SUMMARY
Stress is a well-known risk factor for subsequent alcohol abuse, but the neural mechanisms underlying interactions between stress and alcohol remain largely unknown. Addictive drug reinforcement and stress signaling involve common neural circuitry, including the mesolimbic dopamine system. We demonstrate in rodents that pre-exposure to stress attenuates alcohol-induced dopamine responses and increases alcohol self-administration. The blunted dopamine signaling resulted from ethanol-induced excitation of GABA neurons in the ventral tegmental area. Excitation of GABA neurons was mediated by GABAA receptor activation and involved stress-induced functional down-regulation of the K+, Cl− cotransporter, KCC2. Blocking stress hormone receptors, enhancing KCC2 function, or preventing excitatory GABA signaling by alternative methods all prevented the attenuated alcohol-induced dopamine response and prevented the increased alcohol self-administration. These results demonstrate that stress alters the neural and behavioral responses to alcohol through a neuroendocrine signal that shifts inhibitory GABA transmission towards excitation.
Keywords: alcohol use disorder, neuroendocrine, HPA axis, GABA, reward, mesolimbic, nucleus accumbens, microdialysis, chloride gradient, KCC2
eTOC Blurb
Ostroumov et al. show that stress exposure attenuates alcohol-induced dopamine signals and increases alcohol self-administration in rodents. Stress causes midbrain circuitry changes via neuroendocrine signals that ultimately impair a chloride transporter and shift specific GABA inhibition towards excitation.
INTRODUCTION
Excessive alcohol use is among the leading causes of preventable death worldwide (WHO, 2014). While many variables contribute to the development of alcohol use disorder (AUD), exposure to stressful life events represents a significant risk factor (Keyes et al., 2012). Stress increases alcohol consumption in alcohol dependent and non-dependent populations (Ayer et al., 2011; Tamers et al., 2014; Thomas et al., 2011), and stress is thought to underlie a transition to pathological drug use (Koob and Le Moal, 2005). Animal studies have revealed interactions between stress and ethanol self-administration under certain stressor and drinking paradigms, but some results have been equivocal (Becker et al., 2011; Noori et al., 2014; Spanagel et al., 2014). These discrepancies indicate that the basic neuronal mechanisms linking stress and alcohol use are not well understood.
Stress-induced changes in alcohol use likely arise from an interaction between the stress and reward systems of the brain (Spanagel et al., 2014; Uhart and Wand, 2009). At the cellular level, both stress hormones and ethanol influence the DA system by direct actions on DA neurons or indirectly via changes in excitatory and inhibitory synaptic inputs (Niehaus et al., 2010; Saal et al., 2003). Stress hormone signaling also may alter midbrain GABAA receptor signaling, but the molecular mechanism underlying this adaptation has not been identified (Doyon et al., 2013) and may arise from changes in GABA synthesis, in release, or in expression of specific GABAA receptor subunits (Maguire, 2014). Alternatively, acute stress exposure has been shown to induce a paradoxical shift towards excitatory GABAA receptor signaling within the HPA axis by altering the intracellular anion homeostasis (Hewitt et al., 2009; Sarkar et al., 2011). Given that the GABAA receptor is a target of ethanol, we postulated that alterations in GABAA receptor transmission could contribute to an interaction between stress and ethanol self-administration.
To examine the interaction between stress and ethanol, we exposed drug-naïve rats to stress and then measured their subsequent ethanol intake. Concomitant with increases in ethanol self-administration, we show that acute stress attenuates ethanol-induced DA neuron firing in the VTA and DA release at target regions. These stress hormone-dependent effects were mediated by an increase in VTA GABAergic inhibition onto DA neurons in response to ethanol. Stress induced the functional down-regulation of KCC2, shifting GABAA receptor signaling towards excitation of some downstream GABA neurons. Pharmacological activation of KCC2 restored the GABAergic circuitry and DA neuron signaling and prevented the escalation in ethanol self-administration induced by stress. These results indicate that a shift toward excitatory GABA signaling within the mesolimbic system is associated with increased drinking after exposure to stress.
RESULTS
Stress increases ethanol self-administration via activation of glucocorticoid receptors
We first examined how a single episode of restraint stress alters subsequent ethanol intake measured during daily operant self-administration sessions (Figure 1A). Stable lever pressing for saccharin (0.125%, w/v) was first established followed by the introduction of ethanol (2–4%) into the drinking solution (Doyon et al., 2013). Animals were subjected to restraint stress (1 hr) approximately 15 hrs prior to the first ethanol self-administration session. The 15-hr separation between the stress and ethanol self-administration was chosen to examine the lasting impact on neural circuits, not the immediate proximal influence of the stressor itself (Noori et al., 2014).
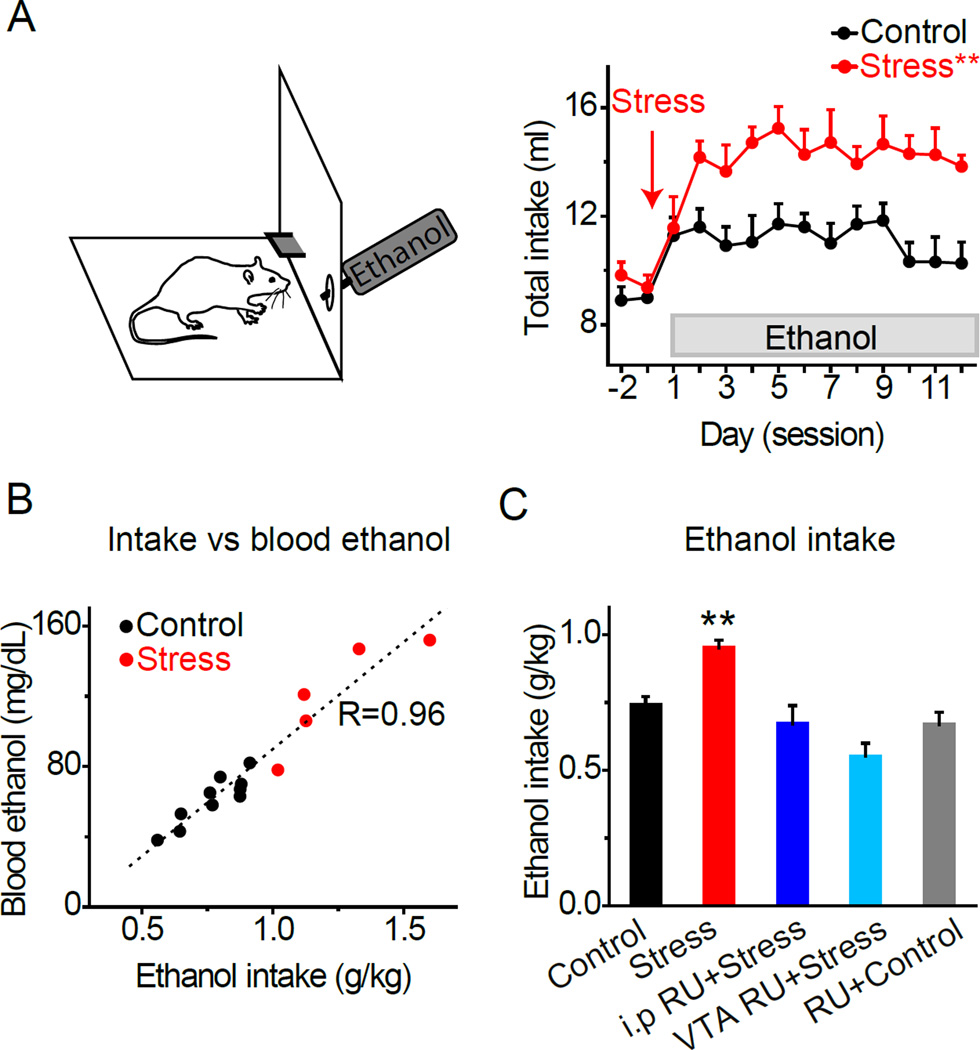
Figure 1. Stress Increases Ethanol Self-administration.
