Abstract
Decreased executive ability elicits racial bias. We clarified the neural correlates of how executive ability contributes to race perception by comparing young adults (YA) to a population with highly variable executive ability: older adults (OA). After replicating work showing higher race bias in OA vs YA and a negative association between bias and executive ability, a subsample of White YA and OA perceived Black and White faces and cars during functional magnetic resonance imaging. YA had higher executive ability than OA, and OA had higher variability in executive ability. When perceiving Black vs White faces, YA exhibited more dorsolateral prefrontal cortex recruitment—a region previously implicated in regulating prejudiced responses—than OA. Moreover, OA with relatively impaired executive ability had more amygdala activity toward Black faces vs OA with relatively intact executive ability, whereas responses to White faces did not differ. Both YA and OA with relatively intact executive ability had stronger amygdala-ventrolateral prefrontal cortex connectivity when perceiving Black vs White faces. These findings are the first to disentangle age from executive ability differences in neural recruitment when perceiving race, potentially informing past behavioral work on aging and race perception.
Keywords: aging, race perception, dorsolateral prefrontal cortex, amygdala, executive ability
Introduction
Executive ability negatively predicts racial bias (Payne, 2005), with research suggesting that increased racial bias evidenced in older vs younger adults (e.g. Gonsalkorale et al., 2009) emerges given their highly variable executive ability (Yilkoski et al., 1999). Racial bias negatively impacts social interactions (Brown, 2011). Although individuals differing in executive ability similarly activate racial stereotypes, those with lower executive ability regulate stereotypes less, eliciting more bias (Payne, 2005). Identifying how executive ability affects processing race information is critical for developing interventions mitigating racial bias.
Viewing Black vs White faces elicits amygdala and prefrontal engagement in White perceivers (for a review, see Amodio, 2014). While increased amygdala activity toward Black faces may reflect increased bias (Phelps et al., 2000), increased prefrontal activity may reflect controlled processing (e.g. Amodio, 2010). Evidencing this possibility, prefrontal activity facilitates racial stereotype regulation (Cunningham et al., 2004; Amodio et al., 2007; Amodio, 2010). Prefrontal regions [anterior cingulate, dorsolateral/ventrolateral prefrontal cortex (vlPFC)] affect unique aspects of cognitive control during race perception (Amodio, 2014), but individual differences in the extent of their engagement underlie behavior (Amodio et al., 2003; Richeson et al., 2003). To investigate how executive ability impacts the neural correlates of race perception, we examined a population with reduced executive ability: older adults (OA). Regulatory decline associated with aging (Mayr et al., 2001) predicts increased racial bias (Gonsalkorale et al., 2009; Stewart et al., 2009; von Hippel et al., 2000). How it affects the processing of race information with age remains unknown.
Regulatory decline with age may disrupt recognizing the need for control, initiating control or maintaining control (see Amodio, 2014). Neuroimaging can elucidate this mechanism because separable neural regions support distinct aspects of control (e.g. Berkman et al., 2012). For instance, anterior cingulate cortex (ACC) engages when signaling the need for control (Botvinick et al., 2001), including in tasks detecting biased attitudes (Stanley et al., 2008). vlPFC contributes to initiating control (Satpute and Lieberman, 2006), including the down-regulation of biased responses (Samson et al., 2005; Satpute and Lieberman, 2006). Dorsolateral prefrontal cortex (dlPFC) engages when maintaining control (Knutson et al., 2007) and controlling stereotypes (Cattaneo et al., 2011), even in tasks de-emphasizing race (e.g. Cunningham et al., 2004).
Comparing young adults (YA) with relatively preserved executive ability, to OAs—with widely variable executive ability (Yilkoski et al., 1999)—can clarify how aging and executive ability affect the neural correlates of race perception. If aging disrupts prefrontal responses toward Black vs White faces suggested to be associated with maintaining control, we would expect more dlPFC activity toward Black vs White faces in YA vs OA. This finding would be consistent with accounts of broadly disrupted dlPFC activity with age (e.g. Kwee and Nakada, 2003). If aging disrupts signaling for (e.g. Pardo et al., 2007) or initiating (e.g. Opitz et al., 2012) control, we would expect more ACC or vlPFC activation, respectively, toward Black vs White faces in YA vs OA. Neural changes could be more nuanced, however, instead reflecting executive ability vs age differences. Comparing groups of OA differing in executive ability can dissociate differences unique to age vs executive ability. Indeed, relatively intact vs impaired OA activate prefrontal regions more when evaluating stigmatized individuals (Krendl et al., 2009). If this extends to processing race, the above-described patterns should emerge for intact vs impaired OA.
To better understand age and executive ability effects on the neural correlates of race perception, we considered their influences on regions linked to different aspects of perceiving race (Amodio, 2014). Prefrontal activity attenuates amygdala engagement (Hariri et al., 2003), and responses toward stigmatized individuals (Krendl et al., 2012). Executive ability could thereby affect amygdala-prefrontal connectivity previously linked to regulating race-based responses (Forbes et al., 2012). If executive ability affects amygdala-prefrontal coupling, YA and relatively intact OA should have increased coupling vs impaired OA when perceiving Black vs White faces. If aging affects amygdala-prefrontal coupling, YA should have stronger coupling than OA. Changes due to executive ability may be further reflected in amygdala responses to race. Specifically, relatively impaired OA vs relatively intact OA and YA might have increased amygdala responses to Black faces, but similar responses to White. Elucidating these differences in the neural correlates of race perception can enhance our understanding of the mechanisms underlying increased racial bias with age (e.g. Gonsalkorale et al., 2009).
Experiment 1a
Aging and race research has revealed age differences in bias, suggesting that relative executive ability underscores this disparity. To connect the present work to this literature, we sought to conceptually replicate findings of more bias with age and a relationship between bias and executive ability (e.g. Gonsalkorale et al., 2009). These relationships widely inform aging-related work on stereotyping and prejudice (von Hippel et al., 2000) and social functioning more broadly (von Hippel, 2007). Because OA behavioral samples (e.g. N = 112 in Stewart et al., 2009) are typically larger than available neuroimaging samples (e.g. N = 17 in Moran et al., 2012), we verified these relationships in larger community-based samples before our neuroimaging investigation. While smaller samples may mask these relationships, larger-scale conceptual replications can verify these effects.
