Abstract
Background
Atherosclerotic renal artery stenosis (ARAS) reduces renal blood flow (RBF), ultimately leading to kidney hypoxia and inflammation. Insulin-like growth factor binding protein-7 (IGFBP-7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) are biomarkers of cell cycle arrest, often increased in ischemic conditions and predictive of acute kidney injury (AKI). This study sought to examine the relationships between renal vein levels of IGFBP-7, TIMP-2, reductions in RBF and postcontrast hypoxia as measured by blood oxygen level–dependent (BOLD) magnetic resonance imaging.
Methods
Renal vein levels of IGFBP-7 and TIMP-2 were obtained in an ARAS cohort (n= 29) scheduled for renal artery stenting and essential hypertensive (EH) healthy controls (n = 32). Cortical and medullary RBFs were measured by multidetector computed tomography (CT) immediately before renal artery stenting and 3 months later. BOLD imaging was performed before and 3 months after stenting in all patients, and a subgroup (N = 12) underwent repeat BOLD imaging 24 h after CT/stenting to examine postcontrast/procedure levels of hypoxia.
Results
Preintervention IGFBP-7 and TIMP-2 levels were elevated in ARAS compared with EH (18.5 ± 2.0 versus 15.7 ± 1.5 and 97.4 ± 23.1 versus 62.7 ± 9.2 ng/mL, respectively; P< 0.0001); baseline IGFBP-7 correlated inversely with hypoxia developing 24 h after contrast injection (r = −0.73, P< 0.0001) and with prestent cortical blood flow (r = −0.59, P= 0.004).
Conclusion
These data demonstrate elevated IGFBP-7 and TIMP-2 levels in ARAS as a function of the degree of reduced RBF. Elevated baseline IGFBP-7 levels were associated with protection against postimaging hypoxia, consistent with ‘ischemic preconditioning’. Despite contrast injection and stenting, AKI in these high-risk ARAS subjects with elevated IGFBP-7/TIMP-2 was rare and did not affect long-term kidney function.
Keywords: contrast, ischemia, renal artery stenosis, revascularization, stent
INTRODUCTION
Atherosclerotic renal artery stenosis (ARAS) produces lumen occlusion, eventually lowering kidney perfusion and accelerating hypertension. Although the kidney is abundantly perfused due to its filtration function and can tolerate moderate reductions in blood flow, severe ARAS elicits complex biological responses that eventually lead to chronic kidney injury [1–3]. High-grade ARAS leads to cortical and medullary hypoxia [4–6] and activates inflammatory pathways and tissue fibrosis in both experimental and human studies [7]. Advanced ARAS is associated with reduced glomerular filtration rate (GFR) and elevated renal vein levels of neutrophil gelatinase associated lipocalin (NGAL), tumor necrosis factor α (TNF-α) and other injury biomarkers from the poststenotic kidney [6, 8, 9]. Little is known regarding the presence and levels of cell cycle arrest markers in this disorder, such as insulin-like growth factor binding protein-7 (IGFBP-7) or tissue inhibitor of metalloproteinases-2 (TIMP-2), which have been validated as urinary biomarkers for acute kidney injury (AKI) in intensive care settings with acutely ill patients [10]. A complex relationship appears to exist between these markers and subsequent kidney damage. While an increase in IGFBP-7 and TIMP-2 after cardiopulmonary bypass predicts a higher rate of AKI, studies of remote ischemic preconditioning suggest that individuals with an increase in TIMP-2 and IGFBP7 after the conditioning procedure itself are those most likely to be protected from AKI after surgery [11]. We have recently shown that transient elevation of TIMP-2 and IGFBP-7 after stenting and contrast in ARAS is associated with a reduction in NGAL [12]. While urinary values for these biomarkers are predictive of developing AKI, some authors suggest that transient cell cycle arrest can protect the kidney from more severe tubular injury [13, 14].
Clinical investigation of ARAS commonly requires exposure to contrast agents during imaging and/or angiography [15]. Human studies of blood flow and tissue oxygenation in ARAS from our laboratory employ a standardized protocol that includes central injection of iodinated contrast medium (CM) as part of a multidetector computed tomography (MDCT) examination immediately prior to stent revascularization [16]. Exposure to intravascular iodinated CM has been associated with an increased risk for AKI [17], usually designated ‘contrast-induced nephropathy’ (CIN). Experimental studies indicate that CM produces transient reductions in RBF [18, 19] and can produce AKI when combined with volume depletion and/or other events [20]. Risk factors for CIN include advanced age, preexisting renal dysfunction and diabetes mellitus [21]. The potential toxicity from exposure to CM combined with renovascular stenting remains poorly understood.
Blood oxygen level–dependent (BOLD) magnetic resonance imaging (MRI) depicts changes in renal oxygenation in humans noninvasively by measuring rates of magnetic dipole relaxation [R2* (The rate at which the phase coherence of magnetization in the transverse plane is lost following an initial radiofrequency pulse and is the inverse of the characteristic relaxation time T2*.)] that reflect local levels of deoxyhemoglobin within the kidney [5, 22–24]. We have previously shown that R2* levels are higher in stenotic kidneys compared with contralateral kidneys in ARAS and kidneys of hypertensive patients [5].
In this study, we examined the effect of ARAS on IGFBP-7 and TIMP-2 and evaluated tissue oxygenation using BOLD MRI before and 24 h and 3 months after contrast imaging and stenting. Our hypotheses were as follows: (1) reduced blood flow to stenotic kidneys would be associated with increased plasma levels of IGFBP-7 and TIMP-2, and (2) combined contrast infusion and stenting procedures would induce AKI in ARAS. We also sought to evaluate circulating markers including plasma levels of NGAL, kidney injury molecule (KIM-1), TNF-α, IGFBP-7 and TIMP-2 at 24 h and 3 months after stenting in ARAS patients when compared with patients with essential hypertension (EH) without stenting undergoing an identical inpatient imaging protocol.
