Abstract
Rumination is associated with increased default-mode network (DMN) activity and functional connectivity (FC) in depressed and healthy individuals. In this study, we sought to examine the relationship between self-reported rumination and resting-state FC in the DMN and cognitive control networks in 25 remitted depressed patients and 25 matched healthy controls using independent component and seed-based analyses. We also explored potential group differences in the global pattern of resting-state FC. Healthy subjects with increased levels of rumination exhibited increased anterior DMN connectivity with the posterior DMN and the dorsal attention network and low connectivity within the anterior DMN. On the other hand, remitted depressed ruminators patients were associated with the opposite FC pattern in these regions. Based on global FC patterns, a support vector machine algorithm correctly classified 92% of the subjects into their respective group by a leave-one-out cross-validation. Whole-brain FC analysis also revealed a group by rumination interaction effect within the DMN. The present findings highlight the different functional roles of the anterior and the posterior DMN, and provide novel insights into the underlying neural mechanisms leading to depression relapse.
Keywords: rumination, default-mode network, depression, functional connectivity
Introduction
Rumination involves a persistent, passive focus on negative self-relevant information, is implicated in the development, maintenance and worsening of depressive symptoms (Nolen-Hoeksema et al., 2008) and confers increased risk for relapse in remitted major depressive disorder (MDD) individuals (Michalak et al., 2011).
Rumination has been strongly linked to the default-mode network (DMN), a network encompassing medial prefrontal cortex (PFC), ventral anterior cingulate cortex (ACC), precuneus, posterior cingulate cortex (PCC) and bilateral angular gyri (Buckner et al., 2008). Rumination-induction has been associated with increased DMN functional connectivity (FC) and hyperactivity in MDD patients (Lemogne et al., 2009; Cooney et al., 2010; Kessler et al., 2011; Berman et al., 2014) and healthy subjects (Kross et al., 2009; Freton et al., 2014). In addition, resting-state studies have shown a positive correlation between self-reported rumination and DMN FC in depressed patients (Zhu et al., 2012) or in both healthy and depressed individuals (Berman et al., 2011).
A large body of evidence advocates that the DMN is decomposed into functionally specialized subsystems (Andrews-Hanna et al., 2010). The anterior DMN component (i.e. medial PFC and ventral ACC) has been implicated in identifying stimuli as self-salient (Gusnard et al., 2001; Schmitz and Johnson, 2007), whereas the posterior DMN component (i.e. precuneus/PCC and bilateral angular gyrus) together with the parahippocampal gyrus are involved in autobiographical search and memory retrieval (Northoff, 2007; Sestieri et al., 2011). Depression-related impairments have been more frequently reported in the anterior DMN component (Whitfield-Gabrieli and Ford, 2012) suggesting that maladaptive rumination in depressed patients is more likely to implicate this DMN component (Hamilton et al., 2011; Zhu et al., 2012).
The switch from internally focused mental activities, such as rumination, to externally focused goal-directed tasks is accompanied by deactivation of the DMN and recruitment of cognitive control networks such as the dorsal attention network (DAN), frontoparietal (FPN) and salient network (SN) (Fornito et al., 2012; Cocchi et al., 2013; Liang et al., 2015). The antagonistic relationship between DMN and cognitive control networks may influence the ability to exert cognitive control over ruminative thought and may determine the type of rumination (Marchetti et al., 2012; Nejad et al., 2013). In line with this view, studies in depression reported deficient recruitment of cognitive control networks during inhibition of ruminative thoughts (Carew et al., 2013) and a relationship between increased trait rumination and hypo-connectivity between subgenual ACC and lateral PFC at rest (Connolly et al., 2013). Taken together, these findings suggest that functional interactions between DMN and cognitive control networks may play an important role in rumination.
Given that rumination is an important cognitive vulnerability factor for depression (Nolen-Hoeksema et al., 2008) and that post-treatment levels of rumination can predict depression relapse (Michalak et al., 2011), it appears critical to investigate the neural underpinnings of rumination in MDD patients during remission.
Substantial evidence has shown alterations in resting-state DMN FC in depression (Zhu et al., 2012; Kaiser et al., 2015) as well as increased rumination and potentially different content of ruminative thoughts in MDD patients compared to healthy subjects (Treynor et al., 2003; Nolen-Hoeksema et al., 2008). In light of this evidence, we hypothesized that self-reported trait rumination may differentially predict the pattern of resting-state FC within the DMN and between the DMN and cognitive control networks for remitted MDD patients and healthy controls.
To test these hypotheses, we examined the relationship between self-reported trait rumination and resting-state FC within the DMN and between the DMN and cognitive control networks. We opted for an independent component analysis (ICA) approach with prior information about the spatial maps of the networks of interest (constrained ICA) (Lin et al., 2010) in order to independently assess FC among the anterior and posterior DMN. In addition, we compared the ICA findings with the results of conventional seed-based analyses to extend the interpretability of our findings.
A limitation of the aforementioned approaches is that they provide no information about how brain areas outside the networks of interest are connected with each other. Recent evidence has shown substantial alterations in whole-brain resting-state FC in acutely depressed patients that may be related to rumination (Berman et al., 2014) and may constitute potential biomarkers for clinical diagnosis (Zeng et al., 2012). We thus investigated whether global FC patterns can reliably distinguish remitted depressed patients from healthy subjects and sought to identify the functional connections in the whole brain that are more affected in the patients and are related to rumination.
Materials and methods
Participants
Twenty-seven remitted patients with MDD and 27 healthy controls matched for age, and gender participated in the study. Two patients and two control subjects had to be excluded from the analysis due to excessive movement artifacts, leaving 25 remitted MDD patients and 25 healthy controls for data analyses (Table 1).
Table 1.