A) Rats self-administered saccharin prior to fading ethanol into the drinking solution. Rats were subjected to a single restraint stress 15–20 hrs before the first ethanol exposure (red arrow). Daily fluid intake was measured in control and stressed rats. Stressed rats showed greater ethanol intake compared to unstressed control rats. **Significantly different from the control group by ANOVA with repeated measures, p < 0.01, n = 16–19 rats/group.
B) Ethanol intake (g/kg) vs blood ethanol levels (mg/dl). Blood ethanol was measured immediately after the self-administration session in control (black) and stressed (red) animals. A regression analysis showed a significant and positive correlation between ethanol intake and blood ethanol levels, F(1,13) = 162.7, p < 0.01.
C) Mean daily ethanol intake over the first 7 self-administration sessions. Stressed rats (red bar) consumed significantly more ethanol (g/kg) compared to control rats (black bar). Blockade of glucocorticoid receptors with RU486 systemically (dark blue, 40 mg/kg, i.p.) or locally in the VTA (light blue, 20 ng/1 ml) prior to stress prevented increases in ethanol intake, n = 10, 14. RU486 administered systemically or intra-VTA to control animals did not alter ethanol intake, n = 9, gray bar. **Significantly different from all groups by t-test, p < 0.01.
Pre-exposure to stress caused a significant and long-lasting increase in ethanol self-administration compared to the non-stressed control group (Figure 1A): group: F(1,21) = 19.32, p < 0.01. Blood-ethanol levels were measured in a subset of animals and were correlated with ethanol intake (Figure 1B). Stressed rats showed significantly higher blood-ethanol levels (120.8 ± 13.6 mg/dl, n = 5) than non-stressed rats (61.3 ± 4.3 mg/dl, n = 10, p < 0.01). Mean intake of ethanol over the first 7 days was 0.74 ± 0.03 g/kg for the control group (n = 19) and 0.95 ± 0.03 g/kg for the stressed group (n = 16, p < 0.01) (Figure 1C, black and red bars). Elevated drinking after stress was also observed at higher ethanol concentrations (7–10%) over a 3-week period (Figure S1A, B). Therefore, acute restraint stress induced robust changes in the acquisition and maintenance of ethanol-drinking behavior.
Restraint stress is known to increase circulating glucocorticoid levels (Pitman et al., 1988). To determine whether the effect of stress on drinking was mediated by stress hormone signaling, a separate group of rats was pretreated with RU486 (a glucocorticoid receptor antagonist) prior to stress. Pretreatment with systemic RU486 prevented the stress from increasing subsequent ethanol self-administration (Figure 1C, dark blue bar; 0.67 ± 0.07 g/kg). To determine whether stress hormone signaling acted locally within the DA system (Spanagel et al., 2014; Uhart and Wand, 2009), we microinfused RU486 bilaterally into the VTA prior to stress exposure. Intra-VTA administration of RU486 (Figure S1C) prevented the stress from increasing subsequent ethanol self-administration (Figure 1C, light blue bar; 0.55 ± 0.05 g/kg), revealing that the effect of stress requires glucocorticoid receptor activation within the VTA. In non-stressed control rats, RU486 did not significantly influence ethanol intake when administered systemically or by local microinfusion (Figure 1C, gray bar on the right; 0.67 ± 0.04 g/kg), demonstrating a selective effect of RU486 on stress-induced drinking. Additional control experiments with saccharin and palatable food suggest that the effect of stress we observe was specific to ethanol self-administration (see Supplemental Experimental Procedures).
Stress attenuates ethanol-induced DA activity in vivo via glucocorticoid receptor signaling
Because ethanol self-administration involves DA signaling in the nucleus accumbens (NAc) (Gonzales et al., 2004), we hypothesized that stress might also alter ethanol-induced DA release in the NAc. To test this hypothesis, rats were subjected to 1-hr restraint stress 15 hrs prior to ethanol exposure, and microdialysis samples were collected to measure changes in the extracellular DA concentration in response to ethanol administration (Figure 2A). We observed a sustained increase in DA levels in the control group (Figure 2B, black trace), but stressed animals showed a blunted DA response to ethanol (Figure 2B, red trace): group × time: F(10,160) = 2.79, p < 0.01. No significant differences in baseline DA levels were detected between control and stress groups: 2.0 ± 0.2 nM in control vs. 2.1 ± 0.2 nM after stress.
Figure 2. Stress Attenuates Ethanol-induced DA Signaling In Vivo.
A) Animals were exposed to a 1-hr restraint stress and microdialysis experiments were conducted 15 hrs later.
B) Time course of DA release in the NAc following in vivo ethanol administration in control rats (black), in stressed rats (red), and in rats injected with RU486 (i.p.) prior to stress exposure (grey). Ethanol (1.5 g/kg) was injected i.v. during the 5-min period (shaded vertical grey bar). *Significantly different from the control group and from the RU486+Stress group by ANOVA with repeated measures, p < 0.05, n = 7–9 rats/group.
C) The spontaneous firing rate of VTA DA neurons was measured in vivo using single-unit recordings in anesthetized animals.
D) Putative DA neuron recording sites in the VTA for control (black) and stressed animals (red).
E) Representative recordings from putative DA neurons before and after ethanol administration (0.6–1.5 g/kg) in the control (black) and stressed (red) groups. No significant differences in the mean basal firing rate were detected between control and stressed groups: 6.3 ± 0.7 Hz in control vs. 8.0 ± 1.0 Hz after stress, n = 8–13, p > 0.05.
F) In the control group (black), ethanol increased the firing rate of putative DA neurons. In the stressed group (red), ethanol failed to increase the firing rate of putative DA neurons. **Significantly different from the control group by t-test, p < 0.01, n = 8–13 rats/group.
Next, we tested whether glucocorticoid receptor activation mediated the stress- induced decrease in ethanol-evoked DA release. Systemic pretreatment with RU486 prevented the inhibitory effect of stress on ethanol-induced DA release (Figure 2B, grey data). The distribution of the microdialysis probe placements within the NAc is shown in Figure S2.
Ethanol stimulates DA release in the NAc by increasing the firing rate of VTA DA neurons (Foddai et al., 2004). To determine whether restrained stress altered DA neuron firing rate in vivo, we conducted single unit recordings of VTA DA neurons in anesthetized rats 15 hrs after stress (Figure 2C). DA neurons were recorded in the lateral VTA (Figure 2D) and were identified based on their electrophysiological and pharmacological properties (Figure S3A, B).
The spontaneous firing rate of VTA DA neurons was measured before and after intravenous infusion of ethanol (0.6–1.5 g/kg). Ethanol administration induced an increase in the spontaneous firing rate of VTA DA neurons in control animals (128.6 ± 7.6%). In contrast, stressed animals failed to demonstrate a significant firing-rate increase upon ethanol administration (102.3 ± 3.1%) (Figures 2E and F; n = 8–13, p < 0.01). Together with the microdialysis experiments, these data indicate that ethanol-induced DA signaling was blunted after exposure to stress.
