Abstract
Neural habituation allows familiar information to be ignored in favor of salient or novel stimuli. In contrast, failure to rapidly habituate likely reflects deficits in the ability to learn that an environment is predictable, familiar and safe. Differences in habituation rate may underlie individual differences in the tendency to approach or avoid novelty; however, many questions remain unanswered. Given the importance of adaptive social functioning, here we tested whether habituation differences to social stimuli are associated with differences in social fearfulness, a trait that ranges from low social fear—the adaptive tendency to approach novel social stimuli—to high social fear—the maladaptive tendency to avoid novel social stimuli. Higher social fearfulness was associated with slower habituation across regions of the social brain, including the hippocampus, amygdala, ventromedial prefrontal cortex, medial orbitofrontal cortex, fusiform face area, primary visual cortex, and extrastriate visual cortex. Interestingly, habituation differences were driven by sustained amygdala-visual cortex interactions, but not deficient amygdala-prefrontal cortex interactions. Together, these findings provide evidence that a failure to filter social stimuli is associated with a key social trait. In light of the link between social fear and dysfunction, individual differences in habituation may provide an important neurobiological marker for risk for psychiatric illness, such as social anxiety disorder.
Keywords: social anxiety disorder, functional connectivity, amygdala, hippocampus, visual cortex
Introduction
Humans constantly experience vast amounts of incoming sensory information. However, processing resources are inherently limited, thus the ability to rapidly filter unimportant information is a crucial skill necessary for survival. Habituation—the reduction in response to a stimulus over repeated exposures—is a fundamental learning mechanism that plays an important role in filtering our vast sensory environment (Ramaswami, 2014). When novel stimuli are encountered, processing resources are directed in order to rapidly determine whether the stimulus requires a response; that is, whether the novel stimulus signals a potential threat or reward. As an individual learns that a stimulus lacks meaningful consequences, habituation occurs rapidly at both the neural and behavioral level.
Although habituation is a fundamental process, individual differences in habituation appear as early as infancy (Bushnell, 1982; Snyder and Keil, 2008) and these differences have been proposed to fundamentally underlie individual differences in mood and anxiety (Davidson, 2002; Schuyler et al., 2012). Rapid habituation is thought to signal safety and familiarity (Wilson and Rolls, 1993; Fried et al., 1997; Dubois et al., 1999; Wright et al., 2001; Gonsalves et al., 2005) and a failure to rapidly habituate to neutral stimuli may trigger feelings of uncertainty or unfamiliarity (Wilson and Rolls, 1993; Fried et al., 1997; Dubois et al., 1999; Wright et al., 2001; Gonsalves et al., 2005). As rapid habituation is adaptive, failure to rapidly habituate may be considered maladaptive, as processing resources are being inappropriately allocated to neutral, safe stimuli, potentially leading to inappropriate fear and anxiety in safe situations.
Humans show extensive behavioral differences in latency to approach a novel neutral person—a measure of behavioral habituation or inhibited temperament—and these differences are associated with substantially heightened risk for social anxiety (Clauss and Blackford, 2012). Through a series of studies, our lab has begun to investigate the link between neural habituation and inhibited temperament using an extreme groups approach (Blackford et al., 2011, 2013). These studies have shown strong evidence that two key neural substrates are the amygdala and hippocampus, regions centrally implicated in threat processing (LeDoux et al., 1988; Leussis and Bolivar, 2006) and familiarity (Wilson and Rolls, 1993; Fried et al., 1997). In the uninhibited group (approaches novelty) both regions show the expected pattern of rapid habituation (Phan et al., 2003; Britton et al., 2008); in contrast, in the inhibited group (avoids novelty), the amygdala and hippocampus show a sustained activation, or failure to habituate (Blackford et al., 2013). Other studies have linked habituation failure to increased anxiety behaviors (Leussis and Bolivar, 2006; Ousdal et al., 2014), and to psychiatric disorders involving prominent social functioning deficits, such as autism (Kleinhans et al., 2009, 2015) and schizophrenia (Holt et al., 2005; Williams et al., 2013). Together, these findings suggest that individual differences in habituation may have broad implications for understanding social function and psychiatric illness.
Despite strong evidence for individual differences in habituation, many fundamental questions remain unanswered. First, does rate of habituation vary across the continuum of social function, or are deficits only observed at the extreme ends, as found in initial studies? Dimensional studies, which focus on understanding the neurobiology of specific traits that span the range from normal to pathological, are essential in identifying neurobiological mechanisms that inform the treatment and diagnosis of psychiatric disorders. Second, are habituation differences isolated to the medial temporal lobe—as previously observed—or found in other regions involved in social processing? The amygdala is a critical brain region involved in detection and processing of threat (Davis, 1997; Lang et al., 2000; Öhman, 2005) and is a central hub in the social processing network (Mathew and Ho, 2006; Furmark, 2009; Freitas-Ferrari et al., 2010; Miskovic and Schmidt, 2012); however, many other brain regions are involved in social information processing (Miskovic and Schmidt, 2012). Third, are habituation differences specific to certain brain regions or do they result from alterations in connectivity across brain regions? Given the amygdala’s widespread connections with both inhibitory connections from prefrontal cortex and reciprocal excitatory connections with visual cortex, we wondered whether failures in habituation might reflect imbalances in amygdala functional connectivity across the social brain. Disrupted functional connectivity has been consistently associated with increased anxiety and anxiety disorders (Etkin and Wager, 2007; Etkin, 2010), including social anxiety disorder (Etkin and Wager, 2007; Goldin et al., 2009; Freitas-Ferrari et al., 2010; Miskovic and Schmidt, 2012); however, no previous studies have investigated fundamental differences in habituation of functional connectivity over time. Finally, does initial response to a novel stimulus also vary with differences in social function? Magnitude of initial response and duration of response to a sensory stimulus support distinct components of an individual’s response style; while magnitude of initial response is associated with differences in detection of novelty (Wilson and Rolls, 1993; Fried et al., 1997), habituation is associated with familiarity. Preliminary evidence from our lab suggests that both amplitude and duration of response are disrupted in inhibited temperament (Blackford et al., 2009). Separation of these two fundamental processes has the potential to inform whether one specific process, or both, contributes to psychiatric illness.
