Abstract
Given the spike in risky behaviors that accompanies adolescence, the need to examine the processes and contextual factors that influence disinhibition for adolescents is of great import. Using an emotionally salient cognitive control task, we examined how socially appetitive and aversive cues differentially affect behavioral inhibition across development. In Study 1 (N = 94, ages 8–30 years), we found that socially appetitive cues were particularly detrimental to inhibition, a finding driven by our adolescent sample. In Study 2 (N = 35, ages 12–17 years), we sought to explore the neural processes implicated in suboptimal inhibition during adolescence. Replicating our behavioral findings from Study 1, socially appetitive cues again caused detriments to inhibition compared with socially aversive cues. At the neural level, increased activation in affective regions (amygdala and ventral striatum) while viewing socially appetitive relative to socially aversive cues was correlated with increases in disinhibition. Furthermore, both whole-brain and functional connectivity analyses suggest recruitment of affective and social-detection networks (fusiform, bilateral temporoparietal junction) may account for the increased focus on appetitive relative to aversive cues. Together, our findings suggest that adolescents show detriments in inhibition to socially appetitive contexts, which is related to increased recruitment of affective and social processing neural regions.
Keywords: emotion–regulation, adolescence, inhibition, social context
Introduction
Adolescence is widely understood as a time of transition. In addition to many physical and hormonal changes (Dorn, 2006), adolescents undergo significant change in their cognitive abilities, self-awareness and social identity (Rutter and Rutter, 1993). Adolescents’ personal identification, understanding of social complexities and successful adherence to social norms become increasingly important and central to daily life (Baumeister and Leary, 1995; Blakemore and Choudhury, 2006), as does the growing reliance on peer relationships (Brown, 2004). In fact, adolescence is a developmental period across species where the mere presence of peers affects decision-making processes (Sisk and Foster, 2004; Sato et al., 2008; Spear, 2009). At a time when fitting in with one’s peers is of great import, the ability to pick up, process and utilize social information is key.
Overlapping with this change in social identity is a theorized social reorientation of the brain—a period of neural growth and plasticity triggered at the onset of puberty that gears processing toward socially salient aspects of the environment (Nelson et al., 2005, 2016). The social reorientation process serves the function of focusing adolescents’ attention to salient socioemotional information in their environment (Nelson et al., 2005, 2016). Social information may receive preferential attention to guide adolescents to branch out and form stronger bonds with their peers relative to their family (Crone and Dahl, 2012; Nelson et al., 2016). Several neurobiological changes, such as increased recruitment of emotion and reward processing regions of the brain coincide with this increased salience of peers (Nelson et al., 2005; Guyer et al., 2012). This heightened responsivity in emotion processing regions is thought to be key in assessing both positive and negative salient features in the social environment (Nelson et al., 2005; Yang et al., 2007) and cooccurs with adolescents’ responsivity to peer cues (Chein et al., 2011; Blakemore and Robbins, 2012; Knoll et al., 2015).
In light of this developmental change, it is interesting to note that much of the prior work examining adolescent decision-making has occurred within a social vacuum (Beyth-Marom et al., 1993; Reyna and Farley, 2006), although several studies have begun to examine developmental changes in decision-making in a social context by examining the role of peer presence on risk taking (Chein et al., 2011), cognitive control in the context of emotional faces (Somerville et al., 2011) or emotion processing and links to risk taking (Pfeifer et al., 2011). Studies set in the lab, divorced from social contexts, have found that adolescents have comparable reasoning ability to adults (Beyth-Marom et al., 1993; Millstein and Halpern-Felsher, 2002; Reyna and Farley, 2006). Yet much data suggest adolescents still engage in higher rates of risky behavior and poorer cognitive control, particularly in the presence of their peers (Gardner and Steinberg, 2005), a phenomenon even found in rodents (Logue et al., 2014). In part, the discrepancy in these findings may be due to intact cognitive abilities that are subverted only in specific contexts. Examining decision-making within a social vacuum is likely to yield results inconsistent with decision-making processes that adolescents must use in everyday life (Steinberg, 2007).
Although social relationships are important across many developmental phases, motivated social engagement is directed toward caregivers in infancy, toward playmates during the pre-teen years, toward larger peer groups during adolescence and toward potential mates in adulthood (see Nelson et al., 2016). Given the increased importance of peer relationships for adolescents, social contexts may be particularly impactful in affecting decision-making processes. That is, when salient cues are present (e.g. presence of social stimuli), poorer employment of emotion regulation strategies (i.e. ‘hot’ cognition) and reduced behavioral inhibition are likely to follow (Metcalfe and Mischel, 1999). Recognizing that adolescents, more so than children or adults, may be engaging in riskier decision-making in the presence of social cues, paradigms with salient social input may explain how and when adolescents’ behavioral choices are likely to be suboptimal. Furthermore, understanding mechanistically how adolescents’ cognitive processing is affected in social contexts will yield insight into factors that influence and derail adolescents’ emotional and cognitive systems (Casey et al., 2008).
Prior research has indicated the ventral striatum (Zink et al., 2004) and amygdala (Cunningham and Brosch, 2012) as particularly sensitive to salient cues. The ventral striatum has been largely implicated in the processing of rewards (O’Doherty et al., 2004), as well as socially appetitive stimuli (Somerville et al., 2011), and tends to be more active among adolescents than children or adults in the presence of peers (Chein et al., 2011). Importantly, some work has shown that ventral striatum activation is positively correlated with reduced inhibition to positive affect during adolescence but not childhood or adulthood (Somerville et al., 2011). The amygdala, while originally thought as primarily responsible in fear processing (Davis, 1992), is now more generally implicated in detecting emotional salience (Anderson and Phelps, 2001) and signaling the presence of individually relevant information (Stillman et al., 2015). This is in large part due to findings implicating the amygdala in reward (Mahler and Berridge, 2009), social-cognition processing (Adolphs, 2010), and coding for stimuli that are both positively and negatively valenced (Zald, 2003). Although the amygdala is important in redirecting attentional resources to emotional stimuli for all age groups, research suggests that adolescents show heightened amygdala activation relative to adults during emotional arousal (Ernst et al., 2005).