Stress attenuates ethanol-induced DA firing ex vivo
To examine the cellular mechanisms of the blunted DA signaling after stress, we measured the ex vivo responses of VTA DA neurons to ethanol (Figure 3A). Midbrain horizontal slices containing the VTA were cut 15 hrs after restraint stress, and patch clamp cell-attached recordings were performed on DA neurons. Putative DA neurons were recorded from the lateral VTA and were identified based on established electrophysiological criteria (Figure S3C–E). In 34 of 36 cells displaying these electrophysiological properties, the DA phenotype was confirmed by tyrosine hydroxylase staining (Figure 3B)
Figure 3. Stress and Corticosterone Attenuate Ethanol-induced DA Neuron Firing Rates via Enhanced GABA Release Ex Vivo.
A) Spontaneous firing rate of VTA DA neurons was measured using the cell-attached configuration, and the whole-cell configuration was used to identify DA neurons electrophysiologically and histochemically upon termination of the experiment.
B) Neurobiotin-labeled putative DA neurons were immuno-positive for tyrosine hydroxylase (TH, red stain).
C) Representative recordings from DA neurons before and after bath ethanol administration in the control and stressed (red) groups. No significant differences in mean basal firing rate were detected before ethanol: 2.3 ± 0.2 Hz in control vs. 2.0 ± 0.2 Hz after stress, n = 10–12.
D) Normalized spontaneous firing rates of VTA DA neurons following ethanol (grey horizontal bar) in unstressed control group (black) and in rats exposed to stress 15-hrs prior to cutting the slice (red). **Significantly different from the control by ANOVA with repeated measures, p < 0.01, n = 10–12 cells/group.
E) Glutamatergic receptor antagonists (DNQX and AP5) did not prevent ethanol-induced attenuation of DA cell firing after exposure to stress (red) and showed a lower firing rate than unstressed controls (black). **Significantly different from the control by ANOVA with repeated measures, p < 0.01, n = 7–8 cells/group.
F) The GABAA receptor antagonist, picrotoxin, prevented ethanol’s attenuation of VTA DA neuron firing rates after stress (red) and showed a response similar to the unstressed controls (black), n = 9–15 cells/group.
G) Spontaneous inhibitory postsynaptic currents (sIPSC) onto VTA DA neurons were recorded using the whole-cell patch clamp configuration. No significant differences were detected in the mean basal sIPSC frequency or amplitude between stressed and control groups before ethanol: frequency, 2.4 ± 0.6 Hz in control vs. 2.3 ± 0.3 Hz after stress; amplitude, 26.2 ± 4.0 pA in control vs. 30.8 ± 3.6 pA after stress, p > 0.05, n = 8–10.
H) Representative recordings of sIPSCs before and after ethanol administration in the control (black) and stressed (red) groups.
I) Mean changes in the sIPSC frequency after ethanol application in VTA DA neurons. DA neurons from stressed animals (red) demonstrated a significantly increased ethanol-mediated sIPSC frequency compared to neurons from unstressed controls (black). Systemic inhibition of glucocorticoid receptors with RU486 (40 mg/kg) prior to stress prevented elevated sIPSC frequency (gray). Incubation of VTA slices from control animals with corticosterone increased ethanol-mediated sIPSC frequency in DA neurons up to stress levels (dark blue). Co-incubation with RU486 prevented this increase (light blue). Incubation of brain slices with RU486 and/or corticosterone did not alter basal parameters of sIPSCs (data not shown). Across all groups, ethanol application did not produce significant changes in the sIPSC amplitudes (data not shown, n = 6–10, p > 0.05). **Significantly different from control and RU486-treated groups by t-test, p < 0.01, n = 6–10 cells/group.
Bath application of 50-mM ethanol increased the spontaneous firing rate of DA neurons from unstressed control rats (Figure 3C and D, black data), but DA cells from stressed animals failed to show significant increases in firing rate upon ethanol application (Figure 3C and D, red data): group × time: F(10,200) = 3.55, p < 0.01.
Stress attenuates ethanol-induced DA neuron firing rates via enhanced GABA release ex vivo
Reduced DA activity after stress could be mediated through decreased excitation or increased inhibition of DA neurons. To probe the relative contribution of excitatory neurotransmission, we recorded ethanol-induced DA activity during inhibition of ionotropic glutamate receptors with DNQX (20 µM) and AP5 (50 µM), which inhibit AMPA and NMDA receptors respectively. When comparing control and stressed groups, the basal firing rate of DA neurons was not significantly altered by bath application of DNQX and AP5. Upon ethanol application, DA neurons from stressed animals again showed reduced DA activity compared to controls (Figure 3E): group × time: F(10,130) = 6, p < 0.01, indicating that this effect of stress was not mediated via changes in glutamatergic neurotransmission.
Next, we tested the contribution of GABAergic signaling by comparing ethanol-induced VTA DA neuron firing between control and stress groups in the presence of picrotoxin, a GABAA receptor antagonist. Picrotoxin (50 µM) did not significantly alter the basal firing rate between the control and stress groups. However, upon application of ethanol, the presence of picrotoxin prevented the blunted DA responses to ethanol observed after stress (Figure 3F, red trace). This finding suggests that stress reduced DA responses to ethanol via changes in GABAergic neurotransmission.
Altered inhibitory control of DA neurons after stress could be mediated through changes in presynaptic neurotransmitter release or postsynaptic receptor responses. To determine the site of adaptation for GABAergic neurotransmission, whole-cell patch clamp recordings of VTA DA neurons were performed and spontaneous inhibitory postsynaptic currents (sIPSC) were measured in the presence of ethanol (Figure 3G). In control animals, bath-applied ethanol produced a small increase in sIPSC frequency (116.1 ± 5.0%). In contrast, DA neurons from stressed animals showed significantly greater ethanol-induced potentiation of sIPSC frequency compared to the control response (171.5 ± 7.2%) (Figures 3H and I, black and red data; n = 8–10, p < 0.01). Systemic injection of RU486 prior to stress prevented the stress-mediated increase in sIPSC frequency observed after ethanol application (Figure 3I, grey bar): 110.6 ± 4.5%, n = 8.
To demonstrate the direct action of glucocorticoids in mediating the effects of stress, brain slices from control rats were incubated in corticosterone (1 µM) for 1 hr (Pitman et al., 1988). DA neurons from control animals treated with corticosterone showed a potentiation of ethanol-induced sIPSC frequency that was indistinguishable from stressed animals (Figure 3I, dark blue bar): 182.7 ± 10.7%, n = 8. Importantly, incubation with RU486 prevented this corticosterone-mediated increase in sIPSC frequency (Figure 3I, light blue bar): 114.5 ± 5.5%, n = 6. Increased frequency, but not amplitude, of sIPSCs suggests that the change caused by stress and corticosterone resides with the presynaptic neuron (i.e., the GABA neuron) not with the postsynaptic neuron (i.e., the DA neuron) (see Fig. 3G).