METHODS
Patient selection
Patients identified with EH (n = 32) or ARAS (n = 29), scheduled for renal revascularization for clinical indications (including resistant hypertension, progressive decline in kidney function or episodes of circulatory congestion) seen between January 2008 to April 2014 participated in this study during a 3-day inpatient protocol. ARAS patients returned to repeat the protocol 3 months after renal artery revascularization in the clinical research unit of Saint Mary's Hospital (Rochester, MN), as previously described [25]. A subgroup (Group B) consisted of 12 ARAS patients subjected to the same overall protocol extending for an additional day for repeat BOLD imaging 24 h after the CM injection and stenting procedure as described below. Since 10 patients had bilateral stenosis, 39 poststenotic kidneys stented (STKs) were available for analysis. For each subject with bilateral stenosis and EH, a single kidney was used for statistical analysis. Dietary intake was regulated at 150 mEq of sodium with an isocaloric diet prepared on site. Patients with ARAS were identified using criteria similar to those used for recruitment in the Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) Trial with cross-sectional luminal occlusion of at least 60% but with the requirement of serum creatinine <2.5 mg/dL [26]. Informed, written consent was obtained as approved by the institutional review board of the Mayo Clinic. The severity of renal artery stenosis was confirmed by Doppler ultrasound measurements in the affected artery and quantitative vascular imaging using CT images, as described below. Patients continued previous medications, and all received agents blocking the renin–angiotensin system during these studies (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers). ARAS patients returned for repeat measurements 3–4 months after renal revascularization.
Renal function and blood pressure measurements
The first study day included measurement of GFR by iothalamate clearance [iothalamate meglumine (Conray, Mallinckrodt)] after oral hydration (20 mL/kg) over three 30-min timed collection periods, as described previously [27, 28]. Single-kidney GFR was determined by apportioning the measured iothalamate clearance to the percentage of blood flow for each kidney. Blood pressure was measured by automated oscillometric recordings including three values taken three times daily (an automated oscillometric unit, Omron blood pressure and measured blood pressure at 5, 7 and 9 min after a 5-min rest).
Tissue oxygenation determined by BOLD MRI
On the second day, BOLD MRI examinations were performed on a GE Signa HDxt 3.0 T system (GE Medical Systems, Waukesha, WI) using a 12-channel torso phased array coil before and 15 min after intravenous injection of furosemide (20 mg), as previously described [24, 25]. Furosemide was used to assess the tubular function, and it is known to inhibit the adenosine triphosphate–dependent sodium potassium chloride cotransporter, leading to a decrease in oxygen consumption of the kidney. The BOLD MRI examination was repeated in a subset of 12 ARAS patients (Group B) 24 h after stenting and in all ARAS patients 3 months after revascularization.
MRI data analysis
Analysis of BOLD data from coronal/axial images was performed by drawing parenchymal regions of interest (ROIs) on two to four slices through the midpole hilar region of each kidney on representative T2*-weighted images and then transferring the ROI to the corresponding R2* parametric image. Two ROIs were traced: one that selected the renal cortex (large segment) for estimation of cortical R2*, and the second, which included the entire kidney slice, including both cortex and medulla while excluding the renal collecting system and any incidental renal cysts to determine the fractional tissue hypoxia of the entire slice, as previously described [5].
Cortical and medullary perfusion and blood flow measured by MDCT
On the third study day, the common femoral vein was cannulated with a 6F sheath and blood samples drawn from the right and left renal veins with a 5F Cobra 2 catheter with side holes (Cook, Inc., Bloomington, IN, USA) for NGAL, KIM-1, IGFBP-7, TIMP-2 and TNF-α, as described below. A 5F straight catheter was then advanced into the right atrium for central venous injection of contrast for flow studies using MDCT. MDCT imaging was obtained using a dual-source 64-slice helical MDCT scanner (SOMATOM Definition, Siemens Medical Solutions) after a bolus injection of iohexol 350 (0.5 mL/kg up to a maximum of 40 mL) using a power injector during respiratory suspension. Perfusion scans were performed as previously described [6]. The total amount of contrast agent [iohexol (Novaplus, Omnipaque 350 mg I/mL, GE Healthcare, Princeton, NJ, USA; 500 mg I/kg] used for CT imaging was ∼100 mL, and the average amount of contrast used for angiography was 60 mL iodixanol (VISIPAQUE 320 mg I/mL, GE Healthcare).
CT data analysis
MDCT images were reconstructed and displayed with the Analyze software package (Biomedical Imaging Resource, Mayo Clinic, MN, USA). ROIs were selected from cross-sectional images of the aorta, renal cortex and medulla. Average tissue attenuation in each region was plotted over time and fitted by curve-fitting algorithms to obtain measures of renal function as described previously [16]. Cortical and medullary volumes were calculated by Analyze and RBF as the sum of the products of cortical and medullary perfusions and corresponding volumes [16].
RENAL VEIN AND PLASMA SAMPLING
Blood samples for NGAL, KIM-1, IGFBP-7 and TIMP-2 and TNF-α were obtained before the CT and stenting from the renal and peripheral veins of all patients, as previously described [29, 30]. Samples were stored at −80°C until measurement. Collected samples were centrifuged and the supernatant was stored. NGAL, KIM-1, IGFBP-7 and TIMP-2 (ng/mL) were tested by ELISA according to the manufacturer's protocol (NGAL BioPorto Diagnostics, KIT 036; KIM-1 and IGFBP-7: R&D Systems, DKM100 and DY1334-05, respectively; TIMP-2: Sigma Aldrich, RAB0472-1KT). Levels of TNF-α were measured by luminex (Millipore, MPXHCYTO-60K). Signals were read by the Bio-plex 200 system (Bio-Rad). All measurements were performed by a single investigator blinded to the clinical data.
