Abstract
The cross-generational transmission of mammalian sociality, initiated by the parent’s postpartum brain plasticity and species-typical behavior that buttress offspring’s socialization, has not been studied in humans. In this longitudinal study, we measured brain response of 45 primary-caregiving parents to their infant’s stimuli, observed parent–infant interactions, and assayed parental oxytocin (OT). Intra- and inter-network connectivity were computed in three main networks of the human parental brain: core limbic, embodied simulation and mentalizing. During preschool, two key child social competencies were observed: emotion regulation and socialization. Parent’s network integrity in infancy predicted preschoolers’ social outcomes, with subcortical and cortical network integrity foreshadowing simple evolutionary-based regulatory tactics vs complex self-regulatory strategies and advanced socialization. Parent–infant synchrony mediated the links between connectivity of the parent’s embodied simulation network and preschoolers' ability to use cognitive/executive emotion regulation strategies, highlighting the inherently dyadic nature of this network and its long-term effects on tuning young to social life. Parent’s inter-network core limbic-embodied simulation connectivity predicted children’s OT as moderated by parental OT. Findings challenge solipsistic neuroscience perspectives by demonstrating how the parent–offspring interface enables the brain of one human to profoundly impact long-term adaptation of another.
Keywords: parental brain, parent-infant synchrony, oxytocin, embodied simulation, mentalizing
Introduction
A key question in the development of social species is how young become members of the social group and learn to function competently within the social milieu. In highly social species, such as ants or bees, young enter social groups via reciprocal exchanges of sensory stimuli with conspecifics underpinned by coordination of neuro-hormonal processes (Wilson, 2012). In mammals, the infant’s first social experiences occur within the parent–infant bond and similarly coordinate brain and behavior into a process of biobehavioral synchrony (Feldman, 2012b, 2015b)—the coupling of parent’s and infant’s physiology and behavior during social contact—which carries profound effects for offspring’s adaptation (Kundakovic and Champagne, 2015). Plasticity of the parental brain in infancy, indicated by increased activations of key brain areas implicated in mammalian parenting accompanied by structural changes in the parent’s brain, initiates the cross-generational sequence of mammalian sociality (Stolzenberg and Numan, 2011; Feldman, 2015b). Research in rodents has shown that the postpartum marks the period of highest plasticity in the adult brain (Cohen and Mizrahi, 2015), when areas of the parent’s brain, including the hypothalamus, basolateral amygdala, parietal cortex and prefrontal cortex, partly triggered by oxytocin (OT) release, become sensitized to infant cues and initiate the expression of species-typical parental behavior (Featherstone et al., 2000; Lambert et al., 2011). These parenting behaviors, in turn, organize the infant’s brain and behavior toward life within the social ecology (Weaver et al., 2004; Numan and Young, 2016). Imaging studies of human parents’ brain show activations in parallel areas in response to infant stimuli, similarly associated with OT and the human-specific parenting repertoire (Rilling and Young, 2014; Swain et al., 2014; Feldman, 2015b; Kim et al., 2016). Yet, the cross-generational sequence of mammalian sociality; initiated by adaptation of the parent’s brain in infancy, continuing with the expression of parenting behavior, and culminating in social adaptation of juveniles, has not been tested in humans. Describing such sequence in humans may provide evidence that the parent–infant interface, the junction of evolutionary progress, marks a unique context where one human’s brain can profoundly affect another human’s social adaptation.
Research on the parental brain described three main networks underpinning human parenting; core limbic, embodied-simulation/empathy and mentalizing (Rilling and Young, 2014; Swain et al., 2014; Feldman, 2015b; Kim et al., 2016). Studies on the parental brain in rodents detail the critical role of a core-limbic network, including the MPOA of the hypothalamus, amygdala and subcortical dopamine reward system. Primed by OT surge during parturition and sensitized by sensory stimuli from infant and nest, these structures consolidate into a mammalian parenting network that subserves parenting (Dulac et al., 2014, Numan and Young, 2015). In humans, all imaging studies of infant visuals show that this highly conserved core-limbic network activates with parents’ exposure to their infants’ pictures (Atzil et al., 2011; Barrett and Fleming, 2011; Swain, 2011b, 2012; Abraham et al., 2014; Feldman, 2015b). However, in humans, the ancient limbic network is connected via multiple ascending and descending projections to two main cingulo-insular and fronto-temporo-parietal networks that support the flexibility, person-specificity and future-directedness of human parenting (Swain, 2011a; Rilling and Young, 2014). The embodied-simulation/empathy network consists of structures in the anterior insular-cingulate cortex that enable the parent to resonate with infant state and emotion, ground experience in the present moment, and afford perceptual-motor coupling of infant action in the parent’s brain via mirror mechanisms (Craig, 2009; Gallese, 2014). The mentalizing network, including fronto-temporo-parietal structures, supports social attribution and theory-of-mind abilities (Spunt and Lieberman, 2013) and enables parents to understand infants’ nonverbal communications and infer infant intentions from actions (Frith and Frith, 2006, 2012).
The two cortical networks, embodied-simulation and mentalization, which jointly comprise the human social brain, serve multiple cognitive and associative functions and are superimposed upon the ancient limbic circuit to create the ‘human parental caregiving network’ that integrates immediate motivational responses with the interoceptive and conscious aspects of human parenting (Swain, 2008; Feldman, 2015b, Kim et al., 2015). The complexity of the human parental brain and its integration of primitive with later-evolving structures lend support to the hypothesis that the cross-generational transfer of human sociality likely combines mammalian-general features with uniquely human brain networks and complex parent–child exchanges, to facilitate both survival-related and executive-mental child social outcomes.
Developmental studies identified two key competencies that enable preschool children, as they enter the social world, to socially participate with non-kin adults and peers: emotion regulation and socialization (Kochanska et al., 1995; Eisenberg and Morris, 2002). Most crucial is emotion regulation, defined as the ability to manage states of increased negative or positive arousal to facilitate adaptation or goal-directed activity (Cole et al., 2004). Research mainly focused on the regulation of negative emotions, addressing the development of emotion regulation from simple tactics in infancy to more complex strategies in preschool that require higher socio-cognitive competencies, such as executive attention or symbolic communications (Fox and Calkins, 2003). Emergence of these complex regulatory abilities follows the development of anterior insula and ACC connectivity at 3–4 years and the increase in Von Economo neurons, projection cells found mainly in human cingulo-insular cortex that provide the rapid, efficient connectivity needed for mature self-regulation (Allman et al., 2005; Posner et al., 2014). Regulation of positive emotions, the enhancement and maintenance of positive affect, draws on simpler mechanisms like mimicking or self-soothing, which comprise part of the infant’s self-regulatory repertoire from birth, serve an adaptive survival function, and are underpinned by brainstem-limbic system connectivity (Burgdorf and Panksepp, 2006). Extant research indicated that emotion-regulation abilities are critical for social adaptation and that preschoolers’ ability to regulate emotions is a stable individual trait that predicts cognitive, social-emotional, life-success and mental-health outcomes throughout life (Eisenberg et al. 2000; Moffitt et al., 2011).
The second key ability, socialization, defined as children’s willing compliance with adult commands and internalization of social rules, undergoes significant maturation during the preschool years and similarly predicts long-term social adjustment, mental health and adult employment (Kochanska et al., 1995; Daly et al., 2015). Importantly, although preschoolers' emotion regulation and socialization may provide an index of lifetime adaptation, their roots lie in parent–infant synchrony (Feldman, 2007b; Calkins and Hill, 2011). Synchronous interactions, which build online from the parent’s and child’s nonverbal signals, tune the child to social dialogue and provide critical inputs for organizing the brain basis of intersubjectivity, a prerequisite of human social life (Feldman, 2007c, 2012a; Gallese, 2014).