Stress increases ethanol-induced VTA GABA neuron firing rate ex vivo and in vivo
Patch-clamp recordings indicated that stress induced long-term alterations in GABAergic inputs onto VTA DA neurons. Local VTA GABA neurons can modulate VTA DA neuron activity (Creed et al., 2014). To examine whether stress altered the effects of ethanol on local VTA GABA neurons, we performed electrophysiological recordings of these cells ex vivo (Figure 4A). Putative VTA GABA neurons in slices were recorded in the lateral VTA and identified by a combination of factors including small somata size (< 20 µm), high pacemaker-like firing rate (> 7 Hz), and the lack of Ih-current (Figure 4B) (Korotkova et al., 2006; Margolis et al., 2006). Importantly, 47 of 49 VTA neurons with these properties were not immunoreactive for TH (Figure 4C). We measured ethanol’s effect on VTA GABA neuron spontaneous firing rate in a cell-attached configuration. Bath-application of ethanol on VTA slices from control animals produced a marginal increase of GABA neuron firing rate (Figure 4D, black trace). A significantly higher ethanol-induced increase in GABA neuron firing rate was observed following stress, compared to non-stressed controls (Figure 4D, red trace): group × time: F(10,160) = 5.89, p < 0.01.
Figure 4. Stress Increases Ethanol-induced GABA Neuron Firing Rates Ex Vivo.
A) Spontaneous firing rate of VTA GABA neurons was measured using the cell-attached configuration, and the whole-cell configuration was used to identify GABA neurons electrophysiologically and histochemically upon termination of the recording.
B) VTA GABA neurons displayed fast spontaneous firing rates (> 7 Hz) and lack of Ih current.
C) Neurobiotin-labeled VTA neurons with these electrophysiological properties were immuno-negative for tyrosine hydroxylase (TH), consistent with a non-DA phenotype.
D) VTA GABA neurons from stressed rats (red) showed a greater increase in firing rate following ethanol application (grey horizontal bar) than did GABA neurons from the unstressed controls (black). **Significantly different from the control by ANOVA with repeated measures, p < 0.01, n = 9 cells/group.
E) Glutamatergic receptor antagonists (DNQX and AP5) did not prevent the enhanced firing rate of VTA GABA neurons from stressed animals in response to ethanol. **Significantly different from the control by ANOVA with repeated measures, p < 0.01, n = 6–7 cells/group.
F) GABAA receptor antagonist, picrotoxin, prevented the ethanol-induced increase of GABA neuron firing rates after stress (red), and the firing rate was similar to the unstressed controls (black), n = 7–12 cells/group. The basal firing of VTA GABA neurons was not significantly different between control and stress groups (before ethanol): 11.1 ± 1.4 Hz vs. 11.3 ± 1.3 Hz, p > 0.05, n = 9.
Qualitatively similar effects of stress on ethanol-induced GABA neuron firing were observed during in vivo single unit electrophysiological recordings in anesthetized rats. Putative GABA neurons in the lateral VTA (Figure S4A) were identified based on their electrophysiological and pharmacological properties (Figure S4B, C). Whereas i.v. administration of ethanol (0.6–1.5 g/kg) slightly decreased the firing rate of VTA GABA neurons in control animals (88.7 ± 5.2%, n = 6), the same doses of ethanol significantly increased spontaneous activity after stress (124.5 ± 8.1%, n = 6, p < 0.01) (Figure S4D–F.
As was previously examined in VTA DA neurons (Figures 3E and F), we probed for changes in excitatory and inhibitory input onto VTA GABA neurons ex vivo. After application of ethanol, inhibition of ionotropic glutamate receptors with DNQX and AP5 did not prevent the enhanced GABA cell firing rate observed after stress, indicating that this effect was not mediated via changes in glutamatergic neurotransmission (Figure 4E): group × time: F(10,110) = 11.19, p < 0.01. However, blocking GABAA receptors with picrotoxin prevented the enhanced firing rate of VTA GABA neurons observed after stress (Figure 4F, red trace). This result suggests that, in stressed animals, ethanol increased VTA GABA neuron firing rate via GABAA receptors.
Stress promotes excitatory GABA input onto VTA GABA neurons ex vivo
Based on our findings in Figure 4F, we hypothesized that GABA inputs onto VTA GABA neurons produced excitatory responses. To test this, we measured VTA GABA neuron firing rates in response to repetitive stimulation of synaptic GABAA receptor inputs with ionotropic glutamate receptors inhibited (Figure 5A). Upon electrical stimulation (20 Hz for 1 s), GABA neurons from control animals showed decreased firing (Figures 5B and C, black data), indicative of GABAergic inhibition of the recorded GABA neuron. In marked contrast, slices from stressed animals showed increased GABA neuron firing after GABAA receptor stimulation (Figures 5B and C, red data): group × time: F(29,464) = 10.03, p < 0.01. This finding directly demonstrates excitation mediated by high frequency stimulation of GABAA receptors. Importantly, this effect was blocked by picrotoxin (Figures 5D upper and E, Stress + picrotoxin), providing further confirmation that the observed excitation of VTA GABA neurons following stress was mediated by GABAA receptors. In control animals, similar GABAA receptor mediated excitation was also observed following 1 hr incubation of brain slices in corticosterone (Figure 5E, blue bar, Cort), suggesting that prolonged exposure to corticosteroids is sufficient to promote excitatory GABAA transmission onto VTA GABA neurons.
Figure 5. Stress Promotes GABA-mediated Excitation of VTA GABA Neurons Ex Vivo.
A) VTA GABA neurons were recorded in a cell-attached configuration before and after electrical stimulation of GABAA receptor inputs. To isolate GABAA receptor inputs, AMPA, NMDA, and GABAB receptors were inhibited with antagonists: DNQX, AP5 and CGP55845 respectively.
B) Representative GABA neuron recording from a control animal demonstrated decreased firing rate in response to stimulation of GABAergic input (black). Similar stimulation enhanced the firing rate of VTA GABA neurons from stressed animals (red). For display, the traces were filtered and stimulus artifacts were removed.
C) Mean changes in VTA GABA neuron firing rate from control (black) and stressed (red) rat slices following repetitive stimulation of synaptic GABA inputs. **Significantly different from the control by ANOVA with repeated measures, p < 0.01, n = 8–10 cells/group.
D) Representative VTA GABA neuron recording from a stressed rat demonstrated that in the presence of picrotoxin (upper) or acetazolamide (ACTZ, lower), repetitive stimulation of GABA inputs failed to increase the firing rate.
E) Normalized mean changes in the firing rates of VTA GABA neurons in response to stimulation for each treatment group. Values were averaged over 5 s immediately following termination of the stimulation. In controls, GABAA receptor-mediated increase in the firing rate was observed after slice incubation with corticosterone (blue). Significantly different from the control by t-test (p < 0.05, *), n = 8–10 cells/group or (p < 0.01, **), n = 6–8 cells/group.
Next, we tested whether the stress-induced transition in GABAA receptor signaling was also observed in VTA DA neurons. Upon electrical stimulation of GABAA receptor input (20 Hz, 1 s), DA neurons from control and stressed animals showed decreased firing (Figure S5A and B), indicating that the direct GABA input onto DA neurons remains inhibitory.
It has been reported (Staley et al., 1995) that GABAA receptor-mediated excitation can be prevented by application of acetazolamide, an inhibitor of carbonic anhydrase. Based on these findings, we postulated that acetazolamide would prevent the transition from GABAA receptor-mediated inhibition to excitation of GABA neurons observed after stress. Bath application of acetazolamide (10 µM) did not change basal GABA neuron firing rate between control and stress groups, nor did it change control responses to repetitive stimulation. However, repetitive stimulation in the presence of acetazolamide blocked the increase in GABA neuron firing after stress (Figures 5D bottom and E, Stress + ACTZ).
