Abstract
Melatonin, or 5-methoxy-N-acetyltryptamine, is synthesized and released by the pineal gland and locally in the retina following a circadian rhythm, with low levels during the day and elevated levels at night. Melatonin activates two high-affinity G protein–coupled receptors, termed MT1 and MT2, to exert beneficial actions in sleep and circadian abnormality, mood disorders, learning and memory, neuroprotection, drug abuse, and cancer. Progress in understanding the role of melatonin receptors in the modulation of sleep and circadian rhythms has led to the discovery of a novel class of melatonin agonists for treating insomnia, circadian rhythms, mood disorders, and cancer. This review describes the pharmacological properties of a slow-release melatonin preparation (i.e., Circadin®) and synthetic ligands (i.e., agomelatine, ramelteon, tasimelteon), with emphasis on identifying specific therapeutic effects mediated through MT1 and MT2 receptor activation. Discovery of selective ligands targeting the MT1 or the MT2 melatonin receptors may promote the development of novel and more efficacious therapeutic agents.
Keywords: sleep disorders, circadian rhythm disorders, depression, cancer, drugs of abuse, neuroprotection
INTRODUCTION
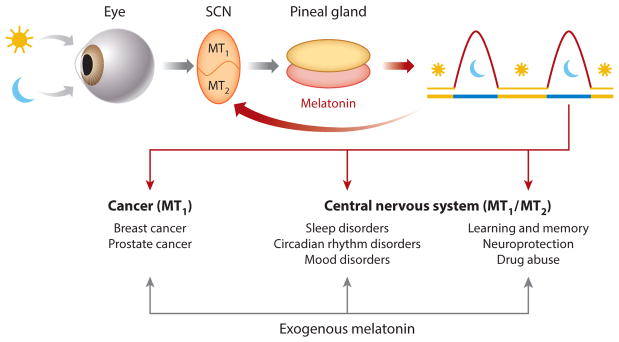
The rhythmic release of melatonin (5-methoxy-N-acetyltryptamine) from the pineal gland (for a review, see Reference 1) and retina (2) helps coordinate circadian rhythms and neuroendocrine processes via activation of two G protein–coupled receptors, termed MT1 and MT2 (3) (Figure 1). The circadian production of pineal melatonin is controlled by endogenous oscillators within the suprachiasmatic nucleus (SCN) and entrained by daily and seasonal changes in the environmental light-dark cycle (4). Endogenous melatonin released from the pineal gland at night may feedback onto the SCN and activate MT1 and MT2 receptors to phase shift local and overt circadian rhythms (Figure 1). This review thus focuses on the actions of exogenous melatonin and melatonin drugs on functional melatonin receptors associated with therapeutic effects, specifically those affecting the central nervous system and cancer (Figure 1). Emphasis is placed on drugs currently on the market targeting MT1 and MT2 melatonin receptors for the treatment of insomnia, circadian sleep disorders, major depression, and cancer (Table 1). Furthermore, we discuss MT1 and/or MT2 receptor–mediated responses to be considered as potential targets for the treatment of learning and memory deficits, neurodegeneration, and drug addiction.
Figure 1.
Therapeutic implications of exogenous melatonin. Melatonin production in the pineal gland and locally in the retina follows a circadian rhythm, with the highest levels produced during the dark phase. The rhythmic production of melatonin is controlled by endogenous circadian oscillations and entrained by the light-dark cycle with a 24-h period. Pineal melatonin activates MT1 and MT2 melatonin receptors in the SCN, discrete brain areas, and peripheral tissues to signal photoperiodic information and regulate physiological functions. Exogenous melatonin modulates processes and responses in the central nervous system via activation of the MT1 and/or MT2 melatonin receptors. Further melatonin activation of MT1 receptors decreases breast and prostate cancer cell growth. Abbreviation: SCN, suprachiasmatic nucleus.
Table 1.
Clinical and preclinical effects of currently marketed drugs targeting MT1 and MT2 melatonin receptors
| Marketed melatonin receptor agonists | Approved use | Affinity and functional responses | Clinical studies | Preclinical studies | ||
|---|---|---|---|---|---|---|
| MT1 (Ki)a | MT2 (Ki)a | 5-HT2C (Ki)b | ||||
|
Melatonin Circadin® (Neurin) 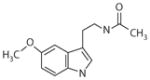
|
Insomnia in the elderly | 0.08 nM | 0.38 nM | NE | Insomnia in the elderly (65) Sleep disorders in children with neurodevelopment abnormalities (67) Circadian rhythms entrainment in blind subjects with non-24-h sleep-wake disorders (50, 52, 68) Seasonal affective disorder (53) Breast and prostate cancer (135, 137, 150) |
Inhibition of SCN neuronal firing (MT1) (9, 14) Phase shift of circadian rhythms in primates (49) and rodents (MT1) (61–64) Modulation of REM (MT1) and NREM (MT2) sleep (30, 76, 77) Antidepressant-like effects in rodent model of depression (81–85) Inhibition of LTP (primarily MT2) (110, 111) Neuroprotection in Huntington’s disease (MT1) (118) and ischemic strokes (MT2) (120, 121) in rodent models Oncostatic properties in cell lines and rodent models of breast and prostate cancer (MT1) (140, 142, 145, 150) |
|
Ramelteon Rozerem® (Takeda) 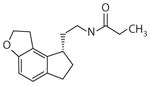
|
Primary chronic insomnia | 0.014 nM | 0.11 nM | NE | Primary chronic insomnia (71) Phase advance when given before bedtime (72) |
Accelerates re-entrainment of circadian rhythms after an abrupt phase advance of dark onset in rats (73) Phase advances neuronal firing rhythms in mouse SCN slices (MT1 and MT2) (62, 63) Phase advances onset of circadian activity rhythms in C3H/HeN mice (MT1) (62, 63) |
|
Tasimelteon Hetlioz® (Vanda) |
Non-24-h sleep-wake disorder in totally blind individuals | 0.30 nM | 0.07 nM | NE | Sleep-wake disorder in totally blind individuals (74) Transient insomnia after phase shift (40) |
Phase shifts circadian activity rhythms (75) |
|
Agomelatine Valdoxan® (Servier) 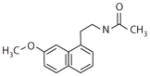
|
Major depressive disorder | 0.01 nM | 0.12 nM | 708 nM | Major depressive disorder (37) Improves disturbed sleep patterns and circadian/seasonal rhythm entrainment (99–101) |
Increases adult hippocampal neurogenesis (91, 92, 103) |
Abbreviations: 5-HT2C, serotonin; LTP, long-term potentiation; NE, no effect; NREM, non-REM; REM, rapid eye movement; SCN, suprachiasmatic nucleus.
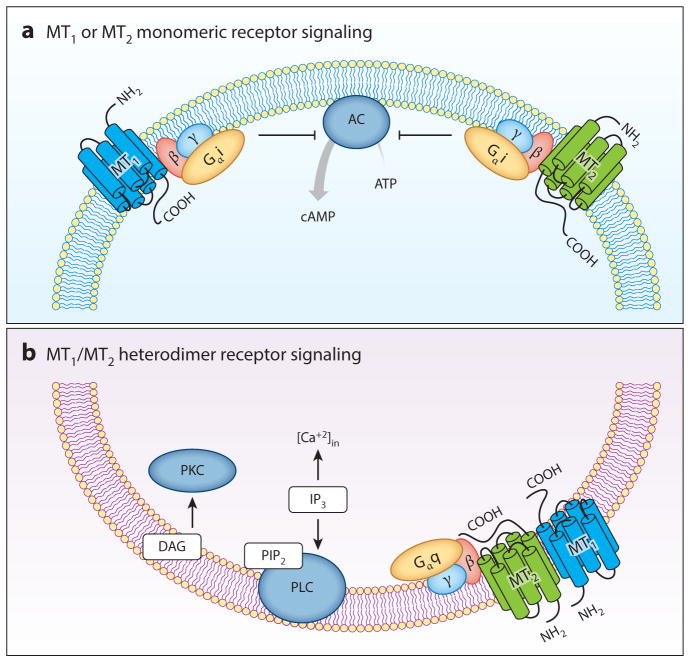
Melatonin acts on the MT1 (formerly called Mel1a or ML1A) and MT2 (formerly called Mel1b or ML1B) receptors (3) (Figure 2). The human MT1 and MT2 (hMT1 and hMT2) melatonin receptors show distinct molecular structures (5), pharmacological characteristics (3, 6, 7), and chromosomal sites (4q35.1 for MT1, 11q21–q22 for MT2) (8). Human MT1 and MT2 receptors are 350 and 362 amino acids long, respectively, with molecular weights of 39–40 kDa and 55% amino acid homology overall (70% within the transmembrane domains) (5). The MT1 melatonin receptor couples to pertussis toxin–sensitive Gi and –insensitive Gq/11 G proteins and inhibits forskolin-stimulated cAMP, protein kinase A signaling, and CREB phosphorylation. The MT1 receptor also increases phosphorylation of mitogen-activated protein kinase 1/2 and extracellular signal–regulated kinase 1/2, as well as increasing potassium conductance through Kir inwardly rectifying channels. MT2 melatonin receptor activation inhibits both forskolin-stimulated cAMP production and cGMP formation, activates protein kinase C (PKC) in the SCN, and decreases calcium-dependent dopamine release in the retina (3).
Figure 2.