The central question guiding the current study is whether and how the human parental brain evolved to facilitate key social abilities in human children, as in other mammals. We assessed parent’s brain response to own-infant cues using functional magnetic resonance imaging (fMRI) among primary-caregiving first-time mothers and fathers, computed intra- and inter- functional connectivity in the aforementioned three networks based on parents’ brain response to their own infant, measured parental OT levels and videotaped parent–infant interactions which were coded offline for parent–infant synchrony. Four years later we revisited families, observing children in a battery of well-validated emotion-regulation and socialization paradigms that were micro-coded offline to create measures of negative and positive emotional expression, use of simple and complex regulatory strategies and self-regulated socialization. OT levels were re-assessed in parent and child.
Recent studies in rodents (Bales and Saltzman, 2016) and humans (Abraham et al., 2014) demonstrate that different pathways to the parental brain exist in mothers and fathers; yet, fathering utilizes similar neural networks as mothering, and no differences in brain activation levels were found in the three aforementioned networks between primary-caregiving mothers and fathers. We applied a network perspective to the parental brain, in light of recent models in social neuroscience (Stanley and Adolphs, 2013; Dulac et al., 2014; Raz et al., 2014) that highlight the need to shift from focusing on activations of discrete structures to coordination of neural circuits that support social life. Thus, theorizing that network connectivity provides a robust index of system functionality, we measured connectivity in each network of the ‘human parental caregiving network’—core-limbic, embodied-simulation and mentalizing—and used these connectivity indices to predict child outcomes.
We hypothesized that network connectivity in the parent’s brain in infancy would shape the child’s long-term social competencies both directly (Hypothesis 1) and indirectly via parent–infant synchrony (Hypothesis 2). We expected that integrity of the core-limbic network would support children’s ability to use simple, evolutionary-based regulatory strategies, possibly in joy-related contexts, whereas integrity of the parent’s cortical networks would support advanced self-regulatory/socialization outcomes that draw on later-evolving executive and inhibitory brain functions. Our special focus centered on the parent’s embodied-simulation network, which directs the parent to ‘here-and-now’ bodily exchanges, integrates online biological and behavioral signals and engages overlapping neural circuits between two individuals (Hasson et al., 2012; Decety, 2015; Hasson and Frith, 2016). Such brain-to-brain overlap afforded by the embodied-simulation network may trigger the expression of parent–infant behavioral synchrony that would tune the infant’s brain to life within the social world. Finally, based on recent research highlighting the critical role of OT as modulator of functional connectivity within the social brain (Sokolowski and Corbin, 2012; Bethlehem et al., 2013), and the cross-generational transmission of OT functionality in both humans and other mammals (Meaney, 2001; Feldman et al., 2010), we expected that connectivity among the three networks may support the development of the child's OT system, as mediated by parental OT (Hypothesis 3).
Materials and methods
Participants
A total of 45 first-time primary-caregiving parents raising their infant within a committed two-parent family participated in the study [mean age: 36.4 years ± 6.87 s.d.]. These included 20 heterosexual primary-caregiving biological mothers and 25 homosexual primary-caregiving biological fathers raising the infant without maternal involvement since birth through surrogacy. Two parents chose not to participate in the follow-up home visit; therefore, we excluded their fMRI data. Infants [mean age at Time 1: 10.95 months ±6.87 s.d.; mean age at Time 2: 40.22 months ± 4.45 s.d.] were all born at term and were healthy since birth (Supplementary Table S1). In all families, parents were healthy with no history of mental illness, and both parents shared housework and childcare responsibilities. Participants received compensation for their time and gave written informed consent. The study received approval from the Institutional Review Board.
Procedure
The experimental procedure included three sessions with each family. In the first, we visited families at home (Time 1 = Infancy), salivary samples were collected for parental OT, and parent was videotaped interacting with the infant. In the second session, several days later, primary-caregiving parent underwent functional brain scanning with the individually tailored home videotapes used as fMRI stimuli. In the third session (Time 2 = Preschool), when children reached preschool age, we re-visited families at home, salivary samples were collected from parent and child for OT, and preschoolers underwent testing with the Stanford-Binet Intelligence Scale (Thorndike et al., 2003). Visit also included parent–child interactions and several child emotion-regulation procedures with a stranger videotaped for later coding when parent was in the room. We carefully selected well-validated emotion-regulation procedures that tap children's negative and positive emotional expression and their use of simple and complex emotion-regulatory strategies, as follows:
Parent-child free play—Parent and child engaged in a 7-min free play session with preselected toys used to elicit play at this age (Feldman, 2007a). Instructions were ‘Play with your child as you typically do’.
Self-regulated socialization—Toy pick-up task (compliance situation). We employed a well-validated paradigm to assess children's self-regulated socialization and parent's discipline techniques (Kochanska et al., 2001). The parent received a cart and instructions to have the child pick up the toys following the free play. The toy pick-up lasted 8 min or until task was completed.
Regulation of negative emotions—Masks. In this procedure, adapted from the LAB-TAB (Goldsmith and Rothbart, 1996), child sits in front of the experimenter who puts on four increasingly fear-eliciting masks: rabbit, lion, alligator and monster. The experimenter wore each mask for 15 s.
Regulation of positive emotions—Bubbles. Similarly adapted from the LAB-TAB (Goldsmith and Rothbart, 1996), the experimenter blew soap bubbles for the child to play for 5 min.
OT collection and determination
Saliva was collected using Salivettes (Sarstedt, Rommelsdorf, Germany). Samples were stored at −20 °C until centrifuged twice, 2 days apart, at 4 °C at 1500g for 20 min. Liquid samples were kept at −80 °C, lyophilized for 10 days, and stored at 20 °C. On the assay day, the dry samples were reconstituted in water and concentrated X 4, before immunoassay. OT was assayed by ELISA (Enzo® (NY, USA) with careful sample preparation; samples were centrifuged twice; delicate lyophilization maintained constant refrigeration to slows the drying; and samples were reconstituted in water prior to assay. Measurements were performed in duplicate and the concentrations of samples were calculated using Matlab-7 according to relevant standard curves. The intra-assay and inter-assay coefficients of variability are <19.1%.
Coding
We used global rating scales for parent–child interaction at the two time-points and micro-coding for child social outcomes—emotion regulation and socialization.
Parent–child interactions (Times 1 and 2) were coded using the Coding Interactive Behavior (CIB) Manual (Feldman, 1998). This CIB is a well-validated global rating system for adult-child interactions includes 42 scales, which aggregate into theoretically meaningful constructs. The system has been validated in multiple studies and shows good psychometric properties including test–retest reliability, individual stability across long developmental epochs and construct validity (for review, see Feldman, 2012b). In infancy (Time 1), we used the parent–infant synchrony construct was used in infancy to index the central behavioral expression of attuned human caregiving. Codes describe the expression of the human species-typical parental behavior (parent gaze, positive affect, ‘motherese’ vocalization, affectionate touch) and their coordination with the infant’s signals (mutual adaptation, dyadic reciprocity, fluency of the interaction and the degree to which it provides a supportive presence for infant play and exploration (Feldman, 2007c). For the human parental behavior construct, we used the four CIB scales that index the human parental caregiving repertoire which appears immediately after birth in the gaze, affect, vocal and touch modalities. Codes describe parents’ expression of warm and positive affect, gaze at infant, provision of affective touch and high-pitched ‘motherese’ vocalizations. In preschool (Time 2), we used the child social engagement construct. This construct includes seven scales that measure the degree of child social involvement, including child alertness, social initiation, affection toward parent, symbolic play, vocalizations, gaze maintenance and positive affect. Trained raters blind to all other information the coding. Inter-rater reliability, measured on 20% of the sample, was intraclass r = 0.94 (range =0.87–0.99).