Demographic and clinical characteristics of participants
| Characteristics | HC n = 25 | MDD n = 25 | Statistics | P-value |
|---|---|---|---|---|
| Gender ratio (female/male) | 17/8 | 18/7 | Chi2(1) = 0.095 | P = 0.758 |
| Mean movement per subject (mm) | 0.0044 | 0.0052 | t(48) = −0.77 | P = 0.445 |
| Age. Mean (SD) | 42.52 (11.18) | 42.82 (11.18) | t(48) = 0.097 | P = 0.923 |
| Rumination CERQ. Mean (SD) | 8.12 (2.27) | 10.04 (3.24) | t(48) = 2.420 | P = 0.019 |
| Current symptoms | ||||
| HAM-D. Mean (SD) | 0.04 (.20) | 0.88 (1.33) | t(48) = 4.203 | P = 0.000 |
| BDI. Mean (SD) | 1.12 (1.58) | 6.68 (6.42) | t(48) = 3.116 | P = 0.003 |
| Clinical characteristics | ||||
| No. of depressive episodes. Mean (SD) | – | 3.40 (2.02) | – | – |
| Illness duration, years. Mean (SD) | – | 12.40 (8.42) | – | – |
| Age at illness onset, years. Mean (SD) | – | 29.00 (11.57) | – | – |
| Time in remission, years. Mean (SD) | – | 6.04 (17.57) | – | – |
| Medication [subjects] | ||||
| Antidepressants | – | 7 | – | – |
| Mood stabilizers | – | 6 | – | – |
| Antipsychotics | – | 3 | – | – |
| None | 25 | 13 | – | – |
| Medication load. mean (SD) # | 0 | 1.64 (2.10) | – | – |
HAM-D, Hamilton Depression Rating Scale; BDI, Beck Depression Inventory.
Patients were recruited at the Central Institute of Mental Health in Mannheim, Germany, through local psychotherapists, and patient support groups. Diagnoses of MDD and potential comorbid mental disorders were assessed with the Structured Clinical Interview for DSM-IV, SCID-I and -II (First et al., 1995), and were conducted by trained psychologists. None of the patients currently fulfilled the criteria for any other mental disorder. Healthy participants were free of any past or present mental disorder. Exclusion criteria for all participants were, age <18 or >65, neurological disorder or head trauma with unconsciousness, current and lifetime alcohol or substance dependence, and common MRI exclusion criteria.
Remission from a depressive episode was defined as a score <6 on the Hamilton Depression Rating Scale (Hamilton, 1960) for at least 8 weeks. The medication status (i.e. type and dose) of all patients had been stable during the past 6 months. Antidepressant medication load was calculated according to Sackeim (2001) and was included as a covariate in all analyses. Variables describing the clinical course of the disease such as the number of past depressive episodes, the age at illness onset, disease duration and the time in remission were acquired for every patient (Table 1) and were also included as covariates in an exploratory regression analysis.
The ethics committee of the University Heidelberg approved the study and all participants provided written informed according to the Declaration of Helsinki.
Questionnaires
Rumination was assessed using the rumination subscale of the German version of the Cognitive Emotion Regulation Questionnaire (CERQ) (Garnefski and Kraaij, 2007). Residual mood symptoms were assessed by the Beck Depression Inventory (BDI) (Beck et al., 1974) and by the Hamilton Depression Rating Scale (Hamilton, 1960).
Image acquisition
Data were acquired on a 3-T whole body scanner (Magnetom Trio, Siemens Medical Solutions, Erlangen, Germany). During resting-state, we acquired 40 gradient-echo T2*-weighted slices (slice thickness = 2.3 mm) per volume with the following parameters: TR = 2.7 s, flip angle = 100°, TE = 27 ms, field of view = 220 × 220 mm2, matrix = 96 × 96, voxel size = 2.3 mm × 2.3 mm × 2.3 mm and a slice gap of 0.7 mm. Participants were instructed to lie still with their eyes closed and not to fall asleep for 5 min. 120 whole-brain volumes were acquired.
Image preprocessing
All imaging preprocessing steps were conducted using statistical parametric mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Functional images were slice-time corrected, and realigned. The images were then spatially normalized to a standard template (Montreal Neurological Institute, Montreal, QC, Canada), resampled to 2.3 mm cubic voxels, and spatially smoothed by convolution with an isotropic Gaussian kernel (full-width at half-maximum = 6.9 mm).
Group ICA
ICA was carried out using the Group ICA of fMRI Toolbox (GIFT, http://mialab.mrn.org/software/gift/). We used constrained ICA incorporating prior information about spatial patterns into standard blind ICA (Lin et al., 2010; Goulden et al., 2014). The main advantages of the constrained ICA algorithm is the automatic extraction of the networks of interest which overcomes the problem of estimating the optimal number of components and identifying neural networks based on the spatial pattern of each independent component (IC). The latter is especially important for this study in which anterior and posterior components of the DMN are extracted as separate ICs. Templates of the networks of interest, namely the anterior DMN, posterior DMN, FPN, DAN and SN, were derived from Allen et al.’s (2011) study which was based on a large number of healthy individuals (n = 603). Constrained ICA automatically extracted the five networks of interest. Group-level ICs (both spatial maps and time courses) of these networks were then back-reconstructed for each subject using the GICA method (Erhardt et al., 2011). The time course of each IC represents the average pattern of coherent brain activity in the network and the intensity of this pattern of brain activity across the voxels is expressed in the associated spatial map. Visual inspection of the spatial maps of the ICs revealed great spatial overlap between ICs and the corresponding templates. Examination of the spectral characteristics of the five ICs confirmed that the time courses are dominated by frequency fluctuations inside the 0.01–0.1 Hz window (Cordes et al., 2001).
To explore the effect of segmenting the DMN into two components, we repeated the constrained ICA, this time representing the DMN (i.e. anterior and posterior DMN) as one component.
Within-network connectivity analysis
After collapsing the two groups, one-sample t-tests were carried out on the subject-specific z-maps of each network of interest. To restrict the analysis only within the core nodes of each network, binary masks of the statistical maps obtained from the one-sample t-tests were created by applying a family-wise error (FWE)-corrected threshold at P < 0.001.