Melatonin receptor signaling. (a) MT1 or MT2 monomeric receptor signaling. Both MT1 and MT2 melatonin receptors couple to pertussis toxin–sensitive Gαi, β, γand –insensitive Gq, β, and γproteins, inhibiting forskolin-stimulated cAMP, protein kinase A signaling, and CREB phosphorylation (see Reference 3). (b) MT1/MT2 heterodimer receptor signaling. Human MT1 and MT2 receptors form homo- and hetero-oligomers between themselves, altering the pharmacological properties of the individual receptors. Formation densities of the MT1/MT2 heterodimer and the MT2/MT2 homodimer are similar and 3- to 4-fold lower than the MT1/MT1 homodimer. Native functional MT1/MT2 heterodimers have been characterized in mouse rod photoreceptors, where they mediate the enhancement of scotopic light sensitivity by melatonin through a heterodimer-specific PLC and PKC pathway (21). Abbreviations: AC, adenylyl cyclase; DAG, diacylglycerol; IP3, inositol 1,4,5-trisphosphate; PIP2, phosphatidylinositol-4,5-bisphosphate; PKC, protein kinase C; PLC, phospholipase C. Figure modified with permission from Reference 151.
Assessing the pharmacology and function of melatonin receptors is challenging, as their native binding site densities in animal tissues are low or undetectable, particularly for the MT2 receptor (9). The MT1 and MT2 receptor sites have been localized to discrete areas of the rodent and human nervous system—including the SCN, cerebellum, thalamus, hippocampus, as well as peripheral tissues—using receptor autoradiography with 2-[125I]-iodomelatonin, in situ mRNA hybridization techniques, and immunohistochemistry (10–13). MT1 and MT2 receptor function has been established using mice with genetic deletion of either the MT1 or MT2 melatonin receptor (9, 14) or pharmacological approaches through the use of prototype competitive melatonin receptor antagonists such as luzindole and 4-phenyl-2-propionamidotetralin (4P-PDOT) (15, 16).
A ligand is considered selective on a specific receptor when its affinity, potency, or both are at least 100-fold higher with respect to the other receptor type under consideration (3). Luzindole, a nonselective ligand with 15- to 25-fold higher affinity for the MT2 melatonin receptor (6), and 4P-PDOT, a selective MT2 ligand with 300- to 1,500-fold higher affinity for this receptor (6), are considered the gold standards for pharmacological characterization of melatonin receptors. Luzindole and 4P-PDOT competitively block MT1 melatonin receptors at concentrations of 300 nM or more, and both act as inverse agonists in systems endowed with constitutively active MT1 receptors (17–19). N-butanoyl-2-(2-methoxy-6H-isoindolo[2,1-a]indol-11-yl)-ethanamine (IIK7) is a selective MT2 melatonin receptor agonist at recombinant hMT2 receptors with an affinity ratio of approximately 90 when compared with hMT1 (20). However, its affinity for the mouse MT2 receptor is 1,000-fold higher than for the MT1 receptor (21), suggesting considerable species differences in melatonin receptor pharmacology.
Researchers have been successful at discovering high-affinity and selective ligands for the MT2 receptor (3); however, there is a lack of selective MT1 receptor ligands with high efficacy (22). The MT1 selective ligands available are either antagonists or partial agonists with high selectivity for human recombinant MT1 receptors in competition for 2-[125I]-iodomelatonin binding; this selectivity is significantly reduced in functional studies (23). S26131, a dimer formed by linking two molecules of agomelatine, shows over 200-fold higher affinity for MT1 and blocks melatonin-mediated stimulation of 35S-GTPγS binding to receptors expressed in heterologous mammalian cells with less than 30-fold selectivity (23). None of these selective MT1 antagonists have been tested in vivo.
The efficacy of ligands targeting G protein–coupled receptors in target tissues is dependent on the cellular milieu, G protein type and/or scaffolding molecules, spare receptors, and homodimers and/or heterodimer formation, as well as receptor structure among various species (24, 25). These changes in efficacy lead to species-dependent fluctuations in in vivo ligand selectivity. For example, the affinity of our prototype competitive melatonin receptor antagonist luzindole (MT1/MT2-nonselective) and 4P-PDOT (MT2-selective) varies considerably between human, ovine, rat, and mouse receptors (26–28). 4P-PDOT is considered a selective ligand on hMT2 receptors when compared with hMT1; however, the aforementioned properties make selective occupancy of MT2 receptors in vivo difficult, yet authors often conclude that in vivo functional responses are mediated through MT2 receptors using 4P-PDOT doses that will raise drug blood concentrations to readily occupy the MT1 receptors as well.
When establishing ligand efficacy and selectivity, investigators must also consider the observation that simultaneous activation of both MT1 and MT2 melatonin receptors expressed in the same or different cells could lead to additive, synergistic, or opposing responses that amplify or diminish each other (29–31). Although the minimal functional unit seems to be monomeric, we know from bioluminescence resonance energy transfer experiments that hMT1 and hMT2 receptors form homo- and hetero-oligomers between themselves and other G protein–coupled receptors. Formation of MT1/MT2 heterodimers is 3- to 4-fold lower than the formation of MT1/MT1 homodimers and is similar to the formation of MT2/MT2 homodimers (32) (Figure 2). These dimers are functionally relevant and distinct from receptor monomers, as demonstrated by Baba and colleagues (21). These authors reported MT1/MT2 heterodimers in mouse rod photoreceptors modulating light sensitivity via a phospholipase C (PLC)/PKC pathway not normally triggered by activation of monomeric units (21) (Figure 2). Monomeric forms of the MT1 or MT2 receptors, however, are differentially desensitized by exposure to melatonin depending on concentration (physiological versus supraphysiological), time of exposure (e.g., short versus long) (33–35), cellular background (7), and receptor state (quiescent versus constitutive) (33–35). Thus, ligands with higher affinity or selectivity for the MT1 or the MT2 receptor could conceivably tip the MT1/MT2 heterodimeric receptor selectivity balance, providing a therapeutic advantage over melatonin and current nonselective synthetic ligands by potentiating or facilitating responses mediated by the target receptor.
MT1 and MT2 receptors also form heterodimers with the serotonin (5-HT2C) receptor (36). This is of particular interest in understanding the antidepressant mechanism of agomelatine as both a melatonin receptor agonist and a 5-HT2C receptor antagonist (36, 37). The formation of MT2/5-HT2C heterodimers is more efficient than the formation of MT1/5-HT2C heterodimers and 5-HT2C homodimers. The MT2/5-HT2C receptor heterodimer exhibits a Gi signaling response to melatonin and agomelatine, i.e., inhibition of forskolin-stimulated cAMP production (36). However, melatonin transactivates the Gq pathway through MT2/5-HT2C heterodimers increasing inositol phosphate production. Interestingly, melatonin receptor antagonists, luzindole, and 4P-PDOT also transactivate the 5-HT2C receptor–mediated Gq pathway. Moreover, MT1 and 5-HT2C heterodimeric receptor formation increases 5-HT2C receptor trafficking to the cell surface and potentiates β-arrestin recruitment induced by melatonin and 5-HT (36). Thus, melatonin receptor heterodimerization (Figure 2) with 5-HT2C receptor and possibly other receptor types generates additional signaling responses with therapeutic potential that should be explored as new melatonin-like drugs are developed.
Melatonin [Circadin® (38)] and several melatonin analogues are currently used clinically {i.e., ramelteon/Rozerem® [insomnia (39)], agomelatine/Valdoxan® [depression (37)], and tasimelteon/Hetlioz® [non-24-h sleep-wake disorder (40, 41)]} (Table 1). These compounds modulate circadian rhythms and neuroendocrine function in rodents and humans. However, all are nonselective agonists and compete for hMT1 and hMT2 receptors with almost equal affinities (6, 42, 43). Ramelteon shows a 10-fold higher affinity for the hMT1 than the hMT2 melatonin receptor and a 17-fold higher affinity than melatonin (42). Agomelatine and tasimelteon have a slightly higher affinity for the hMT2 receptor even though both are nonselective agonists (7, 41). Ramelteon (42), agomelatine (44), and tasimelteon (41) have remarkable selectivity for melatonin receptors, as they show no affinity for many other G protein–coupled receptors, enzymes, and neurotransmitter channels (42, 45, 46). The only exception is agomelatine, which shows high affinity for MT1 and MT2 melatonin receptors but is also an antagonist at the 5-HT2C serotonin receptor, a property believed to contribute to its antidepressant effects (for a review, see Reference 37). The following sections describe the therapeutic potential of melatonin, agomelatine, ramelteon, and tasimelteon in the treatment of insomnia and circadian sleep disorders, depression, and cancer, and they discuss potential new melatonin receptor targets to treat conditions affecting the central nervous system.
MELATONIN RECEPTORS AS THERAPEUTIC TARGETS IN THE CENTRAL NERVOUS SYSTEM
Sleep Disorders
In humans and nonhuman primates, acute melatonin treatment promotes sleep onset, maintenance, or both and induces sleep-like brain waves independent of time of day (47–49). Melatonin and related analogues phase shift circadian rhythms when given at clock-sensitive times following phase response curves (PRCs) conserved in mammals (50, 51). Melatonin can be used effectively to entrain free-running circadian rhythms in totally blind individuals not perceiving light (52) and to phase advance delayed rhythms in individuals suffering from seasonal affective disorders, with concurrent improvement of depression scores (53). These two distinct effects of melatonin (i.e., sleep promotion and phase shift of circadian rhythms) resulting from actions at melatonin receptors in the central nervous system are reviewed below in relation to the therapeutic targets of marketed melatonin drugs (Table 1).
Insomnia, or sleep-wake disorder, is defined as difficulty initiating and/or maintaining sleep or as nonrestorative sleep and is generally associated with daytime impairment or distress. Insomnia is a highly prevalent disorder affecting about 10% of the world’s population (54, 55). Current treatments for insomnia include benzodiazepines and related nonbenzodiazepine drugs, with considerable side effects resulting in impaired cognitive and psychomotor skills, increased risk of falls, rebound, and dependence or abuse potential (56). The search for molecules with improved safety profiles led to the development of a slow-release melatonin preparation (i.e., Circadin) (38) and synthetic melatonin analogues (i.e., ramelteon, tasimelteon, agomelatine) (37, 39–41) (Table 1). Insomnia is related to other comorbid disorders, particularly mood and circadian rhythm disorders (56). Melatonin and the synthetic melatonin agonists are generally devoid of the common side effects frequently observed with sleep medication (i.e., impairment of learning, memory, or motor function).