Together, the ventral striatum and amygdala are significant pieces of what Nelson et al. (2005) refer to as the affective node of the brain. In adolescence, the affective node is thought to show increased responsivity to social stimuli during decision-making processes, often at the cost of regulatory activation (e.g. the prefrontal cortex). With development, connections between regulatory regions and the affective node increase, allowing for better emotion–regulation (Casey, 2015). Examining how regions in the affective node functionally connect to other brain regions—including regions utilized as inhibitory networks such as the prefrontal cortex or regions implicated in the detection and processing of social information such as the temporal parietal junction (TPJ) or fusiform gyrus—will be key in understanding how social contexts affect decision-making processes in adolescents.
In this study, we used fMRI to examine how social cues affect individuals’ emotion regulation in the presence of appetitive (i.e. peer acceptance) and aversive (i.e. peer rejection) contexts. We conducted two studies using a novel version of a cognitive control task. Participants completed a go-nogo task, coupled with socioemotional stimuli, in which the go-nogo stimuli (letters) were superimposed on socially appetitive and aversive scenes. The task was modified based on a prior emotion–regulation task (Cohen-Gilbert and Thomas, 2013) which used images from the IAPS database (Lang et al., 1999). Although this prior study found that aversive stimuli caused greater disinhibition than positive stimuli, the IAPS photos are not necessarily reflective of normative social cues for adolescents. To remedy this, our manipulation utilized more ecologically relevant stimuli, which included both socially appetitive scenes (e.g. teenagers laughing together) and socially aversive scenes (e.g. a child being left out by his peers). In Study 1, we examined inhibitory failure rates in children, adolescents, young adults and adult participants to determine if there are developmental differences in disinhibition as a function of social context. In Study 2, we used fMRI to examine just in adolescents how appetitive and aversive social contexts may differentially impact affective, regulatory and social information networks.
Methods
Participants
Participants were recruited from the community and included 130 individuals, 94 who participated in the behavioral study (Study 1) and 36 who participated in the fMRI study (Study 2). Informed consent/assent was obtained for all participants and the University’s Institutional Review Board approved all study procedures and materials.
Participants in Study 1 included 94 individuals (43 female), ranging in age from 8 to 30 years. To examine differences in performance based on age, we divided the sample into four age groups representing children, who were all elementary school-age (ages 6–11 years), adolescents who were all middle and high school-age (ages 12–17 years), young adults who were college freshman (all age 18 years) and older adults (ages 21–30 years). Participants in Study 2 included an independent sample of 36 adolescents (ages 12–17 years). One participant was excluded due to excessive movement in all functional scan runs, resulting in 35 adolescents in the sample (Table 1).
Table 1.
Participant information for Study 1 (behavioral comparison across age groups) and Study 2 (fMRI study of an independent sample of adolescents)
| Age group | N | Mean age | s.d. | # of females |
|---|---|---|---|---|
| Study 1 | ||||
| Children | 19 | 10.1 | 1.1 | 9 |
| Adolescents | 27 | 14.8 | 2.3 | 18 |
| Young adults | 31 | 18 | 0 | 15 |
| Adults | 17 | 21.6 | 2.7 | 9 |
| Study 2 | ||||
| Adolescents | 35 | 15.3 | 1.3 | 20 |
Social go-nogo task
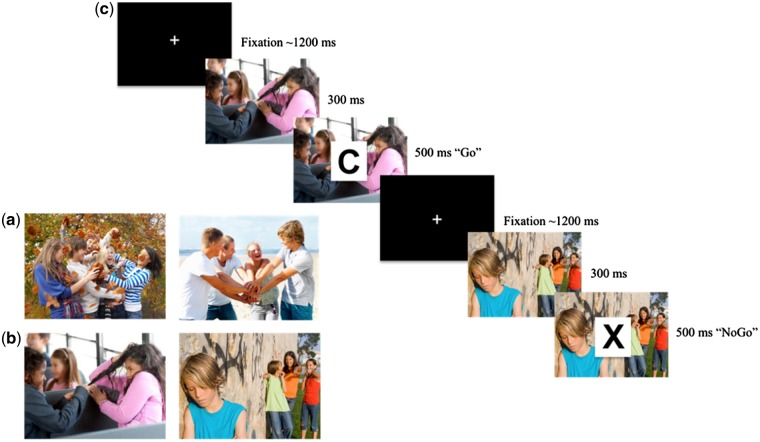
Participants completed a go-nogo task, coupled with socioemotional stimuli, which we modified based on a prior emotion–regulation task (Cohen-Gilbert and Thomas, 2013). Participants were first presented with an image for 300 ms, depicting either socially appetitive or socially aversive scenes. After this short stimulus presentation, a letter was superimposed on top of the scene for 500 ms, during which participants were instructed to hit a response button as fast as possible for every letter except when presented with the letter ‘X’ during which they were told to withhold the button response. The ‘go’ trials were presented throughout each block on ∼72% of the trials and the ‘nogo’ X trials were presented throughout each block on ∼28% of the trials. Participants completed four blocks of the socially appetitive condition (Figure 1a) and four blocks of the socially aversive condition (Figure 1b), each with 25 trials per block. Each block was exclusive, with block presentation order randomized for each participant. Each condition (socially appetitive, socially aversive) consisted of equal number of trials (n = 100 total), allowing for direct comparison across condition type. Participants were given 500 ms to respond per trial, with a jittered intertrial interval averaging 1200 ms. Effective cognitive control was measured via successfully inhibiting the button press on no-go trials. To index behavioral disinhibition, we calculated the percent of false alarms (i.e. pressing on no-go trials when responses should be inhibited) during the socially appetitive and socially aversive conditions, as this has been routinely measured to assess disinhibition in prior fMRI go-nogo paradigms (Ahmadi et al., 2013; Kreusch et al., 2013).
Fig. 1.
Examples of scenes in the socially appetitive (a) and socially aversive (b) go-nogo task. (c) Participants observe social scenes and are then shown a letter, presented rapidly with a jittered fixation between each trial. Participants push a button as quickly as possible to all letters (Go) except for X (No-go), during which they must withhold the button response.