Statistical analysis
Results were expressed as mean and standard deviation (SD) or median (range), as appropriate. Qualitative variables were expressed as number (percentage). Comparisons between independent groups with essential hypertension or ARAS were performed using a two-sample t-test with unequal variance (or the Wilcoxon rank sum test for skewed data) and a χ2 test or Fisher's exact test for categorical variables, as appropriate. Comparisons between stenotic kidneys within the same individuals and repeated measurements within individuals after revascularization were performed using a paired t-test (or Wilcoxon's signed-rank test for skewed data). A significance level of 0.05 was accepted. Multivariate Spearman rank correlation analysis was used to test for correlations between basal injury markers, GFR, cortical blood flow and percentage change in renal hypoxia, while univariable models were used to show the unadjusted association. A regression model was developed to predict the percent change in renal hypoxia levels using a forward stepwise selection method [including age, sex, body mass index (BMI), cortical blood flow, GFR and biomarkers] with 0.25 to enter the model and 0.05 to stay in the model. Statistical analysis was performed using the JMP software package (version 8.0; SAS Institute, Cary, NC, USA).
RESULTS
Demographic comparison between ARAS and EH patients
Demographic and clinical features for 29 patients in the ARAS group (10 with bilateral renal artery stenosis) and 32 EH patients are summarized in Table 1. Age, weight, BMI and most biochemical values were not different between groups. ARAS patients were more commonly treated with statins. Serum creatinine and systolic blood pressure were higher in patients with ARAS, while iothalamate clearance (GFR) was lower.
Table 1.
Clinical, laboratory and demographic data of hypertensive and ARAS patients
| EH (n = 32) | ARAS (n = 29) | P-value | |
|---|---|---|---|
| Gender (% male)a | 59 | 66 | 0.79 |
| Age (years) | 63.1 ± 16.3 | 69 ± 8.1 | 0.11 |
| Creatinine (mg/dL) | 0.96 ± 0.26 | 1.59 ± 0.42 | <0.0001 |
| Iothalamate clearance GFR (mL/min) | 87.9 ± 24.3 | 58.4 ± 28.8 | <0.0001 |
| Degree of arterial stenosisb | 7.1 (2.8, 16.6) | 70 (64.5, 74.2) | <0.0001 |
| ACEI or ARB (yes/no)a | 31/1 | 29/0 | |
| Statins (yes)a | 16 (50%) | 24 (83%) | 0.01 |
| Number of anti-HTN drugsb | 4 (2–4) | 4 (3–5) | 0.38 |
| SBP (mmHg) | 135 ± 18.9 | 150.4 ± 19.4 | 0.003 |
| DBP (mmHg) | 71.3 ± 12.3 | 71.5 ± 11.5 | 0.97 |
| Weight (kg) | 78.3 ± 15.6 | 86.6 ± 22.1 | 0.09 |
| BMI (kg/m2) | 27.4 ± 4.3 | 29.85 ± 6.8 | 0.09 |
| Hematocrit (%) | 40.3 ± 4.1 | 37.7 ± 3.4 | 0.01 |
| Total cholesterol (mg/dL) | 182.8 ± 30.7 | 174.5 ± 41.4 | 0.4 |
| TG (mg/dL) | |||
| Mean | 130.6 ± 57.8 | 177.1 ± 87.7 | 0.09 |
| Median (IQR)b | 106 (93–456) | 168 (96–216) | |
| HDL (mg/dL) | 52.29 ± 12.4 | 45.4 ± 18 | 0.1 |
| LDL (mg/dL) | 103.7 ± 23.6 | 96.3 ± 28.6 | 0.07 |
| Microalbumin (mg/24 h)b | 19 (13, 29) | 22.5 (10.3, 46) | 0.4 |
Data presented as mean ± SD unless otherwise noted. GFR, glomerular filtration rate; anti-HTN, antihypertensive; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; SBP, systolic blood pressure; DBP, diastolic blood pressure; ARAS, atherosclerotic renal artery stenosis; EH, essential hypertension.
aFisher's exact test or Pearson's χ2 test (categorical data).
bMedian [interquartile range (IQR)] reported due to skewed data. P-value obtained from Student's t-test or Wilcoxon's rank sum test (not normally distributed data).
RBF and GFR in ARAS STKs increased after revascularization, while kidney volume remained unchanged
Results from quantitative MDCT measurements of hemodynamics of individual poststenotic kidneys subjected to stenting and EH kidneys are summarized in Table 2. One kidney per subject (randomly selected in the EH group and ARAS patients with bilateral disease) was used for analysis. The total volume of poststenotic kidneys was reduced (EH, 145.9 ± 36 versus STK, 114.9 ± 46.6 mL; P = 0.007), primarily due to a reduction in cortical volume when compared with EH kidneys. Three months after technically successful stent revascularization, total kidney volume remained unchanged but was no longer lower than that in EH. Both baseline cortical and medullary perfusions (flow per unit tissue volume) were reduced in the STK compared with kidneys from EH. Measurements taken 3 months after renal artery stenting demonstrated an increase in cortical perfusion (2.27 ± 0.6 versus 2.65 ± 0.75 mL/min/mL of tissue; P = 0.05) but no change in medullary perfusion. Whole-kidney blood flow was reduced in the STK when compared with EH kidneys, with partial restoration after revascularization. Single-kidney iothalamate GFR (mL/min/kidney) in STKs was lower than that in EH kidneys, and increased slightly after stent revascularization (25.45 ± 14.7 versus 29.69 ± 17.01 mL/min/kidney; P = 0.01), although it remained below single-kidney GFR from EH subjects.
