Abstract
Being able to infer the thoughts, feelings and intentions of those around us is indispensable in order to function in a social world. Despite growing interest in social cognition and its neural underpinnings, the factors that contribute to successful mental state attribution remain unclear. Current knowledge is limited because the most widely used tasks suffer from two main constraints: (i) They fail to capture individual variability due to ceiling effects and (ii) they use highly simplistic, often artificial stimuli inapt to mirror real-world socio-cognitive demands. In the present study, we address these problems by employing complex depictions of naturalistic social interactions that vary in both valence (positive vs negative) and ambiguity (high vs low). Thirty-eight healthy participants (20 female) made mental state judgments while brain responses were obtained using functional magnetic resonance imaging (fMRI). Accuracy varied based on valence and ambiguity conditions and women were more accurate than men with highly ambiguous social stimuli. Activity of the orbitofrontal cortex predicted performance in the high ambiguity condition. The results shed light on subtle differences in mentalizing abilities and associated neural activity.
Keywords: social cognition, OFC, individual differences, theory of mind, naturalistic stimuli
Introduction
Humans are remarkably versed at reading what is on another person’s mind. When encountering a group of people, with just a quick glance one can often grasp the overall mood and sense signs of either rapport or tension. This process requires both emotion recognition and theory of mind (ToM) and provides us the information we need to modify our own behavioral response. Hence, the ability to infer other peoples’ intentions, thoughts and emotions (i.e. their internal mental states, henceforth referred to as mental state attribution) is critical for navigating our social environment including our capacity to form and maintain social bonds (Sasson et al., 2013). Over the past two decades, research on socio-cognitive processes underlying mental state attribution has grown exponentially, reflecting an increased appreciation for their significance for various major life outcomes. Mental state attribution is crucial for social competence (Couture et al., 2006) which in turn is a critical predictor of key endpoints across multiple domains including education and employment (Denham et al., 2013; Jones et al., 2015) as well as mental (Kawachi and Berkman, 2001; Ciarrochi, 2002; Carter et al., 2010; Jones et al., 2015) and physical health (Callaghan, 1993; Uchino, 2006; Holt-Lunstad et al., 2010).
Given the importance of socio-cognitive skills, surprisingly little is known about individual differences in the ability to make adequate mental state judgments. While there is evidence for social cognition deficits in several clinical populations including Autism Spectrum Disorders (ASD; Baron-Cohen et al., 2000; Klin et al., 2002) and schizophrenia (Bellack et al., 1990; Mueser et al., 1996), behavioral data on normal variation in healthy, neurotypical adults is still scarce.
Evidence from both self-report measures and brain imaging data, on the other hand, robustly point to considerable variance in social cognition (Hooker et al., 2010; Wagner et al., 2011; Regenbogen et al., 2015). Self-report data predominantly indicate an advantage for women with regard to mindreading abilities (e.g. Baron-Cohen and Wheelwright, 2004; Rueckert and Naybar, 2008), yet, this finding needs further substantiation through performance data (Grimshaw et al., 2004; Krach et al., 2009; Derntl et al., 2010). Similarly, a growing body of work points to differences in the neural processing of social stimuli within healthy populations (e.g. Rueckert and Naybar, 2008; Schulte-Rüther et al., 2008; Spreckelmeyer et al., 2009; Hooker et al., 2010; Yucel et al., 2015). The network which underlies our capacity to infer the internal states of others using cognitive processes (i.e. as opposed to affect sharing) is referred to as ToM or mentalizing network and subsumes the medial prefrontal cortex (mPFC), the precuneus, the temporo-parietal junction (TPJ) and the temporal poles (Frith and Frith, 2003; Saxe et al., 2006; Van Overwalle and Baetens, 2009; for a meta-analysis see Bzdok et al., 2012). Previous research by Wagner et al. (2011) illustrates that even in the absence of an explicit ToM task, the degree to which people activate this network in response to social stimuli varies as a function of self-reported empathy levels. Furthermore, structural imaging studies demonstrate that grey matter volume in structures which are essential for affective ToM (i.e. inferring emotional states rather than beliefs) namely the amygdala and the orbitofrontal cortex (OFC, as shown for instance by lesion studies: Adolphs et al., 1998; Anderson et al., 1999; Stone et al., 2003) are positively correlated with the size of a person’s social network (Powell et al., 2012; Bickart et al., 2014). Taken together, these findings point to considerable heterogeneity of socio-cognitive abilities that currently is not properly reflected in performance data of most social cognition tasks. Consequently, there is limited insight into brain-behavior relationships, i.e. how neural activity observed during mental state attribution relates to the validity of the judgment made.
A major limitation for research on normal variation is that most studies use highly simplistic stimuli and fail to capture the demands posed by real-life social encounters. Therefore, the majority of these tasks produce ceiling effects in behavior responses (Dodell-Feder et al., 2013; Chiu et al., 2015; Henry et al., 2015). While, on the one hand, they allow for tightly controlled experiments, overly simplistic models also come at an important cost: They are bound to be artificial, because the target processes (e.g. reading a facial expression) do not occur as stand-alone events in real life, but are closely tied in with other cues and contextual information. Notably, social context influences how we perceive emotions (Kret and de Gelder, 2010). Hence, showing interactions of several people rather than an isolated facial expression will result in greater ecological validity and increases ToM-related neural activity compared to viewing a single person (Iacoboni et al., 2004). Similarly, body language contributes significantly to our overall assessment of a person’s internal state. In contrast to facial expressions, bodily expressions can be read from a distance and convey a unique, more immediate type of information (de Gelder, 2009) which is taken into account in real-life social situations. Outside the laboratory, the difficulty of mental state attribution often lies in the fact that emotions and mental states are merely insinuated. Hence, research can benefit from more ambiguous, lower-intensity stimuli that mimic real-life demands more closely (Chiu et al., 2015). Supporting this view, Leppanen and Nelson (2009) demonstrated that participants find emotion recognition more challenging the closer the stimulus’ resemblance to a real face. Moreover, striking implications come from studies that compared authentic social stimuli to professional reenactments or posed versions of the same stimulus. These studies found that authenticity significantly affects neural processing in key mentalizing regions including the mPFC and also left no doubt regarding participants’ ability to distinguish between real and posed recordings of emotional prosody at a rate greatly above chance level (Drolet et al., 2012; Pinkham et al., 2014). The fact that accuracy of authenticity judgments not only varied substantially among individuals (69–93%), but also predicted the size of the neural responses in key ToM regions (mPFC, precuneus; Pinkham et al., 2014) raises several important questions: (i) Is the ability to identify ‘true’ expressions of emotions already a reflection of a person’s socio-cognitive skills? (ii) What does it mean for the interpretation of results when part of the sample treated the stimuli as being authentic, while others thought of them as being fake? Even though these findings are based on auditory experiments, they may translate to other modalities including visual stimuli. Hence, in order to avoid potential confounding variables, research can benefit from using authentic stimuli where feasible.
In the present study we used a newly developed paradigm, the Social Detection Task, which was designed to overcome limitations of previous research by employing photographs of naturalistic human social interactions of varying degrees of ambiguity. Our aim was to use this task to (i) shed light on the neural correlates of mental state attribution in a healthy, neurotypical adult population and (ii) to explore individual differences in mental state attribution including gender effects.