Child emotion regulation and socialization. The Masks and Bubbles paradigms, tapping child emotion regulation, were each micro-coded for the child’s expression of positive emotionality (positive affect, positive vocalizations and laughter) and negative emotionality (negative affect, withdrawal, crying/yelling and protest). Two types of regulatory behaviors were micro-coded, consistent with prior research (Feldman et al, 2011; Hirschler-Guttenberg et al., 2015; Ostfeld-Etzion et al., 2015): Simple regulatory behaviors—included behaviors aiming solely at self-regulation that clearly display the child’s regulatory effort, such as physical self-soothing (e.g. thumb-sucking), verbal self-soothing (e.g. ‘That’s okay’) and proximity seeking. Complex regulatory behaviors—included complex behaviors that are not inherently self-regulatory but may be used for emotion regulation during moments of increased stress, such as substitutive-symbolic play, functional play, using executive skills to divert attention or talking to parent or experimenter.
The Toy pick-up paradigm, aimed to index child socialization, was micro-coded, consistent with prior research (Kochanska and Aksan, 1995; Feldman et al., 1999) for child self-regulated compliance and parent’s discipline techniques. Parent codes included warm control—parent shows positive affect while providing consistent limits, uses tactics such as encouragement, redirection of attention, or praise, negotiates with the child, explains and suggests, and shows empathy in order to keep child on task, harsh control—parent uses physical force, insults, yelling, or manipulations, no control—parent provides no structure, lets child do as he or she pleases with no attention to task, may pick up the toys for child. Coding was conducted on a computerized system (The Observer, Noldus Information Technology, Wageningen, The Netherlands). Two blind trained observerscoded while the tape progressed at normal speed, shifting to slow motion when shift in behavior occurred. Coders were trained to 90% reliability. Inter-rater reliability, measured on 20% of the sample, was intraclass r = 0.91, for the pick-up procedure, r = 0.86 for the masks, and r = 0.89 for the bubbles. Proportion and frequency variables were used.
fMRI data acquisition and analyses
Imaging was performed on a GE-3T Sigma Horizon echo-speed scanner with a resonant gradient echoplanar imaging system. Functional T2*-weighted images were obtained using field of view = 220 mm, matrix size = 96 × 96, repetition time = 3000 ms, echo time = 35 ms, flip angle = 90°, acquisition orientation of the fourth ventricle plane, 39 axial slices of 3-mm thickness, and gap = 0. In addition, each functional scan was accompanied by a three-dimensional (3D) anatomical scan using anatomical 3D sequence spoiled gradient (SPGR) echo sequences that were obtained with high-resolution of 1 × 1 × 1 mm. fMRI data were analyzed with the BrainVoyager analysis package (version 2.1; Brain Innovation).
fMRI data preprocessing
The first six volumes, before signal stabilization, were discarded to allow for T1 equilibrium. Preprocessing of functional scans included 3D motion correction, slice scan time correction, spatial smoothing [a full width at half maximum 4-mm Gaussian Kernel], linear trend removal and high-pass filtering (fast Fourier transform based with a cutoff of two cycles per time course). The functional images were then superimposed on 2D anatomical images (a 3D SPGR echo sequence, field of view = 220 mm, matrix size = 96 × 96, axial slices of 3 mm thickness, gap = 0) and incorporated into the 3D datasets through trilinear interpolation. The complete dataset was transformed into Talairach space.
fMRI experimental design
While lying in the scanner, participants were instructed to watch a series of attachment-related video vignettes presented on the screen. For ecological validity, we examined parents’ brain response to natural interactions and attachment-related stimuli videotaped in the home environment, the context where parental–infant bonding takes place. All videos included multi-modal, dynamic and realistic stimuli. Each parent’s video set was individually tailored, comprising three 2-min infant- and parent-related videos with alternating rest fixation periods of 15 or 18 s between stimuli, preceded by a 1-min rest with fixation period. For the NCI analysis we used a 2 min vignette of each parent interacting with her/his own infant (‘Self–Infant Interaction’). Stimuli were counterbalanced and were randomly presented in three different order patterns. To ensure our results describe parents’ networks integrity stimulated specifically by observing themselves interacting with their infant, we computed NCI analysis for a 2 min ‘unfamiliar parent–infant interaction’ condition (in which the parent was of the same sex as the participant) and found no significant links to any child social outcomes. In addition, we found specific associations between child social outcomes and a parental network, but not with the other network, analyses which provide convincing evidence for the specific role these parental networks play during infancy in supporting children’s long-term social development. To ensure that parents and infants’ affective states did not differ between participants, we selected only clips in which the infants and the parents were in neutral or positive affective states, as coded using the CIB rating system.
Computation of network cohesion indices
To analyzing the dynamic functional network connectivity of the three brain networks of interest, we used a NCI index (for details, see Raz et al., 2012, 2014) probing the dynamics of coordination both within defined network (intra-network cohesion index; intra-NCI) and between networks (inter-network cohesion index; inter-NCI). Cohesion is measured here in a way that reflects both the strength of the average correlations between signals in a group of regions and the variation around this average, with higher values for correlations that are narrowly distributed around a high average. First, the average signal of each region-of-interest (ROI) was extracted using a Gaussian mask with 3 mm radius around the seed coordinates in a selected time window of 114 s (38 TRs), the ‘Self–Infant Interaction’ condition, incorporating a hemodynamic delay of two TRs. Next, for each network, and participant, the set of all pairwise Pearson correlations was computed at the selected time-window as follows:
| (1) |
Thus, the NCI resulted from a right tailed Student’s t-test with a null hypothesis of µR = 0 performed on the population of the Fisher Z-transformed coefficients. In this test, the t-statistic serves as a probe for the connectivity within the network with high values when the mean correlation is high and variance is low. Inter-NCI was calculated in the same manner, except that the population of the t-tested pairwise correlations includes pairs of ROIs in different networks.
Definition of networks of interest
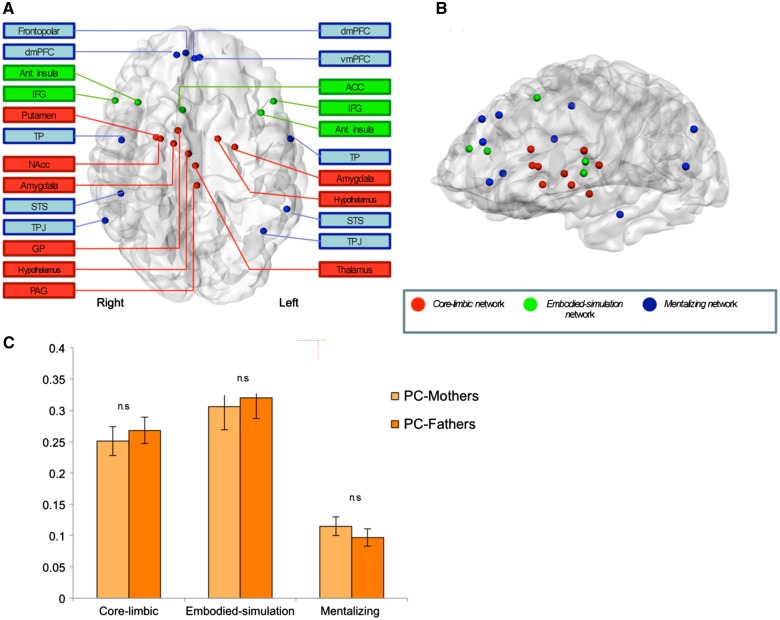
Relevant comprehensive and updated meta-analyses of neuroimaging studies were used to define core-limbic, embodied-simulation/empathy and mentalizing networks (Figure 2 and Supplementary Table S2). The three chosen networks: core-limbic, embodied-simulation/empathy and mentalizing, have been repeatedly shown in studies on the parental brain to underpin human parenting and all brain structures within these networks have been found in previous research to activate in response to infant cues (auditory, visual or multimodal infant stimuli, such as infant crying, pictures or movies, often comprising ‘own’ infant to standard infant or control condition) (Swain, 2008; Rilling and Young, 2014; Swain et al., 2014; Feldman, 2015b; Kim et al., 2016). The core-limbic network was defined on the basis of a meta-analysis that clustered emotion-related brain structures according to their co-activity across studies (Kober et al., 2008). The embodied-simulation/empathy network was defined on the basis of recent meta-analyses on empathy (Fan et al., 2011) and mirror properties (Molenberghs et al., 2012). The mentalizing network was based on in which participants were instructed to infer others’ intentions, thoughts and future actions (Bzdok et al., 2012). Montreal Neurological Institute (MNI) to Talairach transformations were performed using a Lancaster transformation (Lancaster et al., 2007).