About 80 million Americans suffer from some form of circadian rhythm disorder resulting in depressive mood or sleep alterations, as reported by the US National Institutes of Health. Melatonin and melatonin receptor agonists find therapeutic applications in various circadian disorders including jet lag, shift work, delayed-sleep and advance-sleep phase syndrome, seasonal affective and non-24-h sleep-wake disorder, and major depression (37, 53, 57, 58). The mammalian SCN expresses MT1 and MT2 receptors as demonstrated using 2-[125I]-iodomelatonin and in situ hybridization with mRNA probes (3, 59). Melatonin receptor activation decreases neuronal firing through activation of the MT1 receptor in the SCN and areas of the limbic system, which may mediate the sleep-promoting properties of melatonin (9, 14).
Activation of melatonin receptors in the SCN plays a pivotal role in coordinating the phase and amplitude of circadian rhythms throughout the body (60). In humans and mice, melatonin phase advances and delays circadian rhythms, following a PRC with two well-defined periods of sensitivity in late afternoon [advance at circadian time (CT) 8–11] and early morning (delay at CT0–3), respectively (50–52, 61, 62). These periods of sensitivity to melatonin and melatonin agonists (e.g., ramelteon) are conserved in humans (52), nonhuman primates (49), and rodents (61–63). The phase shift of circadian rhythms by melatonin is mediated through activation of the MT1 melatonin receptor, as demonstrated using mice with genetic deletion of the MT1 receptor in a model of circadian re-entrainment (64).
Understanding the hypnotic and chronobiotic actions at melatonin receptors in the central nervous system led to the development of novel melatonin formulations and the synthesis of new drugs with melatonin receptor agonist properties for the treatment of primary insomnia and circadian disorders (see Table 1). Circadin is a prolonged-release melatonin formulation that mimics the physiological profile of melatonin secretion. It improves sleep quality and latency, as well as daytime function, in elderly insomnia patients (65–67). Circadin also improves sleep quality in children with sleep-wake cycle disorders (67) and in blind adults suffering from non-24-h sleep-wake disorders (68). The clinical use of Circadin is not associated with memory impairment or decreased vigilance and has no significant withdrawal symptoms (56).
Ramelteon, a high-affinity MT1 and MT2 melatonin receptor agonist (42), promotes sleep in various mammalian species, including humans, without causing learning, memory, or motor function impairment or inducing reward (69, 70). In subjects with primary chronic insomnia, ramelteon shows a significant reduction in latency to persistent sleep and an increase in total sleep time, with no apparent next-day residual effects (71). In addition, repeated ramelteon treatment before bedtime phase advances circadian rhythm (72). Ramelteon also entrains circadian rhythms after an abrupt phase advance of dark onset (73), and it phase advances circadian rhythms of neuronal firing in the rat SCN brain slice when applied at CT10 (CT12 is the onset of activity for rodents) (60, 63). Activation of either the MT1 or MT2 receptors by ramelteon (10 pM) phase advances the peak of neuronal firing rhythms in C3H/HeN mouse SCN brain slices (63). Further ramelteon phase advances overt circadian activity rhythms in vivo by activating the MT1 melatonin receptor, as identical shifts are observed in wild-type and MT2 knockout C3H/HeN mice (63). Together, these findings suggest that ramelteon-mediated phase advances of circadian activity rhythms in vivo are mediated through activation of MT1 melatonin receptors within the SCN, as previously demonstrated in C57BL/6 mice (64).
Tasimelteon was developed for the treatment of non-24-h sleep-wake disorder, a circadian rhythm disorder affecting totally blind individuals who are unable to entrain their master body clock to the 24-h light-dark cycle (74). Tasimelteon is a high-affinity, non-selective MT1/MT2 receptor agonist with no detectable affinity for many G protein–coupled receptors and enzymes (41). This melatonin analogue phase shifts circadian rhythms in the rat with a potency comparable to that of melatonin (75). Phase III clinical trials examined the effects of tasimelteon and a placebo on transient insomnia after a 5-h phase shift of the sleep-wake cycle. Tasimelteon significantly shifted the endogenous melatonin rhythm, reduced sleep latency, and increased sleep efficiency and wake after sleep onset (i.e., sleep maintenance) with no observable side effects (40). In summary, tasimelteon modulates sleep and circadian rhythms, providing a highly effective treatment for a range of symptoms associated with transmeridian air travel, shift work, and other circadian-rhythm sleep disorders, including those observed in blind people.
The melatonin agonists noted above (Table 1) are nonselective at the MT1 and MT2 melatonin receptor ligands. Recent studies investigated the specific role played by each melatonin receptor type in the modulation of sleep architecture (30, 76). The selective MT2 partial agonist UCM765 decreased the latency to and increased the amount of non–rapid eye movement sleep (NREM). These effects were blocked by the MT2 selective receptor antagonist 4P-PDOT (30). Furthermore, the selective MT2 agonist IIK7 produced similar effects on the latency and amount of NREM (76), suggesting that the MT2 melatonin receptor mediates some of the effects of melatonin agonists on sleep architecture. Interestingly, sleep recordings in mice with genetic deletion of MT1, MT2, or both receptors suggest that each receptor regulates different aspects of the vigilance state, namely NREM for the MT2 and REM for the MT1 receptor (77).
Major Depressive Disorder
Major depressive disorder remains the most prevalent mental disorder in the United States and is the leading cause of disability, affecting an estimated 100 million adults worldwide (78). The disorder is characterized by a constellation of symptoms that affect mood, anxiety, neurochemical balance, sleep patterns, circadian and/or seasonal rhythm entrainment, and increased neuronal atrophy. Current treatments primarily include tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs), known for increasing extracellular concentrations of monoamine neurotransmitters. These antidepressants, however, do not treat sleep disturbances or circadian and/or seasonal rhythm dysfunctions associated with depressive disorders. Additionally, long-term use leads to unwanted side effects such as sexual dysfunction, weight gain, and a cluster of cognitive, autonomic, and motor signs that constitute the serotonin syndrome (79, 80). Thus, researchers urgently need to develop antidepressants with novel mechanisms of action and reduced side effects.
MT1 and MT2 melatonin receptors are important targets for the development of novel antidepressants. Early indications of melatonin receptor involvement in depressive-like behaviors were based on antidepressant-like effects of melatonin and its agonists in rodent models of learned helplessness. The melatonin-mediated antidepressant-like effects in the forced swim test were blocked by luzindole, suggesting the potential involvement of MT1 and MT2 receptors (81, 82). Chronic melatonin treatment also enhances hippocampal neurogenesis in rodent models, which is considered an important process for antidepressant efficacy (83–85). Mice with deletion of the MT1 receptor show increased depressive-like behaviors in the forced swim test (86, 87). MT1 receptor immunoreactivity is upregulated in patients with major depressive disorder, suggesting this receptor type is a potential target for treating some symptoms of depression (88). In summary, melatonin agonists, particularly through activation of the MT1 receptor, may be effective in attenuating the symptoms, neurochemical changes, or both associated with the clinical manifestations of depressive disorders.
The clinical efficacy of melatonin as an antidepressant therapy is limited at best (89). However, interest in targeting melatonin receptors to treat the symptoms of mood disorders was reinvigorated by the discovery of agomelatine, a novel antidepressant that acts through synergistic actions at the melatonin receptors and 5-HT2C receptor (36, 37). In clinical studies, agomelatine is as effective as and better tolerated than SSRIs (i.e., paroxetine, fluoxetine, sertraline) and serotonin/norepinephrine reuptake inhibitors (i.e., Venlafaxine) (90). Agomelatine not only relieves depressed mood, neurochemical imbalance, anxiety, and neuronal atrophy (91–93) but also improves disturbed sleep patterns and circadian/seasonal rhythm entrainment (37, 43). The antidepressant-like effects of agomelatine are not mimicked by individual treatment with melatonin agonists or 5-HT2C antagonists, suggesting the need for simultaneous actions at both receptors for effective antidepressant-like effects (94).
Interestingly, luzindole shows antidepressant-like activity in the forced swimming test through actions at the MT2 receptor, as mice lacking this receptor do not exhibit this effect (95, 96). Chronic luzindole administration also increases hippocampal neurogenesis in mice (97). The antidepressant-like effects of luzindole, a melatonin receptor antagonist, may appear contradictory to that of MT1/MT2 melatonin receptor agonists such as agomelatine. However, MT2 agonist–mediated desensitization in response to agonist stimulation may lead to a net decrease in its receptor signaling over time, hence mimicking the action of a receptor antagonist (34, 96). In support of this hypothesis, melatonin exerts antidepressant-like effects and increases hippocampal neurogenesis only upon chronic administration (83, 84). We suggest that the rapid MT2 receptor desensitization kinetics could facilitate melatonin-mediated antidepressant-like effects following treatment with an MT1/MT2 receptor agonist (33, 35).
Sleep and circadian disturbances are key symptoms of major depressive disorder, coinciding with onsets of depressive episodes and correlating with depressive severity (98). The ability of agomelatine to normalize disturbances in sleep and circadian rhythms holds a great therapeutic advantage over SSRIs and TCAs, which often exacerbate sleep and circadian disorders. In clinical studies, agomelatine phase advances rhythms in core body temperature when given in the late afternoon (99, 100). Also, agomelatine improves sleep and circadian rest-activity rhythms compared to venlafaxine in patients with depression (101). The chronobiotic properties of agomelatine are likely to be mediated primarily through its agonist action at melatonin receptors in the SCN.