Subsequently, subject-specific mean z scores expressing the intensity of the average pattern of coherent brain activity were extracted within each network. The extracted values represent mean connectivity within a network and were imported into SPSS v22.0 for further statistical analysis. The same procedure was followed for the second ICA where the DMN (i.e. anterior and posterior component) was represented as one component.
Between-networks connectivity analysis
The subject-specific time course of each IC was band-pass filtered with cut-off frequencies of 0.01–0.1 Hz (Cordes et al., 2001) and was subjected to functional network connectivity (FNC) analysis (http://mialab.mrn.org/software). FNC analysis estimates the Pearson’s correlation coefficient between pairs of IC time courses with a maximal lagged correlation approach (i.e. −3 to +3 s lag) (Jafri et al., 2008). Pairwise correlations were computed between the time courses of the two DMN components as well as between the DMN components and FPN, DAN and SN for each subject and were imported into SPSS v22.0 for further statistical analysis. The same procedure was followed for the second ICA this time replacing the two DMN components with the time course of the entire DMN.
Statistical analysis
Hierarchical multiple linear regression analysis was conducted to investigate whether within-network and between-networks FC patterns can be predicted by group (i.e. MDD or healthy controls), levels of trait rumination, or an interaction of these two factors (i.e. group by rumination interaction effect). A dummy variable coding for group membership was entered in the first step of the regression analysis, rumination was entered in the second step, and a group by rumination interaction term was used as the last predictor. The interaction term was created by multiplying the group variable with the centered scores of rumination to avoid possible problems with multicollinearity. As dependent variables, we used the output of the within-network and the between-networks connectivity analysis which included two within-network variables (i.e. anterior and posterior DMN) and seven between-networks variables (i.e. all the possible pairs between the two DMN components and cognitive control networks and the anterior-posterior DMN pair). In total, nine multiple regression tests were conducted, one for each dependent variable. Statistical significance was adjusted for multiple testing by applying Bonferroni–Holm correction. Regression diagnostics were performed to test for collinearity, normality, outliers and leverage.
To test for the specificity of the observed effects, the same procedure was repeated one more time forcing age, gender and mean movement per subject in the first block of predictors. In addition, we explored whether variables that correlate with rumination such as BDI, CERQ self-blame, CERQ other-blame and CERQ catastrophizing could explain the rumination effects.
Within each group, post hoc Pearson’s correlation tests between rumination and FC measures were performed to identify the direction of interaction effects. Within the patient group, partial correlations were performed to explore whether significant correlations between rumination and FC measures are confounded by clinical variables such as medication load, the number of past depressive episodes, the age at illness onset, disease duration and the time in remission.
The regression analysis was repeated using as input the FC variables derived from the second constrained ICA that considered DMN as one component.
Seed-based analysis in the DMN
Functional connectivity analysis, using a seed-based approach, was conducted with the ‘conn’ toolbox v13.1 (Whitfield-Gabrieli and Nieto-Castanon, 2012). This method allows for interpretation of anti-correlations as there is no regression of the global signal. In addition, the confounding effect of physiological and other spurious sources of noises from white matter and cerebral spinal fluid and the movement-related parameters (six dimensions with their first-order derivative) were removed and temporal filtering was applied.
A sphere of 10 mm radius in the medial PFC was defined as the seed region around peak coordinates selected from the literature (Fox et al., 2005; Whitfield-Gabrieli et al., 2009). Pearson’s correlation coefficients between the BOLD time course from the seed region-of-interest (ROI) and each voxel in the brain were converted to normally distributed z-scores using Fisher’s transform. Similar to the ICA analysis, we tested for a group effect, a main rumination effect and a group by rumination interaction effect. Significant effects were reported if they survived a stringent cluster-extent threshold at FWE-corrected P < 0.05 and voxel-wise threshold at uncorrected P < 0.001. Post hoc tests examining the relationship between rumination and FC in each group separately were restricted to voxels that displayed a significant group by rumination interaction effect.
Whole-brain functional connectivity analysis
A seed-based approach was also employed to explore global resting-state FC patterns. The preprocessed images were segmented into 116 regions according to the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002) using the software WFU_PickAtlas (version 2.0, http://www.ansir.wfubmc.edu) (Maldjian et al., 2003). In the present analysis, we extracted the average fMRI time series from the 90 regions that correspond to cerebral regions. Pearson’s correlation coefficient was used to assess FC between each pair of regions creating a 90 × 90 symmetric matrix. The lower triangle and the diagonal elements of this matrix were removed leaving (90 × 89)/2 = 4005 elements as the feature space for group classification.
Identification of features with high discriminative power
We used the Kendall tau rank coefficient to assess the relevance of each feature to group classification (Zeng et al., 2012). This correlation coefficient is based on the comparison of each feature between all the possible pairs of subjects among the two groups. A positive tau coefficient indicates that the FC of a certain pair of regions increases in the patient group compared to the control group. A negative coefficient indicates that this feature is decreased in the patient group. We subsequently selected those features with coefficients over a threshold as the final feature set for group classification.
Support vector classification, performance evaluation and permutation tests
Support vector machines with linear kernel function were employed to solve the group classification problem using only the features with high discriminative power. A leave-one-out cross validation procedure was used to estimate the generalization rate, the sensitivity and the specificity of our classifier. The sensitivity represents the proportion of patients correctly predicted, while the specificity represents the proportion of controls correctly predicted. The overall proportion of samples correctly predicted is evaluated by the generalization rate.
In order to assess the performance of our classifier, permutations tests were employed to measure the statistical significance of the observed generalization rate (Golland and Fischl, 2003). The class labels of the training data were randomly permuted prior to training (10 000 permutations) and cross-validation was performed on the permuted training set. We hypothesized that the classification trained on the real class labels was reliable when the generalization rate GR0 exceeded the 95% of the generalization rates produced by the randomly relabeled data.
