Abstract
Generalized anxiety disorder (GAD) is characterized by excessive worry, autonomic dysregulation and functional amygdala dysconnectivity, yet these illness markers have rarely been considered together, nor their interrelationship tested longitudinally. We hypothesized that an individual’s capacity for emotion regulation predicts longer-term changes in amygdala functional connectivity, supporting the modification of GAD core symptoms. Sixteen patients with GAD (14 women) and individually matched controls were studied at two time points separated by 1 year. Resting-state fMRI data and concurrent measurement of vagally mediated heart rate variability were obtained before and after the induction of perseverative cognition. A greater rise in levels of worry following the induction predicted a stronger reduction in connectivity between right amygdala and ventromedial prefrontal cortex, and enhanced coupling between left amygdala and ventral tegmental area at follow-up. Similarly, amplified physiological responses to the induction predicted increased connectivity between right amygdala and thalamus. Longitudinal shifts in a distinct set of functional connectivity scores were associated with concomitant changes in GAD symptomatology over the course of the year. Results highlight the prognostic value of indices of emotional dysregulation and emphasize the integral role of the amygdala as a critical hub in functional neural circuitry underlying the progression of GAD symptomatology.
Keywords: generalized anxiety disorder, longitudinal, heart rate variability, amygdala functional connectivity, perseverative cognition, emotion regulation
Introduction
Generalized anxiety disorder (GAD) is a chronic condition characterized by excessive and uncontrollable worry, alongside symptoms of autonomic dysregulation, restlessness and muscle tension (American Psychiatric Association).
The “emotion dysregulation model” (Behar et al., 2009) explains GAD symptoms as persistent emotional hyperarousal (Mennin et al., 2005). Patients with GAD implement maladaptive emotion regulation strategies, making unsuccessful attempts to either minimize or over-control emotions. Within this scenario, worry plays a fundamental role as an ineffective strategy to cope with and manage emotions (Behar et al., 2009).
A consequence of this persistent emotional hyperarousal is autonomic dysregulation, which is often observed in GAD (Chalmers et al., 2014 for a meta-analysis) as a reduction in heart rate variability (HRV). HRV indexes the parasympathetic regulation of the heart via the vagus nerve and reflects the degree to which cardiac activity can be modulated to meet changing behavioural and emotional demands (Thayer and Lane, 2000). Tonic HRV is closely linked to individual differences in emotional responsivity and capacity for self-regulation (Thayer et al., 2009). Importantly, symptoms of worry and rumination, encapsulated within the transdiagnostic concept of perseverative cognition (Brosschot et al., 2006) are associated with chronic (e.g., Brosschot et al., 2007; Pieper et al., 2010; Ottaviani et al., 2015a, b) and phasic (e.g. Ottaviani et al., 2009, 2014; Ottaviani and Shapiro, 2011) reductions in HRV in patients with GAD (Chalmers et al., 2014) and in healthy individuals (Ottaviani et al., 2016). To date, despite this intrinsic relationship between worry and autonomic dysregulation as core features of GAD, only one previous study tests for a common underlying neurobiology for these primary symptoms (Makovac et al., in press). This study combined neuroimaging (resting-state functional magnetic resonance imaging; rsfMRI) with peripheral physiological monitoring to endorse the hypothesis that disrupted functional connectivity across amygdala-prefrontal networks underlies core features of GAD. Shared neural correlates of (dispositional and state) worry and accompanying autonomic dysregulation, further points to a common mechanism underpinning these affective and physiological manifestations of GAD (Makovac et al., in press).
Other published studies that characterize the neurobiology of GAD mainly focus on the role of the amygdala (Etkin et al., 2009), consistent with a large body of evidence implicating this brain centre in fear and threat processing (LeDoux, 2003) and also in autonomic regulation (LeDoux, 2000; Critchley et al., 2002; Makovac et al., in press). In GAD, aberrant connectivity of the amygdala is observed with prefrontal (Monk et al., 2008; Etkin et al., 2009), insula and anterior cingulate cortices (ACC) (Etkin et al., 2010; Blair et al., 2012). Taken together, this literature suggests amygdala functional dysconnectivity as a promising biomarker for GAD.
Deficits in emotion regulation represent a phenotypic correlate of anxiety disorders (Amstadter, 2008) that helps explain the maintenance of both autonomic dysregulation and excessive worry in GAD. Given the likely contributing role of the amygdala, we first hypothesized that indices of emotion regulation capacity would predict changes in amygdala functional connectivity over time. In line with a dimensional view of psychopathology, we expected these effects to be present in both GAD and (to a lesser extent) in healthy individuals. To date, few longitudinal studies have been conducted on brain functional connectivity. Our hypothesis is supported by the observation in children that amygdala connectivity longitudinally predicts anxiety symptoms and emotion regulation skills (Pagliaccio et al., 2015). In this study, we measured the cognitive (i.e. amount of worrisome and ruminative thoughts) and autonomic responses to a perseverative cognition induction as indices of the capacity for emotion regulation in patients with GAD and in healthy participants. The rationale for considering both perseverative cognition and autonomic dysregulation as indices of emotion regulation capacity originates from the first being recognized as a maladaptive emotion regulation strategy, particularly in GAD (Aldao et al., 2010 for a review) and the second being associated with emotion regulation capacity for the reason that vagally mediated HRV represents an index of inhibitory control (Williams et al., 2015). We tested whether these baseline responses to worry induction carried prognostic value in predicting long-term changes in amygdala connectivity with cortical and subcortical centres apparent 1 year later.
The second hypothesis of this study was that functional connectivity changes would reflect alterations in anxiety symptomatology (levels of anxiety, worry and autonomic dysregulation). This hypothesis was driven by an established finding; that amygdala functional connectivity can predict shifts between the manic and depressed phases of bipolar disorder (Cerullo et al., 2012). Moreover, the degree of amygdala connectivity predicts medication response and clinical improvement in paediatric bipolar disorder regardless of medication type (Wegbreit et al., 2011), further supporting this as treatment-relevant biomarker of symptom expression.