Another important hallmark of depressive disorders is decreased hippocampal neurogenesis resulting from decreased expression of neurotrophic factors (102). Neurogenesis denotes a morphogenetic process that comprises distinct successive steps of progenitor cell proliferation, survival of immature neurons, and neuronal differentiation (103). Chronic agomelatine administration promotes brain-derived neurotrophic factor expression, leading to a net increase in hippocampal neurogenesis in rodents (92, 104). Melatonin and 5-HT2C antagonists appear to differentially modulate the various stages of adult hippocampal neurogenesis, potentially through heterodimer formation. Chronic administration of 5-HT2C receptor antagonists increases neural progenitor cell proliferation in rodent hippocampus (93), whereas chronic melatonin administration increases neuronal differentiation and immature neuron survival (83, 84, 93).
Learning and Memory
Age-related dementias such as Alzheimer’s disease diminish memory and cognition in approximately 5.4 million Americans, indicating a need for new nootropic therapeutics (105). The clinical significance of the melatonin receptors in dementia is strongly supported by postmortem histology studies in hippocampus from Alzheimer’s disease patients showing increased MT1 and decreased MT2 receptor immunoreactivity (106, 107). Memory formation is driven by long-term potentiation (LTP) in the hippocampus, a process by which associations between neurons are cooperatively and selectively strengthened by increased synaptic activity through N-methyl-D-aspartate (NMDA)- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA)-type glutamate receptors (108, 109).
Melatonin inhibits LTP in the CA1 dendritic layer of the Schaffer collaterals of mouse hippocampal brain slices (110). This inhibition of LTP was observed in MT2 knockout but not MT1 knockout mice, revealing the unique requirement of the MT2 receptor for this response. Accordingly, Larson and colleagues (111) demonstrated a significantly reduced baseline level of LTP in hippocampus from MT2 knockout C3H/HeN mice compared to wild-type mice. This link to LTP implies a role for melatonin in memory formation (110). Indeed, melatonin suppresses long-term memory formation in zebrafish, with melatonin receptors being implicated by reversal of the effect via the melatonin receptor antagonists luzindole and K-185 (112). MT2 knockout mice show learning deficits in the elevated plus maze (111), whereas MT1/MT2 double knockout mice show improved spatial and reference learning and memory performance as well as basic memory function, in addition to displaying increased LTP and memory-related neuronal signaling (113). In humans, exogenous melatonin has been linked to decreased activation of the left hippocampus during an autobiographical memory task, and subjects with higher levels of endogenous melatonin demonstrated increased activation of the left parahippocampus (114). Although this study did not address memory performance, it proposes a compelling role for melatonin’s contribution to hippocampal activation in humans. Together, these studies suggest an important role for melatonin and its receptors in memory formation, most likely stemming from the underlying process of LTP. Therefore, a melatonin receptor antagonist may lead to improvements in memory-based tasks and may be useful in a prophylactic or therapeutic role in patients showing signs of Alzheimer’s disease.
Neuroprotection
Evidence suggests a role for melatonin in protection against neurodegeneration, apoptosis, and ischemia/reperfusion injury (115). The neuroprotective effects of melatonin are often attributed to its free radical–scavenging properties (116); however, recent evidence suggests that activation of the MT1 and/or MT2 melatonin receptors may also play a role. In hippocampal slice cultures deprived of oxygen and glucose, melatonin reduces reactive oxygen species to near basal levels, an effect blocked by luzindole (117). This melatonin receptor function may involve the induction of antioxidant genes, such as superoxide dismutase and catalase, through receptor-mediated transcriptional signaling. Thus, melatonin receptors may be viable targets for novel agents capable of counteracting the oxidative stress components of neuroinflammatory processes.
Melatonin inhibits mitochondrial cell death pathways in a mutant striatal cell model of Huntington’s disease. Moreover, melatonin inhibits cell death in cell lines as well as primary cerebrocortical and primary striatal neuronal cultures, an effect reversed by luzindole (118). This effect is likely mediated through the MT1 receptor, as knockdown of this receptor sensitizes cultured neurons to cell death, whereas overexpression is protective (118). In the R6/2 mouse model of Huntington’s disease, melatonin slows disease progression and blocks the mitochondrial cell death pathway. Lower levels of the MT1 but not the MT2 melatonin receptor are observed in brains of R6/2 mice, a deficit that can be partially reversed by treatment with melatonin (118). These findings imply that development of selective MT1 receptor agonists might lead to neuroprotective therapeutics capable of treating patients with Huntington’s disease.
In contrast, activation of MT2 receptors has been linked to the protection melatonin affords against neuronal damage that follows ischemic strokes (119). Melatonin treatment in vivo protects against ischemia-related neuronal death in the CA1 region of the hippocampus of the Mongolian gerbil and is associated with increased MT2 immunoreactivity and protein levels (120). Similarly, melatonin treatment protects against ischemia/reperfusion injury in a mouse model of stroke via receptor-mediated mechanisms blocked by 4P-PDOT or luzindole (121). Furthermore, melatonin promotes neurogenesis and cell proliferation via an MT2 receptor–dependent mechanism (121). Together, these data suggest a role for the MT2 receptor in mediating the neuroprotective effects of melatonin following ischemia/reperfusion, as well as an association with a robust increase in neurogenesis.
Drugs of Abuse
Use of illicit drugs is widespread and costly in the United States, with approximately 24.6 million people reporting usage within the past year (122) and a monetary cost in excess of $700 billion per year stemming from increased health-care costs, crime, and loss of productivity (123). However, the development of effective and safe therapies to prevent or counteract the detrimental effects of drug abuse is lacking. Melatonin has been linked to modulation of drug-induced sensitization and reward (124–128). Recent evidence, including data from our laboratory and others, suggests that melatonin receptors modulate responses to drugs of abuse in two well-established models of addictive liability, i.e., locomotor sensitization (124, 125, 127) and conditioned place preference (126, 128).
Locomotor sensitization denotes a progressive increase in ambulatory response following repeated administration of a psychostimulant, which in turn reflects accumulating neuroadaptations in the brain (129). Cocaine induces locomotor sensitization in C3H/HeN mice during the light phase (12-h light-dark cycle), when melatonin levels are low (130), but this effect is absent at night (127) during peak melatonin levels. This diurnal variation is not observed in C57BL/6J mice (127), which are considered melatonin-deficient owing to a truncation of the arylalkylamine N-acetyltransferase gene (131). These findings suggest a potential link between nocturnal melatonin and the lack of sensitization during the dark phase.
Researchers have linked melatonin receptors to sensitization induced by the psychostimulant methamphetamine. The development and expression of locomotor sensitization to methamphetamine requires the presence of either MT1 or MT2 receptors, as sensitization is significantly reduced only in knockout mice lacking both receptor types (124). In contrast, repeated methamphetamine treatments during the dark phase increase the expression of locomotor sensitization MT1 knockout mice but not their wild-type and MT2 knockout counterparts (124). However, genetic MT1 receptor deletion completely abolishes the expression of locomotor sensitization in C57BL/6 mice pretreated with a single methamphetamine injection (125). These findings in C3H/HeN and C57BL/6 mice appear to be at odds concerning the functional role of the MT1 receptor in sensitization. The discrepancy, however, could be attributed to differential levels of endogenous melatonin in the two mouse strains, leading to a difference in the relative level of ligand-dependent versus constitutive MT1 receptor activity and hence its functional manifestation at the behavioral level. Taken together, data from locomotor sensitization studies imply a unique role for the MT1 receptor in regulating circadian sensitivity to the psychostimulant properties of methamphetamine and could be targeted to block the onset of addiction-favoring maladaptations in the brain.
Researchers have also linked melatonin to the rewarding properties of drugs of abuse, as evidenced by studies using the conditioned place preference paradigm. C3H/HeN mice develop a place preference for cocaine during the light phase, which is significantly decreased during the dark phase (128). This diurnal variation is abolished by pinealectomy (128). Systemic or intracerebral administration of melatonin to KM mice abrogates the expression of morphine-induced conditioned place preference in a dose-dependent manner, as evidenced by a significant decrease in preference for morphine following treatment with either 25 or 50 mg/kg melatonin (132). These effects are reversed through treatment with luzindole or the selective MT2 antagonist K-185 (132). Together, these results suggest that melatonin abrogates the rewarding properties of morphine through the MT2 receptor (132). Results from our laboratory revealed a strong place preference in methamphetamine-treated wild-type C3H/HeN mice during the mid-light phase, which was abrogated during the peak melatonin period of the mid-dark phase (126). Knockout mice lacking either the MT1 or MT2 receptor exhibited sharply reduced place preference for methamphetamine at both times of day (126). Thus, melatonin appears to act through its receptors to inhibit methamphetamine-induced reward, and antagonists of the MT1 and MT2 receptors may have the capacity to block the induction of the methamphetamine-induced reward sensation.
MELATONIN RECEPTOR–MEDIATED ONCOSTATIC EFFECTS IN BREAST AND PROSTATE CANCER
In humans, the nocturnal serum concentration of endogenous melatonin is inversely associated with the risk of breast, lung, and cervical cancers (133). Melatonin treatment enhances the efficacy of chemotherapy in patients with lung, breast, gastrointestinal tract, head, and neck cancers (134). In addition to its oncostatic effect, melatonin decreases anxiety, depression, and toxicity associated with chemotherapy (134, 135). Breast and prostate cancer account for an estimated 30% of new cancer cases and 26% of cancer-caused death in the United States (136). Melatonin is emerging as a safe and effective treatment for breast and prostate cancer, either alone or in combination with other therapies. Here we summarize the oncostatic properties of melatonin and its receptors in breast and prostate cancers models, with particular focus on the MT1 receptor.
