Abstract
The presence of anti-p53 antibody in serum is a biomarker for cancer. However, its high sensitivity detection is still an issue in cancer diagnosis. To tackle this challenge, we used fd phage, a human-safe bacteria-specific virus nanofiber that can be mass-produced by infecting host bacteria in an error-free manner, and genetically engineered it to display a peptide capable of recognizing and capturing anti-p53 antibody on its side wall. We employed the resultant phage nanofibers as a capture probe to develop a modified version of the enzyme-linked immunosorbent assay (ELISA) method, termed phage-ELISA. We compared it to the traditional ELISA method for the detection of anti-p53 antibody, p53-ELISA, which uses recombinant wild-type p53 protein to capture anti-p53 antibody. We applied phage-ELISA to detect anti-p53 antibody in an experimental group of 316 patients with various types of malignant tumors. We found that a detection rate of 17.7% (56 positive cases) was achieved by phage-ELISA, which was comparable to the detection rate of 20.6% for p53-ELISA (65 positive cases). However, when both phage and p53 were combined to form antibody-capturing probes for phage/p53-ELISA, a detection rate of 30.4% (96 positive cases) was achieved. Our work showed that owing to the combined capture of the anti-p53 antibody by both phage nanofibers and p53, the phage/p53-ELISA achieved the highest diagnostic accuracy and detection efficiency for the anti-p53 antibody in patients with various types of cancers. Our work suggests that a combination of nanofibers and antigens, both of which capture antibody, could lead to increased detection sensitivity, which is useful for applications in the life sciences, clinical medicine, and environmental sciences.
Keywords: phage, virus, protein, nanofibers, cancer diagnosis
1 Introduction
Mutations in the tumor suppressor gene p53 are one of the most common genetic alternations found in human cancers [1]. Alteration or inactivation of p53 by mutation or through interactions with oncogene products can lead to cancer [2]. In the serum of healthy subjects, the presence of p53 protein and anti-p53 antibody is extremely rare. However, anti-p53 antibody has been detected in sera from patients with various types of cancers [3]. Thus, anti-p53 antibody in sera has been used as a cancer biomarker [4]. Nonetheless, anti-p53 antibody has only been detected in about 20%–40% of cancer patients with a p53 mutation, and it remains puzzling why this antibody is expressed at such a low rate [3]. One possible reason is that the methods currently available might not be sensitive enough to detect anti-p53 antibody present in serum. Therefore, an improved method for the detection of serum anti-p53 antibody, displaying higher efficiency and accuracy, is needed for cancer diagnosis.
In the past, anti-p53 antibody was detected by immunoprecipitation or western blot [5]. However, these methods are laborious and their sensitivity is low. Compared with these methods, enzyme-linked immunosorbent assay (ELISA) techniques can assay a larger number of samples in a given time. ELISA is also more sensitive, straightforward, and quantitative. This technique has revolutionized immunology and is commonly used in biomedicine and agriculture for the detection of disease markers and allergens. Hence, more recently, ELISA has been applied to detect anti-p53 antibody, however, with limited detection rate.