Materials and methods
Participants
Forty-two right-handed volunteers (mean age = 23.88 ± 3.35, age range 18–30; 21 female) with no history of psychiatric or neurological disorders participated in the study. The study was approved by the Ethics Committee of the Medical Faculty of the RWTH Aachen University. Participants were pre-screened for MR-eligibility, provided written informed consent and were compensated for participation. Four subjects were subsequently excluded from the analysis due to excessive head motion during fMRI scanning (one subject) or insufficient task performance (i.e. > 40% missed trials (one subject) or < 60% correct trials (two subjects)). The final sample consisted of 38 people (mean age = 24.14 years, age range 18–30; 20 female).
Measures
Questionnaires
All participants completed the full-length version of the Social Skills Inventory (Inventar sozialer Kompetenzen, Kanning, 2009), a 108-item self-report instrument designed to assess a broad spectrum of social competences comprising four subscales: (i) social orientation, (ii) assertiveness, (iii) self-management and (iv) social reflection. High test–retest reliability (0.80–0.87) and internal consistency (0.69–0.90) have been reported for this instrument (Kanning, 2009). To shed light on subjects’ social skill level from a different angle, we further included the Lubben Social Network Scale (Lubben, 1988). Participants gave estimates of their social network size and the amount of social support they receive by indicating, for example, how many friends they talk to in a normal week. The subscales ‘family’ and ‘friends’ of the abbreviated version (Lubben et al., 2006) were used. Social network size is positively linked to both trait empathy (Trobst et al., 1994), and grey matter volume of several key structures within the social brain (Bickart et al., 2011; Lewis et al., 2011; Powell et al., 2012).
Stimuli and task
During scanning, participants saw photographs of real-life social interactions from two to four people (two: 80%, three: 13%, four: 7%), which were taken in a complex naturalistic environment. The social interactions took place in various daily life situations (e.g. shopping, eating at a restaurant, waiting for the bus) that most people have experienced. To account for the broad spectrum of interpersonal relationships that social encounters can be imbedded in, stimuli included interactions between romantic partners, friends, coworkers, salesclerks and costumers and family members. All interactions occurred naturally at a time when protagonists were unaware of being photographed so as to ensure authenticity of the emotional expression. The photos depicted people of different age, gender and ethnicity. In all pictures, individuals were shown either in full or at least from the hip upward to include information from body language.
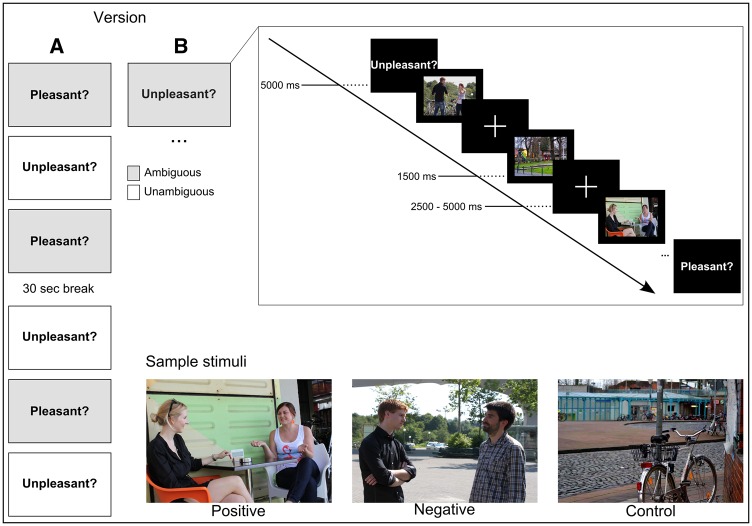
Based on a rating (n = 23, 14 female), sixty images of social interactions consistently described as positive or negative (as opposed to neutral) on a 9-point Likert scale were preselected from a pool of initially 281 images. In order to confirm suitability for the assigned valence category, the final picture set was evaluated again by a separate, larger group of raters (n = 37, 19 female). Excellent inter-rater agreement (calculated as the mean absolute agreement in a two-way random model) was found (ICC(2,37) = 0.986). In addition to valence, the pictures also varied with regard to the level of ambiguity of the social scene. Scenes rated as highly positive or negative were defined as unambiguous (i.e. easy) items, whereas scenes in which the valence was more subtle were defined as more ambiguous (i.e. difficult) items. The final picture set consisted of 30 ambiguous and 30 unambiguous items. Participants were instructed to judge the valence of the social situation from the perspective of those depicted in the scene. For control trials, comparable scenes not containing people were used and participants were asked to evaluate the valence of the location instead. In each of the six blocks, a total of 15 images (5 positive, 5 negative, 5 control) were presented in an event-related fashion. At the beginning of each block the corresponding instruction (either ‘is this scene pleasant?’ or ‘is this scene unpleasant?’) was shown for 5000 ms (Figure 1). The terms ‘pleasant’ and ‘unpleasant’ (rather than ‘positive’ and ‘negative’) were used to refer to the social interactions in a way they might be described as in every-day language and thus would sound more natural to participants. Moreover, less strong labels were deliberately chosen as they provide a more apt description of the subtle emotional stimuli used here. Stimuli were presented in a pseudorandomized order for 1500 ms each and were followed by an inter-trial interval (ITI) varying between 2500 and 5000 ms (mean = 3750 ms) during which a fixation cross appeared. Subjects were asked to answer with ‘yes’ or ‘no’ via a key press on a Lumitouch response panel with their right index and middle finger, respectively. All participants completed a practice run with similar pictures not included in the experiment to familiarize with the task before scanning. Statistical analyses were conducted using IBM SPSS 20.
Fig. 1.
Experimental Design. During fMRI scanning, participants judged the valence of 30 positive and 30 negative social scenes and 30 non-social control scenes. At the beginning of each run an instruction screen delivered the question to be answered in this run (either ‘scene pleasant?’ or ‘scene unpleasant?’) Each run consisted of 5 stimuli from each category (positive, negative, control) with half of the runs using unambiguous and half ambiguous stimuli.
Image acquisition
Functional imaging was performed on a 3-Tesla Magnetom Prisma fit scanner (Siemens, Erlangen, Germany) using a standard 32-channel Radio Frequency head coil. A T2*-weighted echo planar imaging (EPI) sequence (matrix size = 64 × 64; field of view = 224 mm; repetition time = 2.2 s; echo time = 30 ms; flip angle = 77°) was used to acquire 36 axial slices (thickness 3.5 mm, inter-slice gap 0.4 mm). For each participant, a total of 360 whole-brain volumes were acquired in one session, lasting approximately 15 min. Each session began with six dummy scans to allow the magnetization to stabilize, which were later dropped from the analysis. Subsequent to the experiment, a high-resolution T1-weighted scan was acquired for every subject using a standard MP-RAGE sequence (1 mm × 1 mm × 1 mm, 176 slices). Stimulus presentation and recording of subject responses was controlled through Presentation 16.3 software (Neurobehavioral Systems, Inc., San Francisco, CA). Visual stimuli were presented on a 24” screen (Cambridge Research Systems) within the scanner bore which the subject saw via a mirror mounted to the head coil.
Data processing and analysis
Preprocessing and statistical analysis of fMRI data was performed using SPM 12 software. All volumes were corrected for acquisition time delay between slices and realigned to the mean EPI image using rigid body transformation. The T1-weighted image was coregistered to the mean EPI image and normalized to MNI space using the default SPM 12 template. The resulting parameters were then used for spatial normalization of the functional data. Lastly, EPIs were resampled (voxel size = 2 mm × 2 mm × 2 mm) and spatially smoothed with a 3D Gaussian kernel of 4 mm full-width at half-maximum.