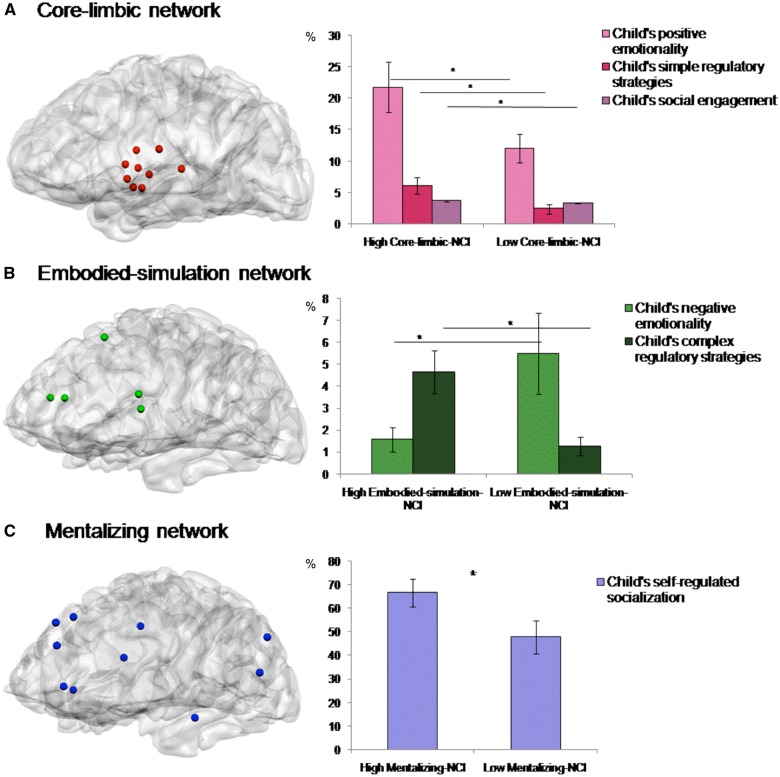
Fig. 2.
Relations between parent's intra-NCIs and child's emotion regulation, emotionality and social behaviors (Time 2). Proportion values (±SE; represented by error bars at the top) are presented for: child’s simple regulatory behaviors and positive emotionality during positive-emotion-eliciting paradigm and child’s social behavior during parent–child interaction (A); child’s complex regulatory behaviors and negative emotionality during negative-emotion-eliciting paradigm (B); and child’s self-regulatory socialization during socialization situation (C). n = 45. * P< 0.05.
Results
Associations between parental behaviors and child social outcomes
Consistent with ethological perspectives, we first sought to examine associations between parental behaviors in infancy and child’s social outcomes during the preschool period. Since no differences emerged between preschoolers reared by primary-caregiving mothers vs fathers on any preschool variable (Supplementary Table S2), we collapsed the two groups. The Human parental behavior construct, including affective touch, ‘motherese’ vocalization, gaze and positive affect—a construct that parallels the licking-and-grooming of other mammals—was longitudinally related to the child’s use of simple regulatory strategies in preschool (r = 0.395, P = 0.007). Parent–infant synchrony in infancy was longitudinally associated with the child's employment of complex regulatory strategies in preschool (r = 0.505, P = 0.0001). Finally, higher parental warm control behavior, defining the integration of warm affect with clear limits, was associated with child’s self-regulated socialization in preschool (r = 0.352, P = 0.018).
Direct links between parent’s intra-network integrity and child social outcomes
We defined the three networks of the parental brain on the basis of prior research (Figure 1A and B; Supplementary Table S3). To dynamically examine parents’ functional connectivity within networks as activated while viewing their videotaped interactions with their infant, we applied network cohesion analysis (NCI; Raz et al., 2012, 2014) to derive intra-network indices. No differences emerged in intra-connectivity between primary-caregiving mothers and fathers in any of the three networks: Core-limbic-NCI, F1,43 = 0.281, P = 0.599; Embodied-simulation-NCI, F1,43 = 0.08, P = 0.778; Mentalizing-NCI, F1,43 = 0.821, P = 0.370 (Figure 1C). Thus, we collapsed the mother and father groups. To test direct links between parental brain and child outcomes (Hypothesis 1), ANOVAs measured differences in child outcomes at Time 2 among parents with high vs low NCI in each network using the median split. Results indicate that preschoolers raised by parents with greater connectivity in the core-limbic network (Figure 2A; median = 0.264) exhibited more positive emotionality (F1,43 = 4.38, P = 0.037), readily employed simple regulatory strategies such as mimicking and self-soothing (F1,43 = 6.15, P = 0.017), and displayed greater social engagement during interaction with their parents (F1,43 = 5.49, P = 0.023). Second, as predicted, preschoolers whose parents exhibited higher connectivity of the embodied-simulation network (Figure 2B; median = 0.288) exhibited less negative emotionality (F1,43 = 4.30, P = 0.043) and utilized complex regulatory strategies to regulate the negative context (F1,43 = 6.88, P = 0.012), including executive attention and symbolic communication. Finally, preschoolers of parents with greater connectivity of the mentalizing network (Figure 2C; median = 0.108) displayed greater self-regulated socialization when asked to comply to adults’ requests (F1,43 = 4.27, P = 0.041) (Supplementary Tables S4–S6).
Fig. 1.
Location of ROIs comprising the core-limbic (red), embodied-simulation (green) and mentalizing (blue) networks, from coronal (A) and sagittal (B) views. dmPFC, dorsomedial prefrontal cortex; Ant. insula, anterior insula; IFG, inferior frontal gyrus; TP, temporal pole; NAcc, nucleus accumbens; GP, globuspallidus; STS, superior temporal sulcus; TPJ, temporoparietal junction; PAG, periaqueductal gray; vmPFC, ventromedial prefrontal cortex; ACC, anterior cingulated cortex. L, left; R, right. The bar graph (C) presents the three networks’ intra-NCI values (±SE; represented by error bars at top) activated by parents’ viewing of their interactions with infants at Time 1, for primary-caregiving mothers (PC-Mothers; n = 20, bright orange) and primary-caregiving fathers (PC-Fathers; n = 25, dark orange).