Materials and methods
We compared race Implicit Association Test (IAT) (Greenwald and Banaji, 1995; Greenwald et al., 2003) data from 83 White YA (Mage = 20.04, s.d. = 2.13, 49 female) and 86 OA (Mage = 70.44 years, s.d. = 6.72, 53 female) who completed various lab experiments (Table 1). Subjects provided written informed consent and the Indiana University IRB approved data collection. The IAT followed the seven-block protocol described in Greenwald et al. (2003). Participants viewed 12 images of male faces (six Black and six White) and 12 words (six pleasant and six unpleasant) and categorized them in stereotypically congruent (e.g. a pleasant word with a White face) or incongruent (e.g. a pleasant word with a Black face) ways. Congruent and incongruent blocks were pseudorandomly presented (e.g. some participants saw the congruent vs incongruent block first). After removing trials <200 ms and over 3000 ms, we calculated IAT D, which divides the reaction time difference between incongruent and congruent blocks by the standard deviation of latencies (Greenwald et al., 2003), as a measure of implicit bias.
Table 1.
Means (standard deviations) of demographic and questionnaire information
| YA | OA | t | P | |
|---|---|---|---|---|
| Experiment 1a | ||||
| Years of education | 13.33 (1.49) | 16.50 (3.10) | 3.18 | < 0.001 |
| IAT | 0.30 (.33) | 0.43 (.46) | 2.19 | 0.03 |
| N (female//total) | 49/83 | 53/86 | ||
| % self-reported political affiliations |
|
|
||
| Experiment 1b (subset of study 1a) | ||||
| Years of education | 14.66 (1.33) | 16.69 (3.18) | 2.46 | 0.02 |
| Shipley vocabulary | 32.50 (2.85) | 35.23 (6.70) | 1.56 | 0.13 |
| Executive ability | 0.60 (0.50) | 0.21 (0.60) | 2.33 | 0.02 |
| IAT | 0.48 (0.24) | 0.47 (0.40) | <1 | 0.92 |
| N (female/total) | 8/16 | 22/39 | ||
| % Self-reported political affiliations |
|
|
We next examined White YA (N = 25, Mage = 19.12 s.d.=0.97, 16 female) and OA (N = 27, Mage = 71.44, s.d.= 6.63, 16 female) performance on the Wisconsin Card Sorting Task (WCST). The WCST measures cognitive flexibility and validly assesses age differences in executive ability (Rhodes, 2004) and prefrontal function (Ridderinkhof et al., 2002). We correlated number of categories achieved, which approximates executive ability (Glisky et al., 1995), with IAT D.
Results and discussion
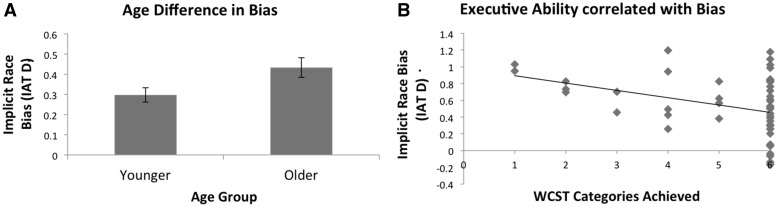
OA (M=0.43, s.d.=0.46) had more bias than YA (M=0.30, s.d.=0.33), t(167)=2.19, P=0.03, d=0.34 (Figure 1a). Bias and executive ability correlated in the entire sample, rs(50) = −0.32, P=0.02 (Figure 1b), and within OA alone, rs(25) = −0.65, P<0.001. Like prior work (Payne, 2005), individuals with less executive ability had more bias. These findings reflect previously shown age-bias and executive ability-bias relationships. Investigating how age and executive ability impact the neural correlates of race perception in a subset of these individuals may further inform these findings.
Fig. 1.
Providing critical conceptual replications of past behavioral work, Experiment 1a showed that OA expressed more implicit race bias than young (A), while bias correlated with executive ability (B). Error bars represent standard errors.
Experiment 1b
Methods
Participants
Sixteen YA (Mage = 21.31 years, s.d. = 1.78, age range = 19–26, 8 female) and 39 OA (Mage = 69.51 years, s.d. = 5.64, age range = 60–82, 22 female) completed the fMRI study. Aside from one OA (whose IAT data were lost due to computer error), these participants were a subset of those analyzed for Experiment 1a. The same IAT scores, collected on the scanning date, were used in both experiments. Experiment 1b participants underwent fMRI on the basis of their willingness and ability to undergo fMRI. Participants were right-handed, did not have conditions potentially impacting cognitive function or brain activity, and provided written informed consent. The Indiana University IRB approved this study.
Participants completed the race IAT, Shipley vocabulary (Shipley, 1986), and a well-validated executive ability battery (Glisky et al., 1995) (Table 1) consisting of five subtests: WCST, FAS word fluency, mental arithmetic (Wechsler Adult Intelligence Scale—Revised), and mental control and backward digit span (Wechsler Memory Scale—Revised). Executive ability was scored using established conventions (see Glisky et al., 1995 for details). We were unable to obtain vocabulary data from four OA. We assessed composite executive ability because a single task could bias fMRI findings toward deficits in regions whose activity maps onto a specific task vs broader differences. OA had Mini-Mental State Examination scores >26 (M = 28.74, s.d. = 1.07) (Folstein et al., 1975).
Stimuli and task
Participants viewed 20 grayscale Black upright faces, Black inverted faces, White upright faces, White inverted faces, black upright cars, black inverted cars, white upright cars and white inverted cars. Because inverted stimuli were of interest for a separate study, they will not be discussed in depth. However, because these images conveyed race information, they were included in our first-level design matrix. This allowed us to verify that inverted faces did not affect activity in our regions of interest (i.e. amygdala and prefrontal cortex). If inversion did not impact activity, then we could collapse across upright and inverted faces to look at overall age differences in race perception. There were no main effects of or interactions with inversion in lateral PFC or amygdala in our analyses of age and executive ability [see analysis of variance (ANOVA) models below], suggesting inversion did not influence our areas of interest. All analyses collapsed across upright and inverted faces.1
In an event-related design, images were presented pseudorandomly one time each, with no more than two of the same image type sequentially. All eight conditions were represented in all runs. Half of the images appeared on the right side of the display, and half on the left. It was equally probable that images from all conditions would appear on either side. Participants indicated on which side pictures appeared via button press (e.g. Cunningham et al., 2004). Periods of jitter, in the form of a fixation cross at the display’s center, ranged from zero to three timepoints. A random number generator determined the order of jitter. Participant responses were monitored to ensure attention during the task.