Here, we tested for individual differences in neural habituation across the dimension of social fearfulness, a prototypical trait encompassing both adaptive and maladaptive social function (Furmark, 2002; Schneier et al., 2002; Stein et al., 2004; Miskovic and Schmidt, 2012). We used functional magnetic resonance imaging (fMRI) and a novel “repeated faces” task to assess neural habituation in a structurally and functionally interconnected network of regions involved in social processing (Stefanacci et al., 1996; Akirav and Richter-Levin, 1999; Iidaka et al., 2001; Ghashghaei and Barbas, 2002; Amaral et al., 2003; Phelps, 2004; Gabbott et al., 2005; Muñoz and Insausti, 2005; Quirk and Beer, 2006; Mohedano-Moriano et al., 2007; Roberts et al., 2007; Wager et al., 2009), including the amygdala, hippocampus, ventromedial prefrontal cortex (vmPFC), medial orbitalfrontal cortex (mOFC), fusiform face area (FFA), primary visual cortex (V1) and extrastriate cortex. We first examined habituation of response within individual regions. As the amygdala is a central hub of the social processing network, we next conducted functional connectivity analyses between the amygdala and each region.
Materials and methods
Study participants
Thirty-two participants between the ages of 18–25 were recruited from the Vanderbilt University community and surrounding Nashville area using advertisements and recruitment databases. To ensure recruitment across the continuum, enrollment was stratified. Because the social fearfulness assessment (SHY-SR, detailed below) is lengthy, we chose a short measure with excellent psychometric properties and normative data for the screening—the Revised Cheek and Buss Shyness Scale (RCBS). The RCBS measures shyness—a construct that closely parallels the physiological, cognitive and behavioral correlates of social fearfulness (Heiser et al., 2003)—and includes only 13 items and has normative data (Hopko et al., 2005), providing clear guidelines for stratification. The internal consistency of the RCBS is excellent (α = 0.90) (Hopko et al., 2005) and was excellent in this sample (α = 0.87).
Participants were excluded for (i) past or current psychiatric illness based on the Structured Clinical Interview for the DSM-IV (Spitzer et al., 1992) with the exception of untreated social anxiety disorder (n = 2)—social anxiety is expected at the extreme end of the social fearfulness dimension individuals; (ii) current use of psychoactive medications (previous 6 months); (iii) significant medical or neurological illness; (iv) pregnancy; (v) developmental disability or intellectual deficit; (vi) head injury resulting in loss of consciousness; or (vii) any conditions that preclude MR scanning (e.g. metal implants). All participants had normal or corrected to normal vision.
Following informed consent, participants completed two study visits. The first visit included a structured clinical interview and completion of questionnaires. Social fearfulness was assessed using the Social Anxiety Spectrum Self-Report (SHY-SR) (Dell’Osso et al., 2002, 2014). The SHY-SR is a 168-item questionnaire specifically developed to assess the dimension of social anxiety and fearfulness, including clinical and sub-clinical symptoms, as well as atypical presentations and isolated symptoms. The SHY-SR has good validity and reliability (Dell’Osso et al., 2002), and the internal consistency in this sample was high (α = 0.98). As expected the RCBS and SHY-SR scores were highly correlated (r = 0.68, P < 0.001) and the stratified recruitment was successful; participants’ scores spanned the continuum of social fearfulness, ranging from 3 (low social fear) to 120 (high social fear). At the second study visit participants completed a magnetic resonance imaging (MRI) scan. From the 32 individuals consented for the study, three individuals were excluded from the analysis for: beginning a psychoactive medication between the first and second study visit (n = 1); at the request of the participant (n = 1); or for technical error/malfunction (n = 1), resulting in a final sample of 29 participants. There were no associations between the social fearfulness and age, sex, ethnicity, or handedness (Table 1).
Table 1.
Participant characteristics
| Mean | S.D. | |
|---|---|---|
| Age (years) | 22 | 2 |
| Social fearfulness (SHY-SR) | 56 | 34 |
| Trait anxiety (STAI-Trait) | 35 | 12 |
| Depression (BDI-II) | 7 | 6 |
| % | ||
| Gender (% female) | 45% | |
| Race (% Caucasian) | 72% | |
| Handedness (% right) | 79% |
State-Trait Anxiety Inventory-Trait (STAI); Beck Depression Inventory (BDI).
The Vanderbilt Institutional Review Board approved the study and written informed consent was obtained following a complete description of the study.
