Abstract
Mind-wandering, an ubiquitous expression of humans’ mental life, reflects a drift of attention away from the current task towards self-generated thoughts, and has been associated with activity in the brain default network. To date, however, little is understood about the contribution of individual nodes of this network to mind-wandering. Here, we investigated whether the ventromedial prefrontal cortex (vmPFC) is critically involved in mind-wandering, by studying the propensity to mind-wander in patients with lesion to the vmPFC (vmPFC patients), control patients with lesions not involving the vmPFC, and healthy individuals. Participants performed three tasks varying in cognitive demands while their thoughts were periodically sampled, and a self-report scale of daydreaming in daily life. vmPFC patients exhibited reduced mind-wandering rates across tasks, and claimed less frequent daydreaming, than both healthy and brain-damaged controls. vmPFC damage reduced off-task thoughts related to the future, while it promoted those about the present. These results indicate that vmPFC critically supports mind-wandering, possibly by helping to construct future-related scenarios and thoughts that have the potential to draw attention inward, away from the ongoing tasks.
Keywords: ventromedial prefrontal cortex, mind-wandering, default mode network, mental time travel
Introduction
As the Italian novelist Tiziano Scarpa (2012) recently noted, ‘the present is made of one billion people who think of something quite different. The sum of their thoughts is the present’. This paradox captures a characteristic aspect of human cognition: it is often disengaged from processing of external events, yet constantly concerned with the shaping of reality. This is apparent during mind-wandering. Mind-wandering occurs when attention shifts away from an ongoing task or events in the external environment towards self-generated thoughts that are often completely unrelated to the current perceptual reality, and focused instead on current concerns, memories and anticipated future experiences (Antrobus et al., 1966; Smallwood and Schooler, 2015; Maillet and Schacter, 2016). Common examples are planning the next vacation in Greece while washing the dishes, or replaying mentally your last meeting with a friend while attending a class. Humans spend about 25–50% of their daily lives mind-wandering (Kane et al., 2007; Killingsworth and Gilbert, 2010). This activity is not costless: mind-wandering reduces processing of external events (Barron et al., 2011; Kam and Handy, 2014), disturbs performance in the task at hand (Smallwood et al., 2007; Mcvay and Kane, 2010; Franklin et al., 2011), and generally results in bad mood (Killingsworth and Gilbert, 2010). Yet, mind-wandering may be adaptive: it promotes self-reflection (Smallwood et al., 2011b), mood regulation (Ruby et al., 2013), and creativity (Baird et al., 2012, but see Smeekens and Kane, 2016). Moreover, given that self-generated thoughts during mind-wandering tend to be future-oriented (Smallwood et al., 2009; Baird et al., 2011), mind-wandering may contribute to instill prospection into daily life, acting as a device to escape the here and now. Indeed, temporal discounting, the tendency to devalue a reward as the delay until its delivery increases, often associated with impulsivity, is reduced in individuals prone to mind-wandering (Smallwood et al., 2013).
Given the pervasiveness of mind-wandering and its important psychological function, much research is being devoted to reveal its neural and cognitive bases. Functional neuroimaging (fMRI) evidence indicates that mind-wandering is associated with activity in the ‘default network’, a set of interconnected brain regions, including the dorsal and ventral medial prefrontal cortex, the posterior cingulate cortex, the medial and lateral temporal lobe and the angular gyrus bilaterally, whose activity is enhanced during relatively passive states (as compared with goal-directed tasks) and internally focused thought (Mason et al., 2007; Buckner et al., 2008; Christoff et al., 2009; Stawarczyk, et al., 2011; Mittner et al., 2014). Mind-wandering is also associated with activity in regions of the ‘executive network’, including the dorsolateral and ventrolateral prefrontal cortex and the anterior cingulate cortex (Christoff et al., 2009; see, for a meta-analysis, Fox et al., 2015). Activity in the default network and the executive network is positively correlated during mind-wandering, suggesting these networks govern different, complementary component processes of mind-wandering (Christoff, 2012; see also Smallwood et al., 2012). Activity in the default network has been linked to the production of the mental content that commonly populates mind-wandering episodes, which generally consists of remembered or simulated experiences involving the self and others (Smallwood and Schooler, 2015). Indeed, remembering the past, envisioning the future, and conceiving the thoughts of other people all activate multiple regions within the default network (Addis et al., 2007; Buckner and Carroll, 2007; Schacter et al., 2012). Moreover, reducing the effective connectivity between nodes of the default network (posterior cingulate cortex, parietal cortex and medial prefrontal cortex) with transcranial direct current stimulation inhibits mind-wandering (Kajimura et al., 2016), and deep transcranial magnetic stimulation over the medial prefrontal cortex before rest reduces self-related mental processing (Gruberger et al., 2015), which is typical of mind-wandering (Andrews-Hanna et al., 2010a).
Although fMRI studies have detected activity in the default network during mind-wandering, it is not clear whether activity in individual nodes of this network is crucial for the experience of mind-wandering. Here, we focus on the ventromedial prefrontal cortex (vmPFC). There are several reasons to suspect that vmPFC may be a crucial neural substrate of mind-wandering. vmPFC is a core component of the default network (Andrews-Hanna et al., 2010b; Spreng and Grady, 2010), and it is consistently engaged in association with mind-wandering (Mason et al., 2007; Christoff et al., 2009; Fox et al., 2015). The thickness of medial prefrontal cortex regions is positively related to individuals’ tendency to mind-wander under low-demanding conditions (Bernhardt et al., 2014). Moreover, patients with lesions to the prefrontal cortex are consistently reported as not interested in, or unable to, daydream and introspect (Ackerly and Benton, 1948; Wheeler et al., 1997), ‘stimulus bound’ (Knight et al., 1995), and ‘stuck in the present moment’ (Ingvar, 1985). More recent research has shown that patients with lesion to the vmPFC are impaired at remembering past events and constructing novel events (Bertossi et al., 2016a,b), make ‘shortsighted’ choices during decision-making (Bechara et al., 1994; Knight and Grabowecky, 1995), and exhibit steep temporal discounting of future rewards (Sellitto et al., 2010), further suggesting these patients may have difficulties at conceiving events alternative to the here and now. To test whether vmPFC is critically involved in mind-wandering, we investigated the frequency and phenomenological qualities of mind-wandering in patients with lesions to the vmPFC, control patients with lesions outside the vmPFC, and healthy controls. Based on fMRI evidence (Fox et al., 2015), we predicted that vmPFC patients would exhibit a reduction in the proclivity to engage in mind-wandering compared with healthy and brain-damaged controls.