Data acquisition and analysis
Whole-brain imaging was performed on a Siemens 3.0T TIM Trio MRI scanner at the Indiana University Imaging Research Facility. Anatomical images were acquired with a high-resolution 3-D magnetization prepared rapid gradient echo sequence (192 slices, TE = 2.67 ms, TR = 2000 ms, flip angle = 9°, 1 × 1 × 1 mm voxels). Functional images were collected over three runs of 121 timepoints each, using a fast field echo-planar sequence sensitive to blood oxygen level dependent contrast (T2*; 40 slices obtained in an interleaved even order with 3.3 mm thickness and 0 mm skip, TR = 2500 ms, TE = 30 ms, flip angle = 70°).
Preprocessing and analyses of functional data were conducted in SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were slice-time corrected, realigned to correct for motion, normalized to the MNI (Montreal Neurological Institute) template, and smoothed using an 8-mm full-width-half-max Gaussian kernel. Data were resampled to 3 mm-isotropic voxels in a 96 × 96 matrix. A general linear model incorporating the image types (Black/White and upright/inverted faces and cars) and covariates of no interest (a session mean, a linear trend and six movement parameters derived from realignment corrections) computed parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel. Events were modeled as a boxcar with durations of one trial (2.5 s). Relevant parameter estimates were included in a group level analysis, treating participants as a random effect.
Given our hypotheses regarding prefrontal cortex, we constrained our analyses by an anatomically defined mask (bilateral frontal lobe, which comprised prefrontal cortex extending to motor cortex) from the WFU Pickatlas (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003, 2004). A 2 (Age Group: Young, Old) × 2 (Race: Black, White) × 2 (Inversion: Upright, Inverted) ANOVA model assessed age effects on prefrontal response to race. More specifically, we examined Age Group × Race interactions to identify prefrontal regions where younger and OA neural responses differed with respect to race. We then created t-contrasts to decompose emergent interactions [(Young > Old for Black > White) and (Old > Young for Black > White)]. For instance, (Young > Old for Black > White) revealed prefrontal activations greater for YA than OA when viewing Black vs White faces. Given our interest in the amygdala, we also extracted parameter estimates from anatomically defined amygdala regions and entered these values into two 2 (Age Group: Young, Old) × 2 (Race: Black, White) ANOVAs. See Supplementary Materials for an exploratory whole-brain analysis between YA and OA that primarily revealed Age × Race interactions in prefrontal cortex.
To examine potential differences in prefrontal activity due to executive ability differences, we created a 2 (Executive Ability: Relatively Intact Old, Relatively Impaired Old) × 2 (Race: Black, White) × 2 (Inversion: Upright, Inverted) ANOVA. We created t-contrasts [(Impaired > Intact for Black > White; note that this contrast eliminated potential age effects); (Intact > Impaired for Black > White); (Impaired > Young for Black > White)] to decompose potential interactions between executive ability and race perception. Given our a priori interest in the amygdala, we also extracted parameter estimates from the anatomically defined amygdala regions and entered these values into two 2 (Executive Ability: Relatively Intact Old, Relatively Impaired Old) × 2 (Race: Black, White) ANOVAs.
We categorized OA as relatively impaired or intact by a median split of executive ability (Table 2), which is a common approach in aging and executive ability research (Fjell et al., 2006; Krendl et al., 2009; West et al., 2010; Krendl and Wolford, 2013). Given the little neuroimaging work on aging and processing race, this approach allows us to initially identify broad group differences in neural function, and is useful because the smaller sample sizes of OA available for neuroimaging may prohibit the detection of nuanced differences. Relatively impaired OA had lower executive ability than relatively intact OA, t(37)=6.88, P<0.001. YA and relatively intact OA had similar executive ability, t < 1, P = 0.72. Relatively impaired and intact OA did not differ in years of education, t(37) = 1.64, P = 0.11, suggesting education level would not confound executive ability findings.
Table 2.
Means (standard deviations) of executive ability and education for YA, relatively intact OA, and relatively impaired OA in Experiment 1b
| YA (N = 16) | Relatively intact OA (N = 19) | Relatively impaired OA (N = 20) | |
|---|---|---|---|
| Executive ability | 0.60 (0.50) | 0.66 (0.45) | −0.22 (0.35) |
| Years of education | 14.66 (1.33) | 17.00 (2.49) | 15.7 (2.45) |
We characterized activations emerging from prefrontal regions by extracting parameter estimates of each condition relative to baseline averaged across a 6 mm-sphere centered on peaks identified from t-contrasts decomposing interactions in neural activity. We entered parameter estimates into ANOVAs to characterize interactions and to plot them, where relevant. To examine whether our effects were race-specific, we characterized the same activations with the parameter estimates for black and white cars. Peak coordinates in prefrontal cortex were identified by an extent threshold of 13 contiguous voxels (re-sampled) exceeding a voxel-wise threshold of P<0.005. One-thousand Monte Carlo simulations indicated this provided a corrected experiment-wise threshold of P<0.05 (for details, see Slotnick et al., 2003). Brodmann areas were obtained with MRIcron (Rorden and Brett, 2000).
Psychophysiological interactions
Psychophysiological interaction (PPI) analyses performed using gPPI (McLaren et al., 2012), examined functional connectivity from a left amygdala seed to prefrontal cortex using the frontal lobe mask described above. The deconvolved time series from a 6 mm radius sphere around the seed was extracted from each participant. First-level images were entered into two-sample t-tests to identify age and executive ability differences in regions coupled with the amygdala when perceiving Black vs White faces. We selected the amygdala seed because it was the only emergent activation from our amygdala analyses sensitive to age or executive ability (see below). The center coordinate was identified from contrasting Impaired > Intact OA when perceiving Black vs White faces using a threshold consistent with related work [P<0.01 and k = 5; (Rule et al., 2013)].
Results
Differences in bias
Unlike Experiment 1a, which critically had larger samples, YA (M = 0.48, s.d. = 0.24) and OA (M = 0.47, s.d. = 0.40) did not differ in bias (IAT D), P = 0.92. Bias among relatively intact (M = 0.41, s.d. = 0.32) and impaired (M = 0.52, s.d. = 0.46) OA was consistent with the expected pattern.
