
- Lenfant C., Torrance J., English E., Finch C. A., Reynafarje C., Ramos J. and Faura J. (1968). Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J. Clin. Invest. 47, 2652-2656. 10.1172/JCI105948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C., Ways P., Aucutt C. and Cruz J. (1969). Effect of chronic hypoxic hypoxia on the O2-Hb dissociation curve and respiratory gas transport in man. Respir. Physiol. 7, 7-29. 10.1016/0034-5687(69)90066-8 [DOI] [PubMed] [Google Scholar]
- Lenfant C., Torrance J. D. and Reynafar C. (1971). Shift of the O2-Hb dissociation curve at altitude: mechanism and effect. J. Appl. Physiol. 30, 625-631. [DOI] [PubMed] [Google Scholar]
- León-Velarde F., de Muizon C., Palacios J. A., Clark D. and Monge C. (1996). Hemoglobin affinity and structure in high-altitude and sea-level carnivores from Peru. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 113, 407-411. 10.1016/0300-9629(95)02083-7 [DOI] [PubMed] [Google Scholar]
- Liddington R., Derewenda Z., Dodson G. and Harris D. (1988). Structure of the liganded T state of haemoglobin identifies the origin of cooperative oxygen binding. Nature 331, 725-728. 10.1038/331725a0 [DOI] [PubMed] [Google Scholar]
- Lutz P. L. (1980). On the oxygen affinity of bird blood. Am. Zool. 20, 187-198. 10.1093/icb/20.1.187 [DOI] [PubMed] [Google Scholar]
- Mairbäurl H. (1994). Red blood cell function in hypoxia at altitude and exercise. Int. J. Sports Med. 15, 51-63. 10.1055/s-2007-1021020 [DOI] [PubMed] [Google Scholar]
- Mairbäurl H. and Weber R. E. (2012). Oxygen transport by hemoglobin. Compr. Physiol. 2, 1463-1489. 10.1002/cphy.c080113 [DOI] [PubMed] [Google Scholar]
- Mairbäurl H., Schobersberger W., Hasibeder W., Schwaberger G., Gaesser G. and Tanaka K. R. (1986). Regulation of red cell 2,3-DPG and Hb-O2-affinity during acute exercise. Eur. J. Appl. Physiol. Occup. Physiol. 55, 174-180. 10.1007/BF00715001 [DOI] [PubMed] [Google Scholar]
- Mairbäurl H., Oelz O. and Bartsch P. (1993). Interactions between Hb, Mg, DPG, ATP, and Cl determine the change in Hb-O2 affinity at high-altitude. J. Appl. Physiol. 74, 40-48. [DOI] [PubMed] [Google Scholar]
- McGuire J. A., Witt C. C., Remsen J. V. Jr., Corl A., Rabosky D. L., Altshuler D. L. and Dudley R. (2014). Molecular phylogenetics and the diversification of hummingbirds. Curr. Biol. 24, 910-916. 10.1016/j.cub.2014.03.016 [DOI] [PubMed] [Google Scholar]
- Monge C. and León-Velarde F. (1991). Physiological adaptation to high-altitude - oxygen-transport in mammals and birds. Physiol. Rev. 71, 1135-1172. [DOI] [PubMed] [Google Scholar]
- Natarajan C., Inoguchi N., Weber R. E., Fago A., Moriyama H. and Storz J. F. (2013). Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340, 1324-1327. 10.1126/science.1236862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C., Hoffmann F. G., Lanier H. C., Wolf C. J., Cheviron Z. A., Spangler M. L., Weber R. E., Fago A. and Storz J. F. (2015a). Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol. Biol. Evol. 32, 978-997. 10.1093/molbev/msu403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C., Projecto-Garcia J., Moriyama H., Weber R. E., Muñoz-Fuentes V., Green A. J., Kopuchian C., Tubaro P. L., Alza L., Bulgarella M. et al. (2015b). Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genet. 11, e1005681 10.1371/journal.pgen.1005681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C., Hoffmann F. G., Weber R. E., Fago A., Witt C. C. and Storz J. F. (2016). Predictable convergence in hemoglobin function has unpredictable molecular underpinnings. Science. 10.1126/science/aaf9070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigen A. M., Manning J. M. and Alben J. O. (1980). Oxygen-linked binding sites for inorganic anions to hemoglobin. J. Biol. Chem. 255, 5525-5529. [PubMed] [Google Scholar]