Materials and methods
Participants
Participants included 18 patients with brain damage and 20 healthy individuals (see Table 1 for demographic information). Patients were recruited at the Centre for Studies and Research in Cognitive Neuroscience, Cesena, Italy. Patients were selected on the basis of the location of their lesion evident on MRI or CT scans. Seven patients had lesions involving the vmPFC (vmPFC patients). Lesions were the results of the rupture of an aneurysm of the anterior communicating artery in all cases. Lesions were bilateral in all cases, though often asymmetrically so. Lesion analysis, performed with MRIcro (Rorden and Brett, 2000), showed that the region of maximal lesion overlap in vmPFC patients involved BA 10, BA 11 and BA 32, though two patients also had minimal damage to BA 46 and BA 47 (see Figure 1). Eleven patients had lesions not involving vmPFC (control patients). In this group, lesions were unilateral in nine cases (five in the left hemisphere), and bilateral in two cases, and were all caused by stroke. Lesion sites mainly included the occipital cortex, extending into the paraventricular occipital area and the posterior forceps (BA 17, BA 18, BA 19; 10 cases). In 2 out of the 11 patients, the lesion included areas beyond the occipital cortex. These were the premotor cortex (BA 6, in both cases), the lateral temporal cortex (BAs 20–22, in both cases), the angular gyrus (BA 39, in one case) and the cerebellum (in both cases) (see Supplementary Materials for more detail on lesion analysis). Included patients were tested at least 3 months after the brain insult, were not receiving psychoactive drugs, and had no other diagnosis likely to affect cognition (e.g. significant psychiatric disease or alcohol abuse). There was no significant difference in lesion volume between vmPFC patients and control patients (58.79 vs 33.66 cc, t-test: t = 1.61; P = 0.13). The healthy control group included 20 individuals matched to patients on mean age and education. Control participants were not taking psychoactive drugs, and were free of current or past psychiatric or neurological illness as determined by history. Participants gave informed consent, according to the Declaration of Helsinki (International Committee of Medical Journal Editors, 1991) and the Ethical Committee of the Department of Psychology, University of Bologna.
Table 1.
Participants’ demographic and clinical information
| Group | n | Age (Years) | Education (Years) | Sex | 2-back task (accuracy) | Weigl (accuracy) | SRM | PF | DS |
|---|---|---|---|---|---|---|---|---|---|
| vmPFC patients | 7 | 56.00 (3.01) | 11.71 (1.21) | 6 M; 1 F | 0.52 (0.12) | 8.43 (1.49) | 34.29 (3.14) | 26.57 (2.29) | 5.57 (3.14) |
| CP | 11 | 55.36 (3.20) | 10.64 (1.30) | 10 M; 1 F | 0.66 (0.10) | 11.56 (1.18) | 33.27 (3.30) | 34.33 (2.60) | 5.70 (3.60) |
| HC | 20 | 52.85 (2.53) | 11.50 (0.69) | 20 M | 0.80 (0.05) | 11.50 (0.47) |
Note. vmPFC patients, patients with lesions to the ventromedial prefrontal cortex; CP, control patients; HC, healthy controls; M, male; F, female; SRM, Standard Raven Matrices; PF, phonemic fluency; DS, digit span. For SRM, PF and DS, we report the mean uncorrected scores and, in parentheses, the mean equivalent score (with 0 = pathological performance, 1 = borderline performance, 2–4 = normal performance). For age, education, two-back and Weigl’s accuracy, the values in parentheses are SEM.
Fig. 1.
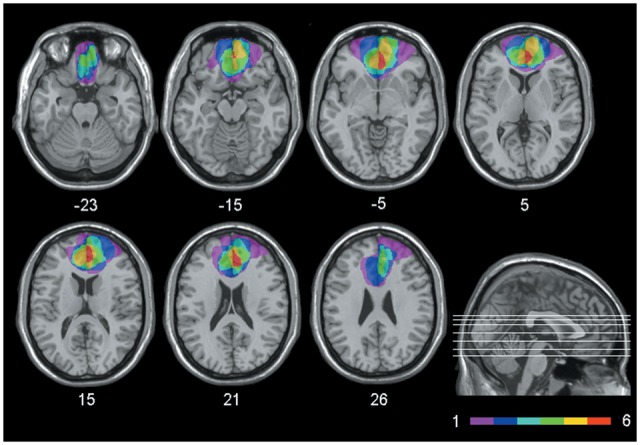
Extent and overlap of brain lesions. The figure represents vmPFC patients’ lesions projected on the same seven axial slices of the standard Montreal Neurological Institute brain. The white horizontal lines on the sagittal view are the positions of the axial slices, and the white numbers under the axial views are the z-coordinates of each slice. The color bar indicates the number of overlapping lesions. Maximal overlap occurs in BA 10, 11 and 32. The left hemisphere is on the left side.
Patients’ general cognitive functioning was generally preserved, as indicated by the scores they obtained in the Raven Standard Matrices, the phonemic fluency test, and the digit span test, which were within the normal range in all cases, and similar between patient groups (t-test: t < 0.83, P > 0.41 in all cases). vmPFC patients showed a marginally lower working memory (WM) and cognitive flexibility performance than the control groups as assessed with a two-back task (P = 0.08) and the Weigl Test (P = 0.06), respectively. Other aspects of executive functioning, as well as short- and long-term memory, were within the normal limits (see Supplementary Materials for more detail on patients' cognitive profile). In previous studies, however, we have shown that vmPFC patients (including a subset of patients described here) have impaired episodic (autobiographical) memory and future thinking (Bertossi et al., 2016a,b).
Mind-wandering assessment
Participants underwent three computerized tasks varying in cognitive demands and conduciveness to mind-wandering: a WM task, a choice reaction time task (CRT) and a Passive task modified from previous studies (Smallwood et al., 2009, 2011b). The WM task required monitoring a series of digits (1–8), presented in black ink and appearing in the center of the screen (non-target stimuli). Non-target stimuli presentation rate was 1 item every 1500 ms, followed by a 2000 ms fixation cross. Interspersed with the digit presentation were question marks presented in green ink (target stimuli), appearing for 2000 ms. The appearance of a green question mark cued participants to report whether the previous digit was even or odd using one of two keys. In the CRT task, individuals saw a similar stream of digits, but this time target stimuli were digits presented in green ink, which cued participants to report whether the current number was even or odd. In both tasks, 156 non-targets and 25 targets were presented (see also Smallwood et al., 2011b). In the Passive task, participants were told to merely observe a series of 181 digits appearing in the center of the screen in a black ink. Non-target and target stimuli were arranged in five blocks, containing ∼31 non-targets and 5 targets each (36 non-targets in the Passive tasks), whose order was randomized for each participant.