Table 2.
Multidetector CT measurements of individual kidney volume, tissue perfusion, blood flow and iothalamate filtration
| Single kidney | EH (N = 32) | STK (N = 29) |
|
|---|---|---|---|
| Baseline | 3 Months | ||
| Total kidney volume (CT) (mL) | 145.9 ± 36 | 114.9 ± 46.6* | 125.9 ± 49.9 |
| Cortical volume (mL) | 96.7 ± 29 | 70.4 ± 34.5* | 78.4 ± 34.6* |
| Medullary volume (mL) | 49 ± 14.6 | 44.5 ± 18.3 | 47.56 ± 18.6 |
| Cortical perfusion (mL/min/mL of tissue) | 3.4 ± 0.99 | 2.27 ± 0.6* | 2.65 ± 0.75#,* |
| Medullary perfusion (mL/min/mL of tissue) | 1.3 ± 0.48 | 0.93 ± 0.28* | 0.94 ± 0.3* |
| Total RBF (mL/min) | 399 ± 174 | 208.1 ± 114.4* | 255.4 ± 148.2#,* |
| Cortical flow (mL/min) | 331 ± 161 | 164.4 ± 94.02* | 208 ± 126.8#,* |
| Medullary flow (mL/min) | 63 ± 25 | 43.6 ± 28.9* | 47.36 ± 29.8* |
| Single-kidney GFR (mL/min/kidney) | 44.3 ± 13.5 | 25.45 ± 14.7* | 29.69 ± 17.01#,* |
Data presented as mean ± SD. *P-value <0.05 versus EH.
#P-value ≤0.05 versus baseline.
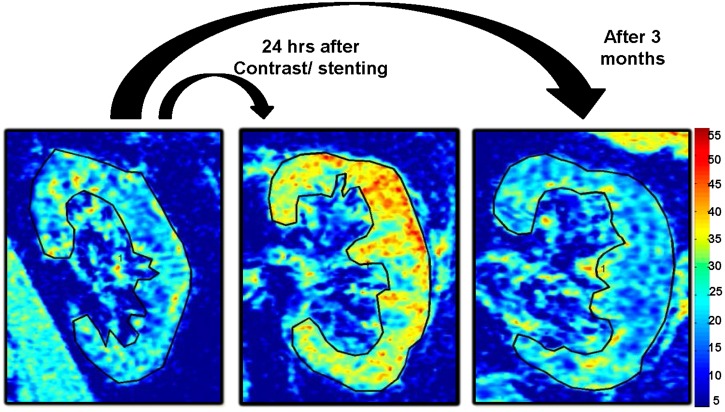
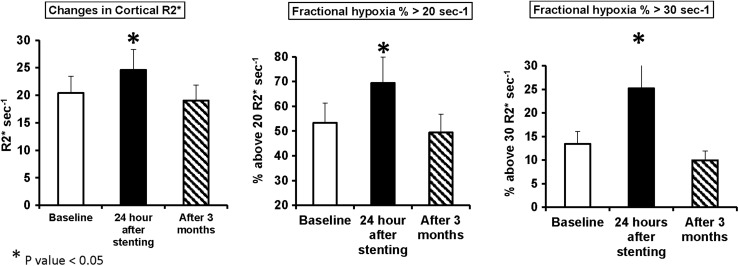
Widespread tissue hypoxia developed 24 h after contrast imaging and stent renal revascularization in ARAS patients and resolved by 3 months
Changes after 24 h in renal hypoxia levels defined both by cortical R2* values and fractional hypoxia (which represents mainly the medulla; %R2* >20 and 30/s) are summarized in Supplementary data, Table S1 and representative coronal R2* parametric maps illustrating the change in hypoxia in ARAS kidney before and after revascularization in Figure 1. Fifty percent of kidneys developed hypoxia after 24 h and, on average, cortical R2* levels rose (baseline, 20.37 ± 4.36 versus 24 h, 24.59 ± 6.7/s; P = 0.01) and renal hypoxia levels increased 24 h after contrast imaging and stenting in Group B but fell to baseline levels when remeasured 3 months after renal artery stenting (Figure 2). Prestenting basal and postfurosemide fractional hypoxia levels were higher in STK than in EH kidneys (19.2 versus 8.7% R2* >30/s; P = 0.001) (Supplementary data, Table S2). Levels of fractional tissue hypoxia fell after furosemide administration, but remained above those of EH.
FIGURE 1:
Example of R2* parametric maps (coronal plane) for a subject with ARAS at baseline and 24 h and 3 months after contrast imaging and renal stenting, obtained using the same color scale for R2*, demonstrating the transient widespread tissue hypoxia developing 24 h after contrast imaging and renal stenting.
FIGURE 2:
Early and late changes in tissue oxygenation after combined imaging angiography and renal revascularization for ARAS (n = 12): cortical R2* and fractional hypoxia (%R2* >20 and 30/s) increased significantly after stent revascularization and angiography. The hypoxia levels fell to baseline levels after 3 months.