A random-effects, event-related statistical analysis was carried out in a two-level procedure. At first-level, a separate general linear model (GLM) was specified for each subject including 6 separate regressors (3 valence × 2 ambiguity) of interest. For all stimulus events, task-related changes in blood oxygenation level dependent (BOLD) signal were estimated for each voxel by modelling the onset of the stimulus event as δ-function convolved with the canonical SPM12 hemodynamic response function. To reduce error variance, a pause between blocks, the six block-wise instructions and the six realignment parameters from motion correction were modeled as regressors of no interest. At group-level, a random-effects analysis (2 × 3 × 2 ANOVA) with the between-subjects factor gender and the within-subject factors condition (positive, negative, control) and ambiguity (high, low) was carried out.
Results were followed up by correlational analyses to test for brain-behavior relationships of 3 brain regions (bilateral amygdala, OFC) which have been implicated in specifically moderating affective ToM. To control for multiple comparisons, the Dubey and Armitage-Parmar procedure was used which takes into account inter-correlations among variables (Sankoh et al., 1997).
Results
Two stimuli from the difficult negative category were not included in the following analyses as they were answered incorrectly by > 50% of our sample contrasting the verdict of the raters preceding stimulus selection. This inconsistency may be due to the difference in time people were given to examine the scenes (ratings: unlimited vs experiment: 1500 ms). All analyses reported subsequently were carried out using the remaining 30 positive, 28 negative and 30 control scenes.
Behavioral results
Performance
Accuracy was calculated as the percentage of correct items out of the total number of items (hits + false alarms) completed by each subject. Thus, accuracy values are adjusted for individual response bias. Overall performance ranged from 61% to 100% of accuracy (M = 87%, s.d. = 8) for positive and negative items combined. A repeated measures ANOVA with valence (positive, negative) and ambiguity (high, low) as within-subject factor and gender as between-subject factor revealed a main effect of ambiguity [F(1,36) = 34.14, P < 0.001] in the expected direction, yielding lower performance in the more ambiguous condition (M = 83%, s.d. = 12) than in the unambiguous one (M = 93%, s.d. = 9). A main effect was also found for valence [F(1,36) = 38.1, P < 0.001] with more correct answers for positive (M = 96%, s.d. = 6) than for negative scenes (M = 79%, s.d. = 16). Furthermore, we found a trend for a main effect of gender, yielding higher overall performance for women (M = 90%, s.d. = 7) than men (M = 84% s.d. = 7), which failed to reach statistical significance [F(1,36) = 3.98, P = 0.054].In addition, we found a gender x ambiguity interaction [F(1,36)=4.24, P = 0.047] such that women achieved higher performance rates for ambiguous scenes (M = 87%, s.d. = 10) than men (M = 78%, s.d. = 12). Another interaction effect was evident for valence x ambiguity [F(1,36)=16.37, P < 0.001]. For negative scenes, there was a greater gap between accuracy for ambiguous (M = 67%, s.d. = 26) and unambiguous scenes (M = 88%, s.d. = 13) than for positive scenes (ambiguous: M = 94%, s.d. = 9; unambiguous: M = 97%, s.d. = 5). Non-social control scenes did not contain interactions and thus were not comparable to the social scenes in terms of accuracy.
Response times (RTs)
A 3 × 2 repeated measures ANOVA with the factors condition (positive, negative, control) and gender revealed a main effect for condition [F(2,70) = 34.57, P < 0.001]. Subjects responded fastest for positive scenes (M = 992.33, s.d. = 91.93), followed by negative scenes (M = 1101.45, s.d. = 79.05) and slowest in the control condition (M = 1046.24, s.d. = 98.61). In a separate repeated measures ANOVA with the within-subject factors ambiguity (high, low) and valence (positive, negative) and gender as the between-subject factor, a main effect of ambiguity [F(1,36) = 65.64, P < 0.001] was found, demonstrating that subjects took more time to respond to ambiguous (M = 1052.56, s.d. = 98.81) than unambiguous scenes (M = 1023.11, s.d. = 92.67) as well as a main effect of valence [F(1,36) = 9.26, P = 0.004] indicating shorter RTs for positive (M = 994.450, s.d. = 94.26) than negative (M = 1120.75, s.d. = 83.38) social scenes. No gender differences were observed [F(1,36) = 0.2, P = 0.656]. A valence x ambiguity interaction [F(1,36) = 15.52, P < 0.001] further revealed that the difference in response times between positive and negative scenes was more pronounced for ambiguous (positive: M = 1004.6, s.d. = 117.61; negative: M = 1167.32, s.d. = 111.41) than unambiguous scenes (positive: M = 984.31, s.d. = 115.41; negative: M = 1074.17, s.d. = 92.6).
Personality measures
No gender differences were observed in any of the personality measures with the exception of the ‘family’ dimension of the LSNS [t(36)=−4.01, P < 0.001], for which women (M = 39.75, s.d. = 11.47) received higher scores than men (M = 27.39, s.d. = 6.59). For correlational analyses of questionnaire data and performance indicators, the significance level was set to P < 0.05 (two-sided) and results have to be regarded as exploratory. Interestingly, the more socially orientated subjects described themselves as, the higher was their performance when determining the valence of more ambiguous social scenes [r = 0.36, P = 0.026]. Similarly, we found that the bigger a person’s social network, the faster he or she was able to perform said task [r = 0.35, P = 0.033] in the more ambiguous category.
Imaging results
All of the following analyses were conducted at a whole-brain level. Results were corrected for multiple comparisons using family-wise-error (FEW) correction. For a full summary of brain activations observed in the contrasts described below, see Table 1.
Table 1.
Brain activations during experimental and control conditions, whole-brain analysis, full-factorial ANOVA, P(FWE) < 0.05 cluster corrected for multiple comparisons.