Social stimuli
The scenes used in the socially appetitive and aversive condition represented either images of appetitive social scenes or aversive social scenes and were collected using Google image searches with keywords such as ‘bullying’ and ‘victimization’ or ‘social acceptance’ and ‘celebration’. We selected images based on (i) their relevance to either social inclusion or social exclusion and (ii) the clarity of the social interaction (i.e. one central person being excluded and expressing sadness). We attempted to balance the number of photos that included children, teens and adults, so that any effect seen would not be specific to participants’ specific inclusion in an age group. To ensure that the appetitive and aversive images were equivalent in terms of valence, physiological impact and social impact, we had a separate sample of college students norm these images (n = 35, 18 females; M age = 19.26, s.d. = 1.01). Students rated all 200 images using a 9-point Likert scale to indicate how they felt when viewing the pictures (emotional valence: 1 = unhappy to 9 = happy; physiological impact: 1 = anxious to 9 = calm; social impact: 1 = submissive to 9 = dominant). A rating of 5 indicates a neutral response. The wording for questions and descriptions for each dimension was modified from the IAPS rating manual (Lang et al., 1999). The adjusted mean rating (distance from neutral) for the socially aversive and socially appetitive stimuli was similar for emotional valence (socially aversive: M = −2.38, s.d. = 1.24; socially appetitive: M = 2.37, s.d. = 1.26), physiological impact (socially aversive: M = −1.89, s.d. = 1.49; socially appetitive: M = 1.91, s.d. = 1.61) and social impact (socially aversive: M = −1.25, s.d. = 1.77; socially appetitive: M = 1.48, s.d. = 1.59). The absolute mean ratings between the socially appetitive and socially aversive stimuli were significantly different [emotional impact: t(34) = 24.56, P < 0.0001; physiological impact, t(34) = 17.68, P < 0.0001; social impact, t(34) = 8.42, P < 0.0001]. In addition, the absolute mean ratings were all significantly different from neutral [socially appetitive: emotional valence t(34) = 22.41; physiological impact t(34) = 13.08; social impact t(34) = 8.44] and socially aversive: emotional valence t(34) = 20.02; physiological impact t(34) = 11.97; social impact t(34) = 6.06 (P < 0.0001).
fMRI acquisition
fMRI data were collected using a 3T Siemens Trio MRI Scanner. Structural scans included a T2*weighted, matched-bandwidth, high-resolution, anatomical scan (TR = 4 s; TE = 64 ms; FOV = 230; matrix = 192 × 192; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1.9 s; TE = 2.3 ms; FOV = 230; matrix = 256 × 256; sagittal plane; slice thickness = 1 mm; 192 slices). Each condition of the go-nogo task included 120 T2-weighted echo-planar images (EPIs) [slice thickness = 3 mm; 38 slices; TR = 2 s; TE = 25 ms; matrix = 92 × 92; FOV = 230 mm; voxel size 3 × 3 × 3 mm]. The orientation for the T2 and functional scans were oblique axial to optimize coverage area and reduce signal dropout.
Data preprocessing and analysis
We conducted analyses using Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Specifically, functional images were spatially realigned to correct for movement (no participant exceeded 3 mm of maximum image to image motion in any direction for more than 5% of their EPIs), which were then coregistered onto each participant’s high-resolution MPRAGE. After segmentation into cerebrospinal fluid, gray matter and white matter, a normalization transformation matrix was applied to the functional and T2 structural images. This process places each participant’s data within the standard stereotactic space set forth by the Montreal Neurological Institute. Normalized functional data were smoothed with an 8 mm Gaussian kernel, full-width-at-half maximum, to optimize signal-to-noise ratio. High-pass temporal filtering (128 s) was applied to remove low-frequency drift. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model order of 1.
At the individual level, a fixed-effects analysis was modeled as a block design which contained all ‘go’, ‘no-go’ ‘false alarm’ and ‘failed hit’ trials for each condition (socially appetitive, socially aversive), but with each individual trial modeled (duration = 800 ms), so null events (e.g. jittered intertrial-intervals) were not explicitly modeled as part of the condition. The parameter estimates from the general linear model were used to create linear contrast images comparing each of the conditions of interest at the group level. Random effects, whole-brain analyses were conducted to examine group effects of condition type on neural activation. In addition, we were particularly interested in examining how individuals’ tendency for reduced inhibitory control would map onto neural activation, so we conducted whole-brain regression analyses in which we correlated each participant’s false alarm rate (percent false alarm) per condition (socially appetitive, socially aversive) onto the contrasts of interest. To calculate how differences in inhibitory performance were associated with neural processing, we calculated the behavioral difference score in false alarm rate (i.e. socially appetitive false alarms minus socially aversive false alarms) and regressed this index onto the contrast comparing the two social contexts (socially appetitive > socially aversive).
Finally, to examine functional connectivity, we conducted psychophysiologal interaction (PPI) analyses by extracting the functional time course within our a priori seed regions (amygdala, ventral striatum). We ran a generalized form of the context-dependent PPI (gPPI; McLaren et al., 2012), which extracts the deconvolved time series from each seed region for each participant (creating the physiological variable), then convolves each trial type with the canonical HRF (creating the psychological regressor) and finally multiplies the time series from each psychological regressor with the physiological variable to create the PPI interaction terms. These interactions identified regions that covaried in a task-dependent manner with both the striatum and the amygdala.
To correct for multiple comparisons, we conducted a Monte Carlo simulation, using 3dClustSim in the AFNI software package (Ward, 2000). We investigated activity within a mask of a priori brain regions, which included affective node regions (ventral striatum, amygdala), social-detection node regions (inferior occipital gyrus, fusiform gyrus, anterior temporal lobe, temporal pole, supramarginal gyrus, angular gyrus, inferior parietal lobule) and regulatory node regions [dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, anterior cingulate cortex)]. These regions of interest (ROIs) were created structurally based on prior studies using similar tasks and the same structural ROIs (see Telzer et al., 2015) or ROIs based on structures within the Automated Anatomical Labeling atlas (Tzourio-Mazoyer et al., 2002) using the WFUpickatlas (Maldjian et al., 2003). The mask included 10 503 voxels. Results of the 3dClustSim indicated a voxel-wise threshold of P < 0.005 combined with a minimum cluster size of 17 voxels, corresponding to P < 0.05, family wise error (FWE) corrected. Regions falling outside of this mask were set at a threshold of P < 0.005 combined with a minimum cluster size of 42 contiguous voxels, based on a 3dClustSim for the whole brain, corresponding to P < 0.05 FWE corrected.