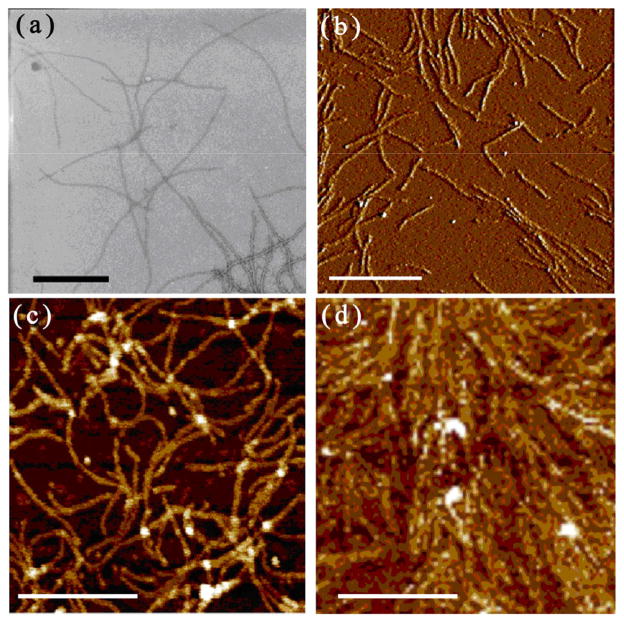
Phages are viruses that specifically infect bacteria but are not toxic to humans. Their use in several nano-material applications has attracted research interest from diverse fields including genetics, molecular biology, materials science, and medicine [6–8]. Fd phage is a nanofiber-like virus (Figs. 1(a) and 1(b)) composed of a circular single-stranded DNA (ssDNA), which is surrounded by a cylinder of coat proteins. Its side walls consist of about 4,000 copies of the major coat protein, pVIII [9]. The two distal tips of the nanofibers are each composed of approximately five copies of minor coat proteins termed pIII, pVI, pVII, and pIX [10, 11]. Since the coat proteins are encoded by the DNA within the phage, the surface of the phage can be genetically modified by fusing a foreign peptide to the N-terminal end of the coat proteins [12, 13].
Figure 1.
TEM (a) and AFM (b) image of anti-p53-binding phage nanofibers before use in ELISA. (c) AFM image of phage nanofibers coated in phage-ELISA plate. (d) AFM image of phage-p53 complex coated in phage/p53-ELISA plate. Black bar = 500 nm. White bar = 1 μm.
There are several types of ELISA tests. One of the most commonly used ELISA methods is called indirect ELISA, which is used to determine the presence of a specific antibody. The indirect ELISA system involves two types of antibodies, namely primary and secondary antibodies. The primary antibody, also known as the target antibody, binds to target antigen while the secondary antibody specifically recognizes the primary antibody. As a result, target antigen, target antibody, and the secondary antibody form a sandwich structure. The secondary antibody is usually tagged with an enzyme, and when the enzyme substrate, a chromogen reagent, is added to the system, color will be developed and the intensity of the color is directly proportional to the amount of bound target antibody. The success of the ELISA method depends on whether the antigen can capture the target antibody efficiently. The traditional ELISA, when applied to the detection of anti-p53 antibody, uses wild type p53 protein as an antigen. This method is termed p53-ELISA. In this study we applied an anti-p53-binding phage, which displays a peptide (SQAMDDLMLS, termed SQ) derived from p53 protein but capable of binding anti-53 antibody on the major coat [14]. We used it as an antigen in two ways. One is to directly use the anti-p53-binding phage (Fig. 1(c)) and the resultant ELISA is termed phage-ELISA. The other is to use a mixture of anti-p53-binding phage and p53 protein (Fig. 1(d)) and the resultant ELISA is termed phage/p53-ELISA (Scheme 1). We found that the phage/p53-ELISA showed a significantly improved detection efficiency for anti-p53 antibody in the serum of cancer patients, leading to a more accurate diagnosis of cancer. It is known that p53 tends to be positively charged and can naturally bind electrostatically to DNA [15]. Because fd phage tends to be negatively charged due to the presence of anionic coat proteins [16], p53 and phage can electrostatically interact to form a complex (Fig. 1(d)) and collectively bind to anti-p53 antibody with a higher antibody-capturing efficiency than phage or p53 alone. Thus as expected, we found that phage/p53-ELISA exhibited a higher detection rate than phage-ELISA or p53-ELISA alone.
Scheme 1.
General principle of using SQ-displaying phage for the detection of anti-p53 antibody. Filamentous phages, which were genetically engineered to display the peptide SQ on the side wall, were mixed with p53 protein to form a complex (Fig. 1(d)) on the plate for the detection of serum anti-p53 antibody. HRP = horseradish peroxidase, TMB = etramethylbenzidine.