| Cluster P(FWE) | Cluster size (number of voxels) | Peak Z | x | y | z | Region | R/L |
|---|---|---|---|---|---|---|---|
| Main effect of condition Social > Non-social scenes | |||||||
| 4676 | Inf | 52 | -70 | 4 | Middle Temporal Gyrus | R | |
| Inf | 46 | -60 | 16 | Middle Temporal Gyrus (extending to the TPJ) | R | ||
| Inf | 46 | -74 | 0 | Middle Occipital Gyrus | R | ||
| 2417 | Inf | -50 | -74 | 10 | Middle Occipital Gyrus | L | |
| Inf | -42 | -54 | -18 | Fusiform Gyrus | L | ||
| Inf | -54 | -48 | 14 | Middle Temporal Gyrus | L | ||
| 2724 | Inf | 4 | -60 | 32 | Precuneus | R | |
| Inf | 4 | -90 | 16 | Cuneus | L | ||
| Inf | 4 | -52 | 46 | Precuneus | R | ||
| 418 | Inf | 6 | 54 | 24 | Superior Medial Gyrus | R | |
| 6.17 | 6 | 62 | 22 | Superior Medial Gyrus | R | ||
| 410 | Inf | -18 | -74 | -34 | Cerebellum | L | |
| 6.95 | -10 | -80 | -40 | Cerebellum | L | ||
| 6.23 | -14 | -70 | -24 | Cerebellum | L | ||
| 9 | 7.64 | 22 | -6 | -14 | Amygdala | R | |
| 133 | 7.49 | 4 | 42 | -16 | Rectal Gyrus | R | |
| 6.12 | 4 | 56 | -6 | Medial Orbitofrontal Gyrus | R | ||
| 250 | 7.25 | 40 | 2 | 40 | Precentral Gyrus | R | |
| 19 | 7.08 | 46 | -16 | -24 | Inferior Temporal Gyrus | R | |
| 5.97 | 44 | -22 | -18 | Fusiform Gyrus | R | ||
| 189 | 6.55 | 36 | 18 | 26 | IFG (p. Triangularis) | R | |
| 6.19 | 50 | 24 | 16 | IFG (p. Triangularis) | R | ||
| 5.89 | 54 | 20 | 28 | IFG (p. Opercularis) | R | ||
| 12 | 6.52 | 14 | -30 | 2 | Thalamus | R | |
| 7 | 6.24 | -20 | -6 | -14 | Amygdala | L | |
| 25 | 6.2 | 4 | -12 | 8 | Thalamus | R | |
| 42 | 5.64 | -60 | -10 | -8 | Middle Temporal Gyrus | L | |
| 5.56 | -52 | -10 | -12 | Middle Temporal Gyrus | L | ||
| 6 | 5.33 | 44 | 20 | -30 | Temporal Pole | R | |
| 11 | 5.25 | 2 | -16 | 36 | Middle Cingulate Cortex | R | |
| Non-social > Social scenes | |||||||
| 2934 | Inf | 26 | -40 | -10 | Parahippocampal Gyrus | R | |
| Inf | -30 | -46 | -8 | Fusiform Gyrus | L | ||
| Inf | -26 | -64 | -12 | Lingual Gyrus | L | ||
| 907 | Inf | 40 | -80 | 30 | Middle Occipital Gyrus | R | |
| Inf | 32 | -80 | 18 | Middle Occipital Gyrus | R | ||
| 6.53 | 28 | -64 | 36 | Superior Occipital Gyrus | R | ||
| 962 | Inf | -34 | -86 | 22 | Middle Occipital Gyrus | L | |
| 7.27 | -30 | -90 | 10 | Middle Occipital Gyrus | L | ||
| 6.94 | -20 | -68 | 42 | Superior Parietal Lobule | L | ||
| 383 | Inf | 20 | -54 | 18 | Calcarine gyrus | R | |
| Inf | 12 | -52 | 12 | Precuneus | R | ||
| 335 | Inf | -18 | -58 | 18 | Cuneus | L | |
| 7.17 | -8 | -50 | 6 | Calcarine gyrus | L | ||
| 81 | 7.34 | -52 | -60 | -10 | Inferior Temporal Gyrus | L | |
| 39 | 6.45 | 26 | 8 | 54 | Superior Frontal Gyrus | R | |
| 26 | 6.21 | -22 | 6 | 52 | Middle Frontal Gyrus | L | |
| 6 | 5.12 | 22 | -72 | 54 | Superior Parietal Lobule | R | |
| Negative > Positive social scenes | |||||||
| 124 | 6.71 | -30 | 26 | -6 | Insula Lobe | L | |
| 5.46 | -46 | 24 | -6 | IFG (p. Orbitalis) | L | ||
| 10 | 5.98 | 48 | 32 | -8 | IFG (p. Orbitalis) | R | |
| 29 | 5.64 | -4 | 50 | 34 | Superior Medial Gyrus | L | |
| 14 | 5.39 | 32 | 26 | -2 | Insula Lobe | R | |
| 7 | 5.11 | -4 | 22 | 40 | Superior Medial Gyrus | L | |
| Positive > Negative social scenes | |||||||
| 13 | 5.21 | -54 | -30 | 26 | Supramarginal Gyrus | L | |
| 5.11 | -62 | -32 | 28 | Supramarginal Gyrus | L | ||
| Main effect of gender Female > Male | |||||||
| 33 | 7.21 | -20 | -82 | -8 | Lingual Gyrus | L | |
| 12 | 6.11 | 12 | -82 | -6 | Lingual Gyrus | R | |
| 37 | 6.05 | -20 | -52 | 2 | Precuneus | L | |
| 5.74 | -24 | -62 | 8 | Calcarine Gyrus | L | ||
| 15 | 5.67 | -16 | -72 | 10 | Calcarine Gyrus | L | |
| 8 | 5.33 | 20 | -52 | 0 | Lingual Gyrus | R | |
| 11 | 5.27 | 14 | -84 | 14 | Calcarine Gyrus | R | |
| 8 | 5.15 | -6 | -84 | 8 | V1 | L |
R. Right Hemisphere; L. Left Hemisphere
Main effect of condition (social vs non-social scenes)
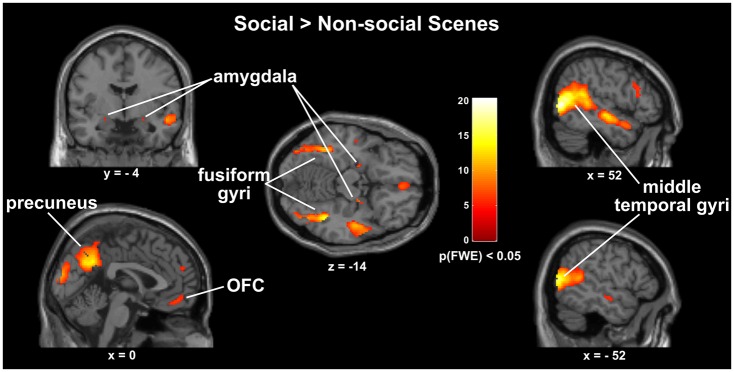
Whole brain analyses revealed several brain areas that show a main effect of condition. As seen in post-hoc t-tests, several of these regions showed greater activity when subjects evaluated the valence of social interactions. They include key nodes of the mentalizing network such as the rTPJ, the right middle temporal gyrus (rMTG), the STS and the precuneus as well as the mPFC where our analysis detected a dorsal and a separate ventral cluster located in the orbital portion of the mPFC and extending into the rectal gyrus. In the social > non-social contrast we also found greater activation in areas associated with the perception of faces and bodies, namely the bilateral fusiform and middle temporal gyri. Our analyses further revealed greater activity in the bilateral amygdala (Figure 2). The opposite contrast non-social vs social scenes yielded a large cluster in the right parahippocampal gyrus. Our analysis further detected activation of several visual and parietal areas as well as clusters in the inferior temporal gyrus, the right superior frontal gyrus, and the left middle frontal gyrus. When comparing social scenes of opposite valence, significantly greater neural activity was observed in the anterior insula cortices and parts of the inferior frontal gyri (IFG) of both hemispheres when subjects were examining negative social interactions as compared to positive ones. In the reverse contrast, the only region to show greater activity for positive social interactions was the left supramarginal gyrus which among several other functions has also been implicated in understanding gestures and bodily postures (Becchio et al., 2012).
Fig. 2.
Brain activations observed in the Social > Non-social contrast, whole-brain analysis, full-factorial ANOVA, P(FWE) < 0.05 cluster corrected for multiple comparisons.
Main effect of ambiguity (ambiguous vs Unambiguous social scenes)
No significant differences were found when contrasting ambiguous and unambiguous social scenes.
Main effect of gender
Whole-brain analyses yielded several occipital and parietal areas involved in the encoding and retrieval of complex visual information including clusters in the bilateral lingual gyri which women activated to a greater degree than men across all conditions [P(FWE) < 0.05, Table 1]. Conversely, the opposite contrast did not show any brain regions, which were more active in men than women.