Across tasks, mind-wandering was assessed through the presentation of five thought probes, one for each block. Thought probes were presented visually, as a series of three screens. First, participants were required to rate, on a 7-point Likert scale, the degree to which, immediately before the probe, their attention was on-task, i.e. focused on the task being performed, vs off-task, i.e. focused on something unrelated to the task (1–completely on-task, 7–completely off-task). If participants provided an answer from 2 to 7, meaning they had, to some extent, experienced task-unrelated thoughts, they were required to classify their thoughts into one of five categories: (i) Past (i.e. the thought pertained to the past; e.g. ‘The week-end in Rome was the best’), (ii Present (i.e. the thought pertained to the present; e.g. ‘I wonder what my wife is doing now’), (iii) Future (i.e. the thought pertained to the future; e.g. ‘I need to see the dentist later’), (iv) Time not clear (i.e. the thought was not easily classified into time categories, e.g. ‘I’m lucky to have a friend like him’) or (v) Unaware (i.e. the participant is not aware of the content of her/his thought). In a following screen, participants additionally specified whether their thoughts were (i) Self-related (i.e. the thought mainly pertained to the self; e.g. ‘I am so going to bed after this’), (ii) Other-related (i.e. the thought mainly pertained to other people; e.g. ‘My son is growing up so fast’) or (iii) Unrelated to people (i.e. the thought did not involve people; e.g. ‘The new car was a good deal’). Several measures were adopted to make sure that participants understood the instructions. We explained the concept of mind-wandering, provided examples of on-task and off-task thoughts, and had participants classify other peoples’ thoughts as on-task vs off-task (see Supplementary Materials). The three tasks were administered in three different testing sessions, each lasting ∼12 min, in a counterbalanced order. The three testing sessions took place, in general, on different days. Five patients (four control patients and one vmPFC patient), who were unavailable to come to the lab three times, performed two of the three tasks on the same day, with a 30-min break in between.
Imaginal processes inventory
Participants completed the daydreaming and night dreaming frequency scales of the Imaginal Processes Inventory (IPI, Singer and Antrobus, 1963), a questionnaire designed to examine individual differences in inner mental life. In a series of 12 daydreaming and 12 night dreaming items, individuals rated the frequency with which they experienced daydreaming in their daily life (i.e. Daydreaming frequency scale: e.g. ‘Whenever I have time on my hand I daydream’, 1—Never, 5—Always), and night dreaming (i.e. Night dreaming frequency scale; e.g. ‘A night’s sleep for me contains a dream’, 1—Rarely, 5—Once a night). In each scale, the score ranges from 12 to 60, with higher scores indicating higher propensity toward daydreaming and night dreaming.
Results
WM and CRT tasks: accuracy and reaction times
Table 2 portrays accuracy (number of correct odd/even responses) and reaction times for correct responses (RTs) by Group (vmPFC patients, control patients, and healthy controls) and Task (CRT, WM). A Group × Task analysis of variance (ANOVA) on accuracy showed no significant main effect or interaction (p = 0.45, partial η2 = 0.03 in all cases). The same ANOVA on RTs for correct responses revealed a main effect of Group [F(2,35) = 5.04, P = 0.01, partial η2 = 0.22], which was qualified by a significant Group × Task interaction [F(2,35) = 3.38, P = 0.04, partial η2 = 0.16]. Newman-Keuls post-hoc tests showed that in the WM task vmPFC patients were slower than healthy controls (P = 0.005) and control patients (P = 0.04), whereas no difference emerged between control patients and healthy controls (P = 0.53). Group differences in RTs in the CRT task were not significant (P > 0.21 in all cases). Thus, vmPFC patients evinced a weaker working-memory performance than the control groups, in line with the results in the two-back task (Supplementary Materials).
Table 2.
Accuracy and reaction times in the CRT and WM tasks
| CRT | WM | |||
|---|---|---|---|---|
| Group | Number of correct responses | RTs | Number of correct responses | RTs |
| vmPFC patients | 23.29 (0.68) | 1147.44 (106.81) | 23.00 (0.53) | 1321.11 (106.59) |
| CP | 23.27 (0.88) | 1070.67 (78.55) | 22.27 (1.06) | 1006.64 (92.00) |
| HC | 23.55 (0.65) | 921.06 (47.58) | 23.60 (0.56) | 883.03(61.17) |
Note. vmPFC patients, patients with lesions to the ventromedial prefrontal cortex; CP, control patients; HC, healthy controls; RTs, Reaction times for correct responses. In parenthesis: the SEM.