In breast cancer, melatonin displays an oncostatic effect both in vivo and in vitro. Coadministration of melatonin and tamoxifen reduces lesion damage size and increases survival rates in breast cancer patients resistant to tamoxifen treatment alone (135, 137). Decreases in endogenous melatonin levels by exposure to light at night (such as what shift workers are exposed to) significantly increases breast cancer risk (138). Decreasing endogenous melatonin levels through exposure to constant light or pinealectomy significantly increases the incidence of mammary tumor formation in rodents. Exogenous melatonin supplementation reverses this process (139). Cancer signaling markers in human breast cancer xenografts implanted in nude rats are significantly increased by perfusion with melatonin-deficient blood obtained by daytime sampling or by exposure to light at night from healthy premenopausal women (140). In contrast, melatonin-rich blood collected at night inhibits human breast cancer growth, which is blocked by a melatonin receptor antagonist (S20928) (140), suggesting nocturnal endogenous serum melatonin inhibits breast cancer growth by receptor-mediated mechanisms.
Cell culture model systems illustrate the conditions and molecular signaling mechanisms involved in melatonin receptor–mediated modulation of cancerous tumor development and growth. Low nanomolar concentrations of melatonin, which are within the range of the nocturnal physiological serum level, inhibit proliferation of estrogen receptor α–positive MCF-7 human breast cancer cells by 30–50% (141). AMMTC, an MT1 and MT2 receptor agonist, inhibits MCF-7 cancer cell proliferation. This antiproliferative effect of melatonin in MCF-7 cells is blocked by CBPT, an MT1 and MT2 receptor antagonist (142). Together, these systematic pharmacological studies demonstrate that the antiproliferative effect of melatonin in MCF-7 cells is melatonin receptor–mediated.
Molecular studies demonstrate that mRNA for the MT1 receptor is expressed in breast cancer cell lines and human breast cancer specimens (142–144). MT2 melatonin receptor mRNA is either absent or expressed at a low density. The MT1 receptor mRNA expression level varies among different breast cancer cell lines and primary breast tumors and is modulated by exogenous estrogen and melatonin treatment (143). Interestingly, MT1 receptor overexpression inhibits MCF-7 cell proliferation and enhances the sensitivity of MCF-7 cells to the antiproliferative effect of melatonin (145, 146). The antiproliferative effect of MT1 receptor overexpression in MCF-7 cells in the absence of exogenous melatonin suggests the presence of constitutively active MT1 receptors.
Studies linking melatonin and prostate cancer show a direct antiproliferative effect of melatonin in human prostate cancer cell lines and nude mice (147, 148). Melatonin also slows the early stages of tumor development in castrated prostate cancer patients, as shown by a 23% reduction in the prostate-specific antigen doubling rate (149). Luzindole but not 4P-PDOT blocks the antiproliferative effect of melatonin on hormone-refractory 22Rv1 human prostate cancer cells, suggesting an MT1 receptor–mediated effect (150).
CONCLUSIONS
This review describes the latest advancements on the role of melatonin receptors as therapeutic targets for the development of drugs aimed at the treatment of sleep and circadian disorders, depression, and cancer. The development of new drugs targeting the MT1 and MT2 melatonin receptors is the next challenge for the field. Described below are potential therapeutic targets for the melatonin receptor types.
Sleep and Circadian Rhythm Disorders
Research has firmly established that melatonin and melatonin ligands promote sleep in nocturnal and diurnal species independently of time of day. Activation of melatonin receptors in the SCN phase shift circadian rhythms at specific times of day following a PRC that is conserved among mammals. A slow-release melatonin preparation (i.e., Circadin) and synthetic melatonin ligands (i.e., ramelteon, agomelatine, tasimelteon) are marketed for the treatment of circadian sleep disorders, insomnia, and depression. Discovery of MT1 or MT2 melatonin receptor–selective drugs may improve efficacy as compared to nonselective ligands and lead to the discovery of new therapeutic targets.
Depressive Disorders
Agomelatine ameliorates symptoms of major depression by a combined action of MT1 and/or MT2 receptor activation and 5-HT2C receptor blockade. Adjuvant melatonin therapy with current antidepressants may thus have additional benefits for depressed patients. Importantly, researchers must remember that these two classes of agents have different processes for modulating hippocampal neurogenesis, sleep or circadian disruption, and synaptic connectivity in the prefrontal cortex. The MT2 receptor appears to be the most promising new target for the development of new antidepressants, as selective ligands of this receptor modulate antidepressant-like effects.
Learning and Memory
Deletion or blockade of the melatonin receptors results in increased LTP as well as improved performance in learning and memory tasks. Therefore, melatonin receptor antagonists may promote increased learning and memory and may thus be useful in ameliorating the symptoms of dementia, particularly Alzheimer’s disease.
Neuroprotection
Melatonin inhibits mitochondrial cell death pathways via the MT1 receptor, whereas action through the MT2 receptor protects against ischemia/reperfusion injury. Therefore, melatonin itself and melatonin receptor agonists may be advantageous in protecting against cell death observed in diseases such as Huntington’s disease, promoting neurogenesis and protecting against injury following stroke-related ischemia/reperfusion.
Drugs of Abuse
Decreased responses to drugs of abuse are observed during the dark phase, when melatonin is at its peak. Deletion of MT1 and/or MT2 melatonin receptors results in abrogation of methamphetamine-induced locomotor sensitization and reward. Thus, melatonin receptor antagonists may provide useful treatments in ameliorating the symptoms of drug abuse.
Cancer
At the cellular level, melatonin and its receptors are poised to influence the pathology of cancer. The MT1 melatonin receptor mediates the oncostatic effect of melatonin in breast and prostate cancer models. Interestingly, the MT1 receptor may be constitutively active in breast cancer cells and intrinsically inhibits cancer cell proliferation. As such, selective MT1 receptor agonists may be effective in the treatment of breast and prostate cancer, alone or as an adjunct to currently available therapeutic approaches.
Acknowledgments
Work described in this review was supported in part by the US National Institutes of Health grants MH42922, MH52585, DA02870, and NS061068 to M.L.D. We thank all former and current members of our laboratory and all collaborators who contributed to the published work discussed in this review.
Glossary
- Melatonin
a molecule produced in the pineal gland following a circadian rhythm with high levels at night
- Circadian rhythms
the endogenous rhythms generated in constant conditions (i.e., in the absence of external cues) with a defined amplitude and period
- Melatonin receptors
G protein–coupled melatonin receptors, termed MT1 and MT2
- Receptor antagonist
a ligand that produces no response and blocks the response of an agonist
- Inverse agonist
a ligand that binds to a receptor and induces a response opposite that of the agonist in the absence of activating ligands
- Receptor agonist
a chemical that binds and activates a receptor to produce a maximal biological response
- Partial agonist
a ligand that activates a given receptor, reaching partial efficacy relative to full agonists even at maximal receptor occupancy
- Circadian time (CT)
standard time based on the free-running period; activity onset defined as CT0 or CT12 for diurnal or nocturnal species, respectively
- Diurnal variation
fluctuations in the level of a substance or behavior with a defined peak and trough across a 24-h light-dark cycle
Footnotes
RELATED RESOURCES
Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, et al. 2015. Melatonin receptors. IUPHAR/BPS Guide to PHARMACOLOGY, accessed Sept. 2. http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=39
DISCLOSURE STATEMENT
M.L.D. had received research support through an investigator-initiated research grant from Takeda Pharmaceuticals North America; had served as a consultant and/or speaker for Glaxo Wellcome, Institut de Recherches Internationales Servier, Eli Lilly and Company, Pfizer Inc., Shire Pharmaceuticals Group, Johnson & Johnson Pharmaceutical Research & Development, Vanda Pharmaceuticals, Takeda Pharmaceuticals North America, Takeda Pharmaceutical Company Limited, Novartis Pharmaceutical Corporation, Forest Laboratories Inc., and Adolor Corporation; and had solicited unrestricted educational grants awarded to Northwestern University from Takeda Pharmaceuticals North America. J.L., S.J.C., A.J.H., E.B.A.B., and M.P.G. report no conflict of interest.
Contributor Information
Jiabei Liu, Email: jl284@buffalo.edu.
Shannon J. Clough, Email: shannonc@buffalo.edu.
Anthony J. Hutchinson, Email: ah64@buffalo.edu.
Ekue B. Adamah-Biassi, Email: eba@buffalo.edu.
Marina Popovska-Gorevski, Email: mgorevsk@buffalo.edu.
Margarita L. Dubocovich, Email: mdubo@buffalo.edu.