Brain behavior relationships
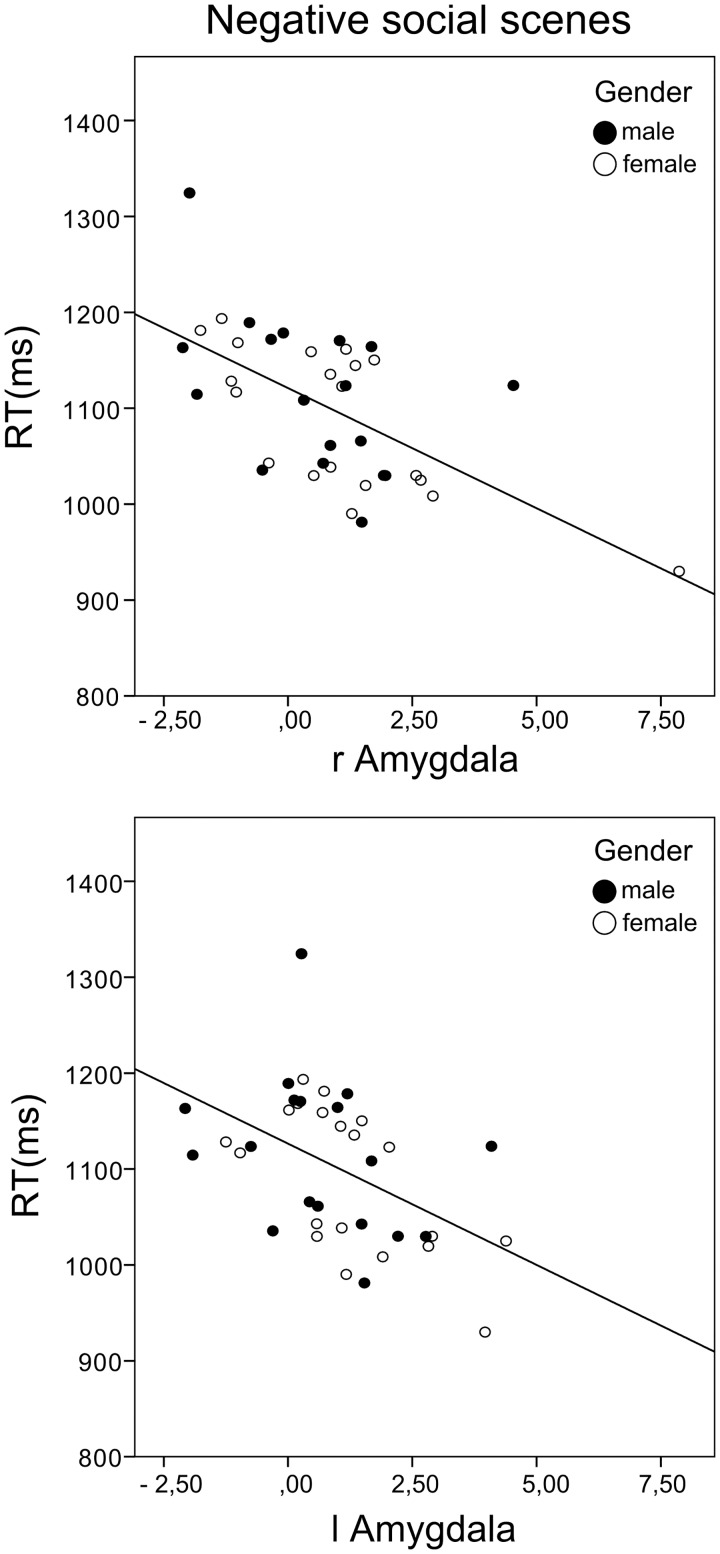
Activation of the medial OFC was positively correlated with subjects’ performance when judging the valence of ambiguous social scenes [r = 0.349, Padj = 0.042, Figure 3]. Amygdala activation was not linked to accuracy levels for ambiguous scenes. However, activation of both the left and right amygdala was inversely related to response times for negative social scenes [RH: r=−0.477, Padj = 0.003, LH: r=−0.605, Padj = 0.000, Figure 4], but not positive ones, while OFC activation was not linked to RTs. Parameter estimates were derived from clusters which showed greater activation in the social than the non-social condition (OFC:133 voxels, Amygdala: RH: 9 voxels, LH: 7 voxels).
Fig. 3.
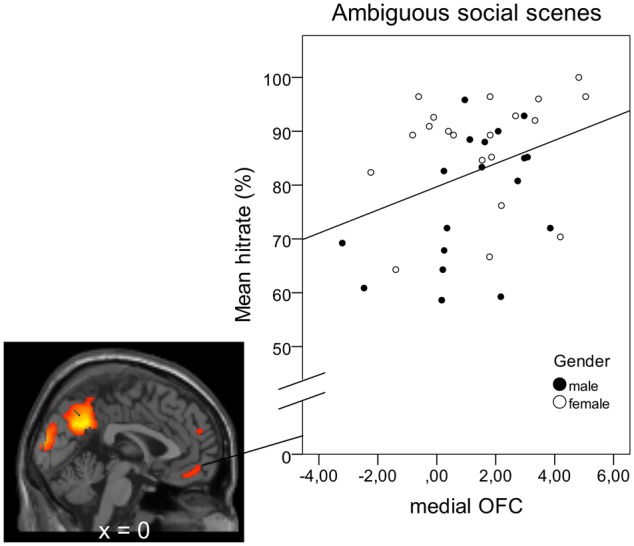
Correlation between OFC activation during ambiguous social scenes and accuracy of mental state judgments in this category: r = 0.349, P = 0.032, two-tailed. Parameter estimates were derived from a 133 voxel cluster (peak activation: x = 4, y = 56, z= −6, P(FWE) < 0.05) from the Social > Non-social contrast.
Fig. 4.
Correlation between bilateral amygdala activation during evaluation of negative social scenes and response times (RH: r = −0.477, P= 0.002, two-tailed, LH: r = −0.605, P = 0 .000, two-tailed). Parameter estimates were derived from clusters found in the Social > Non-social contrast.
Discussion
Here, we investigated the neural correlates of mental state attribution in a population of healthy, neurotypical adults using complex naturalistic depictions of real-life human social interactions. When evaluating the valence of social interactions as contrasted with comparable scenes not showing people, activity in key mentalizing areas was observed which are reliably activated when people engage in social thought (Völlm et al., 2006; Van Overwalle and Baetens, 2009). These areas include the rTPJ, the right middle temporal gyrus (rMTG), the STS and the precuneus as well as two distinct areas within the mPFC (i.e. a dorsal and an orbital cluster) which corresponds to findings from previous studies (Wagner et al., 2011; Corradi-Dell'Acqua et al., 2015). Conversely, when subjects were presented with pictures of places as opposed to human social interactions, greater activation of the parahippocampal gyrus which is involved in the encoding and recognition of environmental scenes (Epstein and Kanwisher, 1998; Ishai et al., 1999) was found.
Despite their subtlety, the stimuli of the Social Detection Task provoked distinct neural responses for social scenes of different valence. Activation of the anterior insula, which is frequently observed in the context of negative emotions (Lamm and Singer, 2010; Beatty et al., 2014; Kashdan et al., 2014) was significantly greater when subjects assessed unpleasant social interactions. Amygdala activation, on the other hand, was detected for social scenes irrespective of valence, supporting the view of the amygdala as a social salience region that responds to aversive and positive emotional content alike (Liberzon et al., 2003; Groppe et al., 2013). Amygdala-driven alertness in the face of negative social information additionally facilitated motor responses, as bilateral amygdala activation was inversely linked to response time for negative scenes.