- Nikinmaa M. (2001). Haemoglobin function in vertebrates: evolutionary changes in cellular regulation in hypoxia. Respir. Physiol. 128, 317-329. 10.1016/S0034-5687(01)00309-7 [DOI] [PubMed] [Google Scholar]
- O'Donnell S., Mandaro R., Schuster T. M. and Arnone A. (1979). X-ray diffraction and solutions studies of specifically carbamylated human hemoglobin A - Evidence for the location of a proton-linked and oxygen-linked chloride binding site at valine 1α. J. Biol. Chem. 254, 2204-2208. [PubMed] [Google Scholar]
- Opazo J. C., Hoffmann F. G. and Storz J. F. (2008a). Genomic evidence for independent origins of β-like globin genes in monotremes and therian mammals. Proc. Natl. Acad. Sci. USA 105, 1590-1595. 10.1073/pnas.0710531105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo J. C., Hoffmann F. G. and Storz J. F. (2008b). Differential loss of embryonic globin genes during the radiation of placental mammals. Proc. Natl. Acad. Sci. USA 105, 12950-12955. 10.1073/pnas.0804392105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo J. C., Sloan A. M., Campbell K. L. and Storz J. F. (2009). Origin and ascendancy of a chimeric fusion gene: the β/δ-globin gene of paenungulate mammals. Mol. Biol. Evol. 26, 1469-1478. 10.1093/molbev/msp064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo J. C., Hoffmann F. G., Natarajan C., Witt C. C., Berenbrink M. and Storz J. F. (2015). Gene turnover in the avian globin gene families and evolutionary changes in hemoglobin isoform expression. Mol. Biol. Evol. 32, 871-887. 10.1093/molbev/msu341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostojic H., Cifuentes V. and Monge C. (2002). Hemoglobin affinity in Andean rodents. Biol. Res. 35, 27-30. 10.4067/S0716-97602002000100005 [DOI] [PubMed] [Google Scholar]
- Pack-Mabien A. V. and Imran H. (2013). Benefits of delayed fetal hemoglobin (HbF) switching in sickle cell disease (SCD): a case report and review of the literature. J. Pediatr. Hematol. Oncol. 35, e347-e349. 10.1097/MPH.0b013e3182880dc8 [DOI] [PubMed] [Google Scholar]
- Penney D. and Thomas M. (1975). Hematological alterations and response to acute hypobaric stress. J. Appl. Physiol. 39, 1034-1037. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. (1970). Stereochemistry of cooperative effects in haemoglobin. Nature 228, 726-734. 10.1038/228726a0 [DOI] [PubMed] [Google Scholar]
- Perutz M. F. (1979). Regulation of oxygen affinity of hemoglobin: Influence of structure of the globin on the heme iron. Annu. Rev. Biochem. 48, 327-386. 10.1146/annurev.bi.48.070179.001551 [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Fermi G., Luisi B., Shaanan B. and Liddington R. C. (1987). Stereochemistry of cooperative mechanisms in hemoglobin. Cold Spring Harb. Symp. Quant. Biol. 52, 555-565. 10.1101/SQB.1987.052.01.063 [DOI] [PubMed] [Google Scholar]
- Petschow D., Wurdinger I., Baumann R., Duhm J., Braunitzer G. and Bauer C. (1977). Causes of high blood O2 affinity of animals living at high-altitude. J. Appl. Physiol. 42, 139-143. [DOI] [PubMed] [Google Scholar]
- Powell F. L. and Hopkins S. R. (2010). Vertebrate life at high altitude. In Respiratory Physiology of Vertebrates (ed. Nilsson G. E.), pp. 265-299. Cambridge: Cambridge University Press. [Google Scholar]
- Projecto-Garcia J., Natarajan C., Moriyama H., Weber R. E., Fago A., Cheviron Z. A., Dudley R., McGuire J. A., Witt C. C. and Storz J. F. (2013). Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc. Natl. Acad. Sci. USA 110, 20669-20674. 10.1073/pnas.1315456110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S. and Guest G. M. (1941). Distribution of acid-soluble phosphorus in the blood cells of various vertebrates. J. Biol. Chem. 138, 269-282. [Google Scholar]