Identifying age effects on the neural correlates of race perception
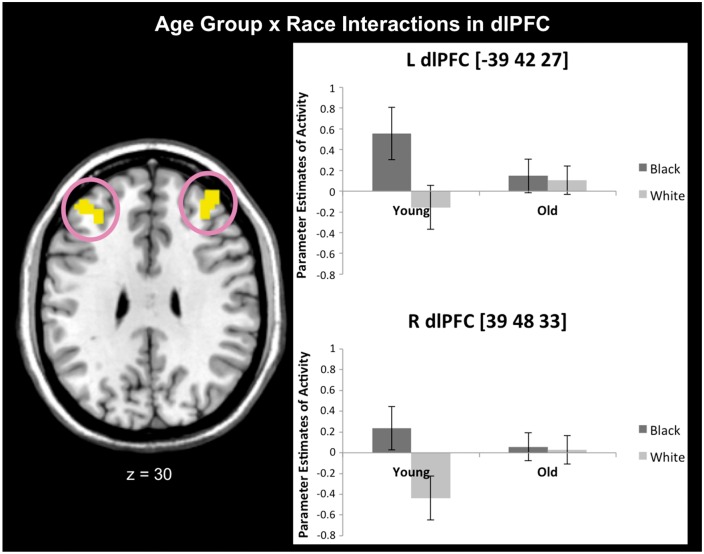
Interactions between Age Group and Race emerged in bilateral dlPFC (Table 3). T-tests showed greater dlPFC activation toward Black vs White faces in YA vs OA (Table 3 and Figure 2). Parameter estimates for characterization purposes showed YA activated dlPFC for Black over White faces, (tright(15) = 3.22, P = 0.01; tleft(15) = 2.77, P = 0.01), no dissociations in OA, (Ps>0.76) and confirmed the interactions (Fright(1, 53) = 8.19, P = 0.01, ηp2 = 0.13; Fleft(1, 53) = 6.09, P = 0.02, ηp2 = 0.10). To verify that these findings were due to age vs executive ability, we contrasted YA with relatively intact OA (these groups differed in age, but not executive ability) when perceiving Black vs White faces. This analysis revealed similar prefrontal activations as when examining all OA (see Supplementary Materials). No Age Group × Color interactions emerged in these dlPFC activations when parameter estimates from cars were examined, Ps > 0.20. In addition to being age-specific, the dlPFC findings were race-specific. No Age Group × Race interactions emerged in the amygdala when examining extracted parameter estimates, Ps > 0.54.
Table 3.
Age Group × Race interactions from the GLM analysis (Experiment 1b)
| Age Group × Race | ||||
| Region | BA | Cluster size (k) | F | MNI coordinates |
| L dorsolateral prefrontal cortex | 46 | 26 | 12.22 | 39, 48, 33 |
| R dorsolateral prefrontal cortex | 46 | 17 | 9.82 | −39, 42, 27 |
| Age Group × Race: Young > Old for Black > White | ||||
| Region | BA | Cluster size (k) | t | MNI coordinates |
| L middle cingulate gyrus | 24/31 | 15 | 3.52 | −9, −24, 45 |
| R dorsolateral prefrontal cortex | 46 | 41 | 3.50 | 39, 48, 33 |
| R dorsolateral prefrontal cortex | 46 | 3.24 | 36, 42, 27 | |
| L dorsolateral prefrontal cortex | 46 | 52 | 3.13 | −39, 42, 27 |
| Age Group × Race: Old > Young for Black > White | ||||
| No significant regions | ||||
Note: Regions listed without a cluster size are subsumed by the cluster above.
Fig. 2.
Age × Race interactions emerged in bilateral dlPFC activity in Experiment 1b. Replicating prior work, YA had greater activation toward Black vs White faces. However, no dissociations emerged for OA. Error bars represent standard errors.
Identifying executive ability effects on the neural correlates of race perception
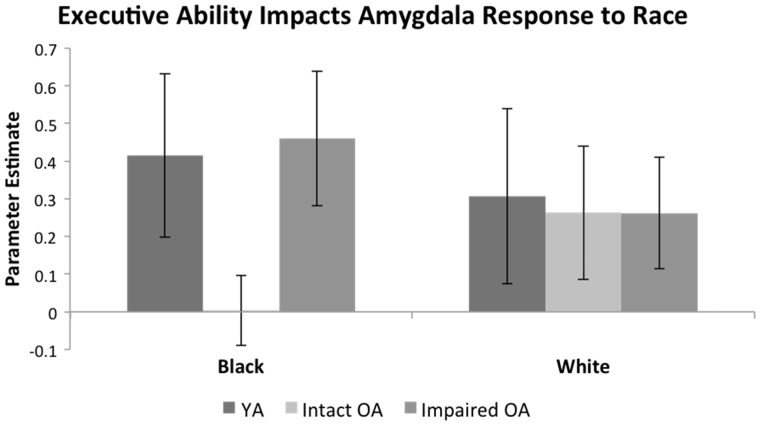
Left amygdala parameter estimates elicited an Executive Ability × Race interaction, F(1, 37)=4.78, P=0.04, ηp2=0.11 (Figure 3). Relatively impaired OA had greater activity toward Black faces vs relatively intact OA, t(37)=2.24, P=0.03, with no difference for White faces, P=0.99. This left amygdala activity pattern was race-specific. In left amygdala parameter estimates from relatively intact and impaired OA for black and white cars, no Executive Ability × Color interaction emerged, P=0.20.
Fig. 3.
Executive ability impacted amygdala activity among OA in Experiment 1b. OA with relatively intact executive ability had decreased amygdala activation toward Black faces relative to relatively impaired OA. Interestingly, YA did not differ from relatively impaired OA in response to Black faces. Responses to White faces were similar among YA and OA regardless of executive ability. Error bars represent standard errors.
To clarify if this effect was specific to OA with high executive ability vs higher executive ability more broadly, we conducted a second 2 (Executive Ability: YA, Relatively intact OA) × 2 (Race: Black, White) ANOVA on parameter estimates extracted from the left amygdala. No interaction emerged, P=0.16, although YA vs relatively intact OA appeared to have greater activation to Black faces. No interaction emerged for YA vs relatively impaired OA, P = 0.70. No Executive Ability × Race interactions emerged in right amygdala or prefrontal cortex.