With regard to brain-behavior relationships, our data provide the first evidence linking functional OFC activity to levels of accuracy in mental state attribution. According to the social brain hypothesis (Dunbar, 1998, 2009), this phylogenetically young region evolved to allow primates and in particular humans to manage their unusually complex social networks—a notion supported by tight correlations between OFC grey matter volume and social network size (Powell et al., 2012) as well as socio-cognitive competence (Powell et al., 2010; Scheuerecker et al., 2010) in humans. The OFC shares extensive connections to the amygdala (Bzdok et al., 2013; Bickart et al., 2014) and has been implicated specifically in ‘affective’ ToM (i.e. reasoning about someone else’s feelings; Shamay-Tsoory et al., 2006; Shamay-Tsoory et al., 2010) as well as in social approach–avoidance behaviors (Roelofs et al., 2009). Hence, it is often thought to subserve more low-level, automatic social inference, whereas the DMPFC is associated with inferring higher-order cognitive states (i.e. goals, intentions; Corradi-Dell'Acqua et al., 2015). In line with this view, performance in the present study, in which subjects rapidly judged the pleasant or unpleasant nature of a social encounter, was linked to OFC but not DMPFC activation.
Accuracy levels of valence judgments varied substantially between individuals (ranging from 61% to 100%), even though the experiment was carried out in a socio-demographically homogeneous sample of healthy participants. This was largely achieved by including stimuli showing more subtle social cues to which subjects responded very differently. Higher performance in this more difficult category was associated with greater social orientation which refers to someone’s readiness to approach others with an open-minded, positive attitude. Being confident at reading nonverbal signals may enable people to experience social situations with ease, allowing them to maintain a curious, receptive mindset. This corresponds to our observation that people with larger social networks took less time to make valence judgments when only subtle cues were available. More correct answers were given for positive than negative items which may be a reflection of the fact that by cultural standards, positive emotions are more openly displayed in social settings than negative ones. Hence, this information may be slightly easier to extract from the stimuli used here.
In line with self-report data (Baron-Cohen and Wheelwright, 2004; Rueckert and Naybar, 2008; Derntl et al., 2010), women exhibited greater mindreading abilities, in particular when social signals were less obvious. This increased sensitivity to non-verbal social information is not surprising given that differences in the attention to social stimuli emerge very early in infancy. Girls have been shown to make more eye contact than boys as early as 12 months of age (Lutchmaya et al., 2002), hence they are likely to develop more fine-tuned socio-perceptive skills as a result of increased learning opportunities. Using the Reading the Mind in the Eyes Task (Baron-Cohen et al., 1997; Baron-Cohen et al., 2001), one of the few ToM measures known to produce variability in performance, several studies have reported a small advantage for women (for a meta-analysis see Kirkland et al., 2013). However, it is not clear whether these results also reflect gender differences in verbal abilities (e.g. Hyde and Linn, 1988) because performance in the RMET has been shown to correlate substantially with verbal IQ (Peterson and Miller, 2012). In contrast, the present task requires only minimal language skills, therefore its results are potentially less susceptible to differences in subjects’ verbal abilities. This could serve as an advantage when assessing gender differences as well as clinical populations in which language skills vary considerably and should be addressed in future studies.
Social cognition deficits are a core symptom of a number of psychiatric disorders including schizophrenia (Mueser et al., 1996; Couture et al., 2006) and ASD (Adolphs et al., 2001; Schultz et al., 2003; Schultz, 2005) and have come into the focus as a viable target for treatment (Pinkham et al., 2014). The promise that lies in this approach is well illustrated by a recent meta-analysis (Fett et al., 2011) wherein social cognition was identified as an even stronger predictor of real-life social functioning than general cognitive abilities. In order to evaluate treatment efficacy of pharmacological or psycho-social interventions, it is essential to properly quantify the target variable(s). Therefore, measures like the Social Detection Task which generates variance regarding the accuracy of the mental state judgments subjects make will advance the development of effective clinical interventions. In a similar vein, subtle mindreading deficits may carry valuable potential as a marker for mental illness (Derntl and Habel, 2011) as observed, for example, in a population genetically at-risk for schizophrenia whose emotion recognition deficits—although only marginal—were strongly linked to prodromal psychopathology (Eack et al., 2010). This example highlights the necessity to capture even subtle impairments as they may have farther-reaching implications. In addition to that, understanding the neural mechanisms that underlie differences in social perception will advance treatments tailored to specific subgroups of patients especially in heterogeneous disorders such as ASD (McPartland et al., 2011; Dichter, 2012; McPartland and Pelphrey, 2012).
Individual differences in performance were most pronounced in the ambiguous (i.e. more subtle) condition, suggesting that future research will benefit from using less clear-cut socio-emotional stimuli. In addition to that, the stimuli used here deviate from those used in most standard tasks in numerous ways: (i) non-verbal cues are embedded in a social context, (ii) targets’ internal states are authentic, (iii) stimuli include body language, (iv) social interactions occurred in natural settings (i.e. before a visually complex background requiring a selection of relevant information). We thereby address many of the concerns raised in recent works (de Gelder, 2009; Zaki and Ochsner, 2009; Zaki and Ochsner, 2012; Henry et al., 2015) calling for ecologically valid tasks. In keeping with this idea, the present study included images from a range of different real-life scenarios with each individual only appearing once. Hence, the images varied in several aspects (e.g. age and gender of those depicted) which increased generalizability, yet, from an experimental perspective, added undesired variation and can be considered a limitation of this study. Given the inevitable trade-off between internal and ecological validity, however, the aim of the present study was to put emphasis on the latter.
Further limitations of the present study include the use of still images as opposed to dynamic and multimodal stimuli due to constraints in experimental design. Ultimately, our goal is to implement dynamic naturalistic stimuli. Despite their static nature, the use of authentic, more subtle emotional stimuli can be seen as an important step forward toward increasing ecological validity within the boundaries of an fMRI experiment. Due to the lack of eye gaze data, the present study cannot answer the question of whether increased fixation on socially relevant features underlies higher accuracy in mental state attribution. This problem is being addressed in an ongoing research project of our group in which we also included participants with varying social skill levels as to avoid the restrictions created by a highly homogenous sample.
Taken together, using authentic more ambiguous social information, we were able to uncover subtle differences in the ability to read non-verbal social cues in a healthy population which could be linked to activation of the OFC. These findings exemplify the need for social cognition tasks which generate variance in performance in order to quantify normative and divergent behaviors – a notion further highlighted by recent efforts to include a social cognition domain (Gur and Gur, 2016) into the NIMH Research Domain Criteria (RDoC; e.g. Insel, 2014) in order to establish a translational framework for mental health research.
Funding
This study was supported by the Deutsche Forschungs-gemeinschaft (DFG, IRTG 1328) and the START-funding program of the RWTH Aachen Medical Faculty.