Relationship between whole-brain functional connectivity and rumination
Similar to the ICA and seed-based analysis, we tested for a group effect, a main rumination effect and a group by rumination interaction effect on the whole-brain functional connectivity patterns. Significant effects were reported if they survived a stringent seed-ROI connections threshold at FDR-corrected P < 0.05 and a correction for the number of seeds at uncorrected P < 0.05.
Results
Descriptive statistics
The demographic and clinical characteristics of the two groups of participants are shown in Table 1. Age and gender did not differ significantly between groups but remitted MDD patients exhibited higher levels of rumination compared to healthy controls (Table 1).
Predictors of within- and between-network FC
The dummy variable coding for group membership did not significantly predict any of the dependent variables. Self-reported levels of rumination did not significantly predict within-network or between-networks FC. In the third regression step, a group by rumination interaction effect significantly predicted connectivity within the anterior DMN (Table 2), anterior–posterior DMN FC (Table 3), and anterior DMN–DAN FC (Table 4). Post hoc Pearson’s correlation tests (Figure 1) revealed that increased rumination in healthy individuals were associated with low connectivity within the anterior DMN (r = −0.430, P = 0.032), increased FC between anterior and posterior DMN (r = 0.423, P = 0.035) and a trend level increase in anterior DMN–DAN connectivity (r = 0.366, P = 0.072). On the other hand, increased trait rumination in remitted MDD patients corresponds to a pattern of strong connectivity within the anterior DMN (r = 0.439, P = 0.028), low FC between anterior and posterior DMN (r = −0.460, P = 0.021) and decreased FC between anterior DMN-DAN (r = −0.491, P = 0.013). Interestingly, the removal of the two remitted MDD patients that reported very high rumination levels did not alter the significance of the group by rumination interaction effects on the connectivity within the anterior DMN (b = −0.022, t(44) = −2.92, P = 0.005), between the anterior–posterior DMN (b = 0.053, t(44) = 3.34, P = 0.002) and between the anterior DMN–DAN (b = 0.045, t(44) = 3.73, P = 0.001).
Table 2.
Stepwise hierarchical regression analysis predicting FC within the anterior DMN (n = 50)
| Coefficients | Model summary | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | t | P-value | ΔR2 | ΔF | Δp | Total R2 (adj) | F | P-value | |
| Step 1 | |||||||||
| Group | 0.27 | 1.94 | 0.058 | 0.073 | 3.77 | 0.058 | 0.054 | 3.77 | 0.058 |
| Step 2 | |||||||||
| Group | 0.31 | 2.08 | 0.043 | ||||||
| CERQ Rumination | 0.12 | 0.78 | 0.440 | 0.012 | 0.60 | 0.440 | 0.046 | 2.17 | 0.125 |
| Step 3 | |||||||||
| Group | 0.26 | 1.88 | 0.066 | ||||||
| CERQ Rumination | −0.04 | −0.27 | 0.784 | ||||||
| Group by rumination interaction term | −0.43 | −3.16 | 0.003 | 0.164 | 10.02 | 0.003 | 0.199 | 5.07 | 0.004 |
Table 3.
Stepwise hierarchical regression analysis predicting FC between the anterior DMN and the posterior DMN (n = 50)
| Coefficients | Model summary | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | t | P-value | ΔR2 | ΔF | Δp | Total R2 (adj) | F | P-value | |
| Step 1 | |||||||||
| Group | −0.06 | −0.44 | 0.663 | 0.004 | 0.19 | 0.663 | −0.017 | 0.19 | 0.663 |
| Step 2 | |||||||||
| Group | −0.11 | −0.71 | 0.480 | ||||||
| CERQ rumination | −0.13 | −0.90 | 0.371 | 0.017 | 0.81 | 0.371 | −0.021 | 0.50 | 0.608 |
| Step 3 | |||||||||
| Group | −0.06 | −0.39 | 0.696 | ||||||
| CERQ rumination | 0.02 | 0.16 | 0.870 | ||||||
| Group by rumination interaction term | 0.45 | 3.22 | 0.002 | 0.180 | 10.36 | 0.002 | 0.149 | 3.86 | 0.015 |
Table 4.
Stepwise hierarchical regression analysis predicting FC between the anterior DMN and DAN (n = 50).
| Coefficients | Model summary | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | t | P-value | ΔR2 | ΔF | Δp | Total R2 (adj) | F | P-value | |
| Step 1 | |||||||||
| Group | 0.16 | 1.13 | 0.262 | 0.026 | 1.28 | 0.262 | 0.026 | 1.29 | 0.262 |
| Step 2 | |||||||||
| Group | 0.19 | 1.24 | 0.220 | ||||||
| CERQ Rumination | −0.08 | −0.54 | 0.586 | 0.006 | 0.3 | 0.586 | 0.032 | 0.78 | 0.462 |
| Step 3 | |||||||||
| Group | 0.13 | 0.97 | 0.333 | ||||||
| CERQ Rumination | 0.07 | 0.48 | 0.634 | ||||||
| Group by rumination interaction term | 0.43 | 3.06 | 0.004 | 0.163 | 9.34 | 0.004 | 0.143 | 3.73 | 0.018 |
Fig. 1.