- Rapoport I., Berger H., Elsner R. and Rapoport S. (1977). pH-dependent changes of 2,3-bisphosphoglycerate in human red cells during transitional and steady states in vitro. Eur. J. Biochem. 73, 421-427. 10.1111/j.1432-1033.1977.tb11333.x [DOI] [PubMed] [Google Scholar]
- Revsbech I. G., Tufts D. M., Projecto-Garcia J., Moriyama H., Weber R. E., Storz J. F. and Fago A. (2013). Hemoglobin function and allosteric regulation in semi-fossorial rodents (family Sciuridae) with different altitudinal ranges. J. Exp. Biol. 216, 4264-4271. 10.1242/jeb.091397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynafarje C., Faura J., Villavicencio D., Curaca A., Reynafarje B., Oyola L., Contreras L., Vallenas E. and Faura A. (1975). Oxygen transport of hemoglobin in high-altitude animals (Camelidae). J. Appl. Physiol. 38, 806-810. [DOI] [PubMed] [Google Scholar]
- Runck A. M., Moriyama H. and Storz J. F. (2009). Evolution of duplicated β-globin genes and the structural basis of hemoglobin isoform differentiation in Mus. Mol. Biol. Evol. 26, 2521-2532. 10.1093/molbev/msp165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runck A. M., Weber R. E., Fago A. and Storz J. F. (2010). Evolutionary and functional properties of a two-locus β-globin polymorphism in Indian house mice. Genetics 184, 1121-1131. 10.1534/genetics.109.113506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaja M. and Winslow R. M. (1979). The separate effects of H+ and 2,3-DPG on the oxygen equilibrium curve of human blood. Br. J. Haematol. 41, 373-381. 10.1111/j.1365-2141.1979.tb05870.x [DOI] [PubMed] [Google Scholar]
- Samaja M., Di Prampero P. E. and Cerretelli P. (1986). The role of 2,3-DPG in the oxygen transport at altitude. Respir. Physiol. 64, 191-202. 10.1016/0034-5687(86)90041-1 [DOI] [PubMed] [Google Scholar]
- Samaja M., Mariani C., Prestini A. and Cerretelli P. (1997). Acid-base balance and O2 transport at high altitude. Acta Physiol. Scand. 159, 249-256. 10.1046/j.1365-201X.1997.574342000.x [DOI] [PubMed] [Google Scholar]
- Samaja M., Crespi T., Guazzi M. and Vandegriff K. D. (2003). Oxygen transport in blood at high altitude: role of the hemoglobin-oxygen affinity and impact of the phenomena related to hemoglobin allosterism and red cell function. Eur. J. Appl. Physiol. 90, 351-359. 10.1007/s00421-003-0954-8 [DOI] [PubMed] [Google Scholar]
- Scott G. R. (2011). Elevated performance: the unique physiology of birds that fly at high altitudes. J. Exp. Biol. 214, 2455-2462. 10.1242/jeb.052548 [DOI] [PubMed] [Google Scholar]
- Scott G. R. and Milsom W. K. (2006). Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir. Physiol. Neurobiol. 154, 284-301. 10.1016/j.resp.2006.02.012 [DOI] [PubMed] [Google Scholar]
- Snyder L. R. G., Born S. and Lechner A. J. (1982). Blood oxygen affinity in high- and low-altitude populations of the deer mouse. Respir. Physiol. 48, 89-105. 10.1016/0034-5687(82)90052-4 [DOI] [PubMed] [Google Scholar]
- Stein J. C. and Ellsworth M. L. (1993). Capillary oxygen-transport during severe hypoxia - Role of hemoglobin-oxygen affinity. J. Appl. Physiol. 75, 1601-1607. [DOI] [PubMed] [Google Scholar]
- Storz J. F. (2007). Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J. Mammal. 88, 24-31. 10.1644/06-MAMM-S-199R1.1 [DOI] [Google Scholar]