The length of the follow-up has been chosen for specific reasons: (i) it is the same time-lag examined by the few existing longitudinal studies conducted on amygdala functional connectivity, based on which we formulated our hypotheses (Pagliaccio et al., 2015; Cerullo et al., 2012); (ii) it is usually considered as an adequate time frame to evaluate the test–retest reliability of questionnaires assessing anxiety symptoms (e.g. Knappe et al., 2014) and (iii) it is a relatively standard time interval to monitor symptom changes after treatment in GAD (e.g. Bradford et al., 2011).
To our knowledge, ours is the first longitudinal study to test the effect of emotion regulation capacity on functional changes in neural connectivity, combining rsfMRI with simultaneous autonomic monitoring and symptom rating to detail the role of amygdala connectivity as a neurofunctional marker of the progression of anxiety symptoms.
Methods
Participants
At the beginning (time 0), 40 individuals were recruited by public advertisement to take part in the study, which was approved by the National Research Ethics Service (NRES) for the UK National Health Service (NHS) with university sponsorship granted via the Brighton and Sussex Medical School Research Governance and Ethics Committee. After 1-year (time 1), eight participants had dropped-out of follow up (three GAD and five healthy controls; HC). Thus, the final sample undergoing both assessments encompassed 16 patients (14 women; mean age = 29.62 ± 7.51 years) who met diagnostic criteria for GAD and 16 HC (13 women; mean age = 28.12 ± 10.11 years). No significant differences emerged between participants who dropped out and those who did not in terms of gender (χ2 = 0.14; P = 0.71), BMI (t = 0.09; P = 0.93), years of education (t = 1.51; P = 0.17), physical fitness (t = 2.00; P = 0.09) and nicotine (t = 0.43; P = 0.68) and alcohol (t = 0.76; P = 0.47) consumption. However, participants who dropped-out were significantly older than those who did not (t = 2.80; P = 0.03).
All participants were right-handed, native English speakers and had normal or corrected-to-normal vision. Exclusion criteria were: age younger than 18 years, prior history of head injury, major medical neurological or psychiatric disorder (other than GAD for the patient group), cognitive impairment, history of substance or alcohol abuse or dependence, diagnosis of heart disease, obesity (body mass index >30 kg/m2), pregnancy, claustrophobia or other general MRI exclusions. None of our participants had a formal diagnosis of co-morbid major depressive disorder (see Makovac et al., in press for further details). Two GAD participants were included who used long-term medications (one citalopram, one pregabalin) at both sessions of the study. All other patients and controls were medication free.
Between the two fMRI scans, six participants (three HC and three GAD) reported to have started psychotherapy or other interventions that might have influenced their wellbeing. Among HC, one participant started counselling and two participants started mindfulness-based yoga. Among GAD, two participants started cognitive-behavioural therapy and one started mindfulness-based yoga. No significant differences emerged between participants who started such interventions and those who did not in terms of anxiety (t = 0.11; P = 0.68) and HRV (t = 0.17; P = 0.74). However, participants who started those interventions had significantly higher increases in levels of worry from time 0 to time 1 compared with those who did not (t = 6.84; P = 0.01).
All participants provided written informed consent.
Procedure
At time 0, the Structured Clinical Interview for DSM-IV (SCID) was first administered to both patient and controls to confirm/exclude the diagnosis of GAD. Participants then completed a series of online sociodemographic and dispositional traits questionnaires. Participants were subsequently familiarized with the neuroimaging environment, connected to the physiological recording equipment and underwent the MRI protocol. Participants went through the same procedure about 1 year after the first scan (average time between sessions = 10.5 ± 2.2 months).
Questionnaires
At time 0, all participants completed a set of questions accessing sociodemographic, and lifestyle (nicotine, alcohol and caffeine consumption, physical activity) information. Core symptoms of GAD (i.e. levels of anxiety and worry) were assessed at both time points by the State-Trait Anxiety Inventory (STAI; Spielberger, 1983) and the Penn State Worry Questionnaire (PSWQ; Meyer et al., 1990).
FMRI design
At time 0, participants underwent a series of four 5-min resting-state periods, each followed by a 6-min easy visuomotor tracking task. During the resting-state periods participants were instructed to rest with their eyes open without thinking of anything and not falling asleep. After the second or third resting block, randomly, participants underwent a recorded verbal induction procedure designed to engender perseverative cognition. At the end of each resting-state period, participants rated their thoughts over the preceding period using visuo-analogue scales (VAS). At time 1, participants underwent one 5-min block of resting-state acquisition, followed by three blocks of visuomotor tracking task, without any induction procedure. Data obtained during the task blocks at time 0 and time 1, go beyond the scope of this study and will not be presented here.
Perseverative cognition induction
“Next I would like you to recall an episode that happened in the past year that made you feel sad, anxious, or stressed or something that may happen in the future that worries you. Then, I would like you to think about this episode in detail, for example about its possible causes, consequences, and your feelings about it. Please keep thinking about this until the end of the next tracking task. Thank you. Please take as much time as you need to recall the episode and press the button whenever you are ready”.
Visual analogue scales (VAS)
At time 0, levels of perseverative cognition occurring prior to and following the induction were assessed. Participants were asked to rate on a visual analogue 100-point scale: “how much, for the duration of the previous resting period, they were distracted by ruminative or worrisome thoughts” (see Makovac et al., in press for a full description of the VAS administered at time 0).
Physiological data processing
At both time points, HR was monitored using MRI-compatible finger pulse oximetry (8600FO; Nonin Medical) recorded digitally as physiological waveforms at a sample rate of 1000 Hz (via a CED power 1401, using Spike2 v7 software; Cambridge Electronic, Design CED). Inter-beat-intervals values were visually inspected and potential artefacts were manually removed. To this pulse data, we applied the root mean square successive difference (RMSSD), which is a reliable parameter for assessing vagally mediated HRV (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). RMSSD was derived using RHRV 4.0 analysis software (http://rhrv.r-forge.r-project.org/) for the duration of each resting-state scanning period. At time 0, attention was given to measures before (pre) and after (post) the perseverative cognition induction. For correlational analyses, we calculated the shift in RMSSD from pre- to post-induction at time 0 (ΔHRV [post-induction− pre-induction]) and the shift in resting RMSSD from time 0 (i.e. pre-induction) to Time 1 (ΔHRV [time 1−time 0]).