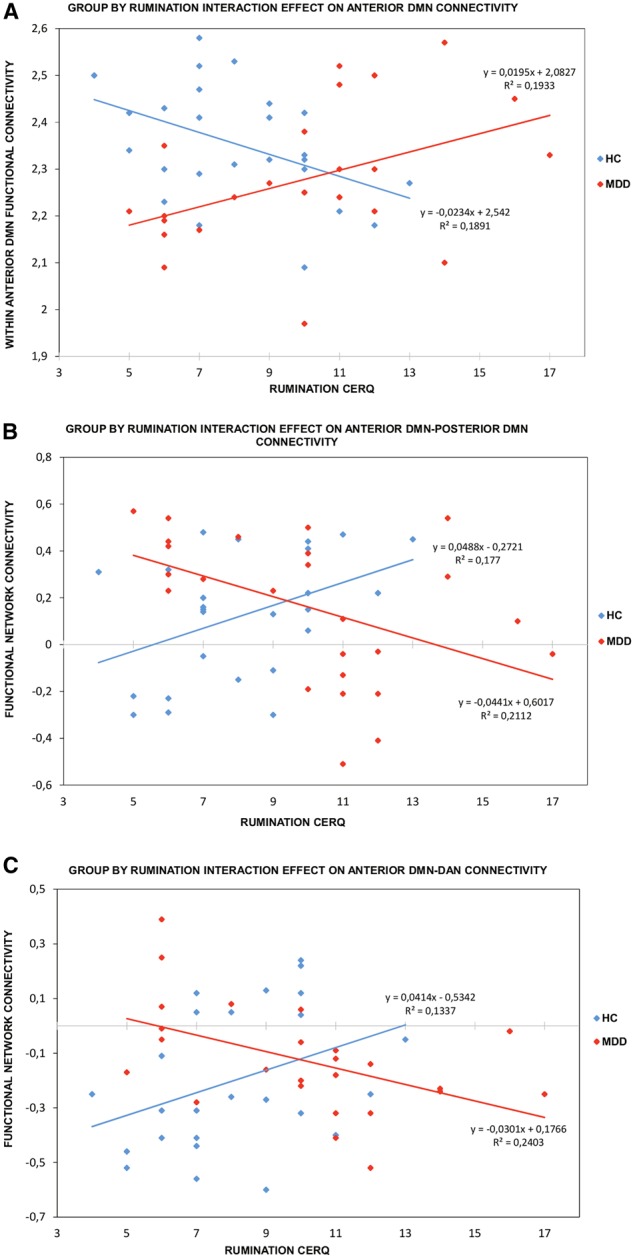
Correlations between self-reported rumination, assessed by CERQ, with FC within the anterior DMN (A), with anterior DMN–posterior DMN FNC (B), or with anterior DMN–DAN FNC (C) separately for the healthy control group (blue) and MDD group (red).
Controlling for variables such as age, gender and mean movement per subject in the first step of the regression model did not alter the group by rumination interaction effects. Furthermore, variables that correlate with rumination such as BDI score, CERQ self-blame, CERQ other-blame and CERQ catastrophizing did not alter the interaction effects. Age and variables that correlate with rumination were also included in the regression model to test for group by measure interaction effects. No group by measure interaction effect was significant highlighting the specificity of the rumination effect. In the patient group, partial correlations using clinical variables and medication load as covariates did not alter the significant correlations between rumination and FC pattern.
The same procedure as described above was carried out for the second constrained ICA (i.e. one component for both the anterior and posterior DMN) and revealed no group effect, rumination effect, or group by rumination interaction effect on resting-state FC.
Seed-based analysis in the DMN
Similar to the ICA, the seed-based analysis demonstrated a group by rumination interaction effect on connectivity between medial PFC and two clusters in the posterior DMN, namely the precuneus/PCC (peak at x = −8 y = −60 z = 40; 236 voxels) and the right angular gyrus (peak at x = 50 y = −60 z = 38; k = 315 voxels) (see Supplementary Figure 1). Post hoc tests revealed a positive correlation between rumination and medial PFC-precuneus/PCC FC (Z(46) = 4.26, P < 0.05 FWE corrected; 48 voxels) and medial PFC-right angular gyrus FC (Z(46) = 3.73, P < 0.05 FWE corrected; 83 voxels) in healthy controls. In remitted MDD patients, self-reported rumination correlated negatively with medial PFC-precuneus/PCC connectivity (Z(46) = 3.59, P < 0.05 FWE corrected; 110 voxels) and medial PFC-right angular gyrus FC (Z(46) = 3.12, P < 0.05 FWE corrected; 18 voxels).
Whole-brain functional connectivity
Using the 400 most discriminating functional connections, the linear support vector machine classifier achieved a generalization rate of 92%, sensitivity of 96% and specificity of 88%. The permutation distribution of the generalization rate indicated that the classifier learned the relationship between the data and the labels with a probability of being wrong of less than 0.0001 (Figure 2A).
Fig. 2.
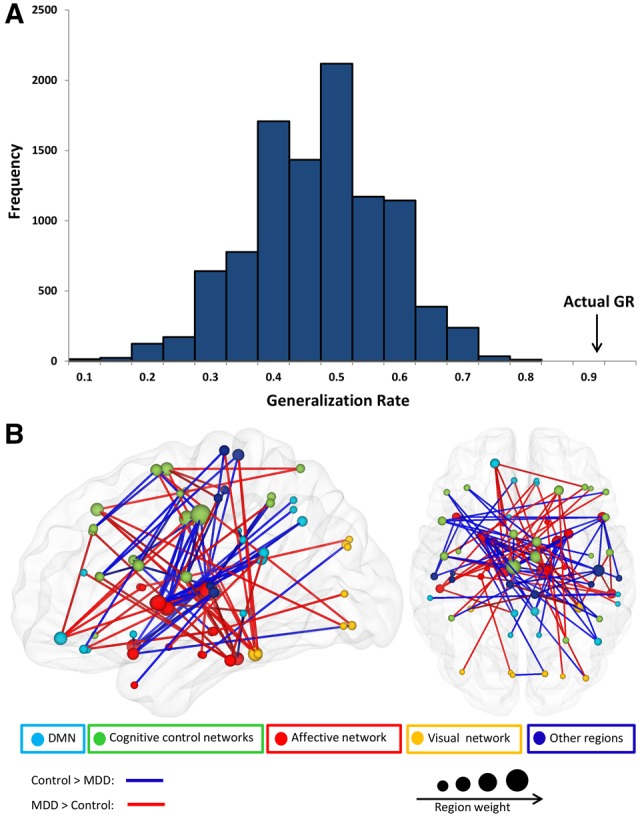
(A) Permutation distribution of the generalization rate after random permutation of the class labels (10000 times). The actual generalization rate indicates that the classifier learned to discriminate between the two groups (p<.0001). (B) Three-dimensional visualization of the 100 most discriminating functional connections predicting group classification. The plotted lines represent abnormally increased (red) or decreased (blue) connectivity in MDD patients. The nodes are color-coded by affiliation to different networks and the region weight is reflected on the size of the nodes.