Results
Study 1
Behavioral results
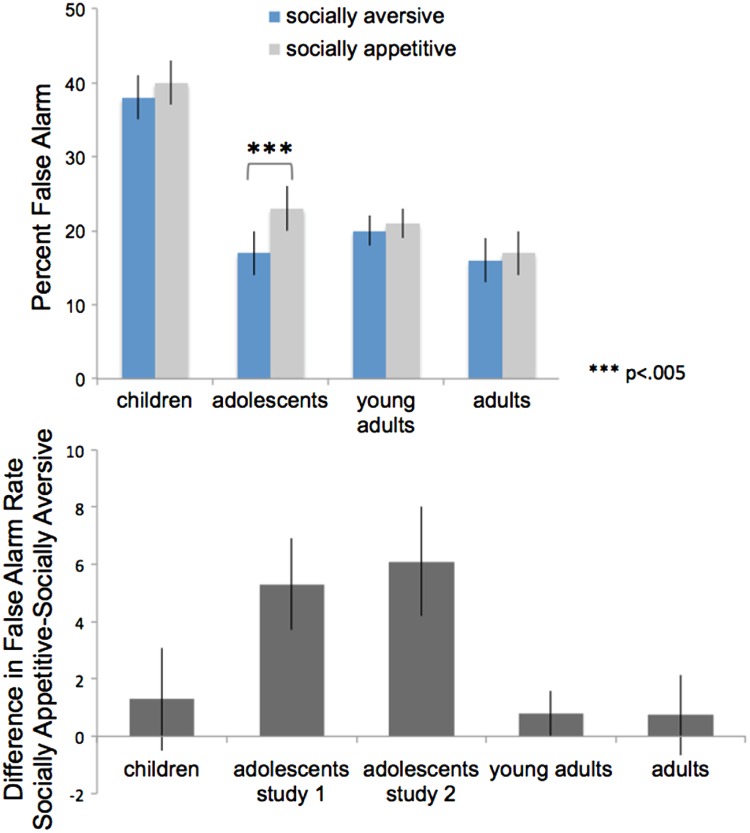
To test for age differences in false alarm rates to socially appetitive versus socially aversive blocks, we conducted a 2 × 4 repeated measures analysis of variance with one within subject variable (condition: appetitive, aversive) and one between subject variable (age group: children, adolescents, young adults, adults). Results revealed a significant main effect of condition [F(1,90) = 5.36, P = 0.023], which was qualified by an age × condition interaction [F(3,90) = 2.81, P = 0.044]. To probe this interaction, we conducted paired samples t-tests within each age group. As shown in the top panel of Figure 2, adolescents made significantly more false alarms during the socially appetitive condition compared with the socially aversive condition, t(26) = 3.3, P = 0.003, whereas no other age group showed a significant difference in false alarm rates between conditions [children: t(19) = 0.58, ns; young adults: t(30) = 0.06, ns, adults: t(16) = 0.50, ns]. For descriptive purposes, we calculated and plotted the difference in false alarm rates (socially appetitive-socially aversive). As shown in the bottom panel of Figure 2, appetitive social stimuli derail individuals’ performance more so than aversive social stimuli, an effect that is specific to adolescents, suggesting that adolescence is a sensitive period for the processing of socially positive stimuli.
Fig. 2.
Adolescents demonstrate significantly more false alarms in the presence of socially appetitive compared with socially aversive stimuli, whereas children, young adults and adults do not differentially perform under the two conditions.
Study 2
Given that adolescents specifically showed altered behavioral performance in the presence of socially appetitive cues, we wanted to examine how neural activation and connectivity patterns may prompt disinhibition in this age range. To address this question, we examined in Study 2 the neural correlates of this behavioral effect in an independent adolescent sample, where participants completed the identical social go-nogo task from Study 1 during an fMRI scan.
Behavioral results
We first conducted a paired samples t-test to compare the false alarm rates of adolescents during the socially appetitive and socially aversive blocks of the go-nogo task. Replicating the findings from Study 1 (Figure 2, bottom panel), we found that adolescents made significantly more false alarms during the socially appetitive condition (M = 32%, SE = 2.2%) compared with the socially aversive condition (M = 25%, SE = 2.1%), t(34) = 3.38, P = 0.002, suggesting that appetitive social stimuli derail adolescents’ performance more so than aversive social stimuli.
Neuroimaging results
Main effects
We first conducted whole-brain t-tests, comparing the contrast socially appetitive > socially aversive. We found greater activation in the inferior parietal lobule in the appetitive condition compared with the aversive condition (Table 2). No regions showed greater activation in the aversive condition relative to the appetitive condition.
Table 2.
Brain activation patterns for the socially appetitive > socially aversive blocksa
| Anatomical region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Main effects | ||||||
| L inferior parietal lobule | 40 | −36 | −46 | 55 | 3.72 | 54 |
| Correlated with false alarm rate | ||||||
| Bilateral ventral striatum | −6 | 2 | 7 | 3.41 | 32 | |
| L amygdala | −24 | 2 | −23 | 2.80 | 35 | |
| Midbrain | 3 | −22 | −17 | 4.00 | 177 | |
| L inferior parietal lobule | 40 | −33 | −28 | 43 | 4.31 | 209 |
| Medial prefrontal cortex | 8 | −3 | 26 | 49 | 4.01 | 84 |
Note. L and R refer to left and right hemispheres; BA refers to the Brodmann area; x, y and z refer to Talairach coordinates; t refers to the t score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster.