References
- Adolphs R., Sears L., Piven J. (2001). Abnormal Processing of Social Information from Faces in Autism. Journal of Cognitive Neuroscience, 13(2), 232–40. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Tranel D., Damasio A.R. (1998). The human amygdala in social judgment. Nature, 393(6684), 470–4. [DOI] [PubMed] [Google Scholar]
- Anderson S.W., Bechara A., Damasio H., Tranel D., Damasio A.R. (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience, 2(11), 1032–7. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Jolliffe T., Mortimore C., Robertson M. (1997). Another advanced test of theory of mind: evidence from very high functioning adults with autism or asperger syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines, 38(7), 813–22. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Ring H.A., Bullmore E.T., Wheelwright S., Ashwin C., Williams S.C. (2000). The amygdala theory of autism. Neuroscience and Biobehavioral Reviews, 24(3), 355–64. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders, 34(2), 163–75. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Hill J., Raste Y., Plumb I. (2001). The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry and Allied Disciplines, 42(2), 241–51. [PubMed] [Google Scholar]
- Beatty E.L., Vartanian O., Muller-Gass A., Robertson J.A., Mandel D.R., Stergiopoulos S. (2014). Neuroanatomical correlates of categorizing emotional valence. Neuroreport, 25(11), 854–9. [DOI] [PubMed] [Google Scholar]
- Becchio C., Cavallo A., Begliomini C., Sartori L., Feltrin G., Castiello U. (2012). Social grasping: From mirroring to mentalizing. Neuroimage, 61(1), 240–8. [DOI] [PubMed] [Google Scholar]
- Bellack A.S., Morrison R.L., Wixted J.T., Mueser K.T. (1990). An analysis of social competence in schizophrenia. British Journal of Psychiatry, 156, 809–18. [DOI] [PubMed] [Google Scholar]
- Bickart K.C., Dickerson B.C., Barrett L.F. (2014). The amygdala as a hub in brain networks that support social life. Neuropsychologia, 63, 235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart K.C., Wright C.I., Dautoff R.J., Dickerson B.C., Barrett L.F. (2011). Amygdala volume and social network size in humans. Nature Neuroscience, 14(2), 163–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Langner R., Schilbach L., et al. (2013). Segregation of the human medial prefrontal cortex in social cognition. Frontiers in Human Neuroscience, 7, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Schilbach L., Vogeley K., et al. (2012). Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Structure & Function 217(4), 783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan P.M.J. (1993). Social support and health: a review. Journal of Advanced Nursing, 18(2), 203–10. [DOI] [PubMed] [Google Scholar]
- Carter A.S., Wagmiller R.J., Gray S.A., McCarthy K.J., Horwitz S.M., Briggs-Gowan M.J. (2010). Prevalence of DSM-IV disorder in a representative, healthy birth cohort at school entry: sociodemographic risks and social adaptation. Journal of the American Academy of Child and Adolescent Psychiatry, 49(7), 686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I., Gfrorer R.I., Piguet O., Berres M., Monsch A.U., Sollberger M. (2015). “Now I see it, now I don’t”: Determining Threshold Levels of Facial Emotion Recognition for Use in Patient Populations. Journal of the International Neuropsychological Society, 21(7), 568–72. [DOI] [PubMed] [Google Scholar]
- Ciarrochi J.S.G., Deane F.P., Heaven P.C.L. (2002). Relations between social and emotional competence and mental health: a construct validation study. Personality and Individual Difference, 35(8), 1947–63. [Google Scholar]
- Corradi-Dell’Acqua C., Turri F., Kaufmann L., Clément F., Schwartz S. (2015). How the brain predicts people’s behavior in relation to rules and desires. Evidence of a medio-prefrontal dissociation. Cortex, 70, 21–34. [DOI] [PubMed] [Google Scholar]
- Couture S.M., Penn D.L., Roberts D.L. (2006). The Functional Significance of Social Cognition in Schizophrenia: a Review. Schizophrenia Bulletin, 32(Suppl 1), 44–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B. (2009). Why bodies? Twelve reasons for including bodily expressions in affective neuroscience. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 364(1535), 3475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham S.A., Kalb S., Way E., Warren-Khot H., Rhoades B.L., Bassett H.H. (2013). Social and emotional information processing in preschoolers: Indicator of early school success? Early Child Development and Care, 183(5), 667–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B., Finkelmeyer A., Eickhoff S., et al. (2010). Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology, 35(1), 67–82. [DOI] [PubMed] [Google Scholar]
- Derntl B., Habel U. (2011). Deficits in social cognition: a marker for psychiatric disorders?. European Archives of Psychiatry and Clinical Neuroscience, 261(2), 145–9. [DOI] [PubMed] [Google Scholar]
- Dichter G.S. (2012). Functional magnetic resonance imaging of autism spectrum disorders. Dialogues in Clinical Neuroscience, 14(3), 319–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodell-Feder D., Lincoln S.H., Coulson J.P., Hooker C.I. (2013). Using fiction to assess mental state understanding: a new task for assessing theory of mind in adults. PloS One 8(11), e81279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M., Schubotz R.I., Fischer J. (2012). Authenticity affects the recognition of emotions in speech: behavioral and fMRI evidence. Cognitive, Affective & Behavioral Neuroscience, 12(1), 140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar R.I.M. (1998). The social brain hypothesis. Evolutionary Anthropology: Issues, News, and Reviews, 6(5), 178–90. [Google Scholar]
- Dunbar R.I.M. (2009). The social brain hypothesis and its implications for social evolution. Annals of Human Biology, 36(5), 562–72. [DOI] [PubMed] [Google Scholar]
- Eack S.M., Mermon D.E., Montrose D.M., et al. (2010). Social Cognition Deficits Among Individuals at Familial High Risk for Schizophrenia. Schizophrenia Bulletin, 36(6), 1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R., Kanwisher N. (1998). A cortical representation of the local visual environment. Nature, 392(6676), 598–601. [DOI] [PubMed] [Google Scholar]
- Fett A.K.J., Viechtbauer W., Dominguez MdG., Penn D.L., van Os J., Krabbendam L. (2011). The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience and Biobehavioral Reviews, 35(3), 573–88. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society B: Biological Sciences, 358(1431), 459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimshaw G.M., Bulman-Fleming M.B., Ngo C. (2004). A signal-detection analysis of sex differences in the perception of emotional faces. Brain and Cognition, 54(3), 248–50. [DOI] [PubMed] [Google Scholar]
- Groppe S.E., Gossen A., Rademacher L., et al. (2013). Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biological Psychiatry, 74(3), 172–9. [DOI] [PubMed] [Google Scholar]
- Gur R.C., Gur R.E. (2016). Social cognition as an RDoC domain. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(1), 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J.D., Cowana D.G., Leeb T., Sachdev P.S. (2015). Recent trends in testing social cognition. Current Opinion, 28(2), 133–40. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J., Smith T.B., Layton J.B. (2010). Social relationships and mortality risk: a meta-analytic review. PLoS Medicine, 7(7), e1000316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Verosky S.C., Germine L.T., Knight R.T., D’Esposito M. (2010). Neural activity during social signal perception correlates with self-reported empathy. Brain Research, 1308, 100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J.S., Linn M.C. (1988). Gender differences in verbal ability: a meta-analysis. Psychological Bulletin, 104(1), 53–69. [Google Scholar]
- Iacoboni M., Lieberman M.D., Knowlton B.J., et al. (2004). Watching social interactions produces dorsomedial prefrontal and medial parietal BOLD fMRI signal increases compared to a resting baseline. Neuroimage, 21(3), 1167–73. [DOI] [PubMed] [Google Scholar]
- Insel T.R. (2014). The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. American Journal of Psychiatry, 171(4), 395–7. [DOI] [PubMed] [Google Scholar]