Baseline levels of IGFBP-7 and NGAL were inversely correlated with renal hypoxia 24 h after contrast injection
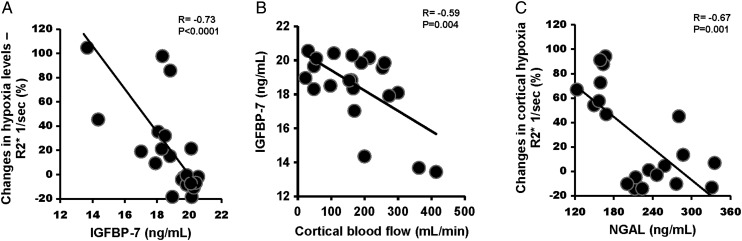
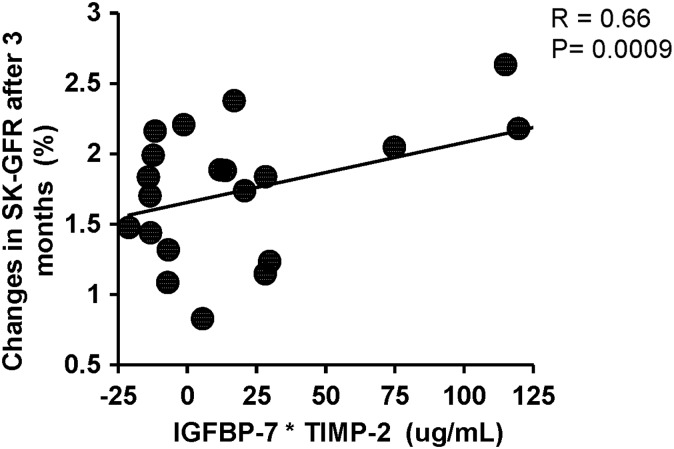
Renal vein baseline levels of IGFBP-7 and TIMP-2, NGAL, MCP-1, KIM-1 and TNF-α were elevated in ARAS compared with EH (Table 3). Baseline levels of renal vein IGFBP-7 and NGAL correlated inversely with the percent change in renal hypoxia both in the cortex and medulla 24 h after the contrast/stenting (Figure 3A and C) (r = −0.73, P < 0.0001 and r = −0.67, P = 0.001, respectively). Baseline renal vein levels of IGFBP-7 correlated inversely with cortical blood flow (r = −0.59, P = 0.004) (Figure 3B) even after adjustment for GFR (r = −0.44, P = 0.04). TIMP-2 levels correlated inversely only with the percent change in cortical hypoxia (R2*) (r = −0.45, P = 0.03) after adjustment for GFR. Importantly, baseline renal vein levels of IGFBP-7* TIMP-2 correlated directly with the percentage change in single-kidney GFR 3 months after stenting (r = 0.66, P= 0.0009) (Figure 5).
Table 3.
Renal vein levels of NGAL, MCP-1, TNF-α, IGFBP-7 and TIMP-2 in ARAS were higher than in essential hypertension
| EH | STK baseline | 3 months | |
|---|---|---|---|
| MCP-1 (pg/mL) | 154.7 ± 71.6 | 572.7 ± 170.5* | 634.3 ± 222.1* |
| TNF-α (pg/mL) | 4.7 ± 4.2 | 15.7 ± 7.6* | 16.1 ± 9.3* |
| NGAL (ng/mL) | 69.7 ± 34.3 | 205.9 ± 49.8* | 242.9 ± 79.8* |
| IGFBP-7 (ng/mL) | 15.7 ± 1.5 | 18.5 ± 1.9* | 17.9 ± 2.3* |
| TIMP-2 (ng/mL) | 62.7 ± 9.2 | 95.7 ± 21.3* | 103.1 ± 33.9* |
Data presented as mean ± SD. *P-value <0.0001 versus EH [Wilcoxon/Kruskal–Wallis tests (rank sums)].
FIGURE 3:
(A) Relationship between baseline renal vein levels of IGFBP-2 (ng/mL) and the percentage change of R2* after 24 h of contrast injection and stenting (r = −0.73). (B) Relationship between baseline renal vein levels of IGFBP-2 (ng/mL) and single-kidney cortical blood flow (mL/min) (r = − 0.59). (C) Relationship between baseline renal vein levels of NGAL (NG/mL) and the percentage change of cortical R2* after 24 h of contrast injection and stenting (r = −0.67).
FIGURE 5:
Relationship between baseline levels of IGFBP-7* TIMP-2 and the observed relative (%) change in single-kidney GFR 3 months after stenting (r= 0.66, P= 0.0009).
Despite transient renal hypoxia immediately after imaging, biomarkers and GFR were unchanged 3 months after contrast injection and renal artery stenting in ARAS patients
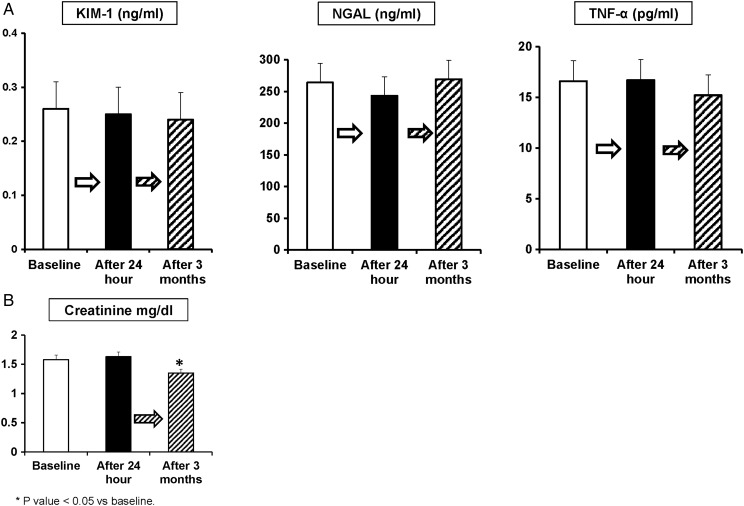
Peripheral vein levels of these markers (KIM-1, NGAL and TNF-α) remained unchanged 24 h and 3 months after renal revascularization (Figure 4a). Four patients had a transient increase in serum creatinine levels >0.3 mg/dL 24 h after contrast imaging and stenting: two patients had 0.4, one 0.5 and one had 0.6 mg/dL changes. Creatinine levels fell to baseline levels or below by 3 months (1.58 ± 0.43–1.35 ± 0.37; P < 0.05) (Figure 4b). Values for eGFR reflect corresponding increases in glomerular filtration for this cohort (38.16 ± 12.2–42.8 ± 13.9; P = 0.003).