Identifying impacts of age and executive ability on amygdala-prefrontal connectivity
Relatively intact and relatively impaired OA differed in left amygdala response, but not PFC recruitment, toward Black vs White faces. To examine why this might be, we identified prefrontal regions functionally coupled with a left amygdala seed when perceiving Black vs White faces. Because left amygdala activation was sensitive to race based on OA executive ability, we characterized a seed region by contrasting relatively impaired to intact OA left amygdala activity toward Black vs White faces. This yielded an activation (k = 9, t = 2.75, P=0.003) around coordinates −24, −3, −15. We used a 6 mm radius spherical seed around these coordinates in our PPI analyses.
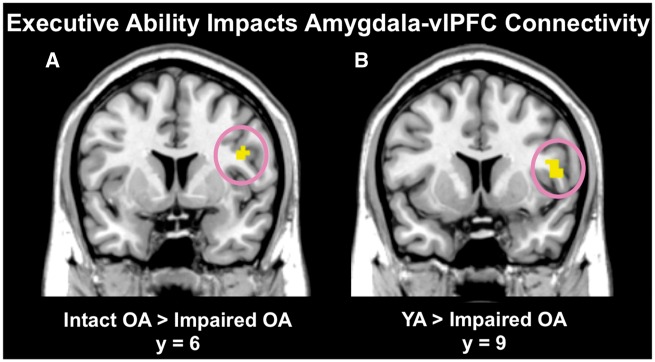
We examined executive ability effects on amygdala-prefrontal connectivity during race perception by comparing relatively intact vs impaired OA in a between-groups t-test. Relatively intact OA had stronger connectivity between left amygdala and a specific peak in vlPFC (BA 44; k = 12, t = 3.41, P=0.001, peak MNI coordinates: 45, 6, 24) than relatively impaired OA when perceiving Black vs White faces (Figure 4a). Comparing connectivity between relatively impaired vs intact OA revealed a left superior frontal gyrus peak (BA 8; k = 15, t = 3.19, P=0.001, peak MNI coordinates: −21, 30, 54).
Fig. 4.
Executive ability impacted the extent of amygdala-vlPFC connectivity when perceiving Black vs White faces in Experiment 1b. OA with relatively intact executive ability (A) and YA (B) had stronger functional connectivity between amygdala and vlPFC relative to relatively impaired OA when perceiving Black vs White faces. Error bars represent standard errors.
To verify that less executive ability corresponded with reduced amygdala-vlPFC connectivity when perceiving race, we compared YA to relatively impaired and intact OA. YA had stronger amygdala-vlPFC connectivity than relatively impaired OA (BA 44/48; k = 38, t = 3.83, P<0.001, peak MNI coordinates: 51, 9, 9) (Figure 4b) when perceiving Black vs White faces. No regions emerged in the reverse contrast (relatively impaired OA > YA), and when comparing YA to relatively intact OA. No overall age differences in amygdala-prefrontal connectivity emerged.
Discussion
This work clarified how age and executive ability affect the neural correlates of race perception by comparing YA to OA, a population with highly variable executive ability. We reveal age effects in dlPFC activation toward Black vs White faces, consistent with an account of aging disrupting the ability to maintain controlled responses to race. Further, OA with relatively intact executive ability had lower amygdala responses to race facilitated by increased connectivity between amygdala and vlPFC, a region linked to initiating control, when perceiving Black vs White faces.
Age differences in dlPFC activity may reflect broadly found cohort differences (e.g. Kwee and Nakada, 2003). That both groups of OA had similar dlPFC engagement toward White and Black faces may reflect increased difficulty maintaining the suppression of racial stereotypes (Stanley et al., 2008; Kubota et al., 2012), which potentially contributes to the relationship between bias and executive ability in Experiment 1a. Indeed, increased dlPFC activation corresponds with suppressing stereotypic attitudes (Knutson et al., 2007). This interpretation was bolstered by the Age × Race interaction from our whole-brain ANOVA primarily revealing frontal activations (see Supplementary Materials). Although the present task did not connect biased behavior to prefrontal activity, this could be due to several factors. For instance, bias is associated with prefrontal activity on tasks where participants explicitly evaluate race (e.g. Lieberman et al., 2005), which was not the case in the current study. Further, the OA cohort in the imaging study were relatively high functioning, perhaps concealing a mediating effect of executive ability on age-related dlPFC engagement and expressed bias. The neural differences revealed here may potentially preclude larger behavioral differences evident with ongoing regulatory decline. Future research should assess this possibility using behavioral and imaging methods.
Our finding is consistent with work demonstrating that OA show reduced dlPFC activity in tasks requiring the maintenance of cognitive control (e.g. Esposito et al., 1999; Rypma et al., 2001; Kwee and Nakada, 2003), but extends this prior work to include activity related to race perception. Indeed, that dlPFC activity often correlates with anti-outgroup bias suggests its role in maintaining control over biased attitudes (Richeson et al., 2003; Knutson et al., 2007). Guided by the present findings and past research (e.g. Amodio et al., 2008), future work should assess how age differences in dlPFC activity during race perception impact different aspects of behavior in response to race.
Differences in OA executive ability contribute to bias (e.g. Gonsalkorale et al., 2009). Our data identify a potential mechanism for this effect. Specifically, OA with relatively impaired vs intact executive ability had heightened amygdala response to Black faces, but similar responses toward White faces. Black faces trigger arousing negative associations (Stephan et al., 2002) and affect (Hendricks and Bootzin, 1976), as well as threat-related amygdala activation (Chekroud et al., 2014). More amygdala response to Black faces in relatively impaired vs intact OA is consistent with work showing increased bias given decreased executive ability with age (e.g. von Hippel et al., 2000; Radvansky et al., 2010), as well as work showing that OA with higher regulatory ability attend less to amygdala-responsive negative information to optimize positive affect and mood (Mather and Carstensen, 2005). Speculatively, less amygdala activity toward Black faces in relatively intact OA may reflect work suggesting less amygdala reactivity in OA vs YA toward negative images is a way to improve affective experiences (Mather et al., 2004). Similar activation toward White faces could, speculatively, reflect comparable salience of these faces regardless of executive ability.
Moreover, although OA with relatively intact vs impaired executive ability did not differ in their prefrontal activation toward Black vs White faces, they had increased amygdala-vlPFC connectivity. People use control to regulate responses to emotionally evocative stimuli that activates the amygdala (Hariri et al., 2003; Ochsner and Gross, 2005). Declines in the functional connectivity between the amygdala and prefrontal cortex, and not prefrontal activation alone, may be associated with increased amygdala response to Black vs White faces.