MRI acquisition and pre-processing
MRI images were acquired on a 1.5-Tesla Siemens Magnetom Avanto scanner (Siemens AG, Munich, Germany). Functional datasets used T2*weighted echoplanar imaging (EPI) sensitive to Blood oxygenation level dependent (BOLD) signal (TR = 2.52 s, TE = 43 ms, flip-angle 90°, 34 slices, 3-mm slice thickness, 192 mm FOV, voxel size 3 × 3 × 3 mm).
Data were pre-processed using Statistical Parametric Mapping (Wellcome Department of Imaging Neuroscience; SPM8, http://www.fil.ion.ucl.ac.uk/spm/), and in-house software implemented in Matlab (The Mathworks Inc, Natick, MA, USA). For each participant, the first four volumes of the fMRI series were discarded to allow for T1 equilibration effects. The pre-processing steps included correction for head motion, compensation for slice-dependent time shifts, normalization to the EPI template in standard space (MNI) coordinates provided with SPM8, and, to better account for inter-subject variability, smoothing with a3D Gaussian Kernel with 8-mm3 full-width at half maximum. The global temporal drift was removed using a third-order polynomial fit. To remove other potential sources of bias, data were further filtered regressing against the realignment parameters, and the mean white matter and cerebrospinal fluid signal averaged over all voxels. Then, all images were filtered by a phase-insensitive band-pass filter (pass band 0.01–0.08 Hz) to reduce the effect of low frequency drift and high-frequency physiological noise.
As global signal removal can potentially change functional connectivity distributions and result in increased negative correlations (Saad et al., 2012), it was avoided in our pre-processing.
Statistical analyses
Questionnaire, behavioural and HRV analyses
All data are expressed as means (±SD). Differences at P ≤ 0.05 are regarded as significant. Data analysis was performed with SPSS 22.0 for Windows (SPSS Inc, USA).
Baseline differences between groups and the effectiveness of the induction in enhancing levels of perseverative cognition were tested elsewhere and will not be repeated here (Makovac et al., in press). Here, the post- minus pre-induction change scores of (i) levels of perseverative cognition (VAS: worrying or ruminating?) and (ii) HRV measures have been calculated and adopted in subsequent correlational analysis.
Seed-based fMRI analysis
Anatomical ROIs were constructed using an anatomical toolbox in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) for bilateral amygdala. The average resting-state fMRI time-series over the ROIs were extracted for each participant and for each scan at time 0 (pre-induction resting-state block) and time 1.
The difference in amygdala connectivity between time 1 and time 0 was estimated for each subject in a first level SPM analysis by regressing the fMRI data against the corresponding ROI time series, and extracting the [time 0 < time 1] contrast. The pre-induction resting-state block at time 0 and the only resting state block gathered at time 1 have been used for the analysis of resting state functional connectivity changes in the amygdala.
To test for associations between amygdala connectivity and cognitive and autonomic measures of emotion regulation, a t-test was run at second level on these contrast images, with Group (GAD vs HC) as the factor and ΔVAS and ΔHRV as covariates of interest. To determine significance within a priori regions (functional connectivity) for which we had strong hypotheses, we restricted our analyses to these regions using an anatomically derived (AAL atlas) partial brain mask of entire prefrontal cortex/frontal lobe. Statistical threshold was set to P < 0.05—FWE-corrected at cluster level (cluster size defined using uncorrected voxel-level threshold P < 0.005). For completeness, results outside our ROI which might be highly informative of the GAD condition are also reported.
For illustrative purposes, parameter estimates for regions that showed significant correlations with the cognitive and autonomic measures of emotion regulation were extracted and plotted.
Results
Neither at time 0 (see Makovac et al., in press) nor at time 1 (see Table S1 in the Supplementary Results) group differences emerged for any of the assessed sociodemographic and lifestyle variables; therefore, these were not included as covariates in the subsequent analyses. Table 1 shows the correlations between the main variables of the study at the two time points.
Table 1.
Means and correlations between the key variables of the study at time 0 and time 1 in GAD patients and healthy participants
| Mean ± SD | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| 1. STAI T0 | 45.09 ± 13.10 | 1 | 0.88** | 0.00 | 0.87** | 0.79** | 0.02 |
| 2. PSWQ T0 | 54.59 ± 17.27 | 1 | −0.11 | 0.75** | 0.86** | −0.52 | |
| 3. HRV T0 | 65.17 ± 43.77 | 1 | −0.15 | −0.23 | 0.53** | ||
| 4. STAI T1 | 44.44 ± 12.36 | 1 | 0.79** | 0.14 | |||
| 5. PSWQ T1 | 53.25 ± 17.74 | 1 | 0.14 | ||||
| 6. HRV T1 | 61.63 ± 34.19 | 1 |
STAI, State Trait Anxiety Inventory; PSWQ, Penn State Worry Questionnaire; HRV, Heart Rate Variability; T0, Time 0; T1, Time 1; ** = P < 0.01.