LITERATURE CITED
- 1.Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–58. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 2.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–21. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 3.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–80. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foulkes NS, Cassone-Corsi P, Borjigin J, Snyder SH. Rhythmic transcription: the molecular basis of circadian melatonin synthesis. Trends Neurosci. 1997;20:487–92. doi: 10.1016/s0166-2236(97)01109-0. [DOI] [PubMed] [Google Scholar]
- 5.Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci. 1996;17:100–2. doi: 10.1016/0165-6147(96)10005-5. [DOI] [PubMed] [Google Scholar]
- 6.Dubocovich ML, Masana MI, Iacob S, Sauri DM. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;355:365–75. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- 7.Audinot V, Mailliet F, Lahaye-Brasseur C, Bonnaud A, Le Gall A, et al. New selective ligands of human cloned melatonin MT1 and MT2 receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2003;367:553–61. doi: 10.1007/s00210-003-0751-2. [DOI] [PubMed] [Google Scholar]
- 8.Slaugenhaupt SA, Roca AL, Liebert CB, Altherr MR, Gusella JF, Reppert SM. Mapping of the gene for the Mel1a-melatonin receptor to human chromosome 4 (MTNR1A) and mouse chromosome 8 (Mtnr1a) Genomics. 1995;27:355–57. doi: 10.1006/geno.1995.1056. [DOI] [PubMed] [Google Scholar]
- 9.Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 10.Al-Ghoul WM, Herman MD, Dubocovich ML. Melatonin receptor subtype expression in human cerebellum. NeuroReport. 1998;9:4063–68. doi: 10.1097/00001756-199812210-00011. [DOI] [PubMed] [Google Scholar]
- 11.Weaver DR, Rivkees SA, Reppert SM. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J Neurosci. 1989;9:2581–90. doi: 10.1523/JNEUROSCI.09-07-02581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM. The melatonin receptor in the human brain: cloning experiments and distribution studies. Mol Brain Res. 1996;39:117–26. doi: 10.1016/0169-328x(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 13.Adamah-Biassi EB, Zhang Y, Jung H, Vissapragada S, Miller RJ, Dubocovich ML. Distribution of MT1 melatonin receptor promoter-driven RFP expression in the brains of BAC C3H/HeN transgenic mice. J Histochem Cytochem. 2014;62:70–84. doi: 10.1369/0022155413507453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, et al. Targeted disruption of the mouse Mel1b melatonin receptor. Mol Cell Biol. 2003;23:1054–60. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubocovich ML. Luzindole (N-0774): a novel melatonin receptor antagonist. J Pharmacol Exp Ther. 1988;246:902–10. [PubMed] [Google Scholar]
- 16.Dubocovich ML. Melatonin receptor agonists and antagonists. In: Claassen V, editor. Trends in Drug Research. Amsterdam: Elsevier; 1993. pp. 285–91. [Google Scholar]
- 17.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–20. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 18.Dubocovich ML, Masana M. The efficacy of melatonin receptor analogues is dependent on the level of human melatonin receptor subtype expression. In: Touitou Y, editor. Biological Clocks, Mechanisms and Applications. Amsterdam: Elsevier; 1998. pp. 289–93. [Google Scholar]
- 19.Browning C, Beresford I, Fraser N, Giles H. Pharmacological characterization of human recombinant melatonin mt1 and MT2 receptors. Br J Pharmacol. 2000;129:877–86. doi: 10.1038/sj.bjp.0703130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugden D, Yeh LK, Teh MT. Design of subtype selective melatonin receptor agonists and antagonists. Reprod Nutr Dev. 1999;39:335–44. doi: 10.1051/rnd:19990306. [DOI] [PubMed] [Google Scholar]
- 21.Baba K, Benleulmi-Chaachoua A, Journe AS, Kamal M, Guillaume JL, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013;6:ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivara S, Pala D, Lodola A, Mor M, Lucini V, et al. MT1-selective melatonin receptor ligands: synthesis, pharmacological evaluation, and molecular dynamics investigation of N-{[(3-O-Substituted)anilino]alkyl}amides. ChemMedChem. 2012;7:1954–64. doi: 10.1002/cmdc.201200303. [DOI] [PubMed] [Google Scholar]
- 23.Descamps-François C, Yous S, Chavatte P, Audinot V, Bonnaud A, et al. Design and synthesis of naphthalenic dimers as selective MT1 melatoninergic ligands. J Med Chem. 2003;46:1127–29. doi: 10.1021/jm0255872. [DOI] [PubMed] [Google Scholar]
- 24.Kenakin TP. Orthosteric drug antagonism. In: Kenakin TP, editor. A Pharmacology Primer: Techniques for More Effective and Strategic Drug Discovery. New York: Academic; 2014. pp. 119–53. [Google Scholar]
- 25.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 26.Audinot V, Bonnaud A, Grandcolas L, Rodriguez M, Nagel N, et al. Molecular cloning and pharmacological characterization of rat melatonin MT1 and MT2 receptors. Biochem Pharmacol. 2008;75:2007–19. doi: 10.1016/j.bcp.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Cogé F, Guenin SP, Fery I, Migaud M, Devavry S, et al. The end of a myth: cloning and characterization of the ovine melatonin MT2 receptor. Brit J Pharmacol. 2009;158:1248–62. doi: 10.1111/j.1476-5381.2009.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailliet F, Audinot V, Malpaux B, Bonnaud A, Delagrange P, et al. Molecular pharmacology of the ovine melatonin receptor: comparison with recombinant human MT1 and MT2 receptors. Biochem Pharmacol. 2004;67:667–77. doi: 10.1016/j.bcp.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 29.Doolen S, Krause DN, Dubocovich ML, Duckles SP. Melatonin mediates two distinct responses in vascular smooth muscle. Eur J Pharmacol. 1998;345:67–69. doi: 10.1016/s0014-2999(98)00064-8. [DOI] [PubMed] [Google Scholar]
- 30.Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, et al. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31:18439–52. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan Q, Man H-Y, Liu F, Braunton J, Niznik HB, et al. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999;2:401–3. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- 32.Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66:312–21. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- 33.Gerdin MJ, Masana MI, Dubocovich ML. Melatonin-mediated regulation of human MT1 melatonin receptors expressed in mammalian cells. Biochem Pharmacol. 2004;67:2023–30. doi: 10.1016/j.bcp.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Gerdin MJ, Masana MI, Rivera-Bermúdez MA, Hudson RL, Earnest DJ, et al. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004;18:1646–56. doi: 10.1096/fj.03-1339com. [DOI] [PubMed] [Google Scholar]
- 35.Kokkola T, Vaittinen M, Laitinen JT. Inverse agonist exposure enhances ligand binding and G protein activation of the human MT1 melatonin receptor, but leads to receptor down-regulation. J Pineal Res. 2007;43:255–62. doi: 10.1111/j.1600-079X.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- 36.Kamal M, Gbahou F, Guillaume JL, Daulat AM, Benleulmi-Chaachoua A, et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J Biol Chem. 2015;290:11537–46. doi: 10.1074/jbc.M114.559542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Bodinat C, Guardiola-Lemaitre B, Mocaër E, Renard P, Muñoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9:628–42. doi: 10.1038/nrd3140. [DOI] [PubMed] [Google Scholar]
- 38.Paulis L, Simko F, Laudon M. Cardiovascular effects of melatonin receptor agonists. Expert Opin Investig Drugs. 2012;21:1661–78. doi: 10.1517/13543784.2012.714771. [DOI] [PubMed] [Google Scholar]
- 39.Mini LJ, Wang-Weigand S, Zhang J. Self-reported efficacy and tolerability of ramelteon 8 mg in older adults experiencing severe sleep-onset difficulty. Am J Geriatr Pharmacother. 2007;5:177–84. doi: 10.1016/j.amjopharm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Rajaratnam SMW, Polymeropoulos MH, Fisher DM, Roth T, Scott C, et al. Melatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Lancet. 2009;373:482–91. doi: 10.1016/S0140-6736(08)61812-7. [DOI] [PubMed] [Google Scholar]
- 41.Lavedan C, Forsberg M, Gentile AJ. Tasimelteon: a selective and unique receptor binding profile. Neuropharmacology. 2015;91:142–47. doi: 10.1016/j.neuropharm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301–10. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Millan MJ, Gobert A, Lejeune F, Dekeyne A, Newman-Tancredi A, et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2c receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003;306:954–64. doi: 10.1124/jpet.103.051797. [DOI] [PubMed] [Google Scholar]
- 44.Ying S-W, Rusak B, Delagrange P, Mocaër E, Renard P, Guardiola-Lemaître B. Melatonin analogues as agonists and antagonists in the circadian system and other brain areas. Eur J Pharmacol. 1996;296:33–42. doi: 10.1016/0014-2999(95)00684-2. [DOI] [PubMed] [Google Scholar]
- 45.Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–19. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Nishiyama K, Nishikawa H, Kato K, Miyamoto M, Tsukamoto T, Hirai K. Pharmacological characterization of M-II, the major human metabolite of ramelteon. Pharmacology. 2014;93:197–201. doi: 10.1159/000362459. [DOI] [PubMed] [Google Scholar]
- 47.Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9:25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Cajochen C, Kräuchi K, von Arx MA, Möri D, Graw P, Wirz-Justice A. Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neurosci Lett. 1996;207:209–13. doi: 10.1016/0304-3940(96)12517-9. [DOI] [PubMed] [Google Scholar]
- 49.Zhdanova IV. Melatonin as a hypnotic: pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Lewy AJ, Ahmed S, Jackson JML, Sack RL. Melatonin shifts human circadian-rhythms according to a phase response curve. Chronobiol Int. 1992;9:380–92. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 51.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocr Metab. 2010;95:3325–31. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewy AJ, Emens JS, Lefler BJ, Yuhas K, Jackman AR. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int. 2005;22:1093–106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- 53.Lewy AJ, Bauer VK, Cutler NL, Sack RL. Melatonin treatment of winter depression: a pilot study. Psychiat Res. 1998;77:57–61. doi: 10.1016/s0165-1781(97)00128-5. [DOI] [PubMed] [Google Scholar]