That executive ability impacted amygdala-prefrontal connectivity may inform behavioral work connecting bias to executive ability (e.g. Experiment 1a, Payne, 2005) and work showing executive ability to specifically impact OA bias (e.g. Gonsalkorale et al., 2009). More executive ability during race perception may facilitate enhanced communication from the amygdala [which signals negative associations with Black faces as compared with White; (Chekroud et al., 2014)] to vlPFC [which signals the initiation of control, and the regulation of race-related responses (Cunningham et al., 2003, 2004; Lieberman et al., 2005)]. Indeed, increased amygdala response to Black vs White faces has been previously linked to increased bias in YA (Phelps et al., 2000). Although relatively intact and impaired OA had less dlPFC activity to race relative to YA, intact OA may potentially compensate for this deficit through increased amygdala-vlPFC activity. Indeed, amygdala-vlPFC coupling plays a dynamic role in reducing negative affect (Banks et al., 2007) and regulating affective responses during race perception (Lieberman et al., 2005). Reduced amygdala-vlPFC functional coupling may be associated with increased amygdala response to Black vs White faces in relatively impaired OA. Future research can be designed to examine the nature of this potential relationship.
Unexpectedly, YA amygdala response to race did not differ from relatively impaired OA. Prior work has similarly shown that relatively intact OA exhibit increased prefrontal activity relative to relatively impaired OA and YA when perceiving stigmatized individuals (Krendl et al., 2009). While relatively intact OA may display similar behavior to YA, the neural mechanisms that give rise to these behaviors may differ (Cabeza et al., 2002; Park and Reuter-Lorenz, 2009). Intact OA may simply use a different strategy than YA or impaired OA when perceiving race. Speculatively, executive ability may help OA subvert emotional responses by, for instance, attending less to negative information to optimize positive affect (Mather and Carstensen, 2005). In contrast, given their broad focus on knowledge acquisition (Carstensen et al., 1999), YA may suppress responses once they occur. Lending behavioral support to this possibility, OA with higher executive ability, but not YA, show more gaze following to faces with positive vs negative subtle emotional cues (Petrican et al., 2012). Future research should more directly disentangle this possibility, as the present task was designed to assess if vs why activation differences occur.
The present study used YA in comparison to OA to elucidate roles of age and executive ability on race perception. A limitation of this approach is that these individuals may differ on factors other than their executive ability. However, the intact and impaired OA were matched on years of education and were from the same geographic location, and no participants reported illnesses potentially affecting cognitive function (e.g. stroke, diabetes, recent heart attacks). By controlling for factors potentially related to bias (e.g. concerns for impression management, life experience, attitudes), research (e.g. von Hippel et al., 2000; Gonsalkorale et al., 2009) has shown that OA express more bias than YA because of declining executive ability vs holding stronger associations. Moreover, relationships between bias regulation and executive ability have been demonstrated in YA by manipulating divided attention (Krendl, 2016). Comparing OA to YA is thus appropriate to disentangle effects of age and executive ability on race perception.
Although Experiment 1a demonstrated higher bias in OA vs YA, replicating numerous similar findings (e.g. von Hippel et al., 2000; Gonsalkorale et al., 2009), this relationship was not observed in Experiment 1b. Examining means across samples revealed higher bias in the YA in Experiment 1b vs 1a, t(81) = 2.52, P = 0.01. One possible explanation for this finding is that individual differences within YA potentially contributed to this disparity. For instance, individual differences in empathy (Pettigrew and Tropp, 2008) and personality dimensions such as openness (Sibley and Duckitt, 2008) predict prejudice. An examination of an independent sample of YA with comparable implicit bias (M = 0.48, s.d. = 0.33) as the YA in Experiment 1b indeed showed similar relationships.2 Although we did not collect these measures from the YA in Studies 1a and 1b, these additional data suggest that the YA in 1b could have differed from the 1a sample in similar ways, ultimately affecting their bias. These differences may simply reflect natural variation within YA, and be further complicated by factors such as intergroup contact that impact both empathy and prejudice (Pettigrew and Tropp, 2008), rather than a systematic difference based on whether individuals volunteer for fMRI.
Further, it is worth noting that although YA in Experiment 1a were about evenly split among self-reported conservative and liberal political affiliations, a larger proportion of the YA in Experiment 1b had self-reported conservative (vs liberal) political affiliations (Table 1). That people with more conservative political affiliations (e.g. Republican vs Democrat) express less favorable attitudes toward stigmatized groups (for a review, see Jost et al., 2009) could, speculatively, contribute to why the smaller sample of YA in Experiment 1b did not differ from a OA in bias. Natural variation based on these and other individual differences may also affect OA behavior and neural response beyond effects of aging and executive function. Future neuroimaging work using larger and more variable samples of YA and OA may therefore aim to disentangle how these individual differences may affect prejudice, and whether they differ across age groups.
However, levels of bias obtained for relatively intact and impaired OA occurred in the expected pattern. OA participating in neuroimaging studies are a self-selected sample and often highly educated and active. This may make differences in bias difficult to detect in the smaller samples of OA volunteering for neuroimaging. However, the present study suggests that neural change in response to race due to age and executive ability may occur before behavioral change (i.e. prejudiced behavior) manifests. Speculatively, significant neural change may mark the origins of increased prejudice due to age and executive ability. It will be important for future research to consider this possibility, perhaps through longitudinal work to actively track patterns of neural and behavioral change.
By comparing YA to a population with relatively compromised executive ability, we broaden our understanding of how differences in age and executive ability uniquely impact the neural correlates of race perception. Given the shifting demographic landscape of the USA, these data inform our understanding of how OA specifically perceive race relative to YA. Executive ability appears to influence amygdala responses to other- vs same-race individuals, and also impacts amygdala-prefrontal connectivity during race perception. By identifying age-related processing differences in perceiving race, we may begin to develop new strategies aimed at mitigating bias with age.
Supplementary data
Supplementary data are available at SCAN online.
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number F32AG051304 to B.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest. None declared.
Supplementary Material
Footnotes
Although neural data did not evidence inversion effects in the regions of interest, inversion may nevertheless affect bias-related behavior. Future work should directly address this idea in behavioral paradigms.
IAT D negatively correlated with empathic concern (Interpersonal Reactivity Index; Davis, 1980), r(28)=-0.47, P=0.01, and openness (Big Five; Goldberg, 1999), r(28)=-0.39, P=0.04, in a separate sample of 30 YA.