Experimental paradigm
Repeated faces task
We designed a “repeated faces” task to investigate neural habituation to social stimuli (Figure 1). At the beginning of the repeated faces task, participants were told ‘In this study faces will appear in the middle of the screen. Your job is to stay focused on the screen and look at each face. The faces will flash quickly’. Participants were shown a series of 32 face identities over eight blocks, with each face identity shown a total of 1, 3, 5, or 7 times, for a total of 128 face presentations. The repeated faces task was comprised of 2 fMRI runs, with each run lasting approximately 4 m, 50 s. Each functional run began with a 10 s fixation crosshair followed by four blocks of faces. Blocks consisted of 16 faces followed by a 10 s fixation crosshair. Each face lasted a total 1 s followed by a black screen shown for 2–4 s. Faces were shown in pseudorandom order using a jittered, event-related design to maximize fMRI signal measurement efficiency (Friston et al., 1999). Stimulus jitter was randomly distributed across presentations. The repeated faces task was presented using E-Prime software (Version 2.0, Psychology Software Tools, Pittsburgh, PA, USA). Face stimuli were derived from two standard sets of human face images with neutral-valenced expressions (Lundqvist et al., 1998; Gur et al., 2001). To ensure that faces were perceived as “neutral”, arousal and valence ratings were collected for each face stimulus following all MRI scanning procedures. Detailed face stimuli and rating procedures can be found in the Supplementary Methods.
Fig. 1.
Repeated faces task design. There were a total of 32 neutral face stimuli presented in this task. Each stimulus was presented either 1 time, 3 times, 5 times, or 7 times. Stimuli were presented in pseudo-random order for 1 s followed by a black screen for 2–4 s.
MRI acquisition and processing
Structural and functional MRI data were collected using a 3 T Philips scanner equipped with a 32-channel head coil (Philips Healthcare, Inc., Best, The Netherlands). Detailed acquisition and processing procedures can be found in the Supplementary Methods. In brief, MRI data were analyzed using statistical parametric mapping (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) and MATLAB (Version 7.10 64-bit, The MathWorks, Inc., Natick, MA, USA), including preprocessing for slice time correction, realignment to the mean, spatially normalization into standard stereotactic space (MNI T1 template), and smoothing with a 6 mm FWHM Gaussian kernel.
Data analysis
Regions of interest (ROIs)
Our goal was to test whether rate of neural habituation in the social brain varied dimensionally across the spectrum of social fearfulness, including adaptive and maladaptive states—;therefore, to limit type I error, we selected seven regions previously shown to be involved in social information processing. ROIs included the: (i) amygdala; (ii) hippocampus; (iii) vmPFC; (iv) medial orbitofrontal cortex; (v) FFA; (vi) V1; and (vii) extrastriate visual cortex. Mask selection details can be found in the Supplementary Methods.
Initial amplitude and habituation slopes
We defined initial amplitude of response as the magnitude of signal during the first presentation of a face. Percent signal change for first face presentations was extracted for each ROI using MarsBar (Brett et al., 2002). Initial amplitudes in the left and right hemispheres were highly correlated across ROIs; to increase statistical power and minimize type I error, data from left and right ROIs were averaged.
Habituation is highly dependent on initial amplitude—that is, there is more opportunity for signal to attenuate over time if signal is initially high. Because were interested in examining differences in rate of habituation independent of differences in initial amplitude, we calculated a normalized habituation slope (b'), independent of initial amplitude differences, for each participant (Montagu, 1963; Plichta et al., 2014). We first extracted percent signal change from each ROI using MarsBar. As percent signal change in the left and right hemispheres were highly correlated across ROIs, data from left and right ROIs were averaged. Habituation slope (b') values were calculated for each participant using linear regression analysis (see Supplementary Methods for details).
We tested for correlations between social fearfulness and both aspects of response to faces: initial amplitude of response to first face presentations; and habituation of response across repeated faces (first to seventh). Correlation results were considered significant at α ≤ 0.05. Based on previous findings from our lab (Avery et al., 2016), showing a non-linear habituation pattern where the strongest differences in behavior occurred between the third and fifth face presentation, we performed planned secondary analyses testing associations between social fearfulness and habituation to faces within three discrete repetition windows: first to third presentation; third to fifth presentation; fifth to seventh presentation. Results were considered significant at α ≤ 0.0167 (0.05/3), Bonferroni-corrected for multiple comparisons. SAS software (Version 9.3, SAS Institute Inc., Cary, NC, USA) was used to perform all statistical analyses.
Functional connectivity
A generalized psychophysiological interaction analysis (gPPI; McLaren et al., 2012) was used to examine habituation of functional connectivity during repeated face presentations. gPPI analyses were conducted between the amygdala (seed region) and each ROI. Average amygdala connectivity data were extracted for each ROI and a normalized habituation of connectivity slope (b’) was calculated for each participant (Montagu, 1963; Plichta et al., 2014). Because percent signal change in the left and right hemispheres were highly correlated, signal was averaged across hemispheres and b' connectivity slopes were calculated bilaterally. As with the habituation analyses, we tested for correlations between social fearfulness and both initial amygdala connectivity to first face presentations and habituation of amygdala connectivity to repeated faces.