FIGURE 4:
(A) Plasma levels of KIM-1, NGAL and TNF-α measured at baseline and 24 h and 3 months after contrast imaging and renal stenting show no change. Despite the transient hypoxia, the AKI markers showed no evidence of acute changes during this period after imaging and stenting. (B) No consistent change in serum creatinine developed the day after contrast imaging and renal stenting. Mean serum creatinine levels fell 3 months after renal stenting (1.58 ± 0.43–1.35 ± 0.37; P < 0.05).
DISCUSSION
This study demonstrates for the first time baseline elevations of renal vein IGFBP-7 and TIMP-2 levels in ARAS patients with reduced RBF and single-kidney GFR. Central venous contrast injection and stenting were followed by widespread increases in R2* consistent with hypoxia affecting both cortical and medullary regions 24 h later in some patients. Remarkably, this intense, transient hypoxic signal correlated inversely with baseline levels of NGAL and IGFBP-7; both were elevated at baseline compared with similar-age patients with essential hypertension. This hypoxic signal resolved after 3 months with no evident loss of GFR in the affected kidneys. Patients with higher levels of TIMP-2 and IGFBP-7 tended to respond better to revascularization (Figure 5). Despite this degree of hypoxia 24 h after contrast and stenting, no sustained changes in serum creatinine or plasma levels of NGAL, KIM-1, TNF-α, IGFBP-7 or TIMP-2 in ARAS occurred during this interval. Temporary increaes in serum creatinine (>0.3 mg/dL) were observed in only four subjects but were resolved by the time of restudy 3 months later. Levels of cortical blood flow, perfusion and single-kidney GFR in poststenotic kidneys rose moderately by 3 months after revascularization, although medullary flows remained reduced and renal vein biomarkers were unchanged 3 months after renal revascularization.
The inverse relationship between baseline biomarker levels in the renal veins and changes in R2* after imaging merits particular attention and underscores the complex relationship between these markers, AKI and subsequent ‘renoprotection’. We interpret these elevated baseline levels as possibly reflecting the effects of ongoing, episodic reductions in perfusion to the poststenotic kidney. Repeated reductions in blood flow represent a clinical example of ‘ischemic preconditioning’. Renal ischemic preconditioning in animal models is usually produced by inducing temporary total renal artery occlusion and can protect the kidney from subsequent prolonged ischemic insults [31]. Repetitive periods of myocardial ischemia cause no harm to cardiac tissue [32]. More recently, ‘remote’ ischemic preconditioning using repetitive, transient tourniquet occlusion to arm blood flow has been shown to prevent contrast-induced AKI in high-risk patients and reduces the development of AKI after cardiac surgery [33, 34]. Importantly, remote ischemic preconditioning was most effective in patients that developed increased levels of IGFBP-7 and TIMP-2 during the conditioning procedure in that study [11]. Our results suggest further that increased levels of renal vein biomarkers reflecting G1 cell cycle arrest within epithelial cells including TIMP-2 and IGFBP-7 were associated with relative protection from subsequent prolonged changes in BOLD signal both in the cortex and medullary regions [12, 35]. Importantly, these markers were higher in patients with reduced RBF and predicted the changes in single-kidney GFR after 3 months of stenting. In a similar pattern, NGAL can be protective against acute ischemic injury, although it is most often utilized as a biomarker for AKI [36].
Our results using BOLD MRI suggest that residual accumulation of deoxyhemoglobin after contrast imaging can persist for at least 24–27 h and extend results observed in experimental rat models that demonstrate renal hypoxia measured by BOLD MRI after injection of iodinated contrast [37]. Studies in the rat suggest that these changes were associated with AKI as measured by NGAL levels [38]. However, another study from the same authors reported that AKI after renal hypoxia in rats after contrast injection did not occur when the rats were pretreated with furosemide [39]. Our study extends these observations to human kidneys that tolerated procedure-related hypoxia after instrumentation without sustained changes in NGAL, KIM-1 or TNF-α.
Remarkably, a recent study of human subjects with total renal artery clamp occlusion for periods between 30 and 60 min during surgery reported minimal kidney injury as determined by levels of creatinine, plasma biomarkers or tissue biopsy [40]. Temporary ischemic changes in mitochondria were evident by electron microscopy with minor tubular injury that began to recover after reflow [40]. These data are consistent with our observations that major tissue hypoxia in the human kidney after 24 h may not result in sustained tissue injury, unlike that observed in experimental models. A growing body of evidence indicates that repeated ischemia and activation of chronic injury signaling pathways may induce natural defenses such as bioenergetic downregulation and temporary cell cycle arrest [11, 41]. These defenses may be the substrate for ‘ischemic preconditioning’ that can protect the kidney during subsequent injury or ischemic stress [42, 43]. While serum creatinine is frequently used to define AKI, it is recognized as an insensitive and delayed marker to identify renal injury [44]. Urinary NGAL, KIM-1, TIMP-2 and IGFBP-7 have been proposed as more sensitive and specific biomarkers of AKI with prognostic value [10, 45–47]. Remote ischemic preconditioning induces the release of IGFBP-7 and TIMP-2 that predicts the protective effect of this intervention from subsequent kidney injury induced by cardiac surgery. Patients with higher levels of IGFBP7 and TIMP-2 before cardiac surgery had a reduced rate of AKI compared with patients with lower IGFBP7 and TIMP-2 concentrations [11, 33]. Our results demonstrate that renal vein levels for these markers of G1 cell cycle arrest in the kidney are elevated in patients with chronic renal ischemia and that higher levels were associated with reduced acute changes in tissue hypoxia after contrast imaging and stenting.