2 Experimental
2.1 Patients and controls
A total of 316 patients diagnosed with various types of cancers and treated at Jilin Tumor Hospital, Changchun, Jilin, China, were enrolled in this study. Serum samples were obtained before the patients received any treatment and stored at −40 °C until use. Of all patients, 90 presented with lung tumors, 63 with breast tumors, 30 with colorectal tumors, and 133 with other types of tumors. A population of 400 healthy controls who received routine physical examinations constituted the control group. None of the controls had any personal history of cancer at the time of routine examination. The recruitment of and sample collection of patients and control individuals were performed following the guidelines of protocols approved by the Institutional Review Boards. Informed consent was obtained from all participants.
2.2 Preparation of recombinant wild-type p53 protein and anti-p53-binding phage
Wild-type human p53 protein with an N-terminal 6-His tag was overexpressed in E. coli and purified according to previously published methods [14]. The purity and integrity of the p53 protein was verified by SDS-PAGE on a 15% acrylamide gel followed by Coomassie brilliant blue staining. A peptide comprising the 37–46 amino acid domain located at the N-terminus of the p53 protein, known as SQ peptide, was displayed on the major coat of fd phages following a previously reported protocol [14]. Briefly, two complementary DNA fragments encoding this peptide were synthesized: 5′-GGAGG GTTCT CAAGC TATGG ATGAT TTAAT GTTAT CTCCA T-3′ and 5′-CGATG GAGAT AACAT TAAAT CATCC ATAGC TTGAG AACCC TCCGC-3′. The oligonucleotide encoding the SQ peptide was cloned into the pfd88 plasmid; then, hybrid phage was produced by infecting NM522 cells with the modified phage pfd88-SQ bearing the SQ peptide. Expression of the inserted peptide was verified by SDS-PAGE on 20% acrylamide gels followed by silver staining. The peptide retained its ability to bind anti-p53 antibody.
2.3 Silver staining of SDS-PAGE gels
Immediately after the electrophoretic run was terminated, the gel was placed in fixation solution I (50% methanol/5% acetic acid) and II (5% methanol/5% acetic acid), each time for 1 h. In between and afterwards, the gel was washed twice for 1 min in deionized water. Cold silver staining solution (1% AgNO3) was added to the gel, which was shaken for 30 min to allow the silver ions to bind to the protein. After staining was completed, the staining solution was poured off and the gel was rinsed with a large volume of deionized water for 1 min to remove the excess of unbound silver ions. The gel was shortly rinsed with developing solution (4% Na2CO3/0.6% methanol), and a fresh developing solution was added to the gel to develop the protein image. The development was stopped as soon as the desired intensity was reached.
2.4 Western blot analysis of wild-type p53 protein and anti-p53-binding phage
Both p53 protein and the peptide-displaying phage were purified by electrophoresis and subsequently transferred to a nitrocellulose membrane (GE Healthcare Bio-sciences, Pittsburgh, PA, USA). The filters were cut into strips, which were then blocked overnight at 4 °C in a blocking buffer (5% nonfat milk in tris-buffered saline (TBS)) to block nonspecific binding sites. After washing with TBS-Tween (TBST), each strip was incubated for 1 h at 37 °C with anti-p53 antibody-positive sera from cancer patients or anti-p53 polyclonal antibodies (prepared in our laboratory). After the strips were washed three times, the peroxidase-conjugated goat anti-human IgG or goat anti-rabbit IgG (Sigma) was added, followed by incubation for 1 h at 37 °C. Thereafter, the strips were stained with the chromogen 3-amino-9-ethylcarbozole (AEC; AMRESCO, Solon, OH, USA). Negative controls were carried out in parallel.
2.5 Transmission electron microscopy (TEM) and atomic force microscopy (AFM) analysis
TEM and AFM images of anti-p53-binding phage nanofibers were captured with a Zeiss 10A microscope and Bruker Bioscope Catalyst, respectively.