Neural activation correlated with reduced inhibition
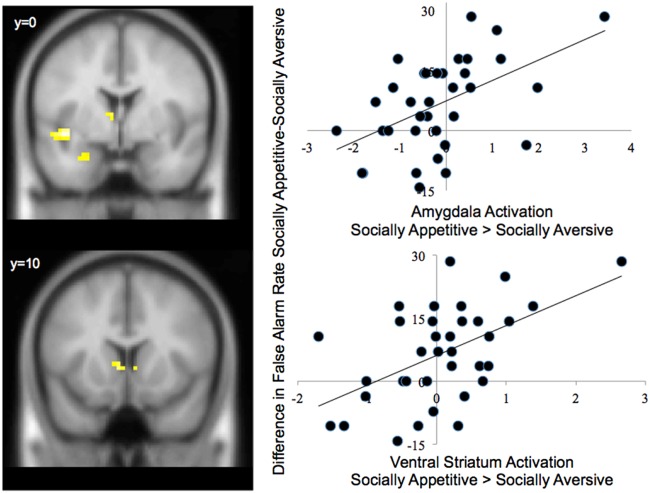
Next, we examined how adolescents’ false alarm rates correlated with neural activation during the socially appetitive and socially aversive conditions. To this end, we ran whole-brain regression analyses in which we regressed differences in false alarm rates (socially appetitive false alarms—socially aversive false alarms) onto neural activation during the socially appetitive > socially aversive conditions. We found significant clusters in the left amygdala and the ventral striatum. Specifically, increased false alarm rates in the socially appetitive condition compared with the socially aversive condition was associated with greater activation in the left amygdala and ventral striatum in the socially appetitive condition compared with the socially aversive condition (Figure 3 and Table 2).
Fig. 3.
Differences in false alarm rate to socially appetitive relative to socially aversive cues correlates with increased amygdala and ventral striatum activation in the socially appetitive > socially aversive blocks. The x-axis represents parameter estimates of signal intensity from each cluster of activation.
Functional connectivity main effects
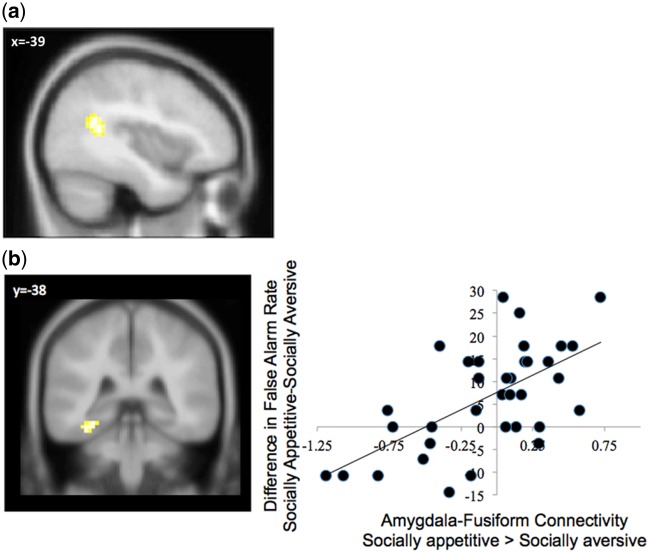
We examined functional coupling with our a priori seed regions in the affective node (amygdala, ventral striatum) to the socially appetitive > socially aversive condition, which allowed us to see which regions were acting in tandem with the affective node while viewing appetitive social stimuli relative to aversive social stimuli. The amygdala was significantly coupled with the bilateral TPJ for socially appetitive > socially aversive cues (Figure 4a and Table 3). No regions showed significant connectivity with the amygdala in the aversive condition relative to the appetitive condition. With the ventral striatum seed, PPI analyses did not reveal any significant coupling.
Fig. 4.
PPI analyses of the amygdala seed during the socially appetitive > socially aversive blocks: (a) main effects and (b) correlation of false alarm rate. The x-axis represents parameter estimates of signal intensity from the fusiform cluster, representing connectivity with the amygdala.
Table 3.
Psychophysiological interaction (PPI) analyses of the amygdala seed for the socially appetitive > socially aversive blocks
| Anatomical region | BA | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Main effects | ||||||
| L temporoparietal junction | 39 | −39 | −49 | 16 | 4.46 | 121 |
| R temporoparietal junction | 39 | 39 | −46 | 25 | 3.74 | 67 |
| Correlated with false alarm rate | ||||||
| L fusiform gyrus | 37 | −30 | −37 | −14 | 3.82 | 32 |
Note. L and R refer to left and right hemispheres; BA refers to the Brodmann area; x, y and z refer to Talairach coordinates; t refers to the t score at those coordinates (local maxima); k refers to the number of voxels in each significant cluster. Regions marked with the same superscript letter are part of the same cluster.
Functional connectivity correlated with reduced inhibition
Finally, we examined functional coupling with our a priori seed regions in the affective node (amygdala, ventral striatum) in relation to disinhibition. We regressed the differences in false alarm rate (socially appetitive false alarms—socially aversive false alarms) onto our PPI analysis comparing the socially appetitive > socially aversive conditions. With the amygdala as the seed region, PPI analyses yielded a single cluster in the left fusiform gyrus (Figure 4b and Table 3). These findings implicate an amygdala–fusiform circuit showing significantly greater coordinated activity to socially appetitive relative to socially aversive scenes as a function of failed inhibition to the socially appetitive relative to aversive scenes. No regions showed significant connectivity with the amygdala in the aversive condition relative to the appetitive condition. With the ventral striatum seed, PPI analyses did not reveal any significant coupling when correlated with false alarm rates in either direction.
Discussion
Adolescence is a period where suboptimal decision-making may be normative in certain circumstances, as reflected by increased rates of risky behaviors (Brener et al., 2013). One of the fundamental changes that may contribute to this suboptimal decision-making is the increased focus on social relationships (Brown et al., 1986). We examined how social cues differentially affect adolescents’ behavior relative to other age groups, and specifically how social cues may reduce inhibitory control in the adolescent brain. Although both positively and negatively valenced social cues likely impair behavioral inhibition, the greatest decrements were seen in the presence of socially appetitive cues specifically in adolescents, which were accompanied by increased activation in the amygdala and ventral striatum during socially appetitive contexts.