Cognitive responses to the induction of perseverative cognition at time 0 predict changes in functional connectivity from time 0 to time 1
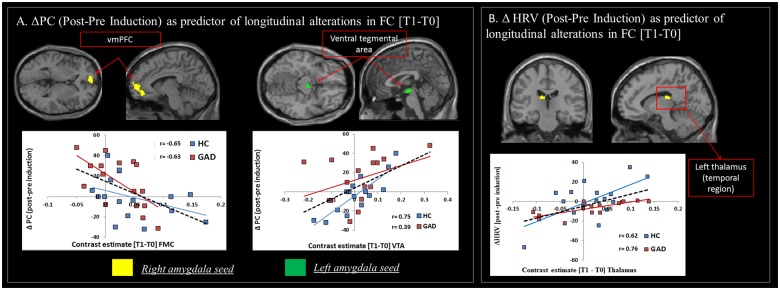
Changes in state levels of perseverative cognition (VAS) after the induction predicted longitudinal alterations in functional connectivity from time 0 to time 1 across both groups of GAD and HC. As illustrated in Table 2(1), two significant associations emerged:
A negative correlation between pre- to post-induction shift in state levels of perseverative cognition (post-induction− pre-induction) and the change in functional connectivity [time 1−time 0] between right amygdala and ventromedial prefrontal cortex (vmPFC) (r = −0.60, P < 0.001). This finding indicates that individuals who reported a stronger increase in levels of ruminative and worrisome thoughts after the induction at time 0 exhibited the strongest reduction in connectivity between these two regions after 1 year. The correlation was driven by a strong negative correlation in the group of GAD compared to HC (Figure 1A, left panel).
A positive correlation between pre- to post-induction changes in state levels of perseverative cognition and longitudinal changes in functional connectivity between left amygdala and ventral tegmental area (VTA) across both groups (r = 0.51, P < 0.01). This observation indicated that individuals with stronger increases in levels of perseverative cognition after the induction were characterized by increased connectivity between these two regions at follow-up (Figure 1A, right panel).
Table 2.
Brain areas showing significant correlations between amygdala connectivity changes over time and cognitive (1) and autonomic (2) responses to the induction and longitudinal modifications in symptoms of anxiety (3a) and autonomic dysregulation (3b) in generalized anxiety disorder patients and healthy participants
| Brain region | Seed | Side | Cluster | Voxel | ||
|---|---|---|---|---|---|---|
| k | P FWE | Z | MNI xyz | |||
| (1) ΔPC (post–pre induction) as predictor of FC longitudinal alterations [T1–T0] | ||||||
| vmPFC | Right amygdala | L | 430 | 0.001 | 3.94 | −12 44 −12 |
| VTA | Left amygdala | 250 | 0.018 | 3.64 | 2 −10 −14 | |
| (2) Δ HRV (post–pre induction) as predictor of FC longitudinal alterations [T1–T0] | ||||||
| Thalamus (temporal region) | Right amygdala | L | 247 | 0.021 | 4.38 | −10 −22 18 |
| (3) Correlation between FC longitudinal alterations [T1-T0] and changes in anxiety symptoms | ||||||
| (3a) Anxiety (ΔSTAI [T1–T0]) | ||||||
| Middle frontal gyrus | Right amygdala | R | 438 | 0.000 | 4.39 | 38 10 62 |
| dlPFC | Right amygdala | R | 318 | 0.000 | 5.04 | 24 48 8 |
| PCC | Right amygdala | L | 292 | 0.000 | 3.94 | 0 −54 28 |
| (3b) HRV (ΔHRV [T1–T0]) | ||||||
| Insula | Right amygdala | L | 545 | 0.008 | 3.85 | −38 8 −2 |
| Temporal/angular gyrus | Right amygdala | R | 224 | 0.032 | 3.72 | 62 −52 12 |
PC, perseverative cognition; STAI, State Trait Anxiety Inventory; PSWQ, Penn State Worry Questionnaire; HRV, Heart Rate Variability; T0, Time 0; T1, Time 1.
Fig. 1.
Cognitive and autonomic responses to the induction of perseverative cognition at time 0 predict changes in functional connectivity from time 0 to time 1. (A) Associations between Δ perseverative cognition (ΔPC) (post–pre induction) and the shift in functional connectivity [Time 1–Time 0] between: right amygdala and vmPFC (negative correlation; left panel) and left amygdala and VTA (positive correlation; right panel). (B) Positive correlation across groups between ΔHRV (post–pre induction) and the shift in functional connectivity [Time 1–Time 0] between right amygdala and temporal thalamus. Note. Correlational plots are for illustrative purposes only. The dashed black line indicates the regression line across both groups; coloured (blue and red) lines indicate the regression line for the each group, separately, and are represented for illustrative purposes only (i.e. to elucidate the influence of each group individual slope on the overall regression).
Physiological (HRV) responses to the induction of perseverative cognition at time 0 predict changes in functional connectivity from time 0 to time 1
A positive correlation was observed between the pre- to post-induction shift in HRV [post–pre] values and longitudinal changes in functional connectivity [time 1–time 0] between right amygdala and the temporal region of the thalamus (r = 0.56, P < 0.001). This observation indicates that individuals with the strongest decrease in HRV after the induction at time 0 showed the greatest loss in connectivity between right amygdala and thalamus at 1-year follow-up (Table 2(2), Figure 1B).