- 54.Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clin Cornerstone. 2003;5:5–15. doi: 10.1016/s1098-3597(03)90031-7. [DOI] [PubMed] [Google Scholar]
- 55.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 56.Laudon M, Frydman-Marom A. Therapeutic effects of melatonin receptor agonists on sleep and comorbid disorders. Int J Mol Sci. 2014;15:15924–50. doi: 10.3390/ijms150915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu L, Zee PC. Circadian rhythm sleep disorders. Neurol Clin. 2012;30:1167–91. doi: 10.1016/j.ncl.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–78. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 59.Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML. Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–18. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- 60.Dubocovich ML. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Benloucif S, Dubocovich ML. Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms. 1996;11:113–25. doi: 10.1177/074873049601100204. [DOI] [PubMed] [Google Scholar]
- 62.Rawashdeh O, Hudson RL, Stepien I, Dubocovich ML. Circadian periods of sensitivity for ramelteon on the onset of running-wheel activity and the peak of suprachiasmatic nucleus neuronal firing rhythms in C3H/HeN mice. Chronobiol Int. 2011;28:31–38. doi: 10.3109/07420528.2010.532894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dubocovich M, Hudson RL, Smith MR. Use of mice with genetic deletion of MT1 and/or MT2 receptors to unravel the receptor type mediating phase shift of circadian rhythms by ramelteon. Presented at Annu. Conf. Am. Coll. Neuropsychopharmacol. (ACNP) Session II 177; Dec. 9–13; Boca Raton, FL. 2007. [Google Scholar]
- 64.Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005;39:113–20. doi: 10.1111/j.1600-079X.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 65.Garfinkel D, Laudon M, Nof D, Zisapel N. Improvement of sleep quality in elderly people by controlled-release melatonin. Lancet. 1995;346:541–44. doi: 10.1016/s0140-6736(95)91382-3. [DOI] [PubMed] [Google Scholar]
- 66.Leger D, Laudon M, Zisapel N. Nocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy. Am J Med. 2004;116:91–95. doi: 10.1016/j.amjmed.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 67.De Leersnyder H, Zisapel N, Laudon M. Prolonged-release melatonin for children with neurodevelopmental disorders. Pediatr Neurol. 2011;45:23–26. doi: 10.1016/j.pediatrneurol.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 68.Roth T, Nir T, Zisapel N. Prolonged release melatonin for improving sleep in totally blind subjects: a pilot placebo-controlled multicenter trial. Nat Sci Sleep. 2015;7:13–23. doi: 10.2147/NSS.S71838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyamoto M, Nishikawa H, Doken Y, Hirai K, Uchikawa O, Ohkawa S. The sleep-promoting action of ramelteon (TAK-375) in freely moving cats. Sleep. 2004;27:1319–25. doi: 10.1093/sleep/27.7.1319. [DOI] [PubMed] [Google Scholar]
- 70.Yukuhiro N, Kimura H, Nishikawa H, Ohkawa S, Yoshikubo S, Miyamoto M. Effects of ramelteon (TAK-375) on nocturnal sleep in freely moving monkeys. Brain Res. 2004;1027:59–66. doi: 10.1016/j.brainres.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 71.Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep. 2005;28:303–7. [PubMed] [Google Scholar]
- 72.Richardson GS, Zee PC, Wang-Weigand S, Rodriguez L, Peng X. Circadian phase-shifting effects of repeated ramelteon administration in healthy adults. J Clin Sleep Med. 2008;4:456–61. [PMC free article] [PubMed] [Google Scholar]
- 73.Hirai K, Kita M, Ohta H, Nishikawa H, Fujiwara Y, et al. Ramelteon (TAK-375) accelerates reentrainment of circadian rhythm after a phase advance of the light-dark cycle in rats. J Biol Rhythms. 2005;20:27–37. doi: 10.1177/0748730404269890. [DOI] [PubMed] [Google Scholar]
- 74.Stahl SM. Mechanism of action of tasimelteon in non-24 sleep-wake syndrome: treatment for a circadian rhythm disorder in blind patients. CNS Spectrums. 2014;19:475–78. doi: 10.1017/S1092852914000637. [DOI] [PubMed] [Google Scholar]
- 75.Mattson RJ, Catt JD, Keavy D, Sloan CP, Epperson J, et al. Indanyl piperazines as melatonergic MT2 selective agents. Bioorg Med Chem Lett. 2003;13:1199–202. doi: 10.1016/s0960-894x(03)00090-8. [DOI] [PubMed] [Google Scholar]
- 76.Fisher SP, Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett. 2009;457:93–96. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Comai S, Ochoa-Sanchez R, Gobbi G. Sleep-wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav Brain Res. 2013;243:231–38. doi: 10.1016/j.bbr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 78.Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 79.Williams JW, Mulrow CD, Chiquette E, Noel PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med. 2000;132:743–56. doi: 10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- 80.Anderson IM, Tomenson BM. Treatment discontinuation with selective serotonin reuptake inhibitors compared with tricyclic antidepressants: a metaanalysis. Br Med J. 1995;310:1433–38. doi: 10.1136/bmj.310.6992.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Micale V, Arezzi A, Rampello L, Drago F. Melatonin affects the immobility time of rats in the forced swim test: the role of serotonin neurotransmission. Eur Neuropsychopharm. 2006;16:538–45. doi: 10.1016/j.euroneuro.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Overstreet DH, Pucilowski O, Retton MC, Delagrange P, Guardiola-Lemaitre B. Effects of melatonin receptor ligands on swim test immobility. NeuroReport. 1998;9:249–53. doi: 10.1097/00001756-199801260-00014. [DOI] [PubMed] [Google Scholar]
- 83.Liu JB, Somera-Molina KC, Hudson RL, Dubocovich ML. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J Pineal Res. 2013;54:222–31. doi: 10.1111/jpi.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramírez-Rodriguez G, Ortiz-López L, Dominguez-Alonso A, Benítez-King GA, Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011;50:29–37. doi: 10.1111/j.1600-079X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 85.Ramírez-Rodriguez G, Klempin F, Babu H, Benítez-King G, Kempermann G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology. 2009;34:2180–91. doi: 10.1038/npp.2009.46. [DOI] [PubMed] [Google Scholar]
- 86.Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ. Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull. 2006;68:425–29. doi: 10.1016/j.brainresbull.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 87.Adamah-Biassi EB, Hudson RL, Dubocovich ML. Genetic deletion of MT1 melatonin receptors alters spontaneous behavioral rhythms in male and female C57BL/6 mice. Hormones Behav. 2014;66:619–27. doi: 10.1016/j.yhbeh.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y-H, Ursinus J, Zhou J-N, Scheer FAJL, Bao A-M, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013;148:357–67. doi: 10.1016/j.jad.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 89.Hansen MV, Danielsen AK, Hageman I, Rosenberg J, Gogenur I. The therapeutic or prophylactic effect of exogenous melatonin against depression and depressive symptoms: a systematic review and meta-analysis. Eur Neuropsychopharm. 2014;24:1719–28. doi: 10.1016/j.euroneuro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–31. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- 91.Bertaina-Anglade V, Drieu la Rochelle C, Boyer P-A, Mocaër E. Antidepressant-like effects of agomelatine (S 20098) in the learned helplessness model. Behav Pharmacol. 2006;17:703–13. doi: 10.1097/FBP.0b013e3280116e5c. [DOI] [PubMed] [Google Scholar]
- 92.Banasr M, Soumier A, Hery M, Mocaër E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol Psychiatry. 2006;59:1087–96. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 93.Soumier A, Banasr M, Lortet S, Masmejean F, Bernard N, et al. Mechanisms contributing to the phase-dependent regulation of neurogenesis by the novel antidepressant, agomelatine, in the adult rat hippocampus. Neuropsychopharmacology. 2009;34:2390–403. doi: 10.1038/npp.2009.72. [DOI] [PubMed] [Google Scholar]
- 94.Schmelting B, Corbach-Soehle S, Kohlhause S, Schlumbohm C, Fluegge G, Fuchs E. Agomelatine in the tree shrew model of depression: effects on stress-induced nocturnal hyperthermia and hormonal status. Eur Neuropsychopharm. 2014;24:437–47. doi: 10.1016/j.euroneuro.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 95.Dubocovich ML, Mogilnicka E, Areso PM. Antidepressant-like activity of the melatonin receptor antagonist, luzindole (N-0774), in the mouse behavioral despair test. Eur J Pharmacol. 1990;182:313–25. doi: 10.1016/0014-2999(90)90290-m. [DOI] [PubMed] [Google Scholar]
- 96.Sumaya IC, Masana MI, Dubocovich ML. The antidepressant-like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J Pineal Res. 2005;39:170–77. doi: 10.1111/j.1600-079X.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 97.Dubocovich ML, Sumaya IC, Zelivyanskaya ML, Soto C, Stepien I. Antidepressant-like effect in the swimming test and increased cell proliferation/survival in hippocampus of adult C3H/HeN mice chronically treated with the melatonin (MLT) receptor ligand luzindole. Neuropsychopharmacology. 2006;31:S172. [Google Scholar]
- 98.Souêtre E, Salvati E, Belugou JL, Pringuey D, Candito M, et al. Circadian-rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiat Res. 1989;28:263–78. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 99.Kräuchi K, Cajochen C, Möri D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am J Physiol. 1997;272:R1178–88. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- 100.Leproult R, Van Onderbergen A, L’Hermite-Balériaux M, Van Cauter E, Copinschi G. Phase-shifts of 24-h rhythms of hormonal release and body temperature following early evening administration of the melatonin agonist agomelatine in healthy older men. Clin Endocrinol. 2005;63:298–304. doi: 10.1111/j.1365-2265.2005.02341.x. [DOI] [PubMed] [Google Scholar]
- 101.Lemoine P, Guilleminault C, Alvarez E. Improvement in subjective sleep in major depressive disorder with a novel antidepressant, agomelatine: randomized, double-blind comparison with venlafaxine. J Clin Psychiatry. 2007;68:1723–32. doi: 10.4088/jcp.v68n1112. [DOI] [PubMed] [Google Scholar]
- 102.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 103.Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–91. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 104.Racagni G, Riva MA, Molteni R, Musazzi L, Calabrese F, et al. Mode of action of agomelatine: synergy between melatonergic and 5-HT2C receptors. World J Biol Psychiatry. 2011;12:574–87. doi: 10.3109/15622975.2011.595823. [DOI] [PubMed] [Google Scholar]