Behaviorally, adolescents showed compromised inhibition particularly in the context of appetitive social scenes, whereas children, young adults and adults did not differ in their disinhibition across appetitive and aversive contexts. In line with recent work (Somerville et al., 2011), the positive affect demonstrated in social scenes may steer adolescents to focus more on social information, rather than prepare them to inhibit a behavioral response. Contrary to traditional narratives about seeking peer acceptance, where considering how social contexts affect adolescent decision-making often focused on the role of antisocial peer influence swaying decision-making processes (Brown et al., 1986; Baumeister and Leary, 1995; Jenkins, 1995; La Greca et al., 2001), our results suggest an alternative pathway where adolescents may disinhibit because they are focused on positive social feedback rather than regulating their behaviors.
The social reorientation of the brain acts to refocus adolescents’ attention toward appetitive social features in their environment, especially in relation to peers and potential mates (Nelson et al., 2005, 2016). If successful in these endeavors, increased desire for social connection and the subsequent ability to engage others often leads to the establishment of meaningful social connections (Rubin et al., 2006). Paradoxically, the same disinhibition that allows adolescents to branch out and connect with their peers is potentially the same process leading them toward the propensity to engage in negative behaviors, such as risk taking. Adolescence is a period of heightened substance use experimentation, risky sexual practices and reckless behaviors (Brener et al., 2013). The types of settings that provide adolescents with access to these risks are likely to be social in nature and therefore involve cues from peers. As evidenced by our study, these cues result in disinhibition. Whether or not this disinhibition results in a negative or positive outcome will be determined by context.
At the neural level, we observed significant intraparietal lobule (IPL) activation during the socially appetitive > socially aversive block in our adolescent sample. This finding is interesting as it broadly fits into the social-detection network as defined by Nelson et al. (2005) and has also been implicated as a key region involved in salience monitoring (Goulden et al., 2014). Our PPI analysis also corroborated an increased social information processing account, as we observed significant coactivation between the amygdala and bilateral TPJ, a region implicated in social cognition and theorized to undergo significant change during adolescence (Mills et al., 2014). Specifically, prior research has shown that the TPJ is involved in attentional selection (Himmelbach et al., 2006) and ‘theory of mind’ processing (Saxe and Kanwisher, 2003), suggesting that adolescents may show relative decrements in the presence of socially appetitive cues because of excessive cognition surrounding these salient cues. Observing significant activation in the IPL and coactivation of the TPJ with the amygdala fits nicely with the social reorientation account of adolescence, where socially appetitive stimuli, relative to socially aversive stimuli, as a function of salience may recruit greater neural resources toward social processing regions and away from regulatory regions (Nelson et al., 2005).
We also examined how participants’ reduced inhibition related to neural activation during the socially appetitive contexts relative to socially aversive contexts. Adolescents who showed greater false alarms to the socially appetitive relative to aversive condition also showed greater ventral striatum and amygdala activation. The ventral striatum and amygdala play a key role in processing the affective social components of the environment and indicating salience (Zink et al., 2004; Cunningham and Brosch, 2012; Stillman et al., 2015). In the context of how hot cognitive processing is particularly adept at curbing inhibitory mechanisms, these affective regions are likely to be heavily involved in explaining poor behavioral control. We observed that increased activation in both the amygdala and ventral striatum when viewing appetitive relative to aversive social cues were associated with increased disinhibition to appetitive relative to aversive social cues. This suggests that while social information processing may generally impair adolescents’ decision-making processes, socially appetitive cues may provide greater relative impact than socially aversive cues. Adolescents who showed heightened activation patterns in the affective node toward appetitive social cues may be particularly at-risk for reduced behavioral inhibition.
In addition, we found that greater tendencies toward failed inhibition to appetitive relative to aversive social cues correlated with amygdala–fusiform connectivity during socially appetitive versus socially aversive contexts. The fusiform is considered to be part of the detection node, responsible for processing social information (Nelson et al., 2005). Recent work has suggested the fusiform is involved in emotion processing, highlighting its role in emotional reactivity (McRae et al., 2012). For example, emotional facial displays elicit heightened activation in the fusiform relative to neutral expressions (Monroe et al., 2013). Although an inability or difficulty in processing social information cues may be involved with suboptimal social functioning (Pierce et al., 2001; Schultz, 2005), our findings suggest processing social information too much (i.e. amygdala–fusiform connectivity) may also be deleterious. Further exploration should be directed at understanding whether amygdala activation is solely guiding attentional resources toward the processing of social cues and away from regulatory mechanisms or if hyperactive social processing is recruiting affective regions. Given the identification of the fusiform as part of the social-detection node (Nelson et al., 2005) and as a potential indicator of emotional reactivity (McRae et al., 2012), we find the amygdala–fusiform connection to be an interesting candidate for studying emotion dysregulation. Recent work has shown that amygdala–fusiform connectivity is implicated when presented with threat cues in a learning paradigm (Molapour et al., 2015). That our amygdala–fusiform connectivity was greater to the socially appetitive scenes relative to the aversive ones is of note, as it suggests that the amygdala–fusiform connection, rather than indicating the valence of the situation may be more indicative of the salience of the situation. When presented with threat, the amygdala–fusiform connection may serve to guide attention toward potential harm; meanwhile in a non-threatening environment, the amygdala–fusiform connectivity may naturally appear in the presence of appetitive social cues. Thus, the amygdala–fusiform connection appears to be a marker of increased salience and, based on our results, likely to result in compromised inhibition.
Of note, we did not find that the amygdala or ventral striatum were differentially connected to regions implicated in the regulatory node (i.e. prefrontal cortex, PFC), nor were frontal regions particularly associated with differences in behavioral performance. Prior work has suggested that immature connectivity between the frontal lobe with limbic regions is normative during adolescence (Somerville et al., 2011) and improves as adolescents age (Hare et al., 2008). Our lack of finding regulatory neural responses suggests that failed inhibition in socially appetitive contexts may occur via hyperactivation in affective regions and resources being directed toward social processing networks rather than dysregulated activation in prefrontal regions. These findings highlight the need to further explore the role of how both appetitive social rewards (e.g. acceptance) and aversive rewards (dangerous/risky situations) differentially affect cognitive processing. Recent work has started to examine how activation in affective processing regions relate to a number of peer-related adolescent phenomenon, such as responses to peer rejection (Sebastian et al., 2011), as well as both increased (Pfeifer et al., 2011) and decreased (Chein et al., 2011) resistance to peer influence. Our study builds on this research by looking at how the mere presence of social cues can impact disinhibition, a component of great import implicated in a large array of detrimental and advantageous behaviors. Our results, coupled with the aforementioned studies, suggest that affective regions may drive effects for a litany of (dys)functional processes, and by examining both activation and functional connectivity patterns, we can gain great insight into why adolescents act the way they do in a variety of situations.