Changes in functional connectivity from time 0 to time 1 reflect changes in GAD symptomatology (anxiety, worry and autonomic dysfunction)
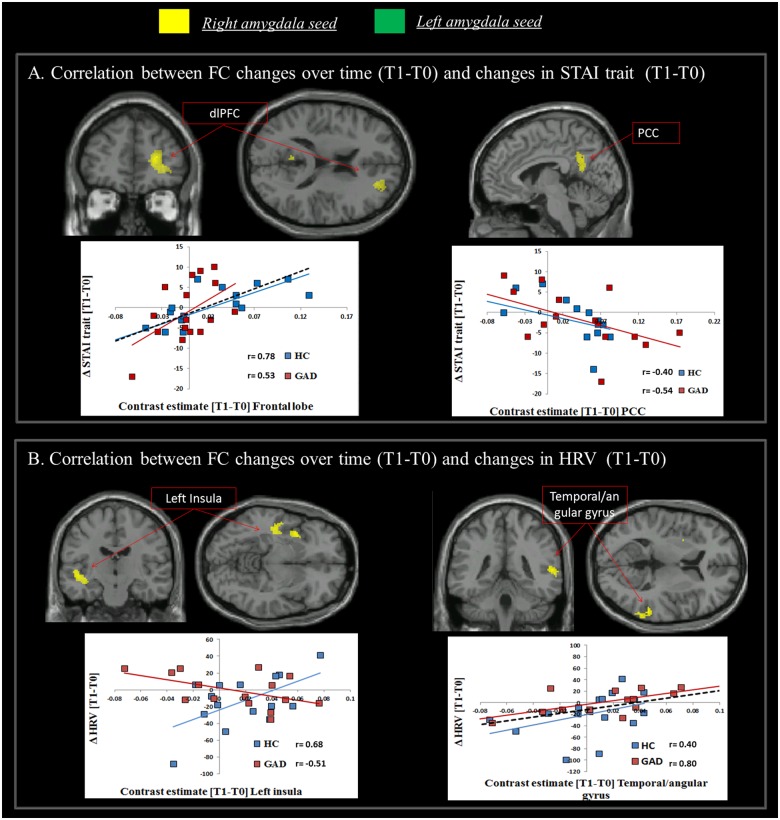
As to the core symptom of anxiety, a positive correlation emerged across both groups between the change in STAI score over time (ΔSTAI [time 1–time 0]) and the shift in connectivity [time 1–time 0] between right amygdala and dorso-lateral PFC (dlPFC) (r = 0.61, P < 0.001). This shows that individuals who reported an increase in trait anxiety after 1 year also manifested an increase in functional connectivity between these brain areas. A negative correlation was observed between the shift in STAI scores over time and the change in functional connectivity between right amygdala and the posterior cingulate cortex (PCC) (r = −0.45, P < 0.01), indicating greater disconnection between these two regions in individuals reporting increases in anxiety symptoms after 1 year (Table 2(3a), Figure 2A).
Fig. 2.
Changes in functional connectivity from time 0 to time 1 reflect changes in GAD symptomatology (anxiety and autonomic dysfunction). A significant correlation was observed between: (A) ΔSTAI [time 1–time 0] and the shift in functional connectivity [time 1–time 0] between right amygdala and dlPFC (positive correlation) and the PCC (negative correlation); (B) ΔHRV [time 1–time 0] and the shift in functional connectivity [time 1–time 0] between right amygdala and left insula (significant interaction indicating a positive correlation in the group of HC and a negative correlation in GAD) and right temporal/angular gyrus area (positive correlation across both groups). Note. The correlational plots are for illustrative purposes only. The dashed black line indicates the regression line across both groups, coloured (blue and red) lines indicate the regression line for each group, separately, and are represented for illustrative purposes only (i.e. to elucidate the influence of each group individual slope on the overall regression).
Given the overlap, results for the PSWQ are provided in S1, Table S2, and Figure S1 in the Supplementary Results.
With respect to autonomic dysregulation, a group × ΔHRV [time 1–time 0] interaction was significant for functional connectivity alterations [time 1–time 0] between right amygdala and left insula. In patients with GAD, a longitudinal decrease in HRV was associated with an increase in connectivity between right amygdala and left insula (r = −0.51, P <0.05). The opposite pattern was evident in HC (r = 0.68, P < 0.01). Both groups showed a positive correlation between Δ HRV and change in the functional connectivity between right amygdala and right temporal/angular gyrus (r = 0.50, P < 0.001) (Table 2(3b), Figure 2B). Here, HRV reductions from time 0 to time 1 were associated with decreased connectivity between these areas.
Additional analyses
For completeness, additional analyses on baseline levels of subjective and physiological responses to the induction and amygdala functional connectivity (time 0) as potential predictors of symptoms progression after 1 year were performed. In summary, results confirmed the role of HRV (S2, Supplementary Results) and baseline amygdala functional connectivity (S3, Figure S2, Supplementary Results) in predicting changes in levels of anxiety and autonomic dysregulation over the course of the year.
Discussion
We combined rsfMRI with cognitive and physiological assessment at two time points, to quantify longitudinally alterations in amygdala functional connectivity with the rest of the brain in GAD. Autonomic and behavioural indices of emotion regulation capacities were acquired at time 0 before and after a perseverative cognition induction, and were tested for their prognostic value on longer-term changes in amygdala connectivity. Moreover, we tested the hypothesis that changes derived subtracting amygdala functional connectivity at time 0 to that at time 1 correspond to changes in symptomatology (levels of anxiety, worry and autonomic dysregulation).
Our data indicate that the cognitive and autonomic response to an emotion regulation task has a prognostic value on the functional organization of emotion-relevant circuitry within the brain after 1 year. In both patients and controls, subjective increases in the amount of ruminative and worrisome thoughts experienced after the worry induction predicted a decrease in functional connectivity between right amygdala and vmPFC and an increase in connectivity between left amygdala and the VTA up to 1 year later. Prefrontal cortex hypo-activation during emotion regulation is a recognized feature in GAD (Ball et al., 2013) and earlier rsfMRI studies highlight a perturbation of amygdala-prefrontal functional connectivity underlying the core features of GAD (Hilbert et al., 2014). A decrease in functional connectivity between amygdala and PFC is in fact reported in adults (Etkin et al., 2009) and adolescents with GAD (Monk et al., 2008; Roy et al., 2013). Here, we extend these observations by demonstrating that a heightened reaction to a perseverative cognition induction has a prognostic value in predicting a longitudinal alteration within the amygdala-PFC network, highlighting a potential target for early intervention. Importantly, subregions of PFC are extremely active at rest and contribute to the so-called default mode network (DMN), a system directly implicated in mind-wandering and related self-generated thoughts. Plausibly, whenever mind wandering takes the form of perseverative cognition, prefrontal inhibition is tuned down, permitting an increase in physiological activity (Thayer and Lane, 2009). Given the pervasiveness of worry in GAD, it is suggested that such chronic and persistent physiological activation (Ottaviani et al., 2016) may ultimately lead to somatic health problems (Perseverative Cognition Hypothesis; Brosschot et al., 2006) including the increased cardiovascular risk that characterizes this clinical population (Larsen and Christenfeld, 2009). Therefore, the identification of biobehavioural markers longitudinally associated with alterations within this network is important not only to predict the psychological evolution of anxiety symptoms, but also prevent its negative physical consequences impacting the somatic health of GAD patients.