References
- Amodio D. (2010). Coordinated roles of motivation and perception in the regulation of intergroup responses: frontal cortical asymmetry effects on the P2 event-related potential and behavior. Journal of Cognitive Neuroscience, 22(11), 2609–17. [DOI] [PubMed] [Google Scholar]
- Amodio D. (2014). The neuroscience of prejudice and stereotyping. Nature Reviews Neuroscience, 15(10), 670–82. [DOI] [PubMed] [Google Scholar]
- Amodio D., Devine P., Harmon-Jones E. (2007). A dynamic model of guilt: implications for motivation and self-regulation in the context of prejudice. Psychological Science, 18, 524–30. [DOI] [PubMed] [Google Scholar]
- Amodio D., Devine P., Harmon-Jones E. (2008). Individual differences in the regulation of intergroup bias: the role of conflict monitoring and neural signals for control. Journal of Personality and Social Psychology, 94(1), 60–74. [DOI] [PubMed] [Google Scholar]
- Amodio D., Harmon-Jones E., Devine P. (2003). Individual differences in the activation and control of affective race bias as assessed by startle eyeblink response and self-report. Journal of Personality and Social Psychology, 84(4), 738–53. [DOI] [PubMed] [Google Scholar]
- Banks S., Eddy K., Angstadt M., Nathan P., Phan K. (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E., Falk E., Lieberman M. (2012). Interactive effects of three core goal pursuit processes on brain control systems: goal maintenance, performance monitoring, and response inhibition. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Braver T., Barch D., Carter C., Cohen J. (2001). Conflict monitoring and cognitive control. Psychological Review, 108(3), 624–52. [DOI] [PubMed] [Google Scholar]
- Brown R. (2011). Prejudice: Its Social Psychology: Oxford, England: John Wiley & Sons. [Google Scholar]
- Cabeza R., Anderson A., Locantore J., McIntosh A. (2002). Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage, 17(3), 1394–402. [DOI] [PubMed] [Google Scholar]
- Carstensen L., Isaacowitz D., Charles S. (1999). Taking time seriously: a theory of socioemotional selectivity. American Psychologist, 54(3), 165–81. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z., Mattavelli G., Platania E., Papagno C. (2011). The role of the prefrontal cortex in controlling gender-stereotypical associations: a TMS investigation. NeuroImage, 56(3), 1839–46. [DOI] [PubMed] [Google Scholar]
- Chekroud A., Everett J., Bridge H., Hewstone M. (2014). A review of neuroimaging studies of race-related prejudice: does amygdala response reflect threat? Frontiers in Human Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W., Johnson M., Gatenby J., Gore J., Banaji M. (2003). Neural components of social evaluation. Journal of Personality and Social Psychology, 85(4), 639–49. [DOI] [PubMed] [Google Scholar]
- Cunningham W., Johnson M., Raye C., Gatenby J., Gore J., Banaji M. (2004). Separable neural components in the processing of black and white faces. Psychological Science, 15(12), 806–13. [DOI] [PubMed] [Google Scholar]
- Davis M. (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10, 85. [Google Scholar]
- Esposito G., Kirkby B., Van Horn J., Ellmore T., Berman K. (1999). Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain, 122, 963–79. [DOI] [PubMed] [Google Scholar]
- Fjell A., Walhovd K., Reinvang I., et al. (2006). Selective increase of coritical thickness in high-performing elderly–structural indices of optimal cognitive aging. NeuroImage, 29, 984–94. [DOI] [PubMed] [Google Scholar]
- Folstein M., Folstein S., McHugh P. (1975). Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Jounral of Psychiatric Research, 12(3), 189–98. [DOI] [PubMed] [Google Scholar]
- Forbes C., Cox C., Schmader T., Ryan L. (2012). Negative stereotype activataion alters interaction between neural correlates of arousal, inhibition, and cognitive control. Social Cognitive and Affective Neuroscience, 7, 771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky E., Polster M., Routhieaux B. (1995). Double dissociation between item and source memory. Neuropsychology, 9(2), 229–35. [Google Scholar]
- Goldberg L. (1999). A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models In: Mervielde I., Deary F., De Fruyt F., Ostendorf F., editors. Personality Psychology in Europe, Vol. 7, pp. 7–28. Tilburg, Netherlands: Tilburg University Press. [Google Scholar]
- Gonsalkorale K., Sherman J., Klauer K. (2009). Aging and prejudice: diminished regulation of automatic race bias among older adults. Journal of Experimental Social Psychology, 45, 410–4. [Google Scholar]
- Greenwald A., Banaji M. (1995). Implicit social cognition: attitudes, self-esteem, and stereotypes. Journal of Personality and Social Psychology, 102, 4–27. [DOI] [PubMed] [Google Scholar]
- Greenwald A., Nosek B., Banaji M. (2003). Understanding and using the Implicit Association Test: an improved scoring algorithm. Journal of Personality and Social Psychology, 85, 197–216. [DOI] [PubMed] [Google Scholar]
- Hariri A., Mattay V., Tessitore A., Fera F., Weinberger D. (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53(6), 494–501. [DOI] [PubMed] [Google Scholar]
- Hendricks M., Bootzin R. (1976). Race and sex as stimuli for negative affect and physical avoidance. The Journal of Social Psychology, 98(1), 111–20. [Google Scholar]
- Jost J., Federico C., Napier J. (2009). Political ideology: its structure, function, and elective affinities. Annual Review of Psychology, 60, 307–37. [DOI] [PubMed] [Google Scholar]
- Knutson K., Mah L., Manly C., Grafman J. (2007). Neural correlates of automatic beliefs about gender and race. Human Brain Mapping, 28, 915–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl A. (2016). Reduced cognitive capacity impairs older adults' ability to explicitly regulate negative bias to stigmatized individuals. Experimental Aging Research. [DOI] [PubMed] [Google Scholar]
- Krendl A., Heatherton T., Kensinger E. (2009). Aging minds and twisting attitudes: an fMRI investigation of age differences in inhibiting prejudice. Psychology and Aging, 24(3), 530–41. [DOI] [PubMed] [Google Scholar]
- Krendl A., Kensinger E., Ambady N. (2012). How does the brain regulate negative bias to stigma? Social Cognitive and Affective Neuroscience, 7(6), 715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl A., Wolford G. (2013). Cognitive decline and older adults' perception of stigma controllability. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 68(3), 333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota J., Banaji M., Phelps E. (2012). The neuroscience of race. Nature Neuroscience, 15(7), 940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwee I., Nakada T. (2003). Dorsolateral prefrontal lobe activation declines significantly with age: functional NIRS study. Journal of Neurology, 250(5), 525–9. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Hariri A., Jarcho J., Eisenberger N., Bookheimer S. (2005). An fMRI investigation of race-related amygdala activity in African-American and Caucasian-American individuals. Nature Neuroscience, 8, 720–2. [DOI] [PubMed] [Google Scholar]