2.6 Establishment of ELISA procedures for the detection of serum anti-p53 Antibody
2.6.1 p53-ELISA
Polystyrene 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated overnight at 4 °C with 50 μL of recombinant p53 protein at a concentration of 5 μg·mL −1 dissolved in 0.05 M carbonate buffer (pH 9.6). Plates were subsequently washed three times with 1X Phosphate Buffered Saline Tween-20 (PBST) and then twice with PBS. Excess binding sites were blocked using 200 μL of blocking buffer (5% nonfat milk dissolved in PBS). After the wells were washed, 50 μL of serum diluted with a ratio of 1/200 in blocking buffer was added and incubated for 1 h at 37 °C. The plates were washed and then incubated with 50 μL of peroxidase-conjugated goat anti-human IgG antibody diluted at a ratio of 1/5,000 for 45 min at 37 °C, and washed again. The peroxidase activity retained in the wells was assayed by the addition of 100 μL of tetramethylbenzidine (TMB; AMRESCO, Solon, OH, USA) solution. The reaction was stopped by adding 50 μL of 2 N H2SO4 per well and the absorbance in each well was measured at 450 nm on a microtiter plate reader (Multiskan Ascent, Labsystems, Finland). All samples were measured in triplicate and the mean of the triplicate values was taken as the final readout.
The cut-off value designating positive reactions was conventionally defined as the mean absorbance of the 400 normal human sera + 2 standard deviations [17–19]. Each ELISA run included 2 control sera samples to reduce the differences caused by analyzing samples at different time periods [20]. The average of the 2 normal controls was used in each run to normalize all absorbance values.
2.6.2 Phage-ELISA
Polystyrene 96-well microtiter plates were coated with 50 μL of anti-p53-binding phage at a concentration of 60 μg·mL −1 in 0.05 M carbonate buffer (pH 9.6) overnight at 4 °C. At the same time, the sera were diluted at a ratio of 1/200 in the blocking buffer and incubated in 10 μg·mL −1 wild-type phage overnight to reduce nonspecific reaction. The rest of the ELISA procedure was the same as for the p53-ELISA procedure described above.
2.6.3 Phage/p53-ELISA
The ELISA procedure was the same as the phage ELISA, except that the plates were coated with a mixture of 25 μL p53 protein at a concentration of 5 μg·mL −1 and 25 μL anti-p53-binding phage at a concentration of 60 μg·mL −1.
2.7 Statistical Analysis
SPSS software version 13.0 was used for data recording and statistical analysis. Differences between malignant cases and normal controls were assessed by χ2-test. To assess the diagnostic performance of each ELISA method with regard to the discrimination of cancer patients from normal controls, we constructed receiver operating characteristic (ROC) curves and calculated the area under the curve (AUC). In this study, the AUC values of ROC curves were generated by using the results of the three ELISA methods from the 400 healthy controls and the 316 cancer patients. The detection data were analyzed using the SPSS software. ROC curves and the AUC value were obtained using this software.
3 Results and discussion
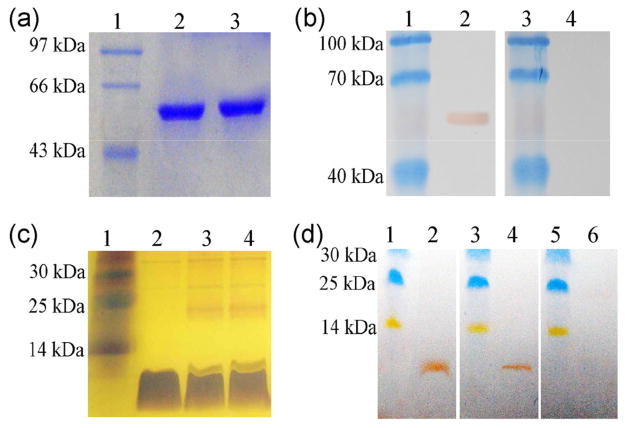
The (His)6-p53 fusion protein was purified by using metal-affinity chromatography. As shown in Fig. 2(a), the fusion protein was purified to at least 90% purity when analyzed. Figure 2(b) reveals that the p53 fusion protein reacted with both anti-p53 monoclonal antibody and anti-p53 antibody-positive sera from cancer patients. These results indicate that the full-length p53 protein could be used as an antigen for the detection of p53 antibody in cancer patients. The peptide SQ was successfully displayed on the surface of filamentous phages (Fig. 2(c)). Figure 2(d) shows that the phage-displayed peptide reacted with anti-p53 polyclonal antibodies and anti-p53 antibody-positive sera from cancer patients. These results also demonstrate that the phage-displayed peptide can be used as an antigen for the detection of p53 antibody in cancer patients.