An increase in worrisome and ruminative thoughts after the induction at time 0 also predicted augmented functional connectivity between left amygdala and the VTA over time. The VTA has distributed connections across forebrain regions involved in emotional and cognitive processes, including hippocampus (Lisman and Grace, 2005), nucleus accumbens, amygdala and PFC (Roeper, 2013). Importantly, the VTA projections release of dopamine, a neurmodulator that shapes both appetitive motivational behaviours and acts potently on mechanisms underlying states of fear and anxiety. Dopaminergic neurotransmission influences each phase of memory for affective information, i.e. formation, expression, retrieval and extinction (Pezze and Feldon, 2004). In mice, the conditioning to a cue that predicts an aversive situation is impaired if the excitatory responses of dopamine neurons are altered, resulting in the development a generalized anxiety-like phenotype. In contrast, dopamine also facilitates contingency awareness during threat-learning, which is critical for the prevention of generalized anxiety (Zweifel et al., 2011). In humans, increased functional connectivity between the VTA and vmPFC is linked to adaptive emotion regulation (Mulej Bratec et al., 2015). Within our own data from people with and without GAD, we observed the longitudinal strengthening of connectivity between VTA and amygdala in participants who showed the most pathological response to the induction, pointing to a maladaptive neurobiological restructuring linked to impaired emotion regulation.
Generalized anxiety is not only characterized by repetitive worry but also by impaired autonomic regulation (Chalmers et al., 2014) and these two core symptoms are likely to be strictly intercorrelated. Indeed, chronic reductions in resting-state HRV are associated with worry (Thayer et al., 1996). Consistently, we previously reported a greater drop in HRV after perseverative cognition induction at time 0 in GAD patients compared to HC, that was mediated by lower baseline connectivity between bilateral amygdala and PFC/cingulum in GAD (Etkin et al., 2009; Makovac et al., in press). In this study, we extended these observations by demonstrating that participants with least physiological capacity for emotional regulation (i.e. greatest drop in HRV after the induction at time 0) went on to manifest a functional decoupling of right amygdala with the thalamus over ensuing months. During states of high HRV (increased parasympathetic tone), the thalamus is more strongly coupled to amygdala (along with brainstem, putamen, ACC and dorsolateral prefrontal cortex; Chang et al., 2013). Our data show that such “adaptive” communication between amygdala and thalamus might decrease over time in individuals who exhibit a dysfunctional physiological threat response (exaggerated parasympathetic withdrawal) during perseverative cognition. It is noteworthy that a GABAergic pathway from the amygdala to thalamus projects directly onto orbital cortex, forming a tight tripartite system that shapes the formation of emotions, and tempers the impact of direct amygdala-orbital projections to influence the neuronal representations through which emotions bias affective reasoning (Timbie and Barbas, 2015). Correspondingly, HRV represents a potential biomarker of progressive functional alterations between structures involved in both autonomic responses and anxiety symptoms in GAD. Recent studies already suggest that HRV can be efficient in predicting the risk of developing post-traumatic stress disorder (Shah and Vaccarino, 2015).
Our demonstration that baseline measures of emotional regulation capacity determine, over time, changes in amygdala-centred organization of functional networks across brain carries more importance as such changes are linked to anxiety symptomatology. Across groups, individuals who reported the greatest increase in levels of trait (i.e. how they generally feel) anxiety and worry up to 1 year later, also showed the deepest decrease in functional connectivity between right amygdala and PCC (a major DMN ‘hub’). The functional connection between the PCC and the amygdala is recognized (Stein et al., 2007; Roy et al., 2009), where intrinsic activity within PCC is altered in anxiety disorders (Sylvester et al., 2012; Andreescu et al., 2011) and linked to self-referential thoughts and worry in anxiety (Paulesu et al., 2010).
Contrary to our initial expectation, increases in trait anxiety over the year were associated with augmented amygdala connectivity with dlPFC. However, this result may reflect the differential contributions of the medial and lateral prefrontal cortices in fear and anxiety. In rodents, lesions of the mPFC may decrease anxiety, while lesions of the lateral PFC substantially increase freezing in fear-conditioning paradigms (Lacroix et al., 2000). Similarly, we observed that more worrisome thoughts after the induction predicted a reduction in amygdala functional connectivity with vmPFC over time, while enhanced connectivity with lateral PFC was associated with increased trait anxiety after 1 year. There are earlier observations of this dissociation in humans. A positive increase in functional connectivity of amygdala to middle frontal gyrus, and a decrease in amygdala-vmPFC coupling is observed in high trait anxiety individuals during anticipatory anxiety (Vytal et al., 2014). Moreover, following an effective mindfulness-based therapy, improvements in anxiety scores negatively correlate with changes in functional connectivity of right amygdala to mPFC (Hölzel et al., 2013). Taken together, our current and previous findings add to growing evidence for a dissociable function of lateral and ventromedial PFC coupling with amygdala in anxiety.
The changes in amygdala functional connectivity over time also resulted correlated with changes in resting HRV from time 0 to time 1 with the opposite pattern in GAD and controls. In the case of GAD, a stronger decrease in HRV over time was associated with increased connectivity between right amygdala and left insula whereas the opposite pattern (diminished connectivity) emerged in controls. An increased connectivity between amygdala and insula is already described in GAD, both at rest (Roy et al., 2009) and when engaged in a task (McClure et al., 2007). One influential account of role of the insula in anxiety disorders highlights the region’s contribution to autonomic control (Paulus and Stein, 2006). This region integrates the information about the salience of environmental stimuli with the effects that these stimuli have on the body state (Critchley et al., 2002; Paulus and Stein, 2006; Terasawa et al., 2013; Craig, 2011) and this is assisted by bidirectional connections with the amygdala (Nieuwenhuys, 2012). The insula is also involved in the regulation of autonomic responses, consistent with a functional organization that integrates cardiovascular response patterns in the service of emotional behaviours. These processes might be disrupted in anxious individuals, making it difficult for them to differentiate normal fluctuations in interoceptive stimuli from potentially aversive body signals, resulting in an increased and persisting worry (Paulesu et al., 2010). Thus, insula activation is observed in response to worry in a population suffering from depression and anxiety (Servaas et al., 2014), in paediatric anxiety disorders (Hamm et al., 2014), and in adults with GAD (Hoehn-Saric et al., 2004). Worry induction in elderly GAD participants evoked stronger connectivity between insula and orbitofrontal cortex that diminished during reappraisal (Andreescu et al., 2015). Stronger amygdala-insula functional connectivity is also reported in urban youth exposed to trauma (Thomason et al., 2015) and adults with PTSD (Rabinak et al., 2011), highlighting reproducible involvement of amygdala–insular interaction for anxiety disorders.