Mind-wandering
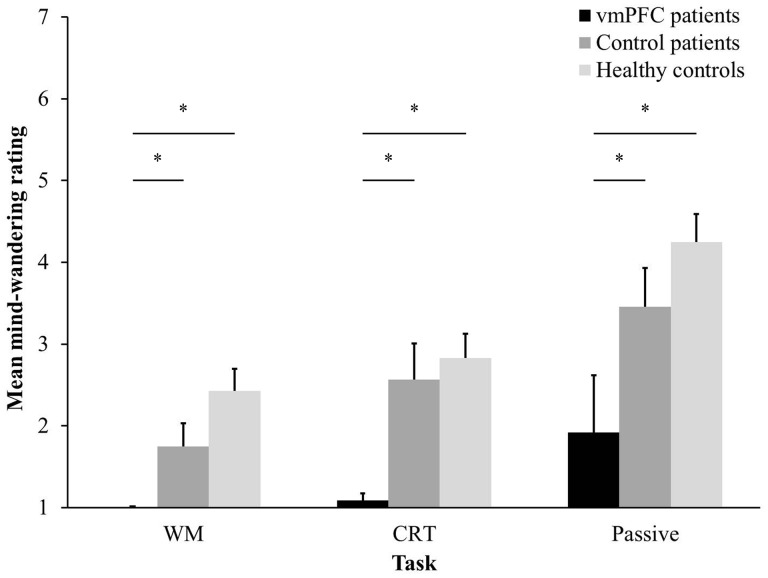
Mind-wandering ratings. As Figure 2 illustrates, mind-wandering ratings were lower in vmPFC patients than in the control groups across tasks. An ANOVA on mean mind-wandering ratings with Group as between-subject factor and Task (WM, CRT, Passive) as within-subject factor showed a main effect of Group [F(2,35) = 6.74, P = 0.003, partial η2 = 0.28]: vmPFC patients evinced lower mind-wandering ratings than healthy controls (P = 0.002) and control patients (P = 0.02), whereas no significant difference emerged between the control groups (P = 0.25). Group differences in mind-wandering were confirmed using more robust, non-parametric Mann-Whitney z-tests: vmPFC patients mind-wandered less than healthy controls (CRT task: z = 3.50, P = 0.0001; WM task: z = 3.55, P = 0.0001; Passive task: z = 2.86, P = 0.003) and control patients (CRT task: z = 2.99, P = 0.002; WM task: z = 2.53, P = 0.03; Passive task: z = 2.42, P = 0.02), with no significant difference between the control groups (P > 0.09 in all cases). The ANOVA also revealed a significant effect of Task [F(2, 70) = 28.74, P < 0.000001, partial η2 = 0.45), such that all participants mind-wandered more in the Passive task than in the CRT task (P = 0.0001), and in the CRT task than in the WM task (P = 0.01) (see Figure 2). No significant interaction emerged (P = 0.29; partial η2 = 0.07). Several studies have shown that mind-wandering is modulated by the complexity of the ongoing task, being less frequent when the external task is more difficult (Teasdale et al., 1993; Kane et al., 2007; Smallwood et al., 2009, 2011b; Levinson et al., 2012). That we observed this normal modulation of mind-wandering in all groups, including vmPFC patients, further suggests that patients complied with task instructions.
Fig. 2.
Mean mind-wandering ratings by participant group and ongoing task. Bars represent SEM. * P < 0.05.
This first set of analyses shows that vmPFC patients mind-wander less than healthy and brain-damaged controls. Four vmPFC patients did not mind-wander at all. We asked these patients whether mind-wandering had occurred far from the thought probes, and therefore had not been recorded. They stated confidently that mind-wandering had never occurred. We also asked a vmPFC patient, G.V., who consistently reported being on-task, to report some of his (on-task) thoughts during the Passive task. He said: ‘No thoughts. I just look at the numbers, I inspect them carefully’, confirming a tendency to stick to external stimuli, as opposed to mind-wander towards internal information.
Because there was a significant difference in RTs in the WM task between vmPFC patients and controls, we ran again the ANOVA on mind-wandering ratings adding RTs in the WM task as a covariate. The effect of the covariate was significant [F(1,34) = 5.63, β = 0.34, P = 0.02, partial η2 = 0.14], such that, in general, a strong tendency to mind-wander was associated with long RTs in the WM task. The effect of Group remained highly significant [F(2,34) = 10.45; P = 0.0003, partial η2 = 0.38], with vmPFC patients showing less mind-wandering than both healthy (p = 0.001) and control patients (P = 0.01), and no difference between the control groups (P = 0.22). No other significant main effect or interaction emerged (P ≥ 0.15, partial η2 < 0.09 in all cases). The effect of Group in the ANCOVA remained significant also if we added accuracy in the 2-back task and in the Weigl Test as two additional covariates [F(2,28) = 7.42; P = 0.003, partial η2 = 0.35], with vmPFC patients exhibiting less mind-wandering than healthy (P = 0.002) and brain-damaged controls (P = 0.04), and no difference between the control groups (P = 0.12). No other significant main effect or interaction emerged (P > 0.12, partial η2 ≤ 0.12 in all cases). These findings suggest that vmPFC patients' abnormally low mind-wander rates were not explained by problems in WM and cognitive flexibility.
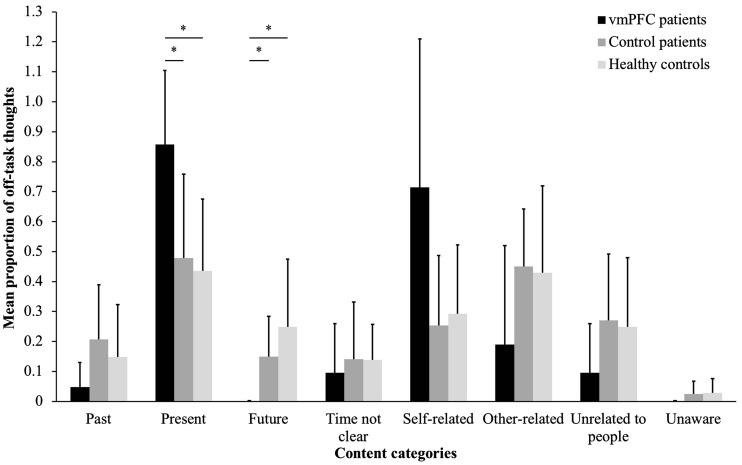
Content of mind-wandering.We investigated whether, beyond reducing the tendency to mind-wander, vmPFC damage altered the content of mind-wandering episodes. We compared across groups the number of off-task thoughts (collapsing across the WM, CRT and Passive tasks, but similar results were obtained considering the tasks separately) belonging to each content category separately (i.e. Past, Present, Future, Time not clear, Self-related, Other-related, Unrelated to people, Unaware), using non-parametric tests (as the data were non-normally distributed). We used Kruskal–Wallis one-way ANOVAs to assess group differences. Subsequently, to determine which group difference drove the main effect, we used Mann-Whitney’s z-tests as post-hoc tests. As Table 3 shows, vmPFC patients tended to show fewer mind-wandering episodes across content categories. Strikingly, they never reported future-related thoughts. Kruskal-Wallis ANOVAs run on each content category yielded statistically significant group differences only for the Future category (χ2 = 8.81, P = 0.01) and the Past category (χ2 = 7.48; P = 0.02), highlighting a more drastic reduction in mind-wandering towards the past and the future following vmPFC damage. Post-hoc Mann-Whitney’s tests confirmed that vmPFC patients experienced fewer future-related and past-related thoughts than healthy controls (future: z = 3.22, P = 0.001; past: z = 2.57, P = 0.01) and control patients (future: z = 2.80, P = 0.008; past: z = 2.53, P = 0.01), with no difference between the control groups (P > 0.14 in both cases). All other Kruskal-Wallis ANOVAs yielded non-significant results (P > 0.065 in all cases). In patients who did report mind-wandering (n = 3), we computed the ratio between the number of thoughts for each content category and the total number of mind-wandering episodes claimed, thus obtaining a clearer index of the ‘quality’ of mind-wandering independent of quantity. This analysis confirmed that, during mind-wandering, vmPFC patients experienced a lower proportion of future-related thoughts than both control groups (vmPFC patients vs healthy controls: z = 2.22, P = 0.03; vmPFC patients vs control patients: z = 1.94, P = 0.05), accompanied by a higher proportion of thoughts focused on the present (vmPFC patients vs healthy controls: z = −2.06, P = 0.04; vmPFC patients vs. control patients: z = −1.97, P = 0.04) (see Figure 3). Differences between the control groups were, in both cases, non-significant, as were group differences in the proportion of thoughts from the remaining content categories (P > 0.09 in all cases). Thus, not only did vmPFC damage reduce the tendency to mind-wander; it also altered its temporal focus. Off-task thoughts towards the past and the future were the most impaired. Even when vmPFC patients engaged in mind-wandering, their thoughts were confined to the present.