Results
Initial amplitude
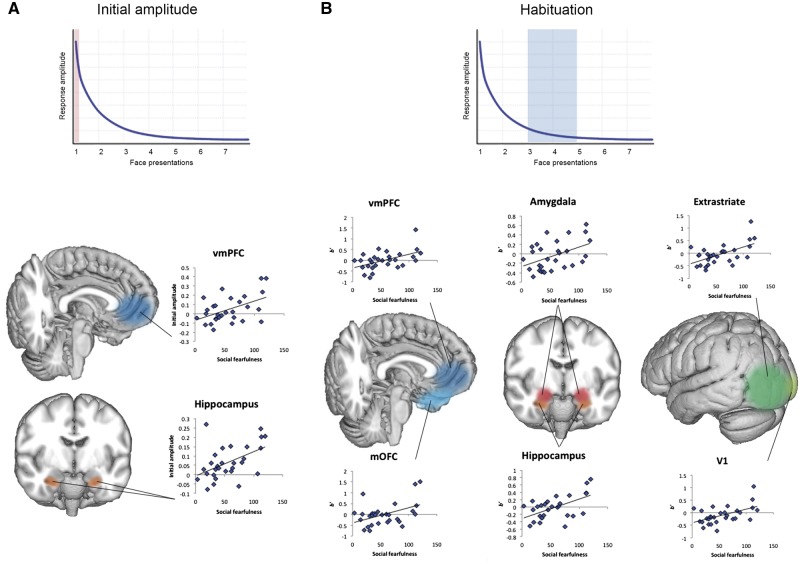
We performed correlations analyses to determine whether neural response to the first face presentation differed across the social fearfulness spectrum. Social fearfulness was correlated with heightened initial amplitude to first face presentations in two regions, the hippocampus and the vmPFC (hippocampus, r = 0.49, r2 = 0.24, P = 0.008; vmPFC, r = 0.48, r2 = 0.23, P = 0.008; Figure 2). Individuals with higher social fearfulness scores had heightened hippocampal and vmPFC responses to first face presentations. There were no correlations between social fearfulness and smaller initial amplitudes. Mean initial response values are included in Supplementary Table S1.
Fig. 2.
Differences in neural response to faces. (A) Social fearfulness was correlated with higher initial amplitudes to first face presentations in the hippocampus and vmPFC. (B) Social fearfulness was also correlated with higher b′ slope values between the third and fifth face exposure, indicating a slower rate of habituation, in the amygdala, hippocampus, vmPFC, mOFC, V1 and extrastriate cortex.
Habituation
Next we examined the relationship between social fearfulness and the rate of habituation from the first to the seventh face presentation. Social fearfulness was correlated with habituation in the hippocampus (Table 2). Individuals with higher social fearfulness scores had higher b' slopes, demonstrating sustained signal across repeated face presentations.
Table 2.
Significant correlations between social fearfulness and habituation to faces
| Region | Habituation | ||
|---|---|---|---|
| First to seventh | First to third | Third to fifth | |
| V1 | – | – | 0.52 |
| Extrastriate | – | 0.43 | 0.57 |
| Amygdala | – | – | 0.45 |
| Hippocampus | 0.46 | – | 0.56 |
| mOFC | – | – | 0.44 |
| vmPFC | – | – | 0.51 |
| Amygdala connectivity | |||
| First to seventh | First to third | Third to fifth | |
| V1 | – | – | 0.45 |
| Extrastriate | – | – | 0.49 |
Note: correlation values (r) are presented; shaded area indicates secondary tests; overall habituation (b', first to seventh) results were considered significant at α ≤ 0.05; secondary habituation results were considered significant at α ≤ 0.0167.
Because habituation changes may be more discrete, we also conducted planned secondary analyses of habituation within specific windows (e.g., first to third presentation). These analyses revealed prominent habituation differences between the third and fifth face presentation across a majority of brain regions, including: V1, extrastriate cortex, amygdala, hippocampus, mOFC and vmPFC. Across all of these regions, higher social fearfulness scores were correlated with slower habituation (Table 2; Figure 2). For the measure of early habituation (first to third presentation), social fearfulness was only correlated with habituation in the extrastriate cortex (Table 2). In line with our previous behavioral results (Avery et al., 2016), there were no habituation differences during the late viewing window (fifth to seventh presentation). Also, the FFA did not show any differences in habituation. Mean habituation values are included in Supplementary Table S1.
Amygdala connectivity
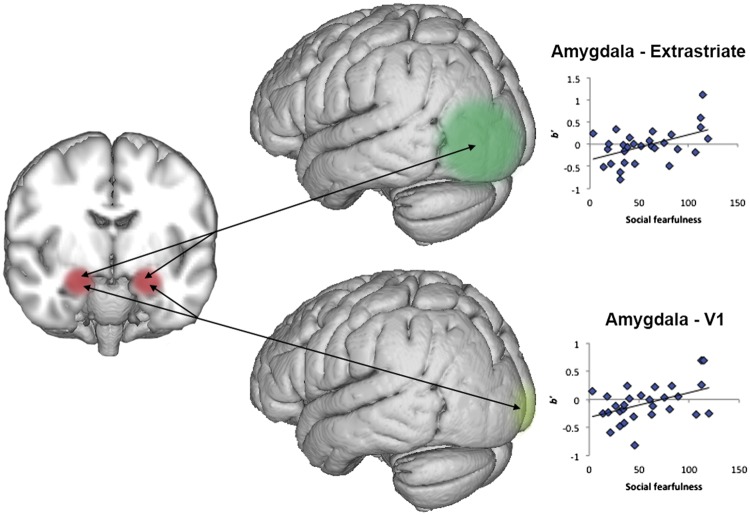
The amygdala is overwhelmingly implicated in social anxiety and is a central hub in the social processing network. To determine whether initial amplitude and habituation differences were associated with functional interactions with the amygdala, or rather reflected localized processing, we examined amygdala functional connectivity. Although social fearfulness was not correlated with connectivity during the initial response or habituation across the entire time period, there were significant connectivity differences between the third and fifth presentations in both V1 and extrastriate cortex. Higher social fearfulness scores were correlated with slower habituation of amygdala connectivity with both regions (Table 2; Figure 3). There were no differences during other face viewing windows.