Indirect effects of parent's intra-network integrity on child outcomes
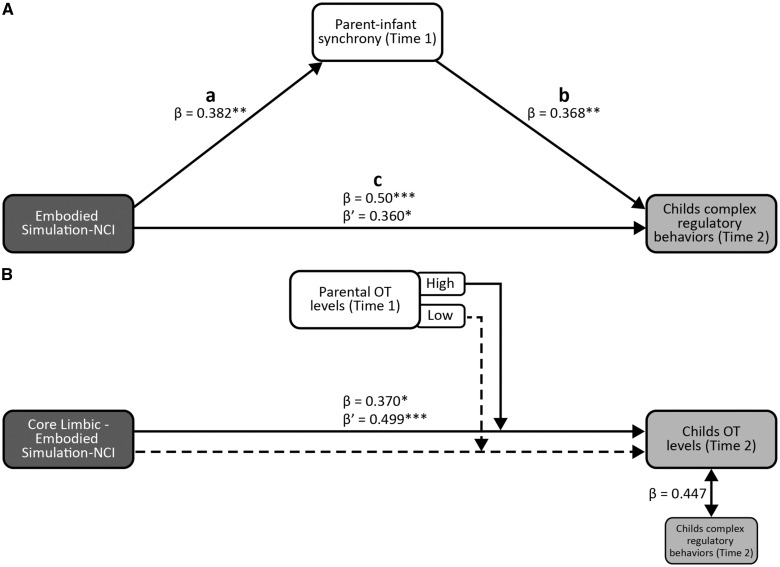
To test Hypothesis 2 on indirect effects via parenting behavior, we examined whether parental behavior mediated the relationship between parent’s intra-NCIs and child’s social outcomes for each network separately. As predicted, using Sobel's (1982) test, we found partial mediation by parent–infant synchronyonly for the link between parent’s embodied-simulation-NCI and preschoolers' complex regulatory behaviors (z = 2.210, P = 0.027; Figure 3). Baron and Kenny’s (1986; see Figure 3A) steps were computed. In Step 1, associations between the predictor (parent’s embodied-simulation-NCI) and outcome (child complex regulation) was found significant (path C; ß = 0.5, t = 3.785, P = 0.001). In Step 2, associations between predictor and mediator (parent–infant synchrony) were significant (path A; ß = 0.382, t = 2.707, P = 0.009). In Step 3, associations between mediator and outcome controlling for predictor were significant (path B, ß = 368, t = 2.764, P = 0.008). In Step 4, we association predictor and the outcome, controlling for mediator were significant (ß’=0.360, t = 2.704, P = 0.01). Sobel’s test for mediation, z = 2.210, P < 0.05, indicated a significant indirect effect of parent’s embodied-simulation-NCI on the child’s use of complex regulatory behaviors 4 years later, partially mediated by parent–infant synchrony.
Fig. 3.
Direct and indirect effects of parent’s-NCI on child’s outcomes (Time 2). Standardized regression coefficient for the relations between parent’s embodied-simulation-NCI and child’s complex self-regulation as partially mediated by parent-infant synchrony. Path c shows the standardized regression coefficient for the total (β) and direct (β') effects of embodied-simulation-NC Ion child's complex regulatory behavior (A). Moderation model of parental OT levels on core limbic and embodied simulation-Inter-NCI and child’s OT levels. Path b Model shows the standardized regression coefficient for the interaction (β) and direct (β') effects of core (there was no space) limbic-embodied simulation-Inter-NCI on child's OT levels. Examination of the interaction showed that under condition of high parental OT levels a significant positive correlation was found between parents’core limbic-embodied simulation-inter-NCI and child’s OT (solid line) and was not found under low parental OT levels (broken line). In addition, higher child’s OT levels were positively associated with more frequent child use of complex regulatory strategies. (B). *P< 0.05; **P< 0.01; ***P< 0.001.
Associations between parent’s inter-network connectivity and child OT
Finally, to provide a system-level perspective on the parental brain and its effects on children, we assessed inter-connectivity (inter-NCI’s) between networks comprising the ‘human parental caregiving network’. Results indicate that the connectivity between parent’s core-limbic and embodied simulation-inter-NCI in infancy predicted children’s OT levels at preschool (Hypothesis 3) (r = 0.499, P = 0.0001). Interestingly, we found that parent's OT level in infancy moderated the relation between parent's core limbic-embodied simulation-NCI and child's OT levels (R2 Total = 0.367; F1,41 = 7.909, P = 0.001; Figure 3B; Supplementary Table S7). A hierarchical multiple regression predicting children’s OT by parent’s core limbic-embodied simulation–NCI, parent’s OT, and their interaction showed children’s OT was independently predicted by parent’s core limbic-embodied simulation-NCI and the interaction of this connectivity with parent’s OT. Under condition of high parental OT levels (above and below the median split, median = 29.34) significant positive correlation emerged between parents’ core limbic-embodied simulation-NCI and child's OT levels (r = 0.677, P = 0.0001), but such correlation was not found under low parent’s OT (r = 0.05, P = 0.831). Higher child’s OT was associated with more frequent child use of complex regulatory strategies (r = 0.447, P = 0.002).
Discussion
This study provides the first evidence that functionality of the human parental brain in infancy, as expressed in network integrity, bears long-term impact on key social abilities in human children and on the neurohormonal substrate that supports mammalian sociality. We found that this effect was both direct and mediated by context-specific and skill-specific parental behavior and parents’ hormones, reflecting the specificity and complexity of human social functions. Our study is the first to use integrity indices in the three main networks comprising the ‘human parental caregiving network’ to test functionality of the human parental brain. This is also the first study to follow parents and infants across the first 4 years of life to examine long-term effects of the parent's brain adaptation in infancy on the social outcomes of juveniles as measured by direct observations of key social competencies and hormonal assessments. We found specific links between network integrity in the parent’s brain in infancy and key social competencies in human children, similar to that found in other mammals. Specifically, integrity of the parent’s subcortical network was associated with the child's expression of positive emotionality and use of simple, mammalian-general regulatory behavior, whereas integrity of the parent’s cortical networks was linked with the development of human-specific sociocognitive skills, including complex emotion regulation and self-regulated socialization. Finally, functional connectivity between the parent's subcortical and embodied-simulation networks was longitudinally linked with the child’s OT levels. OT is implicated in multiple social abilities in children, adolescents and adults, including social reciprocity, empathy, trust, mind-reading and emotion detection (Bartz et al., 2011; Feldman et al., 2013; Gordon et al., 2013; De Dreu and Kret, 2016). Overall, our findings underscore one pathway by which the neurobiology of parenting shapes the social development of human children.
It has long been suggested that the parent–infant interface provides the template for species continuity and evolutionary change via reciprocal social behavior (Tinbergen, 1963). Our findings provide evidence for such cross-generational mechanisms in humans by charting longitudinal links from network integrity of the parent’s brain in infancy and two critical child social competencies in preschool-aged children as measured by observation of social behavior toward a non-kin conspecific. These critical social competencies—emotion regulation and socialization—are individually stable and predict the individual's lifetime adaptation to the social world, from social cognition to adults’ employment history (Moffitt et al., 2011; Fox and Pine, 2012; Daly et al., 2015). Overall, our findings show that the integrity of the human parental brain in the postpartum confers evolutionary advantage to human children similar to that described for other mammals (Rilling and Young, 2014; Nguyen et al., 2015). The complexity and multifinality of the human brain and social behavior is thus reflected in our study’s findings.
Consistent with animal studies that demonstrate the critical role of early parental behavior for social development of the young (Meaney, 2001; Braun and Champagne, 2014), our results describe the links between human parental behavior and young children’s emotion regulation and socialization. The human parental postpartum repertoire, comprising the non-verbal building blocks of human social exchange in the gaze, affect, vocal and touch modalities, was longitudinally related to children's use of regulatory strategies to manage moments of high arousal. This is consistent with animal studies indicating that early somatosensory and olfactory experiences bear lifetime effects on offspring (Lovic et al., 2001). In addition, parent–infant synchrony, the coupling of parent and child's social signals, intentions and communications, predicted the child's use of complex regulatory strategies. Such findings are in line with longitudinal studies indicating that the experience of interactive synchrony in the first months of life shapes a host of human-specific functions in children and adolescents, including complex socialization, symbolic competence and the capacity for empathy (Feldman, 2007b; Calkins and Hill, 2011). Finally, the parent's warm-control discipline tactics correlated with child self-regulated socialization, according with perspectives that emphasize the importance of the parent's warmth and clear limits for child socialization (Kochanska et al., 1995; Feldman and Klein, 2003).