Table 3.
Mean number of off-task thoughts by participant group and content category (collapsed across WM, CRT and Passive tasks)
| Group | Past | Present | Future | Time not clear | Self-related | Other-related | Unrelated to people | Unaware |
|---|---|---|---|---|---|---|---|---|
| vmPFC patients | 0.14 (0.14) | 1.00 (0.58) | 0 | 0.29 (0.29) | 0.57 (0.30) | 0.57 (0.57) | 0.29 (0.29) | 0 |
| CP | 1.91 (0.49) | 4.00 (0.71) | 1.45 (0.39) | 1.45 (0.54) | 2.36 (0.70) | 3.91 (0.62) | 2.54 (0.65) | 0.27 (0.14) |
| HC | 1.65 (0.36) | 4.70 (0.66) | 2.85 (0.57) | 1.50 (0.33) | 3.25 (0.68) | 4.75 (0.67) | 2.70 (0.61) | 0.35 (0.13) |
Note. vmPFC patients, patients with lesions to the ventromedial prefrontal cortex; CP, control patients; HC, healthy controls. In parenthesis: the SEM.
Fig. 3.
Mean proportion of thoughts for each content category out of the total number of off-task thoughts claimed (collapsed across tasks) by participant group. Bars represent SEM. * P ≤ 0.05.
Night dreaming and daydreaming frequency subscales
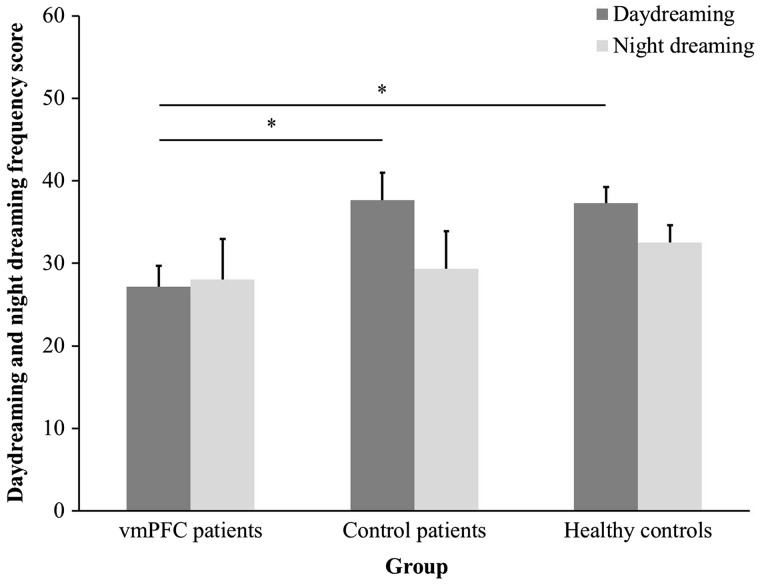
Figure 4 shows daydreaming and night dreaming scores by participant group. An ANOVA on daydreaming frequency scores with Group as a between-subject factor yielded a significant effect of Group [F(2,35) = 3.53; P = 0.04, partial η2 = 0.17]. Newman-Keuls post-hoc tests revealed that vmPFC patients reported less frequent daydream than healthy controls (P = 0.02) and control patients (P = 0.03), with no difference between the control groups (P = 0.93). The same ANOVA on night dreaming frequency scores revealed no significant effect of Group (P = 0.62, partial η2 = 0.03). These results indicate reduced self-reported daydreaming, but not night dreaming, in vmPFC patients.
Fig. 4.
Daydreaming and night dreaming frequency scores from the IPI by participant group. Bars represent SEM.* P < 0.05.
Discussion
This study investigated the neural correlates of mind-wandering, an extremely frequent (and likely necessary) expression of humans’ mental life that complements externally-driven forms of cognition. Previous fMRI studies have pointed to the default network as an important neural substrate of mind-wandering, with prominent loci of activations in the vmPFC (Fox et al., 2015). Drawing brain-behavior inferences in cognitive neuroscience, however, requires converging methods. Lesion studies, in particular, have the potential to causally relate brain activity with behavior and function, and constrain the interpretation of its functional meaning in a way that is not possible with neuroimaging data alone. Here, patients with lesions to the vmPFC exhibited a reduced propensity to mind-wander during three experimental tasks, and claimed less frequent daydreaming in daily life than healthy individuals. Notably, a reduction in spontaneous forms of cognition is not a general consequence of brain damage. Indeed, control patients with lesions mainly affecting the occipital cortex, which is not part of the default network, showed the same tendency to mind-wander and daydream than healthy controls. The results are also unlikely to depend on problems understanding concepts like mind-wandering, on-task thought, and off-task thought on the vmPFC patients’ part. vmPFC patients, indeed, correctly spotted instances of mind-wandering produced by others (see Supplementary Materials), and their tendency to mind-wander, though scant, was modulated by the demands of the ongoing task, as it was in the control groups. Moreover, vmPFC patients reported similar night dreaming rates than the control groups. This finding suggests that vmPFC patients can, to some extent, reflect on inner experiences, and do not generally underreport any type of inner experience. We prefer to not over-interpret the asymmetry in daydreaming vs. night dreaming in vmPFC patients based on a single measure. There is initial evidence, however, that despite several commonalities, night dreams and daydreams differ in both content and neural correlates (see Fox et al., 2013), which may relate to this finding.