Fig. 3.
Differences in habituation of amygdala functional connectivity. Social fearfulness was correlated with sustained signaling between the amygdala and two visual cortex regions, the extrastriate cortex and V1, between the third and fifth face exposure.
Discussion
In this study we tested the hypothesis that rate of neural habituation to faces is associated with individual differences in social fearfulness. We found widespread differences in habituation across the social brain—social fearfulness was associated with slower habituation in the amygdala, hippocampus, vmPFC, mOFC, extrastriate cortex and V1. Habituation differences were driven by sustained functional connectivity between the amygdala and visual cortex, indicating that sustained processing in early visual regions contributes to social function. Surprisingly, we did not find evidence for a failure of medial prefrontal cortex regulation over the amygdala, suggesting that habituation differences are largely influenced by lower-level sensory circuits. Social fearfulness was also associated with elevated initial response to faces in a subset of regions, indicating heightened novelty detection. These findings reveal a compelling brain-behavior link—differences in a fundamental sensory filtering and learning mechanism is associated broadly with social function and may underlie social dysfunction.
We found extensive differences in habituation across the continuum of social fearfulness. Our findings extend our previous findings of habituation differences in extreme groups (e.g., shyness; Avery et al., 2016; Blackford et al., 2011, 2013). The results from this study provide the first evidence that neural habituation varies across the entire spectrum of social fearfulness. In addition to replicating our previous amygdala and hippocampal findings, we now show evidence for habituation differences in the vmPFC, mOFC, V1 and extrastriate cortex. Habituation provides a critical neuronal code for familiarity and safety (Wilson and Rolls, 1993; Fried et al., 1997; Dubois et al., 1999; Wright et al., 2001; Gonsalves et al., 2005) and our findings indicate that this code is altered across multiple regions comprising the social brain. This pattern of sustained signaling contributes to individual variation in social function.
Intriguingly, we found that failure to habituate was driven by sustained amygdala-visuocortical interactions and not by amygdalo-frontal interactions. Although deficient top-down prefrontal regulation of the amygdala is a prominent finding in studies of social anxiety disorder (Milad et al., 2006; Rauch et al., 2006; Akirav and Maroun, 2007; Liao et al., 2010; Sladky et al., 2013), the current findings suggest that prefrontal inhibition of the amygdala is not critical for habituation. Neuronal signals for salience are evident even in early visual regions, such as V1 and extrastriate cortex, with increased activity likely directed through regulation by the amygdala (Kastner and Ungerleider, 2000; Ousdal et al., 2014). Additionally, recent studies have supported the notion of heightened sensory processing in social anxiety, with the amygdala appearing to drive the increased functional interactions with visual cortex (Liao et al., 2010; Sladky et al., 2013). Our findings add to compelling preliminary evidence that feedback between lower-level visual regions and the amygdala may play a crucial role in social function. We also show for the first time that amygdala-visuocortical interactions are sustained in people with higher levels of social fear, suggesting a mechanism for preliminary correlations with social function. Based on these findings we propose that basic sensory gating deficits contribute to the increased feelings of unfamiliarity and threat in social settings characteristic of people with high social fear and social anxiety.
In addition to habituation differences, elevated initial responses were correlated with social fearfulness in two regions—the hippocampus and vmPFC. The hippocampus and vmPFC play well-described roles in novelty detection (Wilson and Rolls, 1993; Fried et al., 1997; Rutishauser et al., 2006; Blackford et al., 2010) and orienting response (Hendrickson et al., 1969; Quirk and Beer, 2006; Hartley and Phelps, 2010; Ray and Zald, 2012; Motzkin et al., 2014; Riga et al., 2014). Social anxiety has long been linked with increased neural response to novel faces (Freitas-Ferrari et al., 2010). However, the standard technique of averaging fMRI signal over time confounds the two components of neural response—initial response to novelty and habituation—making it impossible to determine whether regions differ in one or both components. Here, by systematically isolating initial responses to faces from habituation, we show that the hippocampus and vmPFC have both higher initial responses to faces and slower habituation, suggesting roles in both components. In contrast, we did not find an association between social fearfulness and heightened initial response in the amygdala, a region commonly associated with increased novelty detection in social anxiety disorder. Instead, we show social fearfulness differences only in amygdala habituation, suggesting a selective role for the amygdala in sensory filtering differences. Novelty response and habituation are separable neural mechanisms that rely on distinct neural circuits and may have different implications for treatment; future studies would benefit from parsing these two fundamental components of neural response to stimuli.