- 105.Thies W, Bleiler L. 2012 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2012;8:131–68. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 106.Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, et al. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J Pineal Res. 2005;38:10–16. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 107.Savaskan E, Olivieri G, Meier F, Brydon L, Jockers R, et al. Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer’s disease patients. J Pineal Res. 2002;32:59–62. doi: 10.1034/j.1600-079x.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 108.Cooke SF, Bliss TVP. Plasticity in the human central nervous system. Brain. 2006;129:1659–73. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 109.Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 110.Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–37. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Larson J, Jessen RE, Uz T, Arslan AD, Kurtuncu M, et al. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett. 2006;393:23–26. doi: 10.1016/j.neulet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 112.Rawashdeh O, de Borsetti NH, Roman G, Cahill GM. Melatonin suppresses nighttime memory formation in zebrafish. Science. 2007;318:1144–46. doi: 10.1126/science.1148564. [DOI] [PubMed] [Google Scholar]
- 113.O’Neal-Moffitt G, Pilli J, Kumar SS, Olcese J. Genetic deletion of MT1/MT2 melatonin receptors enhances murine cognitive and motor performance. Neuroscience. 2014;277:506–21. doi: 10.1016/j.neuroscience.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 114.Gorfine T, Zisapel N. Melatonin and the human hippocampus, a time dependent interplay. J Pineal Res. 2007;43:80–86. doi: 10.1111/j.1600-079X.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 115.Tan DX, Manchester LC, Sainz RM, Mayo JC, Leon J, Reiter RJ. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production: a hypothesis. Endocrine. 2005;27:149–58. doi: 10.1385/endo:27:2:149. [DOI] [PubMed] [Google Scholar]
- 116.Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res. 2013;54:245–57. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 117.Parada E, Buendia I, León R, Negredo P, Romero A, et al. Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J Pineal Res. 2014;56:204–12. doi: 10.1111/jpi.12113. [DOI] [PubMed] [Google Scholar]
- 118.Wang X, Sirianni A, Pei Z, Cormier K, Smith K, et al. The melatonin MT1 receptor axis modulates mutant huntingtin-mediated toxicity. J Neurosci. 2011;31:14496–507. doi: 10.1523/JNEUROSCI.3059-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2014;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 120.Lee CH, Yoo K-Y, Choi JH, Park OK, Hwang IK, et al. Melatonin’s protective action against ischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. J Neurosci Res. 2010;88:2630–40. doi: 10.1002/jnr.22430. [DOI] [PubMed] [Google Scholar]
- 121.Chern C-M, Liao J-F, Wang Y-H, Shen Y-C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic Biol Med. 2012;52:1634–47. doi: 10.1016/j.freeradbiomed.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 122.Subst. Abus. Ment. Health Serv. Adm. Results of the 2012 national survey on drug use and health: summary of national findings. US Dep. Health Hum. Serv; Rockville, MD: 2013. NSDUH Series H-46, HHS Publ. No. (SMA) 13–4795. http://www.samhsa.gov/data/sites/default/files/NSDUHresults2012/NSDUHresults2012.pdf. [Google Scholar]
- 123.Natl. Inst. Drug Abus. Drugs, brains, and behavior: the science of addiction. Natl. Inst. Health Publ. No. 14-5605; Bethesda, MD: 2007. http://www.drugabuse.gov/sites/default/files/soa_2014.pdf. [Google Scholar]
- 124.Hutchinson AJ, Hudson RL, Dubocovich ML. Genetic deletion of MT1 and MT2 melatonin receptors differentially abrogates the development and expression of methamphetamine-induced locomotor sensitization during the day and the night in C3H/HeN mice. J Pineal Res. 2012;53:399–409. doi: 10.1111/j.1600-079X.2012.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hutchinson AJ, Ma J, Liu J, Hudson RL, Dubocovich ML. Role of MT1 melatonin receptors in methamphetamine-induced locomotor sensitization in C57BL/6 mice. Psychopharmacology. 2014;231:257–67. doi: 10.1007/s00213-013-3228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clough SJ, Hutchinson AJ, Hudson RL, Dubocovich ML. Genetic deletion of the MT1 or MT2 melatonin receptors abrogates methamphetamine-induced reward in C3H/HeN mice. Physiol Behav. 2014;132:79–86. doi: 10.1016/j.physbeh.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–75. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- 128.Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol. 2004;489:203–5. doi: 10.1016/j.ejphar.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 129.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 130.Masana MI, Benloucif S, Dubocovich ML. Circadian rhythm of mt1 melatonin receptor expression in the suprachiasmatic nucleus of the C3H/HeN mouse. J Pineal Res. 2000;28:185–92. doi: 10.1034/j.1600-079x.2001.280309.x. [DOI] [PubMed] [Google Scholar]
- 131.Roseboom PH, Namboodiri MAA, Zimonjic DB, Popescu NC, Rodriguez IR, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–97. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 132.Han J, Xu Y, Yu C-X, Shen J, Wei Y-M. Melatonin reverses the expression of morphine-induced conditioned place preference through its receptors within central nervous system in mice. Eur J Pharmacol. 2008;594:125–31. doi: 10.1016/j.ejphar.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 133.Witt-Enderby PA, Radio NM, Doctor JS, Davis VL. Therapeutic treatments potentially mediated by melatonin receptors: potential clinical uses in the prevention of osteoporosis, cancer and as an adjuvant therapy. J Pineal Res. 2006;41:297–305. doi: 10.1111/j.1600-079X.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 134.Lissoni P, Barni S, Mandala M, Ardizzoia A, Paolorossi F, et al. Decreased toxicity and increased efficacy of cancer chemotherapy using the pineal hormone melatonin in metastatic solid tumour patients with poor clinical status. Eur J Cancer. 1999;35:1688–92. doi: 10.1016/s0959-8049(99)00159-8. [DOI] [PubMed] [Google Scholar]
- 135.Lissoni P, Barni S, Meregalli S, Fossati V, Cazzaniga M, et al. Modulation of cancer endocrine therapy by melatonin: a Phase II study of tamoxifen plus melatonin in metastatic breast-cancer patients progressing under tamoxifen alone. Brit J Cancer. 1995;71:854–56. doi: 10.1038/bjc.1995.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 137.Lissoni P, Ardizzoia A, Barni S, Paolorossi F, Tancini G, et al. A randomized study of tamoxifen alone versus tamoxifen plus melatonin in estrogen receptor-negative heavily pretreated metastatic breast-cancer patients. Oncol Rep. 1995;2:871–73. doi: 10.3892/or.2.5.871. [DOI] [PubMed] [Google Scholar]
- 138.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 139.Shah PN, Mhatre MC, Kothari LS. Effect of melatonin on mammary carcinogenesis in intact and pinealectomized rats in varying photoperiods. Cancer Res. 1984;44:3403–7. [PubMed] [Google Scholar]
- 140.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–84. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 141.Blask DE, Sauer LA, Dauchy RT, Holowachuk EW, Ruhoff MS, Kopff HS. Melatonin inhibition of cancer growth in vivo involves suppression of tumor fatty acid metabolism via melatonin receptor-mediated signal transduction events. Cancer Res. 1999;59:4693–701. [PubMed] [Google Scholar]
- 142.Ram PT, Dai J, Yuan L, Dong C, Kiefer TL, et al. Involvement of the mt1 melatonin receptor in human breast cancer. Cancer Lett. 2002;179:141–50. doi: 10.1016/s0304-3835(01)00873-4. [DOI] [PubMed] [Google Scholar]
- 143.Lai L, Yuan L, Cheng Q, Dong CM, Mao LL, Hill SM. Alteration of the MT1 melatonin receptor gene and its expression in primary human breast tumors and breast cancer cell lines. Breast Cancer Res Treat. 2009;118:293–305. doi: 10.1007/s10549-008-0220-1. [DOI] [PubMed] [Google Scholar]
- 144.Rögelsperger O, Ekmekcioglu C, Jäger W, Klimpfinger M, Königsberg R, et al. Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens. J Pineal Res. 2009;46:422–32. doi: 10.1111/j.1600-079X.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 145.Yuan L, Collins AR, Dai J, Dubocovich ML, Hill SM. MT1 melatonin receptor overexpression enhances the growth suppressive effect of melatonin in human breast cancer cells. Mol Cell Endocrinol. 2002;192:147–56. doi: 10.1016/s0303-7207(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 146.Collins A, Yuan L, Kiefer TL, Cheng Q, Lai L, Hill SM. Overexpression of the MT1 melatonin receptor in MCF-7 human breast cancer cells inhibits mammary tumor formation in nude mice. Cancer Lett. 2003;189:49–57. doi: 10.1016/s0304-3835(02)00502-5. [DOI] [PubMed] [Google Scholar]
- 147.Xi SC, Tam PC, Brown GM, Pang SF, Shiu SYW. Potential involvement of mt1 receptor and attenuated sex steroid-induced calcium influx in the direct anti-proliferative action of melatonin on androgen-responsive LNCaP human prostate cancer cells. J Pineal Res. 2000;29:172–83. doi: 10.1034/j.1600-079x.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 148.Xi SC, Siu SWF, Fong SW, Shiu SYW. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate. 2001;46:52–61. doi: 10.1002/1097-0045(200101)46:1<52::aid-pros1008>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 149.Shiu SYW, Law IC, Lau KW, Tam PC, Yip AWC, Ng WT. Melatonin slowed the early biochemical progression of hormone-refractory prostate cancer in a patient whose prostate tumor tissue expressed MT1 receptor subtype. J Pineal Res. 2003;35:177–82. doi: 10.1034/j.1600-079x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 150.Tam CW, Mo CW, Yao K-M, Shiu SYW. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: implications for prostate cancer chemoprevention. J Pineal Res. 2007;42:191–202. doi: 10.1111/j.1600-079X.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 151.Clough SJ, Hutchinson AJ, Dubocovich ML. Melatonin receptors as modulators of methamphetamine mediated behaviors. In: Preedy V, editor. The Neuropathology of Drug Addictions and Substance Misuse. New York: Academic; 2016. In press. [Google Scholar]