- Storz J. F. (2016a). Causes of molecular convergence and parallelism in protein evolution. Nat. Rev. Genet. 17, 239-250. 10.1038/nrg.2016.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F. (2016b). Gene duplication and evolutionary innovations in hemoglobin-oxygen transport. Physiology 31, 223-232. 10.1152/physiol.00060.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F. and Moriyama H. (2008). Mechanisms of hemoglobin adaptation to high altitude hypoxia. High Alt. Med. Biol. 9, 148-157. 10.1089/ham.2007.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Baze M., Waite J. L., Hoffmann F. G., Opazo J. C. and Hayes J. P. (2007). Complex signatures of selection and gene conversion in the duplicated globin genes of house mice. Genetics 177, 481-500. 10.1534/genetics.107.078550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Sabatino S. J., Kelly J. K., Ferrand N., Moriyama H., Weber R. E. and Fago A. (2009). Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc. Natl. Acad. Sci. USA 106, 14450-14455. 10.1073/pnas.0905224106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Runck A. M., Moriyama H., Weber R. E. and Fago A. (2010a). Genetic differences in hemoglobin function between highland and lowland deer mice. J. Exp. Biol. 213, 2565-2574. 10.1242/jeb.042598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Scott G. R. and Cheviron Z. A. (2010b). Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125-4136. 10.1242/jeb.048181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Hoffmann F. G., Opazo J. C., Sanger T. J. and Moriyama H. (2011a). Developmental regulation of hemoglobin synthesis in the green anole lizard Anolis carolinensis. J. Exp. Biol. 214, 575-581. 10.1242/jeb.050443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Opazo J. C. and Hoffmann F. G. (2011b). Phylogenetic diversification of the globin gene superfamily in chordates. IUBMB Life 63, 313-322. 10.1002/iub.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Opazo J. C. and Hoffmann F. G. (2013). Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Mol. Phylogenet. Evol. 66, 469-478. 10.1016/j.ympev.2012.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Bridgham J. T., Kelly S. A. and Garland T. Jr. (2015a). Genetic approaches in comparative and evolutionary physiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R197-R214. 10.1152/ajpregu.00100.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J. F., Natarajan C., Moriyama H., Hoffmann F. G., Wang T., Fago A., Malte H., Overgaard J. and Weber R. E. (2015b). Oxygenation properties and isoform diversity of snake hemoglobins. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1178-R1191. 10.1152/ajpregu.00327.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance J. D., Lenfant C., Cruz J. and Marticorena E. (1970/71). Oxygen transport mechanisms in residents at high altitude. Respir. Physiol. 11, 1-15. 10.1016/0034-5687(70)90098-8 [DOI] [PubMed] [Google Scholar]
- Tufts D. M., Revsbech I. G., Cheviron Z. A., Weber R. E., Fago A. and Storz J. F. (2013). Phenotypic plasticity in blood-oxygen transport in highland and lowland deer mice. J. Exp. Biol. 216, 1167-1173. 10.1242/jeb.079848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufts D. M., Natarajan C., Revsbech I. G., Projecto-Garcia J., Hoffmann F. G., Weber R. E., Fago A., Moriyama H. and Storz J. F. (2015). Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol. Biol. Evol. 32, 287-298. 10.1093/molbev/msu311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F. and Hoofd L. J. C. (1973). Advantage or disadvantage of a decrease of blood oxygen affinity for tissue oxygen supply at hypoxia - theoretical study comparing man and rat. Pflügers Arch. 342, 185-197. 10.1007/BF00591367 [DOI] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F. and Ringnalda B. E. M. (1978a). Blood gases at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflügers Arch. 376, 7-13. 10.1007/BF00585241 [DOI] [PubMed] [Google Scholar]