Differences in habituation may contribute broadly to maladaptive social behavior—habituation failures have been noted in disorders marked by impaired social function, including autism (Kleinhans et al., 2009) and schizophrenia (Holt et al., 2005; Williams et al., 2013), with rate of habituation associated with severity of social impairments in autism (Kleinhans et al., 2009). However, habituation findings in patients social anxiety disorder are currently unclear (Campbell et al., 2007; Sladky et al., 2012), with differences in task demands likely contributing to variability across studies. However, overall findings suggest that the study of habituation across the spectrum of individual differences, including both adaptive and maladaptive traits, is well-suited to bridge the gap between basic neural processing differences and the experience of human psychopathology.
While reduced habituation suggests disrupted learning in individuals with high social fear, the question remains whether disrupted neural habituation is associated with overt deficits in behavior. Preliminary work from our lab provides a tantalizing link between habituation and face recognition memory. We studied the association between social inhibition and recognition memory using a similar “repeated faces” task; recognition memory was tested for faces seen 1, 3, 5 and 7 times. While all individuals showed memory improvements with increased exposures to a face, higher inhibition scores were associated with smaller memory benefits from repeated exposure (Avery et al., 2016). We propose that the inability to rapidly habituate to faces reflects a learning deficit that leads to overt impairment in face recognition.
The current study findings should be considered in the context of several limitations. First, we only examined habituation for one through seven presentations. The participants with the highest social fear failed to habituate after seven face presentations; however, it is unknown whether they would eventually habituate (delay) or whether they would fail to habituate (deficit). Whether there is a delay or deficit may provide critical information about underlying processes; therefore, future studies should examine habituation with additional exposures (>7). Second, although many studies of social anxiety have used fearful faces, in this study we only examined neutral faces. It will be critical for future studies to determine whether emotion modulates habituation rate and whether these differences are associated with social fearfulness. Last, although participants recruited from the university and from the broader Nashville community were demographically similar, the majority of study participants were recruited from the university community, which may affect the generalizability of findings.
In conclusion, this is the first study to establish that widespread differences in neural habituation underlie a fundamental social trait, social fearfulness. These findings are an essential step in defining a dimensional biological marker for social function, which may help determine individuals most at risk for development of functional impairments. Understanding individual variation in habituation may also help determine appropriate interventions for social anxiety disorder. For example, exposure therapy is one of the most successful treatments for the reduction of social fears and habituation is improved by successful exposure therapy (Leutgeb et al., 2009; Matthews et al., 2015). Thus, exposure-based learning may alter fundamental sensory filtering processes in the brain. Further studies are needed to determine whether improved habituation is the critical mechanism underlying successful reduction of social fear and social anxiety.
Supplementary data
Supplementary data are available at SCAN online.
Funding
Research was supported in part by funding from the National Institutes of Mental Health (1F31MH102008 to S.N.A.; K01MH083052 to J.U.B.) and the Vanderbilt Institute for Clinical and Translational Research (VR5715 to S.N.A.). Portions of this work were presented at the Society for Biological Psychiatry Annual Meeting, New York, NY, May 2014.
Conflict of interest. None declared.
Supplementary Material
References
- Akirav I., Maroun M. (2007). The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plasticity, 2007, 30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I., Richter-Levin G. (1999). Priming stimulation in the basolateral amygdala modulates synaptic plasticity in the rat dentate gyrus. Neuroscience Letters, 270, 83–6. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Behniea H., Kelly J.L. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience, 118, 1099–120. [DOI] [PubMed] [Google Scholar]
- Avery S.N., VanDerKlok R.M., Heckers S., Blackford J.U. (2016). Impaired face recognition is associated with social inhibition. Psychiatry Research, 236, 53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford J.U., Allen A.H., Cowan R.L., Avery S.N. (2013). Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience, 8, 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford J.U., Avery S.N., Cowan R.L., Shelton R.C., Zald D.H. (2011). Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience, 6, 621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford J.U., Avery S.N., Shelton R.C., Zald D.H. (2009). Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience, 10, 145.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford J.U., Buckholtz J.W., Avery S.N., Zald D.H. (2010). A unique role for the human amygdala in novelty detection. NeuroImage, 50, 1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.L.L., Valabregue R., Poline J.B. (2002). Region of interest analysis using an SPM toolbox [abstract] Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2-6, 2002, Sendai, Japan. NeuroImage, 16, abstract 497. [Google Scholar]
- Britton J.C., Shin L.M., Barrett L.F., Rauch S.L., Wright C.I. (2008). Amygdala and fusiform gyrus temporal dynamics: responses to negative facial expressions. BMC Neuroscience, 9, 44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell I.W. (1982). Discrimination of faces by young infants. Journal of Experimental Child Psychology, 33, 298–308. [DOI] [PubMed] [Google Scholar]