- Ishai A., Ungerleider L.G., Martin A., Schouten J.L., Haxby J.V. (1999). Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences 96(16), 9379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.E., Greenberg M., Crowley M. (2015). Early Social-Emotional Functioning and Public Health: The Relationship Between Kindergarten Social Competence and Future Wellness. American Journal of Public Health, 105(11), 2283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning U.P. (2009) Inventar Sozialer Kompetenzen. Goettingen: Hogrefe Verlag. [Google Scholar]
- Kashdan T.B., Dewall C.N., Masten C.L., et al. (2014). Who is most vulnerable to social rejection? The toxic combination of low self-esteem and lack of negative emotion differentiation on neural responses to rejection. PloS One, 9(3), e90651.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I., Berkman L.F. (2001). Social ties and mental health. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 78(3), 458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland R.A., Peterson E., Baker C.A., Miller S., Pulos S. (2013). Meta-analysis Reveals Adult Female Superiority in “Reading the Mind in the Eyes Test”. North American Journal of Psychology 15(1), 121–46. [Google Scholar]
- Klin A., Jones W., Schultz R., Volkmar F., Cohen D. (2002). Defining and Quantifying the Social Phenotype in Autism. American Journal of Psychiatry, 159(6), 895–908. [DOI] [PubMed] [Google Scholar]
- Krach S., Blumel I., Marjoram D., et al. (2009). Are women better mindreaders? Sex differences in neural correlates of mentalizing detected with functional MRI. BMC Neuroscience, 4(10), 9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kret M., de Gelder B. (2010). Social context influences recognition of bodily expressions. Experimental Brain Research, 203(1), 169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Singer T. (2010). The role of anterior insular cortex in social emotions. Brain Structure & Function, 214(5–6), 579–91. [DOI] [PubMed] [Google Scholar]
- Leppanen J.M., Nelson C.A. (2009). Tuning the developing brain to social signals of emotions. Nature Reviews: Neuroscience, 10(1), 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.A., Rezaie R., Brown R., Roberts N., Dunbar R.I.M. (2011). Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage, 57(4), 1624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Phan K.L., Decker L.R., Taylor S.F. (2003). Extended amygdala and emotional salience: a pet activation study of positive and negative affect. Neuropsychopharmacology, 28(4), 726–33. [DOI] [PubMed] [Google Scholar]
- Lubben J. (1988). Assessing social networks among elderly populations. Family and Community Health, 11, 42–52. [Google Scholar]
- Lubben J., Blozik E., Gillmann G., et al. (2006). Performance of an Abbreviated Version of the Lubben Social Network Scale Among Three European Community-Dwelling Older Adult Populations. The Gerontologist, 46(4), 503–13. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S., Baron-Cohen S., Raggatt P. (2002). Foetal testosterone and eye contact in 12-month-old human infants. Infant Behavior and Development, 25(3), 327–35. [Google Scholar]
- McPartland J.C., Coffman M., Pelphrey K.A. (2011). Recent advances in understanding the neural bases of autism spectrum disorder. Current Opinion in Pediatrics, 23(6), 628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J.C., Pelphrey K.A. (2012). The implications of social neuroscience for social disability. Journal of Autism and Developmental Disorders, 42(6), 1256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser K.T., Doonan R., Penn D.L., et al. (1996). Emotion recognition and social competence in chronic schizophrenia. Journal of Abnormal Psychology, 105(2), 271–5. [DOI] [PubMed] [Google Scholar]
- Peterson E., Miller S.F. (2012). The eyes test as a measure of individual differences: how much of the variance reflects verbal iq? Frontiers in Psychology, 3, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E., Penn D.L., Green M.F., Buck B., Healey K., Harvey P.D. (2014). The social cognition psychometric evaluation study: results of the expert survey and rand panel. Schizophrenia Bulletin, 40(4), 813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J., Lewis P.A., Roberts N., Garcia-Finana M., Dunbar R.I. (2012). Orbital prefrontal cortex volume predicts social network size: an imaging study of individual differences in humans. Proceedings: Biological Sciences, 279(1736), 2157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.L., Lewis P.A., Dunbar R.I., Garcia-Finana M., Roberts N. (2010). Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia, 48(12), 3554–62. [DOI] [PubMed] [Google Scholar]
- Regenbogen C., Kellermann T., Seubert J., et al. (2015). Neural responses to dynamic multimodal stimuli and pathology-specific impairments of social cognition in schizophrenia and depression. The British Journal of Psychiatry, 206(3), 198–205. [DOI] [PubMed] [Google Scholar]
- Roelofs K., Minelli A., Mars R.B., van Peer J., Toni I. (2009). On the neural control of social emotional behavior. Social Cognitive and Affective Neuroscience, 4(1), 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert L., Naybar N. (2008). Gender differences in empathy: the role of the right hemisphere. Brain and Cognition, 67(2), 162–7. [DOI] [PubMed] [Google Scholar]
- Sankoh A.J., Huque M.F., Dubey S.D. (1997). Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine, 16(22), 2529–42. [DOI] [PubMed] [Google Scholar]
- Sasson N.J., Nowlin R.B., Pinkham A.E. (2013). Social cognition, social skill, and the broad autism phenotype. Autism 17(6), 655–67. [DOI] [PubMed] [Google Scholar]
- Saxe R., Moran J.M., Scholz J., Gabrieli J. (2006). Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience 1(3), 229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuerecker J., Meisenzahl E.M., Koutsouleris N., et al. (2010). Orbitofrontal volume reductions during emotion recognition in patients with major depression. Journal of Psychiatry and Neuroscience, 35(5), 311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M., Markowitsch H.J., Shah N.J., Fink G.R., Piefke M. (2008). Gender differences in brain networks supporting empathy. Neuroimage, 42(1), 393–403. [DOI] [PubMed] [Google Scholar]
- Schultz R.T. (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(2–3), 125–41. [DOI] [PubMed] [Google Scholar]
- Schultz R.T., Grelotti D.J., Klin A., et al. (2003). The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 358(1430), 415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Harari H., Aharon-Peretz J., Levkovitz Y. (2010). The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex, 46(5), 668–77. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Tibi-Elhanany Y., Aharon-Peretz J. (2006). The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Social Neuroscience, 1(3–4), 149–66. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer K.N., Krach S., Kohls G., et al. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone V.E., Baron-Cohen S., Calder A., Keane J., Young A. (2003). Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia, 41(2), 209–20. [DOI] [PubMed] [Google Scholar]
- Trobst K.K., Collins R.L., Embree J.M. (1994). The role of emotion in social support provision: gender, empathy and expressions of distress. Journal of Social and Personal Relationships, 11(1), 45–62. [Google Scholar]
- Uchino B.N. (2006). Social support and health: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29(4), 377–87. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F., Baetens K. (2009). Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage, 48(3), 564–84. [DOI] [PubMed] [Google Scholar]
- Völlm B.A., Taylor A.N., Richardson P., et al. (2006). Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage, 29(1), 90–8. [DOI] [PubMed] [Google Scholar]
- Wagner D.D., Kelley W.M., Heatherton T.F. (2011). Individual differences in the spontaneous recruitment of brain regions supporting mental state understanding when viewing natural social scenes. Cerebral Cortex, 21(12), 2788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel G.H., Belger A., Bizzell J., Parlier M., Adolphs R., Piven J. (2015). Abnormal neural activation to faces in the parents of children with autism. Cerebral Cortex, 25(12), 4653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Ochsner K. (2009). The need for a cognitive neuroscience of naturalistic social cognition. Annals of the New York Academy of Sciences, 1167, 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Ochsner K.N. (2012). The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience, 15(5), 675–80. [DOI] [PubMed] [Google Scholar]