Thus, our results point to a significant reduction of mind-wandering following vmPFC damage, indicating that the vmPFC is critically involved in mind-wandering, consistent with fMRI evidence (Mason et al., 2007; Christoff et al., 2009; Andrews-Hanna et al., 2010a; Fox et al., 2015). It is important to bear in mind that even the lesion method has limitations that reduce the certainty of brain-behavior causality inferences, such as the phenomenon of diaschisis, the fact that we typically do not have a measure of patients' behavior before the brain insult, and the fact that natural lesions may extend beyond the area of interest, as was the case in two of our vmPFC patients (but removing these patients from the analyses did not change the results) (see Rosenbaum et al., 2014 for a discussion). That said, a parsimonious account of the fact that vmPFC is strongly activated during mind-wandering in fMRI studies and its damage is associated with a reduction of mind-wandering is that vmPFC is actively involved in the production of mind-wandering.
We propose two mechanisms by which vmPFC may contribute to mind-wandering. vmPFC is part of the ‘medial temporal lobe (MTL)-subsystem’ of the default network, and is functionally connected with the MTL during construction of mental simulations based on memory (Andrews-Hanna et al., 2010b; see also Benoit and Schacter, 2015). Consistently, vmPFC patients are impaired at remembering past events and imagining future events (Bertossi et al., 2016a,b). As anticipated, mind-wandering occurs when attention shifts from the external ongoing task towards inner contents. If, however, vmPFC patients have lost the neural machinery needed to construct vivid scenarios and events alternative to perceptual reality, there would be no internal event capable to surpass the saliency of ongoing external events, resulting in low mind-wandering rates. On this view, vmPFC damage would downregulate mind-wandering directly, by reducing the ‘material’ that mind-wandering episodes are made of. An alternative possibility is that vmPFC damage reduced meta-awareness, i.e. one’s explicit knowledge of the current contents of thought (Schooler et al., 2011), and, in turn, the frequency with which patients became aware of (and reported) mind-wandering (Smallwood and Schooler, 2015). Indeed, anterior and medial regions of prefrontal cortex, damaged in our vmPFC patients, have previously been implicated in meta-awareness of one’s own mental contents (Gilbert et al., 2006; Fleming and Dolan, 2012). Moreover, regions implicated in mind-wandering, including vmPFC, are differentially engaged by unaware vs. aware mind-wandering (Christoff et al., 2009). We cannot determine to what extent vmPFC patients’ reduced self-report of mind-wandering depended on impaired construction or conscious experience of mind-wandering episodes, but we favor the first interpretation for the following reasons. First, vmPFC patients were externally probed to report mind-wandering, which minimizes demands on meta-awareness. Moreover, vmPFC damage reduced most dramatically mind-wandering episodes focusing on future and past events, consistent with vmPFC’s involvement in event (re-)construction (Bertossi et al., 2016b; see also Stawarczyk and D’Argembeau, 2015), and not those in which participants claimed unawareness of the contents of mind-wandering, as one would expect in case of a prominent impairment of meta-awareness. Future studies using indirect (e.g. physiological) indices of mind-wandering will clarify whether lack of meta-awareness contributed to reduced mind-wandering in vmPFC patients (Franklin et al., 2011; Smallwood et al., 2011a).
Recently, Axelrod et al. (2015) have shown increased propensity to mind-wander following anodal (excitatory) tDCS over the dorsolateral prefrontal cortex (dlPFC). Despite using different methodologies, Axelrod et al.’s (2015) study and ours converge in showing that prefrontal cortex regions are crucial for mind-wandering. One important question is whether the contributions of vmPFC and dlPFC during mind-wandering can be differentiated. In Axelrod et al.’s study, in addition to mind-wandering, tDCS over dlPFC enhanced performance in the ongoing external task. The authors, therefore, reasoned that dlPFC stimulation had increased the capacity of the executive system, resulting in improved performance along with enhanced mind-wandering. There is evidence, indeed, that the executive system supports some components of mind-wandering (Smallwood and Schooler, 2006; Levinson et al., 2012; see also Kane et al., 2007). In contrast, even though our patients were mildly impaired in tests of WM and cognitive flexibility, we have no evidence that vmPFC damage reduced mind-wandering by hampering executive functioning. First, vmPFC patients evinced extremely low mind-wandering ratings even in the Passive task, which had minimal cognitive demands, if not at all. Moreover, group differences in mind-wandering held after controlling for WM and cognitive flexibility performance. Interestingly, tDCS over a control site in the occipital cortex had no effect on mind-wandering (Axelrod et al., 2015), in line with our result of normal mind-wandering in control patients with lesions mainly affecting the occipital cortex. These findings reinforce the idea that a reduction of mind-wandering is not merely the effect of damage to (or interference on) any brain region but, more likely, of damage inflicted on large-scale networks governing the emergence of mind-wandering, including the default network and the executive network (Fox et al., 2015). The vmPFC and dlPFC are key nodes of these networks, respectively.
The default network has been thought of as a neural device to escape the here and now (Buckner and Carroll, 2007). This study provides support to this conceptualization, showing that, even while engaged in a tedious task with minimal demands, vmPFC patients’ mind tends to stick to the task, rather than take the chance to wander towards alternative scenarios. This finding aligns to previous evidence that vmPFC patients are impaired at constructing past and future events voluntarily (Moscovitch and Melo, 1997; Levine, 2004; Bertossi et al., 2016a,b), and discount future rewards steeply, indicating preference for immediacy (Sellitto et al., 2010). A recent study has demonstrated that cortical thickness in medial prefrontal regions predicts both the proclivity to mind-wander and the ability to resist immediate temptation in a temporal discounting task (Bernhardt et al., 2014). Reduced mind-wandering, impaired event construction, and steep temporal discounting following vmPFC damage are likely to be different expressions of the same underlying deficit in transcending the present to re-/pre-experience alternative events (Buckner and Carroll, 2007; Hassabis and Maguire, 2009; Schacter et al., 2012). Indeed, Stawarczyk and D’Argembeau (2015) found that the medial prefrontal cortex is engaged during mind-wandering as well as episodic future thinking and personal goal processing.