This study has limitations. It was not a randomized study, but it enrolled patients selected for revascularization based on clinical criteria. Diabetics were excluded, and most of the patients were males. Revascularization and contrast injection were performed as part of a single procedure, so we could not specify the role of either or both regarding the hypoxia after 24 h. Our control group was comprised of subjects with EH of similar age, rather than ‘normal’ individuals, yet the EH group did include healthy individuals with normal kidney hemodynamics and function. Individuals with ARAS had lower GFR, although they were limited to serum creatinine levels <2.5 mg/dL. Thirty percent of patients had bilateral stenosis with slightly more renal injury. The BOLD MRI parameter R2* as a surrogate marker for tissue partial pressure of oxygen has some limitations. It can be affected by variations in R2 (= 1/T2) because, for example, of changes in water content [48]. Although the second BOLD MRI was done 24 h after contrast administration, we cannot exclude the possibility that contrast retention within the kidney could affect R2 [49]. Studies of water loading in normal volunteers demonstrated a change in cortical R2 of 0.72/s, while R2* fell by 1.36/s. Cortical changes in R2* in our patients were considerably greater (averaging 4.2/s), making it likely that R2 changes alone would be a minor contributor. Future studies should include R2 mapping to allow for better interpretation of changes observed with R2* [48].
CONCLUSION
Our results indicate that combined central contrast injection and renal revascularization were followed by widespread elevations of R2* suggesting tissue hypoxia lasting at least 24 h, but did not induce measurable or sustained AKI, as reflected by changes in circulating biomarkers or GFR. Patients with chronic renal ischemia from ARAS had elevated renal vein levels of cell cycle arrest markers IGFBP-7 and TIMP-2, in addition to NGAL. These markers correlated inversely with the degree of hypoxic change at 24 h. These data are consistent with preconditioning and resistance to developing AKI during these imaging procedures. Early hypoxic changes were transient and resolved by 3 months. These data underscore the capacity of the human kidney to adapt to transient hypoxic changes without producing injury or damage, even in older patients with reduced GFR.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGEMENTS
This project was supported by Award Number PO1HL85307 from the National Heart, Lung and Blood Institute (NHLBI), R01 DK100081 and DK 73608 from the National Institute for Digestive, Diabetic and Kidney Diseases (NIDDK) and NIH/NCRR CTSA Grant UL1 RR024150 and a clinical study grant from Stealth Biopharmaceuticals. Our studies were also supported by funds from the Center of Regenerative Medicine at the Mayo Clinic. Furthermore, we appreciate the generous philanthropic support of William and Karen Eby, as well as the charitable foundation in their names. The content is solely the responsibility of the authors and does not represent the official views of the NHLBI, NIDDK or the National Institutes of Health.
REFERENCES
- 1.Chade AR. Renovascular disease, microcirculation, and the progression of renal injury: role of angiogenesis. Am J Physiol Regul Integr Comp Physiol 2011; 300: R783–R790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52: 196–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saad A, Herrmann SM, Textor SC. Chronic renal ischemia in humans: can cell therapy repair the kidney in occlusive renovascular disease? Physiology (Bethesda) 2015; 30: 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gloviczki ML, Glockner JF, Crane JA et al. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 2011; 58: 1066–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saad A, Crane J, Glockner JF et al. Human renovascular disease: estimating fractional tissue hypoxia to analyze blood oxygen level-dependent MR. Radiology 2013; 268: 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad A, Herrmann SM, Crane J et al. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 2013; 6: 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gloviczki ML, Keddis MT, Garovic VD et al. TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol 2013; 8: 546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eirin A, Gloviczki ML, Tang H et al. Chronic renovascular hypertension is associated with elevated levels of neutrophil gelatinase-associated lipocalin. Nephrol Dial Transplant 2012; 27: 4153–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eirin A, Gloviczki ML, Tang H et al. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 2013; 34: 540–548a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashani K, Al-Khafaji A, Ardiles T et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 2013; 17: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarbock A, Schmidt C, Van Aken H et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA 2015; 313: 2133–2141 [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Saad A, Herrmann SM et al. Changes in inflammatory biomarkers after renal revascularization in atherosclerotic renal artery stenosis. Nephrol Dial Transpl 2016; 31: 1437–1443doi:10.1093/ndt/gfv448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ronco C. Cell-cycle arrest biomarkers: the light at the end of the acute kidney injury tunnel. Nephrol Dial Transplant 2016; 31: 3–5 [DOI] [PubMed] [Google Scholar]
- 14.Kellum JA, Chawla LS. Cell-cycle arrest and acute kidney injury: the light and the dark sides. Nephrol Dial Transplant 2016; 31: 16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung CM, Hegarty J, Kalra PA. Dilemmas in the management of renal artery stenosis. Br Med Bull 2005; 73–74: 35–55 [DOI] [PubMed] [Google Scholar]