Integrity of the parent’s core-limbic network, which supports mammalian parenting (Numan, 2012; Kumi and Numan, 2014) and is characterized by high postpartum plasticity (Kim et al., 2010; Leuner et al., 2010; Abraham et al., 2014; Royle et al., 2014), was found to predict the child’s greater skills at maintaining positive emotionality by employing simple, mammalian-general strategies. These results, which highlight some cross-generational origins, parallel findings on increases in c-Fos expression in the limbic system of juvenile rats during positive social play (van Kerkhof et al., 2013). In humans, research has shown that the regulation of negative and positive emotions is underpinned by distinct mechanisms (Feldman, 2003; Hirschler-Guttenberg et al., 2015) and that positive emotionality and social engagement in preschool predict a host of positive outcomes and greater success in social groups throughout life (Porges, 2003; Dougherty et al., 2010).
In contrast, the more complex human-specific social skills, such as attention manipulation, symbolization in the service of emotion regulation and internalization of social rules that build on sociocognitive skills and theory-of-mind capacities, depended on the integrity of the parent's embodied-simulation and mentalizing networks. These networks enable humans to share intentions and experiences during online social interactions (Frith and Frith, 2012) and their functioning has been shown to motivate empathic-related prosocial behavior in everyday life (Rameson et al., 2011). Preschoolers of parents with greater embodied-simulation connectivity displayed lower negative emotionality, which has been linked with greater social competence (Eisenberg et al., 2000), and greater use of complex regulatory strategies, abilities that characterize children with advanced social skills and lower psychopathology (Hirschler-Guttenberg et al., 2015; Ostfeld-Etzion et al. 2015). Finally, preschoolers of parents who displayed higher connectivity of the mentalizing network exhibited higher self-regulated socialization. Self-regulated socialization in the preschool years develops on the basis of synchronous parent–child interactions in infancy and supports, in turn, the emergence of higher-order social competencies in later childhood and adolescence (Kochanska et al., 1995; Feldman et al., 1999; Feldman, 2007b).
Of special interest is the finding that parent–infant synchrony mediated the longitudinal link between integrity of the parent’s embodied-simulation network in infancy and preschoolers’ complex emotion-regulation skills. This accords with biobehavioral perspectives suggesting that the brain’s embodied-simulation network builds upon early social experiences (Keysers and Gazzola, 2007; Gallese, 2014), particularly those involving the coordination of visual and motor signals into a joint brain-to-brain unit (Hasson et al., 2015). The embodied-simulation network is dyadic in nature and contains cellular and molecular mechanisms that bind two brains through parallel activity and integrate first-person experience and third-person observation (Keysers et al., 2013; Decety, 2015). It integrates online biological and behavioral signals between social partners (Gallese, 2015), thus potentially shaping the infant's social brain and the use of complex behavior to navigate the social world. Parent–infant synchrony provides the first practice of such intersubjective mechanisms through the partners' online matching of sensory-motor social cues during moments of social contact (Feldman, 2012a). Parent–infant synchrony in infancy provides the foundation for the child’s social development and shapes the lifelong capacity to regulate stress, modulate arousal and engage in coordinated social interactions with intimate partners and strangers, abilities which are critical for human participation in social life (Feldman, 2015a). Research has shown that parent–infant synchrony predicts the development of children emotion regulation, attachment security, physiological organization and empathy across childhood and adolescence (Feldman, 2007a, 2015a; Beebe et al., 2010). Moreover, the experience of biobehavioral synchrony, the coupling of physiology and behavior in parent and child, organizes the infant’s physiological systems, including the OT system, to enable parenting in the next generation, thereby supporting the cross-generation transmission of human attachment (Feldman et al., 2010). Our findings may suggest that embodied-simulation mechanisms in the parent’s brain utilize synchronous interactions to tune the infant’s brain to social life possibly via mechanisms that involve dynamic coordination between two brains (Ames et al., 2014; Hasson and Frith, 2016). With the maturation of cingular and frontal cortices’ connectivity, this early tuning enables children to reach more complex social outcomes, such as the internalization of social rules, behavior inhibition and use of symbolic acts to regulate emotions.
In addition to intra-network connectivity, we found that inter-network connectivity between the parent’s subcortical and embodied-simulation network was longitudinally associated with the development of children’s OT response, as mediated by the parent’s OT in infancy. This is consistent with recent brain studies demonstrating enhanced functional connectivity between the amygdala and the ACC and anterior insula under OT administration (Bos et al., 2012; Riem et al., 2012; Rilling et al., 2012). Findings is also consistent with research in animal models (Ross and Young, 2009) showing that connectivity between limbic and cortical networks in the parent’s brain shaped children’s OT response, which in turn supported maturation of the child’s social competencies. Overall, our results are consistent with multiple perspectives highlighting the integrative nature of this ancient peptidergic system and its reorganization by early attachment experiences (Ludwig and Leng, 2006; Feldman et al., 2016).
This study should be considered in light of its limitations. First, it is possible that other unmeasured factor, such as shared genes, contributed to the longitudinal effects. In addition, our sample includes parents of middle- to high-socioeconomic backgrounds with no reported parental psychopathology and future studies are need to generalize the findings to other populations, including parents at higher risk.
Overall, our findings challenge the solipsistic viewpoint guiding most human neuroscience research and highlight the embedded nature of the human brain. We further suggest that the human parent–infant interface provides the prototypical context for such embeddedness and that it carries profound effects on offspring’s survival and thriving. Much further research integrating brain, hormones and behavior in the context of the parent–infant unit is required to better understand the dynamic coupling of parent’s and infant’s brains and its long-term effects on the development of infant social adaptation. Our findings may provide a first step toward addressing the gap between individuals by describing how intersubjectivity implements in the parent’s brain and transmits to offspring to facilitate greater adaptation to human social life.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
Acknowledgements
Special thanks to Dr Gal Raz (the Tel Aviv Center for Brain Function) for providing the scripts and overall guidance for the network analysis using the NCI algorithm and to shimrit solnik for invaluable assistance with the NCI implementation. Supported by the German-Israeli Foundation (1114-105.2/2010), the Simms-Mann Foundation, and the Irving B. Harris Foundation, and the I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation (grant No. 51/11).to RF. Thanks for the funding of the Sagol network foundation (to TH), the Israel Centers of Research Excellence for cognitive studies (no. 51/11, to TH), and the Israeli Ministry of Science, Technology and Space (Grant no. 3-11170, to TH.).