There are several limitations in our study. First, it is hard to determine if our neural effects are specific to adolescents given that we do not have a child or adult comparison group in Study 2. Although the same behavioral pattern for adolescents was replicated in both Studies 1 and 2, it would be beneficial to include children and adults to determine whether the neural effects are adolescent specific. Thus, future research should continue to examine how socially appetitive and aversive cues impair neurocognitive processes across a wide age range. Second, the photos used were normed using a young-adult sample. Although past work has suggested that adolescents and adults may not read aversive and appetitive stimuli in a significantly differential manner (McManis et al., 2001), future work should continue examining physiological and subjective experiences of these age groups to social inclusion and social exclusion cues. Finally, our design of using a go-nogo task may have impacted our behavioral effects. For example, it is possible that part of the reason why appetitive stimuli may be particularly problematic for disinhibition is that they may be activating an approach orientation, while the aversive stimuli activate a withdrawal response. Past research examining behavioral responses to congruent and incongruent task demands have been shown to be different (Roelofs et al., 2005), so future examinations should utilize paradigms that can better account for the disruptive effects of social cues that are not confounded by task design.
Our results may provide insight into the mechanisms at play for understanding adolescent disinhibition; specifically, how neural resources may be disproportionately drawn to social-detection regions relative to control regions—an important consideration when discussing why adolescents may become disinhibited in the presence of peers. By manipulating social context cues, we examined how ‘hot’ cognition impacted adolescents’ ability to engage inhibitory mechanisms in both socially appetitive and socially aversive contexts. As many examinations into adolescent decision-making may not provide social cues, we contend the discrepancy between adolescent perceptions of risk—which is on par with adults—and adolescent decision-making ability—which is more likely to be suboptimal and often indicates reduced poor inhibitory control in risky situations—is partially driven by an increased focus on emotionally salient social cues. Emotion processing regions of the brain (the amygdala and ventral striatum) were correlated with this inhibitory breakdown. The greatest failure in behavioral inhibition was found when adolescents were presented with socially appetitive cues, suggesting increased influence of social information and reward processing during this developmental period. Our study represents one of the first forays directly comparing common yet salient social situations and how they may differentially influence activation and coupling patterns during hot cognitive processing.
Acknowledgements
The authors thank the members of the Developmental Social Neuroscience Lab at the University of Illinois. They also thank Nicholas Ichien and Inge Karosevica for collecting the data. They greatly appreciate the assistance of the Biomedical Imaging Center.
Funding
This paper was partially supported by grants from the National Institutes of Health (R01DA039923 to E.H.T.) and generous funds from the Department of Psychology at the University of Illinois.
Conflict of interest. None declared.
References
- Adolphs R. (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191(1), 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi A., Pearlson G.D., Meda S.A., et al. (2013). Influence of alcohol use on neural response to go/no-go task in college drinkers. Neuropsychopharmacology, 38(11), 2197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.K., Phelps E.A. (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411(6835), 305–9. [DOI] [PubMed] [Google Scholar]
- Baumeister R.F., Leary M.R. (1995). The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117(3), 497.. [PubMed] [Google Scholar]
- Beyth-Marom R., Austin L., Fischhoff B., Palmgren C., Jacobs-Quadrel M. (1993). Perceived consequences of risky behaviors: adults and adolescents. Developmental Psychology, 29(3), 549. [Google Scholar]
- Blakemore S.J., Choudhury S. (2006). Development of the adolescent brain: implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, 47(3–4), 296–312. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Robbins T.W. (2012). Decision-making in the adolescent brain. Nature Neuroscience, 15(9), 1184–91. [DOI] [PubMed] [Google Scholar]
- Brener N.D., Kann L., Shanklin S., et al. (2013). Methodology of the youth risk behavior surveillance system—2013. Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports, 62(1), 1–20. [PubMed] [Google Scholar]
- Brown B.B. (2004). Adolescents’ relationships with peers In Lerner R., Steinberg L., editors. Handbook of Adolescent Psychology, 2nd edn, pp. 363–94, New York: Wiley. [Google Scholar]
- Brown B.B., Eicher S.A., Petrie S. (1986). The importance of peer group (“crowd”) affiliation in adolescence. Journal of Adolescence, 9(1), 73–96. [DOI] [PubMed] [Google Scholar]
- Casey B.J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124(1), 111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert J.E., Thomas K.M. (2013). Inhibitory control during emotional distraction across adolescence and early adulthood. Child Development, 84(6), 1954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–50. [DOI] [PubMed] [Google Scholar]
- Cunningham W.A., Brosch T. (2012). Motivational salience amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science, 21(1), 54–9. [Google Scholar]
- Davis M. (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15(1), 353–75. [DOI] [PubMed] [Google Scholar]
- Dorn L.D. (2006). Measuring puberty. Journal of Adolescent Health, 39(5), 625–6. [DOI] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., et al. (2005). Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage, 25(4), 1279–91. [DOI] [PubMed] [Google Scholar]
- Gardner M., Steinberg L. (2005). Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Developmental Psychology, 41(4), 625–35. [DOI] [PubMed] [Google Scholar]
- Goulden N., Khusnulina A., Davis N.J., et al. (2014). The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage, 99, 180–90. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Choate V.R., Pine D.S., Nelson E.E. (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach M., Erb M., Karnath H.O. (2006). Exploring the visual world: the neural substrate of spatial orienting. Neuroimage, 32(4), 1747–59. [DOI] [PubMed] [Google Scholar]
- Jenkins J.E. (1995). The influence of peer affiliation and student activities on adolescent drug involvement. Adolescence, 31(122), 297–306. [PubMed] [Google Scholar]
- Knoll L.J. Magis-Weinberg L. Speekenbrink M. and Blakemore S.J. (2015). Social influence on risk perception. Psychological Science, 26(5), 583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusch F., Vilenne A., Quertemont E. (2013). Response inhibition toward alcohol-related cues using an alcohol go/no-go task in problem and non-problem drinkers. Addictive Behaviors, 38(10), 2520–8. [DOI] [PubMed] [Google Scholar]
- La Greca A.M., Prinstein M.J., Fetter M.D. (2001). Adolescent peer crowd affiliation: linkages with health-risk behaviors and close friendships. Journal of Pediatric Psychology, 26(3), 131–43. [DOI] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. (1999). International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida.