Lastly, changes in HRV over time were positively correlated with the functional connectivity between right amygdala and areas implicated in memory retrieval and attention, including right middle temporal gyrus and angular gyrus. More specifically, HRV reductions from time 0 to time 1 were associated with similar decreases in resting-state connectivity in regions underlying the perceptual, attentional and working memory deficits associated with trait anxiety (Modi et al., 2015).
One limitation of this study is the relatively small sample size, due to difficulties in asking GAD patients to receive two MRI scans in a year. The prevalence of women in the sample reflects the unequal gender distribution in the GAD and, despite our best attempts at matching patients and controls, it may have biased the results. Another important limitation is that our measures of functional connectivity do not allow determining the direction of causal relationships between brain regions. Limitations notwithstanding, our data provides important new insight into neural mechanisms through which emotional regulation and autonomic dysfunction interact in GAD, and broadens this knowledge by clarifying the prognostic value of measures of emotion regulation on longitudinal functional connectivity alterations. Overall, the present study further validates the use of rsfMRI in the evaluation and monitoring of psychiatric disorders (Woodward and Cascio, 2015). If the robustness of our results will be confirmed by future longitudinal research, differences in connectivity may represent neurofunctional markers of emotion regulation capacities and anxiety symptoms progression.
Supplementary data
Supplementary data are available at SCAN online.
Funding
This work was supported by the Italian Ministry of Health Young Researcher Grant [GR-2010-2312442] to C.O.
Conflict of interest. None declared.
Supplementary Material
References
- Aldao A., Nolen-Hoeksema S., Schweizer S. (2010). Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychological Review 30, 217–37. [DOI] [PubMed] [Google Scholar]
- Amstadter A.B. (2008). Emotion regulation and anxiety disorders. Journal of Anxiety Disorders 22, 211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreescu C., Sheu L.K., Tudorascu D., et al. (2015). Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. American Journal of Geriatric Psychiatry 23, 200–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T.M., Ramsawh H.J., Campbell-Sills L. (2013). Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorder. Psychological Medicine 43, 1475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar E., DiMarco I.D., Hekler E.B., Mohlman J., Staples A.M. (2009). Current theoretical models of generalized anxiety disorder (GAD): conceptual review and treatment implications. Journal of Anxiety Disorders 23, 1011–23. [DOI] [PubMed] [Google Scholar]
- Blair K.S., Geraci M., Smith B.W., et al. (2012). Reduced dorsal anterior cingulate cortical activity during emotional regulation and top-down attentional control in generalized social phobia, generalized anxiety disorder, and comorbid generalized social phobia/generalized anxiety disorder. Biological Psychiatry 72, 476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford A., Cully J., Rhoades H., et al. (2011). Early response to psychotherapy and long-term change in worry symptoms in older adults with generalized anxiety disorder. American Journal of Geriatric Psychiatry 19, 347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot J.F., Gerin W., Thayer J.F. (2006). The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation and health. Journal of Psychosomatic Research 60, 113–24. [DOI] [PubMed] [Google Scholar]
- Brosschot J.F., Van Dijk E., Thayer J.F. (2007). Daily worry is related to low heart rate variability during waking and the subsequent nocturnal sleep period. International Journal of Psychophysiology 63, 39–47. [DOI] [PubMed] [Google Scholar]
- Cerullo M.A., Fleck D.E., Eliassen J.C., et al. (2012). A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disorder 14, 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers J.A., Quintana D.S., Abbott M.J.A., et al. (2014). Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Frontiers in Psychiatry 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Metzger C.D., Glover G.H., et al. (2013). Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences 1225, 72–82. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Dolan R.J. (2002). Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33, 653–63. [DOI] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Hoeft F., et al. (2010). Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. American Journal of Psychiatry 167, 545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A., Prater K.E., Schatzberg A.F., et al. (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry 66, 1361–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm L., Jacobs R.H., Johnson M.W., et al. (2014). Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biology of Mood and Anxiety Disorders 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert K., Lueken U., Beesdo-Baum K. (2014). Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: a systematic review. Journal of Affective Disorders 158, 114–26. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R., Schlund M.W., Wong S.H. (2004). Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Research 131, 11–21. [DOI] [PubMed] [Google Scholar]
- Hölzel B.K., Hoge E.A., Greve D.N., et al. (2013). Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. Neuroimage Clinical 2, 448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe S., Klotsche J., Heyde F, et al. (2014). Test-retest reliability and sensitivity to change of the dimensional anxiety scales for DSM-5. CNS Spectrums 19, 256–67. [DOI] [PubMed] [Google Scholar]
- Lacroix L., Spinelli S., Heidbreder C.A., et al. (2000). Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behavioral Neuroscience 114, 1119–30. [DOI] [PubMed] [Google Scholar]
- Larsen B.A., Christenfeld N.J. (2009). Cardiovascular disease and psychiatric comorbidity: the potential role of perseverative cognition. Cardiovascular Psychiatry and Neurology, 2009, 791017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review in Neuroscience 23, 155–84. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2003). The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology 23, 727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J.E., Grace A.A. (2005). The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 46, 703–13. [DOI] [PubMed] [Google Scholar]
- Makovac E., Meeten F., Watson D., et al. Alterations in amygdala-prefrontal functional connectivity account for excessive worry and autonomic dysregulation in generalized anxiety disorder. Biological Psychiatry (in press). [DOI] [PubMed] [Google Scholar]
- McClure E.B., Monk C.S., Nelson E.E., et al. (2007). Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry 64, 97–106. [DOI] [PubMed] [Google Scholar]
- Mennin D.S., Heimberg R.G., Turk C.L., et al. (2005). Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behavior Research and Therapy 43, 1281–310. [DOI] [PubMed] [Google Scholar]