The majority of the highly discriminating functional connections were diminished in remitted depressed patients compared to controls (54.7%). The diameter of a sphere representing a region (i.e. region weight) was scaled by the corresponding number of occurrence of this region in the highly discriminating functional connections. Among the regions that exhibited greater weights were mainly subcortical areas such as thalamus, and basal ganglia, but also cortical areas in the dorsal ACC and PFC (Figure 2B).
T-tests revealed significantly reduced connections in remitted depressed patients compared with healthy controls between the dorsal ACC and thalamus or basal ganglia (Figure 3). A group by rumination interaction effect was present mainly in regions within the DMN, the inferior frontal gyrus and visual cortex areas (Figure 3).
Fig. 3.
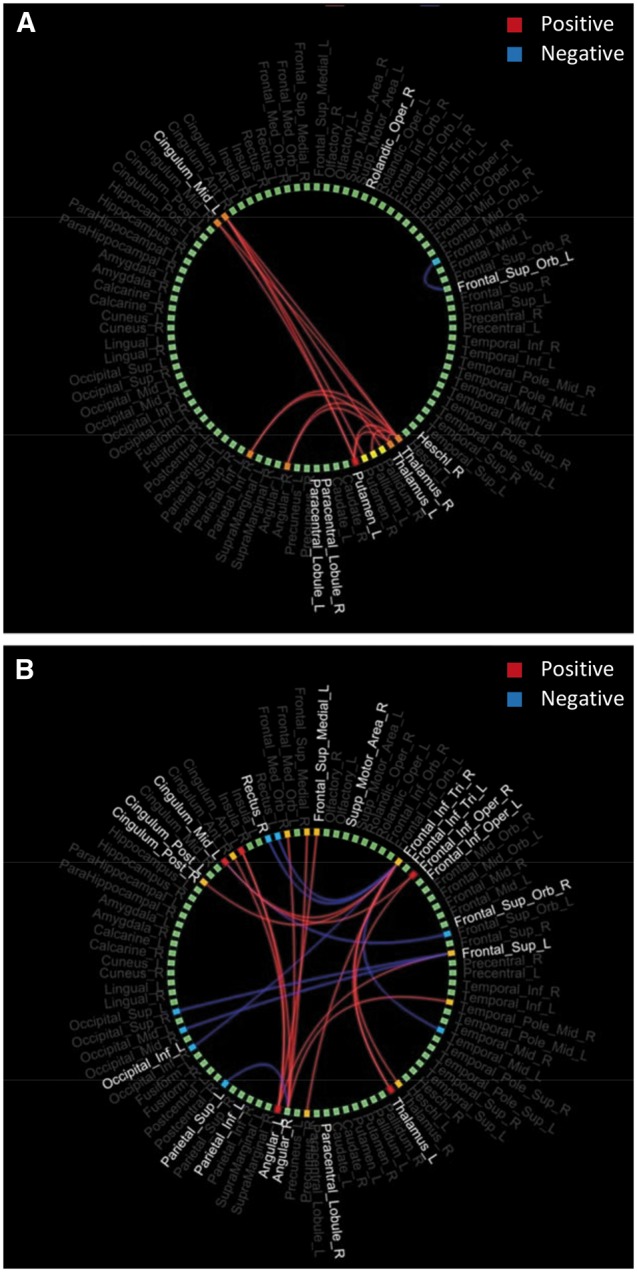
Circle graphs depicting the functional connections that exhibited significant group effects (A) or group by rumination interaction effects (B) in the whole brain. Positive connections (red) represent increased functional connectivity in healthy controls compared to patients (A) or positive correlation between connectivity and rumination in the healthy group and negative in the patient group (B). Blue lines represent the opposite effects.
Discussion
This study extends previous knowledge by showing that healthy subjects and remitted depressed patients displayed different, i.e. opposite relationships between DMN FC and self-reported rumination. Further, functional connectivity within the anterior DMN was oppositely correlated to self-reported rumination than FC between the anterior and posterior DMN as well as between the anterior DMN and DAN. Finally, global resting-state FC correctly classified 92% of the subjects into their respective groups.
The observed dissociation of DMN FC between remitted MDD and healthy individuals based on the level of rumination is partly in line with previous literature. One study also reported an association between self-reported rumination and increased anterior-posterior DMN connectivity in healthy subjects, but unlike our study, also a positive relationship between increased rumination and increased anterior-posterior DMN connectivity in acutely depressed patients (Berman et al., 2011). This latter discrepancy could be attributed to differences between eyes-open and eyes-closed resting conditions or to the fact that Berman et al. concatenated short “rest” periods of some seconds between task blocks instead of using a standard resting-state session. Considering the resting-state literature in depressive patients, our results are quite consistent with previous findings, showing lower anterior-posterior DMN resting-state FC in depressive patients compared with healthy controls (Zhu et al., 2012; Connolly et al., 2013).
This study revealed a second dissociation between the anterior DMN and the anterior–posterior DMN FC based on the levels of rumination. The functional roles of the anterior and the posterior DMN differ substantially (Gusnard et al., 2001; Northoff, 2007; Schmitz and Johnson, 2007; Andrews-Hanna et al., 2010) and medial PFC and ventral ACC are key brain regions involved in the pathophysiology of depression (Northoff, 2007; Drevets et al., 2008a; Lemogne et al., 2009,Whitfield-Gabrieli and Ford, 2012). A recent ICA study showed increased resting-state FC in the anterior DMN and decreased FC in the posterior DMN in first-episode, medication-naïve MDD patients compared with healthy controls (Zhu et al., 2012). Despite the absence of group differences in our remitted MDD group, both studies demonstrated a positive correlation between rumination and anterior DMN FC in the depression group consistent with the role of medial PFC and ventral ACC in self-referential processing and rumination (Drevets et al., 2008b; Johnson et al., 2009; Lemogne et al., 2009).