- Maldjian J., Laurienti P., Burdette J. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage, 21(1), 450–5. [DOI] [PubMed] [Google Scholar]
- Maldjian J., Laurienti P., Burdette J., Kraft R. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Mather M., Canli T., English T., et al. (2004). Amygdala responses to emotionall valenced stimuli in older and younger adults. Psychological Science, 15(4), 259–63. [DOI] [PubMed] [Google Scholar]
- Mather M., Carstensen L. (2005). Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences, 9(10), 496–502. [DOI] [PubMed] [Google Scholar]
- Mayr U., Spieler D., Kliegl R. (2001). Aging and executive control: introduction to this special issue. European Journal of Cognitive Psychology, 13, 1–4. [Google Scholar]
- McLaren D., Ries M., Xu G., Johnson S. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61(4), 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran J., Jolly E., Mitchell J. (2012). Social-cognitive deficits in normal aging. The Journal of Neuroscience, 32(16), 5553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K., Gross J. (2005). The cognitive control of emotion. Trends in Cognitive Neuroscience, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Opitz P., Rauch L., Terry D., Urry H. (2012). Prefrontal mediation of age differences in cognitive reappraisal. Neurobiology of Aging, 33(4), 645–55. [DOI] [PubMed] [Google Scholar]
- Pardo J., Lee J., Sheikh S., et al. (2007). Where the brain grows old, Decline in anterior cingulate and medial prefrontal function with normal aging. NeuroImage, 35(3), 1231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., Reuter-Lorenz P. (2009). The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology, 60, 173–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B. (2005). Conceptualizing control in social cognition: how executive functioning modulates the expression of automatic sterotyping. Journal of Personality and Social Psychology, 89(4), 488–503. [DOI] [PubMed] [Google Scholar]
- Petrican R., English T., Gross J., Grady C., Hai T., Moscovitch M. (2012). Friend or foe? Age moderates time-course specific responsiveness to trustworthiness cues. The Journal of Gerontology Series B: Psychological Sciences and Social Sciences, 68(2), 215–23. [DOI] [PubMed] [Google Scholar]
- Pettigrew T., Tropp L. (2008). How does intergroup contact reduce prejudice? Meta-analytic tests of three mediators. European Journal of Social Psychology, 38(6), 922–34. [Google Scholar]
- Phelps E., O'Connor D., Cunningham W., et al. (2000). Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience, 12(5), 729–38. [DOI] [PubMed] [Google Scholar]
- Radvansky G., Copeland D., von Hippel W. (2010). Stereotype activation, inhibition, and aging. Journal of Experimental Social Psychology, 46, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes M. (2004). Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychology and Aging, 19(3), 482–94. [DOI] [PubMed] [Google Scholar]
- Richeson J., Baird A., Gordon H., et al. (2003). An fMRI investigation of the impact of interracial contact on executive function. Nature Neuroscience, 6(12), 1323–8. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K., Span M., van der Molen M. (2002). Perseverative behavior and adaptive control in older adults: performance monitoring, rule induction, and set shifting. Brain and Cognition, 49(3), 382–401. [DOI] [PubMed] [Google Scholar]
- Rorden C., Brett M. (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. [DOI] [PubMed] [Google Scholar]
- Rule N., Krendl A., Ivcevic Z., Ambady N. (2013). Accuracy and consensus in judgments of trustworthiness from faces: behavioral and neural correlates. Journal of Personality and Social Psychology, 104(3), 409–26. [DOI] [PubMed] [Google Scholar]
- Rypma B., Prabhakaran V., Desmond J., Gabrieli J. (2001). Age differences in prefrontal cortical activity in working memory. Psychology and Aging, 16(3), 371–84. [DOI] [PubMed] [Google Scholar]
- Samson D., Apperly I., Kathirgamanathan U., Humphreys G. (2005). Seeing it my way: a case of a selective deficit in inhibiting self-perspective. Brain, 128(5), 1102–11. [DOI] [PubMed] [Google Scholar]
- Satpute A., Lieberman M. (2006). Integrating automatic and controlled processing into neurocognitive models of social cognition. Brain Research, 1079, 86–97. [DOI] [PubMed] [Google Scholar]
- Shipley W. (1986). Shipley Institute of Living Scale. Los Angeles: Western Psychological Services. [Google Scholar]
- Sibley C., Duckitt J. (2008). Personality and prejudice: a meta-analysis and theoretical review. Personality and Social Psychology Review, 12(3), 248–79. [DOI] [PubMed] [Google Scholar]
- Slotnick S., Moo L., Segal J., Hart J. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17, 75–82. [DOI] [PubMed] [Google Scholar]
- Stanley D., Phelps E., Banaji M. (2008). The neural basis of implicit attitudes. Current Directions in Psychological Science, 17(2), 164–70. [Google Scholar]
- Stephan W., Boniecki K., Ybarra O., et al. (2002). The role of threats in the racial attitudes of Blacks and Whites. Personality and Social Psychology Bulletin, 28(9), 1242–54. [Google Scholar]
- Stewart B., von Hippel W., Radvansky G. (2009). Age, race, and implicit prejudice: using process dissociation to separate the underlying components. Psychological Science, 20(2), 164–8. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- von Hippel W. (2007). Aging, executive functioning, and social control. Current Directions in Psychological Science, 16(5), 240–4. [Google Scholar]
- von Hippel W., Silver L., Lynch M. (2000). Stereotyping against your will: the role of inibitory ability in stereotyping and prejudice among the elderly. Personality and Social Psychology Bulletin, 26, 523–32. [Google Scholar]
- West R., Schwarb H., Johnson M. (2010). The influence of age and individual differences in executive function on stimulus processing in the oddball task. Cortex, 46(4), 550–63. [DOI] [PubMed] [Google Scholar]
- Yilkoski R., Yilkoski A., Keskivaara P., Tilvis R., Sulkava R., Erkinjuntti T. (1999). Heterogeneity of cognitive profiles in aging: successful aging, normal aging, and individuals at risk for cognitive decline. European Journal of Neurology, 6(6), 645–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.