Before concluding, we wish to emphasize that evidence of reduced mind-wandering in vmPFC patients by no means indicates that these patients are less easily distracted than healthy controls. They are less easily distracted by ‘internal’ information, while they may retain a normal (or even increased) tendency to direct attention to task-irrelevant ‘external’ information. In fact, external distractions may be one source of present-related off-task thoughts in our task, which were inflated in vmPFC patients. External and internal distraction can be differentiated at the behavioral and physiological level: they have overlapping but not coincident component processes (Stawarczyk et al., 2014; Unsworth and McMilan, 2014), and also differ in terms of pupillary correlates (Unsworth and Robison, 2016). Stawarczyk et al. (2011) found that the medial prefrontal cortex was more engaged during stimulus-independent (internal) off-task thoughts compared with both task-related (external) interferences and external distractions, in line with our hypothesis. Telling in this respect is also an anecdote: the wife of a vmPFC patient, L.G., reported that he would interrupt her frequently to comment on the appearance, behavior, or clothes of other people during their daily stroll, but rarely to report some thoughts he had, or some ideas or memories that had popped into his mind, further suggesting that vmPFC patients are not easily distracted by mental contents, but can still be distracted by external information.
Future studies will be needed to uncover the interplay between vmPFC and other regions of the default and executive network that enables the mind to wander away from perceptual reality. For the time being, we have shown that vmPFC is necessary to mind-wander, possibly by helping construe events and scenarios alternative to the here and now that we typically ‘visit’ while off-task. This renders vmPFC patients abnormally tied to the external perceptual reality, with a reduced ability to conceive and re/pre-experience what is not present, and to create that unique blend of external and internal information that ultimately shapes our understanding of reality.
Supplementary data
Supplementary data are available at SCAN online.
Acknowledgements
We thank Davide Braghittoni for helping with patients’ recruitment, and Stefania Prostrati and Francesca Petrini for assistance with data collection.
Conflict of interest. None declared.
References
- Ackerly S.S., Benton A.L. (1948). Report of case of bilateral frontal lobe defect. Research Publications - Association for Research in Nervous and Mental Disease 27, 479–504. [PubMed] [Google Scholar]
- Addis D.R., Wong A.T., Schacter D.L. (2007). Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45, 1363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Huang C., Buckner R.L. (2010a). Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology, 104, 322–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010b). Functional-anatomic fractionation of the brain’s default network. Neuron 65, 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus J.S., Singer J.L., Greenberg S. (1966). Studies in the stream of consciousness: experimental enhancement and suppression of spontaneous cognitive processes. Perceptual and Motor Skills 23, 399–417. [Google Scholar]
- Axelrod V., Rees G., Lavidor M., Bar M. (2015). Increasing propensity to mind-wander with transcranial direct current stimulation. Proceedings of the National Academy of Sciences of the United States of America, 112, 3314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Mrazek M.D., Kam J.W., Franklin M.S., Schooler J.W. (2012). Inspired by distraction: mind wandering facilitates creative incubation. Psychological Science, 23, 1117–22. [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Schooler J.W. (2011). Back to the future: autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition, 20, 1604–11. [DOI] [PubMed] [Google Scholar]
- Barron E., Riby L.M., Greer J., Smallwood J. (2011). Absorbed in thought: the effect of mind wandering on the processing of relevant and irrelevant events. Psychological Science, 22, 596–601. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio A.R., Damasio H., Anderson S.W. (1994). Insensitivity to future consequences following damage to human prefrontal cortex. Cognition, 50, 7–15. [DOI] [PubMed] [Google Scholar]
- Benoit R.G., Schacter D.L. (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt B.C., Smallwood J., Tusche A., et al. (2014). Medial prefrontal and anterior cingulate cortical thickness predicts shared individual differences in self-generated thought and temporal discounting. Neuroimage, 90, 290–7. [DOI] [PubMed] [Google Scholar]
- Bertossi E., Aleo F., Braghittoni D., Ciaramelli E. (2016a). Stuck in the here and now: Construction of fictitious and future experiences following ventromedial prefrontal damage. Neuropsychologia, 81, 107–16. [DOI] [PubMed] [Google Scholar]
- Bertossi E., Tesini C., Cappelli A., Ciaramelli E. (2016b). Ventromedial prefrontal cortex damage causes a pervasive impairment in episodic remembering and future thinking. Neuropsychologia. In press. doi: 10.1016/j.neuropsychologia.2016.01.034. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Carroll D.C. (2007). Self-projection and the brain. Trends in Cognitive Sciences, 11, 49–57. [DOI] [PubMed] [Google Scholar]
- Christoff K. (2012). Undirected thought: neural determinants and correlates. Brain Research, 1428, 51–9. [DOI] [PubMed] [Google Scholar]
- Christoff K., Gordon A.M., Smallwood J., Smith R., Schooler J.W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106, 8719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S.M., Dolan R.J. (2012). The neural basis of metacognitive ability. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 1338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K.C., Nijeboer S., Solomonova E., Domhoff G.W., Christoff K. (2013). Dreaming as mind wandering: evidence from functional neuroimaging and first-person content reports. Frontiers in Human Neuroscience, 7, 412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K.C., Spreng R.N., Ellamil M., Andrews-Hanna J.R., Christoff K. (2015). The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage, 111, 611–21. [DOI] [PubMed] [Google Scholar]
- Franklin M.S., Smallwood J., Schooler J.W. (2011). Catching the mind in flight: using behavioral indices to detect mindless reading in real time. Psychonomic Bulletin and Review, 18, 992–7. [DOI] [PubMed] [Google Scholar]
- Gilbert S.J., Spengler S., Simons J.S., et al. (2006). Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. Journal of Cognitive Neuroscience, 18, 932–48. [DOI] [PubMed] [Google Scholar]
- Gruberger M., Levkovitz Y., Hendler T., et al. (2015). I think therefore I am: rest-related prefrontal cortex neural activity is involved in generating the sense of self. Consciousness and Cognition, 33, 414–21. [DOI] [PubMed] [Google Scholar]
- Hassabis D., Maguire E.A. (2009). The construction system of the brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvar D.H. (1985). “Memory of the future”: an essay on the temporal organization of conscious awareness. Human Neurobiology, 4, 127–36. [PubMed] [Google Scholar]
- Kam J.W., Handy T.C. (2014). Differential recruitment of executive resources during mind wandering. Consciousness and Cognition, 26, 51–63. [DOI] [PubMed] [Google Scholar]