The classification of the subjects based on their global FC patterns showed that not only acutely depressed patients (Zeng et al., 2012) but also remitted patients can be reliably distinguished from healthy controls upon these global FC patterns. However, unlike acutely depressed patients, showing robust reduction in whole-brain FC (Berman et al., 2014), remitted MDD patients in our study displayed decreased FC in only 54.7% of the discriminating connections in different networks compared to healthy controls. Relating results from the whole-brain FC with the network-based analysis, we observed converging findings: As the network-based analysis, the whole-brain FC analysis showed no group differences in the DMN, but a significant group by rumination interaction effect within the DMN.
Previous evidence has shown that the content of ruminative thought and the type of rumination may differ among depressed and never depressed individuals (Treynor et al., 2003; Nolen-Hoeksema et al., 2008). We thus posit that the group by rumination interaction effects on DMN FC may reflect the different intensity and/or type of rumination in remitted MDD patients compared with healthy controls during resting-state. In this framework, the observed pattern of FC in healthy ruminators characterized by low connectivity within the anterior DMN and strong interactions between the anterior DMN and the posterior DMN or DAN may reflect an adaptive form of rumination that is associated with lower levels of depressive symptoms (Treynor et al., 2003). The opposite pattern of DMN FC was associated with increased levels of rumination in remitted MDD patients and may underlie a maladaptive type of rumination (i.e. brooding) that focuses on negative thoughts (Joormann et al., 2006). In line with this view, the authors of a recent meta-analysis of resting-state studies in MDD concluded that the abnormal FC between the subgenual ACC and the DMN rather than the abnormal DMN activity reflects negative rumination and interrupts the healthy self-referential processing supported by the core DMN structures (Hamilton et al., 2015). However, this hypothesis should be viewed with caution as the rumination subscale of the CERQ questionnaire cannot distinguish between adaptive and maladaptive forms of rumination. Future studies are warranted to clarify the relationship between the different types of rumination and DMN FC in symptomatic and remitted depressed patients.
Unlike a number of resting-state studies that showed abnormal DMN FC in depression (Zhu et al., 2012; Kaiser et al., 2015), we failed to observe robust group differences in DMN connectivity. This discrepancy suggests that DMN abnormal FC may be state dependent and may be present only in symptomatic patients. Interestingly, an ICA study showed increased resting-state FC in the anterior DMN and decreased FC in the posterior DMN in acutely depressed patients (Zhu et al., 2012). This pattern of abnormal DMN FC in acutely MDD patients is similar to the FC pattern observed in remitted MDD patients with increased tendency to ruminate and is consistent with the view that rumination is a cognitive vulnerability factor for depression relapse (Kuehner and Weber, 1999; Nolen-Hoeksema et al., 2008; Michalak et al., 2011). In light of this evidence and given that remitted MDD patients display higher level of self-reported rumination, the pattern of DMN in remitted depressed ruminators might underlie increased risk for depression relapse. This argument should be viewed within the framework of a recently proposed model postulating that the functional imbalance between the DMN and cognitive control networks in depressed individuals may constitute a neurobiological risk factor for recurrent depression mediated by cognitive deficits such as increased rumination (Hamilton et al., 2011; Marchetti et al., 2012). The identification of such neurobiological marker is important for several reasons: first, they are more objective than self-report measures as they are not dependent on the level of introspection. Second, such neurobiological markers might be identified even before manifestation of the relevant symptoms (here rumination) and third, neurobiological measures (e.g. resting state FC patterns) might be suitable to validate effects observed in self-report studies, thereby supporting the utility of such measures. Nonetheless, longitudinal studies that include records of depression relapse are warranted to further investigate this hypothesis. Confirmation of this hypothesis would provide opportunities for the development of novel objective diagnostic biomarkers that could track the vulnerability to depression relapse and could provide novel neural targets for treatment.
A strength of this study lies in the use of different methodologies (i.e. ICA, seed-based, whole-brain analysis) and the different measures of FC (i.e. FNC or within-network connectivity). We showed that FNC analysis and seed-based analysis can equally well detect relationships between behavioral measures (e.g. rumination) and long-distance FC patterns. Moreover, by performing two different ICAs, we were able to show that FC in the DMN as a whole may differ substantially from FC in the subcomponents of the DMN. This distinction is very important for the interpretation of the results and is often neglected in studies investigating the DMN.
The medication status of the patients may limit the interpretation of the present findings. Twelve patients were receiving pharmacological treatment at the time of the experiment and the rest of them were medicated in the past. Although we found no confounding medication effect in the present analysis, it has been shown that medication can normalize abnormal activity and FC in DMN and cognitive control areas in MDD (Ma, 2015).
In conclusion, we have demonstrated a dissociation of DMN FC in remitted MDD patients and healthy individuals based on the levels of rumination. This dissociation provides indirect evidence for a potential underlying neural mechanism reflecting increased risk for disease relapse in remitted MDD patients.
Supplementary data
Supplementary data are available at SCAN online.
Funding
Funding for this study was provided by a grant to MW by the Deutsche Forschungsgemeinschaft (We3638/3-1).
Conflict of interest. None declared.