Figure 2.
SDS-PAGE and western blot analysis of the wild-type p53 protein and anti-p53-binding phage nanofibers. (a) Samples were analyzed by SDS-PAGE and visualized by Coomassie brilliant blue staining. Lane 1: protein marker, lanes 2 and 3: wild-type p53 protein. (b) Western blot analysis of wild-type p53 protein. Lanes 1 and 3: protein marker; lane 2: probed with one anti-p53 positive serum sample from a cancer patient; lane 4: probed with one anti-p53 negative serum sample from a healthy control. (c) Anti-p53-binding phages were analyzed by SDS-PAGE and silver staining. Lane 1: protein marker; lane 2: major coat protein of wild-type phages; lanes 3 and 4: major coat protein of anti-p53-binding phages. (d) Western blot analysis of anti-p53-binding phage. Lane 1, 3, and 5: protein marker; lane 2: probed with anti-p53 polyclonal antibodies; lane 4: probed with one anti-p53 positive serum sample from a cancer patient; lanes 6: probed with one anti-p53 negative serum sample from a healthy control.
Table 1 lists the results of anti-p53 antibody detection for the two groups using the three different ELISA procedures. The positive rates of anti-p53 antibody in the normal controls and malignant tumor samples were 3.8% versus 20.6% (p53-ELISA), 4.3% versus 17.7% (phage-ELISA), and 4.0% versus 26.0% (phage/p53-ELISA), respectively. This shows that the phage/p53-ELISA method can detect anti-p53 antibody from sera of cancer patients with a higher sensitivity than both phage-ELISA and p53-ELISA separately, which each show a comparable positive rate. As expected, the detection rates of anti-p53 antibody in the malignant tumors were significantly higher than in the normal control group (P < 0.001).
Table 1.
The detection rates of serum anti-p53 antibody in two groups using three different ELISA methods
| Group | Population (rate) with positive anti-p53 antibody in sera
|
||||||
|---|---|---|---|---|---|---|---|
| p53-ELISA | Phage-ELISA | Phage/p53-ELISA | |||||
|
| |||||||
| N | N (%) | Pa | N (%) | Pa | N (%) | Pa | |
| Healthy controls | 400 | 15 ± 1 (3.8) | <0.001 | 17 ± 1 (4.3) | <0.001 | 16 ± 1 (4.0) | <0.001 |
| Cancer patients | 316 | 65 ± 2 (20.6) | 56 ± 1 (17.7) | 82 ± 2 (26.0) | |||
The test method was χ2-test. P < 0.05 was statistically significant.
Figure 3(a) lists the average positive rates of serum anti-p53 antibody in 316 patients with different cancer types using the three different ELISA methods. The average detection rates of p53-ELISA, phage-ELISA, and phage/p53-ELISA as well as the corresponding average cases detected for different types of cancer patients are summarized as follows: 17.8% (16 ± 1 cases), 15.6% (14 ± 1 cases), and 25.6% (23 ± 1 cases) in lung cancer; 15.9% (10 ± 1 cases), 17.5% (11 ± 1 cases), and 22.2% (14 ± 1 cases) in breast cancer; 23.3% (7 ± 1 cases), 20.0% (6 cases), and 33.3% (10 ± 1 cases) in colorectal cancer; 24.1% (32 ± 1 cases), 18.8% (25 ± 1 cases), and 26.3% (35 ± 1 cases) in the group with other types of cancer. These results indicate that the combined phage/p53-ELISA has a sensitivity superior to that of either p53-ELISA or phage-ELISA for each cancer type. The positive cases detected by a combination of all three ELISA methods are 96 (± 3) out of a total of 316 patients (30.4%). However, only 29 (± 1) cases (9.2%) were shared by all of the three methods (Fig. 3(b)). These results suggest that a combination of these three ELISA systems could make the detection of anti -p53 antibody more accurate than using each of the individual ELISA methods alone.