- Campbell D.W., Sareen J., Paulus M.P., Goldin P.R., Stein M.B., Reiss J.P. (2007). Time-Varying amygdala response to emotional faces in generalized social phobia. Biological Psychiatry, 62, 455–63. [DOI] [PubMed] [Google Scholar]
- Clauss J.A., Blackford J.U. (2012). Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 1066–75.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J. (2002). Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry, 51, 68–80. [DOI] [PubMed] [Google Scholar]
- Davis M. (1997). Neurobiology of fear responses: the role of the amygdala. The Journal of Neuropsychiatry and Clinical Neurosciences, 9, 382–402. [DOI] [PubMed] [Google Scholar]
- Dell’Osso L., Abelli M., Pini S., et al. (2014). Dimensional assessment of DSM-5 social anxiety symptoms among university students and its relationship with functional impairment. Neuropsychiatric Disease and Treatment, 10, 1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Osso L., Rucci P., Cassano G.B., et al. (2002). Measuring social anxiety and obsessive-compulsive spectra: comparison of interviews and self-report instruments. Comprehensive Psychiatry, 43, 81–7. [DOI] [PubMed] [Google Scholar]
- Dubois S., Rossion B., Schiltz C., et al. (1999). Effect of familiarity on the processing of human faces. NeuroImage, 9, 278–89. [DOI] [PubMed] [Google Scholar]
- Etkin A. (2010). Functional neuroanatomy of anxiety: a neural circuit perspective. Current Topics in Behavioral Neurosciences, 2, 251–77. [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry, 164, 1476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Ferrari M.C., Hallak J.E.C., Trzesniak C., et al. (2010). Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 34, 565–80. [DOI] [PubMed] [Google Scholar]
- Fried I., MacDonald K.A., Wilson C.L. (1997). Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron, 18, 753–65. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Zarahn E., Josephs O., Henson R.N., Dale A.M. (1999). Stochastic designs in event-related fMRI. NeuroImage, 10, 607–19. [DOI] [PubMed] [Google Scholar]
- Furmark T. (2002). Social phobia: overview of community surveys. Acta Psychiatrica Scandinavica, 105, 84–93. [DOI] [PubMed] [Google Scholar]
- Furmark T. (2009). Neurobiological aspects of social anxiety disorder. The Israel Journal of Psychiatry and Related Sciences, 46, 5–12. [PubMed] [Google Scholar]
- Gabbott P.L.A., Warner T.A., Jays P.R.L., Salway P., Busby S.J. (2005). Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology, 492, 145–77. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. (2002). Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience, 115, 1261–79. [DOI] [PubMed] [Google Scholar]
- Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. (2009). Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry, 66, 170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves B.D., Kahn I., Curran T., Norman K.A., Wagner A.D. (2005). Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron, 47, 751–61. [DOI] [PubMed] [Google Scholar]
- Gur R.C., Ragland J.D., Moberg P.J., et al. (2001). Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 25, 766–76. [DOI] [PubMed] [Google Scholar]
- Hartley C.A., Phelps E.A. (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 35, 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiser N. A., Turner S.M., Beidel D.C. (2003). Shyness: relationship to social phobia and other psychiatric disorders. Behaviour Research and Therapy, 41, 209–21. [DOI] [PubMed] [Google Scholar]
- Hendrickson C.W., Kimble R.J., Kimble D.P. (1969). Hippocampal lesions and the orienting response. Journal of Comparative and Physiological Psychology, 67, 220–7. [DOI] [PubMed] [Google Scholar]
- Holt D.J., Weiss A.P., Rauch S.L., et al. (2005). Sustained activation of the hippocampus in response to fearful faces in schizophrenia. Biological Psychiatry, 57, 1011–9. [DOI] [PubMed] [Google Scholar]
- Hopko D.R., Stowell J., Jones W.H., Armento M.E.A., Cheek J.M. (2005). Psychometric properties of the Revised Cheek and Buss Shyness Scale. Journal of Personality Assessment, 84, 185–92. [DOI] [PubMed] [Google Scholar]
- Iidaka T., Omori M., Murata T., et al. (2001). Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. Journal of Cognitive Neuroscience, 13, 1035–47. [DOI] [PubMed] [Google Scholar]
- Kastner S., Ungerleider L.G. (2000). Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience, 23, 315–41. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Johnson L.C., Richards T., et al. (2009). Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. American Journal of Psychiatry, 166, 467–75. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Greenson J., Dawson G., Aylward E. (2015). Altered dynamics of the fMRI response to faces in individuals with autism. Journal of Autism and Developmental Disorders, 46, 232–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J, Davis M., Ohman A. (2000). Fear and anxiety: animal models and human cognitive psychophysiology. Journal of Affective Disorders, 61, 137–59. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E., Iwata J., Cicchetti P., Reis D.J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 8, 2517–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis M.P., Bolivar V.J. (2006). Habituation in rodents: a review of behavior, neurobiology, and genetics. Neuroscience and Biobehavioral Reviews, 30, 1045–64. [DOI] [PubMed] [Google Scholar]
- Leutgeb V., Schäfer A., Schienle A. (2009). An event-related potential study on exposure therapy for patients suffering from spider phobia. Biological Psychology, 82, 293–300. [DOI] [PubMed] [Google Scholar]
- Liao W., Qiu C., Gentili C., et al. (2010). Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state fMRI study. PLoS One, 5, e15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D., Flykt A., Ohman A., Lundqvist D., Flykt A., Ohman A. (1998). The Karolinska Directed Emotional Faces - KDEF, CD ROM from Department of Clinical Neuroscience, Psychology Section, Karolinska Institutet, Stockholm, Sweden ISBN, 91-630-7164-9.