Acetazolamide prevents stress and ethanol interactions ex vivo and in vivo
Given that stress promoted excitatory GABA input onto VTA GABA neurons, we tested whether this phenomenon mediated the stress-induced alterations in alcohol’s actions. Based on the results of our repetitive stimulation studies (Figures 5D and E), we bath-applied acetazolamide to prevent the changes in GABA and DA neuron firing to ethanol observed after stress. We found that upon application of ethanol, there was no longer a difference in GABA and DA neuron firing rates between control and stressed groups (Figure 6A and B, black and red traces compared to the dotted red lines representing stress without acetazolamide).
Figure 6. Stress Requires Excitatory GABA Shift to Attenuate Ethanol-induced DA Levels and Increase Ethanol Self-administration Ex Vivo and In Vivo.
A) In the presence of bath applied acetazolamide (ACTZ), ethanol-induced VTA GABA neuron firing was no longer enhanced after exposure to stress (red) and was similar to the response of the unstressed controls (black). The effect of stress exposure and ethanol application in the absence of acetazolamide from Figure 4D is shown for comparison (dotted red line), n = 8 cells/group. Note, bath application of acetazolamide on VTA slices (before ethanol) did not produce any significant alterations in the mean basal firing rate of GABA neurons between control and stress groups.
B) In the presence of bath ACTZ, ethanol-induced VTA DA neuron firing was no longer attenuated after exposure to stress (red) and was similar to the response of the unstressed controls (black). The effect of stress exposure and ethanol application in the absence of ACTZ from Figure 3D is shown for comparison (dotted red line), n = 10–14 cells/group. Note, bath application of ACTZ on VTA slices (before ethanol) did not produce any significant alterations in the mean basal firing rate of DA neurons between control and stress groups.
C) Stressed animals received intra-VTA infusion of ACTZ or vehicle prior to the onset of baseline microdialysis sample collection. Subsequent ethanol-induced DA release in the NAc was measured.
D) In contrast to vehicle injection (red), ethanol-induced DA levels in the NAc following ACTZ infusion were not blunted (blue) and were similar to the control response from Figure 2B (dotted black line). **Significantly different from the VTA vehicle group by ANOVA with repeated measure, p < 0.01, n = 7 rats/group.
E) Stressed animals received bilateral intra-VTA infusions of ACTZ or vehicle prior to the onset of each self-administration session.
F) ACTZ-infused stressed animals consumed significantly less ethanol (blue) compared to vehicle-infused stressed animals (red). Ethanol consumption in unstressed control rats from Figure 1C is shown for comparison (dotted horizontal line). *Significantly different from the VTA vehicle group by t-test, p < 0.05, n = 10–14 rats/group.
To prevent stress from changing ethanol-induced DA release in the NAc, we infused acetazolamide into the VTA prior to the microdialysis experiments (Figure 6C). In contrast to the intra-VTA infusion of vehicle, acetazolamide prevented the inhibitory effect of stress on ethanol-induced [DA] (Figure 6D): group × time: F(10,120) = 2.96, p < 0.01. The effect of acetazolamide was indistinguishable from the non-stressed control group (Figure 6D, black dotted trace). Microinfusion of acetazolamide outside the VTA did not reverse the inhibitory effect of stress exposure (Figure S6A). In unstressed control animals, the microinfusion of acetazolamide in the VTA did not alter the DA response to ethanol (data not shown, n = 6, p > 0.05).
To prevent stress from escalating ethanol intake, we bilaterally infused acetazolamide into the VTA prior to each ethanol self-administration session (Figure 6E). Intra-VTA infusion of acetazolamide significantly decreased the average daily ethanol intake in the stressed group (0.67 ± 0.06 g/kg) compared to the stressed group that received intra-VTA infusions of vehicle (0.92 ± 0.08 g/kg) (Figure 6F; n = 10–14, p < 0.05). These data were indistinguishable from the non-stressed control group (Figure 6F, dotted horizontal line). VTA infusions of acetazolamide did not alter ethanol consumption in control animals (Figure S6B). All microinfusion sites of acetazolamide in the VTA are shown in Figure S6C and D.
Stress alters anion homeostasis in VTA GABA neurons ex vivo
We next investigated the causes underlying the increased GABA cell excitation by alcohol. The transition in GABAA receptor signaling (Figure 5B) suggests a depolarizing shift in the GABAA reversal potential (EGABA) in VTA GABA neurons (Hewitt et al., 2009). EGABA is the membrane potential at which evoked IPSCs change their direction from inward to outward. To determine EGABA in VTA GABA neurons, gramicidin-perforated patch-clamp recordings were performed to preserve the intracellular anion concentrations, and GABAA IPSCs were measured at different membrane potentials (Figure 7A). VTA GABA neurons from stressed animals showed a significantly more depolarized EGABA value compared to controls (Figure 7B and C): −63.4 ± 3.1 mV after stress (red data) vs. −88.2 ± 3.3 mV in controls (black data), n = 10, p < 0.01.
Figure 7. Stress Weakens Cl− Extrusion and Decreases the Function of KCC2 in the VTA.
A) GABAergic input onto VTA GABA neurons was recorded using gramicidin perforated patches at different holding potentials to measure stress-induced alterations in anion homeostasis. GABAA IPSCs were evoked by electrical stimulation in the presence of DNQX, AP5, and CGP55845.
B) Representative IPSCs recordings from control (black) and stressed (red) animals at the given holding potentials. The IPSCs reverse direction at the EGABA. For display, the traces were filtered and stimulus artifacts were removed.
C) VTA GABA neurons from stressed animals (red, **, p < 0.01 by t-test) demonstrated a significantly more positive EGABA value compared to neurons from unstressed control animals (black square), n = 10 cells/group.
D) Cl− accumulation was estimated by stimulating repetitive GABAA receptor input. Upon stimulation (20 Hz, Vh = 0 mV), a representative GABA neuron from a control (black) animal demonstrated a minor depression of IPSC amplitude compared to the significantly greater depression seen in a GABA neuron from a stressed animal (red).
E) At 0 mV, VTA GABA neurons from stressed animals (red) demonstrated a significantly higher rate of evoked IPSC amplitude depression compared to control animals (black). **Significantly different from the control by F-test, p < 0.01, n = 10–17 cells/group.
F) Western blot analysis was conducted for total KCC2 and phosphorylated-S940 KCC2 with GAPDH as a loading control. A representative western blot indicates no differences in total KCC2 expression after stress. However, stressed animals showed reduced expression of pS940 KCC2 relative to total KCC2 when compared to non-stressed controls.
G) Densiometric analysis revealed a significant reduction in the ratio of pS940 KCC2 to total KCC2 protein in stressed animals compared to non-stress controls (horizontal dashed line). *Significantly different from the control by t-test, p < 0.05, n = 10 animals/group.
H) Densiometric analysis revealed a significant reduction in the ratio of pS940 KCC2 to total KCC2 protein in corticosterone-incubated slices from controls *Significantly different from the control by t-test, p < 0.05, n = 16 animals/group.