Figure 3.
(a) Phage/p53-ELISA is superior to either p53-ELISA or phage-ELISA with regard to the detection of serum p53 antibody from patients with various types of cancer. (b) Comparison of the detection rates of serum anti-p53 using three different ELISA methods. (c) Comparison of ROC curves of p53-ELISA, phage-ELISA, and mixed-antigen-ELISA. Sera derived from 316 cancer patients and 400 healthy controls were tested by p53-ELISA, phage-ELISA, and phage/p53-ELISA, and ROC curves were calculated.
The performance of one or more diagnostic methods in discriminating disease cases from normal cases is usually evaluated by ROC curve analysis [21, 22]. The ROC curve is a graph of sensitivity (y-axis) vs. specificity (x-axis) [23]. The AUC is a measure of how well a parameter can be distinguished between two diagnostic groups (diseased and normal). In this study, ROC curves were generated by using the results of the three ELISA methods from the 400 healthy controls and the 316 cancer patients. Based on the ROC curves, AUCs, which represent the accuracy of the diagnostic methods, were calculated (Fig. 3(c)). In this data set, phage/p53-ELISA (AUC, 0.728) showed the highest diagnostic accuracy in comparison to p53-ELISA (AUC, 0.675) and phage-ELISA (AUC, 0.648), suggesting that phage/p53-ELISA is a superior method for discriminating between cancer patients and healthy controls.
Antigenic sites of p53 recognized by the serum antibody were studied by Lubin et al. and Schlichtholz et al. using peptide scanning analysis [24, 25]. Their studies demonstrated that the immunodominant epitopes for anti-p53 antibody recognition are located in the highly antigenic amino-terminal region of the p53 protein and that the majority of the antibodies interacted specifically with a 10-mer peptide segment, the SQ peptide in that region. In addition, it has been shown that the natural epitope of proteins could be simulated effectively when the core functioning peptide is displayed on the phage surface, which to some extent improves the solvent exposure of the peptide [26]. Therefore, with the SQ peptide, which is the immunodominant segment of the p53 protein, displayed on the surface of phage nanofibers could act as an ideal substrate for efficient recognition and detection of anti-p53 antibody in the serum. Because the phage-displayed peptide represents the amino-terminal region of the p53 protein [24, 25, 27], the phage can serve as a substitute for the recombinant p53 protein.
Different epitopes of the p53 protein can recognize the serum anti-p53 antibody in cancer patients, which might explain why the positive rate of serum anti-p53 antibody detected by the p53-ELISA was higher than that detected by the phage-ELISA. However, since phage and p53 can electrostatically bind each other to form a complex (Fig. 1(d)) and collectively capture the anti-p53 antibody, the phage/p53-ELISA showed a higher detection efficiency than either phage-ELISA or p53-ELISA. In addition, after p53 is electrostatically bound to the phage nanofibers, the immobilization of p53 on the surface of the phage is likely to enhance the projection of the p53 protein into the analyte phase, in a way similar to a recent study using DNA nano-structures [28, 29], which results in higher detection efficiency by phage/p53-ELISA.
In the current study, we determined the cut-off values of serum anti-p53 antibody by analyzing sera from 400 healthy controls. Previous studies only included a small number of healthy controls [17, 30]. Our three immunoassays had specificity rates around 96% (Table 1), which were comparable to the rates found in another study that used p53 protein as a coating antigen [31]. Because the serum of about 20 healthy individuals also tested positive for antibody specific to p53 protein, intensive follow-up is ongoing to screen the subclinical potential of malignant tumors in these individuals. The fact that the anti-p53 antibody positive detection rate was significantly higher in cancer patients than in healthy subjects (P < 0.001) (Table 1) indicates that our method is reasonably reliable for cancer detection.