References
- Abraham E., Hendler T., Shapira-Lichter I., Kanat-Maymon Y., Zagoory-Sharon O., Feldman R. (2014). Father’s brain is sensitive to childcare experiences. Proceedings of the National Academy of Science United States of America, 111, 9792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman J.M., Watson K.K., Tetreault N.A., Hakeem A.Y. (2005). Intuition and autism: a possible role for Von Economo neurons. Trends in Cognitive Sciences, 9, 367–73. [DOI] [PubMed] [Google Scholar]
- Ames D.L., Honey C.J., Chow M.A., Todorov A., Hasson U. (2014). Contextual alignment of cognitive and neural dynamics. Journal of Cognitive Neuroscience, 27, 655–64. [DOI] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. (2011). Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology, 36, 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales K.L., Saltzman W. (2016). Fathering in rodents: neurobiological substrates and consequences for offspring. Hormones and Behavior, 77, 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J., Fleming A.S. (2011). Annual research review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52, 368–97. [DOI] [PubMed] [Google Scholar]
- Bartz J.A., Zaki J., Bolger N., Ochsner K.N. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences, 15, 301–9. [DOI] [PubMed] [Google Scholar]
- Beebe B., Jaffe J., Markese S., et al. (2010). The origins of 12-month attachment: a microanalysis of 4-month mother–infant interaction. Attachment and Human Development, 12, 3–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem R.A.I., van Honk J., Auyeung B., Baron-Cohen S. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38, 962–74. [DOI] [PubMed] [Google Scholar]
- Bos P.A., Panksepp J., Bluthé R.M., Honk J. V. (2012). Acute effects of steroid hormones and neuropeptides on human social–emotional behavior: a review of single administration studies. Frontiers in Neuroendocrinology, 33, 17–35. [DOI] [PubMed] [Google Scholar]
- Braun K., Champagne F.A. (2014). Paternal influences on offspring development: behavioural and epigenetic pathways. Journal of Neuroendocrinology, 26, 697–706. [DOI] [PubMed] [Google Scholar]
- Burgdorf J., Panksepp J. (2006). The neurobiology of positive emotions. Neuroscience and Biobehavioral Reviews, 30, 173–87. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., et al. (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure and Function, 217(4), 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins S., Hill A. (2007). Caregiver Influences on Emerging Emotion Regulation. In: Gross J.J. editor. Handbook of Emotion Regulation. New York: Guilford Press, 229–48. [Google Scholar]
- Cohen L., Mizrahi A. (2015). Plasticity during motherhood: changes in excitatory and inhibitory layer 2/3 neurons in auditory cortex. The Journal of Neuroscience, 35, 1806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P.M., Martin S.E., Dennis T.A. (2004). Emotion regulation as a scientific construct: methodological challenges and directions for child development research. Child Development, 75, 317–33. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel: now? The anterior insula and human awareness. Nature Reviews. Neuroscience, 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Daly M., Delaney L., Egan M., Baumeister R.F. (2015). Childhood self-control and unemployment throughout the life span evidence from two British cohort studies. Psychological Science, 26, 709–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu C.K.W., Kret M.E. (2016). Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biological Psychiatry, 79, 165–73. [DOI] [PubMed] [Google Scholar]
- Decety J. (2015). The neural pathways, development and functions of empathy. Current Opinion in Behavioral Sciences, 3, 1–6. [Google Scholar]
- Dougherty L.R., Klei, D N., Emilyhayden C., Elizabeth P., Thomas M. (2010). Temperamental positive and negative emotionality and children’s depressive symptoms: a longitudinal prospective study from age three to age ten. Journal of Social and Clinical Psychology, 29, 462–88. [Google Scholar]
- Dulac C., O’Connell L.A., Wu Z. (2014). Neural control of maternal and paternal behaviors. Science, 345, 765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N., Fabes R.A., Guthrie I.K., Reiser M. (2000). Dispositional emotionality and regulation: their role in predicting quality of social functioning. Journal of Personality and Social Psychology, 78, 136–57. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Morris A.S. (2002). Children's emotion-related regulation. Advances in Child Development and Behavior, 30, 189–229. [PubMed] [Google Scholar]
- Fan Y., Duncan N.W., de Greck M., et al. (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews, 35(3), 903–11. [DOI] [PubMed] [Google Scholar]
- Featherstone R.E., Fleming A.S., Ivy G.O. (2000). Plasticity in the maternal circuit: effects of experience and partum condition on brain astroctye number in female rats. Behavioral Neuroscience, 114, 158–72. [DOI] [PubMed] [Google Scholar]
- Feldman R. (1998). Mother-Newborn Coding System Manual. Tel Aviv, Israel: Bar-Ilan University Press. [Google Scholar]
- Feldman R. (2003). Infant–mother and infant–father synchrony: the coregulation of positive arousal. Infant Mental Health Journal, 24, 1–23. [Google Scholar]
- Feldman R. (2007a). Mother-infant synchrony and the development of moral orientation in childhood and adolescence: direct and indirect mechanisms of developmental continuity. American Journal of Orthopsychiatry, 77, 582–97. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2007b). Parent–infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48, 329–54. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2007c). Parent–infant synchrony biological foundations and developmental outcomes. Current Directions in Psychological Science, 16, 340–5. [Google Scholar]
- Feldman R. (2012a). Bio-behavioral synchrony: a model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting, 12, 154–64. [Google Scholar]
- Feldman R. (2012b). Parenting Behavior as the Environment Where Children Grow. In: Mayes L.C., Lewis M., editors. The Cambridge Handbook of Environment in Human Development. New York: Cambridge University Press, 535–67. [Google Scholar]
- Feldman R. (2015a). Sensitive periods in human social development: new insights from research on oxytocin, synchrony, and high-risk parenting. Development and Psychopathology, 27, 369–95. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2015b). The adaptive human parental brain: implications for children’s social development. Trends in Neurosciences, 38, 387–99. [DOI] [PubMed] [Google Scholar]
- Feldman R., Dollberg D., Nadam R. (2011). The expression and regulation of anger in toddlers: relations to maternal behavior and mental representations. Infant Behavior and Development, 34, 310–20. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Influs M., Gutbir T., Ebstein R.P. (2013). Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology, 38, 1154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. (2010). The cross-generation transmission of oxytocin in humans. Hormones and Behavior, 58, 669–76. [DOI] [PubMed] [Google Scholar]
- Feldman R., Greenbaum C.W., Yirmiya N. (1999). Mother-infant affect synchrony as an antecedent of the emergence of self-control. Development and Psychopathology, 35, 223–31. [DOI] [PubMed] [Google Scholar]
- Feldman R., Klein P.S. (2003). Toddlers’ self-regulated compliance to mothers, caregivers, and fathers: implications for theories of socialization. Development and Psychopathology, 39, 680–92. [DOI] [PubMed] [Google Scholar]
- Feldman R., Monakhov M., Pratt M., Ebstein R.P. (2016). Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biological Psychiatry, 79, 174–84. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Calkins S.D. (2003). The development of self-control of emotion: intrinsic and extrinsic influences. Motivation and Emotion, 27, 7–26. [Google Scholar]
- Fox N.A., Pine D.S. (2012). Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50, 531–4. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2012). Mechanisms of social cognition. Annual Review of Psychology, 63, 287–313. [DOI] [PubMed] [Google Scholar]
- Gallese V. (2014). Bodily selves in relation: embodied simulation as second-person perspective on intersubjectivity. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 369, 20130177.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. (2015). Embodied simulation as second-person perspective on intersubjectivity. In: Jan De Vos, Ed Pluth, editors. Neuroscience and Critique: Exploring the Limits of Neurological Turns. New York: Routledge, 188–203. [Google Scholar]
- Goldsmith H.H., Rothbart M.K. (1996). The Laboratory Temperament Assessment Battery: Locomotor Version 3.0. Technical Manual. Madison, WI: University of Wisconsin. [Google Scholar]