A depolarizing shift in EGABA reflects a higher intracellular chloride concentration, which in adult neurons is often mediated by a decrease in Cl− extrusion capacity. During prolonged GABAA receptor stimulation, decreased Cl− extrusion capacity leads to intracellular Cl− accumulation, culminating in the collapse of the Cl− gradient and decreased synaptic GABAA receptor inhibition. To test whether exposure to stress weakens Cl− extrusion in VTA GABA neurons, we applied repetitive GABAA receptor stimulation and measured activity-dependent depression of the IPSCs (Hewitt et al., 2009). The rate of decrease in IPSC amplitude at the conditions that favor Cl− influx (0 mV) depends on Cl− accumulation and activity-dependent synaptic depression. In contrast, GABAA receptor stimulation at the conditions of Cl- efflux (-90 mV) only depends on activity-dependent synaptic depression. Upon electrical stimulation at 20 Hz at a holding potential of −90 mV, stress did not affect the rate of synaptic depression in VTA GABA neurons (Figure S7A and B). In contrast, the decrease of IPSC amplitude at 0 mV occurred significantly faster after stress (Figure 7D and E): F = 39.9, p < 0.01. The differential effect of stress at −90 mV vs. 0 mV indicates that stress increased Cl− accumulation, suggesting a reduced capacity for Cl− extrusion in VTA GABA neurons.
Stress and glucocorticoids induce dephosphorylation of KCC2 at serine 940
Stress-induced reductions in Cl− extrusion capacity have been associated with dephosphorylation of the K+, Cl− cotransporter, KCC2, at serine 940 (S940) (Kahle et al., 2013; Sarkar et al., 2011). To examine stress-induced alterations in KCC2 protein expression and its phosphorylation in the VTA, we performed western blot analysis using an antibody against total KCC2 protein, as well as a phospho-specific antibody against the KCC2 phosphorylation site S940 (Sarkar et al., 2011). Immunoblots revealed two prominent bands (~140 and ~270 kDa) for both total and S940 KCC2 antibodies, indicating the presence of monomeric and dimeric structures of KCC2 protein (Figure 7F) (Hewitt et al., 2009). No significant differences in the expression of total KCC2 protein between control and stressed groups were observed (Figure S7C, red data). In contrast, the ratio of phosphorylated-S940 KCC2 to total KCC2 protein after stress was significantly lower compared to control (Figure 7F, G): 78.3 ± 5.5% for monomer, 79.6 ± 4.7% for dimer. Importantly, as also reported previously (Taylor et al., 2015), immunolabeling analysis in the VTA suggested that KCC2 protein was expressed exclusively on non-DA neurons (data not shown), which is consistent with the presence of another chloride extrusion mechanism in DA neurons (Gulacsi et al., 2003).
To determine whether glucocorticoids mediate the effect of stress on KCC2, VTA slices from control animals were incubated for 1 hr in corticosterone. Similar to in vivo exposure to stress, this treatment did not alter levels of total KCC2 protein in the VTA (Figure S7C, blue data). However, the ratio of phosphorylated-S940 KCC2 to total KCC2 protein was significantly lower after corticosterone incubation (Figure 7H): 75.5 ± 7.6% for monomer, 78.6 ± 9.4% for dimer. Taken together, these results suggest that stress or corticosterone leads to dephosphorylation of KCC2 protein at S940, which decreases KCC2 function and alters anion homeostasis.
Enhanced Cl− extrusion restores anion homeostasis in VTA GABA neurons
Based on our findings in Figure 7, we hypothesized that enhancement of Cl− extrusion would restore normal GABAA receptor-mediated inhibition in VTA GABA neurons from stressed animals. To enhance Cl− extrusion specifically, we used recently developed activators of KCC2, CLP257 and CLP290 (Gagnon et al., 2013). First, we confirmed that CLP257 and CLP290 were effective in VTA GABA neurons by measuring activity-dependent depression of IPSCs (as in Figures 7D, E and S7B). After incubation of VTA slices with CLP257 (>1hr, 5 µM) or CLP290 (>1 hr, 10 µM) the rates of decrease in IPSC amplitude at 0 and −90 mV were similar between control and stressed groups (Figures S8A–D). Given that CLP257 was shown to be rapidly metabolized in vivo, we further tested CLP290, which was demonstrated to have longer bio-availability (Gagnon et al., 2013).
Next, we studied the effect of CLP290 on the stress-induced depolarizing shift in EGABA and conditional GABAA receptor-mediated excitation of GABA neurons observed after stress (as in Figures 7C and 5E, respectively). We found that correcting the Cl− gradient with CLP290 in slices from stressed animals returned the EGABA back to the control value (Figure 8A) and blocked the stimulation-induced increase in GABA neuron firing (Figure S8E).
Figure 8. KCC2 activation in the VTA prevents stress-ethanol interaction.
A) When brain slices were incubated in the KCC2 activator, CLP290, EGABA was hyperpolarized comparably in VTA GABA neurons from control (black) and stressed (red) animals. The effect of stress exposure on EGABA in the absence of CLP290 is shown for comparison (open red square), n = 6 cells/group
B) After CLP290 incubation, ethanol-induced VTA DA neuron firing was no longer attenuated after exposure to stress (red) and was similar to the response of the unstressed controls (black). The effect of stress exposure and ethanol application in the absence of CLP290 is shown for comparison (dotted red line), n = 10 cells/group. Note, incubation of VTA slices with CLP290 (before ethanol) did not produce any significant alterations in the mean basal firing rate of DA neurons between control and stress groups.
C) CLP290 was infused bilaterally intra-VTA (40 µM at 0.5 µl/min). After CLP290 administration, stressed animals consumed significantly less ethanol (blue) compared to vehicle-injected stressed animals (red). Ethanol consumption in unstressed control rats is shown for comparison (dotted horizontal line). *Significantly different from the VTA vehicle group by t-test, p < 0.05, n = 12–13 rats/group.
Enhanced Cl− extrusion prevents stress and ethanol interactions ex vivo and in vivo
Given that stress altered anion homeostasis in VTA GABA neurons, we next determined whether this phenomenon mediated stress-induced alterations in alcohol’s actions. In slices from stressed animals, boosting Cl− extrusion with the KCC2 activator, CLP290, returned the alcohol-induced firing rate of DA neurons to the control value (Figure 8B).
Next, we locally microinfused CLP290 bilaterally into the VTA prior to the first ethanol self-administration session and measured ethanol intake over 7 days (Figure 8C). Intra-VTA infusion of CLP290 (Figure S8F) significantly decreased the average daily ethanol intake in the stressed group back to control levels (0.79 ± 0.05 g/kg) compared to the stressed group that received intra-VTA infusions of vehicle (1.00 ± 0.08 g/kg) (Figure 8C; n = 12–13, p < 0.05). These data were indistinguishable from the non-stressed control group (Figure 8C, dotted horizontal line). VTA infusions of CLP290 did not alter ethanol consumption in control animals (0.84 ± 0.05 g/kg, data not shown).
DISCUSSION
While epidemiological studies consistently report associations between stress and ethanol consumption (Keyes et al., 2012), the underlying neuronal effects have not been well delineated. We found that alterations in GABAA receptor responses on GABAergic neurons of the VTA correlate with an increase in ethanol self-administration induced by temporally-distant, acute stress. After stress, we detected enhanced VTA GABAergic inhibition of DA neurons and reduced mesolimbic DA release in response to ethanol. Blunted DA signaling was mediated by a transition towards excitatory GABAA receptor signaling and was associated with decreased Cl− extrusion capacity in VTA GABA neurons. Stress-induced adaptations were prevented by acetazolamide (Staley et al., 1995) or by CLP290 (Gagnon et al., 2013). The effect of stress on GABA transmission was recapitulated in vitro by corticosterone exposure and was prevented by pharmacological blockade of glucocorticoid receptors (Cadepond et al., 1997). Most importantly, when acetazolamide, CLP290, or RU486 were locally infused in the VTA in vivo, stress no longer increased ethanol self-administration.