4 Conclusions
In summary, this study shows that phage/p53-ELISA can significantly increase the detection rate of anti-p53 antibody in the serum of cancer patients and also had highly discriminative ability between true- and false-positive cases when compared with p53-ELISA or phage-ELISA. The combination of three ELISA methods maximized the identification of positive cases among patients with various types of cancer. This phage/p53-ELISA approach could be applied for antibody detection in numerous scientific fields, including life sciences, environmental analysis, and clinical medicine.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 81028010), Ministry of Science and Technology (No. 2014DFA31740) and the Department of Science and Technology of Jilin Province, China (Nos. 20130206009YY and 20130727034YY). Y. Z., Z. G. J., P. H. Q. and C. B. M. also would like to thank the financial support from National Science Foundation (Nos. CMMI-1234957 and CBET-1512664), National Institutes of Health (Nos. EB015190 and CA200504), Department of Defense Peer Reviewed Medical Research Program (No. W81XWH-12-1-0384), Oklahoma Center for the Advancement of Science and Technology (No. HR14-160) and Oklahoma Center for Adult Stem Cell Research (No. 434003).
References
- 1.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 2.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 3.Soussi T. p53 antibodies in the sera of patients with various types of cancer: A review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 4.Lara JF, Thor AD, Dressler LG, Broadwater G, Bleiweiss IJ, Edgerton S, Cowan D, Goldstein LJ, Martino S, Ingle JN, et al. p53 expression in node- positive breast cancer patients: Results from the cancer and leukemia group B 9344 trial. Clin Cancer Res. 2011;17:5170–5178. doi: 10.1158/1078-0432.CCR-11-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubin R, Zalcman G, Bouchet L, Trédanel J, Legros Y, Cazals D, Hirsch A, Soussi T. Serum p53 antibodies as early markers of lung cancer. Nature Med. 1995;1:701–702. doi: 10.1038/nm0795-701. [DOI] [PubMed] [Google Scholar]
- 6.Mao CB, Liu AH, Cao BR. Virus-based chemical and biological sensing. Angew Chem, Int Ed. 2009;48:6790–6810. doi: 10.1002/anie.200900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Arutyunov D, Szymanski CM, Evoy S. Bacteriophage based probes for pathogen detection. Analyst. 2012;137:3405–3421. doi: 10.1039/c2an35371g. [DOI] [PubMed] [Google Scholar]
- 8.Abbineni G, Modali S, Safiejko-Mroczka B, Petrenko VA, Mao C. Evolutionary selection of new breast cancer cell-targeting peptides and phages with the cell-targeting peptides fully displayed on the major coat and their effects on actin dynamics during cell internalization. Mol Pharm. 2010;7:1629–1642. doi: 10.1021/mp100052y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marvin DA, Hale RD, Nave C, Helmer-Citterich M. Molecular models and structural comparisons of native and mutant class I filamentous bacteriophages: Ff (fd, f1, M13), If1 and IKe. J Mol Biol. 1994;235:260–286. doi: 10.1016/s0022-2836(05)80032-4. [DOI] [PubMed] [Google Scholar]
- 10.Mao CB, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, Georgiou G, Iverson B, Belcher AM. Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires. Science. 2004;303:213–217. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- 11.Smith GP, Petrenko VA. Phage display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 12.Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249:386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]