- Logue S., Chein J., Gould T., Holliday E., Steinberg L. (2014). Adolescent mice, unlike adults, consume more alcohol in the presence of peers than alone. Developmental Science, 17(1), 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S.V., Berridge K.C. (2009). Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. Journal of Neuroscience, 29(20), 6500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61(4), 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManis M.H., Bradley M.M., Berg W.K., Cuthbert B.N., Lang P.J. (2001). Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology, 38(2), 222–31. [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., et al. (2012). The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7(1), 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J., Mischel W. (1999). A hot/cool-system analysis of delay of gratification: dynamics of willpower. Psychological Review, 106(1), 3.. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.J. (2014). Developmental changes in the structure of the social brain in late childhood and adolescence. Social Cognitive and Affective Neuroscience, 9(1), 123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein S.G., Halpern-Felsher B.L. (2002). Perceptions of risk and vulnerability. Journal of Adolescent Health, 31(1), 10–27. [DOI] [PubMed] [Google Scholar]
- Molapour T., Golkar A., Navarrete C.D., Haaker J., Olsson A. (2015). Neural correlates of biased social fear learning and interaction in an intergroup context. NeuroImage, 121, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J.F., Griffin M., Pinkham A., et al. (2013). The fusiform response to faces: explicit versus implicit processing of emotion. Human Brain Mapping, 34(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Jarcho J.M., Guyer A.E. (2016). Social re-orientation and brain development: an expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E., Pine D.S. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(02), 163–74. [DOI] [PubMed] [Google Scholar]
- O’Doherty J., Dayan P., Schultz J., Deichmann R., Friston K., Dolan R.J. (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304(5669), 452–4. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E., et al. (2011). Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69(5), 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K., Müller R.A., Ambrose J., Allen G., Courchesne E. (2001). Face processing occurs outside the fusiform face area in autism: evidence from functional MRI. Brain, 124(10), 2059–73. [DOI] [PubMed] [Google Scholar]
- Reyna V.F., Farley F. (2006). Risk and rationality in adolescent decision making implications for theory, practice, and public policy. Psychological Science in the Public Interest, 7(1), 1–44. [DOI] [PubMed] [Google Scholar]
- Roelofs K., Elzinga B.M., Rotteveel M. (2005). The effects of stress-induced cortisol responses on approach–avoidance behavior. Psychoneuroendocrinology, 30(7), 665–77. [DOI] [PubMed] [Google Scholar]
- Rubin K.H., Bukowski W., Parker J.G. (2006). Peer interactions, relationships and groups In: Eisenberg N.,, Damon W., Lerner R.M., Hoboken N.J., editors. Handbook of Child Psychology. Social, Emotional and Personality Development, 6 edn, pp. 571–645, New York: Wiley. [Google Scholar]
- Rutter M., Rutter M. (1993). Developing Minds: Challenge and Continuity across the Lifespan. London, UK: Penguin Books. [Google Scholar]
- Sato S.M., Schulz K.M., Sisk C.L., Wood R.I. (2008). Adolescents and androgens, receptors and rewards. Hormones and Behavior, 53(5), 647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. (2003). People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. Neuroimage, 19(4), 1835–42. [DOI] [PubMed] [Google Scholar]
- Schultz R.T. (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23(2), 125–41. [DOI] [PubMed] [Google Scholar]
- Sebastian C.L., Tan G.C., Roiser J.P., Viding E., Dumontheil I., Blakemore S.J. (2011). Developmental influences on the neural bases of responses to social rejection: implications of social neuroscience for education. Neuroimage, 57(3), 686–94. [DOI] [PubMed] [Google Scholar]
- Sisk C.L., Foster D.L. (2004). The neural basis of puberty and adolescence. Nature Neuroscience, 7(10), 1040–7. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. (2011). Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience, 23(9), 2123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. (2009). The Behavioral Neuroscience of Adolescence. New York, NY: Norton. [Google Scholar]
- Steinberg L. (2007). Risk taking in adolescence new perspectives from brain and behavioral science. Current Directions in Psychological Science, 16(2), 55–9. [Google Scholar]
- Stillman P.E., van Bavel J.J., Cunningham W.A. (2015). Valence Asymmetries in the human amygdala: Task relevance modulates amygdala responses to positive more than negative affective cues. Journal of Cognitive Neuroscience, 27(4), 842–51. [DOI] [PubMed] [Google Scholar]
- Telzer E.H., Ichien N., Qu Y. (2015). The ties that bind: group membership shapes the neural correlates of in-group favoritism. NeuroImage, 115, 42–51. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–89. [DOI] [PubMed] [Google Scholar]
- Ward B. D. (2000). Simultaneous inference for fMRI data. Retrieved from http://afni-dev.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.ps. [Google Scholar]
- Yang T.T., Simmons A.N., Matthews S.C., et al. (2007). Increased amygdala activation is related to heart rate during emotion processing in adolescent subjects. Neuroscience Letters, 428(2), 109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H. (2003). The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews, 41(1), 88–123. [DOI] [PubMed] [Google Scholar]
- Zink C.F., Pagnoni G., Martin-Skurski M.E., Chappelow J.C., Berns G.S. (2004). Human striatal responses to monetary reward depend on saliency. Neuron, 42(3), 509–17. [DOI] [PubMed] [Google Scholar]