The decreased DA response to ethanol was correlated to the stress event and to the excitatory GABA signaling. Although the DA response was not directly examined as a cause of the increased self-administration, others have reported that enhancing DA signaling exogenously attenuates voluntary drinking in rats (Bass et al., 2013). Furthermore, the correlation between decreased ethanol-induced DA release and increased self-administration has been previously reported in rodent studies (Brodie and Appel, 2000; Doyon et al., 2013; Ramachandra et al., 2007).
The shift towards excitatory GABAA receptor signaling was correlated to the stress event and was required for the stress to cause increased ethanol self-administration (Figures 6 and 8). Although GABAA signaling normally mediates inhibitory synaptic transmission in the adult mammalian nervous system, it can shift towards excitation under certain pathological conditions, including epilepsy, neuropathic pain, and neuronal trauma (De Koninck, 2007). The shift arises from the decreased function of the chloride extrusion pump, KCC2 (Kaila et al., 2014). Upon strong GABAA receptor stimulation, diminished function of KCC2 led to the accumulation of chloride ions inside the cell and subsequent loss of the chloride gradient. Activity-dependent loss of the hyperpolarizing chloride gradient unmasks an outward flux of bicarbonate ions through GABAA receptors, resulting in neuronal depolarization/excitation (Staley et al., 1995). Consistent with this model, we found that the GABAergic circuitry responds as expected in the basal condition, but when GABAA receptors are highly engaged by strong stimulation (Figure 5) or by ethanol (Figures 2, 3, 4), a compromised extrusion capacity leads to a collapse in the Cl− gradient and excitation of GABAergic neurons within the VTA (Figures 5 and 7). This shift towards excitatory GABA may occur elsewhere in the brain after stress, but the increased ethanol self-administration was prevented if this shift was blocked in the VTA. Although an excitatory shift in GABA transmission in the adult brain is usually associated with pathological conditions, similar transitions were found in the HPA axis following stress and in the VTA following chronic exposure to opiates (Hewitt et al., 2009; Laviolette et al., 2004; Sarkar et al., 2011). When our results are taken with the accumulation of evidence in the literature, we speculate that the shift towards excitatory GABA signaling may be a more common phenomenon than is presently appreciated (Astorga et al., 2015; Chung, 2012).
Here we demonstrated that glucocorticoid receptor signaling within the VTA was necessary to increase ethanol self-administration after stress. Moreover, prolonged exposure to corticosterone in vitro (in midbrain slices) was sufficient to induce neuroadaptations associated with in vivo stress. These findings highlight the importance of glucocorticoid signaling within the VTA, but we do not rule out the participation of other stress signaling molecules or hormones, such as CRF, in mediating stress-induced adaptations (Hwa et al., 2016; Ungless et al., 2003). Furthermore, the effect of glucocorticoids on VTA GABA neurons may involve the activity of noradrenaline, glutamate, and glial cells (Coull et al., 2003; Hewitt et al., 2009; Lee et al., 2011; Taylor et al., 2015).
Although animal studies generally support the hypothesis that stress increases ethanol consumption, some results have shown that stress decreases intake or has no effect on it (Becker et al., 2011). These differences arise from a combination of factors, including the type of stressor used, the duration or timing of the stressor, as well as the type of drinking paradigm employed (Noori et al., 2014). An important parameter in our experimental design is that the stress exposure was well separated (15–20 hrs) from the ethanol self-administration, which allowed us to examine the lasting neural circuit consequences of the treatment not the proximal effect of stress itself. In our study, we kept the ethanol content of the drinking solutions relatively low during the acquisition phase, which likely resulted in less variability in self-administration. The rats experienced less of the aversive stimulus cues of ethanol while still achieving significant blood-ethanol levels. In addition, some animals show resilience to the effects of stress (Pfau and Russo, 2015), so it is essential to verify that there is a physiological response to the stressor and to exclude animals that do not show that response.
In summary, we showed that acute stress exposure decreases the sensitivity of the DA system to ethanol and increases subsequent ethanol self-administration. These effects required a shift towards excitatory GABAA signaling in the VTA and were associated with decreased chloride extrusion capacity in VTA GABA neurons. The temporally distant, acute, intense stress experience produced long-lasting neuroadaptations within the mesolimbic systems of the brain that were expressed upon exposure to ethanol. This overall process represents one mechanistic pathway linking life stress experiences to increased alcohol use. However, future work should determine if similar mechanisms also contribute to the effects of stress during chronic alcohol use and relapse.
EXPERIMENTAL PROCEDURES
Additional detail is provided in the Supplemental Experimental Procedures.
Subjects
Male Long-Evans rats (Harlan-Envigo) weighing 300–500 g were used for in vivo studies. All animals were handled at least 5 days prior to the onset of surgery/behavioral testing and were singly housed in a quiet, temperature- and humidity-controlled satellite facility under at 12-hour light/dark cycle. Rats had food and water available ad libitum in their home cage. All procedures were carried out in compliance with guidelines specified by the Institutional Animal Care and Use Committee at University of Pennsylvania.
Drugs and experimental design
Systemic or intra-VTA administration of RU486 occurred 15 min before stress (Saal et al., 2003), whereas intra-VTA microinfusions of acetazolamide or CLP290 occurred 15–30 min before microdialysis or ethanol self-administration.
Stressed animals were subjected to a 1-hour of immobilization in a clear cylindrical Broome-style restrainer. We required that the rats display somatic signs of stress, and the immobilization potently activated the stress hormone systems (Pitman et al., 1988). Restraint stress occurred 15–20 hours prior to ethanol exposure or testing.
Operant ethanol self-administration
Standard operant chambers (Med Associates Inc., St. Albans, VT, USA) were used for the self-administration experiments. Animals were initially water-restricted overnight and trained to lever press for a saccharin solution (0.125%, w/v). Once trained, the animals were no longer water restricted and their baseline saccharin intake was monitored for at least 3 days until intake appeared stable. If the animals underwent surgery, saccharin intake was re-established. The effects of stress on acquisition of ethanol self-administration were then measured. Ethanol was introduced into the saccharin drinking solution in the following way: 2% ethanol on day 1 and 4% ethanol usually for all subsequent days except for additional experiments with 7% and 10% ethanol (Figure S1A, B).
In vivo microdialysis
For in vivo microdialysis (Doyon et al., 2013), animals were habituated to tethering and the microdialysis chambers one day prior to testing. Baseline DA samples were collected (15–30 min), followed by a timed intravenous (i.v.) infusion of ethanol (1.5 g/kg, 20% in sterile saline, v/v) over 5 min. The i.v. route (using a cannula) was chosen to circumvent handling-related disturbances in DA levels associated with i.p. injections. Dialysis samples were analyzed for DA content using high-pressure liquid chromatography (HPLC) coupled to an electrochemical detector.
In vivo electrophysiology
Rats were anesthetized with isoflurane and implanted with a catheter in the jugular vein. Animals were positioned on a stereotaxic apparatus and burr holes were drilled to accommodate recording and ground electrodes. Rat body temperature was maintained throughout the experiment at 37 °C using an isothermal pad.