- 13.Smith GP. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 14.Yu DH, Li JH, Wang YC, Xu JG, Pan PT, Wang L. Serum anti-p53 antibody detection in carcinomas and the predictive values of serum p53 antibodies, carcino-embryonic antigen and carbohydrate antigen 12–5 in the neoadjuvant chemotherapy treatment for III stage non-small cell lung cancer patients. Clin Chim Acta. 2011;412:930–935. doi: 10.1016/j.cca.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Sauer M, Bretz AC, Beinoraviciute-Kellner R, Beitzinger M, Burek C, Rosenwald A, Harms GS, Stiewe T. C-terminal diversity within the p53 family accounts for differences in DNA binding and transcriptional activity. Nucleic Acids Res. 2008;36:1900–1912. doi: 10.1093/nar/gkn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang FK, Cao BR, Mao CB. Bacteriophage bundles with prealigned Ca2+ initiate the oriented nucleation and growth of hydroxylapatite. Chem Mater. 2010;22:3630–3636. doi: 10.1021/cm902727s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson KS, Wong J, Vitonis A, Crum CP, Sluss PM, Labaer J, Cramer D. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:859–868. doi: 10.1158/1055-9965.EPI-09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, Baxendale DM, Reddish MA, Hu MC, Stroop SD, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41:1114–1122. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 19.Chapman CJ, Murray A, McElveen JE, Sahin U, Luxemburger U, Türeci O, Wiewrodt R, Barnes AC, Robertson JF. Autoantibodies in lung cancer: Possibilities for early detection and subsequent cure. Thorax. 2008;63:228–233. doi: 10.1136/thx.2007.083592. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- 21.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 22.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 23.Griner PF, Mayewski RJ, Mushlin AI, Greenland P. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med. 1981;94:557–592. [PubMed] [Google Scholar]
- 24.Lubin R, Schlichtholz B, Bengoufa D, Zalcman G, Trédaniel J, Hirsch A, Caron de Fromentel C, Preudhomme C, Fenaux P, Fournier G, et al. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53: Distribution on primary structure and exposure on protein surface. Cancer Res. 1993;53:5872–5876. [PubMed] [Google Scholar]
- 25.Schlichtholz B, Trédaniel J, Lubin R, Zalcman G, Hirsch A, Soussi T. Analyses of p53 antibodies in sera of patients with lung carcinoma define immunodominant regions in the p53 protein. Brit J Cancer. 1994;69:809–816. doi: 10.1038/bjc.1994.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.di Marzo Veronese F, Willis AE, Boyer-Thompson C, Appella E, Perham RN. Structural mimicry and enhanced immunogenicity of peptide epitopes displayed on filamentous bacteriophage: The V3 loop of HIV-1 gp120. J Mol Biol. 1994;243:167–172. doi: 10.1006/jmbi.1994.1643. [DOI] [PubMed] [Google Scholar]
- 27.Vennegoor CJM, Nijman HW, Drijfhout JW, Vernie L, Verstraeten RA, von Mensdorff-Pouilly S, Hilgers J, Verheijen RHM, Kast WM, Melief CJM, et al. Autoantibodies to p53 in ovarian cancer patients and healthy women: A comparison between whole p53 protein and 18-mer peptides for screening purposes. Cancer Lett. 1997;116:93–101. doi: 10.1016/s0304-3835(97)00168-7. [DOI] [PubMed] [Google Scholar]
- 28.Giovanni M, Setyawati MI, Tay CY, Qian H, Kuan WS, Leong DT. Electrochemical quantification of Escherichia coli with DNA nanostructure. Adv Funct Mater. 2015;25:3840–3846. [Google Scholar]
- 29.Yuan L, Giovanni M, Xie JP, Fan CH, Leong DT. Ultrasensitive IgG quantification using DNA nano-pyramids. NPG Asia Mater. 2014;6:e112. [Google Scholar]
- 30.Ralhan R, Arora S, Chattopadhyay TK, Shukla NK, Mathur M. Circulating p53 antibodies, p53 gene mutational profile and product accumulation in esophageal squamous-cell carcinoma in India. Int J Cancer. 2000;85:791–795. doi: 10.1002/(sici)1097-0215(20000315)85:6<791::aid-ijc9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 31.Shimada H, Ochiai T, Nomura F. Titration of serum p53 antibodies in 1085 patients with various types of malignant tumors: A multiinstitutional analysis by the Japan p53 antibody research group. Cancer. 2003;97:682–689. doi: 10.1002/cncr.11092. [DOI] [PubMed] [Google Scholar]