- Gordon I., Wyk B.C.V., Bennett R.H., et al. (2013). Oxytocin enhances brain function in children with autism. Proceedings of the National Academy of Science United States of America, 110, 20953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Janice C., Christopher J.H. (2015). Hierarchical process memory: memory as an integral component of information processing. Trends in cognitive sciences, 19.6, 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Frith C.D. (2016). Mirroring and beyond: coupled dynamics as a generalized framework for modelling social interactions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371, 20150366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U., Ghazanfar A.A., Galantucci B., Garrod S., Keysers C. (2012). Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends in Cognitive Sciences, 16, 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschler-Guttenberg Y., Feldman R., Ostfeld-Etzion S., Laor N., Golan O. (2015). Self- and co-regulation of anger and fear in preschoolers with autism spectrum disorders: the role of maternal parenting style and temperament. Journal of Autism and Developmental Disorders, 45, 1–11. [DOI] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. (2007). Integrating simulation and theory of mind: from self to social cognition. Trends in Cognitive Sciences, 11, 194–6. [DOI] [PubMed] [Google Scholar]
- Keysers C., Thioux M., Gazzola V. (2013). Mirror neurons system and social cognition. In: Baron-Cohen S., Lombardo M., Tager-Flusberg H., Cohen D., editors. Understanding Other Minds: Perspectives from Developmental Social Neuroscience. Oxford: Oxford University Press, 233–64. [Google Scholar]
- Kim P., Leckman J.F., Mayes L.C., Feldman R., Wang X., Swain J.E. (2010). The plasticity of human maternal brain: longitudinal changes in brain anatomy during the early postpartum period. Behavioral neuroscience, 124(5), 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.E., Torres-Garcia A., Swain J.E. (2015). The parental brain: a neural framework for study of teaching in humans and other animals. The Behavioral and Brain Sciences, 38, 28–9. [DOI] [PubMed] [Google Scholar]
- Kim P., Strathearn L., Swain J.E. (2016). The maternal brain and its plasticity in humans. Hormones and Behavior, 77, 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D., et al. (2008). Functional grouping and corticalsubcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage, 42(2), 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G., Aksan N. (1995). Mother-child mutually positive affect, the quality of child compliance to requests and prohibitions, and maternal control as correlates of early internalization. Child Development, 66, 236–54. [Google Scholar]
- Kochanska G., Aksan N., Koenig A.L. (1995). A longitudinal study of the roots of preschoolers’ conscience: committed compliance and emerging internalization. Child Development, 66, 1752–69. [PubMed] [Google Scholar]
- Kochanska G., Coy K.C., Murray K.T. (2001). The development of self-regulation in the first four years of life. Child Development, 72, 1091–111. [DOI] [PubMed] [Google Scholar]
- Kuroda K.O., Numan M. (2014). The medial preoptic area and the regulation of parental behavior. Neuroscience Bulletin, 30, 863–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M., Champagne F.A. (2015). Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology, 40, 141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert K.G., Franssen C. L., Bardi M., et al. (2011). Characteristic neurobiological patterns differentiate paternal responsiveness in two Peromyscus species. Brain, Behavior and Evolution, 77, 159–75. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutiérrez D., Martinez M., et al. (2007). Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping, 28, 1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B., Glasper E.R., Gould E. (2010). Parenting and plasticity. Trends in Neurosciences, 33, 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovic V., Gonzalez A., Fleming A.S. (2001). Maternally separated rats show deficits in maternal care in adulthood. Developmental Psychobiology, 39, 19–33. [DOI] [PubMed] [Google Scholar]
- Ludwig M., Leng G. (2006). Dendritic peptide release and peptide-dependent behaviours. Nature Reviews. Neuroscience, 7, 126–36. [DOI] [PubMed] [Google Scholar]
- Meaney M.J. (2001). Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience, 24, 1161–92. [DOI] [PubMed] [Google Scholar]
- Moffitt T.E., Arseneault L., Belsky D., et al. (2011). A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Science United States of America, 108, 2693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P., Cunnington R., Mattingley J.B. (2012). Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews, 36, 341–9. [DOI] [PubMed] [Google Scholar]
- Nguyen H.B., Bagot R.C., Diorio J., Wong T.P., Meaney M.J. (2015). Maternal care differentially affects neuronal excitability and synaptic plasticity in the dorsal and ventral hippocampus. Neuropsychopharmacology, 40, 1590–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. (2012). Maternal behavior: neural circuits, stimulus valence, and motivational processes. Parenting, 12, 105–14. [Google Scholar]
- Numan M., Young L.J. (2016). Neural mechanisms of mother–infant bonding and pair bonding: similarities, differences, and broader implications. Hormones and Behavior, 77, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld-Etzion S., Golan O., Hirschler-Guttenberg Y., Zagoory-Sharon O., Feldman R. (2015). Neuroendocrine and behavioral response to social rupture and repair in preschoolers with autism spectrum disorders interacting with mother and father. Molecular Autism, 6, 11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W. (2003). Social engagement and attachment. New York Academy of Sciences, 1008, 31–47. [DOI] [PubMed] [Google Scholar]
- Posner M.I., Rothbart M.K., Sheese B.E., Voelker P. (2014). Developing attention: behavioral and brain mechanisms. Advances in Neuroscience, 2014, e405094.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson L.T., Morelli S.A., Lieberman M.D. (2011). The neural correlates of empathy: experience, automaticity, and prosocial behavior. Journal of Cognitive Neuroscience, 24, 235–45. [DOI] [PubMed] [Google Scholar]
- Raz G., Jacob Y., Gonen T., et al. (2014). Cry for her or cry with her: context-dependent dissociation of two modes of cinematic empathy reflected in network cohesion dynamics. Social Cognitive and Affective Neuroscience, 9, 30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz G., Winetraub Y., Jacob Y., et al. (2012). Portraying emotions at their unfolding: a multilayered approach for probing dynamics of neural networks. NeuroImage, 60, 1448–61. [DOI] [PubMed] [Google Scholar]
- Riem M.M.E., van IJzendoorn M.H., Tops M., Boksem M.A.S., Rombouts S.A.R.B., Bakermans-Kranenburg M.J. (2012). No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology, 37, 1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., DeMarco A.C., Hackett P.D., et al. (2012). Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology, 37, 447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J.K., Young L.J. (2014). The biology of mammalian parenting and its effect on offspring social development. Science, 345, 771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross H.E., Young L.J. (2009). Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in Neuroendocrinology, 30, 534–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle N.J., Russell A.F., Wilson A.J. (2014). The evolution of flexible parenting. Science, 345, 776–81. [DOI] [PubMed] [Google Scholar]
- Sobel M.E. (1982). Asymptotic intervals for indirect effects in structural equations models. In: S. Leinhart, editor Sociological Methodology. San Francisco: Jossey-Bass, 290–312. [Google Scholar]
- Sokolowski K., Corbin J.G. (2012). Wired for behaviors: from development to function of innate limbic system circuitry. Frontiers in Molecular Neuroscience, 5, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R.P., Lieberman M.D. (2013). The busy social brain evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychological Science, 24, 80–6. [DOI] [PubMed] [Google Scholar]
- Stanley D.A., Adolphs R. (2013). Toward a neural basis for social behavior. Neuron, 80, 816–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg D.S., Numan M. (2011). Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neuroscience and Biobehavioral Reviews, 35, 826–47. [DOI] [PubMed] [Google Scholar]
- Swain J.E. (2008). Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry, 5, 28–36. [PMC free article] [PubMed] [Google Scholar]
- Swain J.E. (2011a). Becoming a parent—biobehavioral and brain science perspectives. Current Problems in Pediatric and Adolescent Health Care, 41, 192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain J.E. (2011b). The human parental brain: in vivo neuroimaging. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35, 1242–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain J.E., Konrath S., Brown S.L., et al. (2012). Parenting and beyond: common neurocircuits underlying parental and altruistic caregiving. Parenting, 12, 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain J.E., Kim P., Spicer J., et al. (2014). Approaching the biology of human parental attachment: brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Research, 1580, 78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike R., Hagen E., Sattler J. (2003). The Stanford-Binet Intelligence Scale, 5th edn. Chicago, IL: Riverside Publication Co.. [Google Scholar]
- Tinbergen N. (1963). On aims and methods of ethology. Zeitschrift für Tierpsychologie, 20, 410–33. [Google Scholar]
- van Kerkhof LW, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LJ. (2013). Cellular activation in limbic brain systems during social play behaviour in rats. Brain Structure and Function, 219, 1181–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I.C.G., Cervoni N., Champagne F.A., et al. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7, 847–54. [DOI] [PubMed] [Google Scholar]
- Wilson E.O. (2012). On Human Nature. Cambridge, MA: Harvard University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.