- 16.Daghini E, Primak AN, Chade AR et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 2007; 243: 405–412 [DOI] [PubMed] [Google Scholar]
- 17.Porter GA. Experimental contrast-associated nephropathy and its clinical implications. Am J Cardiol 1990; 66: 18F–22F [DOI] [PubMed] [Google Scholar]
- 18.Liss P, Hansell P, Carlsson PO et al. Iodinated contrast media decrease renomedullary blood flow. A possible cause of contrast media-induced nephropathy. Adv Exp Med Biol 2009; 645: 213–218 [DOI] [PubMed] [Google Scholar]
- 19.Hackstein N, Schneider C, Eichner G et al. Effect of i.v. injection of radiographic contrast media on human renal blood flow. AJR Am J Roentgenol 2007; 188: 1367–1372 [DOI] [PubMed] [Google Scholar]
- 20.Cantley LG, Spokes K, Clark B et al. Role of endothelin and prostaglandins in radiocontrast-induced renal artery constriction. Kidney Int 1993; 44: 1217–1223 [DOI] [PubMed] [Google Scholar]
- 21.Stacul F, van der Molen AJ, Reimer P et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol 2011; 21: 2527–2541 [DOI] [PubMed] [Google Scholar]
- 22.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with bold MRI. Circulation 1996; 94: 3271–3275 [DOI] [PubMed] [Google Scholar]
- 23.Pedersen M, Dissing TH, Morkenborg J et al. Validation of quantitative bold MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int 2005; 67: 2305–2312 [DOI] [PubMed] [Google Scholar]
- 24.Textor SC, Glockner JF, Lerman LO et al. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol 2008; 19: 780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloviczki ML, Glockner J, Gomez SI et al. Comparison of 1.5 and 3 T bold MR to study oxygenation of kidney cortex and medulla in human renovascular disease. Invest Radiol 2009; 44: 566–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy TP, Cooper CJ, Dworkin LD et al. The Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study: rationale and methods. J Vasc Interv Radiol 2005; 16: 1295–1300 [DOI] [PubMed] [Google Scholar]
- 27.Textor SC, Turner ST. Renal vascular response to sodium loading in sons of hypertensive parents. Hypertension 1991; 17: 982–988 [DOI] [PubMed] [Google Scholar]
- 28.Wilson DM, Bergert JH, Larson TS et al. GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis 1997; 30: 646–652 [DOI] [PubMed] [Google Scholar]
- 29.Gloviczki ML, Glockner JF, Lerman LO et al. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension 2010; 55: 961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eirin A, Gloviczki ML, Tang H et al. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 2012; 34: 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joo JD, Kim M, D'Agati VD et al. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol 2006; 17: 3115–3123 [DOI] [PubMed] [Google Scholar]
- 32.Reimer KA, Murry CE, Yamasawa I et al. Four brief periods of myocardial ischemia cause no cumulative ATP loss or necrosis. Am J Physiol 1986; 251: H1306–H1315 [DOI] [PubMed] [Google Scholar]
- 33.Er F, Nia AM, Dopp H et al. Ischemic preconditioning for prevention of contrast medium-induced nephropathy: randomized pilot RenPro Trial (Renal Protection Trial). Circulation 2012; 126: 296–303 [DOI] [PubMed] [Google Scholar]
- 34.McCafferty K, Byrne C, Yaqoob MM. Ischaemic conditioning strategies for the nephrologist: a promise lost in translation? Nephrol Dial Transplant 2014; 29: 1827–1840 [DOI] [PubMed] [Google Scholar]
- 35.Yang QH, Liu DW, Long Y et al. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infect 2009; 58: 459–464 [DOI] [PubMed] [Google Scholar]
- 36.Roudkenar MH, Halabian R, Bahmani P et al. Neutrophil gelatinase-associated lipocalin: a new antioxidant that exerts its cytoprotective effect independent on heme oxygenase-1. Free Radic Res 2011; 45: 810–819 [DOI] [PubMed] [Google Scholar]
- 37.Chen WB, Liang L, Zhang B et al. To evaluate the damage of renal function in Ciaki rats at 3T: using ASL and bold MRI. Biomed Res Int 2015; 2015: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li LP, Lu J, Zhou Y et al. Evaluation of intrarenal oxygenation in iodinated contrast-induced acute kidney injury-susceptible rats by blood oxygen level-dependent magnetic resonance imaging. Invest Radiol 2014; 49: 403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li LP, Thacker J, Lu J et al. Efficacy of preventive interventions for iodinated contrast-induced acute kidney injury evaluated by intrarenal oxygenation as an early marker. Invest Radiol 2014; 49: 647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parekh DJ, Weinberg JM, Ercole B et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol 2013; 24: 506–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 2006; 290: G1083–G1088 [DOI] [PubMed] [Google Scholar]
- 42.Zager RA, Baltes LA, Sharma HM et al. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int 1984; 26: 689–700 [DOI] [PubMed] [Google Scholar]
- 43.Park KM, Byun JY, Kramers C et al. Inducible nitric-oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem 2003; 278: 27256–27266 [DOI] [PubMed] [Google Scholar]
- 44.Star RA. Treatment of acute renal failure. Kidney Int 1998; 54: 1817–1831 [DOI] [PubMed] [Google Scholar]
- 45.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med 2010; 4: 265–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm 2009; 2009: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han WK, Bailly V, Abichandani R et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002; 62: 237–244 [DOI] [PubMed] [Google Scholar]
- 48.Vivier PH, Storey P, Chandarana H et al. Renal blood oxygenation level-dependent imaging: contribution of R2 to R2* values. Invest Radiol 2013; 48: 501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenhard DC, Frisk AL, Lengsfeld P et al. The effect of iodinated contrast agent properties on renal kinetics and oxygenation. Invest Radiol 2013; 48: 175–182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.