- Kane M.J., Brown L.H., McVay J.C., Silvia P.J., Myin-Germeys I., Kwapil T.R. (2007). For whom the mind wanders, and when: an experience-sampling study of working memory and executive control in daily life. Psychological Science, 18, 614–21. [DOI] [PubMed] [Google Scholar]
- Kajimura S., Kochiyama T., Nakai R., Abe N., Nomura M. (2016). Causal relationship between effective connectivity within the default mode network and mind-wandering regulation and facilitation. Neuroimage, 133, 21–30. [DOI] [PubMed] [Google Scholar]
- Killingsworth M.A., Gilbert D.T. (2010). A wandering mind is an unhappy mind. Science, 330, 932.. [DOI] [PubMed] [Google Scholar]
- Knight R.T., Grabowecky M. (1995) Escape from linear time: prefrontal cortex in conscious experience In Gazzaniga M. S., editor. The Cognitive Neurosciences. Cambridge, MA, US: The MIT Press, 1357–71. [Google Scholar]
- Knight R.T., Grabowecky M.F., Scabini D. (1995). Role of human prefrontal cortex in attention control. Advances in Neurology, 66, 21–36. [PubMed] [Google Scholar]
- Levine B. (2004). Autobiographical memory and the self in time: brain lesion effects, functional neuroanatomy, and lifespan development. Brain and Cognition, 55, 54–68. [DOI] [PubMed] [Google Scholar]
- Levinson D.B., Smallwood J., Davidson R.J. (2012). The persistence of thought: evidence for a role of working memory in the maintenance of task-unrelated thinking. Psychological Science, 23, 375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D., Schacter D.L. (2016). From mind wandering to involuntary retrieval: age-related differences in spontaneous cognitive processes. Neuropsychologia, 80, 142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315, 393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcvay J.C., Kane M.J. (2010). Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and Watkins (2008). Psychological Bulletin, 136, 188–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittner M., Boekel W., Tucker A.M., Turner B.M., Heathcote A., Forstmann B.U. (2014). When the brain takes a break: a model-based analysis of mind wandering. Journal of Neuroscience, 34, 16286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M., Melo B. (1997). Strategic retrieval and the frontal lobes: evidence from confabulation and amnesia. Neuropsychologia, 35, 1017–34. [DOI] [PubMed] [Google Scholar]
- Rorden C., Brett M. (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12, 191–200. [DOI] [PubMed] [Google Scholar]
- Rosenbaum R.S., Gilboa A., Moscovitch M. (2014). Case studies continue to illuminate the cognitive neuroscience of memory. Annals of the New York Academy of Sciences, 1316, 105–33. [DOI] [PubMed] [Google Scholar]
- Ruby F.J., Smallwood J., Engen H., Singer T. (2013). How self-generated thought shapes mood – the Relation between mind-wandering and mood depends on the socio-temporal content of thoughts. PLoS One, 8, e77554.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa T. (2012) Le Cose Fondamentali. Milano: Einaudi editore. [Google Scholar]
- Schacter D.L., Addis D.R., Hassabis D., Martin V.C., Spreng R.N., Szpunar K.K. (2012). The future of memory: remembering, imagining, and the brain. Neuron, 76, 677–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler J.W., Smallwood J., Christoff K., Handy T.C., Reichle E.D., Sayette M.A. (2011). Meta-awareness, perceptual decoupling and the wandering mind. Trends in Cognitive Sciences, 15, 319–26. [DOI] [PubMed] [Google Scholar]
- Sellitto M., Ciaramelli E., di Pellegrino G. (2010). Myopic discounting of future rewards after medial orbitofrontal damage in humans. The Journal of Neuroscience, 30, 16429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J.L., Antrobus J.S. (1963). A factor analytic study of daydreaming and conceptually-related cognitive and personality variables. Perceptual and Motor Skills, 17, 187–209. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Brown K., Baird B., Schooler J.W. (2012). Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Research, 1428, 60–70. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Brown K.S., Tipper C., et al. (2011a). Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLoS One, 6, e18298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J., Fishman D.J., Schooler J.W. (2007). Counting the cost of an absent mind: mind wandering as an underrecognized influence on educational performance. Psychonomic Bulletin and Review, 14, 230–6. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Nind L., O’Connor R.C. (2009). When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Consciousness and Cognition, 18, 118–25. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Ruby F.J., Singer T. (2013). Letting go of the present: mind-wandering is associated with reduced delay discounting. Consciousness and Cognition, 22, 1–7. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J.W. (2006). The restless mind. Psychological Bulletin, 132, 946–58. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J.W. (2015). The science of mind wandering: empirically navigating the stream of consciousness. Annual Review of Psychology, 66, 487–518. [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J.W., Turk D.J., Cunningham S.J., Burns P., Macrae C.N. (2011b). Self-reflection and the temporal focus of the wandering mind. Consciousness and Cognition, 20, 1120–6. [DOI] [PubMed] [Google Scholar]
- Smeekens B.A., Kane M.J. (2016). Working memory capacity, mind wandering, and creative cognition: an individual-differences investigation into the benefits of controlled versus spontaneous thought. Psychology of Aesthetics, Creativity, and the Arts, In press. doi: 10.1037/aca0000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Grady C.L. (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22, 1112–23. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D., D’Argembeau A. (2015). Neural correlates of personal goal processing during episodic future thinking and mind-wandering: an ALE meta-analysis. Human Brain Mapping, 36, 2928–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D., Majerus S., Catale C., D'Argembeau A. (2014). Relationships between mind-wandering and attentional control abilities in young adults and adolescents. Acta Psychologica, 148, 25–36. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D., Majerus S., Maquet P., D’Argembeau A. (2011). Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PloS One, 6, e16997.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale J.D., Proctor L., Lloyd C.A., Baddeley A.D. (1993). Working memory and stimulus-independent thought: effects of memory load and presentation rate. European Journal of Cognitive Psychology, 5, 417–33. [Google Scholar]
- Unsworth N., McMillan B.D. (2014). Similarities and differences between mind-wandering and external distraction: a latent variable analysis of lapses of attention and their relation to cognitive abilities. Acta Psychologica, 150, 14–25. [DOI] [PubMed] [Google Scholar]
- Unsworth N., Robison M.K. (2016). Pupillary correlates of lapses of sustained attention. Cognitive, Affective, and Behavioral Neuroscience, 16, 601–15. [DOI] [PubMed] [Google Scholar]
- Wheeler M.A., Stuss D.T., Tulving E. (1997). Toward a theory of episodic memory: the frontal lobe and autonoetic consciousness. Psychonomic Bulletin, 121, 331–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.