- Meyer T.J., Miller M.L., Metzger R.L. (1990). Development and validation of the Penn State Worry Questionnaire. Behavior Research and Therapy 28, 487–95. [DOI] [PubMed] [Google Scholar]
- Modi S., Kumar M., Kumar P., et al. (2015). Aberrant functional connectivity of resting state networks associated with trait anxiety. Psychiatry Research 234, 25–34. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Telzer E.H., Mogg K., et al. (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry 65, 568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulej Bratec S., Xie X., Schmid G., et al. (2015). Cognitive emotion regulation enhances aversive prediction error activity while reducing emotional responses. Neuroimage 123, 138–48. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. (2012). The insular cortex: a review. Progress in Brain Research 195, 123–63. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Borlimi R., Brighetti G., et al. (2014). Worry as an adaptive avoidance strategy in healthy controls but not in pathological worriers. International Journal of Psychophysiology 93, 349–55. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Medea B., Lonigro A., Tarvainen M., Couyoumdjian A. (2015a). Cognitive rigidity is mirrored by autonomic inflexibility in daily life perseverative cognition. Biological Psychology 107, 24–30. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Shahabi L., Tarvainen M., Cook I., Abrams M., Shapiro D. (2015b). Cognitive, behavioral, and autonomic correlates of mind wandering and perseverative cognition in major depression. Frontiers in Neuroscience 8, 433.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C., Shapiro D., Couyoumdjian A. (2013). Flexibility as the key for somatic health: from mind wandering to perseverative cognition. Biological Psychology 94, 38–43. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Shapiro D. (2011). Do we need a stressor to be stressed? Insights from cardiac regulation. Japanese Psychological Research 53, 155–62. [Google Scholar]
- Ottaviani C., Shapiro D., Davydov D.M., Goldstein I.B., Mills P.J. (2009). The autonomic phenotype of rumination. International Journal of Psychophysiology 72, 267–75. [DOI] [PubMed] [Google Scholar]
- Ottaviani C., Thayer J.F., Verkuil B., et al. (2016). Physiological concomitants of perseverative cognition: a systematic review and metaanalysis. Psychological Bulletin 142, 231–59. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., et al. (2015). Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. Journal of Abnormal Psychology 124, 817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E., Sambugaro E., Torti T., et al. (2010). Neural correlates of worry in generalized anxiety disorder and in normal controls: a functional MRI study. Psychological Medicine 40, 117–24. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. (2006). An insular view of anxiety. Biological Psychiatry 60, 383–7. [DOI] [PubMed] [Google Scholar]
- Pezze M.A., Feldon J. (2004). Mesolimbic dopaminergic pathways in fear conditioning. Progress in Neurobiology 74, 301–20. [DOI] [PubMed] [Google Scholar]
- Pieper S., Brosschot J.F., van der Leeden R., et al. (2010). Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosomatic Medicine 72, 570–7. [DOI] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Welsh R.C., et al. (2011). Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers in Psychiatry 2, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J. (2013). Dissecting the diversity of midbrain dopamine neurons. Trends in Neuroscience 36, 336–42. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Fudge J.L., Kelly C., et al. (2013). Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry 52, 290–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., et al. (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45, 614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., et al. (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas M.N., Riese H., Ormel J., Aleman A. (2014). The neural correlates of worry in association with individual differences in neuroticism. Human Brain Mapping 35, 4303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Vaccarino V. (2015). Heart rate variability in the prediction of risk for posttraumatic stress disorder. JAMA Psychiatry 72, 964–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C.D. (1983). State-Trait Anxiety Inventory. A Comprehensive Bibliography. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Stein J.L., Wiedholz L.M., Bassett D.S., et al. (2007). A validated network of effective amygdala connectivity. Neuroimage 36, 736–45. [DOI] [PubMed] [Google Scholar]
- Sylvester C.M., Corbetta M., Raichle M.E., et al. (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neuroscience 35, 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996). Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 93, 1043–65. [PubMed] [Google Scholar]
- Terasawa Y., Shibata M., Moriguchi Y., Umeda S. (2013). Anterior insular cortex mediates bodily sensibility and social anxiety. Social Cognitive and Affective Neuroscience 8, 259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J.F., Friedman B.H., Borkovec T.D. (1996). Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry 39, 255–66. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Hansen A.L., Saus-Rose E., et al. (2009). Heart rate variability, prefrontal neural function and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine 37, 141–53. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders 61, 201–16. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews 33, 81–8. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Marusak H.A., Tocco M.A., et al. (2015). Altered amygdala connectivity in urban youth exposed to trauma. Social Cognitive and Affective Neuroscience 10, 1460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbie C., Barbas H. (2015). Pathways for emotions: specializations in the amygdalar, mediodorsal thalamic, and posterior orbitofrontal network. Journal of Neuroscience 35, 11976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal K.E., Overstreet C., Charney D.R., et al. (2014). Sustained anxiety increases amygdala–dorsomedial prefrontal coupling: a mechanism for maintaining an anxious state in healthy adults. Journal of Psychiatry and Neuroscience 39, 321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegbreit E., Ellis J.A., Nandam A., et al. (2011). Amygdala functional connectivity predicts pharmacotherapy outcome in pediatric bipolar disorder. Brain Connectivity 1, 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.P., Cash C., Rankin C., Bernardi A., Koenig J., Thayer J.F. (2015). Resting heart rate variability predicts self-reported difficulties in emotion regulation: a focus on different facets of emotion regulation. Frontiers in Psychology 6, 261.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N.D., Cascio C.J. (2015). Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry 72, 743–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel L.S., Fadok J.P., Argilli E., et al. (2011). Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nature Neuroscience 14, 620–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.