- Mathew S.J., Ho S. (2006). Etiology and neurobiology of social anxiety disorder. The Journal of Clinical Psychiatry, 67(Suppl 1), 9–13. [PubMed] [Google Scholar]
- Matthews A., Naran N., Kirkby K.C. (2015). Symbolic online exposure for spider fear: habituation of fear, disgust and physiological arousal and predictors of symptom improvement. Journal of Behavior Therapy and Experimental Psychiatry, 47C, 129–37. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61, 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L., Pitman R.K., Quirk G.J. (2006). Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological Psychology, 73, 61–71. [DOI] [PubMed] [Google Scholar]
- Miskovic V., Schmidt L.A. (2012). Social fearfulness in the human brain. Neuroscience and Biobehavioral Reviews, 36, 459–78. [DOI] [PubMed] [Google Scholar]
- Mohedano-Moriano A., Pro-Sistiaga P., Arroyo-Jimenez M.M., et al. (2007). Topographical and laminar distribution of cortical input to the monkey entorhinal cortex. Journal of Anatomy, 211, 250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagu J.D. (1963). Habituation of the psycho-galvanic reflex during serial tests. Journal of Psychosomatic Research, 52, 199–214. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. (2014). Ventromedial prefrontal cortex lesions alter neural and physiological correlates of anticipation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34, 10430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M., Insausti R. (2005). Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis). European Journal of Neuroscience, 22, 1368–88. [DOI] [PubMed] [Google Scholar]
- Öhman A. (2005). The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology, 30, 953–8. [DOI] [PubMed] [Google Scholar]
- Ousdal O.T., Andreassen O.A., Server A., Jensen J. (2014). Increased Amygdala and Visual Cortex Activity and Functional Connectivity towards Stimulus Novelty Is Associated with State Anxiety. PLoS One, 9, e96146.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K.L., Liberzon I., Welsh R.C., Britton J.C., Taylor S.F. (2003). Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 28, 1344–50. [DOI] [PubMed] [Google Scholar]
- Phelps E.A. (2004). Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology, 14, 198–202. [DOI] [PubMed] [Google Scholar]
- Plichta M.M., Grimm O., Morgen K., et al. (2014). Amygdala habituation: a reliable fMRI phenotype. NeuroImage, 103, 383–90. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. (2006). Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology, 16, 723–7. [DOI] [PubMed] [Google Scholar]
- Ramaswami M. (2014). Network plasticity in adaptive filtering and behavioral habituation. Neuron, 82, 1216–29. [DOI] [PubMed] [Google Scholar]
- Rauch S.L., Shin L.M., Phelps E.A. (2006). Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biological Psychiatry, 60, 376–82. [DOI] [PubMed] [Google Scholar]
- Ray R.D., Zald D.H. (2012). Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience & Biobehavioral Reviews, 36, 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riga D., Matos M.R., Glas A., Smit A.B., Spijker S., Van den Oever M.C. (2014). Optogenetic dissection of medial prefrontal cortex circuitry. Frontiers in Systems Neuroscience, 8, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.C., Tomic D.L., Parkinson C.H., et al. (2007). Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. Journal of Comparative Neurology, 502, 86–112. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Mamelak A.N., Schuman E.M. (2006). Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron, 49, 805–13. [DOI] [PubMed] [Google Scholar]
- Schneier F.R., Blanco C., Antia S.X., Liebowitz M.R. (2002). The social anxiety spectrum. The Psychiatric Clinics of North America, 25, 757–74. [DOI] [PubMed] [Google Scholar]
- Schuyler B.S., Kral T.R.A., Jacquart J., et al. (2012). Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Social Cognitive and Affective Neuroscience, 9, 176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Höflich A., Atanelov J., et al. (2012). Increased neural habituation in the amygdala and orbitofrontal cortex in social anxiety disorder revealed by FMRI. PLoS One, 7, e50050.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladky R., Höflich A., Küblböck M., et al. (2013). Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI. Cerebral Cortex; ( New York, N.Y: 1991), 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder K.A., Keil A. (2008). Repetition suppression of induced gamma activity predicts enhanced orienting toward a novel stimulus in 6-month-old infants. Journal of Cognitive Neuroscience, 20, 2137–52. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Williams J.B., Gibbon M., First M.B. (1992). The structured clinical interview for DSM-III-R (SCID). I: history, rationale, and description. Archives of General Psychiatry, 49, 624–9. [DOI] [PubMed] [Google Scholar]
- Stefanacci L., Suzuki W.A., Amaral D.G. (1996). Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. Journal of Comparative Neurology, 375, 552–82. [DOI] [PubMed] [Google Scholar]
- Stein D.J., Ono Y., Tajima O., Muller J.E. (2004). The social anxiety disorder spectrum. The Journal of Clinical Psychiatry, 65(Suppl 1), 27–33. quiz 34–36. [PubMed] [Google Scholar]
- Wager T.D., van Ast V.A., Hughes B.L., Davidson M.L., Lindquist M.A., Ochsner K.N. (2009). Brain mediators of cardiovascular responses to social threat, Part II: prefrontal-subcortical pathways and relationship with anxiety. NeuroImage, 47, 836–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.E., Blackford J.U., Luksik A., Gauthier I., Heckers S. (2013). Reduced habituation in patients with schizophrenia. Schizophrenia Research, 151, 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F.A.W., Rolls E.T. (1993). The effects of stimulus novelty and familiarity on neuronal activity in the amygdala of monkeys performing recognition memory tasks. Experimental Brain Research, 93, 367–82. [DOI] [PubMed] [Google Scholar]
- Wright C.I., Fischer H., Whalen P.J., McInerney S.C., Shin L.M., Rauch S.L. (2001). Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport, 12, 379–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.