- Turek Z., Kreuzer F., Turek-Maischeider M. and Ringnalda B. E. M. (1978b). Blood O2 content, cardiac output, and flow to organs at several levels of oxygenation in rats with a left-shifted blood oxygen dissociation curve. Pflügers Arch. 376, 201-207. 10.1007/BF00584951 [DOI] [PubMed] [Google Scholar]
- Val A. L. (2000). Organic phosphates in the red blood cells of fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 125, 417-435. 10.1016/S1095-6433(00)00184-7 [DOI] [PubMed] [Google Scholar]
- Weber R. E. (1990). Functional significance and structural basis of multiple hemoglobins with special reference to ectothermic vertebrates. In Animal Nutrition and Transport Processes, Vol. 6 (ed. Truchot J.-P. and Lahlou B.), pp. 58-75. Basel, Switzerland: S. Karger. [Google Scholar]
- Weber R. E. (1995). Hemoglobin adaptations to hypoxia and altitude - the phylogenetic perspective. In Hypoxia and the Brain (ed. Sutton J. R., Houston C. S. and Coates G.), pp. 31-44. Burlington, VT: Queen City Printers. [Google Scholar]
- Weber R. E. (1996). Hemoglobin adaptations in Amazonian and temperate fish with special reference to hypoxia, allosteric effectors and functional heterogeneity. In Physiology and Biochemistry of the Fishes of the Amazon (ed. Val A. L., Almeida-Val V. M. F. and Randall D. J.), pp. 75-90. Manaus, Brazil: INPA. [Google Scholar]
- Weber R. E. (2007). High-altitude adaptations in vertebrate hemoglobins. Respir. Physiol. Neurobiol. 158, 132-142. 10.1016/j.resp.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Weber R. E. and Fago A. (2004). Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir. Physiol. Neurobiol. 144, 141-159. 10.1016/j.resp.2004.04.018 [DOI] [PubMed] [Google Scholar]
- Weber R. E. and Jensen F. B. (1988). Functional adaptations in hemoglobins from ectothermic vertebrates. Annu. Rev. Physiol. 50, 161-179. 10.1146/annurev.ph.50.030188.001113 [DOI] [PubMed] [Google Scholar]
- Weber R. E., Hiebl I. and Braunitzer G. (1988a). High altitude and hemoglobin function in the vultures Gyps rueppellii and Aegypius monachus. Biol. Chem. Hoppe Seyler 369, 233-240. 10.1515/bchm3.1988.369.1.233 [DOI] [PubMed] [Google Scholar]
- Weber R. E., Lalthantluanga R. and Braunitzer G. (1988b). Functional characterization of fetal and adult yak hemoglobins: an oxygen binding cascade and its molecular basis. Arch. Biochem. Biophys. 263, 199-203. 10.1016/0003-9861(88)90628-5 [DOI] [PubMed] [Google Scholar]
- West J. B. and Wagner P. D. (1980). Predicted gas exchange on the summit of Mt. Everest. Respir. Physiol. 42, 1-16. 10.1016/0034-5687(80)90100-0 [DOI] [PubMed] [Google Scholar]
- Willford D. C., Hill E. P. and Moores W. Y. (1982). Theoretical analysis of optimal P50. J. Appl. Physiol. 52, 1043-1048. [DOI] [PubMed] [Google Scholar]
- Winslow R. M., Samaja M. and West J. B. (1984). Red cell function at extreme altitudes on Mount Everest. J. Appl. Physiol. 56, 109-116. [DOI] [PubMed] [Google Scholar]
- Woodson R. D. (1988). Evidence that changes in blood oxygen affinity modulate oxygen delivery: implications for control of tissue PO2 gradients. Adv. Exp. Med. Biol. 215, 309-313. 10.1007/978-1-4615-9510-6_36 [DOI] [PubMed] [Google Scholar]
- Yalcin O. and Cabrales P. (2012). Increased hemoglobin O2 affinity protects during acute hypoxia. Am. J. Physiol. Heart Circ. Physiol. 303, H271-H281. 10.1152/ajpheart.00078.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonetani T. and Tsuneshige A. (2003). The global allostery model of hemoglobin: an allosteric mechanism involving homotropic and heterotropic interactions. C. R. Biol. 326, 523-532. 10.1016/S1631-0691(03)00150-1 [DOI] [PubMed] [Google Scholar]