Supplementary Material
References
- Allen E.A., Erhardt E.B., Damaraju E., et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010). Functional-anatomic fractionation of the brain's default network. Neuron 65(4), 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Rial W.Y., Rickels K. (1974). Short form of depression inventory—cross-validation. Psychological Reports 34(3), 1184–6. [PubMed] [Google Scholar]
- Berman M.G., Misic B., Buschkuehl M., et al. (2014). Does resting-state connectivity reflect depressive rumination? A tale of two analyses. Neuroimage 103, 267–79. [DOI] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience 6(5), 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Carew C.L., Milne A.M., Tatham E.L., MacQueen G.M., Hall G.B. (2013). Neural systems underlying thought suppression in young women with, and at-risk, for depression. Behavioral Brain Research 257, 13–24. [DOI] [PubMed] [Google Scholar]
- Cocchi L., Zalesky A., Fornito A., Mattingley J.B. (2013). Dynamic cooperation and competition between brain systems during cognitive control. Trends in Cognitive Science 17(10), 493–501. [DOI] [PubMed] [Google Scholar]
- Connolly C.G., Wu J., Ho T.C., et al. (2013). Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry 74(12), 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugene F., Dennis E.L., Gotlib I.H. (2010). Neural correlates of rumination in depression. Cognitive, Affective & Behavioral Neuroscience 10(4), 470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., et al. (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22(7), 1326–33. [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Furey M.L. (2008a). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function 213(1–2), 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Savitz J., Trimble M. (2008b). The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums 13(8), 663–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt E.B., Rachakonda S., Bedrick E.J., Allen E.A., Adali T., Calhoun V.D. (2011). Comparison of multi-subject ICA methods for analysis of fMRI data. Human Brain Mapping 32(12), 2075–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. (1995). The structured clinical interview for DSM-III-R personality-disorders (SCID-II). 1. Description. Journal of Personality Disorders 9(2), 83–91. [Google Scholar]
- Fornito A., Harrison B.J., Zalesky A., Simons J.S. (2012). Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences of the United States of America 109(31), 12788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America 102(27), 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freton M., Lemogne C., Delaveau P., et al. (2014). The dark side of self-focus: brain activity during self-focus in low and high brooders. Social Cognitive and Affective Neuroscience 9(11), 1808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N., Kraaij V. (2007). The cognitive emotion regulation questionnaire—psychometric features and prospective relationships with depression and anxiety in adults. European Journal of Psychological Assessment 23(3), 141–9. [Google Scholar]
- Golland P., Fischl B. (2003). Permutation tests for classification: towards statistical significance in image-based studies. Information Processing in Medical Imaging 18, 330–41. [DOI] [PubMed] [Google Scholar]
- Goulden N., Khusnulina A., Davis N.J., et al. (2014). The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage 99, 180–90. [DOI] [PubMed] [Google Scholar]
- Gusnard D., Akbudak E., Shulman G., Raichle M.E. (2001). Role of medial prefrontal cortex in a default mode of brain function. Neuroimage 13(6), S414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Chen G., Thomason M.E., Schwartz M.E., Gotlib I.H. (2011). Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Molecular Psychiatry 16(7), 763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Farmer M., Fogelman P., Gotlib I.H. (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biological Psychiatry 78, 224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry 23(1), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M.J., Pearlson G.D., Stevens M., Calhoun V.D. (2008). A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage 39(4), 1666–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.K., Nolen-Hoeksema S., Mitchell K.J., Levin Y. (2009). Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience 4(4), 313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J., Dkane M., Gotlib I.H. (2006). Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavioral Therapy 37(3), 269–80. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72(6), 603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler H., Taubner S., Buchheim A., et al. (2011). Individualized and clinically derived stimuli activate limbic structures in depression: an fMRI study. PLoS One 6(1), e15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E., Davidson M., Weber J., Ochsner K. (2009). Coping with emotions past: the neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry 65(5), 361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C., Weber I. (1999). Responses to depression in unipolar depressed patients: an investigation of Nolen-Hoeksema's response styles theory. Psychological Medicine 29(6), 1323–33. [DOI] [PubMed] [Google Scholar]
- Lemogne C., le Bastard G., Mayberg H., et al. (2009). In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience 4(3), 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zou Q., He Y., Yang Y. (2015). Topologically reorganized connectivity architecture of default-mode, executive-control, and salience networks across working memory task loads. Cerebral Cortex 26, 1501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q.H., Liu J., Zheng Y.R., Liang H., Calhoun V.D. (2010). Semiblind spatial ICA of fMRI using spatial constraints. Human Brain Mapping 31(7), 1076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. (2015). Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Molecular Psychiatry 20(3), 311–9. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E.H., Sonuga-Barke E.J., De Raedt R. (2012). The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychology Review 22(3), 229–51. [DOI] [PubMed] [Google Scholar]
- Michalak J., Holz A., Teismann T. (2011). Rumination as a predictor of relapse in mindfulness-based cognitive therapy for depression. Psychology and Psychotherapy 84(2), 230–6. [DOI] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. (2013). Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience 7, 666.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. (2008). Rethinking rumination. Perspectives on Psychological Science 3(5), 400–24. [DOI] [PubMed] [Google Scholar]
- Northoff G. (2007). Psychopathology and pathophysiology of the self in depression—neuropsychiatric hypothesis. Journal of Affective Disorders 104(1–3), 1–14. [DOI] [PubMed] [Google Scholar]
- Sackeim H.A. (2001). The definition and meaning of treatment-resistant depression. Journal of Clinical Psychiatry 62(Suppl 16), 10–7. [PubMed] [Google Scholar]
- Schmitz T.W., Johnson S.C. (2007). Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews 31(4), 585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C., Corbetta M., Romani G.L., Shulman G.L. (2011). Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. Journal of Neuroscience 31(12), 4407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W., Gonzalez R., Nolen-Hoeksema S. (2003). Rumination reconsidered: a psychometric analysis. Cognitive Therapy and Research 27(3), 247–59. [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connection 2(3), 125–41. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., et al. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America 106(4), 1279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L.L., Shen H., Liu L., et al. (2012). Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 135(Pt 5), 1498–507. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang X., Xiao J., et al. (2012). Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry 71(7), 611–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