Glass electrodes backfilled with 0.5M Na+-acetate and 2% Chicago Sky Blue (5 −15 MΩ) were positioned in the lateral VTA (coordinates: 5.3–6.0 mm posterior from bregma, 0.8–1.4 lateral to midline and 7.5–8.5 ventral to brain surface). Electrical signals were filtered at 0.3- 5 kHz.
DA and GABA neurons were identified in vivo using established electrophysiological and pharmacological criteria (see Supplemental Experimental Procedures). After 6–20 min of stable baseline recording, we infused 0.3 g/kg of ethanol i.v. every 3 mins (for a final dose of 1.5 g/kg) and recorded single-unit activity. Drug-induced changes were calculated as a percent of baseline for each 3-min period. Following ethanol administration, quinpirole and eticlopride were infused (i.v., 0.25 mg/kg) to aid in the identification of VTA neurons. Chicago Sky Blue injections were used to identify the recording sites.
Ex vivo electrophysiology
Horizontal slices (230 µm) containing the VTA were cut (Leica Microsystems) from Long-Evans rats (21–30 days old). The firing rates of VTA DA and GABA neurons were recorded in the cell-attached configuration in passive voltage-follower mode. For synaptic stimulation recordings, a bipolar tungsten-stimulating electrode (World Precision Instruments, Inc) was placed 100–150 µm away from the recording electrode. After each cell-attached experiment, recordings were converted to the whole-cell configuration and Ih current was measured. Most cells were also backfilled with neurobiotin for immuno-identification.
Spontaneous and evoked inhibitory postsynaptic currents were recorded in voltage clamp mode in the whole-cell configuration. Synaptic GABAA inputs were isolated pharmacologically. The liquid junction potential between the bath and the pipette solutions was corrected. Gramicidin perforated-patch recordings were applied to maintain the anionic gradient during reversal potential measurements. Supplemental Experimental Procedures provide the details for the perforated-patch, the western blot, and the immunohistochemical procedures.
Statistical analysis
Analysis of variance (ANOVA) with repeated measures (in SPSS for Windows) was used to analyze the dialysate and the basal DA concentrations, and the DA and GABA neuron firing rates. For analysis of action potential firing, the raw data (in Hz) were converted into percent of basal, and the last three bins (2-min each) before bath application of ethanol were used as the baseline. For analysis of repetitive synaptic stimulation, the last ten bins (0.5-s each) before the stimulus were used as the baseline. Exponential fittings were compared by F-test. A two-tail t-test assuming equal variance was used to assess differences between the mean sIPSC frequency, the mean firing rates, as well as the mean ethanol intake levels. For western blot analysis, a paired t-test was used to compare protein levels from control and stress littermates that were run on the same gel. Significance for all analyses was determined by p < 0.05.
Supplementary Material
HIGHLIGHTS.
Stress increases alcohol self-administration
Stress attenuates alcohol-induced dopamine signals
Stress alters mesolimbic circuit inhibition, shifting GABA towards excitation
Stress hormones impair the Cl− extrusion capacity of the transporter KCC2
Acknowledgments
We thank Drs. Y. De Koninck and A. Castonguay from Laval University for the generous gift of CLP290 and technical assistance. We also thank Dr. Amber Alhadeff, Madison Taormina, Kevin Guan, and Natalie Neale for their help with experiments and Dr. David Connor for helpful discussions. This work was supported by grants from the National Institutes of Health NS21229, DA09411 (JAD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
AO designed and performed ex vivo and in vivo electrophysiological experiments assisted by BAK. AMT and WMD designed and performed in vivo experiments. JSK performed western blot and histology experiments. JAD originated, planned, and oversaw the experiments with AO’s, AMT’s, and WMD’s assistance. Led by JAD, all the authors contributed to write the manuscript.
CITATIONS
- Astorga G, Bao J, Marty A, Augustine GJ, Franconville R, Jalil A, Bradley J, Llano I. An excitatory GABA loop operating in vivo. Front Cell Neurosci. 2015;9:275. doi: 10.3389/fncel.2015.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer LA, Harder VS, Rose GL, Helzer JE. Drinking and stress: an examination of sex and stressor differences using IVR-based daily data. Drug Alcohol Depend. 2011;115:205–212. doi: 10.1016/j.drugalcdep.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci. 2013;7:173. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- Chung L. Recent progress in GABAergic excitation from mature brain. Arch Pharm Res. 2012;35:2035–2044. doi: 10.1007/s12272-012-1202-8. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci. 2014;8:8. doi: 10.3389/fnbeh.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol. 2007;7:93–99. doi: 10.1016/j.coph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Dong Y, Ostroumov A, Thomas AM, Zhang TA, Dani JA. Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron. 2013;79:530–540. doi: 10.1016/j.neuron.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Gagnon M, Bergeron MJ, Lavertu G, Castonguay A, Tripathy S, Bonin RP, Perez-Sanchez J, Boudreau D, Wang B, Dumas L, et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gulacsi A, Lee CR, Sik A, Viitanen T, Kaila K, Tepper JM, Freund TF. Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra. J Neurosci. 2003;23:8237–8246. doi: 10.1523/JNEUROSCI.23-23-08237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology (Berl) 2016;233:681–690. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Deeb TZ, Puskarjov M, Silayeva L, Liang B, Kaila K, Moss SJ. Modulation of neuronal activity by phosphorylation of the K-Cl cotransporter KCC2. Trends Neurosci. 2013;36:726–737. doi: 10.1016/j.tins.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Grant BF, Hasin DS. Stress and alcohol: epidemiologic evidence. Alcohol Res. 2012;34:391–400. doi: 10.35946/arcr.v34.4.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D. Opiate state controls bidirectional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci. 2004;7:160–169. doi: 10.1038/nn1182. [DOI] [PubMed] [Google Scholar]
- Lee HH, Deeb TZ, Walker JA, Davies PA, Moss SJ. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat Neurosci. 2011;14:736–743. doi: 10.1038/nn.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. Stress-induced plasticity of GABAergic inhibition. Front Cell Neurosci. 2014;8:157. doi: 10.3389/fncel.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addict Biol. 2014;19:225–232. doi: 10.1111/adb.12125. [DOI] [PubMed] [Google Scholar]
- Pfau ML, Russo SJ. Peripheral and Central Mechanisms of Stress Resilience. Neurobiol Stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Ramachandra V, Phuc S, Franco AC, Gonzales RA. Ethanol preference is inversely correlated with ethanol-induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1669–1676. doi: 10.1111/j.1530-0277.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sarkar J, Wakefield S, MacKenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31:18198–18210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends Neurosci. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Soldo BL, Proctor WR. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science. 1995;269:977–981. doi: 10.1126/science.7638623. [DOI] [PubMed] [Google Scholar]
- Tamers SL, Okechukwu C, Bohl AA, Gueguen A, Goldberg M, Zins M. The impact of stressful life events on excessive alcohol consumption in the French population: findings from the GAZEL cohort study. PLoS One. 2014;9:e87653. doi: 10.1371/journal.pone.0087653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Castonguay A, Ghogha A, Vayssiere P, Pradhan AA, Xue L, Mehrabani S, Wu J, Levitt P, Olmstead MC, et al. Neuroimmune Regulation of GABAergic Neurons Within the Ventral Tegmental Area During Withdrawal from Chronic Morphine. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology (Berl) 2011;218:19–28. doi: 10.1007/s00213-010-2163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organization Global Status Report on Alcohol and Health. 2014:2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.